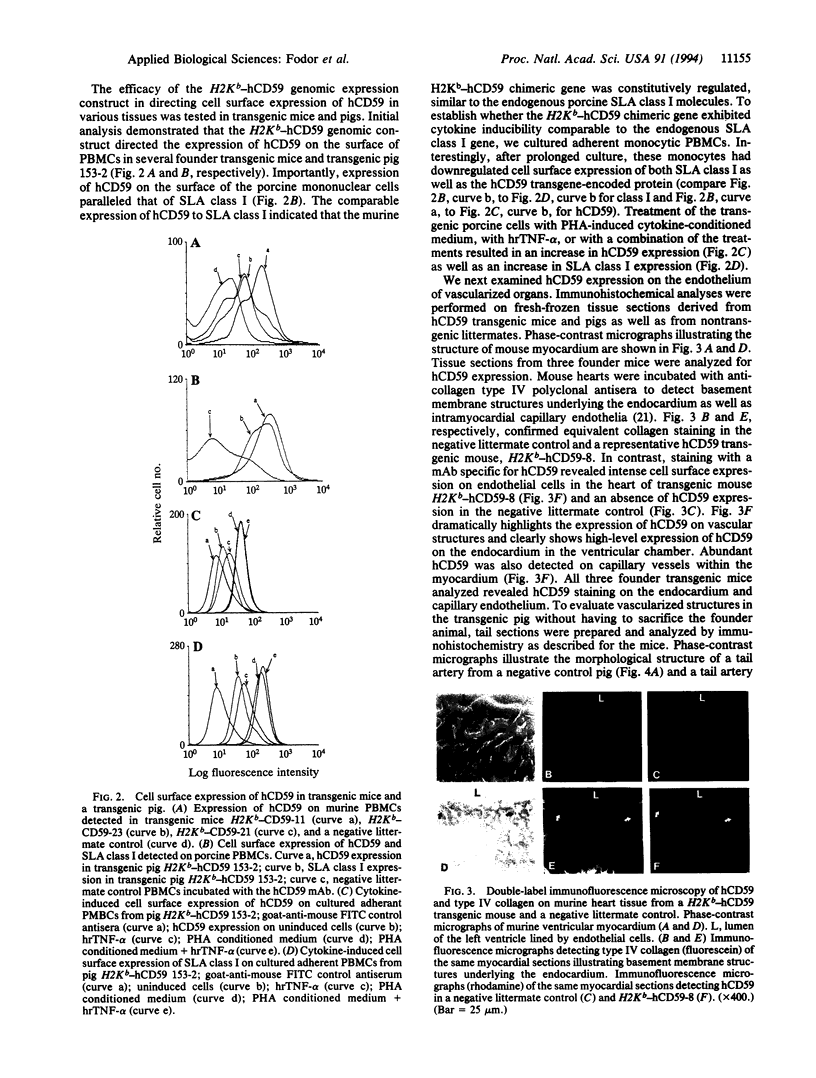
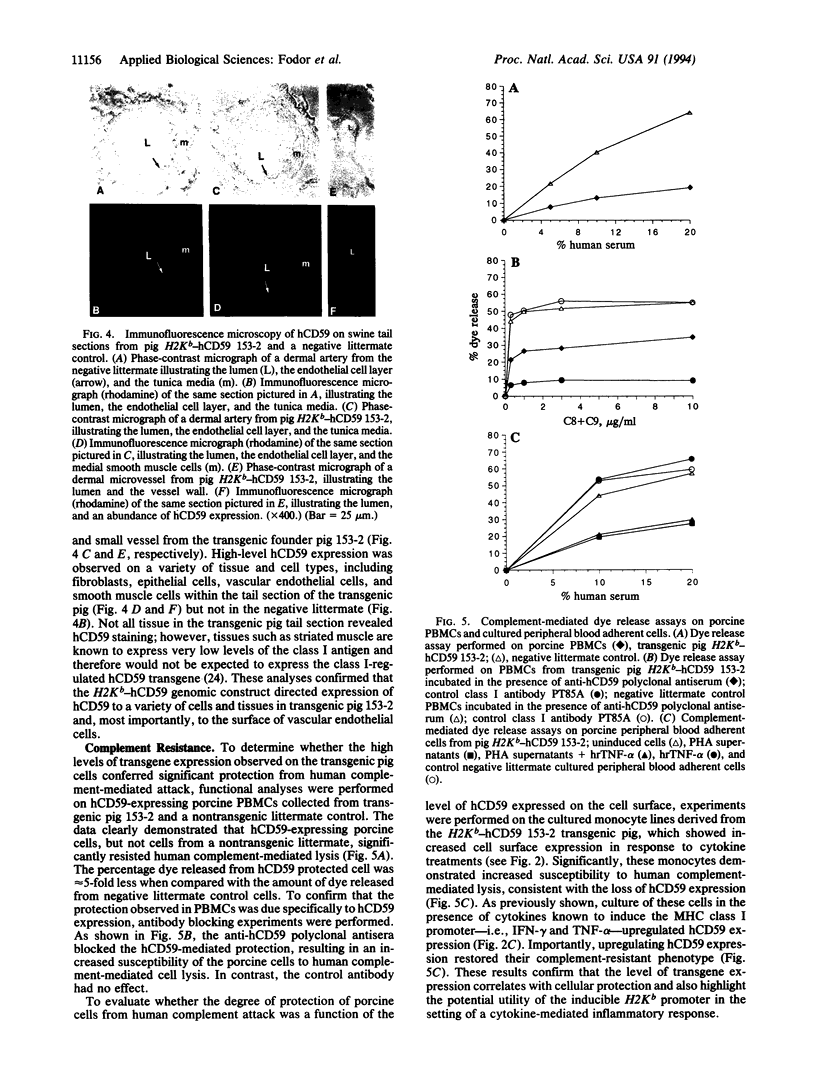
Abstract
The serious shortage of human organs available for transplantation has engendered a heightened interest in the use of animal organs (xenografts) for transplantation. However, the major barrier to successful discordant xenogeneic organ transplantation is the phenomenon of hyperacute rejection. Hyperacute rejection results from the deposition of high-titer preformed antibodies that activate serum complement on the luminal surface of the vascular endothelium, leading to vessel occlusion and graft failure within minutes to hours. Although endogenous membrane-associated complement inhibitors normally protect endothelial cells from autologous complement, they are species restricted and thus confer limited resistance to activated xenogeneic complement. To address the pathogenesis of hyperacute rejection in xenotransplantation, transgenic mice and a transgenic pig were engineered to express the human terminal complement inhibitor hCD59. High-level cell surface expression of hCD59 was achieved in a variety of murine and porcine cell types, most importantly on both large vessel and capillary endothelium. hCD59-expressing porcine cells were significantly resistant to challenge with high-titer anti-porcine antibody and human complement. These experiments demonstrate a strategy for developing a pig-to-primate xenogeneic transplantation model to test whether the expression of a human complement inhibitor in transgenic pigs could render xenogeneic organs resistant to hyperacute rejection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanar M. A., Baldwin A. S., Jr, Flavell R. A., Sharp P. A. A gamma-interferon-induced factor that binds the interferon response sequence of the MHC class I gene, H-2Kb. EMBO J. 1989 Apr;8(4):1139–1144. doi: 10.1002/j.1460-2075.1989.tb03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso A. P., Vercellotti G. M., Fischel R. J., Bolman R. M., Bach F. H., Platt J. L. Mechanism of complement activation in the hyperacute rejection of porcine organs transplanted into primate recipients. Am J Pathol. 1992 May;140(5):1157–1166. [PMC free article] [PubMed] [Google Scholar]
- Dalmasso A. P., Vercellotti G. M., Platt J. L., Bach F. H. Inhibition of complement-mediated endothelial cell cytotoxicity by decay-accelerating factor. Potential for prevention of xenograft hyperacute rejection. Transplantation. 1991 Sep;52(3):530–533. doi: 10.1097/00007890-199109000-00029. [DOI] [PubMed] [Google Scholar]
- Johnson D. R., Pober J. S. Tumor necrosis factor and immune interferon synergistically increase transcription of HLA class I heavy- and light-chain genes in vascular endothelium. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5183–5187. doi: 10.1073/pnas.87.13.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. P., Rollins S. A., Burton W. V., Sims P. J., Bothwell A. L., Squinto S. P., Zavoico G. B. Protection of porcine aortic endothelial cells from complement-mediated cell lysis and activation by recombinant human CD59. Transplantation. 1994 May 27;57(10):1494–1501. [PubMed] [Google Scholar]
- Kimura A., Israël A., Le Bail O., Kourilsky P. Detailed analysis of the mouse H-2Kb promoter: enhancer-like sequences and their role in the regulation of class I gene expression. Cell. 1986 Jan 31;44(2):261–272. doi: 10.1016/0092-8674(86)90760-9. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J. The control of homologous lysis. Immunol Today. 1991 Sep;12(9):312–315. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- Larsen R. D., Rivera-Marrero C. A., Ernst L. K., Cummings R. D., Lowe J. B. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:beta-D-Gal(1,4)-D-GlcNAc alpha(1,3)-galactosyltransferase cDNA. J Biol Chem. 1990 Apr 25;265(12):7055–7061. [PubMed] [Google Scholar]
- Leventhal J. R., Dalmasso A. P., Cromwell J. W., Platt J. L., Manivel C. J., Bolman R. M., 3rd, Matas A. J. Prolongation of cardiac xenograft survival by depletion of complement. Transplantation. 1993 Apr;55(4):857–866. doi: 10.1097/00007890-199304000-00033. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Dreyer B., Pitlick F. A., Furthmayr H. The collagenous components of the subendothelium. Correlation of structure and function. Lab Invest. 1980 Oct;43(4):303–315. [PubMed] [Google Scholar]
- Momburg F., Koch N., Möller P., Moldenhauer G., Hämmerling G. J. In vivo induction of H-2K/D antigens by recombinant interferon-gamma. Eur J Immunol. 1986 May;16(5):551–557. doi: 10.1002/eji.1830160516. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Lindman B. J., Chen H., Spitalnik S. L., Bach F. H. Endothelial cell antigens recognized by xenoreactive human natural antibodies. Transplantation. 1990 Nov;50(5):817–822. doi: 10.1097/00007890-199011000-00015. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Vercellotti G. M., Dalmasso A. P., Matas A. J., Bolman R. M., Najarian J. S., Bach F. H. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990 Dec;11(12):450–457. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- Pruitt S. K., Kirk A. D., Bollinger R. R., Marsh H. C., Jr, Collins B. H., Levin J. L., Mault J. R., Heinle J. S., Ibrahim S., Rudolph A. R. The effect of soluble complement receptor type 1 on hyperacute rejection of porcine xenografts. Transplantation. 1994 Feb;57(3):363–370. doi: 10.1097/00007890-199402150-00009. [DOI] [PubMed] [Google Scholar]
- Rollins S. A., Zhao J., Ninomiya H., Sims P. J. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991 Apr 1;146(7):2345–2351. [PubMed] [Google Scholar]
- Sandrin M. S., Vaughan H. A., Dabkowski P. L., McKenzie I. F. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1-3)Gal epitopes. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11391–11395. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D. S., Ehrlich R., Satz L., Frels W., Bluestone J., Hodes R., Rudikoff S. Structure and expression of class I MHC genes in the miniature swine. Vet Immunol Immunopathol. 1987 Dec;17(1-4):211–221. doi: 10.1016/0165-2427(87)90141-3. [DOI] [PubMed] [Google Scholar]
- Somerville C. A., d'Apice A. J. Future directions in transplantation: xenotransplantation. Kidney Int Suppl. 1993 Jul;42:S112–S121. [PubMed] [Google Scholar]
- Velander W. H., Johnson J. L., Page R. L., Russell C. G., Subramanian A., Wilkins T. D., Gwazdauskas F. C., Pittius C., Drohan W. N. High-level expression of a heterologous protein in the milk of transgenic swine using the cDNA encoding human protein C. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12003–12007. doi: 10.1073/pnas.89.24.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh L. A., Tone M., Waldmann H. Transfection of human CD59 complementary DNA into rat cells confers resistance to human complement. Eur J Immunol. 1991 Mar;21(3):847–850. doi: 10.1002/eji.1830210349. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing M. G., Zajicek J., Seilly D. J., Compston D. A., Lachmann P. J. Oligodendrocytes lack glycolipid anchored proteins which protect them against complement lysis. Restoration of resistance to lysis by incorporation of CD59. Immunology. 1992 May;76(1):140–145. [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Rollins S. A., Maher S. E., Bothwell A. L., Sims P. J. Amplified gene expression in CD59-transfected Chinese hamster ovary cells confers protection against the membrane attack complex of human complement. J Biol Chem. 1991 Jul 15;266(20):13418–13422. [PubMed] [Google Scholar]