Abstract
Background
Executive dysfunction and psychomotor slowing in depressed patients have been associated with poor antidepressant clinical response, but little is known about the value of neurocognitive tests for differential prediction of response.
Methods
This report presents new findings for 70 depressed patients tested on neurocogntive tests before receiving treatment with a SSRI (escitalopram or citalopram), NDRI (bupropion) or dual mechanism therapy including a serotonergic agent, and for 57 healthy controls.
Results
As predicted from previous research, patients who did not respond to a SSRI or dual therapy showed poorer word fluency than responders, whereas this was not seen for patients treated with bupropion alone. Longer choice reaction time (RT) was also found in nonresponders to a SSRI or dual therapy, but the opposite trend was seen for bupropion. Using a combined index of word fluency and RT (with normative performance as a cutoff) yielded differential predictions of response. Equal to or above normal performance predicted good response to a SSRI or dual therapy, with high positive predictive value (90%) and specificity (78%) but lower sensitivity (53%). In contrast, less than normal performance predicted good response to bupropion alone (positive predictive value= 82%; specificity= 67%; sensitivity= 90%).
Limitations
Relatively small sample size, no placebo control, and combining across SSRI alone and dual treatments.
Conclusions
Although findings are preliminary due to small sample size, brief tests of word fluency and psychomotor speed may help identify depressed patients who are unresponsive to a serotonergic agent, but who may respond to bupropion alone.
Keywords: Depression, antidepressants, treatment response, neurocognitive tests, psychomotor speed
1. Introduction
Although a variety of antidepressants are available for treatment of depression, clinicians have no way of knowing in advance whether or not a patient will benefit from treatment with a specific agent. Patients must often endure a prolonged “trial and error” period before finding an effective antidepressant and they may become increasingly hopeless if they fail to benefit and discontinue treatment. Recent findings have raised hopes for identifying cognitive tests for predicting antidepressant clinical response, which would have the distinct advantage of being quick and easy to administer in a doctor's office. Impaired performance on neurocognitive tests of executive function and psychomotor speed has been associated with poor response to antidepressants (Dunkin et al., 2000; Gorlyn et al., 2008; Kalayam & Alexopoulos, 1999). In our initial study (Taylor et al., 2006), unmedicated depressed patients were tested on a battery of neuropsychological tests before receiving a selective serotonin reuptake inhibitor (SSRI) fluoxetine for 12 weeks. Compared to fluoxetine responders (n = 25), nonresponders (n = 12) showed poorer word fluency (p < .001) and Stroop color naming (p < .05), and also tended to perform worse on Stroop word reading and WAIS-III digit symbol subtest (p < .10). Differences between responders and nonresponders were found for these tests assessing verbal fluency and speed of cognitive processing, but were not seen on tests of executive function, e.g., Wisconsin Card Sorting Test. We concluded that psychomotor slowing may identify a subgroup of patients who are unresponsive to SSRI monotherapy and who should receive an alternative treatment.
There is neuroimaging evidence linking psychomotor retardation in depressed patients to dysfunction in dopaminergic striatal areas (Ebert et al., 1996; Martinot et al., 2001) and left dorsolateral prefrontal cortex (Galynker et al., 1998). There is some support for the hypothesis that depressed patients with psychomotor slowing may respond best to antidepressants targeting the noradrenaline or dopamine system (Herrera-Guzmán et al., 2008; Rampello et al., 1991). Herrera-Guzmán et al. found that responders (n=12) to 8 weeks of treatment with the noradrenaline/dopamine reuptake inhibitor (NDRI) bupropion showed poorer pretreatment performance than nonresponders (n=8) on measures of mental processing speed and visual memory. This raises the possibility that neurocognitive tests, particularly those dependent on speed of cognitive processing, may be differential predictors or moderators (Trivedi, 2013) of clinical response to antidepressants that directly act on the serotonin system as opposed to the NDRI bupropion.
The current study evaluated this prediction by testing depressed patients before receiving a SSRI alone (escitalopram or citalopram), NDRI alone (bupropion) or dual mechanism therapy, i.e., combination of SSRI plus NDRI, serotonin-noradrenaline reuptake inhibitor (SNRI) duloxetine or SNRI plus NDRI. We used neurocognitive tests that have shown particular promise for predicting treatment response to a SSRI (Gorlyn et al., 2008; Taylor et al., 2006), and also included a four-choice reaction time (RT) test to further establish the importance of psychomotor speed for predicting treatment response. The pretreatment performance of depressed patients who received a serotonin reuptake inhibitor (SSRI alone or dual therapy including a serotonergic agent) was compared to those who were treated with a NDRI alone. Although little is known about prediction of treatment response to these dual mechanism therapies, differences between responders and nonresponders on electrophysiological measures were the same for patients receiving SSRI monotherapy or dual therapy (Tenke et al., 2011). We therefore predicted that patients who fail to respond to a serotonin reuptake inhibitor (SSRI alone or dual therapy including a serotonergic agent), would show poorer accuracy and slower RTs than patients who respond to these treatments. On the contrary, based on the findings of Herrera-Guzmán et al. (2008), we expected that responders to bupropion alone would show poorer performance than nonresponders to this treatment. Healthy adults were also tested to provide normative data, which may serve as meaningful and stable criteria for predicting clinical response to antidepressants (Tenke et al., 2011).
2. Methods
2.1 Subjects
Depressed outpatients (n=92) were recruited before entering one of four studies at the Depression Evaluation Service at the New York State Psychiatric Institute: (1) 51 were randomly assigned double-blind to treatment with escitalopram, bupropion, or dual therapy with both antidepressants; (2) 13 were randomly assigned to open treatment with citalopram, bupropion, or dual therapy; (3) 13 patients received open (non-randomized) treatment with escitalopram or bupropion; (4) 15 were randomly assigned double-blind to treatment with duloxetine or placebo. Healthy controls (n=57) were recruited from the New York metropolitan area. Only patients and controls in good physical health and who spoke fluent English participated in the study. Participants were excluded for any of the following reasons: significant risk of suicide, current (within last 6 months) drug or alcohol use disorder, bipolar disorder or life-time history of psychotic disorder, seizure disorder, a history of head trauma or other neurological disorder. The diagnostic assessment and treatment of patients was carried out by research psychiatrists as part of ongoing clinical trials. Control participants were screened using the Structured Clinical Interview for DSM-IV Axis I Disorders, Nonpatient Edition (First et al., 1996) to exclude those with Axis I disorders, except for nicotine dependence. Eleven patients dropped out before receiving at least 4 weeks of treatment with an SSRI alone (n=4), SSRI plus bupropion (n=4), bupropion alone (n=2) or duloxetine (n=1) and were not included in this report. Two dropped because of side effects, two moved away, one withdrew consent, and the remainder did not show up for appointments and were lost to follow-up. Their mean baseline score on the Hamilton Rating Scale for Depression (HAM-D17; Hamilton, 1960) was 18.9 (SD=4.9). An additional 6 patients who were not tested on the neurocognitive tests because they were not fluent in English or did not have the time, and 5 patients who received placebo treatment are not included in this report. The remaining 70 patients met DSM-IV criteria for major depressive disorder (MDD, n=41), dysthymia (n=10) or both disorders (n=19). Twelve patients had a comorbid anxiety disorder (n=4 general anxiety disorder, n=4 social phobia, n=2 obsessive-compulsive disorder, n=1 panic disorder and n=1 panic and social phobia disorders). Including pretreatment scores on the State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983) as a covariate in analyses reported below did not affect the significance of differences in performance between responders and nonresponders. All participants were paid $15 per hour for participating. This study was approved by the institutional review board and all participants signed an informed consent form.
Patients were tested after being unmedicated for a minimum of 7 days, but most patients were drug-free for considerably longer or were not previously treated with an antidepressant. Patients then received one of the treatments listed in Table 1. A total of 20 patients received a SSRI alone (escitalopram or citalopram), 18 received a SSRI plus NDRI (bupropion), 9 received a SNRI (duloxetine), and 1 received this SNRI plus NDRI. Patients who received a SSRI alone or one of these dual mechanism treatments were combined into a SSRI/Dual therapy group. This allowed a direct comparison of pretreatment performance of these patients who were treated with a serotonin reuptake inhibitor as opposed to the 22 patients who received only the NDRI bupropion. As shown in the results, differences in word fluency and RT between treatment responders and nonresponders were comparable for patients who received an SSRI alone and those who received a dual mechanism therapy including a serotonergic agent.
Table 1. Antidepressant Treatments.
| Responders | Nonresponders | |||
|---|---|---|---|---|
| n | Dosagea | n | Dosagea | |
| Escilalopram alone | 15 | 40 (10-40) | 4 | 15 (10-20) |
| Citalopram alone | 1 | 60 | 0 | --- |
| Escitalopram | 9 | 30 (10-40) | 6 | 40 (10-40) |
| plus bupropion | 350 (150-450) | 450 (150-450) | ||
| Citalopram | 3 | 40 (20-40) | 0 | --- |
| plus bupropion | 450 (300-450) | |||
| Duloxetine alone | 9 | 60 (30-120) | 0 | |
| Duloxetine | 0 | --- | 1 | 30 |
| plus bupropion | 300 | |||
| Bupropion alone | 12 | 3350 (150-450) | 10 | 450 (150-450) |
Median dosage in milligrams/day (range).
After a baseline evaluation, patients were seen weekly or biweekly by their study psychiatrist for 8-12 weeks or until they discontinued treatment. Clinical response was based on ratings from the HAM-D17 by a psychiatrist who was blind to the neurocognitive test data and to the hypotheses being tested in this study. Patients showing a change in HAM-D17≥50% from baseline assessment to end of treatment trial (or last visit brought forward) were considered responders and all other patients were considered to be nonresponders. Of the patients treated with a serotonergic agent (SSRI or dual therapy), 37 (77.1%) were responders and 11 were nonresponders, and 12 patients treated with a bupropion alone were responders (54.5%) and 10 nonresponders. The dosage levels of antidepressants were comparable in the responder and nonresponder groups (Table 1). The average length of treatment did not differ between treatments. Responders and nonresponder groups did not significantly differ from each other and from healthy controls in gender, age, education or handedness (Table 2). There was a significant difference among groups in self-ratings on the Beck Depression Inventory (Beck et al., 1961), with nonresponders to either treatment having the highest pretreatment depression scores and controls the lowest. There was no difference, however, in pretreatment HAM-D17 ratings between responders and nonresponders, and responders showed the expected lower HAM-D17 scores than nonresponders at end of treatment.
Table 2. Characteristics of Treatment Responders, Nonresponders and Healthy Controls.
| SSRI/Dual | Bupropion | Controls | |||
|---|---|---|---|---|---|
| R | NR | R | NR | ||
| Gender | |||||
| F/M | 23/14 | 7/4 | 6/6 | 5/5 | 31/26 |
| Age (Yrs) | |||||
| Mean | 40.2 | 44.1 | 40.3 | 39.2 | 35.1 |
| SD | 11.9 | 14.4 | 13.1 | 14.1 | 9.8 |
| Education (Yrs) | |||||
| Mean | 16.2 | 14.6 | 15.8 | 17.4 | 15.8 |
| SD | 2.0 | 2.7 | 1.7 | 3.4 | 2.1 |
| Handedness (LQ) | |||||
| Mean | 74.4 | 84.1 | 57.4 | 65.7 | 80.4 |
| SD | 37.0 | 21.0 | 55.1 | 56.4 | 24.6 |
| Anxiety (ST AI)a | |||||
| Mean | 76.2 | 82.4 | 75.4 | 79.7 | 42.8 |
| SD | 11.4 | 9.6 | 9.0 | 12.9 | 8.2 |
| Depression (BDI)b | |||||
| Mean | 21.1 | 28.4 | 21.8 | 26.3 | 1.3 |
| SD | 7.1 | 7.4 | 10.5 | 7.2 | 2.2 |
| HAM-D17 Pre | |||||
| Mean | 15.7 | 16.7 | 15.8 | 17.2 | |
| SD | 4.4 | 3.9 | 3.2 | 4.1 | |
| HAM-D17Postc | |||||
| Mean | 3.7 | 13.4 | 2.7 | 13.7 | |
| SD | 2.7 | 5.5 | 2.3 | 4.4 | |
Abbreviations: R, Responder; NR, Non-Responder; F, Female; M, Male; LQ, Laterality Quotient on the Edinburgh Inventory (Oldfield, 1971); STAI, State-Trait Anxiety Inventory (Spielberger et al., 1983); BDI, Beck Depression Inventory (Beck et al., 1961); HAM-D17, Hamilton Rating Scale for Depression (Hamilton, 1960).
Significant difference among groups in STAI scores, F=97.7, df=4,121, p<.001; R=NR>Controls.
Significant difference among groups in BDI scores, F=109.9, df=4, 22, p<.001; NR>R>Controls.
NR to each treatment have significantly higher post-treatment HAM-D17 scores compared to R, F=117.5, df=1,66, p< .001
2.2 Neurocognitive Tests
The neurocognitive assessments were selected by the authors (GEB & JGK) on the basis of their prior studies in depressed patients (Gorlyn et al., 2008; Keilp et al., 2008; Taylor et al., 2006). Training and supervision of the research assistants who administered the neurocognitive tests was done by one of the authors (JGK), a Clinical Psychologist and Neuropsychologist by training. The non-computerized tests included: (1) word fluency test using a written version of the Controlled Oral Word Association Test (Benton et al., 1983), in which participants had one minute to write down as many words as possible that began with each of three letters (FAS); (2) Wechsler Adult Intelligence Test (WAIS; Wechsler, 1997)- III Digit Symbol test; and (3) The National Adult Reading Test (NART; Bright et al., 2002), which provided a measure of IQ equivalent. Two computerized tests were presented on a Macintosh laptop with PsyScope programming language (Keilp et al., 2005). A 4-choice reaction time task was adapted from Thorne et al. (1985). The participant sees a black screen with 4 white squares arranged in a windowpane pattern. A red “X” appears in one of the squares, and the subject responds by pressing one of four buttons to indicate the position of the X. Following the response, the X disappears and then reappears in the same or different square. The participant is instructed to “catch the X” by pressing the correct buttons as the task progresses. The dependent measure is median reaction time on correct trials. A computerized Stroop test used single item presentation and a button press response (Keilp et al., 2008). Three conditions were given in a blocked fashion in a fixed order: (1) Word Condition—identify the color names in black letters; (2) Color Condition—identify color of a string of four X's displayed in one of three colors; and (3) Color/Word Condition—indentify display color of the stimulus containing an incongruous color name, ignoring the text. Auditory feedback was provided for all responses: correct (beep) and incorrect (Buzz). Word and Color blocks included 45 stimulus trials and Word/Color blocks included 90 trials. Median reaction time on correct trials is the dependent measure.
2.3 Statistical Analyses
Demographic variables in Table 2 (age, education, handedness) were compared among responder, nonresponders and control groups using one-way analyses of variance (ANOVA) and post-hoc Newman-Keuls tests for continuous variables. An ANCOVA was used to evaluate predicted pretreatment differences between responders and nonresponders on each neurocognitive test. Between-subject variables were Treatment (SSRI/Dual Therapy vs. bupropion alone) and Response (responder vs. nonresponder) and covariates were age and pretreatment severity of depression (BDI) and anxiety (STAI). Given a significant Treatment by Response interaction, analyses of simple effects compared performance of responders and nonresponders to each treatment.
3. Results
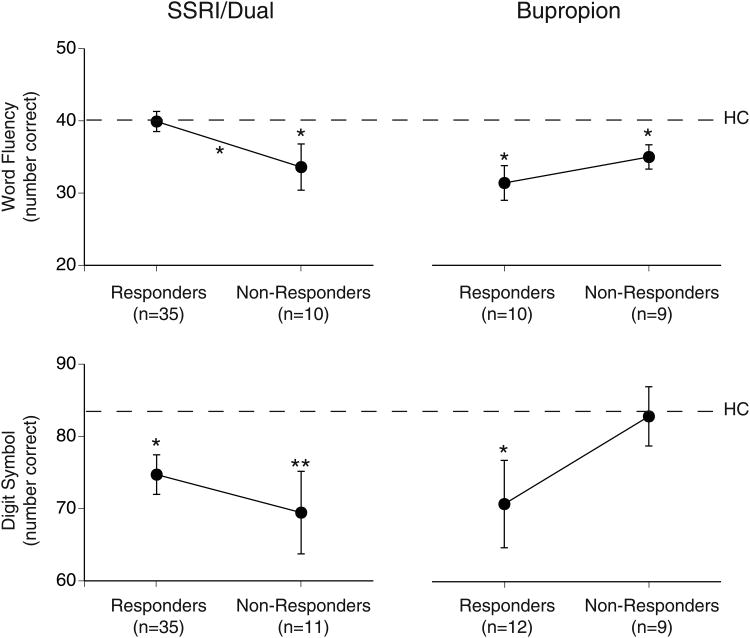
Fig. 1 shows the mean pretreatment accuracy for responders and nonresponders to SSRI/Dual therapy as compared to bupropion alone on the word fluency and digit symbol tests. ANCOVA showed a significant Treatment by Response interaction for word fluency, F=4.87, df=1,57, p=.03. The word fluency for SSRI/Dual responders was the same as found for healthy controls (dashed line), whereas nonresponders had poorer word fluency than responders and healthy controls (p<.05). In contrast, bupropion responders and nonresponders did not differ significantly in word fluency and both groups performed poorer than controls (p<.05). The Treatment by Response interaction for digit symbol test was not significant, F=2.99, df=1,60, p=.09. Although there was no significant difference in digit symbol performance between responders and nonresponders, nonresponders to bupropion showed normal digit symbol performance, but nonresponders to SSRI/Dual (p<.01) and responders to either treatment (p<.05) performed worse than controls. There was no significant difference in NART IQ equivalent between responders and nonresponders to either treatment and no Treatment by Response interaction, F=2.51, df=1,59, p=.12.
Fig. 1.
Mean accuracy on word fluency and digit symbol tests for responders and nonresponders to SSRI/Dual therapy or bupropion alone (error bars= SEM). Dashed line= mean accuracy for 57 healthy controls (HC) and asterisks indicate significant group differences (*p<.05, **p<.01).
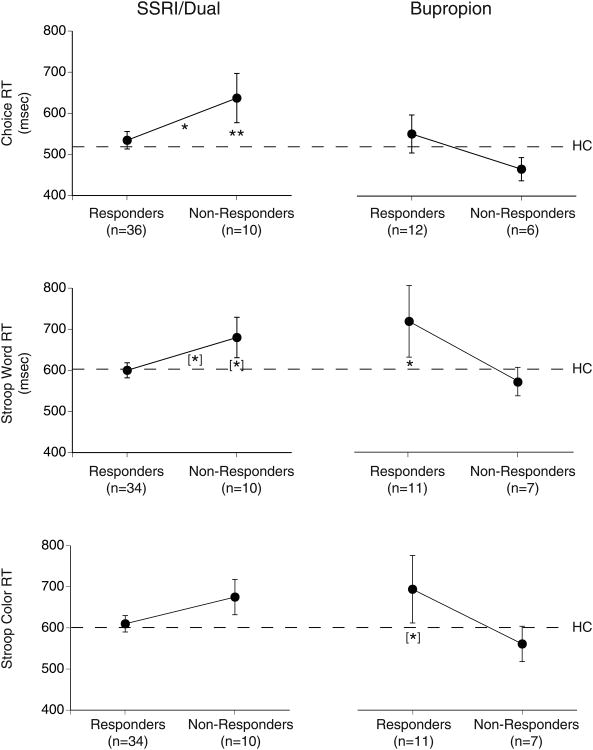
ANCOVA also showed Treatment by Response interactions for the three measures of psychomotor speed: (1) Choice RT, F=4.68, df=1,57, p=.03; (2) Stroop Word RT, F=5.49, df=1,55, p=.02, and (3) Stroop Color RT, F=4.36, df=1,55, p=.04. As shown in Fig. 2, these interactions reflect the opposite difference in RT between responders and nonresponders to SSRI/Dual therapy as opposed to bupropion alone. The longer choice RT in SSRI/Dual nonresponders than in responders (p<.05) and healthy controls (p<.01) is indicative of psychomotor slowing in patients who fail to respond to a serotonergic agent. RT measures on the Stroop Word test show a comparable trend for psychomotor slowing in SSRI/Dual nonresponders compared to responders and controls (p<.10). In contrast, nonresponders to bupropion alone had normal RTs (i.e., equal to or shorter than healthy controls) on all three tests, whereas bupropion responders tended to have longer RTs than controls on the Stroop Word (p<.05) and Color (p<.10) tests. There was no significant difference in Stroop interference effect between responders and nonresponders to either treatment and no Treatment by Response interaction, F=1.44, df=1,55, p=.23.
Fig. 2.
Mean reaction time (RT) on choice RT, Stroop word and Stroop coler tests for responders and nonresponders to SSRI/Dual therapy or bupropion alone (error bars= SEM). Dashed line= mean accuracy for 57 healthy controls (HC) and asterisks indicate significant group differences (*p<.05, **p<.01).
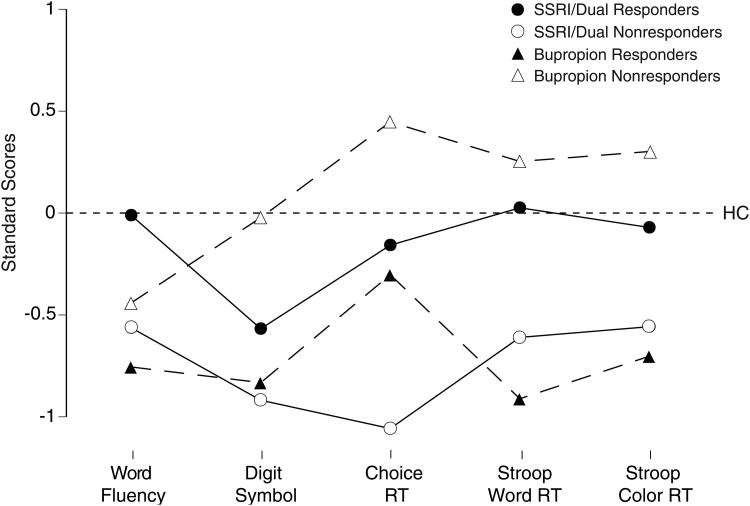
To examine the comparability of findings across tests, performance of patients on each test were converted to standard scores using the mean and standard deviation for healthy controls. The sign of the RT measures were inverted so that negative scores in Fig. 3 reflect poorer performance than controls. Patients who responded to SSRI/Dual treatments had normal performance on all but the Digit Symbol test, whereas SSRI/Dual nonresponders had consistently poorer performance than controls. SSRI/Dual nonresponders showed poorer performance than SSRI/Dual responders on all tests, with moderate effect sizes (Cohen's d) ranging from .51 to .68. In contrast, patients who responded to bupropion alone had consistently poorer performance than controls on all tests, whereas bupropion nonresponders had normal performance on all but the word fluency test. Bupropion responders showed poorer performance than nonresponders on all tests, with moderate effects sizes ranging from .57 to .75.
Fig. 3.
Standard scores (using mean and SD for 57 healthy adults) on 5 tests for responders and nonresponders to SSRI/Dual therapy or bupropion alone.
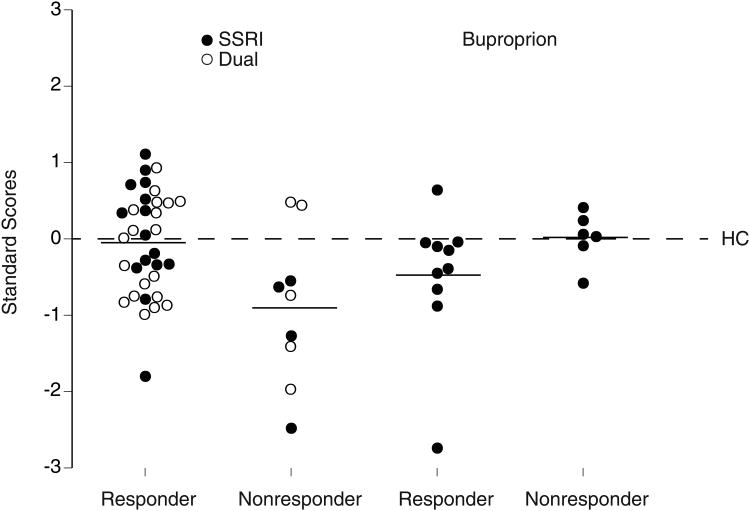
Correlations of word fluency in patients with their performance on the Digit Symbol test (r=.36, p<.01) and RT tests (ranging from r= -.23 to -.28, p<.10) were small, but correlations among the digit symbol, choice RT, Stroop word RT and color RT were relatively high (ranging from r=-.68 to .91, p<.001), reflecting the common psychomotor speed component of these tests. This indicates that word fluency provides some independent information beyond psychomotor speed alone and the combination of scores on this test and a test of psychomotor speed (i.e., choice RT) might yield better predictions of treatment response than either measure alone. We averaged the standard scores on the word fluency and choice RT tests to obtain a combined index of performance and examined its value for predicting treatment response (Fig. 4). An ANCOVA of the these standard scores yielded a significant Treatment by Response interaction, F=7.37, df=1,52, p=.009. The combined index differentiated SSRI/Dual therapy responders and nonresponders (p=.005), with a larger effect size than was seen for either test alone (Cohen's d= 1.00). The opposite difference in combined index between bupropion responders and nonresponders was not statistically significant, but had a moderate effect size of .80. When we repeated the above ANCOVA including only patients who were treated with a SSRI alone or bupropion alone (solid points in Fig. 4), the same Treatment by Response interaction was found, F=6.79, df=1,28, p=.015, and SSRI responders differed significantly from nonresponders (p=.01; large effect size=1.55). Also, an ANCOVA contrasting the combined index scores for patients receiving a SSRI alone (solid points) versus those receiving dual therapy including a serotonergic agent (open points) yielded an overall responder vs. nonresponder difference, F=10.40, df=1,36, p=.003, but no difference between these treatments, F=0.89, df=1,36, p=.35, and no Treatment by Response interaction, F=1.22, df=1,36, p=.28.
Fig. 4.
Combined standard scores (averaged across word fluency and choice RT tests) for individual responders and nonresponders to Dual therapy (○) or monotherapy with SSRI alone or bupropion alone (●).
To further examine the value of the combined index (i.e., averaged word fluency and choice RT standard scores) for predicting treatment response, the mean for healthy controls (combined index of zero in Fig. 4) was used as a cutoff to divide patients into those having performance equal to or greater than controls (predicted to be SSRI/Dual responders) or less than controls (predicted to be SSRI/Dual nonresponders). Eighteen of the 20 patients having combined index scores ≥0 responded to SSRI/Dual treatment (positive predictive value=90%). Also, the percentage of SSRI/Dual nonresponders who had scores <0 was relatively high (specificity=78%). However, the percentage of responders with scores ≥0 was lower (sensitivity=53%). When we included only patients receiving a SSRI alone, the positive predictive value and specificity increased to 100%, but the sensitivity remained essentially the same. For evaluating predictions of response to bupropion alone, patients with a combined index score <0 were predicted to be responders and those ≥0 were predicted to be nonresponders. The indices for predicting response to bupropion were all reasonable, with positive predictive value of 82%, specificity of 67% and sensitivity of 90%.
4. Discussion
We replicated in a new sample our prior finding of reduced pretreatment word fluency in SSRI nonresponders (Taylor et al., 2006). Depressed patients who subsequently failed to respond to a SSRI or dual therapy including a serotonergic agent showed poorer word fluency compared to responders and healthy controls, whereas responders had normal word fluency. Two other studies similarly found a trend for SSRI nonresponders to differ from responders in showing poorer word fluency (Dunkin et al., 2000; Gorlyn et al., 2008). We did not, however, find a difference in the Stroop interference effect, which was found by Dunkin et al. (2000), but not Gorlyn et al. (2008). Moreover, we demonstrated for the first time that nonresponders to a SSRI or dual therapy had longer choice RT than responders and controls. They also tended to have poorer Stroop word reading, which was found by Taylor et al. (2006), but not by Dunkin et al. (2000) or Gorlyn et al. (2008). In a prior study by Kalayam & Alexopoulos (1999), elderly depressed patients who were unresponsive to antidepressants (mostly SSRIs) showed evidence of psychomotor retardation and prolonged latency of the P300 cortical event-related potential. These findings support the hypothesis that psychomotor slowing may identify a subgroup of depressed patients who are unresponsive to a serotonin reuptake inhibitor. In contrast, patients treated with only the NDRI bupropion tended to show the opposite difference in neurocognitive performance between responders and nonresponders. It was the bupropion responders who had poorer pretreatment word fluency and slower RTs on the Stroop test than healthy controls. These findings provide, to our knowledge, the first direct and replicated evidence that neurocogntive tests, particularly those dependent on psychomotor speed, may be of value as differential predictors of clinical response to a SSRI or dual therapy that directly targets the serotonergic system as opposed to the NDRI bupropion. Our findings are consistent with the hypothesis that depressed patients with psychomotor slowing will respond an antidepressant that acts on the noradrenergic or dopaminergic system (Herrera-Guzmán et al., 2008; Rampello et al., 1991). The mechanism of antidepressant action of bupropion is not, however, well understood. Preclinical studies suggest that bupropion alters the firing rate of norepinephrine neurons in rats, but may not be an effective dopamine reuptake inhibitor (El Mansari et al., 2008).
Differences in performance between treatment responders and nonresponders were consistently seen across word fluency, digit symbol, choice RT and Stroop RT tests. Performance of patients on the digit symbol, choice RT and Stroop RT tests were highly correlated reflecting their common psychomotor speed component. One could reduce testing time by including only one of these tests for predicting treatment response. Our 4-choice RT test takes less than 5 minutes to administer and more strongly differentiated SSRI/Dual responders and nonresponders than the Digit Symbol or Stroop tests and might therefore be the best candidate. The word fluency test, however, only weakly correlated with these tests indicating that it taps additional processes, e.g., involving left dorsolateral prefrontal and anterior cingulate activity (Frith et al., 1991; Ravnkilde et al., 2002; Schlosser et al., 1998), and could improve predictions of treatment response when used in combination with the choice RT test. A combined index of standard scores on the word fluency and choice RT tests was evaluated for accuracy of predicting response to SSRI/Dual therapy. Using normative values as a cutoff (i.e., a combined index score of zero) yielded high positive predictive value and specificity. Patients who scored equal to or above normal had a 90% chance of responding to a SSRI or dual therapy with a serotonergic agent. Moreover, less than normal performance identified those who did not respond to these treatments with 78% accuracy. The sensitivity for predicting response to an SSRI or dual therapy was, however, lower (53%) with almost half of responders having less than normal performance. These patients may include a number of “nonspecific” responders who would respond to placebo, and the low combined index scores of these patients predicts good response to bupropion, if given as an alternative. Although the sample size was small, having less than normal performance predicts response to bupropion with high positive predictive value (82%) and sensitivity (90%) but lower specificity (67%). These findings support the potential value of this combined word fluency and choice RT index as a differential predictor of antidepressant response. If confirmed in larger samples, it could have important clinical value because these cognitive tests are noninvasive, quick, and easy to administer.
This study does have some limitations. First, several DSM-IV mood disorders (MDD, dysthymia, double depression) were included, but samples were not large enough to determine whether the predictive value of neurocognitive tests varies across these diagnoses. Second, depressed patients having comorbid anxiety disorders were included. Although co-varying for severity of anxiety had no effect on the findings, future studies should determine whether or not the presence of comorbid anxiety disorders affects the predictive value of neurocognitive tests. Third, the study did not control for nonspecific placebo response. It is not clear, however, that placebo effects could have resulted in the observed Treatment by Response interactions for the word fluency and RT tests, which indicate that the relation between test performance and subsequent clinical response differed for patients receiving a serotonergic antidepressant versus bupropion alone. Fourth, sample sizes were relatively small, particularly for patients treated with bupropion alone, and we combined across patients treated with a SSRI alone and those receiving dual therapy including a serotongeric agent. Although we did not find a significant difference in performance between patients receiving a SSRI alone or dual therapy, predictions may be even stronger for a SSRI. Among patients treated with a SSRI alone, all those who had performance equal to or above the normative mean responded to treatment (100% positive predictive value) and all nonresponders had scores below normal (100% specificity), which agrees with prior findings for word fluency in patients treated with the SSRI fluoxetine (Taylor et al., 2006). Sensitivity of predictions was lower in both our prior and current study suggesting room for improvement. Inclusion of additional behavioral tests, in particular tests of working memory (Gorlyn et al., 2008) or reward learning (Pizzagalli et al., 2005), and electrophysiological (Bruder et al., 2013; Tenke et al., 2011) or neuroimaging measures (Mayberg et al., 1997; Pizzagalli 2011), which have been linked to antidepressant response, might further improve predictions of treatment response. We are currently participating in a multi-site project (EMBARC; Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care), in which behavioral, electrophysiological and neuroimaging measures are being obtained before depressed patients are randomly assigned to treatment with an SSRI or placebo and again after one week of treatment. The combined use of these measures in larger samples of responders and nonresponders should further aid in identifying both moderators and mediators of SSRI response and nonspecific placebo response (Trivedi, 2013).
Acknowledgments
Supported in part by grants MH36295 (GEB) and MH076961 (JWS) from the National Institute of Mental Health.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Ward CH, Mendelson M, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:456–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3rd. AJA Associates; Iowa City: 1983. [Google Scholar]
- Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8:847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Kayser J. Electrophysiological predictors of clinical response to antidepressants. In: Mann JJ, Roose SP, McGrath PJ, editors. The Clinical Handbook for the Management of Mood Disorders. Cambridge University Press; Cambridge, UK: 2013. pp. 380–393. [Google Scholar]
- Dunkin JJ, Leuchter AF, Cook IA, Kasl Godley JE, Abrams M, Rosenberg- Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Loew T, Pirner A. Dopamine and depression--striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacology (Berl) 1996;126:91–94. doi: 10.1007/BF02246416. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Ghanbari R, Janssen S, Blier P. Sustained administration of bupropion alters the neuronal activity of serotonin, norepinephrine but not dopamine neurons in the rat brain. Neuropharmacology. 2008;55:1191–1198. doi: 10.1016/j.neuropharm.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Liddle PF, Frackowiak RS. A PET study of word findings. Neuropsychologia. 1991;29:1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Nonpatient Edition (SCID-NP) Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1996. [Google Scholar]
- Galynker II, Cai J, Ongseng F, Finestone H, Dutta E, Serseni D. Hypofrontality and negative symptoms in major depressive disorder. J Nucl Med. 1998;39:608–612. [PubMed] [Google Scholar]
- Gorlyn M, Keilp JG, Grunebaum MF, Taylor BP, Oquendo MA, Bruder GB, Stewart JW, Mann JJ. Neuropsychological characteristics as predictors of SSRI treatment response in depressed subjects. J Neural Transm. 2008;115:1213–1219. doi: 10.1007/s00702-008-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Guzmán I, Gudayol-Ferré E, Lira-Mandujano J, Herrera-Abarca J, Herrera-Guzmán D, Montoya-Pérez K, Guardia-Olmos J. Cognitive predictors of treatment response to bupropion and cognitive effects of bupropion in patients with major depressive disorder. Psychiatry Res. 2008;160:72–82. doi: 10.1016/j.psychres.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry Res. 2008;159:7–17. doi: 10.1016/j.psychres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Res. 2005;135:191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- Martinot M, Bragulat V, Artiges E, Dolle F, Hinnen F, Jouvent R, Martinot J. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry. 2001;158:314–316. doi: 10.1176/appi.ajp.158.2.314. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampello L, Nicoletti G, Raffaele R. Dopaminergic hypothesis for retarded depression: a symptom profile for predicting therapeutical responses. Acta Psychiatr Scand. 1991;84:552–554. doi: 10.1111/j.1600-0447.1991.tb03193.x. [DOI] [PubMed] [Google Scholar]
- Ravnkilde B, Videbech P, Rosenberg R, Gjedde A, Gade A. Putative tests of frontal lobe function: A PET-study of brain activation during Stroop's test and verbal fluency. J Clin Exp Neuropsychol. 2002;24:534–547. doi: 10.1076/jcen.24.4.534.1033. [DOI] [PubMed] [Google Scholar]
- Schlösser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998;64:492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Ehrlichman H, Quitkin FM. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. Am J Psychiatry. 2006;163:73–78. doi: 10.1176/appi.ajp.163.1.73. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J, Manna CG, Fekri S, Kroppmann CJ, Schaller JD, Alschuler DM, Stewart JW, McGrath PJ, Bruder GE. Current source density measures of EEG alpha predict antidepressant treatment response. Biol Psychiatry. 2011;70:388–394. doi: 10.1016/j.biopsych.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thone DR, Genser SG, Sing HC, Hegge FW. The Walter Reed Performance Assessment Battery. Neurobehav Toxicol Teratol. 1985;7:415–418. [PubMed] [Google Scholar]
- Trivedi MH. Modeling predictors, moderators and mediators of treatment outcome and resistance in depression. Biol Psychiatry. 2013;74:2–4. doi: 10.1016/j.biopsych.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]