Abstract
Background
Non-cardiovascular comorbidities are recognised as independent prognostic factors in selected heart failure (HF) populations, but the evidence on non-selected HF and how comorbid disease severity and change impacts on outcomes has not been synthesised. We identified primary HF comorbidity follow-up studies to compare the impact of non-cardiovascular comorbidity, severity and change on the outcomes of quality of life, all-cause hospital admissions and all-cause mortality.
Methods
Literature databases (Jan 1990–May 2013) were screened using validated strategies and quality appraisal (QUIPS tool). Adjusted hazard ratios for the main HF outcomes were combined using random effects meta-analysis and inclusion of comorbidity in prognostic models was described.
Results
There were 68 primary HF studies covering nine non-cardiovascular comorbidities. Most were on diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD) and renal dysfunction (RD) for the outcome of mortality (93%) and hospital admissions (16%), median follow-up of 4 years. The adjusted associations between HF comorbidity and mortality were DM (HR 1.34; 95% CI 1.2, 1.5), COPD (1.39; 1.2, 1.6) and RD (1.52; 1.3, 1.7). Comorbidity severity increased mortality from moderate to severe disease by an estimated 78%, 42% and 80% respectively. The risk of hospital admissions increased up to 50% for each disease. Few studies or prognostic models included comorbidity change.
Conclusions
Non-cardiovascular comorbidity and severity significantly increases the prognostic risk of poor outcomes in non-selected HF populations but there is a major gap in investigating change in comorbid status over time. The evidence supports a step-change for the inclusion of comorbidity severity in new HF interventions to improve prognostic outcomes.
Keywords: Heart failure, Comorbidity, Prognosis, Systematic review, Meta-analysis
Highlights
-
•
We synthesise the prognosis evidence on non-CVD comorbidity and severity in non-selected HF
-
•
Most studies focused on three comorbid diseases for mortality and admissions and none for QoL
-
•
COPD, diabetes and CKD increased mortality and admission risk in non-selected HF
-
•
Severity studies were few but where available, risk increased with disease severity
-
•
Comorbidity severity is important but has yet to be included in HF prognostic models
1. Introduction
Non-cardiovascular (CVD) comorbidity is common in heart failure (HF) and is well known to influence its prognosis [1,2] which may occur through shared risk factors [3,4] or direct pathophysiological links [5–8]. For non-cardiovascular diseases in HF, the current comorbidity focus has been on conditions such as diabetes and chronic kidney disease (CKD) in selected populations. The range of potential non-cardiovascular comorbidity is most commonly identified in the general non-selected HF population and yet current investigations have focused on distinct prognostic factors associated with aetiology [9] or HF sub-groups selected by ejection fraction [10,11]. To capture the HF spectrum [12] we focused on the general non-selected population to identify the fullest range of non-CVD comorbidity and identified studies which could support the development of better prognostic models.
Current evidence on cardiovascular comorbidity such as hypertension or ischemic heart disease [13–15] has shown that severity is associated with poor HF outcomes [16,17]. Evidence from CKD studies also shows that severity and additionally change in status are important determinants of higher hospital admissions and mortality [18,19]. The combined evidence for cardiovascular comorbidity and CKD severity in HF, generates the hypothesis that weighting of other non-CVD comorbidities by severity and its change over time may provide better risk stratification for identifying patients with the worst prognostic outcomes. This evidence is vital if precise comorbidity measurements are incorporated into practical applications of prognosis and for developing new interventions to improve outcomes. Prognostic models, which incorporate comorbidity severity and change, need to apply to the broad spectrum of HF and to the life course of outcomes from diagnosis and quality of life, to end-stage disease, hospital admissions and death.
We conducted a systematic review to synthesise the current evidence on non-CVD comorbidity in the non-selected general population of HF. We postulated two hypotheses: (i) the prognostic risk estimates for the association between chronic disease comorbidities and quality of life, hospital admissions and mortality outcomes in non-selected HF would differ by comorbid disease and (ii) increasing comorbidity severity and change in HF would be associated with worse quality of life, hospital admissions and mortality outcomes.
2. Methods
A systematic review proposal was registered with PROSPERO (protocol no. CRD42013003605 — www.crd.york.ac.uk/prospero/) and peer reviewed by the Cochrane Prognosis Methods group. The development stages were: (i) literature searching of key databases, (ii) screening and selection of articles using inclusion criteria, (iii) extraction using structured data collection, (iv) quality appraisal of the selected articles and (v) narrative and meta-analyses of the study findings. We used PRISMA [20] and MOOSE [21] international guidelines for reporting the review and meta-analysis (Supplementary material online, Appendices S1.1 and 1.2).
2.1. Literature searches
We searched MEDLINE, EMBASE and CINAHL for studies published between January 1st 1990 and 1st May 2013. Validated search strategies for prognosis follow-up studies [22,23] were combined with HF population and outcome search strings that included quality of life, hospital admission and mortality terms (Supplementary material online, Appendix S2).
The search approaches were validated by the following: (i) 3 cardiology physicians, (ii) comparison with prior HF-focused prognosis systematic reviews with similar inclusion criteria [24,25] and (iii) comparison with key comorbidity prognosis articles. Additional searches included unpublished studies, reference lists and citations, key journals and communication with international HF experts from Europe and the United States.
2.2. Study selection
Eligible cohort studies in adults 18 years and over with de novo or chronic HF and two or more months of follow-up were identified with quality of life, all-cause hospital admissions or all-cause mortality as outcomes. These studies had not selected HF samples by aetiology, ejection fraction or surgical intervention and diagnosis was based on either clinical assessment [26], medical record diagnosis or an administration code. Excluded studies had either used composite outcomes or been published in non-English journals.
Abstract screening identified cohort studies investigating (i) the association between a primary non-cardiovascular disease comorbidity in HF and outcome, labelled ‘chronic disease focus’ prognostic factor studies, (ii) a number of potential prognostic factors including a non-cardiovascular comorbidity as an independent and significant factor, labelled as ‘general’ prognostic factor studies and (iii) prognostic model studies that combined two or more factors, including a non-cardiovascular comorbidity, to estimate risk. RCTs were only included if they were conducted in non-selected HF as defined in this review and where both trial arms were used for the prognosis study, which is the recommended approach given the often negligible effect of the intervention [27]. Two independent reviewers with a third for arbitration selected abstracts for full paper review based on inclusion criteria.
2.3. Definition of comorbidity
Comorbidity was defined as any non-cardiovascular disease in HF based on a clinical diagnosis, administration code or patient self-report. In addition to the comorbidity disease status, we also identified, where possible, indicators of severity which included complications, drugs, healthcare use episodes and physiological markers used in current diagnostic frameworks. In an initial literature scoping exercise, the clear current evidence for comorbidity in HF was for CKD with two completed systematic reviews [28,29], but only studies meeting our study inclusion criteria were included from these reviews or publications subsequent to 2005.
2.4. Data extraction from studies
All available data were extracted using a pre-defined template which included the domains of study characteristics, comorbidity measurement, outcome measures, statistical analysis and results. Study characteristics included source, setting, eligibility, sample size, mean age, methods (design, inclusion/exclusion criteria, follow-up), males (%), ethnicity (%), clinical HF definition, New York Heart Association (NYHA) stages 3 to 4 (%), systolic HF (%) and mean ejection fraction (EF). Comorbidity measures included definition, type, severity or change indicator and prevalence. Outcome measures were all-cause mortality or hospital admission or quality of life. Statistical analysis and results included the regression method, variables for adjustment of confounding, number of events, non-adjusted and adjusted effect estimates and measure of association (odds ratios or hazard ratios). Where characteristics were reported by study sub-groups, whole study group estimates were calculated or extracted.
For the ‘chronic disease focus’ HF prognostic factor studies, significant and non-significant effect estimates were extracted for the most adjusted primary comorbid disease exposure and not additional chronic diseases included as confounders (these confounders are likely to have their own unconsidered confounding). For the ‘general’ prognostic factor and model HF studies, only comorbid chronic diseases that were significant in the final adjusted analyses were extracted as non-significant exposure effects are mostly unreported.
2.5. Quality appraisal
We used the Quality in Prognosis Studies Tool (QUIPS) [30] to evaluate the risk of study bias in six domains: participation, attrition, prognostic factor measurement, outcome measurement, study confounding, statistical analysis and reporting. We recorded selected objective criteria in the QUIPs tool, other internal and external validity markers [31] and criteria for prognostic models to assess the risk of bias for each domain (low, medium and high risk) (Supplementary material online, Appendix S3).
This assessment informed the study inclusion in meta-analysis and sensitivity analysis of combined effects, removing studies with one or more individual domains at high risk. Inter-rater agreement for overall study risk was measured using Cohen's kappa coefficient.
2.6. Statistical analysis
Meta-analysis methods were applied using a specified inclusion format to a sub-set of chronic disease focus HF prognostic factor studies as they had not been selected on the basis of the significance or independence of the comorbidity exposure. Individual study results were reported when meta-analysis was not possible due to low numbers of studies for specific outcomes and summaries of the ‘general’ prognostic factor and model studies by each chronic disease were provided.
The only primary studies identified that included a severity measure were for diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD) and chronic kidney disease (CKD) in HF. The known indicators of severity and change were glycosylated haemoglobin (HbA1c) for DM and forced expiratory volume in 1 s (FEV1) for COPD. The framework for measuring renal function and change in HF studies was based on eGFR or creatinine (Cr). The broader term ‘renal dysfunction’ (RD) could also include normal to sub-threshold renal function levels without underlying and established CKD. So based on guidelines [32] ‘any renal dysfunction’ was defined as eGFR of < 60 mL/min/1.73 m2, with severity level defined as mild (60–89), moderate (30–59) and severe (< 30 mL/min) compared to the reference category. Moderate change in severity was defined as an increase in creatinine (Cr) of ≥ 0.3 mg/dL from hospital baseline to study defined end point and severe increase in severity as an increase in Cr of ≥ 0.5 mg/dL.
Random effects meta-analysis (DerSimonian and Laird method) [33] was used to combine maximally adjusted hazard ratios (HRs) from individual studies using Metan in Stata version 13. This method assumes that the exposure effects differ across studies and takes account of between and within study variation by adjusting the standard errors of the individual studies. Where meta-analysis indicated heterogeneity (I2 ≥ 40%, Chi2; p ≤ 0.1), Galbraith plots [34] and a-priori selected potential effect modifiers (setting, population and exposure definitions and the risk of bias level) were used to perform sensitivity analysis and increase accuracy of estimates by removal of studies. Small study effects and publication bias were investigated by funnel plots and Egger tests (p ≤ 0.1 indicating possible publication bias) [35].
3. Results
3.1. Overall study characteristics
From a total of 10,331 studies (Supplementary material online, Appendix S4), 68 were eligible for the review (Appendix S5 and Table 1). There were 34 ‘chronic disease focus’ studies, 22 ‘general’ studies and 12 prognostic model studies (Supplementary material online, Tables S1.1–3.2).
Table 1.
Overall study characteristics for the 68 included studies.
| ‘Chronic disease focus’ prognostic factor studies |
‘General’ prognostic factor studies | Prognostic model studies | |||||
|---|---|---|---|---|---|---|---|
| Diabetes | COPD | Renal dysfunction | Rheumatoid arthritis | ||||
| Study characteristics | |||||||
| Number of unique studies | 11 | 5 | 17 | 1 | 22 | 12 | |
| Number of participants | 138,953 | 7121 | 102,638 | 955 | 211,077 | 265,573 | |
| Mean study follow-up; months (m), years (y) | 6 m–7.5 y | 2.9–4.5 y | 6 m–6.5 y | 1 y | 6 m–4.7 y | 2.4 m–5.2 y | |
| Location (n) | |||||||
| North America | 4 | 7 | 1 | 7 | 6 | ||
| South America | 1 | 1 | |||||
| Europe | 5 | 5 | 5 | 9 | 6 | ||
| Asia | 4 | 6 | |||||
| Multiple | 1 | ||||||
| Setting (n) | |||||||
| RCT | 6 | 1 | 5 | 1 | 5 | ||
| Hospital | 4 | 3 | 11 | 1 | 16 | 5 | |
| Community | 1 | ||||||
| Hospital/community | 1 | 1 | 1 | 4 | 2 | ||
| Population characteristics | |||||||
| Clinical definition | |||||||
| Clinical diagnosis | 6 | 3 | 14 | 1 | 15 | 7 | |
| Clinical record | 1 | 1 | |||||
| Multiple | 1 | ||||||
| Administration code | 4 | 2 | 2 | 6 | 4 | ||
| Unspecified | 1 | ||||||
| Mean age; years (SD) range | 50 (11)–77 (12) | 70 (12)–80 (IQR75–87) | 62 (15)–80 (median) | 45 | 66 (12)–86 (5) yrs | 66 (11)–79 (6) | |
| Males (%) range | 46–73 | 50–71 | 43–76 | 77 (12) | 40–71 | 34–98 | |
| Systolic HF (study defined) (%) | 47–87 | 25–83 | 36–82 | 51 | 36–79 | 44–90 | |
| Mean ejection fraction; % (SD) range | 27 (14)–47 (13) | 33 (12)–50 (16) | 27 (12)–44 (16) | 44 | 35 (14)–54 (15) | 33 (9)–43 (14) | |
| NYHA (Stage 3/4) (%) | 36–84 | 52–95 | 32–84 | 12–96 | 25–55 | ||
| Exposure characteristics | |||||||
| Prevalence % (range) | 16 (13–47) | 19 (17–35) | Any 49 (39–79) | 11 | Diabetes | (n = 17) 3–61% | (n = 7) 14–36% |
| Mild 43 | COPD | (n = 6) 20–47% | (n = 6) 10–31% | ||||
| Moderate 36 (20–67) | Renal dysfunction | (n = 12) 1–70% | (n = 5) 8–25% | ||||
| Severe 6 (2–33)% | Arthritis | ||||||
| Cancer | (n = 2) 5–29% | (n = 3) 2–9% | |||||
| Dementia | (n = 7) 2–12% | (n = 3) 5–9% | |||||
| Other lung disease | (n = 2) 10–23% | (n = 1) 9% | |||||
| Liver disease | (n = 1) 7% | (n = 3) 1–3% | |||||
| Exposure measure (n) | |||||||
| Status | 9 | 5 | 8 | 1 | 20 | ||
| Severity | 4 | 1 | 10 | 4 | |||
| Severity change | 0 | 7 | 1 | ||||
| Outcomes (n) | |||||||
| All-cause mortality | 11 | 5 | 17 | 1 | 19 | 10 | |
| All-cause hospital admission | 1 | 4 | 4 | 2 | |||
| Quality of life | 0 | 0 | 0 | 0 | 0 | 0 | |
| Risk of bias (%) | |||||||
| Low | 36 | 40 | 29 | 32 | 42 | ||
| Moderate | 64 | 60 | 65 | 1 | 64 | 58 | |
| High | 6 | 5 | |||||
Any renal dysfunction is defined by eGFR < 60 mL/min, mild by eGFR 60–89 mL/min, moderate by eGFR 30–59 mL/min and severe by eGFR < 30 mL/min compared to the highest category. Moderate severity change was defined by an increase in creatinine (Cr) of ≥ 0.3 mg/dL from hospital baseline to study defined end point and severe by an increase in Cr of ≥ 0.5 mg/dL.
The 68 studies were based in 16 countries and 60 had more than 1-year of follow-up, with median follow-up reported in 50 studies of 4 years [interquartile range 1 to 5 years]. Most studies were hospital-based observational studies (60%) and 26% were RCTs. The proportion of HF patients with systolic dysfunction was 58% and for NYHA class 3 or 4 was 51%. Mortality outcome was investigated in 63 studies, hospital admissions in 11 and quality of life in none.
There was a high level of agreement on the overall risk score between the two reviewers (kappa coefficient of 0.86). The overall risk of bias was low in 34% (n = 23) studies, moderate in 63% (n = 43) studies and high in 3% (n = 2) studies (Supplementary material online, Tables S4.1–4.2). The risk domains with low risk scores were outcome measurement and study attrition and high risk scores were study confounding and statistical analysis and reporting (Fig. 1). Nine comorbidities had been investigated in HF, but meta-analysis was only possible for DM, COPD and RD.
Fig. 1.
The risk of bias domain summary results.
3.1.1. DM comorbidity in HF and mortality; meta-analysis
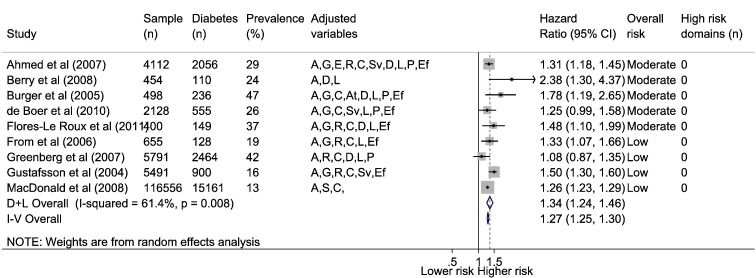
Nine HF and DM focused studies reported all-cause mortality rates with a mean follow-up in individual studies of ≥ 6 months (range 6 months to 7.5 years). Out of 135,402 HF patients (56% with LVSD), 21,455 (16%) had diabetes (range 13 to 47%). Over follow-up crude mortality was 62% in HF patients with and without comorbid diabetes. Using random effects model the combined adjusted mortality risk was hazard ratio (HR) of 1.34 (95% confidence interval 1.24, 1.46) (Fig. 2). Following exclusion of a study from the meta-analysis which included more chronically severe patients [36] identified in a Galbraith plot (Supplementary material online, Fig. S1a), heterogeneity became non-significant (I2 = 35%, p = 0.15). Funnel plot indicated possible publication bias (Egger test p = 0.12) (Supplementary material online, Fig. S2a), but removal of the two smallest studies [37,38], had little effect on the pooled mortality risk estimate (HR 1.31; 1.22, 1.41) (Egger p = 0.43). Significant interactions for higher mortality risk in HF were found between diabetes status and those age 65 years or less [39], females [36,39], non-ischemic HF aetiology (p = 0.013) [40], coronary artery disease [41] and LVSD [42].
Fig. 2.
Association between HF and diabetes comorbidity and all-cause mortality.
Diabetes comorbidity defined by clinical diagnosis, administration code, prescription or patient self-report. Adjusted variables: age (A), gender (G), ethnicity (E), social (S), risk factors (R), comorbidities (C), aetiology (At), heart failure severity (Sv), drugs (D), laboratory (L), physical (P), ejection fraction (Ef).
3.1.2. DM comorbidity severity in HF and mortality
When diabetes was stratified by treatment type, non-significant effects were found for oral and diet treated diabetes (HR 1.38; p = 0.13 and HR 1.33; p = 0.22 respectively), but the mortality effects increased and became significant in the undiagnosed (not treated, HR 1.69; p < 0.01) and insulin treated group (HR 2.11; p < 0.01) [43]. This represents an estimated 78% increase in HR mortality risk from the diet to insulin treated group. Increasing HbA1c levels were also reported as increasing HF mortality risk in one study with an adjusted risk per 1% higher HbA1c of HR 1.14 (1.06, 1.23) [44].
3.1.3. DM comorbidity in HF and mortality; general factor and model studies
The significant associations between DM in HF and all-cause mortality ranged from HR 1.16 to 3.19 in the 14 ‘general’ prognostic factor studies and from HR 1.34 to 2.37 in the 6 prognostic model studies (Supplementary material online, Fig. S3a–b). A significant interaction was found between age and diabetes in one study with younger group aged < 85 years being associated with poorer mortality outcome (p = 0.014) [45]. Only one of the six prognostic models had stratified by treatment severity [46] for insulin prescription (HR 1.8; 1.56, 2.08) and ‘other treatment’ (HR 1.5; 1.34, 1.68), but none included a change measure.
3.1.4. DM comorbidity in HF and hospital admissions
Only one DM chronic disease focused study included all-cause hospital admissions which showed a significant adjusted association (HR 1.28; 1.19, 1.38) [47]. In the 4 ‘general’ prognostic factor and 1 model studies, the range of significant associations with all-cause hospital admissions were from HR 1.13 to 1.53 and HR 1.17 respectively (Supplementary material online, Fig. S3). None of the ‘general’ prognostic factor or model studies for hospital admissions included a comorbidity severity or change measure.
3.1.5. COPD comorbidity in HF and mortality; meta-analysis
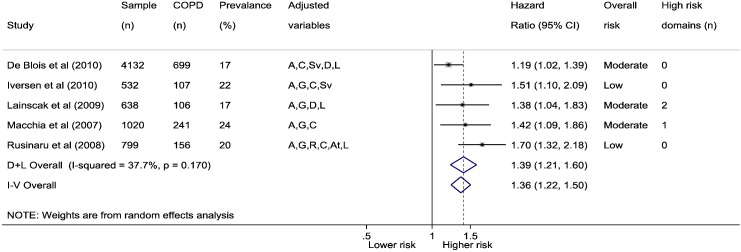
Five COPD focused studies reported adjusted associations with all-cause mortality with individual study mean follow-up ranging from 1 to 8 years. Out of 7121 HF patients (54% with LVSD), 1309 (18%) had COPD (range 17 to 35%). Over follow-up, 48% of HF patients with COPD had died compared to 38% without COPD, and using random effects, the combined adjusted all-cause mortality risk was HR 1.39 (1.21, 1.6) (Fig. 3). There was marginal evidence of heterogeneity in studies (I2 = 38%, p = 0.17) (Supplementary material online, Fig. S1b) and publication bias (Egger test p = 0.12) (Supplementary material online, Fig. S2b).
Fig. 3.
Association between HF and COPD comorbidity and all-cause mortality.
COPD comorbidity defined by clinical diagnosis, administration code or patient self-report. Adjusted variables: age (A), gender (G), ethnicity (E), social (S), risk factors (R), comorbidities (C), aetiology (At), heart failure severity (Sv), drugs (D), laboratory (L), physical (P), ejection fraction (Ef).
3.1.6. COPD comorbidity severity in HF and mortality
One study (N = 532) showed that the association between lower COPD severity and all-cause mortality in HF was reduced, with an increasing FEV1 (per 10% of predicted) being associated with an estimated 14% reduction in death (adjusted HR 0.86; 0.8, 0.92) [48]. The other reported unadjusted estimates were: HR 1.26 (0.9, 1.8) for the moderate COPD severity group and HR 1.68 (1.2, 2.3) for the severe group.
3.1.7. COPD comorbidity in HF and mortality; general factor and model studies
The associations between COPD in HF and all-cause mortality risk ranged from HR 1.24 to 1.7 in the 5 ‘general’ prognostic factor studies and HR 1.23 to 1.6 in the 5 prognostic model studies (Supplementary material online, Fig. S3d). Two of the studies included community patients [49,50] one of which compared the associated risk from COPD on mortality in community patients (HR 1.7; 1.58, 1.82) to hospital patients (HR 1.24; 1.19, 1.31). None of the ‘general’ prognostic factor or model studies for mortality included a comorbidity severity or change measure.
3.1.8. COPD comorbidity in HF and hospital admissions
No HF studies were identified investigating the primary association between COPD and hospital admissions. Only one ‘general’ prognostic factor study and one prognostic model study included COPD to investigate hospital admissions (adjusted HR 1.47; 1.3, 1.7 and odds ratio (OR) 1.14; 1.1, 1.2 respectively) [51,52] (Supplementary material online, Fig. S3e). However neither study had included a COPD comorbidity severity or change measure.
3.1.9. RD comorbidity severity in HF and mortality; meta-analysis
Seven primary studies reported the adjusted association between HF and renal dysfunction and all-cause mortality, with an individual study median follow-up ranging from 20 to 38 months. Out of 69,520 HF patients, 28,596 (41%) had ‘any’ renal dysfunction (range 36 to 70%). Over follow-up, 51% of HF patients with renal dysfunction died compared to 42% of those without renal dysfunction, with a combined adjusted mortality risk of HR 1.52 (1.34, 1.71) (Fig. 4).
Fig. 4.
Association between HF and renal dysfunction comorbidity and all-cause mortality.
Renal dysfunction comorbidity defined by eGFR < 60 mL/min/m2. Adjusted variables: age (A), gender (G), ethnicity (E), social (S), risk factors (R), comorbidities (C), aetiology (At), heart failure severity (Sv), drugs (D), laboratory (L), physical (P), ejection fraction (Ef).
Galbraith plot identified two studies as being heterogeneous (Supplementary material online, Fig. S1c). Following removal of a study with a higher proportion of males (76%) and a lower mean ejection fraction (32%) [18] and another with an older population (mean age, 76 years) and a lower proportion of males (43%) [53], heterogeneity became non-significant (I2 = 0%, p = 0.825). The pooled effect estimate increased (HR 1.62; 1.59, 1.66) and there was no significant evidence of publication bias (Egger test p = 0.56) (Fig. S2c).
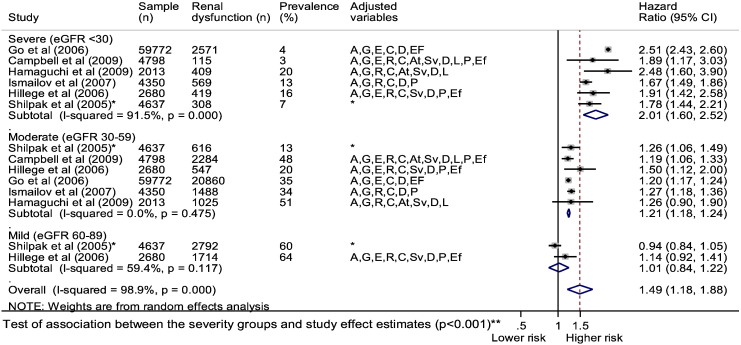
Five RD severity stratified studies reported adjusted associations between HF and all-cause mortality. Out of 64,257 HF patients, 24,349 (38%) had moderate RD and 3784 (6%) had severe RD. Over follow-up, 63% of HF patients with severe and 48% with moderate RD died compared to 42% of patients without RD. Random effects meta-analysis (including an additional study from a prior review) [28] was performed, stratified by severity group (Fig. 5) which showed the following adjusted estimates of association with all-cause mortality: mild renal dysfunction (HR 1.01; 0.84, 1.22), moderate renal dysfunction (1.21; 1.18, 1.24), and severe RD (2.01; 1.60, 2.52). This represents an estimated 80% increase in mortality risk between moderate and severe RD. The association between the severity subgroups and the study effect estimates was significant (p < 0.001). Using meta-regression, when the upper eGFR limit for each study defined severity category (x-axis) was plotted against their associated hazard ratio (y-axis), there was a ‘dose response’ association between HF, reducing eGFR and increasing mortality (Fig. 6).
Fig. 5.
Association between HF and renal dysfunction comorbidity and all-cause mortality stratified by severity.
Adjusted variables: age (A), gender (G), ethnicity (E), social (S), risk factors (R), comorbidities (C), aetiology (At), heart failure severity (Sv), drugs (D), laboratory (L), physical (P), ejection fraction (Ef) *from prior systematic review [28].
**Test of association between the severity subgroups and the study effect estimates was performed using random effects meta-regression with Monte Carlo permutations to calculate the p value.
Fig. 6.
All-cause mortality risk by upper eGFR severity category limit in HF.
All-cause mortality risk plotted against upper eGFR severity category limit (study defined) in HF.
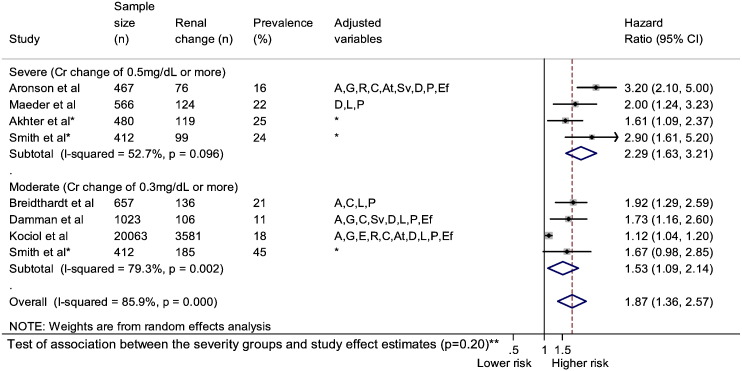
3.1.10. RD comorbidity severity change in HF and mortality; meta-analysis
Five HF studies investigated worsening renal function (WRF) from hospital admission baseline to discharge or study defined endpoint. Random effects meta-analysis (including two further studies from the prior review of renal function change [29]) stratified WRF showed significant HR for mortality which increased from 1.53 (1.09, 2.14) in the moderate change group to 2.29 (1.63, 3.21) in the severe change group (Fig. 7). The association between the severity change subgroup and the study effect estimates was non-significant (p = 0.20) but both change groups were independently and significantly associated with mortality (p < 0.05). One study showed that the monthly percentage reduction in eGFR of ≥ 1% was associated with an adjusted mortality risk of HR 3.6 (2.2, 5.7) [54] and a linear relationship was found between increasing eGFR and all-cause mortality risk in four other studies [55–57].
Fig. 7.
Association between HF and renal dysfunction comorbidity and all-cause mortality stratified by severity change.
Adjusted variables: age (A), gender (G), ethnicity (E), social (S), risk factors (R), comorbidities (C), aetiology (At), heart failure severity (Sv), drugs (D), laboratory (L), physical (P), ejection fraction (Ef) *from previous systematic review [29].
**Test of association between the severity subgroups and the study effect estimates was performed using random effects meta-regression with Monte Carlo permutations to calculate the p value.
3.1.11. RD comorbidity in HF and mortality; general prognostic factor and model studies
The association between HF and RD with all-cause mortality, showed estimates ranging from HR 1.35 to 2.27 in the 11 ‘general’ prognostic factor studies and HR 1.37 to 5.22 in the 4 prognostic model studies (Supplementary material online, Fig. S3f–g). Younger age (< 85 years) was associated with increased mortality risk from RD in HF [45]. RD was included in 4 ‘general’ prognostic factor studies and one model study by a severity indicator and in one ‘general’ factor study by severity change (pre-hospital WRF) [58].
3.1.12. RD comorbidity severity in HF and hospital admissions
Two studies focused on the association between RD severity and the risk of all-cause hospital admissions. In one study, an eGFR of > 53 mL/min compared to < 35 mL/min was associated with a significant reduction in hospital admissions (HR 0.77; 0.56, 1.06) [24]. In another study follow-up, 66% of patients without RD experienced a hospital admission compared to 68% of those with moderate RD (HR 1.16; 1.06, 1.27) and 73% of those with severe RD (HR 1.77; 1.16, 2.69) [18,56]. Only two studies focused on WRF and hospital admissions with significant association in one [59] (HR 1.57; 1.2, 2.2) and non-significant in another [60].
There was one ‘general’ prognostic factor study and one prognostic model study that had identified RD status as a significant factor for hospital admission (adjusted HR 1.32; 1.1, 1.5 and OR 1.09; 1.0, 1.1 respectively) [52,61]. Both studies had not assessed RD severity or change.
3.1.13. HF and other non-CVD comorbid diseases
A single study focused on the association between rheumatoid arthritis and all-cause mortality in HF showing an independent and significant effect (HR 1.89; 1.3, 2.8). Eleven HF ‘general’ prognostic factor and model studies investigated mortality for comorbid arthritis (n = 2), dementia (n = 5), cancer (n = 10), other lung disease (n = 2) or liver disease (n = 3) (the range of estimates is reported in Supplementary material online, Fig. S3h). Three mortality models and one hospital admission HF model study included other diseases such as dementia, cancer and liver disease [49,52,62–63]. All additional diseases had been included by status and not a severity or change indicator.
4. Discussion
This systematic review reports the current prognostic evidence in non-selected HF populations on the impact of non-CVD comorbidity and severity on mortality and hospital admission outcomes. There were 68 primary HF studies covering nine non-CVD comorbid conditions. Around half the studies identified had a chronic disease as the primary comorbidity focus and the other half were a mix of ‘general’ prognostic factor and model studies. Most studies (93%) focused on mortality and only 16% included hospital admissions outcomes. Meta-analysis was only possible for the mortality outcome as there were few primary comorbidity studies for hospital admissions. Furthermore, no prognosis studies on HF comorbidity and quality of life outcome were identified using the study inclusion criteria despite the fact that HF is associated with poor quality of life [64,65] that worsens as the disease progresses [66].
Most of the current evidence is on prevalent comorbidity in HF, which were RD, DM or COPD and five other conditions which included; arthritis, dementia, cancer, other lung disease or liver disease. In the non-selected HF population, non-cardiovascular comorbidity was associated with increasing likelihood of hospital admissions and higher mortality, but the prognostic risk estimates did not differ by the type of disease or the chosen outcomes. In terms of the increasing comorbidity severity and change in HF, there was scarce evidence even in the commonly prevalent conditions of DM and COPD, with exception of RD where worsening renal failure has been investigated. Whilst much of this evidence is highlighting the importance of non-cardiovascular comorbidity status in HF, there is still a lack of evidence on how the fuller range of comorbidity severity and change might be incorporated into HF prognosis. Prognostic models thus far, have incorporated non-cardiovascular comorbidity status as a prognostic indicator for mortality and some for hospital admissions, but the clear evidence gap is around severity and change for each comorbid condition, as well as what happens to the model when patients suffer from multiple non-cardiovascular conditions at the same time.
Whilst comorbidity may have been considered as a confounding factor in quality of life studies or in individual studies for selected HF groups [65,67], there has been no systematic approach to investigating comorbidity, severity and change in quality of life studies. The implications and importance of these evidence gaps are that the full spectrum of HF patients and populations who experience the broadest range of non-cardiovascular comorbidity, do not have the full range of prognostic evidence that applies to the life course of outcomes from diagnosis, change in quality of life, end-stage disease, hospital admissions and finally death.
This review showed that for the examples of DM, COPD and RD there were strong and independent associations with mortality (estimated 34 to 52% higher risk) and hospital admissions (estimated 28 to 77% higher risk) in non-selected HF. The magnitude of association between different chronic disease comorbidities and mortality or admissions in HF was not significantly different and these findings are important for three main reasons. First, it is well recognised that the latency period between chronic disease comorbidity exposure and outcome is likely to be long. In HF, comorbidity exposure often occurs many years prior to the onset of the index HF disease or subsequent to its onset, which leads to later small to moderate exposure effects that can be difficult to detect. Second, comorbidity status is not static and will change over the life course progression of the HF, yet cumulative effects from disease onset to outcomes maybe similar across diseases [68]. Third, HF is a disease that predominately occurs in older people, which means that incorporation of comorbidity in prognosis requires the cumulative assessment of ageing and HF and comorbidity severity and its change over time. The long latency period between comorbidity exposure and outcome and the dynamic status of comorbidity exposure leads to the hypothesis that prognosis approaches that capture a change in exposure status may yield better prognostic estimation.
In terms of this systematic review, the focus was on non-selected HF populations. There have been other reviews [69–71] which have mostly included comorbidities investigated in selected populations. Examples include trial studies such as SOLVD [72], BEST [73], DIG [74] and other observational studies [75–80]. Whilst these reviews summarize the importance of comorbidity in specific heart failure groups we wanted to synthesise the evidence on comorbidity in the broadest population of heart failure, where the evidence is more limited, but where the range of comorbidity is experienced.
5. Strengths and limitations
This systematic review is a comprehensive investigation of comorbidity in non-selected HF which has identified prognostic follow-up studies that focused on comorbidity as a primary prognostic factor or included comorbidity in a ‘general’ prognostic factor or model study. The review methods used validated strategies and were peer reviewed, articles were multi-sourced and there was a structured approach to data extraction and synthesis in line with current guidelines on prognostic studies [20,21]. The inclusion of studies was based on standard definitions for the HF population, non-cardiovascular chronic diseases and the outcomes of quality of life, hospital admissions and mortality, but eligible studies may have been excluded particularly of non-English publications. We performed detailed data extraction and quality appraisal of each study to allow for appropriate quantitative synthesis.
The review focus was on a HF definition that did not select groups by a HF mechanism, ejection fraction or aetiology, as the primary interest was to report the current evidence on the influence of non-cardiovascular comorbidity on outcomes in the broadest HF population. In terms of including eligible HF studies, most studies had used a clinical diagnosis, some had used administration codes but studies based on self-report or diuretic prescription had been excluded. Diagnostic criteria used to define HF may vary, which may influence prognosis estimates, but our approach provides a reasonable perspective on HF and common comorbidity. The comorbidity definition was also based either on clinical criteria in primary studies or combination of large administrative databases or patient self-report. Again this approach to definitions could potentially influence estimates but the synthesis of large samples is likely to provide the best estimation of HF prognosis.
The meta-analysis only included studies where the comorbidity was the primary focus of the investigation, but the quantitative synthesis of observational studies is subject to heterogeneity across studies. Whilst clear inclusion criterion helped to minimise selection issues, Galbraith plots [34] and sensitivity analysis were used to explore the influence of heterogeneity on the estimates of association between the non-cardiovascular comorbidity and HF outcomes. The risk of study bias was assessed using funnel plots and Egger tests [35] and only studies with moderate or low risk of bias were included in the analysis. We also ensured that for the ‘general’ prognostic factor and model studies, where the under-reporting of comorbidity exposures may introduce bias, meta-analysis was not performed. The ability to accurately account for confounding is limited in meta-analysis of observational studies without access to individual patient data. However the consistency of the magnitude of estimates across the different studies identified suggests that the combined estimate takes account of a range of important confounders, but cannot exclude residual confounding.
6. Conclusions
Comorbidity prognosis studies for non-selected HF populations have to date focused on hospital settings and the outcome of mortality, showing that the impact of comorbidities on HF mortality is similar across nine different non-cardiovascular diseases. Much of the evidence is centred on RD, and lesser evidence on DM and COPD, despite the fact that there is a range of common non-cardiovascular conditions that may occur in the older person across the spectrum of HF in the general population. Comorbidity severity change is beginning to be identified as an important indicator of mortality and hospital admissions in non-selected HF, but few prognostic models for HF have taken account of individual comorbidity severity or consider change in influencing prognostic outcomes and the clearest gap is when there are multiple non-cardiovascular conditions in the same HF patient. Further investigation of the interplay between HF and comorbid severity is required and for the range of life course outcomes from diagnosis to death.
Conflicts of interest
None declared.
Acknowledgements
We thank Dr. Lucy Doos for her help in abstract and article selection, the Cochrane Prognosis Methods Group for their protocol review and to the Library team at Keele University.
Footnotes
Funding support: This work was supported by the National Institute for Health Research (NIHR, United Kingdom) Doctoral Fellowship for CA Rushton [grant number NIHR-DRF-2012-05-288]. The study sponsors had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR (UK).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ijcard.2015.05.180.
Supplementary data
Supplementary material.
References
- 1.Braunstein J.B., Anderson G.F., Gerstenblith G. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J. Am. Coll. Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 2.van Deursen V.M., Urso R., Laroche C. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur. J. Heart Fail. 2014;16:103–111. doi: 10.1002/ejhf.30. [DOI] [PubMed] [Google Scholar]
- 3.Kannel W.B., McGee D.L. Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 1979;59:8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M., Bohm C., Pandeya S., Gill J., Levin A., Kiberd B.A. Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am. J. Kidney Dis. 2001;37:484–489. [PubMed] [Google Scholar]
- 5.Lim H., MacFadyen R.J., Lip G.H. Diabetes mellitus, the renin–angiotensin–aldosterone system, and the heart. Arch. Intern. Med. 2004;164:1737–1748. doi: 10.1001/archinte.164.16.1737. [DOI] [PubMed] [Google Scholar]
- 6.Miller J.A. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J. Am. Soc. Nephrol. 1999;10:1778–1785. doi: 10.1681/ASN.V1081778. [DOI] [PubMed] [Google Scholar]
- 7.Han M.K., McLaughlin V.V., Criner G.J., Martinez F.J. Pulmonary diseases and the heart. Circulation. 2007;116:2992–3005. doi: 10.1161/CIRCULATIONAHA.106.685206. [DOI] [PubMed] [Google Scholar]
- 8.Schiffrin E.L., Lipman M.L., Mann J.F. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow G.C., Stough W.G., Abraham W.T., OPTIMIZE-HF Investigators and Hospitals Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J. Am. Coll. Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 10.Sachdeva A., Horwich T.B., Fonarow G.C. Comparison of usefulness of each of five predictors of mortality and urgent transplantation in patients with advanced heart failure. Am. J. Cardiol. 2010;106:830–835. doi: 10.1016/j.amjcard.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 11.O'Connor C.M., Whellan D.J., Wojdyla D. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction the HF-ACTION predictive risk score model. Circ. Heart Fail. 2012;5:63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C., Lin H., Yang H., Kong S., Zhang Q., Lee S.W. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 13.Babayan Z.V., McNamara R.L., Nagajothi N. Predictors of cause-specific hospital readmission in patients with heart failure. Clin. Cardiol. 2003;26:411–418. doi: 10.1002/clc.4960260906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun S., Tu J.V., Wijeysundera H.C. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ. Heart Fail. 2012;1(5):414–421. doi: 10.1161/CIRCHEARTFAILURE.111.964791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg G., Cohen E., Garty M. Outcomes of acute heart failure associated with acute coronary syndrome versus other causes. Acute Card. Care. 2011;13:87–92. doi: 10.3109/17482941.2011.567284. [DOI] [PubMed] [Google Scholar]
- 16.Lee D.S., Austin P.C., Stukel T.A. “Dose-dependent” impact of recurrent cardiac events on mortality in patients with heart failure. Am. J. Med. 2009;122:162–169.e1. doi: 10.1016/j.amjmed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghiade M., Flaherty J.D., Fonarow G.C. Coronary artery disease, coronary revascularization, and outcomes in chronic advanced systolic heart failure. Int. J. Cardiol. 2011;151(1):69–75. doi: 10.1016/j.ijcard.2010.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell R.C., Sui X., Filippatos G. Association of chronic kidney disease with outcomes in chronic heart failure: a propensity-matched study. Nephrol. Dial. Transplant. 2009;24(1):186–193. doi: 10.1093/ndt/gfn445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowie M.R., Komajda M., Murray-Thomas T., Underwood J., Ticho B., POSH Investigators Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH) Eur. Heart J. 2006;27:1216–1222. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 22.Geersing G., Bouwmeester W., Zuithoff P., Spijker R., Leeflang M., Moons K. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLoS ONE. 2012;7:e32844. doi: 10.1371/journal.pone.0032844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilczynski N., Haynes R. Optimal search strategies for detecting clinically sound prognostic studies in EMBASE: an analytic survey. J. Am. Med. Inform. Assoc. 2005;12:481–485. doi: 10.1197/jamia.M1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betihavas V., Davidson P.M., Newton P.J., Frost S.A., Macdonald P.S., Stewart S. What are the factors in risk prediction models for rehospitalisation for adults with chronic heart failure? Aust. Crit. Care. 2012;25:31–40. doi: 10.1016/j.aucc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Ross J.S., Mulvey G.K., Stauffer B. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch. Intern. Med. 2008;168:1371–1386. doi: 10.1001/archinte.168.13.1371. [DOI] [PubMed] [Google Scholar]
- 26.McMurray J.J., Adamopoulos S., Anker S.D., ESC Committee for Practice Guidelines ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 27.Steyerberg E. Springer; New York: 2008. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. [Google Scholar]
- 28.Smith G.L., Lichtman J.H., Bracken M.B. Renal impairment and outcomes in heart failure. Systematic review and meta-analysis. J. Am. Coll. Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 29.Damman K., Navis G., Voors A.A. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J. Card. Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Hayden J., van der Windt D., Cartwright J., Cote P., Bombardier C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 31.Perel P. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. 2008;336:425. doi: 10.1136/bmj.39461.643438.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The National Collaborating Centre for Chronic Conditions . Royal College Physicians; London: 2008. Chronic Kidney Disease: National Clinical Guideline for Early Identification and Management in Adults in Primary and Secondary Care. [PubMed] [Google Scholar]
- 33.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Galbraith R. Graphical display of estimates having differing standard errors. Technometrics. 1988;30:271–281. [Google Scholar]
- 35.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustafsson I., Brendorp B., Seibæk M., Danish Investigators of Arrhythmia and Mortality on Dofetilde Study Group Influence of diabetes and diabetes–gender interaction on the risk of death in patients hospitalized with congestive heart failure. J. Am. Coll. Cardiol. 2004;43:771–777. doi: 10.1016/j.jacc.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 37.Berry C., Brett M., Stevenson K., McMurray J.J., Norrie J. Nature and prognostic importance of abnormal glucose tolerance and diabetes in acute heart failure. Heart. 2008;94:296–304. doi: 10.1136/hrt.2006.110999. [DOI] [PubMed] [Google Scholar]
- 38.Burger A.J., Tsao L., Aronson D. Prognostic impact of diabetes mellitus in patients with acute decompensated heart failure. Am. J. Cardiol. 2005;95:1117–1119. doi: 10.1016/j.amjcard.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald M.R., Jhund P.S., Petrie M.C. Discordant short- and long-term outcomes associated with diabetes in patients with heart failure: importance of age and sex: a population study of 5.1 million people in Scotland. Circ. Heart Fail. 2008;1:234–241. doi: 10.1161/CIRCHEARTFAILURE.108.794008. [DOI] [PubMed] [Google Scholar]
- 40.de Boer R.A., Doehner W., van der Horst I.C.C., SENIORS Investigators Influence of diabetes mellitus and hyperglycemia on prognosis in patients >=70 years old with heart failure and effects of nebivolol (data from the study of effects of nebivolol intervention on outcomes and rehospitalization in seniors with heart failure [SENIORS]) Am. J. Cardiol. 2010;106:78–86.e1. doi: 10.1016/j.amjcard.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 41.From A.M., Leibson C.L., Bursi F. Diabetes in heart failure: prevalence and impact on outcome in the population. Am. J. Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 42.Greenberg B.H., Abraham W.T., Albert N.M. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) Am. Heart J. 2007;154:647–654. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Flores-Le Roux J.A., Comin J., Pedro-Botet J. Seven-year mortality in heart failure patients with undiagnosed diabetes: an observational study. Cardiovasc. Diabetol. 2011;10 doi: 10.1186/1475-2840-10-39. (39-2840-10-39. Published online 2011 May 14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerstein H.C., Swedberg K., Carlsson J., CHARM Program Investigators The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch. Intern. Med. 2008;168:1699–1704. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 45.Mogensen U.M., Ersboll M., Andersen M. Clinical characteristics and major comorbidities in heart failure patients more than 85 years of age compared with younger age groups. Eur. J. Heart Fail. 2011;13:1216–1223. doi: 10.1093/eurjhf/hfr116. [DOI] [PubMed] [Google Scholar]
- 46.Pocock S.J., Wang D., Pfeffer M.A. Predictors of mortality and morbidity in patients with chronic heart failure. Eur. Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed A., Aban I.B., Vaccarino V. A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007;93:1584–1590. doi: 10.1136/hrt.2006.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iversen K.K., Kjaergaard J., Akkan D., ECHOS Lung Function Study Group The prognostic importance of lung function in patients admitted with heart failure. Eur. J. Heart Fail. 2010;12:685–691. doi: 10.1093/eurjhf/hfq050. [DOI] [PubMed] [Google Scholar]
- 49.Senni M., Santilli G., Parrella P. A novel prognostic index to determine the impact of cardiac conditions and co-morbidities on one-year outcome in patients with heart failure. Am. J. Cardiol. 2006;98:1076–1082. doi: 10.1016/j.amjcard.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 50.Ahluwalia S.C., Gross C.P., Chaudhry S.I. Impact of comorbidity on mortality among older persons with advanced heart failure. J. Gen. Intern. Med. 2012;27:513–519. doi: 10.1007/s11606-011-1930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunlay S.M., Redfield M.M., Weston S.A. Hospitalizations after heart failure diagnosis a community perspective. J. Am. Coll. Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L., Porter B., Maynard C. Predicting risk of hospitalization or death among patients with heart failure in the veterans health administration. Am. J. Cardiol. 2012;12(110):1342–1349. doi: 10.1016/j.amjcard.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 53.Ismailov R.M., Goldberg R.J., Lessard D., Spencer F.A. Decompensated heart failure in the setting of kidney dysfunction: a community-wide perspective. Nephron. Clin. Pract. 2007;107:c147–c155. doi: 10.1159/000110035. [DOI] [PubMed] [Google Scholar]
- 54.Olandoski M., De Lima R.R., Da Silva M.M.F. Interaction of anemia and decrease in renal function on survival of patients with heart failure. Int. J. Cardiol. 2012;154:338–340. doi: 10.1016/j.ijcard.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 55.Petretta M., Scopacasa F., Fontanella L. Prognostic value of reduced kidney function and anemia in patients with chronic heart failure. J. Cardiovasc. Med. 2007;8:909–916. doi: 10.2459/JCM.0b013e32801464b6. [DOI] [PubMed] [Google Scholar]
- 56.Gotsman I., Zwas D., Planer D., Admon D., Lotan C., Keren A. The significance of serum urea and renal function in patients with heart failure. Medicine. 2010;89:197–203. doi: 10.1097/MD.0b013e3181e893ee. [DOI] [PubMed] [Google Scholar]
- 57.Hillege H.L., Nitsch D., Pfeffer M.A., Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 58.Fonarow G.C., Abraham W.T., Albert N.M., OPTIMIZE-HF Investigators and Hospitals Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch. Intern. Med. 2008;168:847–854. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 59.Damman K., Jaarsma T., Voors A.A., COACH investigators Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) Eur. J. Heart Fail. 2009;11:847–854. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 60.Kociol R.D., Greiner M.A., Hammill B.G. Long-term outcomes of medicare beneficiaries with worsening renal function during hospitalization for heart failure. Am. J. Cardiol. 2010;105:1786–1793. doi: 10.1016/j.amjcard.2010.01.361. [DOI] [PubMed] [Google Scholar]
- 61.Chaudhry S.I., McAvay G., Chen S. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the cardiovascular health study. J. Am. Coll. Cardiol. 2013;61:635–642. doi: 10.1016/j.jacc.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee D.S., Austin P.C., Rouleau J.L., Liu P.P., Naimark D., Tu J.V. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 63.O'Connor C.M., Abraham W.T., Albert N.M. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) Am. Heart J. 2008;156:662–673. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 64.Blinderman C.D., Homel P., Billings J.A., Portenoy R.K., Tennstedt S.L. Symptom distress and quality of life in patients with advanced congestive heart failure. J. Pain Symptom Manag. 2008;35:594–603. doi: 10.1016/j.jpainsymman.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carson P., Tam S.W., Ghali J.K. Relationship of quality of life scores with baseline characteristics and outcomes in the African-American Heart Failure Trial. J. Card. Fail. 2009;15:835–842. doi: 10.1016/j.cardfail.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 66.Azevedo A., Bettencourt P., Alvelos M., Martins E., Abreu-Lima C., Hense H.W., Barros H. Health-related quality of life and stages of heart failure. Int. J. Cardiol. 2008;129:238–244. doi: 10.1016/j.ijcard.2007.07.091. [DOI] [PubMed] [Google Scholar]
- 67.Allen L.A., Gheorghiade M., Reid K.J. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ. Cardiovasc. Qual. Outcomes. 2011;4:389–398. doi: 10.1161/CIRCOUTCOMES.110.958009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hennekens C., Buring J. Little Brown and Company; Boston/Toronto: 1987. Epidemiology in Medicine. [Google Scholar]
- 69.MacDonald M.R., Petrie M.C., Hawkins N.M. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur. Heart J. 2008;29:1224–1240. doi: 10.1093/eurheartj/ehn156. [DOI] [PubMed] [Google Scholar]
- 70.Hawkins N., Petrie M., Jhund P., Chalmers G., Dunn F., McMurray J. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur. J. Heart Fail. 2009;11:130–139. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damman K., Valente M.A.E., Voors A.A., O'Connor C.M., van Veldhuisen D.J., Hillege H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur. Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 72.Dries D.L., Sweitzer N.K., Drazner M.H., Stevenson L.W., Gersh B.J. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J. Am. Coll. Cardiol. 2001;38:421–428. doi: 10.1016/s0735-1097(01)01408-5. [DOI] [PubMed] [Google Scholar]
- 73.Domanski M., Krause-Steinrauf H., Deedwania P. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J. Am. Coll. Cardiol. 2003;42:914–922. doi: 10.1016/s0735-1097(03)00856-8. [DOI] [PubMed] [Google Scholar]
- 74.Brophy J.M., Dagenais G.R., McSherry F., Williford W., Yusuf S. A multivariate model for predicting mortality in patients with heart failure and systolic dysfunction. Am. J. Med. 2004;116:300–304. doi: 10.1016/j.amjmed.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 75.Kamalesh M., Subramanian U., Sawada S., Eckert G., Temkit M., Tierney W. Decreased survival in diabetic patients with heart failure due to systolic dysfunction. Eur. J. Heart Fail. 2006;8:404–408. doi: 10.1016/j.ejheart.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Smooke S., Horwich T.B., Fonarow G.C. Insulin-treated diabetes is associated with a marked increase in mortality in patients with advanced heart failure. Am. Heart J. 2005;149:168–174. doi: 10.1016/j.ahj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 77.De Groote P., Lamblin N., Mouquet F. Impact of diabetes mellitus on long-term survival in patients with congestive heart failure. Eur. Heart J. 2004;25:656–662. doi: 10.1016/j.ehj.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 78.Berry C., Hogg K., Norrie J., Stevenson K., Brett M., McMurray J. Heart failure with preserved left ventricular systolic function: a hospital cohort study. Heart. 2005;91:907–913. doi: 10.1136/hrt.2004.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harjai K.J., Thompson H.W., Turgut T., Shah M. Simple clinical variables are markers of the propensity for readmission in patients hospitalized with heart failure. Am. J. Cardiol. 2001;87:234–237. doi: 10.1016/s0002-9149(00)01328-x. [DOI] [PubMed] [Google Scholar]
- 80.Parker A.B., Yusuf S., Naylor C.D. The relevance of subgroup-specific treatment effects: the Studies of Left Ventricular Dysfunction (SOLVD) revisited. Am. Heart J. 2002;144:941–947. doi: 10.1067/mhj.2002.126446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.