Abstract
Objective
To examine associations of patient and injury characteristics with outcomes at inpatient rehabilitation discharge and 9 months post-discharge for patients with traumatic brain injury (TBI)
Design
Prospective, longitudinal observational study
Setting
10 inpatient rehabilitation centers (9 US, 1 Canada)
Participants
Consecutive patients (n=2130) enrolled between 2008 and 2011, admitted for inpatient rehabilitation after index TBI injury, and divided into 5 subgroups based on rehabilitation admission FIM Cognitive score
Interventions
Not applicable
Main Outcome Measures
Rehabilitation length of stay, discharge to home, and FIM at discharge and 9 months post-discharge.
Results
Severity indices increased explained variation in outcomes beyond that accounted for by patient characteristics. FIM Motor scores were generally the most predictable. Higher functioning subgroups had more predictable outcomes then subgroups with lower cognitive function at admission. Age at injury, time from injury to rehabilitation admission, and functional independence at rehabilitation admission were the most consistent predictors across all outcomes and subgroups.
Conclusions
Findings from previous studies of the relationships among patient and injury characteristics and rehabilitation outcomes were largely replicated. Discharge outcomes were most strongly associated with injury severity characteristics; while predictors of functional independence at 9 months post-discharge included both patient and injury characteristics.
Keywords: brain injuries, rehabilitation, craniocerebral trauma, outcome assessment (health care)
A plethora of rehabilitation investigations have examined how outcomes of traumatic brain injury (TBI) are affected by pre-injury differences among patients, as well as characteristics of the injury itself. These factors are immutable sources of variance in outcomes and must be accounted for to appreciate the contribution of time, treatment, environment, and other modifiable aspects of the rehabilitation process. While the severity of the TBI holds considerable variance that can be captured via multiple direct and indirect measures, concomitant injuries as well as the health and well-being of the individual at time of injury have been found to contribute important, independent variance to outcomes. However, to-date, studies have not comprehensively examined the amount of variance explained when the full range of potential predictive factors are considered together.
Severity of the index TBI has been measured via multiple behavioral observation scores that capture the extent of altered consciousness (e.g., Glasgow Coma Scale, time to follow commands, length of loss of consciousness, length of post-traumatic amnesia), 1,4-8-13 as well as via indicators of the structural integrity of the brain (e.g., skull fracture, hemorrhage, hematoma, intracranial pressure, midline shift). 1,4-8 Treatments required during acute trauma care (e.g., chemical paralysis, craniotomy, time in the intensive care unit) also may predict later outcomes, though the amount of variance is often small and only evident if indicators of post-trauma status are not included in prediction models. Cause of injury often contains variance related to injury severity because, in general, different causes involve greater (e.g., vehicular crashes) or lesser (e.g., falls) energy exchange with the brain. Cause of injury is also related to age (e.g., very young and very old most likely to fall) and socioeconomic status (e.g., violence related injuries) 9 Intoxication at time of injury has been found to both be associated with greater injury severity and to be protective of it––a puzzling finding made complicated by the influence of intoxication on the behavioral presentation used to judge injury severity. 12 Several variables that capture the patient's status upon admission to rehabilitation appear to be a proxy for the severity of the index injury and hold considerable predictive power for rehabilitation outcomes (e.g., time from injury to rehabilitation admission, functional independence at admission, presence of agitation or other pathognomonic signs). 2,10,11
Injuries to other parts of the body also contribute to outcomes, including facial fractures, injuries to extremities, and organ damage. 4,13 The Comprehensive Severity Index [CSI®] is a disease-specific severity assessment system that combines indices of the index TBI, concomitant injuries, as well as pre-injury morbidities. The CSI generates severity scores using physical exam findings, vital signs, and laboratory results at specified levels of abnormality found in a patient's chart. In the current study, the CSI score was segmented into signs and symptoms directly related to the brain injury versus all remaining severity symptoms. 14
Among patient pre-injury characteristics, age at injury has always accounted for the most variance in outcomes. 1-3 For adolescents and adults generally worse outcomes are associated with older age, a relationship that appears to accelerate among older adults. 15,16 Premorbid compromise to the central nervous systems also contributes to outcomes, particularly prior acquired brain injuries, intellectual impairment, or developmental disability. 17 Among pre-morbid issues, behavioral health problems in particular appear to contribute to later outcomes, including psychiatric and substance use disorders. 18-20 The integrity of the brain pre-injury adds to outcomes, with education often used as an indicator of one's “cognitive reserve”.21,22
Another class of pre-injury characteristics that contributes to outcomes is financial and social capital—one's social, financial, and environmental resources that may mitigate the impact of TBI. Factors that reflect these resources include pre-injury employment, household income, marital status, and primary insurance. 23 Socio-economic status is a component of this class of variables and is associated with access to resources, health status, and behavioral predispositions. Socio-economic status has been studied extensively as a driver of health disparities, which in rehabilitation outcomes research is often inferred from a patient's source of primary insurance.4,24
A detailed account of the design and methods of the TBI practice-based evidence (PBE) study is provided in the initial article in this supplemental issue.14 The current study addressed the question: When considered together how much do non-modifiable pre-injury and injury characteristics explain variations in outcomes at discharge (discharge FIM Motor and Cognitive, length of stay, and discharge to home) and 9 months after discharge (FIM Motor and Cognitive)? We were not trying to build prediction models for future use but were trying to understand the importance of patient and injury factors related to outcomes in this sample.
Methods
Patients with a primary diagnosis of TBI who were consecutively admitted to 10 acute inpatient rehabilitation facilities between October 2008 and September 2011 were enrolled in the TBI-PBE study. The methodology of the study is fully described elsewhere, including participating facilities, patient selection criteria, the validity and reliability of data collection instruments, and a detailed description of the cohort. 14 The 10 rehabilitation programs from which participants were recruited constituted a convenience sample; however, the sample for the study closely resembled the US population of persons 16 and older receiving acute rehabilitation for a primary diagnosis of TBI. 14 The Institutional Review Board at each center approved the study and informed consent was solicited from each participant or his/her proxy.
Participants
All participants in the study (n=2130) had (a) sustained a TBI, defined as damage to brain tissue caused by an external force and evidenced by loss of consciousness, post-traumatic amnesia, skull fracture, or objective neurological findings, (b) received inpatient care at one of the 10 participating facilities, and (c) were at least 14 years of age upon entry into the facility. Homogenous patient subgroups were formed using the FIM™ Cognitive score upon admission to rehabilitation. The 5 subgroups were as follows: scores ≤6 (n=339), 7-10 (n=374), 11-15 (n=495), 16-20 (n=408), and ≥21 (n=504). Ten patients missing admission FIM Cognitive scores were not included in analyses. More details about these subgroups are presented elsewhere. 14
All variables reflecting patient and injury characteristics that were eligible for inclusion in prediction models are shown in Table 1. Only variables actually included in at least one final model are described here. Data were primarily abstracted from the rehabilitation medical record using a standardized methodology employed by abstractors trained to criteria and monitored for quality over the course of the study. 14 Patient variables were comprised of demographic characteristics (e.g., age, gender, race/ethnicity, marital status) and pre-injury health information (body mass index, prior alcohol and drug use, pre-existing co-morbidities). Variables reflecting injury characteristics included direct measures of severity (e.g., Glasgow Coma Scale, post-traumatic amnesia, CSI brain and non-brain scores), indirect measures of injury severity (e.g., functional status upon admission, days from injury to rehabilitation admission), and indications of pathognomonic conditions (e.g., structural damage to the brain, agitation, ataxia)
Table 1. Patient, Injury and Outcome Variables by Admission FIM Cognitive score.
| Admission FIM Cognitive Score* | |||||||
|---|---|---|---|---|---|---|---|
| VariableDescrip | Overall (n=2130) | <=6 (n=339) | 7-10 (n=374) | 11-15 (n=495) | 16-20 (n=408) | >=21 (n=504) | P |
| STEP 1 | |||||||
| Age (mean, SD) | 44.5 (21.3) | 43.0 (21.9) | 42.3 (20.0) | 43.1 (20.9) | 46.9 (21.6) | 46.8 (21.8) | <.001 ‡ |
| Male (%) | 72.5 | 71.7 | 76.5 | 75.8 | 71.3 | 67.7 | 0.018 † |
| Race: black (%) | 15.1 | 15.3 | 13.9 | 16.8 | 15.4 | 13.9 | 0.083 † |
| Race: white non-Hispanic (%) | 74.4 | 77.9 | 73.5 | 73.5 | 74.3 | 73.4 | |
| Race white: Hispanic (%) | 6.2 | 5.0 | 8.0 | 5.7 | 6.6 | 5.8 | |
| Race: other and unknown (%) ‖ | 4.4 | 1.8 | 4.5 | 4.0 | 3.7 | 6.9 | |
| Marital status before injury: Single (%) | 42.6 | 43.7 | 44.9 | 44.8 | 38.2 | 40.9 | 0.267 † |
| Marital status before injury: Married (%) | 36.5 | 36.3 | 35.6 | 35.8 | 37.0 | 37.9 | |
| Marital status before injury: Previously married (%) | 17.5 | 16.2 | 15.5 | 16.2 | 22.5 | 17.1 | |
| Marital status before injury: Other/Unknown§ (%) § | 3.5 | 3.8 | 4.0 | 3.2 | 2.2 | 4.2 | |
| Highest education achieved: some high school, no diploma (%) | 23.0 | 20.4 | 26.2 | 25.3 | 26.5 | 17.5 | 0.008 † |
| Highest education achieved: high school diploma (%) | 25.9 | 25.1 | 27.5 | 28.1 | 25.7 | 22.6 | |
| Highest education achieved: work towards or completed Associate's degree (%) | 16.2 | 15.9 | 13.9 | 14.9 | 17.9 | 18.1 | |
| Highest education achieved: work towards or completed Bachelor's degree (%) | 19.7 | 21.2 | 20.3 | 18.8 | 15.9 | 22.0 | |
| Highest education achieved: work towards or completed Master's/Doctoral degree (%) | 9.7 | 11.5 | 8.0 | 8.3 | 8.3 | 12.1 | |
| Highest education achieved: Unknown (%) | 5.7 | 5.9 | 4.0 | 4.6 | 5.6 | 7.7 | |
| Employment prior to injury: Employed (%) | 47.1 | 45.4 | 48.4 | 47.5 | 43.9 | 49.4 | 0.006 † |
| Employment prior to injury: Employed and Student (%) | 4.0 | 4.1 | 4.0 | 4.4 | 2.9 | 4.4 | |
| Employment prior to injury: Not Employed (%) | 13.3 | 13.6 | 15.8 | 13.7 | 14.2 | 10.3 | |
| Employment prior to injury: Retired (%) | 23.1 | 20.9 | 17.9 | 21.0 | 28.4 | 26.4 | |
| Employment prior to injury: Student (%) | 11.4 | 13.6 | 12.0 | 12.7 | 9.6 | 9.3 | |
| Employment prior to injury: Unknown (%) | 1.1 | 2.4 | 1.9 | 0.6 | 1.0 | 0.2 | |
| Able to drive before injury: Yes (%) | 73.1 | 70.2 | 75.7 | 75.6 | 69.9 | 73.0 | 0.128 † |
| Able to drive before injury: No (%) | 10.8 | 9.4 | 10.7 | 9.5 | 14.0 | 10.5 | |
| Able to drive before injury: Unknown (%) | 16.1 | 20.4 | 13.6 | 14.9 | 16.2 | 16.5 | |
| History of alcohol abuse before injury (%) | 35.6 | 30.4 | 36.9 | 39.0 | 37.3 | 33.7 | 0.091 † |
| History of alcohol use before injury (%) | 44.6 | 38.3 | 50.0 | 48.1 | 44.6 | 41.1 | 0.005 † |
| Drug Use before Injury (%) | 20.5 | 17.7 | 22.5 | 20.8 | 25.0 | 17.1 | 0.024 † |
| Anxiety on admission (%) | 19.5 | 13.0 | 20.9 | 20.6 | 20.8 | 21.2 | 0.023 † |
| Depression on admission (%) | 29.3 | 29.8 | 29.4 | 30.5 | 31.1 | 26.6 | 0.588 † |
| ADHD on admission (%) | 7.3 | 5.9 | 7.0 | 7.9 | 8.3 | 7.1 | 0.744 † |
| Learning disorder on admission (%) | 4.6 | 2.9 | 4.0 | 5.9 | 5.9 | 4.0 | 0.189 † |
| Number of previous brain injuries (mean, SD) | 0.1 (0.4) | 0.1 (0.3) | 0.1 (0.4) | 0.1 (0.4) | 0.2 (0.6) | 0.1 (0.4) | 0.016 ‡ |
| High Cholesterol on admission (%) | 15.8 | 13.0 | 13.1 | 14.1 | 20.6 | 17.7 | 0.009 † |
| Diabetes on admission (%) | 16.3 | 20.6 | 15.0 | 20.4 | 14.0 | 12.7 | 0.001 † |
| Hypertension on admission (%) | 42.5 | 46.3 | 40.4 | 43.4 | 45.1 | 38.9 | 0.153 † |
| Paralysis on admission (%) | 37.7 | 47.2 | 45.2 | 43.6 | 29.4 | 26.2 | <.001 † |
| Renal Failure on admission (%) | 8.3 | 8.6 | 8.3 | 8.1 | 7.6 | 8.7 | 0.979 † |
| Body Mass Index: <16 (%) | 1.4 | 2.4 | 2.1 | 1.6 | 1.0 | 0.4 | |
| Body Mass Index: 16-<=18.5 (%) | 8.5 | 11.8 | 11.0 | 8.1 | 8.1 | 5.2 | |
| Body Mass Index: >18.5-<=25 (%) | 49.7 | 55.5 | 55.1 | 50.5 | 46.3 | 43.1 | <.001 † |
| Body Mass Index: >25-<=30 (%) | 23.6 | 18.0 | 18.7 | 25.1 | 26.5 | 27.6 | |
| Body Mass Index >30-<=35 (%) | 7.9 | 6.5 | 7.5 | 7.9 | 8.3 | 9.1 | |
| Body Mass Index: >35-<=40 (%) | 2.3 | 1.8 | 1.3 | 2.4 | 1.7 | 3.6 | |
| Body Mass Index: >40 (%) | 1.3 | 0.0 | 1.3 | 1.2 | 1.2 | 2.4 | |
| Body Mass Index: Unknown (%) | 5.3 | 4.1 | 2.9 | 3.2 | 6.9 | 8.7 | |
| STEP 2 | |||||||
| Cause of injury: Fall (%) | 31.9 | 28.0 | 26.2 | 30.1 | 35.0 | 38.3 | 0.001 † |
| Cause of injury: Motor vehicle crash (%) | 55.6 | 63.7 | 57.8 | 57.2 | 52.5 | 49.2 | |
| Cause of injury: Sports (%) | 1.8 | 1.2 | 2.4 | 1.0 | 2.2 | 2.4 | |
| Cause of injury: Violence (%) | 7.0 | 4.4 | 7.8 | 8.3 | 7.1 | 7.1 | |
| Cause of injury: Miscellaneous (%) | 3.6 | 2.7 | 5.9 | 3.4 | 3.2 | 3.0 | |
| Alcohol Misuse At Time of Injury (%) | 8.3 | 8.3 | 7.5 | 7.9 | 9.1 | 8.5 | 0.94 † |
| Alcohol Use At Time of Injury (%) | 19.1 | 18.6 | 17.4 | 20.2 | 20.1 | 18.5 | 0.816 † |
| Drug Use At Time of Injury (%) | 6.4 | 7.7 | 8.0 | 5.9 | 6.9 | 4.6 | 0.217 † |
| GCS score immediately after injury or upon arrival in acute care: Mild (13-15) (%) | 14.7 | 6.8 | 9.1 | 11.1 | 17.9 | 25.6 | <.001 † |
| GCS score: Moderate (9-12) (%) | 7.7 | 4.1 | 4.8 | 8.3 | 7.6 | 12.1 | |
| GCS score: Severe (3-8) (%) | 32.3 | 46.0 | 42.0 | 35.4 | 24.5 | 18.8 | |
| GCS score: Intubated or sedated (%) | 12.2 | 12.4 | 15.0 | 11.7 | 13.7 | 8.7 | |
| GCS score: Unknown (%) | 33.0 | 30.7 | 29.1 | 33.5 | 36.3 | 34.7 | |
| PTA cleared prior to rehab admission (%) | 40.0 | 1.2 | 8.0 | 25.7 | 62.5 | 86.5 | <.001 † |
| Facial fracture (%) | 13.6 | 10.3 | 16.8 | 14.3 | 15.9 | 10.9 | 0.02 † |
| Skull closed, contusion/hemorrhage present (%) | 71.1 | 74.9 | 72.7 | 69.7 | 71.6 | 68.8 | 0.045 † |
| Skull closed, no contusion/hemorrhage (%) | 21.6 | 15.3 | 19.5 | 24.0 | 21.6 | 24.6 | |
| Skull open, contusion/hemorrhage present (%) | 7.3 | 9.7 | 7.8 | 6.3 | 6.9 | 6.5 | |
| Epidural hematoma (%) | 8.2 | 8.8 | 8.3 | 8.9 | 6.4 | 7.9 | 0.672 † |
| Intraventricular hemorrhage (%) | 18.6 | 29.2 | 23.8 | 18.0 | 14.2 | 11.7 | <.001 † |
| Subarachnoid hemorrhage (%) | 59.2 | 71.1 | 65.0 | 58.2 | 55.1 | 51.0 | <.001 † |
| Subdural hematoma (%) | 46.8 | 49.3 | 52.1 | 46.5 | 45.6 | 42.9 | 0.075 † |
| Brain stem involved (%) | 5.6 | 7.7 | 6.1 | 5.3 | 4.4 | 5.2 | 0.362 † |
| Injury Side: Bilateral (%) | 64.2 | 64.9 | 68.7 | 61.8 | 64.7 | 62.3 | 0.034 † |
| Injury Side: Left Brain (%) | 18.4 | 22.4 | 16.3 | 20.0 | 16.4 | 17.3 | |
| Injury Side: Right Brain (%) | 17.5 | 12.7 | 15.0 | 18.2 | 18.9 | 20.4 | |
| Midline shift: No midline shift (%) | 30.5 | 22.4 | 23.8 | 26.5 | 35.8 | 40.9 | <.001 † |
| Midline shift: >0-5 mm (%) | 12.4 | 13.6 | 13.6 | 12.5 | 12.5 | 10.9 | |
| Midline shift: >5 mm of midline shift (%) | 12.1 | 13.9 | 17.4 | 11.3 | 11.0 | 8.5 | |
| Midline shift: midline shift NOS (%) | 11.1 | 15.3 | 9.1 | 10.7 | 9.6 | 11.5 | |
| Midline shift: Unknown (%) | 33.9 | 34.8 | 36.1 | 39.0 | 31.1 | 28.2 | |
| Craniectomy before rehabilitation (%) | 7.2 | 12.7 | 9.6 | 6.9 | 2.9 | 5.4 | <.001 † |
| Craniotomy before rehabilitation (%) | 20.0 | 18.6 | 23.5 | 20.6 | 20.1 | 17.9 | 0.299 † |
| Days from injury to rehabilitation admission (mean, SD) | 29.3 (34.3) | 36.5 (38.7) | 34.2 (37.6) | 27.5 (32.3) | 26.0 (33.5) | 24.9 (30.1) | <.001 ‡ |
| Admission FIM Motor Rasch-adjusted (mean, SD) | 33.2 (19.3) | 11.5 (14.5) | 23.2 (16.4) | 34.2 (14.8) | 40.5 (12.5) | 48.3 (15.2) | <.001 ‡ |
| Admission FIM Cog Rasch-adjusted (mean, SD) | 37.2 (19.5) | 2.5 (4.4) | 25.7 (4.8) | 38.4 (3.0) | 47.5 (2.4) | 59.6 (7.7) | <.001 ‡ |
| Admission brain injury component of CSI score (mean, SD) | 45 (23.6) | 72.1 (18.1) | 62.0 (17.6) | 47.8 (14.1) | 33.3 (12.5) | 20.0 (9.8) | <.001 ‡ |
| Admission non-brain injury component of CSI score (mean, SD) | 17 (15) | 19.2 (16.1) | 20.3 (15.2) | 18.1 (14.7) | 16.1 (15.7) | 12.3 (11.9) | <.001 ‡ |
| Average of all ABS scores in the 1st 3 days (mean, SD) | 17.1 (4.3) | 19.1 (5.2) | 19.1 (5.4) | 17.4 (3.8) | 16.0 (3.0) | 14.9 (1.5) | <.001 ‡ |
| Moderate to severe aphasia on admission (%) | 46.5 | 74.3 | 68.2 | 50.3 | 37.3 | 15.1 | <.001 † |
| Moderate to severe ataxia on admission (%) | 15.4 | 21.8 | 21.4 | 17.0 | 12.7 | 6.5 | <.001 † |
| Weight bearing precautions required (%) | 26.0 | 25.1 | 24.3 | 21.8 | 30.4 | 27.8 | 0.038 † |
| OUTCOMES | |||||||
| Rehab length of stay excluding interruptions (mean, SD) | 26.5 (19.9) | 40.4 (27.6) | 32.5 (18.4) | 24.4 (15.4) | 21.0 (15.5) | 19.0 (15.1) | <.001 ‡ |
| Discharge destination-home (%) | 83.9 | 73.7 | 78.1 | 85.7 | 85.0 | 92.3 | <.001 † |
| Discharge FIM Motor Rasch-adjusted (mean, SD) | 55.7 (15.9) | 44.8 (17.5) | 50.7 (13.7) | 56.0 (12.6) | 58.7 (13.2) | 64.4 (15.4) | <.001 ‡ |
| Discharge FIM Cog Rasch-adjusted (mean, SD) | 54.4 (15.1) | 40.2 (18.0) | 47.0 (10.6) | 53.0 (9.4) | 57.9 (8.5) | 68.3 (11.1) | <.001 ‡ |
| 9-Month FIM Motor Rasch-adjusted (mean, SD) | 80.7 (20) | 72.6 (25.0) | 77.7 (20.8) | 82.4 (19.1) | 82.8 (16.8) | 85.4 (16.4) | <.001 ‡ |
| 9-Month FIM Cog Rasch-adjusted (mean, SD) | 76.3 (17.9) | 68.2 (20.6) | 72.0 (18.7) | 76.8 (16.6) | 79.1 (16.2) | 82.3 (14.8) | <.001 ‡ |
Note: Abbreviations: ADHD, Attention deficit hyperactivity disorder; ABS, Agitation Behaviour scale; GCS, Glasgow Coma Scale; CSI, Comprehensive Severity Index;
n=10 patients missing admission FIM cognitive score.
Chi-Square analysis.
Analysis of variance test.
Other/unknown includes 62 Separated status, 2 listed as Significant Other, and 10 with unknown or missing status.
Other and unknown includes 69 Asians, 8 Native Americans, 7 Pacific Islanders, and 3 with unknown race.
Outcomes
Six outcome variables were studied: rehabilitation length of stay (LOS), which excluded days out of the rehabilitation facility resulting from readmission to acute care; discharge destination dichotomized as private residence versus institutional setting; and Rasch-transformed FIM Motor and Cognitive scores at discharge from rehabilitation and 9 months post-discharge.25
Analyses
Analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC). When data were missing, adjustments were made depending on the variable and its intended use in analyses. In some instances, we categorized values simply as “unknown” (and included this category in analysis as a dummy variable representing missingness); in other cases we collapsed a continuous variable with missing data into a categorical one and placed the cases with missing information into one of these categories using corroborating data available. In some circumstances missing data resulted in an observation being excluded from a specific model––final sample sizes are reported for all models.
Descriptive statistics were used to provide frequencies and percentages for categorical variables describing patients, treatments, and outcomes; mean, median, quartiles, and SD were used to summarize continuous measures. Bivariate analyses were conducted to examine differences among FIM Cognitive subgroups. For discrete variables, we used the Fisher's Exact test or chi-square test to determine statistical significance of associations. For continuous variables we used t-tests, analysis of variance (ANOVA), or the Kruskal-Wallis test. A 2-sided p value <0.05 was considered statistically significant.
To assess associations between covariates and outcomes, we used ordinary least squares (OLS) multiple regression or logistic regression. Variables with fewer than 20 valid responses were not allowed to enter models. Variables entering into the regression models were checked for multi-collinearity. If a correlation ≥|0.60| was observed, one of the pair was removed. The most parsimonious models were created for each of the 6 outcomes within each of the 5 admission FIM Cognitive subgroups by allowing only significant (p<.05) variables to remain in the models. We used R2 and c statistics to capture how much variation in an outcome was explained by the predictors.
Covariates were allowed to enter each model in one of 3 blocks: (1) patient characteristics, (2) injury characteristics, and (3) centers participating in the study. We computed the amount of variation explained at each step. The centers participating in the study were included to determine whether a significant source of variance remained unexplained after patient and injury characteristics were included.
Results
Participant Characteristics
The demographic and injury characteristics of the sample are described in the first article in this series. 14 The sample was 73% male, 74% white, 37% married, and 51% employed at the time of injury, with an average age of 45 (SD 21.3) years. Vehicular accidents were the most common cause of injury (56%), followed by falls or being hit by a falling object (32%), violence (7%), and sports (2%). Mean time from injury to rehabilitation admission was 29 days (SD 34 days), and mean rehabilitation length of stay 27 days (SD 20 days). The mean raw FIM at rehabilitation admission was 35 (SD 20 points) for the Motor score and 15 (SD 7 points) for the Cognitive score.
Patient, Injury, and Outcome Characteristics
Table 1 shows how the 5 FIM Cognitive subgroups differed by predictor and outcome variables. Among personal characteristics, few differed significantly (p<.01) across Admission FIM Cognitive subgroups, the exceptions being age, Body Mass Index, diabetes present at admission, employment prior to injury, high cholesterol on admission, highest education achieved, history of alcohol use before injury, and at least some paralysis present on admission. With the exception of paralysis present on admission, which increased monotonically from 26.2% in the highest functioning subgroup to 47.2% in the lowest, most changes across admission FIM Cognitive subgroups were not monotonic. However, the proportion with low BMI decreased as FIM Cognitive at admission increased and high cholesterol was more prevalent in the 2 highest FIM Cognitive subgroups.
Among injury characteristics, most direct measures of TBI severity (e.g., Glasgow Coma Scale score, clearing post-traumatic amnesia by rehabilitation admission, and CSI brain component) showed a monotonic relationship across FIM Cognitive subgroups, as did indirect indicators of severity (time to rehabilitation admission, functional status upon admission) and pathognomonic signs (presence of intraventricular hemorrhage, no midline shift, required craniectomy, and aphasia, ataxia, and agitation upon admission). The CSI non-brain component did not show a montonic relationship but was lower in the 2 highest admission FIM Cognitive subgroups.
All outcomes (except discharge to a private residence in the 16-20 admission FIM Cognitive subgroup) showed a monotonic decrease across FIM Cognitive subgroups––lower functioning patients tended to have longer lengths of stay, smaller proportions discharged to a private residence, and lower Rasch-transformed FIM Motor and Cognitive scores at discharge and 9 months post-discharge.
Ordinary Least Squares and Logistic Regression Results
Table 2 shows the R2 values by Admission FIM Cognitive subgroup for blocks of variables predicting continuous outcomes, and c-statistics for blocks of variables for the dichotomous outcome discharge disposition. In some models patient characteristics (Block 1) alone explained as little as 3% of the variation in continuous discharge outcomes to as much as 23%. The least amount of variance accounted for occurred with discharge and follow-up FIM Cognitive scores, which was due to the restricted range that resulted from using Admission FIM Cognitive to create subgroups. Indeed, it is remarkable how much variance was accounted for within subgroups for the 2 FIM Cognitive outcomes.
Table 2. OLS Regression Model Phases for all Dependent Variables by Admission FIM Cognitive.
| Admission FIM Cognitive Score* | ||||||
|---|---|---|---|---|---|---|
| Dependent Variable | Phase R2/c-Statistic | <=6 | 7-10 | 11-15 | 16-20 | >=21 |
| Rehab Length of Stay (LoS) | N used RSq pat | 0.133 | 0.122 | 0.205 | 0.144 | 0.096 |
| Rehab Length of Stay (LoS) | RSq pat + inj | 0.251 | 0.283 | 0.440 | 0.380 | 0.443 |
| Rehab Length of Stay (LoS) | RSq pat, inj + sites | 0.341 | 0.296 | 0.476 | 0.401 | 0.472 |
| Discharge to home (dcH) | Cstat pat | 0.750 | 0.787 | 0.786 | 0.781 | 0.785 |
| Discharge to home (dcH) | Cstat pat + inj | 0.797 | 0.864 | 0.807 | 0.798 | 0.843 |
| Discharge to home (dcH) | Cstat pat, inj + sites | 0.864 | 0.864 | 0.807 | 0.811 | 0.843 |
| Discharge FIM Motor (dcM) | RSq pat | 0.147 | 0.206 | 0.231 | 0.228 | 0.171 |
| Discharge FIM Motor (dcM) | RSq pat + inj | 0.421 | 0.527 | 0.519 | 0.620 | 0.727 |
| Discharge FIM Motor (dcM) | RSq pat, inj + sites | 0.461 | 0.527 | 0.531 | 0.658 | 0.755 |
| Discharge FIM Cognitive (dcC) | RSq pat | 0.115 | 0.069 | 0.080 | 0.031 | 0.059 |
| Discharge FIM Cognitive (dcC) | RSq pat + inj | 0.307 | 0.238 | 0.177 | 0.157 | 0.420 |
| Discharge FIM Cognitive (dcC) | RSq pat, inj + sites | 0.334 | 0.285 | 0.293 | 0.207 | 0.513 |
| 9-month FIM Motor (fuM) | RSq pat | 0.137 | 0.240 | 0.298 | 0.174 | 0.204 |
| 9-month FIM Motor (fuM) | RSq pat + inj | 0.465 | 0.428 | 0.426 | 0.333 | 0.313 |
| 9-month FIM Motor (fuM) | RSq pat, inj + sites | 0.475 | 0.428 | 0.426 | 0.333 | 0.337 |
| 9-month FIM Cognitive (fuC) | RSq pat | 0.158 | 0.169 | 0.109 | 0.062 | 0.121 |
| 9-month FIM Cognitive (fuC) | RSq pat + inj | 0.237 | 0.269 | 0.149 | 0.126 | 0.198 |
| 9-month FIM Cognitive (fuC) | RSq pat, inj + sites | 0.271 | 0.292 | 0.149 | 0.126 | 0.216 |
When injury characteristics were added to patient variables (Block 2), the variance accounted for increased dramatically. The least improvement associated with adding injury characteristics occurred for 9-month Cognitive FIM scores. As might be expected, discharge outcomes were more predictable than those at 9 months. Interestingly, outcomes at discharge for lower functioning Admission FIM groups were generally less predictable compared to higher functioning subgroups, but this trend reversed for 9-month outcomes with the models for lower functioning subgroups accounting for more variance explained.
In general, adding site (Block 3) into the prediction model added little explanatory power–– generally less than a 10% improvement over patient and injury characteristics alone. However, a small improvement in prediction by adding site was evident for discharge FIM Cognitive.
Supplemental figures 1 through 6 show model details for the 6 outcome variables for each of the 5 admission FIM Cognitive subgroups. These models include patient and injury characteristics only and omit site. Each subgroup model includes 3 columns: the least squares regression coefficient (parameter) estimate of the effect of the variable, the standardized estimate, and the p-value. Additionally, in the right hand columns there are colored cells that represent the associated relative strength of each significant variable's effect on the outcome being modeled. This effect is obtained by multiplying the OLS regression coefficient estimate by the subgroup mean value of that covariate. For example, in the admission FIM Cognitive ≤6 subgroup model predicting rehabilitation LOS, the parameter estimate for admission Rasch-transformed FIM Motor is -0.4 while the effect on LOS is -4.5, indicating that a 1-point increase in admission Rasch-transformed FIM Motor score is associated with a 4.5 day decrease in the patient's rehabilitation LOS, controlling for other variables in the model. Green cells indicate a positive effect and red cells indicate a negative effect on the outcome (note, for rehabilitation length of stay, “positive” means more days). Negative levels of association between an outcome and patient and injury characteristics do not imply the absence of a positive outcome.
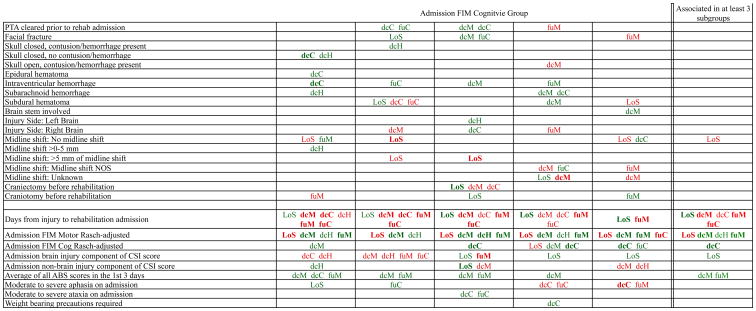
Figure 1 provides a high-level summary of the significant variables for each dependent variable by admission FIM Cognitive subgroup (see detailed results in supplemental figures 1 through 6). Cells containing “LoS” (rehabilitation length of stay), “dcH” (discharge to home), “dcM” (discharge FIM Motor), “dcC” (discharge FIM Cognitive), “fuM” (9-month follow-up FIM Motor), or “fuC” (9-month follow-up FIM Cognitive) indicate that in the final model for the specified dependent variable the covariate in the row is a significant predictor (p<.001 if bolded, and p<.05 if not). Red color indicates a negative association (coefficient), while green indicates a positive one. In every case, the parameter estimate is with all other variables in the model held constant.
Figure 1. Summary of Significant Covariates by Admission FIM Cognitive Score.
* indicates the associations for the 3 FIM Cognitive subgroups are not in the same direction
Footnote. Red values indicate a negative association (coefficient), while green indicates a positive one. Bold values indicate p<0.001. Not bold values indicate p between 0.001 and 0.05. LoS = Length of Stay. dcH = Discharge to home. dcM = Discharge Rasch-adjusted FIM Motor. dcC = Discharge Rasch-adjusted FIM Cognitive. fuM = 9-Month Followup Rasch-adjusted FIM Motor. fuC = 9-Month Followup Rasch-adjusted FIM Cognitive
Excluding LOS due to differences of opinion about whether shorter or longer is “better”, most characteristics showed associations with outcomes that were in the same direction for all admission FIM Cognitive subgroups, either consistently positive or consistently negative. In some cases, a variable for 1 FIM subgroup went in a positive direction while the same variable was negative for a different FIM subgroup, but these instances were few. The only variable that showed significant inconsistency was anxiety upon admission. The presence of anxiety shortened LOS and improved the other 5 outcomes for the lowest functioning subgroup; however, it was associated with poorer follow-up FIM scores for the highest 3 functioning subgroups.
Several patient variables contributed significantly across all or most Admission FIM Cognitive subgroups. Older age resulted in the patient being less likely to be discharged home and lower FIM scores, particularly at follow-up. Not having achieved a high school diploma was associated with lower FIM Cognitive scores at follow-up. Those employed pre-injury had better FIM Motor scores at follow-up. Being retired at injury was also associated with lower FIM Motor scores at discharge, likely due to age but also potentially reflecting premorbid disability. Being unable to drive before injury was associated with lower FIM Cognitive scores at follow-up, perhaps suggesting premorbid functional limitations.
Among injury severity indicators, better Admission FIM Motor score was associated with shorter LOS and greater independence for all 5 subgroups. More days from injury to rehabilitation admission was associated with longer rehabilitation LOS and less independence for all 5 subgroups. Interestingly, greater agitation at admission was associated with higher FIM Motor scores at discharge, and follow-up, for 4 of the 5 groups.
Discussion
The findings of the current study were largely consistent with previous research. As would be expected when severity indices are added to patient characteristics, there is better prediction of outcomes than by either alone. FIM Motor scores were generally the most predictable, while FIM Cognitive scores were the least. However, the use of admission FIM Cognitive scores to define subgroups limited the variance in the models predicting cognitive outcomes. Generally, the higher functioning subgroups had more predictable outcomes than lower functioning ones, the exception being FIM Cognitive scores, for which the opposite was observed. Again the use of FIM Cognitive score at admission to define subgroups affected prediction of FIM Cognitive at follow-up and discharge.
Age was a consistent predictor of discharge to home, as well as of follow up motor and cognitive functioning; in all cases, the higher the age, the worse the outcome. Past studies of the relationship between age and rehabilitation outcomes have been inconsistent, with some finding worse outcomes with higher age, 15,16 while others failed to find age differences. 26,27 Higher age predicted poorer motor outcome at 9-month follow-up in all subgroups, and had a similar effect on cognitive outcome, but only for the lowest 3 levels of admission FIM cognitive score. The negative effect of age on cognitive outcomes appeared to be stronger for persons who started rehabilitation with lower cognitive performance.
Among injury characteristics, days from injury to admission to rehabilitation, as well as the admission FIM Motor score, were the strongest contributors to prediction. Once again, the use of admission FIM Cognitive to define subgroups limited the variance available in regression modeling. Time from injury to rehabilitation admission was significantly associated with all outcomes, although for discharge to home it only explained variance for the lowest functioning subgroup. The strength of this relationship despite all other variables, including indicators of injury severity, being held constant, may suggest that the sooner a patient can start rehabilitation, the better the subsequent outcome.
Admission FIM Motor contributed significantly to the prediction of length of stay, discharge disposition, as well as both discharge and follow-up motor functioning. Despite the high correlation between admission FIM Motor and Cognitive scores, the motor score did not consistently contribute to the prediction of cognitive function.
All the consistent predictors of rehabilitation LOS were injury-related characteristics. The absence of a midline shift in CT scan, as well as higher FIM Motor score at admission were associated with shorter length of stay, while more days from injury to rehabilitation admission, as well as increased brain injury component of the CSI, were associated with longer length of stay. These results are consistent with previous findings. Furthermore, they suggest that these 4 variables may be the most important indicators of the resources required during acute rehabilitation. It is interesting that each contributed significant variance despite the presence of the others, suggesting that direct and proxy indicators of injury severity may be sensitive to different aspects of injury that are important in determining the need for rehabilitation resources.
Across a majority of the admission FIM Cognitive subgroups, only 2 variables consistently contributed variance to the prediction of discharge to home. One reflected patient characteristics while the other captured an injury factor: younger age was associated with being more likely to go home, as was higher admission FIM Motor score. While these 2 variables reflect qualities of the injury or response to injury, their consistency in predicting discharge to home may also be due to their relationship with aspects of the discharge environment that make going home more likely. Younger persons may be more likely to have someone else in the home to assist with functioning in spite of residual deficits. Better motor abilities may reduce the impact of barriers in the built environment that would interfere with the ability to live in a private residence.
The same injury-related characteristics were consistent predictors of motor functioning at both discharge and follow-up: days from injury to rehabilitation admission, FIM Motor score at admission, and agitation at admission. Interestingly, greater average agitation in the first 72 hours of admission was associated with higher FIM Motor scores at discharge and follow-up. While agitation is normally associated with greater impairment, in this case agitation at admission may capture a patient's better residual motor function that allows greater manifestation of agitation. Still, given that FIM Motor at admission and admission agitation scores both explain variance, other explanations for the predictive capability of agitation should be considered. Patient characteristics that contributed to the prediction of motor function may reflect better premorbid motor function: older age and being diabetic were associated with less motor function at follow-up, while being retired was associated with less function at discharge, and being employed with greater function at follow-up.
The only predictor that was consistent for both discharge and follow-up cognitive function was the days from injury to rehabilitation admission. Lower admission FIM Cognitive score was also a predictor of worse cognitive functioning at discharge. At 9-month follow-up, 3 patient characteristics were consistently associated with poorer cognitive function: older age, not having a high school diploma, and not being able to drive pre-injury. These patient characteristics may suggest that the integrity of cognitive functioning pre-injury has a greater influence on cognitive abilities post-discharge than it does on discharge abilities.
Limitations
Though this study used a comprehensive dataset derived from a large sample of TBI patients treated in brain injury rehabilitation units in geographically diverse parts of the country, there were limitations to the available data. Potentially most important, we did not have medical data from the acute care record. Injury and hospital-acquired medical conditions were limited to those noted upon admission to rehabilitation. Some important patient and injury characteristics may not have been included in our analyses, in particular we could not use payer source due to the international make-up of our centers. This latter exclusion may have particularly affected the strength of our models for LOS, as allowed LOS may exert influence on the actual duration.
The large numbers of variables used in modeling presents the potential for unstable results. To minimize instability, predictor variables with fewer than 20 valid responses were disallowed from entering models. We reduced the potential for models to be over-specified by removing one covariate from any pairs that were correlated at r ≥|0.60| and by having at least 5 cases per predictor variable allowed into to a model; in most cases the actual ratio was 10:1 or greater. Finally, to assure that the findings from models we interpreted were stable, we gave the greatest emphasis to relationships that were apparent in multiple of the subgroups created based on admission FIM Cognitive scores; thus, subgroups served to replicate observed associations. This approach increased our confidence in the stability of our findings despite the fact that we were not trying to build prediction models for future use but instead trying to understand the importance of patient and injury factors related to outcomes in this cohort.
Conclusions
This article examined the contribution of patient and injury characteristics to outcomes observed at rehabiliation discharge and 9 months later. Previous studies of these predictors were largely replicated: age at injury, time from injury to rehabilitation admission, and functional status upon admission were the most powerful predictors. Discharge outcomes were more driven by injury severity characteristics, while the prediction of functional independence at 9 months post-discharge also included patient characteristics. Some unexpected findings were observed, including positive associations of agitation at admission and both positive and negative associations of anxiety on admission.
Supplementary Material
Supplemental Figure 1. OLS Regression Models Predicting Rehabilitation Length of Stay by Admission FIM Cognitive score
Supplemental Figure 2. Logistic Regression Models Predicting Discharge Destination Home by Admission FIM Cognitive score
Supplemental Figure 3. OLS Regression Models Predicting Discharge FIM Motor Score Rasch transformed by Admission FIM Cognitive score
Supplemental Figure 4. OLS Regression Models Predicting Discharge FIM Cognitive Score Rasch transformed by Admission FIM Cognitive score
Supplemental Figure 5. OLS Regression Models Predicting 9-Month FIM Motor Score Rasch transformed by Admission FIM Cognitive score
Suplemental Figure 6. OLS Regression Models Predicting 9-Month FIM Cognitive Score Rasch transformed by Admission FIM Cognitive score
Acknowledgments
We gratefully acknowledge the contributions of clinical and research staff at each of the 10 inpatient rehabilitation facilities represented in the study. The study center directors included: John D. Corrigan, PhD and Jennifer Bogner, PhD (Department of Physical Medicine and Rehabilitation, Ohio State University, Columbus, OH); Nora Cullen, MD (Toronto Rehabilitation Institute, Toronto, ON Canada); Cynthia L. Beaulieu, PhD (Brooks Rehabilitation Hospital, Jacksonville, FL); Flora M. Hammond, MD (Carolinas Rehabilitation, Charlotte, NC [now at Indiana University]); David K. Ryser, MD (Neuro Specialty Rehabilitation Unit, Intermountain Medical Center, Salt Lake City, UT); Murray E. Brandstater, MD (Loma Linda University Medical Center, Loma Linda, CA); Marcel P. Dijkers, PhD (Mount Sinai Medical Center, New York, NY); William Garmoe, PhD (Medstar National Rehabilitation Hospital, Washington, DC); James A. Young, MD (Physical Medicine and Rehabilitation, Rush University Medical Center, Chicago, IL); Ronald T. Seel, PhD (Crawford Research Institute, Shepherd Center, Atlanta, GA).
Financial Support: Funding for this study came from the National Institutes of Health, National Center for Medical Rehabilitation Research (grant 1R01HD050439-01), the National Institute on Disability and Rehabilitation Research (grant H133A080023), and the Ontario Neurotrauma Foundation (grant 2007-ABI-ISIS-525).
The opinions contained in this article are those of the authors and should not be construed as an official statement from the National Institutes of Health, National Center for Medical Rehabilitation Research, the National Institute on Disability and Rehabilitation Research, or the Ontario Neurotrauma Foundation.
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated.
Abbreviations
- CSI
Comprehensive Severity Index
- LOS
Length of stay
- TBI
Traumatic brain injury
Footnotes
- - Annual meetings of the American Congress of Rehabilitation Medicine (ACRM) in October 2009 in Denver, CO; October 2011 in Atlanta, GA; October 2012 in Vancouver, BC, Canada.
- - International Brain Injury Association (IBIA) World Congress in March 2010 in Washington, DC; March 2014 in San Francisco, CA.
- -Annual Canadian Association of Physical Medicine and Rehabilitation meetings in May 2010 in Winnipeg, Manitoba.
- -Federal TBI Interagency Conference in June 2011 in Washington, DC.
- -Annual American Academy of Physical Medicine and Rehabilitation meetings in November 2012 in Atlanta, GA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hart T, Kozlowski A, Whyte J, et al. Functional recovery after severe traumatic brain injury: An individual growth curve approach. Arch Phys Med Rehabil. 2014 doi: 10.1016/j.apmr.2014.07.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Leon-Carrion J, Machuca-Murga F, Solis-Marcos I, Leon-Dominguez U, Dominguez-Morales Mdel R. The sooner patients begin neurorehabilitation, the better their functional outcome. Brain Inj. 2013;27(10):1119–1123. doi: 10.3109/02699052.2013.804204. [DOI] [PubMed] [Google Scholar]
- 3.Renner C, Hummelsheim H, Kopczak A, et al. The influence of gender on the injury severity, course and outcome of traumatic brain injury. Brain Inj. 2012;26(11):1360–1371. doi: 10.3109/02699052.2012.667592. [DOI] [PubMed] [Google Scholar]
- 4.Gardizi E, Hanks RA, Millis SR, Figueroa MJ. Comorbidity and insurance as predictors of disability following traumatic brain injury. Arch Phys Med Rehabil. 2014 doi: 10.1016/j.apmr.2014.06.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Sigurdardottir S, Andelic N, Wehling E, et al. Neuropsychological functioning in a national cohort of severe traumatic brain injury: Demographic and acute injury-related predictors. J Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000039. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Perrin PB, Niemeier JP, Mougeot JL, et al. Measures of injury severity and prediction of acute traumatic brain injury outcomes. J Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000026. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Barker MD, Whyte J, Pretz CR, et al. Application and clinical utility of the glasgow coma scale over time: A study employing the NIDRR traumatic brain injury model systems database. J Head Trauma Rehabil. 2014;29(5):400–6. doi: 10.1097/HTR.0b013e31828a0a45. [DOI] [PubMed] [Google Scholar]
- 8.Eastvold AD, Walker WC, Curtiss G, Schwab K, Vanderploeg RD. The differential contributions of posttraumatic amnesia duration and time since injury in prediction of functional outcomes following moderate-to-severe traumatic brain injury. J Head Trauma Rehabil. 2013;28(1):48–58. doi: 10.1097/HTR.0b013e31823c9317. [DOI] [PubMed] [Google Scholar]
- 9.Coronado V. Traumatic brain injury hospitalizations in the US: Trends, costs, and prevention. national hospital discharge survey, 1995-2010. MMWR. in press. [Google Scholar]
- 10.Godbolt AK, Stenberg M, Lindgren M, et al. Associations between care pathways and outcome 1 year after severe traumatic brain injury. J Head Trauma Rehabil. 2014 doi: 10.1097/HTR.0000000000000050. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Avesani R, Carraro E, Armani G, Masiero S. Exploring variables associated with rehabilitation length of stay in brain injuries patients. Eur J Phys Rehabil Med. 2012;48(3):433–441. [PubMed] [Google Scholar]
- 12.Corrigan JD, Mysiw WJ. Substance abuse among persons with TBI. In: Zasler ND, Katz DI, Zafonte RD, Arciniegas DB, Bullock MR, Kreutzer JS, editors. Brain injury medicine: Principles and practice. Second. New York: Demos Medical Publishing; 2012. pp. 1315–1328. [Google Scholar]
- 13.Leong BK, Mazlan M, Abd Rahim RB, Ganesan D. Concomitant injuries and its influence on functional outcome after traumatic brain injury. Disabil Rehabil. 2013;35(18):1546–1551. doi: 10.3109/09638288.2012.748832. [DOI] [PubMed] [Google Scholar]
- 14.Horn SD, Corrigan JD, Bogner J, et al. Traumatic brain injury practice-based evidence study: Design and patients, centers, treatments, and outcomes. Arch Phys Med Rehabil. 2015;(Suppl 1) doi: 10.1016/j.apmr.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz DI, Alexander MP. Traumatic brain injury. predicting course of recovery and outcome for patients admitted to rehabilitation. Arch Neurol. 1994;51(7):661–670. doi: 10.1001/archneur.1994.00540190041013. [DOI] [PubMed] [Google Scholar]
- 16.Cifu DX, Kreutzer JS, Marwitz JH, Rosenthal M, Englander J, High W. Functional outcomes of older adults with traumatic brain injury: A prospective, multicenter analysis. Arch Phys Med Rehabil. 1996;77(9):883–888. doi: 10.1016/s0003-9993(96)90274-9. [DOI] [PubMed] [Google Scholar]
- 17.Corrigan JD, Bogner J, Mellick D, et al. Prior history of traumatic brain injury among persons in the traumatic brain injury model systems national database. Arch Phys Med Rehabil. 2013;94(10):1940–1950. doi: 10.1016/j.apmr.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould KR, Ponsford JL, Johnston L, Schonberger M. Relationship between psychiatric disorders and 1-year psychosocial outcome following traumatic brain injury. J Head Trauma Rehabil. 2011;26(1):79–89. doi: 10.1097/HTR.0b013e3182036799. [DOI] [PubMed] [Google Scholar]
- 19.Schonberger M, Ponsford J, Olver J, Ponsford M, Wirtz M. Prediction of functional and employment outcome 1 year after traumatic brain injury: A structural equation modelling approach. J Neurol Neurosurg Psychiatry. 2011;82(8):936–941. doi: 10.1136/jnnp.2010.210021. [DOI] [PubMed] [Google Scholar]
- 20.Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA. 2010;303(19):1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider EB, Sur S, Raymont V, et al. Functional recovery after moderate/severe traumatic brain injury: A role for cognitive reserve? Neurology. 2014;82(18):1636–1642. doi: 10.1212/WNL.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sumowski JF, Chiaravalloti N, Krch D, Paxton J, Deluca J. Education attenuates the negative impact of traumatic brain injury on cognitive status. Arch Phys Med Rehabil. 2013;94(12):2562–2564. doi: 10.1016/j.apmr.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Ponsford J. Factors contributing to outcome following traumatic brain injury. NeuroRehabilitation. 2013;32(4):803–815. doi: 10.3233/NRE-130904. [DOI] [PubMed] [Google Scholar]
- 24.Heffernan DS, Vera RM, Monaghan SF, et al. Impact of socioethnic factors on outcomes following traumatic brain injury. J Trauma. 2011;70(3):527–534. doi: 10.1097/TA.0b013e31820d0ed7. [DOI] [PubMed] [Google Scholar]
- 25.Heinemann AW, Linacre JM, Wright BD, Hamilton BB, Granger C. Relationships between impairment and physical disability as measured by the functional independence measure. Arch Phys Med Rehabil. 1993;74:566–73. doi: 10.1016/0003-9993(93)90153-2. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen AR, Severinsen K, Nielsen JF. The effect of age on rehabilitation outcome after traumatic brain injury assessed by the Functional Independence Measure (FIM) Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314545171. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Reeder KP, Rosenthal M, Lichtenberg P, Wood D. Impact of age on functional outcome following traumatic brain injury. J Head Trauma Rehabil. 1996;11(3):22–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. OLS Regression Models Predicting Rehabilitation Length of Stay by Admission FIM Cognitive score
Supplemental Figure 2. Logistic Regression Models Predicting Discharge Destination Home by Admission FIM Cognitive score
Supplemental Figure 3. OLS Regression Models Predicting Discharge FIM Motor Score Rasch transformed by Admission FIM Cognitive score
Supplemental Figure 4. OLS Regression Models Predicting Discharge FIM Cognitive Score Rasch transformed by Admission FIM Cognitive score
Supplemental Figure 5. OLS Regression Models Predicting 9-Month FIM Motor Score Rasch transformed by Admission FIM Cognitive score
Suplemental Figure 6. OLS Regression Models Predicting 9-Month FIM Cognitive Score Rasch transformed by Admission FIM Cognitive score