Abstract
Background
The omega-3 fatty acid (ω-3 fatty acid) such as eicosapentaenoic acid (EPA) is currently used in the clinic as a nutritional supplement in the treatment of poly- cystic ovarian syndrome (PCOS). The present study was designed to investigate the ef- fect of EPA on the expression levels of peroxisome proliferator-activated receptor gamma (PPARγ) and cytochrome P450 aromatase (encoded by the CYP-19) in primary cultured granulosa cells (GC) from patients undergoing in vitro fertilization (IVF), and also to compare these effects with those in GC of PCOS patients.
Materials and Methods
In this experimental study, human GC were isolated, pri- mary cultured in vitro, exposed to a range of concentrations of the EPA and in- vestigated with respect to gene expression levels of PPARγ and CYP-19 using real time-polymerase chain reaction (PCR). The participants (n=30) were the patients admitted to the IVF Center in February-March 2013 at Alzahra Hospital, Tabriz, Iran, who were divided into two groups as PCOS (n=15) and non-PCOS (n=15) women (controls).
Results
All doses of the EPA significantly induced PPARγ mRNA gene expression level as compared to the control recombinant follicle stimulating hormone (rFSH) alone condi- tion. High doses of EPA in the presence of rFSH produced a stimulatory effect on expres- sion level of PPARγ (2.15-fold, P=0.001) and a suppressive effect (0.56-fold, P=0.01) on the expression level of CYP-19, only in the PCOS GC.
Conclusion
EPA and FSH signaling pathway affect differentially on the gene ex- pression levels of PPARγ and CYP-19 in PCOS GC. Altered FSH-induced PPARγ activity in PCOS GC may modulate the CYP-19 gene expression in response to EPA, and possibly modulates the subsequent steroidogenesis of these cells.
Keywords: Eicosapentaenoic Acid, PPAR Gamma, Aromatase, Granulosa Cells, Polycystic Ovary Syndrome
Introduction
Polycystic ovarian syndrome (PCOS) is the most commonly occurring cause of female infertility (1). In PCOS, there is an imbalance of female sex hormones, which may lead to ovarian cysts and irregular or absent menstrual cycle. The abnormality has been mainly attributed to the suppression of the follicle stimulating hormone (FSH) secretion by an excess androgen produced from the ovary. Accelerated early follicular growth leads to attenuated FSH responsiveness and the premature luteinisation of granulosa cells (GC). In turn, the development of the dominant follicle is disrupted which causes cystic follicular arrest (2).
The cytochrome P450 aromatase, encoded by the CYP-19 gene, in ovarian GC that converts testosterone to estradiol is induced by FSH during early follicle development. The timely expression of CYP-19 in GC plays a critical role in follicle development. In the CYP-19 knockout mice, antrum formation is arrested at a stage before ovulation and no corpora lutea are formed (3). The follicular arrest of PCOS has been characterized by the lack of in vivo FSH-induced CYP-19 activity in GC (4).
The expressions of CYP-19 is coordinately regulated and efficiently inhibited by thiazolidinediones (TZDs) in human GC obtained from in vitro fertilization (IVF) (5, 6). TZDs are known as agonists of the gamma isoform of the peroxisome proliferatoractivated receptor (PPARγ), a family of nuclear receptors regulating the expression of genes involved in lipid metabolism, insulin sensitivity, and cellular differentiation. PPARγ expression has been found in the GC (7). The PPARγ may regulate the steroidogenesis, thereby contributes to the regulation of ovarian function (8). Previous studies have reported that retinoid X receptor (RXR) response elements are present in the CYP-19; however, no exact region that responds independently to PPARγ has yet been identified (9).
There is a strong indication that omega-3 fatty acids (ω-3 fatty acids) have protective action against PCOS (10). In particular, eicosapentaenoic acid (EPA), a long-chain ω-3 fatty acid (PUFA), is a natural high-affinity ligand for PPARγ. Despite the increasing clinical use, the mechanisms by which EPA exerts its effects is yet relatively unknown. The aim of the present study was to investigate the effects of EPA on gene expression levels of PPARγ and CYP-19 in cultured GC from patients undergoing IVF, and also to compare these effects with those in GC of PCOS patients.
Materials and Methods
This experimental study was approved by the Ethics Committee of Tabriz University of Medical Sciences. All patients gave a written informed consent and their confidentiality and anonymity were protected.
Primary cell culture
Sampling was done by a simple consecutive method covering all patients (n=30) who were admitted to the IVF Center in February-March 2013 at Alzahra Hospital, Tabriz, East Azerbaijan Province, Iran. PCOS were defined as the presence of 12 or more follicles measuring 2-9 mm with clinical (a Ferriman–Gallwey score >7) and/or biochemical hyperandrogenism (total testosterone >3 nmol/l) (11). The participants (n=30) were divided into two groups as PCOS (n=15) and non-PCOS (n=15) women (controls).
Inclusion criteria were no alcohol consumption and no smoking habit. Uterus abnormalities, endometriosis, anovulation, positive history of endocrine disease and inflammatory disorders such as thyroid and adrenal disorders, hormonal treatment, and history of recurrent infections were considered as exclusion criteria in this study. Control group (n=15) included individuals with age- (27.62 ± 4.14 years) and body mass index (BMI)- (25.11 ± 2.57 kg/m2) matched with no evidence of hyperandrogenemia or menstrual irregularities. All patients underwent a standard infertility evaluation, including hormonal testing and assessment of the uterus and fallopian tubes by means of hysterosalpingography. Patients underwent a long gonadotropin-releasing hormone (GnRH) agonist (decapeptyl, Debio Pharm, Geneva, Switzerland)/ FSH-long down regulation protocol as described previously by us (12). GC was isolated from aspirated follicular fluid by hyaluronidase digestion, followed by Percoll gradient centrifugation (13).
Three sets of experiments with both PCOS and control groups were performed. GC was pooled because the number of cells from follicles was insufficient to perform individualized culture. In the experiments, each group composed of GC pooled from 5 women. In total, GC were isolated and pooled from 15 PCOS and 15 control women of reproductive age. The GC were counted with a homocytometer, and approximately 1×106 cells were plated in a 12-well culture plate containing dulbecco’s modified eagle medium/ nutrient mixture/F-12 (DMEM/F12, Cellgro, USA) medium supplemented with 10% fetal bovine serum (FBS), 100 IU/ml penicillin, and 100 μg/ml streptomycin, for 24 hours. Cells were maintained at 37˚C in 5% CO2 in a humidified incubator. EPA (Sigma, St. Louis, MO) was conjugated with bovine serum albumin (BSA) fatty acid-free (Sigma, St. Louis, MO) before treatment (14). GC, after serum starvation overnight, were treated with indicated concentrations of EPA (25-100 μM), both either with or without pretreatment with recombinant (r)FSH (100 ng/mL).
Real-time polymerase chain reaction analysis
Total RNA was isolated using RNX-Plus according to the instructions of the manufacturer. RNA pellets were ethanol-precipitated, washed, and resuspended in sterile ribonuclease-free water. Two μg of total RNA were reverse transcribed into cDNA using SuperScript II reverse transcriptase (Life Technologies, Carlsbad, CA, USA). Real-time polymerase chain reaction (PCR) was carried out using the fluorescent dye SYBR-Green and a Bio-Rad CFX real-time PCR system (BioRad Co, CA, USA). The primers used for qPCR were as follows: PPARγ, 5΄ ATGACAGACCTCAGACAGATTG 3΄ (sense) and 5΄ AATGTTGGCAGTGGCTCACGTG 3΄ (antisense); CYP-19, 5΄ TCTTGGTGTGGAATTATGAG 3΄ (sense) and 5΄ TTGAGGACTTGCTGATAATG 3΄ (antisense); glyceraldehydes 3-phosphate dehydrogenase (GAPDH), 5 AAGCTCATTTCCTGGTATGACG 3 (sense) and 5΄ TCTTCCTCTTGTGCTCTTGCTGG 3΄ (antisense).
Samples were assayed in duplicates. The amount of specific PCR products was normalized to the GAPDH mRNA content, and quantities were expressed as an x-fold difference relative to a control.
Statistical analysis
Values are presented as mean ± standard deviation (SD) of 3 separate experiments done in duplicate. Data in all groups were normally distributed. Statistically significant differences in mean values between groups were assessed by t tests. Analysis of variance test were used for comparing the group means. Calculation of significance between groups was done according to analysis of variance (ANOVA) with post hoc Tukey’s tests for multiple comparisons. Repeated- measures ANOVA was used for measures of response times, and a P value of <0.05 was considered statistically significant.
Results
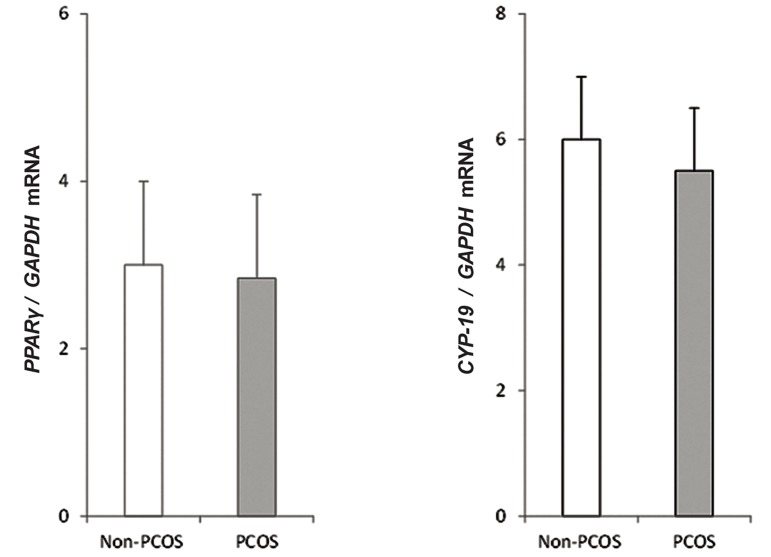
Figure 1 shows the genes expression levels measured by quantitative PCR method in GC from patients with PCOS and non-PCOS women. Primarily, no significant differences were found in the gene expression levels of PPARγ and CYP-19 between the two groups.
Fig.1.
Quantitative analysis of PPARγ (A) and CYP-19 (B) genes expression levels by real-time PCR in GCs from PCOS and non PCOS-women. Each expression level was normalized to the GAPDH levels. The mean ± SD of three independent determinations with cells pooled from 5 women per group per experiment (t test).
PCR; Polymerase chain reaction, GCs; Granulosa cells and PCOS; Polycystic ovarian syndrome.
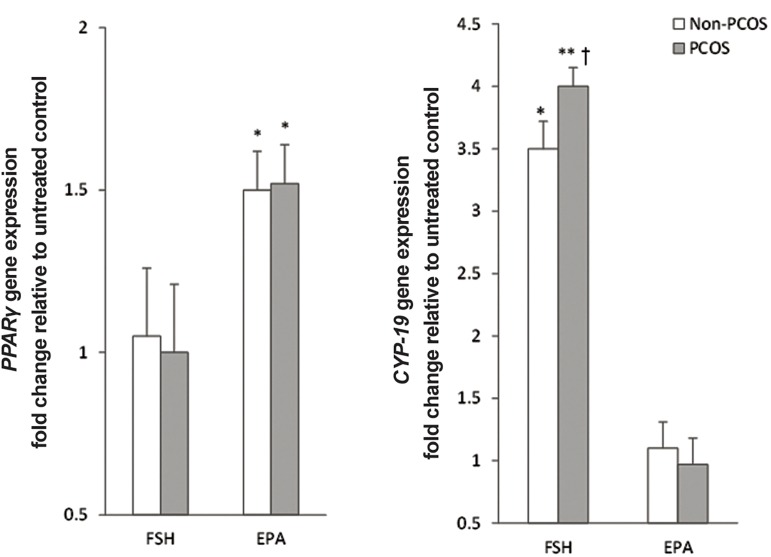
To determine the effect of rFSH stimulation on expression levels of PPARγ and CYP-19, GC was treated with rFSH. Only CYP-19 showed a significant increase in mRNA level (P<0.001, Fig.2), which was more elevated in PCOS than in non- PCOS (mean 4.0-fold vs. 3.5-fold, respectively, P=0.03). In contrast, incubation with EPA alone resulted in comparable upregulation of PPARγ expression level (1.49 ± 0.12 vs. 1.52 ± 0.11, P=0.51) in GCs from non-PCOS and PCOS patients. However, no such changes were observed for CYP-19 expression level in EPA-treated cells (Fig.2).
Fig.2.
Effect of the follicle stimulating hormone (FSH) and eicosapentaenoic acid (EPA) incubation on mRNA expression levels of PPARγ and CYP-19. GCs, after serum starvation, were incubated for 24 hours ± 100 ng/mL FSH or 50 μmol/L EPA. Cell lysates were prepared and analyzed by real-time PCR for genes expression levels. Expression levels of PPARγ (A) and CYP-19 (B) in each lysate were normalized to the amount of GAPDH and represented as fold of untreated control. The mean ± SD of three independent experiments with cells pooled from 5 women per group per experiment (t test).
*; P<0.05 and **; P<0.01 vs. untreated control and †; P<0.05 vs. non-PCOS.
PCR; Polymerase chain reaction, GCs; Granulosa cells and PCOS; Polycystic ovarian syndrome.
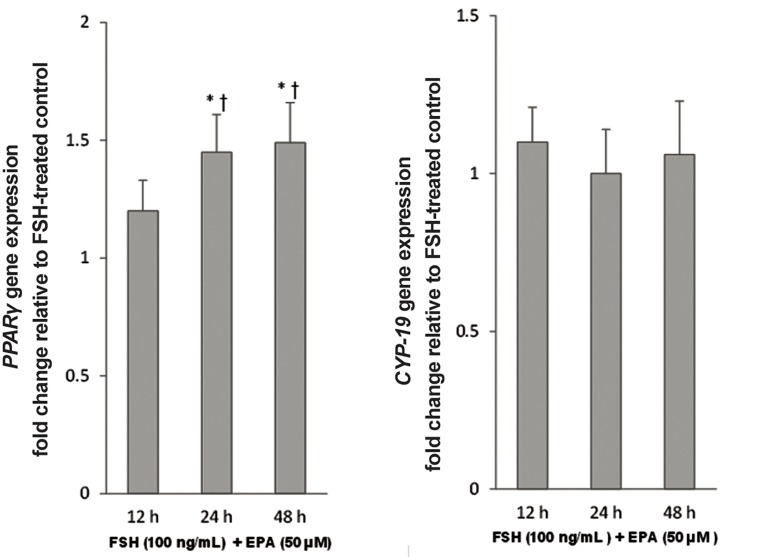
Comparison of control rFSH with the combined rFSH-EPA condition showed a similar response compared to the EPA alone. To optimize the assay, cultured GC from non PCOS women were incubated with the 50 μmol/L EPA and the incubation time ranged from 12 hours to 48 hours. While no significant changes were observed in the expression level of CYP-19, the expression level of PPARγ increased by 30% (P=0.02) after 24 hours. However, later no further changes were observed in the expression levels of both mRNAs (Fig.3).
Fig.3.
Effect of eicosapentaenoic acid (EPA) incubation time on mRNA expression levels of PPARγ and CYP-19. GCs, after serum starvation, were incubated in 100 ng/mL follicle stimulating hormone (FSH) alone or in combination with 50 μmol/L EPA for 12 hours, 24 hours and 48 hours. Cell lysates were prepared and analyzed by real-time PCR for genes expression levels. Expression levels of PPARγ (A) and CYP-19 (B) in each lysate were normalized to the amount of GAPDH and represented as fold of FSH-treated control. The mean ± SD of three independent experiments with cells pooled from 5 women per group per experiment (repeated-measures ANOVA. *; P<0.05 and †; P<0.05 vs. FSH-treated control and 12-hour incubation, respectively).
PCR; Polymerase chain reaction, GCs; Granulosa cells and h; Hours.
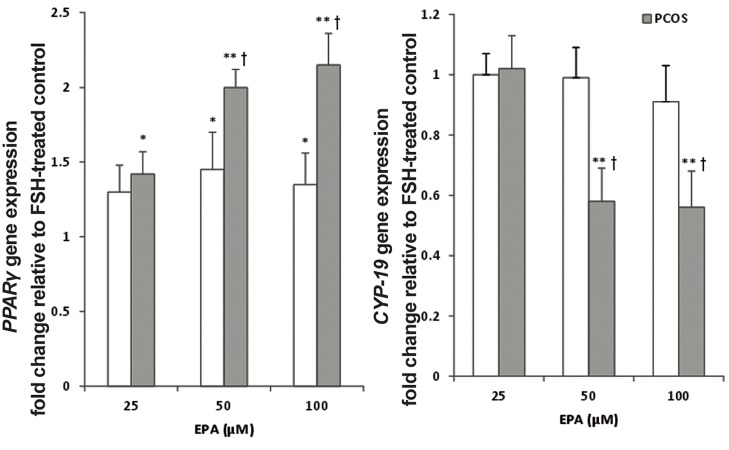
In the next series of experiments, three doses of EPA (0-100 μM) were tested in the presence of rFSH. Treatment of GCs with 50 and 100 μM doses of the EPA significantly increased PPARγ mRNA gene expression level compared to the control rFSH alone condition (P<0.05). PPARγ displayed a larger fold change in the PCOS group than in the non-PCOS group. The magnitude of this difference between non-PCOS and PCOS was more pronounced at the higher doses of EPA (e.g., 1.42- fold at 25 μmol vs. 2.15-fold at 100 μM, P=0.008). Moreover, it was identified that the expression level of CYP-19 was also influenced by the higher doses of EPA in the PCOS GC as compared to the control. The combination of high doses of EPA in the presence of rFSH produced a strong suppressive effect on the CYP-19 gene expression level in the PCOS GC (0.56-fold, P=0.01, Fig.4).
Fig.4.
Effect of different doses of eicosapentaenoic acid (EPA) on expression levels of PPARγ and CYP-19 in follicle stimulating hormone (FSH)-stimulated GCs from PCOS and non-PCOS women. GCs, after serum starvation, were incubated in 100 ng/mL FSH alone or in combination with 25 μmol/L, 50 μmol/L or 100 μmol/L EPA for 24 hours. Cell lysates were prepared and analyzed by real-time PCR for genes expression levels. Expression levels of PPARγ (A) and CYP-19 (B) in each lysate were normalized to the amount of GAPDH and represented as fold of FSH-treated control. The mean ± SD of three independent experiments with cells pooled from 5 women per group per experiment (ANOVA with post hoc Tukey’s test, *; P<0.05, **; P<0.01 vs. FSH-treated control and †; P<0.01 vs. non-PCOS).
PCR; Polymerase chain reaction, GCs; Granulosa cells and PCOS; Polycystic ovarian syndrome.
Discussion
PPAR-γ has been shown to be critically important in multiple biological functions such as fertility (12), while EPA and docosahexanoic acid (DHA) are natural, preferentially-binding ligands for this receptor. It has been shown that EPA and DHA down-regulate activation of NF-κB through increasing both PPAR-γ mRNA levels and protein activity in different types of cells. These effects may be one of the underlying mechanisms for the anti-inflammatory effect of the ω-3 PUFA (15, 16). To the contrary, although no change in PPARγ mRNA expression level has been reported previously in certain types of cells after exposure to EPA (17). Our results demonstrated that there were mRNA expression levels of PPARγ and CYP-19 in pre-ovulatory human GC, and that PPARγ was increased by EPA. This suggests that EPA may elicit important biological responses in GC via activation of PPARγ.
PPARγ is a key transcription factor involved in follicular differentiation (18) and ovarian GC tumor (19). It has been shown that a decrease in expression level of PPARγ in response to luteinizing hormone (LH) is important for ovulation and/ or luteinization. GC differentiation into the corpus luteum in response to the LH surge is accompanied by reduced CYP-19 activity. It has been reported that the expression level of mRNA for PPARγ in follicles is inversely related to the expression level of mRNA for CYP-19 (20). Overexpression of PPARγ in the KGN ovarian granulosa-like tumor cell line reduced FSH-stimulated CYP-19 mRNAs (21). These observations suggest that PPARγ has an inhibitory effect on the CYP-19 activity as well as on ovulation and/or luteinization. The complete disruption of FSH-induced estradiol production by synthetic PPAR-γ agonists in cultured human ovarian cells has been attributed to CYP-19 (5). It has been shown that PPARγ agonists suppress the CYP-19 mRNA expression level in human GC, in a dose-dependent manner, probably via nuclear receptor system PPARγ: RXR heterodimer (22). However, the data reported in the literature about the effects of TZDs on CYP-19 activity in the ovary are controversial. Either no effect (23) or suppressive effects (22) have been shown, which could partly be attributed to a variety of PPARγ independent signaling events (24). Furthermore, no specific data is available regarding the effect of either the synthetic or natural PPARγ agonists on the expression and activity of GC aromatase in PCOS.
As shown herein and reported previously, FSH induces the expression level of CYP-19 (25, 26). In contrast, levels of mRNA for PPARγ were not affected by treatment with rFSH, in agreement with the observations made previously in rats (27). Cotreatment with EPA and rFSH resulted in enhanced PPARγ expression level both in control and PCOS GC. However, altered levels of gene expression in PCOS granulosa in response to the combined drug condition was not similar to that observed in control granulosa. In cultured GC obtained from patients with PCOS, EPA induced a more pronounced effect with rFSH treatment on the mRNA expression level of PPARγ. Furthermore, EPA treatment of PCOS GC remarkably down regulated CYP-19 gene, as compared with non-PCOS patients. Coffler et al. have shown that women with PCOS exhibited dose-dependent GC hyperresponsiveness to FSH and increased production of estradiol (28, 29). The above results implied a possibility that the apparent suppressive effect of EPA on hypersensitivity of PCOS GC to rFSH may be due to a negative regulation of the rFSH signaling by activated PPARγ. Accordingly, CYP-19 down-regulation via induction of PPARγ has recently been noted in GC from subjects undergoing IVF (21).
The deregulated synthesis of estradiol (E2) by PCOS GC has been associated with the arrest of early antral follicle development (30). The GC from PCO antral follicles produce normal or increased E2 amounts in vitro (31), even though follicles in women with PCOS contain low levels of CYP-19 mRNA (32). This would suggest an in vivo blockade of estrogen production by follicular environment in PCOS. This is in accordance with our findings of no statistically significant difference in the expression of CYP-19 in primary culture between GC from patients with PCOS and those from control non-PCOS.
Unlike the response to combination of rFSH and EPA, the gene expression of PPARγ in response to EPA alone was not different between control and PCOS GC. On the other hand, rFSH alone exerted no apparent effect on PPARγ gene expression level in the both control and PCOS GC. Based on these results, the higher EPA-induced PPARγ expression level in PCOS than in control GC may be somewhat explained by concomitant hypersensitivity of PCOS cells to FSH. FSH activates several signaling mechanisms through its surface G proteincoupled receptor (GPCR) such as the MEK and PI3K pathways, which are potentially involved in the regulation of PPARγ-mediated signaling in GCs (33).
Several clinical evidences support the preventive and therapeutic effects of ω-3 fatty acids in menopausal problems (10). Recently, ω-3 fatty acids supplementation has been related to the improvement in insulin sensitivity (34), and less androgenic and atherogenic lipid profiles (35) in women with PCOS. The results of the present study confirmed the potential effect of ω-3 fatty acids on the ovulatory function of PCOS. It is suggested that the modulatory effect of ω-3 fatty acids on the GC steroidogenesis could also play an important role in the oocyte maturation and subsequent ovulation.
Although previous research has shown beneficial effect of PPARγ agonists in PCOS, this is the first study to examine the combined effect of EPA and rFSH on the gene expression levels of PPARγ and CYP-19 in human GC. The small sample size, pooled estimate and lack of assessment of CYP-19 activity may be seen as limitations of this study. However, the regulatory effects were simultaneously analyzed by studying the expression level in control and PCOS GC, which made it possible to identify similarities and differences. Since the preliminary findings of the present study were derived from cultured GC, it remained to confirm the in vivo effect of EPA and to further assess the possible mechanism of action of EPA in the treatment of PCOS.
Conclusion
Our study showed that EPA and FSH signaling pathway affect differentially on the gene expression levels of PPARγ and CYP-19 in PCOS GC. We speculated that altered FSH-induced PPARγ activity in PCOS GC may modulate the CYP-19 gene expression level in response to EPA, and subsequently modulates the steroidogenesis of these cells.
Acknowledgments
This study was conducted as part of a Master’s thesis project no. 90/2-6/4 at the Tabriz University of Medical Sciences, Tabriz, Iran. The research was partially supported by a grant (research project number 5/62/4865) from the Women’s Reproductive Health Research Center of Tabriz University of Medical Sciences. The authors acknowledge the Research Center for Pharmaceutical Nanotechnology at Tabriz University of Medical Sciences for providing research facilities.The authors declare that there is no conflict of interests regarding the publication of this article.
References
- 1.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41–41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10:e3–e3. doi: 10.1017/S1462399408000598. [DOI] [PubMed] [Google Scholar]
- 3.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95(12):6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86(3):1318–1323. doi: 10.1210/jcem.86.3.7318. [DOI] [PubMed] [Google Scholar]
- 5.Seto-Young D, Paliou M, Schlosser J, Avtanski D, Park A, Patel P, et al. Direct thiazolidinedione action in the human ovary: insulin-independent and insulin-sensitizing effects on steroidogenesis and insulin-like growth factor binding protein-1 production. J Clin Endocrinol Metab. 2005;90(11):6099–6105. doi: 10.1210/jc.2005-0469. [DOI] [PubMed] [Google Scholar]
- 6.Seto-Young D, Avtanski D, Parikh G, Suwandhi P, Strizhevsky M, Araki T, et al. Rosiglitazone and pioglitazone inhibit estrogen synthesis in human granulosa cells by interfering with androgen binding to aromatase. Horm Metab Res. 2011;43(4):250–256. doi: 10.1055/s-0030-1270525. [DOI] [PubMed] [Google Scholar]
- 7.Puttabyatappa M, Vandevoort CA, Chaffin CL. hCG-induced down-regulation of PPARgamma and liver X receptors promotes periovulatory progesterone synthesis by macaque granulosa cells. Endocrinology. 2010;151(12):5865–5872. doi: 10.1210/en.2010-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowalewski MP, Dyson MT, Manna PR, Stocco DM. Involvement of peroxisome proliferator-activated receptor gamma in gonadal steroidogenesis and steroidogenic acute regulatory protein expression. Reprod Fertil Dev. 2009;21(7):909–922. doi: 10.1071/RD09027. [DOI] [PubMed] [Google Scholar]
- 9.Fan W, Yanase T, Morinaga H, Mu YM, Nomura M, Okabe T, et al. Activation of peroxisome proliferator-activated receptor- gamma and retinoid X receptor inhibits aromatase transcription via nuclear factor-kappaB. Endocrinology. 2005;146(1):85–92. doi: 10.1210/en.2004-1046. [DOI] [PubMed] [Google Scholar]
- 10.Saldeen P, Saldeen T. Women and omega-3 Fatty acids. Obstet Gynecol Surv. 2004;59(10):722–730. doi: 10.1097/01.ogx.0000140038.70473.96. [DOI] [PubMed] [Google Scholar]
- 11.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Sahmani M, Sakhinia E, Farzadi L, Najafipour R, Darabi M, Mehdizadeh A, et al. Two common polymorphisms in the peroxisome proliferator-activated receptor gamma gene may improve fertilization in IVF. Reprod Biomed Online. 2011;23(3):355–360. doi: 10.1016/j.rbmo.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Bodis J, Koppan M, Kornya L, Tinneberg HR, Torok A. The effect of catecholamines, acetylcholine and histamine on progesterone release by human granulosa cells in a granulosa cell superfusion system. Gynecol Endocrinol. 2002;16(4):259–264. [PubMed] [Google Scholar]
- 14.Igoillo-Esteve M, Marselli L, Cunha DA, Ladriere L, Ortis F, Grieco FA, et al. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia. 2010;53(7):1395–1405. doi: 10.1007/s00125-010-1707-y. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gammadependent mechanism. Kidney Int. 2005;67(3):867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 16.Selvaraj RK, Shanmugasundaram R, Klasing KC. Effects of dietary lutein and PUFA on PPAR and RXR isomer expression in chickens during an inflammatory response. Comp Biochem Physiol A Mol Integr Physiol. 2010;157(3):198–203. doi: 10.1016/j.cbpa.2010.06.172. [DOI] [PubMed] [Google Scholar]
- 17.Coyne GS, Kenny DA, Childs S, Sreenan JM, Waters SM. Dietary n-3 polyunsaturated fatty acids alter the expression of genes involved in prostaglandin biosynthesis in the bovine uterus. Theriogenology. 2008;70(5):772–782. doi: 10.1016/j.theriogenology.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 18.Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function--implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrinol. 2005;3:41–41. doi: 10.1186/1477-7827-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mu YM, Yanase T, Nishi Y, Takayanagi R, Goto K, Nawata H. Combined treatment with specific ligands for PPARgamma:RXR nuclear receptor system markedly inhibits the expression of cytochrome P450arom in human granulosa cancer cells. Mol Cell Endocrinol. 2001;181(1- 2):239–248. doi: 10.1016/s0303-7207(00)00457-3. [DOI] [PubMed] [Google Scholar]
- 20.Komar CM, Curry TE Jr. Inverse relationship between the expression of messenger ribonucleic acid for peroxisome proliferator-activated receptor gamma and P450 side chain cleavage in the rat ovary. Biol Reprod. 2003;69(2):549–555. doi: 10.1095/biolreprod.102.012831. [DOI] [PubMed] [Google Scholar]
- 21.Kwintkiewicz J, Nishi Y, Yanase T, Giudice LC. Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect. 2010;118(3):400–406. doi: 10.1289/ehp.0901161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mu YM, Yanase T, Nishi Y, Waseda N, Oda T, Tanaka A, et al. Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochem Biophys Res Commun. 2000;271(3):710–713. doi: 10.1006/bbrc.2000.2701. [DOI] [PubMed] [Google Scholar]
- 23.Gasic S, Bodenburg Y, Nagamani M, Green A, Urban RJ. Troglitazone inhibits progesterone production in porcine granulosa cells. Endocrinology. 1998;139(12):4962–4966. doi: 10.1210/endo.139.12.6385. [DOI] [PubMed] [Google Scholar]
- 24.Jump DB. The biochemistry of n-3 polyunsaturated fatty acids. J Biol Chem. 2002;277(11):8755–8758. doi: 10.1074/jbc.R100062200. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Rudd MD, Hernandez-Gonzalez I, Gonzalez- Robayna I, Fan HY, Zeleznik AJ, et al. FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol Endocrinol. 2009;23(5):649–661. doi: 10.1210/me.2008-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yong EL, Hillier SG, Turner M, Baird DT, Ng SC, Bongso A, et al. Differential regulation of cholesterol side-chain cleavage (P450scc) and aromatase (P450arom) enzyme mRNA expression by gonadotrophins and cyclic AMP in human granulosa cells. J Mol Endocrinol. 1994;12(2):239–249. doi: 10.1677/jme.0.0120239. [DOI] [PubMed] [Google Scholar]
- 27.Long MJ, Sairam MR, Komar CM. Initiation of the expression of peroxisome proliferator-activated receptor gamma (PPAR gamma) in the rat ovary and the role of FSH. Reprod Biol Endocrinol. 2009;7:145–145. doi: 10.1186/1477-7827-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson GF, Magoffin DA, Cragun JR, Chang RJ. The effects of insulin and insulin-like growth factors-I and -II on estradiol production by granulosa cells of polycystic ovaries. J Clin Endocrinol Metab. 1990;70(4):894–902. doi: 10.1210/jcem-70-4-894. [DOI] [PubMed] [Google Scholar]
- 29.Coffler MS, Patel K, Dahan MH, Malcom PJ, Kawashima T, Deutsch R, et al. Evidence for abnormal granulosa cell responsiveness to follicle-stimulating hormone in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(4):1742–1747. doi: 10.1210/jc.2002-021280. [DOI] [PubMed] [Google Scholar]
- 30.Misajon A, Hutchinson P, Lolatgis N, Trounson AO, Almahbobi G. The mechanism of action of epidermal growth factor and transforming growth factor alpha on aromatase activity in granulosa cells from polycystic ovaries. Mol Hum Reprod. 1999;5(2):96–103. doi: 10.1093/molehr/5.2.96. [DOI] [PubMed] [Google Scholar]
- 31.Almahbobi G, Anderiesz C, Hutchinson P, McFarlane JR, Wood C, Trounson AO. Functional integrity of granulosa cells from polycystic ovaries. Clin Endocrinol (Oxf) 1996;44(5):571–580. doi: 10.1046/j.1365-2265.1996.724545.x. [DOI] [PubMed] [Google Scholar]
- 32.Jakimiuk AJ, Weitsman SR, Brzechffa PR, Magoffin DA. Aromatase mRNA expression in individual follicles from polycystic ovaries. Mol Hum Reprod. 1998;4(1):1–8. doi: 10.1093/molehr/4.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Li Q, Lin H, Yang Q, Wang H, Zhu C. Role of PPARgamma and its gonadotrophic regulation in rat ovarian granulosa cells in vitro. Neuro Endocrinol Lett. 2007;28(3):289–294. [PubMed] [Google Scholar]
- 34.Rafraf M, Mohammadi E, Asghari-Jafarabadi M, Farzadi L. Omega-3 fatty acids improve glucose metabolism without effects on obesity values and serum visfatin levels in women with polycystic ovary syndrome. J Am Coll Nutr. 2012;31(5):361–368. doi: 10.1080/07315724.2012.10720443. [DOI] [PubMed] [Google Scholar]
- 35.Phelan N, O'Connor A, Kyaw TT, Correia N, Boran G, Roche HM, et al. Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: results from a cross-sectional analysis and a randomized, placebo-controlled, crossover trial. Am J Clin Nutr. 2011;93(3):652–662. doi: 10.3945/ajcn.110.005538. [DOI] [PubMed] [Google Scholar]