Abstract
Objectives
This is the first systematic review of the effectiveness of barcoding practices for reducing patient specimen and laboratory testing identification errors.
Design and Methods
The CDC-funded Laboratory Medicine Best Practices Initiative systematic review methods for quality improvement practices were used.
Results
A total of 17 observational studies reporting on barcoding systems are included in the body of evidence; 10 for patient specimens and 7 for point-of-care testing. All 17 studies favored barcoding, with meta-analysis mean odds ratios for barcoding systems of 4.39 (95% CI: 3.05 – 6.32) and for point-of-care testing of 5.93 (95% CI: 5.28 – 6.67).
Conclusions
Barcoding is effective for reducing patient specimen and laboratory testing identification errors in diverse hospital settings and is recommended as an evidence-based “best practice.” The overall strength of evidence rating is high and the effect size rating is substantial. Unpublished studies made an important contribution comprising almost half of the body of evidence.
Keywords: Barcoding, Comparative Effectiveness Research, Healthcare Quality Improvement, Laboratory Medicine, Laboratory Testing Errors, Patient Identification Systems, Patient Safety, Patient Specimen Identification, Specimen Labeling Errors
1.0 Introduction
Reduction of medical errors has been a major national priority since the publication of the Institute of Medicine report To Err is Human [1]. Patient specimen and laboratory testing identification errors have been reported as the leading cause of laboratory errors [2]. Identification (ID) errors may result in patient harm and are completely preventable. Identifying effective strategies for reducing these errors has been identified as a research priority[3], but there are no systematic reviews available providing evidence of effectiveness for quality improvement practices. The purpose of this article is to provide a systematic review that evaluates whether barcoding practices are effective at reducing patient specimen and laboratory testing identification errors. The answer is provided by applying the CDC Laboratory Medicine Best Practices Initiative’s (LMBP) systematic review methods for quality improvement practices and translating the results into evidence-based guidance [4].
Accurate identification of patients, their specimens and laboratory test results linked to them is essential in all healthcare settings for providing effective, safe, timely, efficient, equitable and patient-centered healthcare. Systems to monitor errors in patient specimen and laboratory testing identification are federally regulated [5], and accurate identification is a nationally recognized patient safety priority [6–11]. Although government, accreditation, patient safety, professional and industry organizations require laboratories to establish and follow policies and procedures to ensure accurate identification from specimen collection to result reporting, the guidance provided is largely based on expert opinion. Typical hospital clinical laboratories are responsible for thousands of tests daily, yet there is considerable uncertainty about how to reduce identification errors, and what quality improvement practices are effective [12]. ID error consequences include incorrect, delayed and/or lack of treatment which may cause injury, disability, death, longer lengths of stay, and higher healthcare costs, as well as other patient harm and diverted resources [13–15]. Accurate identification is particularly essential to the safe transfusion of blood products since ID errors put patients at risk for adverse outcomes from blood incompatibility [14].
Quality Gap: Patient Specimen and Laboratory Testing Identification Errors
ID errors involve incorrect matching of patient, specimen and/or test information, all of which should be unequivocally linked to a correct patient identify throughout the entire testing process [7, 13]. There are many causes of ID errors, most of which are associated with human error and under the control of the laboratory [13]. ID errors lack a standardized definitiona and systems for detecting, reporting, measuring and categorizing them and their consequences among laboratories and health care organizations. They are generally considered underreported as the true frequency includes undetected errors [8, 11]. As a consequence, reported ID error results can vary among organizations due to differences in measurement methods and how effective laboratory and clinical staff are in identifying errors [8, 9], which makes it difficult to arrive at conclusions about the true size and variability of the ID error quality gap.
Reported ID error rates of 1% and less are common [3, 10, 16–20], yet are still considered a serious problem since any error has the potential for serious adverse patient consequences. The lowest rates are associated with transfusion medicine and are usually less than 0.1%, followed by the general pathology laboratory at closer to 1%, but as high as 10% [21–26], with even higher rates found in surgical pathology [8, 13, 27]. While errors rates at or very close to 0% have been documented, the upper end of the range could be as high as 50%. The highest rates[23, 27] have been measured by a prospective, direct observation method using surgical specimen requisitions and container labeling with an extensive list of variables included in the ID error definition. Most detected errors do not harm patients since their detection results in the associated erroneous test reports typically not being released by the laboratory [8, 9, 28].
2.0 Methods
This evidence review followed the “A-6 Cycle” systematic review methods for evaluating quality improvement practices funded by the CDC’s Laboratory Medicine Best Practices Initiative (LMBP) and reported in detail elsewhere [4]. This approach is derived from previously validated methods, and designed to transparently evaluate the results of studies of practice effectiveness to support evidence-based best practice recommendations. A review team conducts the systematic review and includes a review coordinator and staff trained to apply the LMBP methods. Guidance is provided by a multi-disciplinary expert panel including at least one LMBP Workgroup member and individuals selected for their diverse perspectives as well as relevant expertise in the topic area, laboratory management, and evidence review methods.b The results are translated into an evidence-based best practice recommendation by the expert panel for approval by the LMBP Workgroup. These methods as applied in this evidence review of barcoding practices are presented below.
2.1 ASK: Review question and analytic framework
The LMBP methods begin with the ASK step which frames at least one review question supported by an analytic framework and PICO elements (population, intervention/practice, comparator, outcome). The question answered by this evidence review is:
Are barcoding practices effective at reducing patient specimen and laboratory testing identification errors?
This review question is addressed in the context of an analytic framework for the quality issue of patient specimen and laboratory testing identification errors (Figure 1). The relevant PICO elements are:
Figure 1.
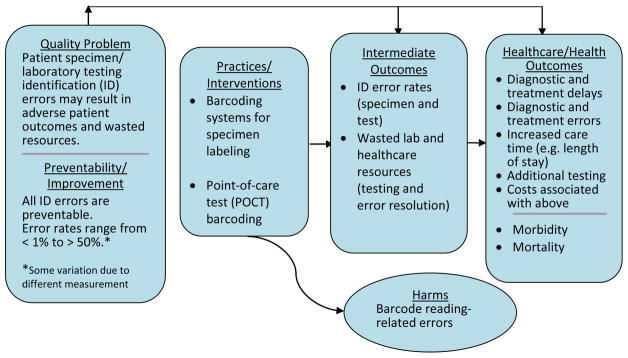
LMBP QI Analytic Framework: Patient specimen and laboratory testing identification errors
Population: all patients in healthcare settings using laboratory or point-of-care testing and their specimens requiring accurate identification for use in a healthcare context
Intervention: barcoding practices defined as laboratory test barcoding systems using barcoded patient identification linked to specimen labels or point-of-care testing
Comparison practice/intervention: non-barcoded identification systems for patients, specimens and laboratory tests
Outcome: specimen and/or laboratory testing identification error rates are the primary and most direct outcome of interest.
The two barcoding practices being evaluated in this review are described below.
Barcoding Systems
Electronic barcoding for identification of patients, specimens and laboratory testing is used to positively establish identification and link specimens and tests to a patient throughout the entire testing process including test ordering, specimen collection, analysis and test result reporting[13]. Barcode scanners are used to confirm patient identity. Other options include barcoded patient wristbands, portable printers to generate labels at the bedside, and use of an interface with a computerized physician order entry (CPOE) system.
Point-of-Care Test Barcoding Systems
Automated patient specimen and laboratory testing identification system use bar-coded patient identification and bar code scanners with a testing device at or close to the patient. Testing devices can interface with laboratory information systems to receive and transmit patient identification and test result information. This practice may include barcoded patient wristbands.
2.2 ACQUIRE: Search for practice effectiveness evidence
The search for studies of barcoding practice effectiveness to reduce patient specimen and laboratory testing ID errors included a systematic search of multiple electronic databases, hand searching of bibliographies from relevant information sources and their bibliographies, provision of references by as well as consultation with experts in the field including members of the expert panel (Appendix A). Additional evidence was obtained by solicitation of unpublished quality improvement studies resulting in submissions to the Laboratory Medicine Best Practices Initiative.c The literature search strategy and terms were developed with the assistance of a research librarian and included a systematic search in August 2011 of three electronic databases (PubMed, Embase and CINAHL) for English language articles from 1995 to 2012 about human subjects. The search contained the following Medical Subject Headings: automatic data processing, blood transfusion, hospitals, laboratories, methods, patient identification systems, patients, and specimen handling as well as these keywords: barcode/bar-code/bar code, labeling errors, laboratory/ies, methods/strategy(ies) reduce patient specimen handling practice/identification errors, patient identification systems errors, pharmaceutical, specimen, and transfusion.
2.3 APPRAISE: Screen and evaluate evidence
The ACQUIRE step search results are reviewed by an initial screening of titles and abstracts using pre-specified inclusion criteria consistent with the ASK step, followed by a full-text review of all eligible effectiveness studies, involving abstracting, standardizing and evaluating study quality using the LMBP methods. Included studies are considered to provide valid and useful information addressing the review question [29] with barcoding effectiveness findings that include at least one ID error outcome measure. To reduce subjectivity and the potential for bias, all screening, abstraction and evaluation is conducted by at least two independent reviewers, and all reviewer discrepancies are resolved through consensus. The effect size for each study was standardized using its reported data and results to calculate an odds ratio (OR)d since the outcome of interest is dichotomous (i.e., correctly identified versus misidentified) and the findings for these practices are typically expressed in terms of rates or percentages. The OR compares the barcoding practice to a non-barcoding practice in terms of the relative odds of a successful outcome (i.e., the patient’s specimen and/or test is correctly identified versus incorrectly identified). Each study is assigned one of three quality ratings (Good, Fair, Poor) and one of three effect size ratings (Substantial, Moderate or Minimal/none).e
2.4 ANALYZE: Evidence review synthesis and results
The individual effectiveness study results from the APPRAISE step are aggregated into two practice-specific bodies of evidence (barcoding systems and POCT barcoding) and then analyzed to produce the systematic review practice effectiveness results for translation into evidence-based recommendations (Recommend, No recommendation for or against, Recommend against). Both qualitative and quantitative analyses are used to assess effect size consistency and patterns of results across studies [30]. Qualitative analysis is used to rate the overall strength of the body of evidence for practice effectiveness (High, Moderate, Suggestive, or Insufficient) Criteria for these ratings are described in detail elsewhere [4, 31]. The qualitative analysis synthesizes the individual studies to convey key study characteristics, results and evaluation findings summarized in a body of evidence table. A quantitative analysis is provided using meta-analysis of the results from similar individual studies to estimate a weighted average effect size and confidence interval using a random-effects modelf with the results presented in a forest plot.
3.0 Evidence review synthesis and results
The ACQUIRE step procedures identified 1,307 separate bibliographic records that were screened for eligibility to contribute evidence of the relation of barcoding with ID error outcomes. The APPRAISE step screening resulted in 1,211 of these records being excluded as off-topic, and 73 being excluded for not meeting effectiveness study inclusion criteria (i.e., not a study, no barcoding practice, no ID error outcome measure) for a total of 23 full-text studies meeting the review inclusion criteria. A systematic review flow diagram in Figure 2 provides a breakdown of the search results. Abstracted and standardized information as well as study quality ratings for the 23 eligible studies are provided in Appendix B containing evidence summary tables preceded by a Body of Evidence table for each practice. Bibliographic reference information for these studies is provided in Appendix C.
Figure 2.
Systematic Review Flow Diagram
The full-text review and evaluation of the 23 eligible studies resulted in the exclusion of 6 studies due to “poor” study quality ratings which did not meet the minimum required LMBP study quality inclusion criteria (4 barcoding systems studies: 3 published and 1 unpublished; 2 point-of-care test barcoding studies: 1 published and 1unpublished). A total of 17 studies are included in this review as evidence of practice effectiveness (8 of which are unpublished submissions): 10 studies for barcoding systems (3 unpublished) and 7 studies for point-of-care test barcoding (5 unpublished). All included studies used observational before-after study designs.
3.1 Barcoding systems practice effectiveness evidence
Of the 10 studies included in the barcoding systems practice body of evidence, 7 were published and 3 were unpublished, and 6 were rated “Good” study quality and 4 were rated “Fair” with summarized information provided in Table 1. The earliest study time period was 1999–2000, and the starting date for 6 of the studies was 2005 or later, with 3 published in 2010. All study sample sizes were very large while the number of identification errors was very small. In all studies both the barcoding and the non-barcoding comparison groups were considerably in excess of 1,000 specimens. All but 2 studies exceeded 10,000 specimens for both groups, and 3 studies exceeded 100,000 specimens. All 10 included studies involved laboratory testing with identification of patient specimens using labels in U.S. hospital settings; 8 studies from clinical pathology laboratories and 2 from surgical/anatomic pathology laboratories (Zarbo 2009 and University of Washington 2009). There was geographic and patient diversity across study settings which included inpatient, outpatient, emergency department and pediatric settings. All hospitals were relatively large, with the smallest exceeding 200 beds. Four studies relied exclusively on inpatient blood specimens and used bedside label printing (Brown 2010, Morrison 2010, LBJ 2009, Unpub A 2009), and two studies relied only on emergency department specimens (Hill 2010, Killeen 2005).
Table 1.
Body of Evidence Summary Table: Barcoding Systems
| Study (Quality and Effect Size Ratings) | Population/Sample | Setting | Time period | Results (Specimen ID Error Rates) |
|---|---|---|---|---|
Bologna 2002
|
All phlebotomies Pre: 69,432 Post: 59,490 |
Valley Hospital, Ridgewood, NJ; >400 beds); 10 care centers | Pre: 1/1999– 12/1999 Post: 1/2000 – 9/2000 |
Incorrect/incomplete specimen label (misidentified) rate: Pre: 0.017% (12/69,432) Post: 0.007% (4/59,490) OR = 2.57 (CI: 0.83 – 7.97) |
Brown 2010
|
Inpatient blood specimens from 6 care units (3 med/surg., intermediate, psychiatry, and obstetrics) a Pre: 456,069 Post: 458,461 |
Howard County General Hospital, Columbia, MD: 227- bed, nonprofit acute care community hospital | Pre: 11/2005 – 10/2006 Post: 11/2006 – 10/2007 |
Specimen labeling error rate (per 10,000 specimens): Pre: 2.26 Post: 0.17 OR = 12.95 (CI: 6.31 – 26.58) |
Hayden 2009
|
All tissue and body fluid test order events (accessions) Pre: 19,247/mo. (1 yr.) Post: 17,793/mo. (1 yr.) |
St. Jude Children’s Research Hospital, Memphis, TN Pediatric oncology hospital |
Pre: 9/2003– 8/2004 Post: 9/2005– 8/2006 |
Mislabeled specimen rate: Pre: 0.032% Post: 0.005% OR = 6.58 (CI: 5.26 –8.22) |
Hill 2010
|
All emergency department specimens sent to any hospital laboratory Pre: 724,465 Post: 334,039 |
Johns Hopkins Hospital, Baltimore, MD; Academic medical center; Adult emergency department | Pre: 9/2004 – 4/2008 Post: 5/2008 – 9/2009 |
Specimen processing error rate (unlabeled, mislabeled, wrong patient specimen or requisition): Pre: 0.42% Post: 0.11% OR = 3.67 (CI: 3.30 – 4.08) |
Kileen 2005
|
All emergency department patient specimens Pre: 22,243 Post: 22,574 |
UCSD Medical Center, San Diego, CA; Academic medical center; Emergency Dept. | Two 6 month periods for Pre and Post – no dates | PSID Error Rate per 1,000 (misidentified, unlabeled, or mislabeled specimens): Pre: 2.56 [CI: 1.94 −3.32] Post: 0.49 [CI: 0.24 −0.87] OR = 5.27 (CI: 2.76 – 10.05) |
Morrison 2010
|
All inpatient phlebotomist blood draws Pre: 181,758 Post: 184,043 |
Brigham and Women’s Hospital, Boston, MA; 777-bed Academic medical center | Pre: 10/2007– 7/2008 Post: 9/2008– 6/2009 |
Labeling error rate per 10,000 (unlabeled and mislabeled): Pre: 5.45 Post: 3.20 OR = 1.70 (CI : 1.23 – 2.35) |
Zarbo 2009
|
All surgical cases/accessions (specimen containers) Pre: 2,694 Post: 2,877 Note: Other outcomes reported require more specimen processingb |
Henry Ford Hospital, Detroit, MI Surgical pathology laboratory gross room |
Two 3- week periods; Pre: 7/2006 Post: 8/2007 |
Surgical cases misidentification rate: Pre: 1.67% Post: 0.63% OR = 2.68 (CI: 1.55 – 4.63) |
| Unpublished | ||||
LBJ 2009
|
LBJ 2009
|
LBJ 2009
|
LBJ 2009
|
LBJ 2009
|
Univ of WA c 2009
|
Univ of WA c 2009
|
Univ of WA c 2009
|
Univ of WA c 2009
|
Univ of WA c 2009
|
Unpub A 2009
|
Unpub A 2009
|
Unpub A 2009
|
Unpub A 2009
|
Unpub A 2009
|
| BODY OF EVIDENCE RATINGS |
# Studies by Quality and Effect Size Ratings 5 Good/Substantial 2 Fair/Substantial 1 Good/Moderate 2 Fair/Moderate |
|||
| Consistency | YES | |||
| Overall Strength | HIGH | |||
Number of blood samples not provided in published article; provided in direct e-mail communication received 7/12/2011 from Susan Neal-Lyman.
Additional measures: specimen parts, tissue cassettes, and histology slide labels
Excluded from meta-analysis
3.1.1 Body of evidence qualitative analysis
As summarized in Table 1, the evidence of practice effectiveness for reducing ID errors indicates consistent improvement associated with barcoding systems compared to non-barcoding practices with a high strength of evidence in hospital settings. The odds ratio for 9 of the 10 barcoding system studies exceeded 2.0 (favoring barcoding), and the 95% confidence interval lower limit exceeded 1.0 for 8 of the 10 studies. The 3 study exceptions (Bologna 2002, Morrison 2010, and LBJ 2009) had the smallest numbers of ID errors in the barcoding and non-barcoding study groups ranging from 0 to 12 which corresponded to very large sample sizes; the smallest being about 25,000 and the rest substantially larger. The odds ratio estimates for all 10 included studies ranged from 1.7 to 147. The unpublished studies’ odds ratios were consistent with those of the published studies with the exception of the uppermost unpublished study odds ratio of 147.
3.1.2 Meta-analysis
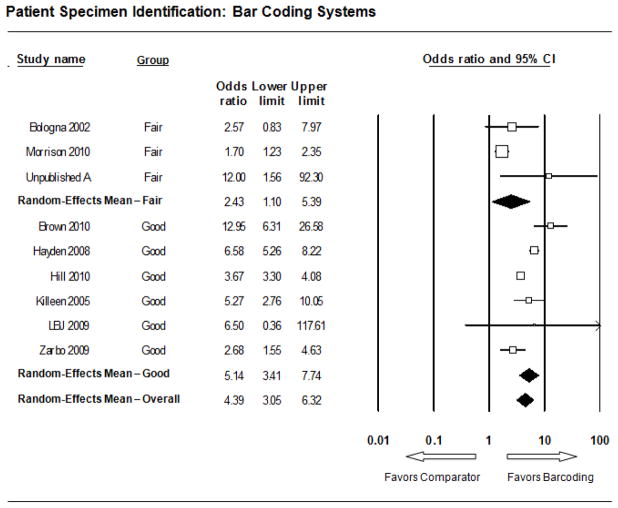
The forest plot in Figure 3 presents the meta-analysis effect size results for barcoding systems. The odds ratio for each of the 9 included studies favors the barcoding system practice over the non-barcoding practice for improving identification error rates indicating a consistent and statistically significant effect. The overall summary effect mean odds ratio was 4.39 (95% CI: 3.05 – 6.32). The higher rated “Good” study quality subgroup summary effect size exceeded that of the lower rated “Fair” quality subgroup with an odds ratio of 5.14 versus 2.43. The lower limit of the confidence interval for both subgroups exceeded 1.0 at 3.41 for the 6 “Good” quality studies but only 1.1 for the 3 “Fair” studies. One of the ten barcoding system practice effectiveness studies was excluded from the meta-analysis (University of Washington 2009) as its ID error rate outcome measure (processed specimen cassettes/blocks rather than specimens in their original containers with labels) and results (OR = 147; 95% CI: 55 – 391) were considered too heterogeneous relative to the other nine included studies.
Figure 3. Meta-Analysis Forest Plot: Barcoding Systems.
Each box represents the mean odds ratio for an individual study indicated to the far left, with the box size proportional to the study sample size. The endpoints of the lines on the left and right sides of the box represent the lower and upper limits, respectively, of the study odds ratio’s 95% confidence interval, with the numerical values provided to the left. The bottom line represents the overall summary effect (or grand mean) for all the studies in the body of evidence along with its confidence interval. In addition, meta-analysis results were tabulated separately for two subgroups using the two study quality ratings “Fair” and “Good.”
Meta-analysis results for barcoding systems show significant statistical heterogeneity which is typical of random effects results. The I2 statistic ranges from 0–100% and estimates the percent of variability in estimates attributable to between study differences. Studies rated “Good” showed somewhat less between-study variation (10.5%) relative to “Fair” studies (15.9%) which had larger estimated odds ratios. This modest attenuation of effect size from “Fair” studies contributes to modest between-study variation (24.8%) in the overall estimate.
3.2. Point-of-care test barcoding practice effectiveness evidence
Of the 7 studies included in the point-of-care barcoding practice effectiveness body of evidence, 2 were published and 5 were unpublished, and 5 were rated “Good” study quality and 2 were rated “Fair” with summarized information provided in Table 2. All of the included studies relied on U.S. hospital inpatient point-of-care glucose tests, with at least 4 studies also including emergency department patient tests. The earliest study time period began at the end of 2002, while the remaining studies began in 2006 or later, with 4 ending in 2011. Like the barcoding systems’ practice effectiveness studies, the point-of-care test barcoding study sample sizes were typically very large with all barcoding and non-barcoding groups exceeding 10,000 tests with one exception (Rao 2005). Several studies had barcoding and/or comparison groups with substantially more than 100,000 tests. Four of the unpublished studies came from separate hospitals within one hospital system (Catholic Health System: Kenmore Mercy Hospital, Mercy Hospital of Buffalo, Sisters of Charity Hospital Buffalo and Sisters of Charity Hospital St. Joseph Campus). As a result, the body of evidence is not as geographically diverse as for barcoding systems, but the study settings may be reasonably representative of diverse hospitalized patient populations.
Table 2.
Body of Evidence Summary Table: Point-of-Care Test Barcoding
| Study (Quality and Effect Size Ratings) | Population/Sample | Setting | Time period | Results (Specimen ID Error Rates) |
|---|---|---|---|---|
Colard 2005
|
All inpatient glucose POCT- approximately 12,000–15,000/mo. (no data provided for study sample) | Saint Luke’s Hospital, Kansas City, MO; 629-bed tertiary care teaching hospital | 11/2002 – 12/2003 Pre: 1 mo. (11/2002) Post: 9 mos. (4–12/2003) |
Patient ID Error rate (monthly) Pre: 9.4% Post: 0.7% OR = 14.72 (CI: 13.47 – 16.08) |
Rao 2005
|
Glucose POCTs for 35 inpatients No Barcoding: 304 Barcoding: 158 |
Massachusetts General Hospital, teaching hospital, > 900 beds | No dates reported No Barcoding: 2 mos. Barcoding: 1 mo. |
Patient ID Error rate No Barcoding: 1.32% Barcoding: 0.00% OR = 4.75 (CI: 0.25 – 88.73) |
| Unpublished | ||||
Geisinger 2009
|
Glucose POCTs for all inpatients; ~18,000/mo.; Baseline sample size not reported (includes ~1/3 barcoding); Barcoding: 106,780 | Geisinger Medical Center, Danville, PA; teaching hospital, > 300 beds. | 1/2004 and 1–6/2009 Baseline: 1 mo. (2004) Barcoding: 6 mos. (2009) |
Patient ID Error rate Baseline: 2.9% Barcoding (full implementation): 0.5% OR = 5.94 (CI: 5.26 −6.71) |
Kenmore Mercy 2011
|
All hospital inpatient and Emergency Dept. POC glucose tests Pre: 79,437 Post: 184,491 |
Kenmore Mercy Hospital, Kenmore, NY; teaching hospital; 100–300 beds | 1/2007 – 5/2011 Pre: 16 mos. Post: 37 mos. |
Patient ID Error rate Pre : 2.16% Post: 0.57% OR = 3.85 (CI: 3.56 – 4.16) |
Mercy Buffalo2011
|
All hospital inpatient and Emergency Dept. POC glucose tests Pre: 249,667 Post: 517,744 |
Mercy Hospital of Buffalo, Buffalo, NY; teaching hospital, >300 beds | 1/2007 – 5/2011 Pre: 17 mos. Post: 36 mos. |
Patient ID error rate Pre : 2.24% Post: 0.44% OR = 5.23 (CI: 4.98 – 5.50) |
Sisters Buffalo 2011
|
All hospital inpatient and Emergency Dept. POC glucose tests Pre: 120,718 Post: 259,787 |
Sisters of Charity Hospital, Buffalo, NY; teaching hospital; 100– 300 beds | 1/2007 – 5/2011 Pre: 17 mos. Post: 36 mos. |
Patient ID error rate Pre : 1.56% Post: 0.42% OR = 3.75 (CI: 3.48 – 4.04) |
Sisters. St. Joseph 2011
|
All hospital inpatient and Emergency Dept. POC glucose tests Pre: 44,932 Post: 182,150 |
Sisters of Charity Hospital St. Joseph’s Campus, Cheektowaga, NY; teaching hospital; 100– 300 beds | 1/2007 – 5/2011 Pre: 11 mos. Post: 42 mos. |
Patient ID error rate Pre : 3.22% Post: 0.54% OR = 6.09 (CI: 5.61 – 6.60) |
| BODY OF EVIDENCE RATINGS |
# Studies by Quality and Effect Size Ratings 5 Good/Substantial 1 Fair/Substantial 1 Fair/Moderate |
|||
| Consistency | YES | |||
| Overall Strength | HIGH | |||
3.2.1 Body of evidence qualitative analysis
As summarized in Table 2, the evidence of practice effectiveness for reducing identification errors indicates consistent and substantial improvement for point-of-care test barcoding compared to non-barcoding (manual entry) practices with a high strength of evidence for point-of-care glucose testing in hospital settings. The point-of-care barcoding practice odds ratio for all 7 of the included studies exceeded 2.0 (favoring the barcoding practice over non-barcoding practices), and the lower limit of the odds ratios’ 95% confidence interval exceeded 1.0 for 6 of the 7 studies. The one study exception (Rao 2005) had the smallest numbers of errors (4 without barcoding; 0 with barcoding) and the smallest total sample size (462) of the included studies. The odds ratio estimates for all the included studies ranged from 3.76 to 14.72.
3.1.2 Meta-analysis
The forest plot in Figure 4 presents the meta-analysis results for the point-of-care test barcoding practice. The overall summary effect mean odds ratio was 5.93 (95% CI: 5.28 – 6.67). The 5 higher rated “Good” quality studies’ subgroup summary effect size was similar: mean odds ratio of 5.83 (95% CI: 3.86 – 8.82). Of the 7 included studies, only one had an odds ratio 95% confidence interval lower limit less than 1.0 (Rao 2005), reflecting the very small number of identification errors in the study (4) as well as a relatively small sample size (462). These meta-analysis results show significant statistical heterogeneity as is typical of random effects results. Most of the point-of-care test barcoding meta-analysis results’ statistical heterogeneity is attributable to within-study variance. The higher rated “Good” study quality subgroup showed modest between-study variation (I2 = 27.8%) while all between-study variation in the fair and overall results can be attributed to chance. At the aggregate level, results for “Fair” and “Good” studies are essentially indistinguishable and can be considered well represented by the overall mean estimate.
Figure 4.
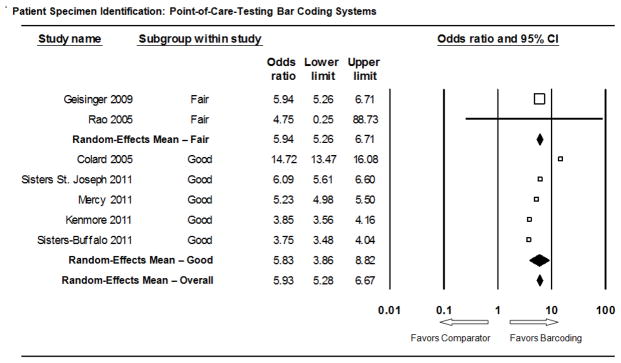
Meta-Analysis Forest Plot: Point-of-Care Test Barcoding
4.0 Discussion
4.1 Additional considerations
4.1.1 Applicability
Barcoding practices demonstrated effectiveness at reducing ID errors for patient specimen and laboratory testing identification is generalizable to most common hospital settings and patient populations, to clinical pathology laboratory testing, and potentially to surgical/anatomic pathology laboratory testing for which there was more limited evidence [28, 32, 33]. Although barcoding effectiveness studies for non-hospital settings and point-of-care testing other than glucose were not included in the body of evidence reviewed, barcoding may be similarly effective at reducing ID errors but no evidence was available to test this hypothesis. Cost, technological requirements and training may be barriers to adoption of barcoding in some non-hospital settings, as well as hospital settings, however this is not clearly supported. In relatively low volume testing settings, limited economies of scale for barcoding technology may present a cost barrier. Non-hospital settings generally do not rely on patient identification armbands typically used in hospital inpatient and emergency departments, but patients may have specimen container or test requisition form labels with identification information that can be barcoded.
4.1.2 Harms
Barcoding technology is not error free. ID errors associated with barcoding practices include those created by the patient identification barcodes themselves [34]. Barcode scanners may misread patient identification barcodes due to incompatibility between the barcode print area size or symbology on patient ID bands or specimen labels with scanner settings. In one study a small number of scanner misreads occurred due to the narrow wrist band curvature of pediatric patients [35]. Other sources of barcoding ID errors included labels being unreadable by a scanner due to label print quality problems, which may indicate a need for label printer maintenance, and degradation of the barcode on the patient ID band from being worn or written on. Studies and articles have also reported scanner failure attributable to low batteries [36]. Even when the scanner works properly, incorrect information such as the wrong patient’s barcode, incorrect barcode information from a patient’s ID band, or non-patient identification barcodes (e.g., medication) can cause ID errors [37, 38]. A specific type of ID error is from scanning incorrect barcode information from a patient ID band with a barcode related to a previous hospitalization or a hospital transfer. Such an episode could include more than one armband and/or multiple patient accounts [37, 39]. Although many potential sources of ID errors associated with barcoding have been identified, these errors appear relatively rare, generally preventable, and likely have only a negligible impact on ID error rates.
4.1.3 Additional benefits
The studies reviewed report other beneficial outcomes associated with barcoding including an observed reduction in misidentified patients [40], unnecessary phlebotomy [40], labor time savings and reduced workflow process time in surgical pathology [32, 33]. Implementing barcoding has been credited with improving identification of those responsible for making ID errors, thus enabling targeted measures to improve performance [37, 40]. Cost savings noted from fewer ID errors associated with barcoding include reductions in specimen recollections, labor to investigate and correct ID errors, length of patient stays and legal issues [41]. Additional benefits to patients from fewer ID errors include avoiding unnecessary discomfort, inconvenience, and treatment delays from recollecting and retesting specimens [40, 42].
4.1.4 Economic evaluation
No patient specimen barcoding practice economic evaluations (cost, cost-effectiveness, or cost-benefit analyses) were found in the search results described in section 2.2.g Completing a resource-related inventory for barcoding practices is beyond the scope of this study but it should include the costs associated with implementing and sustaining the practice (e.g., hardware, software, equipment, supplies and labor requirements as well as resources associated with training, testing, monitoring, and maintenance) and all downstream costs and savings that occur because the intervention was performed [43].
4.1.5 Feasibility of implementation
The evidence reviewed clearly demonstrates the feasibility of adopting barcoding practices in a variety of hospital settings. Nevertheless, each environment is distinctive and implementation requires adequate process development and modification, training, education and testing to achieve full effectiveness. Barcoding process design issues appear more complex for surgical pathology [32, 44] which typically involves more workflow process steps than patient specimens for routine laboratory or point-of-care testing. Many studies on surgical pathology describe the approach used for barcoding-related process changes in detail, along with the accompanying challenges and solutions [32, 36–38, 40, 42, 45]. Key implementation components for making barcoding technology work as intended include adequate training and education, well-designed patient ID bands, and adequate supplies and equipment maintained in good working order (e.g., label printers, computers, batteries, wireless networks) [13]. Shortages and performance issues were noted as problems frustrating staff that can result in using error-prone work around processes [42]. Support and involvement from all relevant departments and leaders including nursing, laboratory and information systems, were identified as critical success factors since no one department typically has full ownership of implementing and using barcoding technology.
4.2 Future research needs
Standardized outcome measures and measurement methods that consistently and reliably detect ID errors are needed for robust evaluation and comparison of QI practices. For more complete and useful assessment of barcoding practices, studies are needed to address its applicability and effectiveness in ambulatory and non-hospital settings, as well as more research evaluating barcoding in surgical pathology and settings known to have relatively higher ID error rates (e.g., emergency departments). Cost-effectiveness studies evaluating investments in potentially expensive ID error reduction technologies such as barcoding are needed. There should be a focus on settings with greater potential ID error impact due to higher rates and/or more serious consequences. Addressing this requires well-constructed data collection and analysis efforts identifying and measuring resources needed for implementation and maintenance of barcoding along with outcomes of interest. Future effectiveness research can be more informative if expected barcoding effectiveness moderators including implementation variables or practice components (e.g., electronic order system interface, bedside labeling, different barcode formats) can be evaluated for their contribution to overall effectiveness. Other benefits and harms of barcoding for patient specimen identification have not been well studied or reported and may be unknown. More information on other potential practice effects is needed to evaluate the full range of consequences and to allow for a comprehensive assessment of its net benefit. In addition, more information is needed about how to maintain and enhance the effectiveness of barcoding over time.
4.3 Limitations
The LMBP systematic review methods are consistent with practice standards for systematic reviews[30], but all such methods are imperfect and include subjective assessments at multiple points that may produce bias. Like most systematic reviews, this one may be subject to publication bias, although this review includes unpublished studies which may mitigate that bias. The restriction to English language studies to satisfy the requirement of multiple reviewers for each study may also introduce bias if barcoding practices differ substantially in international settings. Quality improvement efforts typically differ from research, and are commonly observational studies that rely on natural experiments in realistic practice settings. The major drawback of these uncontrolled designs is that it is not possible to know if measured or unmeasured factors affect the outcomes of interest. Regardless of study design, by gathering evidence from multiple clinical and organizational settings, systematic reviews provide more useful assessments of the totality of evidence for a given QI practice than individual studies [46].
Barcoding and other technology or practice changes may be easier to measure than individual step process changes that may contribute to observed results. Also, these processes are rarely uniform, and are clearly very different for clinical versus surgical pathology specimens, and for point-of-care testing. While these factors may moderate study findings and the observed heterogeneity suggests they are not insignificant, it can be observed that all studies reported support for barcoding.
Some studies comprising the barcoding body of evidence involved less than full implementation for all or a portion of the post-implementation period which would have an expected tendency to understate the impact of barcoding on the reduction in ID error rates. In particular, some studies indicated barcoding “scan rates” of substantially less than 100 percent during the post-implementation period such that the effect of a non-barcoding practice (i.e., manual entry of patient identification information) is reflected in a portion of the post-implementation data. This was noted when provided, however as it was not always clearly or consistently reported it could not be used to adjust effect size estimates. As studies were done within a single institution, there may be many site-specific differences that impact their study results. Many studies were missing information including actual study sample sizes, dates for relevant time periods, and practice implementation and setting characteristics. Another perceived limitation is the inclusion of unpublished studies.
Designing and publishing controlled studies are typically not among the primary objectives of individuals collecting and analyzing quality improvement data relevant to laboratory medicine. In the barcoding body of evidence, both the published and unpublished studies had similar limitations. The LMBP experience to date in reviewing and rating study quality for both published and unpublished studies indicates that peer-reviewed journals do not provide assurance of high study quality.
5.0 Conclusion and Recommendation
On the basis of a high overall strength of evidence of effectiveness, barcoding systems for specimen labeling and point-of-care test barcoding are recommended as best practices to reduce identification errors and improve the accuracy of patient specimen and laboratory testing identification in hospital settings. The high overall strength of evidence is due to sufficient evidence of practice effectiveness from individual studies demonstrating consistent and substantial reduction in patient specimen and laboratory testing-related identification error rates in hospital settings. The findings of barcoding effectiveness are based on 10 studies of specimen barcoding systems and 7 studies of point-of-care test barcoding assessing impact on identification errors. In every study barcoding is associated with a reduction in the identification error rate. The meta-analysis overall summary effect mean odds ratio favoring barcoding is 4.39 (95% confidence interval: 3.05 – 6.32) for barcoding systems and 5.93 (95% confidence interval: 5.28 – 6.67) for point-of-care test barcoding. There was limited evidence of additional benefits and potential harms associated with the use of barcoding for specimen and laboratory testing identification, and any effect of potential harms appears to be very small relative to its overall benefits. All included studies were conducted in hospital settings. No evidence was available for assessing the effectiveness and applicability of barcoding in other laboratory testing settings.
Acknowledgments
Melissa Gustafson of Battelle, Devery Howerton, Malaika Washington, and Barbara Zehnbauer of the Centers for Disease Control and Prevention, the LMBP Patient Specimen Identification Expert Panel and LMBP Workgroup members, and Submitters of unpublished studies
ABBREVIATIONS
- CDC
U.S. Centers for Disease Control and Prevention
- CI
Confidence interval
- ID
Identification
- IOM
Institute of Medicine
- LMBP
Laboratory Medicine Best Practices Initiative
- QI
Quality improvement
GLOSSARY
- Bias
systematic error; threats to validity; tendency to produce results that depart systematically from the ‘true’ results. Unbiased results are internally valid. Four types of bias are selection/allocation, performance, measurement/detection and attrition/exclusion.
- Consistency
The degree to which estimates of effect for specific outcomes are similar across included studies.
- External validity
Generalizability, applicability – extent to which the effects observed in the study are applicable outside of the study to other populations and settings.
- Effect size
A value which reflects the magnitude of the difference in a study’s outcome measure between the group with the intervention/practice being evaluated and its control or comparison group.
- ID errors
Misidentification in matching a patient or a specimen with a laboratory test. ID errors may include specimen/test requisition mismatches (e.g., specimen labeled with another patient’s name, wrong type of specimen, duplicate orders or specimens), mislabeled specimens (sometimes referred to as “wrong blood in tube”), specimen label with partial, missing or incorrect information (e.g., one of two patient identifiers, missing or wrong patient gender, date of birth or middle initial), and unlabeled specimens.[9, 12] Different institutions may use different denominators when expressing ID errors as a rate (e.g., number of specimens, phlebotomies, requisitions, accessions). Some ID error types are more likely to be detected by the laboratory than others (e.g., mismatch versus wrong blood in tube), with those detected typically preventing the release of a test result.[8] Most ID errors are the result of human error, and causes include but are not limited to: laboratory tests ordered on the wrong patient, incorrect or incomplete entry of patient data in the laboratory information system, collection of specimens from the wrong patient, inappropriate labeling of specimens, multiple users of the same label printer, lost identification label on specimens, incorrect identification information on specimen labels, pre-printed labels from different patients, handwritten labels on specimen containers, tissue cassettes and slides, and incorrect entry of patient results in the laboratory information system.[13]
- Internal validity
extent to which the design and conduct of the study are likely to prevent systematic error. Internal validity is a prerequisite for external validity.
- Meta-analysis
The process of using statistical methods to combine quantitatively the results of similar studies in an attempt to allow inferences to be made from the sample of studies and be applied to the population of interest.
- Odds ratio
-
The ratio of two odds of an event from two groups - a treatment or intervention group (a/c) versus a control group (b/d) where a and c represent the number of times the event occurs for the intervention and control group, respectively, using the formula below and the barcoding and comparison practice example table. An OR =1 means the two practices are equally successful (no difference in reducing risk with respect to the outcome evaluated); OR >1 means the barcoding practice is more successful; and OR < 1 means the barcoding practice is less successful. ;
Where pa = a/(a + b), pc = c/(c + d) and a, b, c, and d are proportions in the table below.
Frequencies Proportions Success Failure Success Failure Barcoding Practice a b pa = a/(a + b) pb = b/(a + b) Comparison Practice c d pc = c/(c + d) pd = d/(c + d) - Systematic review
A scientific investigation that focuses on a specific question and that uses explicit, planned scientific methods to identify, select, assess, and summarize the findings of similar but separate studies. It may or may not include a quantitative synthesis of the results from separate studies (meta-analysis).
- Transparency
Methods are explicitly defined, consistently applied, and available for public review so that observers can readily link judgments, decisions, or actions to the data on which they are based. Allows users to assess the strengths and weaknesses of the systematic review and associated guidance and recommendations.
Appendix A. LMBP Patient Specimen Identification Expert Panel Members
Corrine Fantz, PhD (Emory University School of Medicine)
Denise Geiger, PhD (John T. Mather Hospital)
David Hopkins, MD, MPH (Centers for Disease Control and Prevention)
Julie Gayken, MT(ASCP)* (Regions Hospital)
Steven Kahn, PhD (Loyola Medical Center of Chicago)
James Nichols, PhD*(Bay State, Tufts University)
Stephen Raab, MD, PhD* (University of Washington)
Ronald Schifman, MD, MPH (Tucson Veterans Administration Health Center)
Milenko Tanasijevic, MD, MBA* (Harvard Medical School, Brigham and Women’s Hospital, Dana Farber Cancer Institute)
Paul Valenstein, MD, FCAP (St. Joseph Mercy Hospital)
APPENDIX B. Laboratory Medicine Best Practices Body of Evidence Table
TOPIC AREA: Patient Specimen Identification
Practice: Barcoding Systems
| Practice: Bar Coding Systems |
Study Quality Rating | Effect Size Rating | Overall Consistency |
Overall Strength of Body of Evidence |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Practice | Measures | Results | Total | Rating | ||||
|
| |||||||||
| Published | |||||||||
| Bologna 2002 | 3 | 2 | 1 | 1 | 7 | Fair | Moderate | 5 Studies = Good/Substantial | |
| Brown 2010 | 2 | 2 | 2 | 3 | 9 | Good | Substantial | ||
| Fabretti 2011 | 2 | 2 | 0 | 1 | 5 | Poor | N/A | ||
| Hayden. 2008 | 3 | 2 | 1 | 2 | 8 | Good | Substantial | 2 Studies = Fair/Substantial | |
| Hill 2010 | 2 | 2 | 2 | 3 | 9 | Good | Substantial | ||
| Killeen 2005 | 3 | 2 | 1 | 3 | 9 | Good | Substantial | 1 Study = Good/Moderate | |
| Morrison 2010 | 2 | 2 | 2 | 1 | 7 | Fair | Moderate | ||
| Sandler 2005 | 0 | 1 | 1 | 0 | 2 | Poor | N/A | 2 Studies = Fair/Moderate | |
| Turner 2003 | 1 | 1 | 1 | 1 | 4 | Poor | N/A | ||
| Zarbo 2009 | 2 | 2 | 2 | 3 | 9 | Good | Substantial | 4 Studies = Poor - Excluded | |
| Unpublished | |||||||||
| LBJ 2009 | 2 | 2 | 2 | 2 | 8 | Good | Moderate | ||
| U of MN 2009 | 1 | 2 | 2 | 0 | 5 | Poor | N/A | ||
| U of WA* | 2 | 2 | 2 | 1 | 7 | Fair | Substantial | ||
| Unpub A 2009 | 1 | 2 | 1 | 2 | 6 | Fair | Substantial | ||
| *not in meta- analysis | Yes | High | |||||||
|
Bibliographic Information - Author (s) - Yr Published/Submitted - Publication - Author Affiliations - Funding |
Study - Design - Facility/Setting - Time Period - Population/Sample - Comparator - Study bias |
Practice - Description - Duration - Training - Staff/Other Resources - Cost |
Outcome Measures - Description (s) - Recording method |
Results/Findings - Type of Findings - Findings/Effect Size - Stat. Significance/Test(s) - Results/Conclusion Bias |
|---|---|---|---|---|
| Bologna LJ and Mutter M - 2002 - J Healthcare Information Manag. - The Valley Hospital, Ridgewood NJ - Funding: not reported |
- Design: Before-After - Facility/Setting: Valley Hospital, Ridgewood, NJ; > 400- bed community hospital - Time period: 1/1999 – 9/2000 Pre: 1 year (1/1999–12/1999) Post: 9 mos. (1/2000–9/2000) Population/Sample: Number of phlebotomies for 10 units: Pre: 69,432 Post: 59,490 - Comparator: Combination of print and hand-written labels used - Study bias: None noted |
-Description: Barcoding system with portable label printer; labels printed at bedside - Duration: 9 months (1/2000 – 9/2000); ongoing - Training: not discussed - Staff/Other resources: Nursing supervisor, licensed medical practitioners, phlebotomists; portable label printers, BD System Software; bar-coded wristbands - Cost: not reported |
- Description: Error Rates (1) Incorrect/incomplete specimen label (misidentified specimen) (2) Misidentified patients (3) Unnecessary phlebotomy - Recording Method: Pre: Not reported Post: Barcoding system automatically collects via built in reporting capabilities |
- Pretest-Posttest - Findings/Effect Size: Error Rates (10 care centers: (1) Incorrect/incomplete specimen label (misidentified specimen): Pre: 0.017% (12/69,432) Post: 0.007% (4/59,490) Absolute decrease: 0.01% Relative decrease: 58.8% ➢ OR = 2.57 (CI: 0.83 – 7.97) (2) Misidentified patients: 94% reduction, pretest: 0.049% (34/69,432) posttest: 0.003% (2/59,490) ➢ OR = 29 (CI: 4 – 212) (3) Unnecessary phlebotomy: 89% reduction pretest: 0.027% (19/69,432) posttest: 0.003% (2/59,490) ➢ OR = 16.01 - Statistical Significance/Test(s): not discussed - Results/conclusion biases: None noted |
|
Quality Rating: 7 (Fair) (10 point maximum) Effect Size Magnitude Rating: Moderate (Relevance: Direct) |
Study (3 pts maximum): 3 | Practice (2 pts maximum): 2 | Outcome measures (2 pts. maximum): 1; Recording method: Not reported for pre period | Results/findings (3 pts maximum): 1; Appropriateness of statistical analysis: Pre and Post estimates appear to rely on different recording methods |
| - Brown JE [1], Smith N [1], Sherfy B. [2] - 2010 - Journal of Nursing Care Quality [1] Howard County General Hospital, member of Johns Hopkins Medicine [2] University of Maryland Medical Center - Funding: Self-financed *Additional study sample size information not in publication. Source: E-mail communication 7/12/2011 from Susan Neal-Lyman, Johns Hopkins Dept. of Pathology at Howard County General Hospital) |
- Design: Before-after - Facility/Setting: Howard County General Hospital, Columbia, MD: 227-bed, nonprofit acute care community hospital – 6 inpatient units (3 med/surg, intermediate care, psychiatry, and obstetrics) - Time Period: 11/2005 – 10/2007 Pre: 1 yr. (11/2005 – 10/2006) Post: 1 yr. (11/2006–10/2007) - Sample: Inpatient blood specimens – totals* Pre: 456,069 Post: 458,461 - Comparator: Decentralized phlebotomy system with patient care technicians (PCTs) and RNs printing specimen labels at nurse’s station central printer; implemented methods and education to decrease errors. - Study bias: Barcoding implemented in one unit and after one month the other 5 units in post period one noted |
- Description: Specimen positive patient identification system integrated with hospital, lab and physician order entry information systems for printing barcoded specimen labels at the bedside using a portable label printer generated from laboratory orders entered from an order management system -Duration: 12 mos. - Implemented 11/2006 in limited number of units; subsequently expanded hospital-wide over 3 years; ongoing -Training: 1 hour staff training, one week of 24-hour support during implementation, and “extensive” training/educational program -Staff/Other Resources Staff: Patient care technicians (PCTs) and registered nurses Other resources: portable label printers, portable handheld or beside computer including a barcode scanner, patients’ barcoded Identification band - Cost: Not reported |
- Description: Specimen labeling errors/rate (# and %): Wrong patient name or specimens with multiple patient names in one specimen bag Additional qualitative benefits (no data): - Avoiding unnecessary patient discomfort and inconvenience for redrawing specimens - Preventing treatment delays - Eliminating nursing and laboratory staff rework - Recording method: Adverse event reports for specimen labeling errors entered by nursing and lab personnel; paper event- reporting system 2005- 3/2007; afterward web- based reporting |
- Pretest-Posttest - Findings/Effect Size specimen labeling errors per 10,0000 specimens Pre: 2.26 (103/456,069 = 0.0226%) Post: 0.17 (8/458,461 = 0.0017%) Absolute decrease: 0.02% Relative decrease: 92.5% ➢ OR = 12.95 (CI: 6.31 – 26.58) - Stat. Significance/Test(s): None provided in published article for error rates as calculated above using total specimens; Mann-Whitney U Test showed a significant difference in the mean number of errors for the 2 periods (p <.001) - Results/Conclusion Bias: None |
|
Quality Rating (10 point maximum): 9 (Good) Effect Size Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 2; Potential study bias - Barcoding implemented in one unit and then after one month the other 5 units in post period | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 3 |
| Fabbretti G. - 2010 - Pathologica - Infermi Hospital. Rimini, Italy. - Funding: Self-funded |
- Design: Observational, Non- comparative. - Facility/Setting: Infermi Hospital. Rimini, Italy. Pathology Lab.; no additional information reported - Time period: 2009 Post only: 1 year (dates not specified) Population/Sample: Histo-cytological samples. Post: 34,932 - Comparator: Handwritten request forms. - Study bias: None noted |
-Description: Database and lab information system integrated using HL7 messages for patient ID data to be acquired from hospital records and transferred to local database. Examination phase: ID code obtained using barcode reader and automatically printed on cassette. Cutting phase: ID code printed directly on slide. - Duration: 5/2008–2009; ongoing - Training: 2-months immediately preceding implementation. Given in classrooms equipped with PCs to medical and paramedical staff who work in OR; other staff followed. - Staff/Other resources: 8 doctors, 4 biologists, 14 biomedical technicians, 2 admin assistants. IT Dept, Pathology Lab, medical, nursing and administrative staff of various hospitals in Rimini. Two databases and lab information system. - Cost: Not reported |
- Description: Error Rates (1) Misinterpretation of handwritten data on request forms and sample labels (2) Mismatch of patient and/or specimen - Recording Method: Pre: Manual data transcription and handwritten requests forms. Post: Request and labels data recorded twice (printed by machine and on barcode). System identifies patient access by date and test type. |
- Noncomparative - Findings/Effect Size: Error Rate: 0.27% (94/34,932) - Statistical Significance/Test(s): Not reported - Results/conclusion biases: None noted |
|
Quality Rating: 5 (Poor*) (10 point maximum) Effect Size Magnitude Rating: (Relevance: Direct) *0 Outcome Measure rating |
Study (3 pts maximum): 2 - Study Setting: Sufficiently distinctive that results may not be generalizable to other settings/specimens – Surg. path. specimen cassettes |
Practice (2 pts maximum): 2 | Outcome measures (2 pts. maximum): 0; Face Validity -Outcome measure confounded by practice itself (no comparison) | Results/findings (3 pts maximum;: 1; Appropriateness of statistical Analysis- Insufficient data to allow/verify calculation of effect size without comparison practice |
| Hayden RT, Patterson DJ, Jay DW, Cross C, Dotson P, Possel RE, Srivastava DK, Mirro J, and Shenep JL. - 2008 - Journal of Pediatrics - St. Jude’s Children’s Research Hospital (multiple departments), Memphis, TN, USA - Funding: Partly self- financed; and supported by the American Lebanese Syrian Associated Charities |
- Design: Before-after - Facility/Setting: St. Jude Children’s Research Hospital, Memphis, TN ; pediatric cancer center - Time period: 9/2003 – 8/2006; 36 months Pre: 1 year (9/2003 – 8/2004) Staged implementation: 1 year Post: 1 year (9/2005 – 8/2006) - Population/Sample: Accessions of all tissue and body fluid specimens (test ordering events) approximating number of labeled containers. Excludes samples collected during system downtime, off site and by cardiac arrest team. Pre: 19,247 mean accessions per month (1 year) Post: 17,793 mean accessions per month (1 year) - Comparator: Not reported - Study bias: None noted |
- Description: Electronic Positive Patient Identification (EPPID) system with barcoding. Handheld personal digital assistants in each patient, clinic and procedure room with scanner to track and verify clinician entered orders at point of collection; labels printed centrally at nursing station. - Duration: 12 – 24 mos. (staged implementation); ongoing - Training: 3-week training led by nurses. Included in-depth “train-the-trainer” for “super- users; end-user training on routine hardware and software process with hands-on training. Computer-based modules with hands-on training for new staff during employee orientation. - Staff/Other Resources: Nurses (all specimen collections by nursing staff) - Cost: Not reported |
- Description: Mislabeled specimens (# and % of total accessions): mismatches between patient name and. specimen (wrong label used or specimen collected from wrong patient). - Recording Methods: QA data collected (method not specified) based on telephone notifications: 1) nursing alerting lab of labeling error; 2) test results for patients no longer in- house; 3) inquiries to lab about patient results from whom no sample received; 4) lab results discordant with earlier patient results |
- Pretest-Posttest - Findings/Effect Size: Monthly mean mislabeled specimen error rate: Pre: 0.032% Post: 0.005% Absolute decrease: 0.03% Relative decrease: 84.4% ➢ OR = 6.58 (CI: 5.26–8.22) - Statistical Significance/Test(s): p < .001 exact Wilcoxon rank sum test - Results/conclusion biases: None noted |
|
Quality Rating: 8 (Good) (10 point maximum) Effect Size Magnitude Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 3; | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 1; Recording method not specified | Results/findings (3 pts maximum): 2; Appropriateness of statistical analysis: Comparison practice and recording method not specified; may differ for practices |
| Hill PM [1,2], Mareniss D [1,2], Murphy P [2], Gardner H [2], Hsieh Y [1], Levy F [1,2], Kelen GD [1,2] - 2010 - Annals of Emergency Medicine [1] Dept. of Emergency Medicine, Johns Hopkins Univ. School of Med. [2] Johns Hopkins Hospital - Funding: Self-financed |
- Design: Before-after observational cohort study - Facility/Setting: Johns Hopkins Hospital, Baltimore, MD: Large, urban, university- based academic, adult emergency department (ED) with annual census of 57,000 - Time Period: 9/2004 – 9/2009 Pre: 44 mos. (9/2004 – 4/2008) Post: 17 mos. (5/2008 – 9/2009) - Sample: All specimens collected in the ED and sent to any hospital laboratory Pre: 724,465 Post: 345,039 - Comparator: Manual specimen ordering and labeling process; nurse stamps blank labels using embosser with plastic patient id card Study bias: Many post-period errors from manually processed specimens (not barcoded); some from work-arounds, but may include errors that should be excluded (blood bank, tissue, Level 1 trauma and critical care). |
- Description: 2-component intervention: ED electronic documentation and information system integrated with the LIS including physician order entry combined with bar-code technology linked to patient’s identity; physician order entry generates printed barcode specimen labels near patient’s room. Not used for blood bank, tissue, Level I trauma and severe critical care specimens - Duration: 17 months (5/2008- 9/2009); ongoing - Training: Not reported - Staff/Other Resources Staff: Nurses and clinical technicians; Other Resources: Electronic documentation and information system integrated with a laboratory information system and physician order entry system, patient wristbands containing a patient identify barcode, label printers, specimen containers, scanners and labels - Cost: Not reported |
- Description: Specimen processing error rate (total) including 4 separate types: unlabeled/mislabeled/wrong patient specimen or requisition; tabulated monthly -Recording method Monthly monitoring reports from normal quality assurance program clinical pathology information system records. Data from the Pre and Post periods were tabulated monthly. Limitation: Outcome measure includes unlabeled specimens – barcoding practice associated with barcoded labels |
- Pretest-Posttest - Findings/Effect Size: error rate; Pre: 0.42% (3,007/724,465) Post: 0.11% (379/334,039) Absolute decrease: 0.3% Relative decrease: 73.8% ➢ OR = 3.67 (CI: 3.30 – 4.08) - Stat. Significance/Test(s) 0.31% absolute reduction; 95% C.I.: 0.28% to 0.32%; Chi-squared test - Results/Conclusion Bias: None |
|
Quality Rating (10 point maximum): 9 (Good) Effect Size Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 2 Potential study bias - Errors and specimens from non- barcoded specimens not explicitly excluded | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 3 |
| Killeen JP, Chan TC, Jones K, and Guess DA - 2005 - Academic Emergency Medicine - University of California, San Diego, CA, USA - Funding: Self-financed |
- Design: Before-after - Facility/Setting: UCSD Medical Center, San Diego CA, academic medical center emergency department – Time Period: Two 6-month periods (Pre and Post); dates not reported - Sample: All Emergency Department (ED) patients seen during study period (annual census: 40,000) with ancillary ED laboratory tests Total ED laboratory specimens: Pre: 22,243 Post: 22,574 - Comparator: Imprint stamp sticker labels on specimens and paper requisitions -Study limitation: Unclear if 6- month periods being compared are immediately before and after implementation. |
- Description: Barcoding system with electronic requisitions and computerized physician order entry (CPOE). Label printing location not specified. - Duration: 6 months duration; no dates provided -Training: not discussed - Staff/Other Resources: not discussed - Cost: not reported |
- Description: PSID Error Rate - Number of misidentified, unlabeled, or mislabeled specimens per 1,000 specimens - Recording Methods: Prospectively collected – recording method not described |
- Pretest-Posttest - Findings/Effect Size: PSID Error rate Pre : 2.56 per 1,000 [CI: 1.94–3.32] (0.26% = 57/22,243) Post: 0.49 per 1,000 [CI: 0.24–0.87] (0.05% = 11 /22,574) Absolute decrease: 0.2% Relative decrease: 80.8% ➢OR = 5.27 (CI: 2.76–10.05) - Statistical Significance/Test(s): p <.05 ; Chi-square test - Results/conclusion biases: None noted |
|
Quality Rating: 9 (Good) (10 point maximum) Effect Size Magnitude Rating: Substantial (Relevance: Less Direct) |
Study (3 pts maximum): 3 | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 1; Recording method not described | Results/findings (3 pts maximum): 3 |
| - Morrison AP [1,2], Tanasijevic MJ [2], Goonan EM [2], Lobo MM [2], Bates MM[1], Lipsitz SR [1], Bates DW [1], Melanson SEF [2] - 2010 - American Journal of Clinical Pathology - Brigham and Women’s Hospital, Harvard Medical School, Boston, MA [1] Dept. of Medicine [2]Dept. of Pathology, Clinical Laboratories Division - Funding: Self-financed |
- Design: Before-after - Facility/Setting: Brigham and Women’s Hospital, Boston, MA, a 777-bed academic medical center - Time period: 10/2007 – 6/2009 Pre: 10 mos. (10/2007 – 7/2008) Post: 10 mos. (9/2008 – 6/2009) - Sample: All inpatient care phlebotomy service blood collections (about 50% of total inpatient; excludes neonatal ICUs and patients on contact precautions) Pre: 181,758 Post: 184,043 - Comparator: Manually pre- printed patient addressograph labels attached to a correct matched requisition for phlebotomy rounds. - Study Bias: Only phlebotomist collections (15% of ID errors); subsequent use expanded to non-phlebotomists with higher error rates. Post-implementation less than 100% barcoding (reported 85% in 8th month). |
- Description: Barcoding mobile system; handheld computers with barcode scanners, patient barcoded wristbands, mobile printers and integrated wireless radio and interfaced with hospital patient information system. Specimens labeled at bedside (no preprinted labels). No CPOE. - Duration: 18 mos. (8/2008 – 6/2009); ongoing - Training: 1.5 hour group introductory sessions followed by individual training of each phlebotomist accompanied by an experienced user and then individual additional training/education as needed - Staff/Other resources: Initial implementation: 20 handheld systems purchased for a team of 39 inpatient phlebotomists covering 3 daily shifts - Cost: Not reported |
- Description: Incorrectly labeled samples – monthly number and rate: (1) Mislabeled (2) Unlabeled (3) Overall = (1)+ (2) - Recording Method Pre: Lab staff compared patient identifiers on specimen label and requisition form at receipt. Recorded as mislabeled if identifiers did not match or later determined sample from a different patient; without labels recorded as unlabeled. Post: Audit data collected by electronic ID system detected mismatches when scanning patient wristband before specimen collection; created a mismatch alert preventing a wrong- patient sample draw. |
-Pretest-Posttest - Findings/effect size: Overall labeling error rate per 10,000 Pre: 5.45 (95% CI: 4.47 – 6.63) Post: 3.20 (95% CI: 2.48 – 4.14) Absolute decrease: 0.02% Relative decrease: 41.3% ➢ OR = 1.70 (CI : 1.23–2.35) Mislabeled: 43% reduction Pre: 0.030% (55 errors) Post: 0.017% (32 errors) Unlabeled: 38% reduction Pre: 0.024% (44 errors) Post: 0.015% (27 errors) - Stat. Significance Test: Logistic regression used to model rates over time and for statistical significance and confidence interval estimates; before and after changes tested via Wald statistics; p = 0.0013 - Results/Conclusion Bias: None noted |
|
Quality Rating (10 point maximum): 7 (Fair) Effect Size Rating: Moderate (Relevance: Direct) |
Study (3 pts maximum): 2 Potential study bias – phlebotomists only and <100% barcoding may understate effect size. | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2; | Results/findings (3 pts max.): 1; Appropriateness of statistical analysis - Different recording methods pre and post; effect size estimate modeled |
| Sandler SG, Langeberg A, and Dohnalek L. - 2005 - Developmental Biology - Georgetown University Hospital (multiple departments), Washington DC, USA - Funding: Partially from the Greenspring Financial Insurance Limited (GFIL) |
- Design: Non-comparative - Facility: Georgetown Univ. Hospital, Washington, DC; 609-bed, not-for-profit, acute care teaching/research facility. - Study Setting: 18-bed hematology-oncology-bone marrow transplant unit - Time Period: 10/02- no end date provided - Sample: >125 tests, all blood samples and blood components for transfusions. -Comparator: Not reported - Study bias: No time period and number of patients represented by transfusions reported. Focus on nurses who transfuse infrequently. |
- Description: Barcoding system for transfusion linking patients’ wristbands with blood component labels. Consists of the hand-held PC/bar-code scanner with radio frequency port to a portable printer. Duration: 10/02 - ?(no end date) - Training: provided during 1- hour session including written and instruction review on how to use the system. - Staff: Nurses - Cost: not reported |
- Description: (1) Positive Identification rate Percentage of patients, blood samples and blood components for transfusion positively and accurately identified (2) Number of correctly labeled samples - labels for blood sample tubes & certification forms legible with complete information - Recording Method: Electronic medical record |
- Non-comparative time series: - Findings/Effect Size: (1) “All (100%) patients, blood samples, and blood components for transfusion were positively and accurately identified.” (2) “All (100%) bar-code-labeled blood sample tubes and certification forms were legible with complete information. - Stat. Significance/Test(s): None - Results/conclusions biases: Purpose to focus on nurses who transfuse blood infrequently, yet no data presented for these results (suggest that these nurses perform more poorly than nurses who transfuse frequently). Results focused on subjective ratings. |
|
Quality Rating: 2 (Poor) (10 point maximum) Effect Size Magnitude Rating: N/A (Relevance: Direct) |
Study (3 pts maximum): 0; - Study Setting: Sufficiently distinctive that results unlikely generalizable - Bone marrow transplant unit transfusions (−2) - Potential Study Bias: Study time period and sample selection may introduce bias affecting results - Study time period not reported (−1) |
Practice (2 pts maximum): 1; - Adequacy of practice description: Important aspect likely to critically affect implementation of the practice is not well described - No practice duration specified |
Outcome measures (2 pts maximum): 1; Recording method is not adequately described. |
Results/findings (3 pts maximum): 0 - Sample size: Measurement period not reported; sample size likely too small for a robust estimate of practice impact. - Appropriateness of statistical analysis: Data insufficient to allow effect size calculation(non comparative) |
| Turner CL, Casband AC, Murphy MF [1] - Yr Published: 2003 - Transfusion - [1] National Blood Service, John Radcliffe Hospital Oxford, UK - Funding: National Blood Service |
- Design: Observational study - Facility/Setting Oxford Radcliffe Hospital, 1500 bed teaching hospital, Oxford, UK; Setting: Hematology outpatient clinic (later extended to hematology inpatient ward) - Time Period: Not reported - Sample: First unit RBC transfusions: Pre: 51 (48 blood prescribed) Post: 51. (45 blood prescribed) Sample collection: Pre: 30; Post: 30 - Comparator: Standard system without barcoding (checking and administering blood process of 27 steps; sample collection process 17 steps); manual checking/verification of patient wristband and chart information |
- Barcoding system using hand- held computers for scanning barcodes generates barcoded wristbands and labels via portable printer for crossmatch with blood administration process (16 steps) and sample collection and verification process (8 steps) at bedside. - Duration: Not provided - Training: Education/training on transfusion safety and use of the barcode system was provided to staff - Staff: Phlebotomists, blood bank receptionists, IT, blood bank (Note: Staff preferred the new technology once familiar with it) - Cost: Initial equipment/support ~ $1.2 million (US$ 2003) |
- Description: Blood administration % correct performance of blood pack bedside checks (1) Patient ID (name, DOB, sex, hospital #). (2) Cross ref. of blood group, unit #, compatibility label, expiration date, prescription & transfusion report requirements (3) Sample collection: % tubes labeled immediately with hospital #, date, sex, name, DOB - Recording Method: Audits/direct observations |
- Pretest-Posttest: - Findings/Effect Size: (1) Blood admin. patient ID check: Pre: 100% (51 /51) Post: 100% (51 /51) 0% improvement (2) Blood admin. cross reference check: Pre: 9.8% ( 5/51) Post: 41.2% (21 /51) 30.4% improvement p-value : 0.0005 (3) Sample collection labels - patient ID -Pre: 50% (15/30) Post: 100% (30/30) 50% improvement (p-value: <0.0001) - Stat. Significance/Test(s): Exact tests of independent proportions -Biases: Study period not reported, small sample, education and training possible confounders |
|
Quality Rating: 4 ( Poor) (10 point maximum) Effect Size Magnitude Rating: N/A (Relevance: Indirect) |
Study (3 pts maximum): 1; - Study Setting: Sufficiently distinctive that results may not be generalizable – hematology outpatient transfusions - Potential study bias: Time period and sample selection |
Practice (2 pts maximum): 1; - Adequacy of practice description: Important aspect likely to critically affect implementation of the practice is not well described - No practice duration specified |
Outcome measures (2 pts maximum): 1; - Face validity: Process outcomes lack correspondence to evidence review PSID error rate outcome |
Results/findings (3 pts maximum): 1; - Sample size: Measurement period not reported; sample size likely too small for robust estimate. - Uncontrolled deviations: Results not clearly attributable to practice - training may have an impact |
| Zarbo RJ, Tuthill JM, D’Angelo R, Varney R, Mahar B, Neuman C, Ormsby A. - 2009 - Am J Clin Pathol - Department of Pathology, Henry Ford Hospital, Detroit, MI. - Funding: Not reported |
- Design: Before-after - Facility: Henry Ford Hospital, Detroit, MI; anatomic pathology volumes over 80,000 surgical pathology lab cases/year. - Study Setting: Surgical Pathology lab gross room - Time Period: Two 3-week periods: Pre: 7/06; Post: 8/07 - Sample: Surgical cases/accessions (specimen containers): Pre: 2,694; Post: 2,877 Specimen parts: Pre: 4,413; Post: 4,725 Tissue cassettes: Pre: 8,776; Post: 9,167 Histology slides: Pre: 14,270; Post:17,927 - Comparator: Simple-logic, bar-coded slide label only (2006); specimen accessioning and processing completed by manual entry of information and hand written labels on specimen cassettes and slides |
- Barcoding system and process redesign to standardize workflow using a complex- logic, bar-coded pathway tying together 4 work cells to provide computer-readable encoding for identification of parts, and accession, gross dissection, histology/microtomy, and pathology sign-out stations. Also includes manual quality control checks at each station (2007 implementation). No electronic order entry or interface. - Duration: 3 weeks - July 2007 Training: - Group education session, ensuring all staff mem- bers were in unison on the goals and time frame of the data collection and how to use the visual data display - Staff: Surgical pathology, histology and informatics staff - Cost: Not reported |
- Description: Patient specimen identification (PSID) error rates (1) Surgical cases (2) Specimen parts – mismatch between pathology requisition and patient information (3) tissue cassettes – mismatch between cassette ID and lab tag information (4) histology slide labels - Recording Method: Data collected, recorded and defects categorized by 59 surgical pathology personnel ( 21 senior staff and 38 technical staff), using a visual data display collection tool |
- Pretest-Posttest - Findings/Effect Size: PSID error rates (1) Surgical cases/accessions (specimen containers): Pre: 1.67% (45/2,694) Post: 0.63% (18/2,877) Absolute decrease: 1.0% Relative decrease 62.3%; p-value: <.001 ➢ OR = 2.68 (CI: 1.55–4.63) (2) Specimen parts Pre: 0.23% (10/4,413) Post: 0.38% (11/4,725) 0% reduction - not stat. significant (3) Tissue cassettes Pre: 0.057% (5/8,776) Post : 0.055% (5/9,167) 3.5% reduction; not stat. significant (4) Histology slide labels Pre: 0.21%, (30/14,270) Post : 0.01% ( 2/17,927) 95.2% reduction; p-value <.001 - Stat. Significance/Test(s): χ2 tests (Fisher exact test adjusted for small counts and Mantel- Haenszel test) to 2 data sets |
|
Quality Rating: 9 (Good) (10 point maximum) Effect Size Magnitude Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 2; - Study Setting: Sufficiently distinctive that results may not be generalizable to other settings/specimens – Surg. path. workflow redesign |
Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 3 |
LMBP EVIDENCE REVIEW PSID BARCODING SYSTEMS PRACTICE UNPUBLISHED STUDIES
|
Bibliographic Information - Author (s) - Yr Published/Submitted - Publication - Author Affiliations - Funding |
Study - Design - Facility/Setting - Time Period - Population/Sample - Comparator - Study bias |
Practice - Description - Duration - Training - Staff/Other Resources - Cost |
Outcome Measures - Description (s) - Recording method |
Results/Findings - Type of Findings - Findings/Effect Size - Stat. Significance/Test(s) - Results/Conclusion Bias |
|---|---|---|---|---|
| Lyndon B Johnson General Hospital (Heng J) - 2009 - LMBP Unpublished Submission - Lyndon B Johnson General Hospital Core Laboratory, Houston, TX - Funding: Self-financed |
- Design: Before-after - Facility: Lyndon B Johnson hospital, Houston, TX, >300 bed teaching hospital.; > 1,000,000 lab tests annually Study Setting: Laboratory department and all nursing units except ER, ICU, NICU and Outpatient; Lab collects 8,500 specimens/ mo.; 280/day - Time Period: 1/1/2009 – 8/31/2009 Pre: 1/2009 – 4/2009 (4 mos.) Post: 6/2009 – 8/2009 (3 mos.) - Sample: Inpatient blood samples by venipuncture Pre: 41,815 Post: 24,789 - Comparator: Phlebotomists use of a printed draw list and pre-printed specimen label to enter collection information (date/time & ID of patient) - Study bias: Phlebotomists only - low initial/baseline rates |
- Description Barcoding system used by laboratory department only; laboratory phlebotomists print labels from wireless handheld printer and label specimen tubes by the patients’ bedside; in use 24/7. No mention of CPOE. - Duration: 6/1/2009– 10/1/2009; ongoing - Training: Staff training takes 3 hours to learn equipment use - Staff: 20 phlebotomists, IT facility staff for installs and training - Cost: Cost related to training phlebotomists: $14.20 * 3 hours * 20 FTEs =$852. Cost of Collection Manager (hardware, installation, support, and training) = $1 million for district (2 hospitals; 650 and 330 beds respectively). (US$ 2009) |
- Description: Patient specimen identification (PSID) error rate: Number of mislabeled specimens/total number of specimens - Recording Methods: Incident reports Pre: review of occurrence log based on manual forms Post: online application |
- Pretest-Posttest Findings/Effect Size: PSID error rate Pre: 0.012%, (5 / 41, 815) Post: 0.00% (0 / 24,789) Absolute decrease: 0.01% Relative decrease 100.0%; OR = 6.50 (CI: 0.36 117.61) Stat. Significance/Test(s): Proportion successful/ significance not reported Results/conclusion bias: None reported |
|
Quality Rating: 8 (Good) (10 point maximum) Effect Size Magnitude Rating: Moderate (Relevance: Direct) |
Study (3 pts maximum): 2; Potential study bias –Sample selection using phlebotomists only with low initial error rates. | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 2;- Sample sufficiency: Small number of errors reported yields unstable effect size estimate |
| Univ. of Minnesota Medical Center Fairview (Senn C and Bormann P) - 2009 - LMBP Unpublished Submission - Univ. of Minnesota Medical Center Fairview, Acute Care Laboratory - Funding: Self-financed |
- Design: Before-After - Facility/ Setting: UMN Fairview, Minneapolis, MN; >300 bed academic medical center; > 1,000,000 tests/yr. - Study Setting: Clinical lab, EDs, Adult ICUs, Pediatric PCUs - Time Period: Multiple study arms/start dates in various units: Clinical lab: 6/2006–8/2009; Univ. campus ED 7/2006– 8/2009; select PCUs-pilot only: 11/2006–2/2007; Riverside and behavioral ED: 2/2007 –8/2009; Adult ICUs: 2/2008–8/2009. - Sample: 100% of specimen containers; numbers and dates not reported. Total volume: 39,300 / month. - Comparator: No details reported for practice - Study bias: Time period and sample selection methods may introduce bias affecting results |
- Description: Barcoding system for patient ID using hand-held PC to verify specimen labels match wristband prior to labeling specimen tubes at the bedside. No mention of CPOE. - Duration: 3 years; ongoing (staggered implementation as indicated in under Study Period). - Training: ~30 min. for users - Staff: Lab in collaboration with nursing and IT staff to implement; 0.5 FTE maintaining, auditing, problem- solving, validation, etc. - Cost: Start-Up: Design & programming cost: $600,000; hardware : ~ $425,000 Post Start-Up: ~$425,000 for new installations; ~$300,000 for replacement hardware |
- Description: Patient specimen Identification (PSID) error rate: Number of mislabeled specimens, wrong specimen in tube (WSIT) and unlabeled specimens per 10,000 collections - Recording Methods: Pre-implementation: manual error reporting system Post-implementation: electronic reporting system (electronic event tracking logs, and compared to “cancel comments” in lab computer system). Compliance (scan rate) based on monthly 1-day audits of each unit where barcoding implemented. |
- Pretest-Posttest - Findings/Effect Size PSID error rate Units Without Barcoding system: 12.1 errors/10,000 collections. Units with Barcoding system: 0.4 errors/10,000 collections OR = N/A - Stat. Significance/Test(s): None reported Results/conclusion biases: Sample sizes and specific dates not reported. Results not specified by medical unit (i.e., with/without barcoding system vs. those not reported), and different recording methods used for measuring outcomes of practices. |
|
Quality Rating: 5 (Poor:
Results/Findings rating = 0) (10 point maximum) Effect Size Magnitude Rating: N/A (Relevance: Less Direct) |
Study (3 pts maximum): 1; - Potential study bias: Study sample and selection methods may introduce a study bias substantially affecting results - Sample not adequately described and sample selection may be biased (when practice used “compliantly”) | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 0; - Sample sufficiency: Measurement period, # tests and errors not reported (−2) - Appropriateness of statistical analysis: Insufficient data to allow/verify calculation of effect size (−1) and different recording method used for two practices (−2) |
| Univ. of Washington (Schmidt R) - 2009 - LMBP Unpublished Submission - Univ. of Washington, Pathology Laboratory, Seattle, WA - Funding: Self-financed |
- Design: Before-after - Facility: Univ. of Washington, Seattle, WA;,>300 bed academic medical center; > 1,000,000 lab tests annually -Study Setting: Anatomic pathology lab gross room. - Time Period: 12/1/2007 – 8/2009 Pre: 12/2007 11/2008 (1 yr.) Post: 12/2008– 8/2009 (9 mos.) - Sample: Primary cassettes/blocks derived from patient specimens (outpatient biopsies and inpatient surgical) Pre: 85,213 Post: 50,016 - Comparator: Not described - Study bias: None noted for error rate measure. |
-Barcoding system to identify and track all specimens from accessioning to gross station, and just-in-time, single piece workflow system for cassette/block labeling, with specimen barcode read at grossing station and user specifies number of cassettes to produce and then computer prints 2D barcoded cassettes requiring custom software and commercial cassette printers. - Duration: 9 months (12/1/08– 8/2009), ongoing -Training: End User Training for permanent gross room personnel and rotating pathology residents - Staff: Gross room pathologists, path. assistants and residents - Cost: Software custom~$200,000; 4 cassette printers @ $20,000 each = $80,000; Hardware: PCs, barcode readers, label printers, mounting arms = $8,000 (US$ 2008) |
- Description: (1) Primary cassette/ block labeling error rate: # mislabeled specimen cassettes (includes duplicate number, wrong specimen, wrong case, wrong patient) / total pathology specimen cassettes (2) Personnel Savings – estimate of labor hours saved due to practice - Recording Methods: (1) Pre: Estimated from incident reports supplemented by management and user survey Post: Counted directly and documented via incident reports. (2) Survey of gross room personnel – estimates of time saved due to barcoding system |
- Pretest-Posttest - Findings/Effect Size: (1) Cassette/block labeling error rate Pre: 1.16% (988 / 85,213 ) Post: 0.008% (4 / 50,016) Absolute decrease: 1.2% Relative decrease 99.3%; ➢ OR = 147 (CI: 55 – 391) (2) Post-practice: saved 0.75–1.0 FTE gross room personnel (less material handling, less error resolution efforts), not reported over what time period. - Stat. Significance/Test(s): Proportion successful; no statistical analysis/significance reported - Results/conclusion bias: personnel savings based on user recall (no point deduction as bias is for non-effectiveness measure) |
|
Quality Rating: 7 (Fair) (10 point maximum) Effect Size Magnitude Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 2; - Study Setting: Sufficiently distinctive that results may not be generalizable to other settings /specimens – Anat. path. gross room process- derived specimen cassettes | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 1; - Appropriateness of statistical analysis: Different recording methods used during for pre and post periods for measuring and comparing errors (−2) |
| Unpublished Study A – Barcoding System - 2009 - LMBP Unpublished Submission - Large academic medical center, Southern California, USA - Funding: Self-financed |
- Design: Before-after - Facility: Academic medical center in Western U.S.; >300 beds; > 1,000,000 lab tests/yr. - Setting: Inpatient non-ICU units where blood collected by clinical lab phlebotomy team - Time Period: 1/1/06 – 7/31/2009: Pre: 1–12/2006 (1 year); Post: 1/2007–7/2009) - Sample: All phlebotomist inpatient blood specimens (sample size not reported); approx. 33% of 29,000/mo. collected by clinical phlebotomy team (units: neurology, med./ surg., transplant, OB/GYN, oncology, neuropsych, emergency, pediatric, newborn and NICU) Estimated sample: 9,570/mo. (=.0.33 x 29,000); 114,840/year - Comparator: Status quo (no barcode labeling system) |
- Description: Barcoding system with phlebotomists using patient bedside barcode specimen labeling with wireless handheld device and attached mini-barcode label printer. The device can access the patient test orders in real time, collect orders, and print test labels at the patient bedside. CPOE not mentioned. - Duration: 11/06–7/09, ongoing -Training: 3 trainers provided directly from system vendor and 2 FTEs from clinical laboratory IT; no time-related information - Staff: Clinical lab team: 1 FTE phlebotomy supervisor; 20 FTEs for 24/7 blood draws - Cost: Start-up software: $30,000; hardware: $72,000; Annual maintenance: $32,000 (US$ 2006) |
- Description: Annual # of patient specimen Identification (PSID) errors Note: error rate estimated from data provided by submitter as described under “Sample” - Recording Methods: Event reporting system and occurrence management reports/ log |
- Pretest-Posttest - Findings/Effect Size: PSID Error Rate (calculated using above PSID errors using estimated sample size of 114,840/ yr.): Pre: 12/114,840 = 0.010% Post: 1/114,840 = 0.0008% Absolute decrease: 0.01% Relative decrease 92.0% ➢ OR = 12.00 (CI: 1.56 92.3) Total (annual) PSID errors reported Pre (2006): 12 errors Post: 2007: 1 error 2008: 0 errors; 2009: 0 errors (through 7/09). - Stat. Significance/ Test(s): None reported Results/conclusion bias: None noted. |
|
Quality Rating: 6 (Fair) (10 point maximum) Effect Size Magnitude Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 1; Potential study bias: (1) Phlebotomists only (no information non-phlebotomist) (−1); (2) barcoded specimens from 11–12/2006 in Pre period (−1) | Practice (2 pts maximum): 2; | Outcome measures (2 pts maximum): 1; - Recording method: May not accurately capture all instances of the outcome | Results/findings (3 pts maximum): 2; - Sample sufficiency: Small number of errors reported yields unstable effect size estimate |
Laboratory Medicine Best Practices Body of Evidence Table
TOPIC AREA: Patient Specimen Identification
Practice: Point-of-Care Test Barcoding
| Practice: POCT Bar Coding Systems |
Study Quality Rating | Effect Size Rating |
Overall Consistency |
Overall Strength of Body of Evidence |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Practice | Measures | Results | Total | Rating | ||||
|
| |||||||||
| Colard 2005 | 3 | 2 | 1 | 2 | 8 | Good | Substantial | ||
| Nichols et. al 2004 | 2 | 2 | 1 | 0 | 5 | Poor | N/A | ||
| Rao et al. 2005 | 2 | 2 | 1 | 2 | 7 | Fair | Moderate | 5 Studies = Good/Substantial | |
| Unpublished | |||||||||
| Geisinger 2009 | 2 | 2 | 2 | 1 | 7 | Fair | Substantial | 1 Study = Fair/Substantial | |
| Kenmore Mercy | |||||||||
| Hospital 2011 | 3 | 2 | 2 | 3 | 10 | Good | Substantial | 1 Study – Fair/Moderate | |
| Mercy Hospital of | |||||||||
| Buffalo 2011 | 3 | 2 | 2 | 3 | 10 | Good | Substantial | 2 Studies = Poor- Excluded | |
| Sisters of Charity | |||||||||
| Hospital Buffalo 2011 | 3 | 2 | 2 | 3 | 10 | Good | Substantial | ||
| Sisters of Charity | |||||||||
| Hosp. St. Joseph 2011 | 3 | 2 | 2 | 3 | 10 | Good | Substantial | ||
| Unpub B 2009 | 1 | 2 | 2 | 0 | 5 | Poor | N/A | Yes | High |
| Bibliographic Information - Author (s) - Yr Published/Submitted - Publication - Author Affiliations - Funding |
Study - Design - Facility/Setting - Time Period - Sample - Comparator - Study Bias |
Practice - Description - Duration - Training - Staff/Other Resources - Cost |
Outcome Measures - Description (s) - Recording method |
Results/Findings - Type of Findings - Findings/Effect Size - Stat. Significance/Test(s) - Results/Conclusion Bias |
|---|---|---|---|---|
| Colard, DR - 2005 - Point of Care - Department of Pathology, Saint Luke’s Hospital; Kansas City, MO - Funding: not reported |
- Design: Before-after - Facility/Setting: Saint Luke’s Hospital, Kansas City, MO; 629-bed tertiary care teaching hospital for Univ. of Missouri - Kansas City School of Med. - Time Period: 12/2002 – 12/2003 Pre: 1 mo. (11/2002) Post: 9 mos. (4/2003 – 12/2003) Implementation: 4 mos. (12/2002–3/2003); scan rates <75%. - Sample: All inpatient point-of- care glucose tests during study period. No data provided for # tests or tests/mo. Used for study. Before implementation: ~12,000 tests/mo.; at end of study period: >15,000 tests/mo. - Comparator: Manual entry of patient ID and test results with a scripted interface to the LIS. Glucose meter without optional barcode wand. - Study limitation: Possible unexplained sample increase. |
- Description: POCT Barcoding with barcode reader, barcoded patient armbands, direct HL7 interface to LIS. - Duration: 19 mos.; 12/2002– 5/2004; ongoing - Training: Provided to all nursing staff; those with high scan errors received additional training. Due to modifications, additional training required. - Staff/Other Resources: nursing staff (operators); POCT coordinator implement new process change - Cost: Not reported - Additional information: Multiple changes and problems affecting early scan and error rates including change in symbology/reduction in width of bar code (2/2003), new armband with label pouch (3/2003), improper armband assembly, armband assembly training (4/2003). Monthly scan rate ranged from 76% (4/2003) to ≥ 90% (6/2003–12/2003). |
- Description: (1) Patient ID Error Rate - % patient identification error rate for point-of-care blood glucose tests (2) Number of identification errors/unidentified point- of-care blood glucose test results - Recording method: Not described. |
- Pretest-Posttest - Findings/Effect Size (1) ID Error Rate (monthly)* Pre: 9.4% Post: 0.7% Absolute decrease: 8.7% Relative decrease: 92.6% ➢ OR = 14.72 (CI: 13.47 – 16.08) (2) # ID Errors/unidentified test results (monthly) Pre: 404 Post: 25 - Stat. Significance/Test(s): Not reported - Results/Conclusion Bias: Data presented as reported above however after 12/2003 one additional month reported following “additional operator training” - 5/2004: (1) ID Error Rate: 0.18%; (2) 6 errors. Not included as no data reported for 1/2003–4/2004). * Sample size not explicitly reported; effect size calculated based on authors’ monthly test volume estimates in article. |
|
Quality Rating (10 point maximum): 8 (Good) Effect Size Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 3 | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 1; Recording method not adequately described.(−1) | Results/findings (3 pts max.): 2; - Appropriateness of statistical analysis; Does not provide data sufficient to allow/verify calculation of an effect size– sample size (−1) |
| Nichols JH [1,2], Bartholomew C [2], Brunton M [2], Cintron C [2], Elliott S [2], McGirr, J [2], Morsi, D [2], Scott, S, Seipel, J [2] and Sinha D [2] - 2004 - Clinical Leadership Management Review [1] Tufts University School of Medicine [2] Baystate Health System, Springfield, MA. - Funding: Self-financed |
- Design: Before-After - Description: Baystate Health System, integrated delivery network, western MA; 3 hospitals; > 850 beds; (Baystate Med. Ctr., Franklin Med. Ctr., Mary Lane Hospital). - Time Period: 1/ 2002–1/2004 Pre: 10 mos. (1/2002–10/2002) Post: 15 mos. (11/2002– 1/2004) - Sample: All Intensive Care Unit (ICU) blood gas and glucose POCTs for all 3 hospitals; annual system-wide volume ~600,000 glucose and blood gas tests. - Comparator: POCT with manual entry of patient ID and operator code with operator lock out “3-Strike Rule” for ID entry errors (began 6/2002) - Study Bias: Operator problems reading barcoded wristbands in first months -not quantified. |
- Description: POCT Barcoding 5-digit operator code and 9-digit patient account number - Duration: 15 months (11/2002 – 1/2004); ongoing - Training: Not reported - Staff/Other Resources: Nursing and pathology departments - Cost: Not reported |
- Description: # POCT Patient ID errors per month (count): (1) Glucose meter (2) Blood gas - Recording method: POCT devices electronic data collection, POCT program monthly data collection and review as well as POCT coordinators regular compliance monitoring. |
- Pretest-Posttest: - Findings/Effect Size: Identification errors (1)Glucose meter Pre: 26 / month Post: 1 / month (p = 0.0007) (2) Blood gas Pre: 4.6/ month Post: 1.7 / month* (p = 0.048) ➢ OR: N/A due to insufficient data *Included one patient with 11 errors in one month due to scanning barcoded wristband from another hospital - Stat. Significance/Test(s): Not reported; p-values provided - Results/Conclusion Bias: Sample sizes or monthly volumes not provided for ICU POC glucose meter and blood gas tests. |
|
Quality Rating (10 point maximum): 5 (Poor: Results/Findings rating = 0) Effect Size Rating: N/A (Relevance: Direct) |
Study (3 pts maximum): 2 - Potential study bias: Study time period and sample may introduce bias substantially affecting/understating results – Operator problems in post months (−1) | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 1; - Face validity: Monthly error count without test volume is not error rate outcome (−1) | Results/findings (3 pts maximum): 0; - Sample sufficiency: Number of tests not reported (−2); - Appropriateness of statistical analysis: Does not provide data to allow/verify calculation of effect size rate (sample size not reported) (−1) |
| Rao, AC; Burke, DA; and Dighe, AS [1] - 2005 - Point of Care [1] Massachusetts General Hospital - Funding: Self-financed |
- Design: Before-after - Facility/Setting: Massachusetts General, Boston, MA; 900-beds; largest teaching hospital of Harvard Medical School and hospital- based research program - Time Period: No dates reported; 1 month pilot test of barcoding with 2 months of comparator (1 Pretest and 1 Post test) - Sample: 35 inpatients included in pilot test of bar coding only - 462 total glucometry tests: - Pre: 170 (no barcoding) - Pilot: 158 (barcoding) - Post: 134 (no barcoding) - Comparator: Usual Care - POC device with keypad for manual patient ID data entry - Study Bias: Pilot test limited sample |
- Description: POCT Barcoding 2D bar code for patient wristbands; only the medical record number included in the ID bar code which is a unique identifier for each patient. - Duration: 1 month (pilot test only) - Training: Teach nurses, operations coordinators, operations assistants on how to print wristbands and troubleshoot. - Staff/Other Resources: Nurses - Cost: Not reported |
- Desription: Patient ID error rate - % errors in Medical Record Number (MRN) for glucometry tests - Recording method: Verification of MRN; not described |
- Pretest-Posttest - Findings/Effect Size: No Barcoding: 1.32% (4/304)* Barcoding Pilot test: 0% (0/158) *( Pretest and Posttest combined: Pretest: 1.2% (2/170); Posttest: 1.5% ( 2/134)) Absolute decrease: 1.3% Relative decrease: 100.0% ➢ OR = 4.75 (CI: 0.25 – 88.73) (Results for 2 comparator periods pooled) - Stat Significance/ Tests: Difference in error rates was statistically significant by Chi- squared analysis (P<0.005). - Results/Conclusions Biases: Study period is short, 1 month; relatively small sample sizes. |
|
Quality Rating (10 point maximum): 7 (Fair) Effect Size Magnitude Rating: Moderate (Relevance: Direct) |
Study (3 pts maximum): 2; Potential study bias: The study design, time period and sample selection methods may introduce study bias substantially affecting results – limited to 35 inpatients (−1) | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 1; - Recording method not described (-1) | Results/findings (3 pts maximum): 2; - Sample sufficiency: Measurement period and sample size may be too small/insufficient to allow a robust estimate of the impact of a practice (-1) |
LMBP EVIDENCE REVIEW PSID POINT-OF-CARE TEST BARCODING PRACTICE UNPUBLISHED STUDIES
|
Bibliographic Information - Author (s) - Yr Published/Submitted - Publication - Author Affiliations - Funding |
Study - Design - Facility/Setting - Time Period - Population/Sample - Comparator - Study bias |
Practice - Description - Duration - Training - Staff/Other Resources - Cost |
Outcome Measures - Description (s) - Recording method |
Results/Findings - Type of Findings - Findings/Effect Size - Stat. Significance/Test(s) - Results/Conclusion Bias |
|---|---|---|---|---|
| - Geisinger Medical Center (Schuerch C) - 2009 - LMBP Unpublished Submission - Department of Laboratory Medicine, Geisinger Medical Center, Danville, PA - Funding: Self-financed |
- Design: Observational study - Facility/Setting: Geisinger Medical Center, Danville, PA; Teaching hospital; 404 beds Time Period: 1/2004–6/2009 Baseline - early barcoding: 1/2004 (1 mo.)* Barcoding practice (full implementation): 1/2009 – 6/2009 (6 mos.) - Sample: Inpatient point-of- care glucose tests Baseline: ~ 18,000/mo. (avg.) Barcoding: 106,780 - Comparator: Initial stage barcode POCT implementation (2002–2004) compared to full implementation (2007–2009). - Study Bias: Baseline sample data include ~ 1/3 barcoding practice (less-than-full implementation), as error reporting began 1/2004. |
- Description: POCT (point-of- care test) Barcoding with ongoing reporting of barcoding procedure compliance (scan rate) and patient ID errors to nursing management. - Duration: 9/2002- 6/2009; ongoing - Training: Education of nursing staff on new practice guidelines includes one-on-one nursing educators; placing “scan only” on each meter, and laminated scanning guidelines cards were attached to each meter tote. - Staff/Other Resources: Not reported - Cost: Not reported *Following Improvement Committee investigation reporting after low scan rates (<1/3) and high ID errors |
- Description: Patient ID error rate: Monthly # misidentified patients/total glucose POCTs * Monthly average scan rate: # of patient ID wristband barcodes scanned/Total POCT glucose tests Baseline (1/2004): 31.8% Barcoding full implementation (1- 6/2009): 96.7% - Recording method: Information downloaded from all scanning devices for audit reports; documentation of POCT scan rate and error rate |
- Pretest-Posttest - Findings/Effect Size: Patient ID error rate Baseline: 2.9% Barcoding practice (full implementation): 0.5% Absolute decrease: 2.4% Relative decrease: 82.8% ➢OR = 5.94 (CI: 5.26–6.71) - Stat. Significance/Tests: Not reported - Results/Conclusion Bias: Pre and post comparison practices include POCT barcoding; results show effect of improving implementation of POCT barcoding as reflected in average scan rates from 31.8% versus 96.7%. Limitation: comparison based on data collected during notably different time periods (5 yrs apart). |
|
Quality Rating (10 point maximum): 7 (Fair) Effect Size Magnitude Rating : Substantial (Relevance: Direct) |
Study (3 pts maximum): 2; - Potential study bias: Study design, time period and sample may introduce bias affecting/understating effect size - Baseline period includes ~1/3 barcoding (−1). |
Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 |
Results/findings (3 pts max.): 1; -Appropriateness of statistical analysis: Data provided not sufficient to verify calculation of effect size - baseline sample (−1) -Uncontrolled deviations: Results/effect size reported includes barcoding in both baseline (~1/3) and practice samples (−1) |
| Kenmore Mercy Hospital, Catholic Health System (Jarnot J and Weber A) - 2011 - LMBP Unpublished Submission - Kenmore Mercy Hospital; Kenmore, New York - Funding: Self-Funded |
- Design: Before-after - Facility/Setting: Kenmore Mercy Hospital; Kenmore, NY; teaching hospital; 100- 300 beds - Time Period: 1/1/2007 – 5/31/2011 Pre: 16 mos. (1/2007 – 4/2008) Post: 37 mos. (5/2008 – 5/2011) - Sample: All hospital inpatient and Emergency Department POC glucose tests Pre: 79,437 Post: 184,491 - Comparator: Patient wristband with typed patient identifying information (name, date of birth, medical record number) - Study bias: None noted |
- Description: POC glucose tests with barcoded patient ID wristbands with account/billing # - Duration: 4/21/2008 – 5/31/2011; ongoing. - Training: Training, re-training and communication/feedback using data reports; also included internal competition. POC glucose barcoding implemented as an upgrade to pharmacy barcoding which first introduced nurses to the process of scanning a barcode prior to actions. - Staff/Other Resources: Nursing staff - Cost: Not reported |
- Description: Patient ID error rate (%): # Patient ID errors/total # POC glucose tests (Error: Patient ID # from glucometer does not match current patient ) - Recording Method: Glucometer data management system audit of daily testing log flags ID #s not matched to patients. Monthly review of ID errors by the POC department. Comparative statistics provided for each nurse manager. Same recording practice pre- and post-barcoding. |
Pretest-Posttest Findings/Effect Size: Patient ID error rate Pre : 2.16% (1,716/79,437) Post: 0.57% (1,051/184,491) Absolute decrease: 1.6% Relative decrease: 73.6% ➢ OR = 3.85 (CI: 3.56–4.16) - Stat. Significance/Test(s): None reported - Results/Conclusions Bias: None noted |
|
Quality Rating (10 point maximum): 10 (Good) Effect Size Magnitude Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 3 | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 3 |
| Mercy Hospital of Buffalo, Catholic Health System (Jarnot J and Weber A) - 2011 - LMBP Unpublished Submission - Mercy Hospital of Buffalo, Buffalo, New York - Funding: Self-Funded |
- Design: Before-after - Facility/Setting: Mercy Hospital of Buffalo, Buffalo, NY; teaching hospital; > 300 beds - Time Period: 1/1/2007 – 5/31/2011 Pre: 17 mos. (1/2007 – 5/2008) Post: 36 mos. (6/2008 – 5/2011) - Sample: All hospital inpatient and Emergency Department POC glucose tests Pre: 249,667 Post: 517,744 - Comparator: Patient wristband with typed patient identifying information (name, date of birth, medical record number) - Study bias: None noted |
- Description: POC glucose tests with barcoded patient ID wristbands with account/billing # - Duration: 5/28/2008 – 5/31/2011; ongoing - Training: Training, re-training and communication/feedback using data reports; also included internal competition. POC glucose barcoding implemented as an upgrade to pharmacy barcoding which first introduced nurses to the process of scanning a barcode prior to actions. - Staff/Other Resources: Nursing staff - Cost: Not reported |
- Description: Patient ID error rate (%): # Patient ID errors/total # POC glucose tests (Error: Patient ID # from glucometer does not match current patient ) - Recording Method: Glucometer data management system audit of daily testing log flags ID #s not matched to patients. Monthly review of ID errors by the POC department. Comparative statistics provided for each nurse manager. Same recording practice pre- and post-barcoding. |
Pretest-Posttest Findings/Effect Size: Patient ID error rate Pre : 2.24% (5,589/249,667) Post: 0.44% (2,256/517,744) Absolute decrease: 1.8% Relative decrease: 80.4% ➢OR = 5.23 (CI: 4.98 – 5.50) - Stat. Significance/Test(s): None reported - Results/Conclusions Bias: None noted. |
|
Quality Rating (10 point maximum): 10 (Good) Effect Size Magnitude Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 3 | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 3 |
| Sisters of Charity Hospital Buffalo, Catholic Health System (Jarnot J and Weber A) - 2011 - LMBP Unpublished Submission - Sisters of Charity Hospital, Buffalo, New York - Funding: Self-Funded |
- Design: Before-after - Facility/Setting: Sisters of Charity Hospital, Buffalo, NY teaching hospital; 100- 300 beds - Time Period: 1/1/2007 – 5/31/2011 Pre: 17 mos. (1/2007 – 5/2008) Post: 36 mos. (6/2008 – 5/2011) - Sample: All hospital inpatient and Emergency Department POC glucose tests Pre: 120,718 Post: 259,787 - Comparator: Patient wristband with typed patient identifying information (name, date of birth, medical record number) - Study bias: None noted |
- Description: POC glucose tests with barcoded patient ID wristbands with account/billing # - Duration: 5/19/2008 – 5/31/2011; ongoing. - Training: Training, re-training and communication/feedback using data reports; also included internal competition. POC glucose barcoding implemented as an upgrade to pharmacy barcoding which first introduced nurses to the process of scanning a barcode prior to actions. - Staff/Other Resources: Nursing staff - Cost: Not reported |
- Description: Patient ID error rate (%): # Patient ID errors/total # POC glucose tests (Error: Patient ID # from glucometer does not match current patient ) - Recording Method: Glucometer data management system audit of daily testing log flags ID #s not matched to patients. Monthly review of ID errors by the POC department. Comparative statistics provided for each nurse manager. Same recording practice pre- and post-barcoding. |
Pretest-Posttest Findings/Effect Size: Patient ID error rate Pre : 1.56% (1,888/120,718) Post: 0.42% (1,096/259,787) Absolute decrease: 1.1% Relative decrease: 73.1% ➢OR = 3.75 (CI: 3.48 – 4.04) - Stat. Significance/Test(s): None reported - Results/Conclusions Bias: None noted. |
|
Quality Rating (10 point maximum): 10 (Good) Effect Size Magnitude Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 3 | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 3 |
| Sisters of Charity Hospital St. Joseph’s Campus, Catholic Health System (Jarnot J and Weber A) - 2011 - LMBP Unpublished Submission - Sisters of Charity Hospital St. Joseph’s Campus, Cheektowaga, New York - Funding: Self-Funded |
- Design: Before-after - Facility/Setting: Sisters of Charity Hospital St. Joseph’s Campus, Cheektowaga, NY; teaching hospital; 100- 300 beds - Time Period: 1/1/2007 – 5/31/2011 Pre: 11 mos. (1/2007 – 11/2007) Post: 42 mos. (12//2007 – 5/2011) - Sample: All hospital inpatient and Emergency Department POC glucose tests Pre: 44,932 Post: 182,150 - Comparator: Patient wristband with typed patient identifying information (name, date of birth, medical record number) - Study bias: None noted |
- Description: POC glucose tests with barcoded patient ID wristbands with account/billing # - Duration: 11/26/2007 – 5/31/2011; ongoing. - Training: Training, re-training and communication/feedback using data reports; also included internal competition. POC glucose barcoding implemented as an upgrade to pharmacy barcoding which first introduced nurses to the process of scanning a barcode prior to actions. - Staff/Other Resources: Nursing staff - Cost: Not reported |
- Description: Patient ID error rate (%): # Patient ID errors/total # POC glucose tests (Error: Patient ID # from glucometer does not match current patient ) - Recording Method: Glucometer data management system audit of daily testing log flags ID #s not matched to patients. Monthly review of ID errors by the POC department. Comparative statistics provided for each nurse manager. Same recording practice pre- and post-barcoding. |
Pretest-Posttest Findings/Effect Size: Patient ID error rate Pre : 3.22% (1,449/44,932) Post: 0.54% ( 992/182,150) Absolute decrease: 2.7% Relative decrease: 83.2% ➢ OR = 6.09 (CI: 5.61 – 6.60) - Stat. Significance/Test(s): None reported - Results/Conclusions Bias: None noted. |
|
Quality Rating (10 point maximum): 10 (Good) Effect Size Magnitude Rating: Substantial (Relevance: Direct) |
Study (3 pts maximum): 3 | Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 | Results/findings (3 pts maximum): 3 |
| Unpublished Study B – POCT Barcoding (Anonymous) - 2009 - LMBP Unpublished Submission - Midwest Academic Medical Center, Minnesota, USA - Funding: In-house, quality management project |
- Design: Before-after - Facility/Setting: Midwest- MN Pathology Lab, Teaching Hospital with affiliated clinic sites; > 300 beds. - Time Period: 1/1/2009 – 6/30/2009; Pre: 1st Quarter 2009 (1/1/2009 – 3/31/2009) – 3 mos. Post: 2nd Quarter 2009 (4/1/09 – 6/30/09 ) – 3 mos. - Sample: All inpatient and outpatient bedside POC glucose tests; Annual total (2008): 247,000 - Comparator: Manually verify patient armband to glucose work list - Study bias: Barcoding period includes pre-barcoding (4/1/09 – 4/27/09) |
- Description: POCT barcoding for glucose tests by adding a barcode to the patient armband readable by POCT devices. - Duration: 4/28/2009 – 6/30/2009; ongoing - Training: Training needs are modest – time to train all glucose users on new process; support for barcode accessories; on-going training on policy and process updates. - Staff/Other Resources: Time to identify & test new armband- materials barcodes, develop barcodes for user-id and maintain process - Cost: Not reported |
- Description: Patient ID error rate (%): POC glucose test results with either incorrectly entered (miskeyed) patient ID or reported on the wrong patient (two measures combined) - Recording Method: Occurrence reports (numerator); financial reports for total number of tests (denominator) |
Pretest-Posttest Findings/Effect Size: Patient ID error rate Pre : 1.987% Post: 1.381% Absolute decrease: 0.6% Relative decrease: 30.1% ➢ OR = 1.59 (CI: 0.85 – 2.99) (Note: Denominator of rate estimated from data provided by author – not explicitly stated.) - Stat. Significance/Test(s): None reported - Results/Conclusions Bias: Authors state Std Dev and means reported – none provided; denominator info not provided to replicate results. No power or stat. test reported |
|
Quality Rating (10 point maximum): 5 (Poor:
Results/Findings rating = 0 Effect Size Magnitude Rating: N/A (Relevance: Direct) |
Study (3 pts maximum): 1; - Potential study bias: Study Post sample likely to introduce bias substantially affecting/understating effect size - includes 100% non-barcoded tests for 27 days (4/1–4/27) of 3 mo. Period (−2). |
Practice (2 pts maximum): 2 | Outcome measures (2 pts maximum): 2 |
Results/findings (3 pts maximum): 0; - Sample Sufficiency: Sample size (# tests) not reported (−2) - Uncontrolled deviations: Barcoding effect size not clearly attributable to practice: ~1/3 of time period based on comparison practice (−2) |
APPENDIX C. LMBP Barcoding Systematic Review Eligible Studies
Barcoding Systems
Included studies
Published
- Bologna LJ, Mutter M. Life after phlebotomy deployment: Reducing major patient and specimen identification errors. Journal of healthcare information management : JHIM. 2002;16:65–70. [PubMed] [Google Scholar]
- Brown JE, Smith N, Sherfy BR. Decreasing mislabeled laboratory specimens using barcode technology and bedside printers. Journal of nursing care quality. 2010 doi: 10.1097/NCQ.0b013e3181e4e6dd. [DOI] [PubMed] [Google Scholar]
- Hayden RT, Patterson DJ, Jay DW, Cross C, Dotson P, Possel RE, et al. Computer-assisted bar-coding system significantly reduces clinical laboratory specimen identification errors in a pediatric oncology hospital. The Journal of pediatrics. 2008;152:219–24. doi: 10.1016/j.jpeds.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Hill PM, Mareiniss D, Murphy P, Gardner H, Hsieh YH, Levy F, et al. Significant reduction of laboratory specimen labeling errors by implementation of an electronic ordering system paired with a bar-code specimen labeling process. Annals of emergency medicine. 2010;56:630–6. doi: 10.1016/j.annemergmed.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Killeen JP, Chan TC, Jones K, Guss DA. Impact of bar coding technology and computerized physician order entry on reducing laboratory specimen misidentification errors in the emergency department. Annual Meeting of the Society for Academic Emergency Medicine; New York City. 2005; p. 49. [Google Scholar]
- Morrison AP, Tanasijevic MJ, Goonan EM, Lobo MM, Bates MM, Lipsitz SR, et al. Reduction in specimen labeling errors after implementation of a positive patient identification system in phlebotomy. American journal of clinical pathology. 2010;133:870–7. doi: 10.1309/AJCPC95YYMSLLRCX. [DOI] [PubMed] [Google Scholar]
- Zarbo RJ, Tuthill JM, D’Angelo R, Varney R, Mahar B, Neuman C, et al. The henry ford production system: Reduction of surgical pathology in-process misidentification defects by bar code-specified work process standardization. American journal of clinical pathology. 2009;131:468–77. doi: 10.1309/AJCPPTJ3XJY6ZXDB. [DOI] [PubMed] [Google Scholar]
Unpublished
- Heng J, Lyndon B. Johnson General Hospital; 2009. [Google Scholar]
- Schmidt R. University of Washington, School of Medicine; 2009. [Google Scholar]
- Large academic medical center, Southern CA; 2009.
Excluded studies
Published
- Fabbretti G. Risk management: Correct patient and specimen identification in a surgical pathology laboratory. The experience of infermi hospital, rimini, italy. Pathologica. 2010;102:96–101. [PubMed] [Google Scholar]
- Sandler SG, Langeberg A, Dohnalek L. Bar code technology improves positive patient identification and transfusion safety. Developments in biologicals. 2005;120:19–24. [PubMed] [Google Scholar]
- Turner CL, Casbard AC, Murphy MF. Barcode technology: Its role in increasing the safety of blood transfusion. Transfusion. 2003;43:1200–9. doi: 10.1046/j.1537-2995.2003.00428.x. [DOI] [PubMed] [Google Scholar]
Unpublished
- Senn C, Bormann P. University of Minnesota Medical Center Fairview; 2009. [Google Scholar]
Point-of-Care Test Barcoding
Included studies
Published
- Colard DR. Reduction of patient identification errors using technology. Point of Care. 2005;4:61–3. [Google Scholar]
- Rao AC, Burke DA, Dighe AS. Implementation of bar coded wristbands in a large academic medical center: Impact on point of care error rates. Point of Care. 2005;4:119–22. [Google Scholar]
Unpublished
- Schuerch C. Geisinger Medical Center; 2009. [Google Scholar]
- Jarnot J, Weber A. Kenmore Mercy Hospital. 2011. [Google Scholar]
- Jarnot J, Weber A. Mercy Hospital of Buffalo; 2011. [Google Scholar]
- Jarnot J, Weber A. Sisters of Charity Hospital Buffalo; 2011. [Google Scholar]
- Jarnot J, Weber A. Sisters of Charity Hospital St. Joseph’s Campus; 2011. [Google Scholar]
Excluded studies
Published
- Nichols JH, Bartholomew C, Brunton M, Cintron C, Elliott S, McGirr J, et al. Reducing medical errors through barcoding at the point of care. Clinical leadership & management review : the journal of CLMA. 2004;18:328–34. [PubMed] [Google Scholar]
Unpublished
- Midwest Academic Medical Center, Minnesota; 2009.
Footnotes
See Glossary for more detailed information on ID errors and associated terms.
See Appendix A for the LMBP Patient Specimen Identification Expert Panel Members. LMBP Workgroup members are listed at: https://www.futurelabmedicine.org/about/lmbp_workgroup/
More information on submission of unpublished studies to the Laboratory Medicine Best Practices Initiative is available at www.futurelabmedicine.org
See Glossary for more information on odds ratios.
The criteria for a Substantial effect size rating: OR > 2.0 and significantly different from OR =1.0 at p = 0.05 (i.e., the lower limit of the 95 percent confidence interval is > 1.0).
Random-effects model assumes there is no common population effect size for the included studies and the studies’ effect size variation follows a distribution with the studies representing a random sample. This is in contrast to the fixed-effects model which assumes a single population effect size for all studies and that observed differences reflect random variation.
Similarly, additional searches covering the following databases completed in September 2011 identified no economic evaluations: EconLit, Cost Effectiveness Analysis (CEA) Registry, and the U.K. National Health Service (NHS) National Institute for Health Research Centre for Reviews and Dissemination (CRD) Economic Evaluation Database (EED) and Health Technology Assessment (HTA) Database.
Laboratory Medicine Best Practices Workgroup member
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry (CDC/ATSDR).
References
- 1.Kohn LT, Corrigan J, Donaldson MS. To err is human : building a safer health system. National Academy Press; Washington, D.C: 2000. [PubMed] [Google Scholar]
- 2.Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem. 2002;48:691–698. [PubMed] [Google Scholar]
- 3.Makary MA, Epstein J, Pronovost PJ, Millman EA, Hartmann EC, Freischlag JA. Surgical specimen identification errors: a new measure of quality in surgical care. Surgery. 2007;141:450–455. doi: 10.1016/j.surg.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Christenson RH, Snyder SR, Shaw CS, Derzon JH, Black RS, Mass D, Epner P, Favoretto AM, Liebow EB. Laboratory medicine best practices: systematic evidence review and evaluation methods for quality improvement. Clin Chem. 2011;57:816–825. doi: 10.1373/clinchem.2010.157131. [DOI] [PubMed] [Google Scholar]
- 5.CLIA Subpart K - Quality Systems for Nonwaived Testing - General Laboratory Systems - Preanalytic Systems - §493.1242 Standard: Specimen submission, handling, and referral Federal Register, 42 CFR 493.1242.
- 6.National Quality Forum (NQF) Safe Practices for Better Healthcare - 2009 Update: A Consensus Report. Washington, DC: 2007. [Google Scholar]
- 7.Woodcock S, Bettinelli R, Fedraw L, Fischer C, Henricks W, Mann P, Parsons B, Smith M, Wright C. Accuracy in Patient and Sample Identification: Approved Guideline. Wayne, PA: 2010. [Google Scholar]
- 8.Valenstein PN, Sirota RL. Identification errors in pathology and laboratory medicine. Clin Lab Med. 2004;24:979–996. vii. doi: 10.1016/j.cll.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Valenstein PN, Raab SS, Walsh MK. Identification errors involving clinical laboratories: a College of American Pathologists Q-Probes study of patient and specimen identification errors at 120 institutions. Arch Pathol Lab Med. 2006;130:1106–1113. doi: 10.5858/2006-130-1106-IEICL. [DOI] [PubMed] [Google Scholar]
- 10.Wagar EA, Stankovic AK, Raab S, Nakhleh RE, Walsh MK. Specimen labeling errors: a Q-probes analysis of 147 clinical laboratories. Arch Pathol Lab Med. 2008;132:1617–1622. doi: 10.5858/2008-132-1617-SLEAQA. [DOI] [PubMed] [Google Scholar]
- 11.Zarbo RJ, Meier FA, Raab SS. Error detection in anatomic pathology. Arch Pathol Lab Med. 2005;129:1237–1245. doi: 10.5858/2005-129-1237-EDIAP. [DOI] [PubMed] [Google Scholar]
- 12.Wagar EA, Tamashiro L, Yasin B, Hilborne L, Bruckner DA. Patient safety in the clinical laboratory: a longitudinal analysis of specimen identification errors. Arch Pathol Lab Med. 2006;130:1662–1668. doi: 10.5858/2006-130-1662-PSITCL. [DOI] [PubMed] [Google Scholar]
- 13.Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, Vassault AJ, Mattiuzzi C, Plebani M. Causes, consequences, detection,and prevention of identification errors in laboratory diagnostics. Clin Chem Lab Med. 2009;47:143–153. doi: 10.1515/CCLM.2009.045. [DOI] [PubMed] [Google Scholar]
- 14.Lippi G, Plebani M. Identification errors in the blood transfusion laboratory: a still relevant issue for patient safety. Transfus Apher Sci. 2011;44:231–233. doi: 10.1016/j.transci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Woodcock S. First Step to Success: Accurate Patient and Sample Identification. Fall Clinical and Laboratory Standards Institute - Association of Public Health Laboratories Teleconference Series; 2010. [Google Scholar]
- 16.Ansari S, Szallasi A. ‘Wrong blood in tube’: solutions for a persistent problem. Vox Sang. 2011;100:298–302. doi: 10.1111/j.1423-0410.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 17.Dzik WH, Murphy MF, Andreu G, Heddle N, Hogman C, Kekomaki R, Murphy S, Shimizu M, Smit-Sibinga CT. An international study of the performance of sample collection from patients. Vox Sang. 2003;85:40–47. doi: 10.1046/j.1423-0410.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 18.Grimm E, Friedberg RC, Wilkinson DS, AuBuchon JP, Souers RJ, Lehman CM. Blood bank safety practices: mislabeled samples and wrong blood in tube--a Q-Probes analysis of 122 clinical laboratories. Arch Pathol Lab Med. 2010;134:1108–1115. doi: 10.5858/2009-0674-CP.1. [DOI] [PubMed] [Google Scholar]
- 19.Layfield LJ, Anderson GM. Specimen labeling errors in surgical pathology: an 18-month experience. Am J Clin Pathol. 2010;134:466–470. doi: 10.1309/AJCPHLQHJ0S3DFJK. [DOI] [PubMed] [Google Scholar]
- 20.Ricos C, Garcia-Victoria M, de la Fuente B. Quality indicators and specifications for the extra-analytical phases in clinical laboratory management. Clin Chem Lab Med. 2004;42:578–582. doi: 10.1515/CCLM.2004.100. [DOI] [PubMed] [Google Scholar]
- 21.Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem. 2007;53:1338–1342. doi: 10.1373/clinchem.2007.088344. [DOI] [PubMed] [Google Scholar]
- 22.Howanitz PJ, Renner SW, Walsh MK. Continuous wristband monitoring over 2 years decreases identification errors: a College of American Pathologists Q-Tracks Study. Arch Pathol Lab Med. 2002;126:809–815. doi: 10.5858/2002-126-0809-CWMOYD. [DOI] [PubMed] [Google Scholar]
- 23.Khoury M, Burnett L, Mackay MA. Error rates in Australian chemical pathology laboratories. Med J Aust. 1996;165:128–130. [PubMed] [Google Scholar]
- 24.Lippi G, Guidi GC. Risk management in the preanalytical phase of laboratory testing. Clin Chem and Lab Med. 2007;45:720–727. doi: 10.1515/CCLM.2007.167. [DOI] [PubMed] [Google Scholar]
- 25.Renner SW, Howanitz PJ, Bachner P. Wristband identification error reporting in 712 hospitals. A College of American Pathologists’ Q-Probes study of quality issues in transfusion practice. Arch Pathol Lab Med. 1993;117:573–577. [PubMed] [Google Scholar]
- 26.Valenstein P, Meier F. Outpatient order accuracy. A College of American Pathologists Q-Probes study of requisition order entry accuracy in 660 institutions. Arch Pathol Lab Med. 1999;123:1145–1150. doi: 10.5858/1999-123-1145-OOA. [DOI] [PubMed] [Google Scholar]
- 27.Grzybicki D, Bruno S, Zarbo D, Jensen C, Geisinger K, Raab SS. Laboratory Specimen Identification Error Detection: Use of Direct Observation Reveals Large Numbers of Near Miss Events. AcademyHealth Annual Research Meeting; Walt Disney World Swan and Dolphin Resort in Orlando. 2007; p. abstract no. 803. [Google Scholar]
- 28.Fabbretti G. Risk management: correct patient and specimen identification in a surgical pathology laboratory. The experience of Infermi Hospital, Rimini, Italy. Pathologica. 2010;102:96–101. [PubMed] [Google Scholar]
- 29.Norris SL, Atkins D, Bruening W, Fox S, Johnson E, Kane R, Morton SC, Oremus M, Ospina M, Randhawa G, Schoelles K, Shekelle P, Viswanathan M. Observational studies should be considered for inclusion in reviews of comparative effectiveness. J Clin Epidemiol. 2011 doi: 10.1016/j.jclinepi.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Institute of Medicine. Finding What Works in Health Care: Standards for Systematic Reviews. 2011. Committee on Standards for Systematic Reviews of Comparative Effectiveness Research. [Google Scholar]
- 31.CDC Laboratory Medicine Best Practices Team. Laboratory Medicine Best Practices: Developing Systematic Evidence Review and Evaluation Methods for Quality Improvement - Phase 3 Final Technical Report. 2010. [Google Scholar]
- 32.Zarbo RJ, Tuthill JM, D’Angelo R, Varney R, Mahar B, Neuman C, Ormsby A. The Henry Ford Production System: reduction of surgical pathology in-process misidentification defects by bar code-specified work process standardization. Am J Clin Pathol. 2009;131:468–477. doi: 10.1309/AJCPPTJ3XJY6ZXDB. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt R. University of Washington, School of Medicine; 2009. unpublished submission. [Google Scholar]
- 34.Snyder ML, Carter A, Jenkins K, Fantz CR. Patient misidentifications caused by errors in standard bar code technology. Clin Chem. 2010;56:1554–1560. doi: 10.1373/clinchem.2010.150094. [DOI] [PubMed] [Google Scholar]
- 35.Hayden RT, Patterson DJ, Jay DW, Cross C, Dotson P, Possel RE, Srivastava DK, Mirro J, Shenep JL. Computer-assisted bar-coding system significantly reduces clinical laboratory specimen identification errors in a pediatric oncology hospital. J Pediatr. 2008;152:219–224. doi: 10.1016/j.jpeds.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Morrison AP, Tanasijevic MJ, Goonan EM, Lobo MM, Bates MM, Lipsitz SR, Bates DW, Melanson SE. Reduction in specimen labeling errors after implementation of a positive patient identification system in phlebotomy. Am J Clin Pathol. 2010;133:870–877. doi: 10.1309/AJCPC95YYMSLLRCX. [DOI] [PubMed] [Google Scholar]
- 37.Nichols JH, Bartholomew C, Brunton M, Cintron C, Elliott S, McGirr J, Morsi D, Scott S, Seipel J, Sinha D. Reducing medical errors through barcoding at the point of care. Clin Leadersh Manag Rev. 2004;18:328–334. [PubMed] [Google Scholar]
- 38.Hill PM, Mareiniss D, Murphy P, Gardner H, Hsieh YH, Levy F, Kelen GD. Significant reduction of laboratory specimen labeling errors by implementation of an electronic ordering system paired with a bar-code specimen labeling process. Ann Emerg Med. 2010;56:630–636. doi: 10.1016/j.annemergmed.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 39.Elston DM. Opportunities to improve quality in laboratory medicine. Clin Lab Med. 2008;28:173–177. v. doi: 10.1016/j.cll.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Bologna LJ, Mutter M. Life after phlebotomy deployment: reducing major patient and specimen identification errors. J Healthc Inf Manag. 2002;16:65–70. [PubMed] [Google Scholar]
- 41.Bologna LJ, Lind C, Riggs RC. Reducing major identification errors within a deployed phlebotomy process. Clin Leadersh Manag Rev. 2002;16:22–26. [PubMed] [Google Scholar]
- 42.Brown JE, Smith N, Sherfy BR. Decreasing mislabeled laboratory specimens using barcode technology and bedside printers. J Nurs Care Qual. 2010 doi: 10.1097/NCQ.0b013e3181e4e6dd. [DOI] [PubMed] [Google Scholar]
- 43.Owens DK, Qaseem A, Chou R, Shekelle P. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180. doi: 10.7326/0003-4819-154-3-201102010-00007. [DOI] [PubMed] [Google Scholar]
- 44.Grimm EE, Schmidt RA. Reengineered workflow in the anatomic pathology laboratory: costs and benefits. Arch Pathol Lab Med. 2009;133:601–604. doi: 10.5858/133.4.601. [DOI] [PubMed] [Google Scholar]
- 45.Rao AC, Burke DA, Dighe AS. Implementation of bar coded wristbands in a large academic medical center: impact on point of care error rates. Point of Care. 2005;4:119–122. [Google Scholar]
- 46.Shojania KG, Grimshaw JM. Evidence-based quality improvement: the state of the science. Health Aff (Millwood) 2005;24:138–150. doi: 10.1377/hlthaff.24.1.138. [DOI] [PubMed] [Google Scholar]