Abstract
Background
β-hydroxy-β-methylbutyric acid (HMB) is an interesting supplement in sports. However, literature sources present a limited number of studies that verify the efficacy of HMB intake over a longer time period among endurance athletes. For this reason, the aim of this study was to assess the effect of HMB supplementation on physical capacity, body composition and levels of biochemical markers in rowers.
Methods
Sixteen elite male rowers were administered a 12-week HMB supplementation (3×1 gHMB · day−1) and placebo administration (PLA) following the model of a randomised, placebo controlled, double-blind crossover study with a 10 days washout period. Over the course of the experiment, aerobic (maximal oxygen uptake, ventilatory threshold) and anaerobic (anaerobic power indices) capacity were determined, while analyses were conducted on body composition as well as levels of creatine kinase, lactate dehydrogenase, testosterone, cortisol and the T/C ratio. A normal distribution of variables was tested using the paired 2-tailed t-tests; the Mann–Whitney U-test or the Wilcoxon-signed rank test were applied for non-normally distributed variables.
Results
Following HMB supplementation, increased (+2.7 mL · min−1 · kg−1) significantly (p < 0.001) in comparison to its reduction after PLA (−1.0 mL · min−1 · kg−1). In turn, at the ventilatory threshold, a longer time was required to reach this point (+1.2 minHMB vs. −0.2 minPLA, p = 0.012), while threshold load (+0.42 W · kg−1HMB vs. −0.06 W · kg−1PLA, p = 0.002) and threshold heart rate (+9 bpmHMB vs. +1 bpmPLA, p < 0.001) increased. After HMB supplementation, fat mass decreased (−0.9 kgHMB vs. +0.8 kgPLA, p = 0.03). In relation to the initial values after HMB supplementation, the refusal time to continue in the progressive test was extended (p = 0.04), maximum load (p = 0.04) and anaerobic peak power (p = 0.02) increased. However, in relation to the placebo, no differences were observed in anaerobic adaptation or blood marker levels.
Conclusions
The results indicate that HMB intake in endurance training has an advantageous effect on the increase in aerobic capacity and the reduction of fat mass. It may also stimulate an increase in peak anaerobic power, while it seems to have no effect on other indices of anaerobic adaptation and levels of investigated markers in the blood.
Keywords: β-hydroxy-β-methylbutyric acid, Sport supplements, Training support, Adaptation, Rowing
Background
β-hydroxy-β-methylbutyric acid (HMB) – a metabolite of leucine and 2-ketoisocaproic acid – has, for almost 20 years, drawn special attention regarding supplementation support in sports [1–5]. A major advantage of its use suggested in the literature is connected with its anticatabolic action, manifested particularly when there is a high load on the body and when muscle damage is experienced, which may result from the potential effect of HMB on the enhancement of the synthesis or the inhibition of the proteolysis of muscle proteins [2, 5–7]. The observed effect of HMB on the reduction of body mass loss, muscle mass loss, and the degree of cancerous cachexia, as well as slowed neoplasm proliferation, should also be mentioned here [8, 9]. These beneficial effects might be connected with the influence of HMB on the de novo synthesis of cholesterol [5, 7, 10], the expression of insulin-like growth factor 1 (IGF-1) [11, 12], the stimulation of the mTOR kinase pathway [6, 8, 12, 13] or the ubiquitin-proteasome system and caspase activity [7, 9, 14–16]. The possible impact of HMB on the activation of AMPK kinase and Sirt 1, may promote stimulation of mitochondrial biogenesis, higher oxygen consumption, increased efficiency of carbohydrate and fat metabolism, increased lipolysis and fat mass reduction [17, 18].
Studies assessing the effects of HMB in physically active individuals have mainly focused on verifying changes in the state of nutrition, assessing protein synthesis and proteolysis rates, and monitoring hormone levels and selected indices illustrating, for example, the degree of muscle damage and determination of changes in physical capacity [3, 19–22]. Since 1996, studies have been published that claim that HMB uptake may promote advantageous changes in body composition and strength, and reduced levels of muscle damage markers during resistance training [3, 18, 22, 23]. Further, in a meta-analysis by Nissen and Sharp [21], it was found that HMB supplementation for resistance exercise resulted in increased strength and fat-free mass by (net value) 1.4 and 0.28 % per week, respectively, in both trained and untrained individuals.
In contrast, the effect of HMB uptake on physical capacity has rarely been verified in endurance sports. HMB-supplemented cyclists and runners showed an increase in aerobic adaptation, which was assessed by maximal oxygen uptake (), ventilatory threshold (VT), peak oxygen uptake (), time needed to reach , a delay in the onset of blood lactate accumulation (OBLA), and a lower activity of creatine kinase (CK) and lactate dehydrogenase (LDH) [20, 24–26].
However, it’s important to observe that certain studies did not show the effectiveness of HMB in enhancing aerobic capacity [3, 20]. A lack of evidence for the effectiveness of HMB supplementation on changes in body composition, activity of muscle damage markers, or in hormone concentrations was also presented in studies on participants involved in resistance or volleyball training [3, 27–29].
In view of the inconclusive character of the study results conducted to date, and the relatively low number of studies investigating the effectiveness of HMB over a longer period on endurance trained athletes, the aim of this study was to verify the effect of HMB supplementation on physical capacity, body composition and the levels of biochemical markers in elite athletes practicing rowing.
Methods
Subjects
The characteristics of the examined group of athletes are given in Table 1. The experiment involved 16 elite male rowers, aged 20 ± 2 years, with a body weight of 87.3 ± 9.8 kg and a height of 187 ± 5 cm. Athletes were members of the Polish National Team in Rowing and/or rowers occupying high positions in national competitions. The criteria for qualifying for the study included, among others, a good state of health, a valid and current medical certificate confirming their ability to practice sports, at least 5 years of training experience, and a minimum of five rowing training sessions per week. The investigations were conducted from 2009 to 2014 at different times of the year. All athletes declared that they had not undergone changes in their lifestyle, characteristics of training, nutrition, or supplementation, and that they were not using any preparations with potential ergogenic effects, other than those supplied by the authors of this study. Moreover, dietary journals were performed every second week, which proved that athletes did not change their dietary habits during the supplementation period.
Table 1.
Characteristics of the participating rowers (n = 16)
| Study group | ||
|---|---|---|
| Parameter | Means ± SD | Range |
| Age (yr) | 19.5 ± 1.4 | 17.0 – 22.0 |
| Body mass (kg) | 87.3 ± 9.8 | 69.0 – 104.7 |
| Height (cm) | 187 ± 5 | 176 – 194 |
| FFM (kg) | 73.8 ± 6.4 | 63.9 – 83.4 |
| FM (kg) | 13.6 ± 5.3 | 5.1 –21.6 |
| (mL · min−1 · kg−1) | 68.1 ± 6.4 | 58.4 – 75.0 |
| Peak power (W · kg−1) | 11.6 ± 1.1 | 9.9 – 13.2 |
| Years training (yr) | 8.2 ± 2.8 | 6 – 10 |
| Number of training sessions per week (session · week−1) | 8.6 ± 2.7 | 7 – 10 |
| Number of hours of training per week (h · week−1) | 16.8 ± 5.9 | 10 – 24 |
| Energy intake (kcal · kg−1 · day−1)a | 56.1 ± 14.2 | 37.0 – 79.2 |
| Protein intake (g · kg−1 · day−1)a | 1.6 ± 0.2 | 1.3 – 2.0 |
| Carbohydrate intake (g · kg−1 · day−1)a | 6.3 ± 1.4 | 4.9 – 9.3 |
| Fat intake (g · kg−1 · day−1)a | 1.7 ± 0.4 | 1.2 – 2.4 |
FFM fat-free mass, FM fat mass; maximal oxygen uptake
aMean energy and macronutrient intake during the supplementation period
In accordance with the Declaration of Helsinki, all the participants expressed their free and conscious consent to participate in the research procedures. The consent of the Bioethics Committee at Poznań University of Medicine Sciences was obtained for this study (decision no. 584/09 of 18 June 2009).
Experimental design
Characteristics of the administered supplementation
The effect of HMB supplementation was assessed in randomised, crossover, double-blind tests (Fig. 1). The experimental procedure for each athlete included a 12-week supplementation with an HMB preparation and a 12-week placebo administration. Upon determining experiment qualification, the athletes were subjected to the randomisation procedure (based upon lean body mass) and assigned either to the group receiving the HMB preparation in the first 12 weeks of the trial or to the group receiving the placebo. After 12 weeks, a 10-day washout period was implemented, which was similar in other studies and sufficient given the kinetics of HMB absorption and excretion from the body [24, 30, 31]. After the washout period, a crossover exchange of the preparations administered to the groups was applied.
Fig. 1.
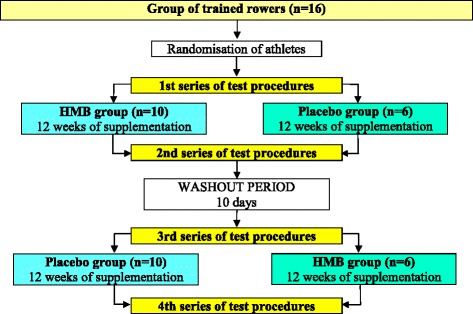
A flow chart of the study design
The experiments were conducted using a preparation of calcium salt of HMB produced by Olimp Laboratories. A single capsule contained 1250 mg of Ca-HMB, which corresponds to 1000 mg HMB. The producer also prepared a placebo preparation containing maltodextrin. The tested group of athletes was administered three capsules of the assigned preparation per day in three doses as follows: upon waking, immediately after training, and before sleep. On non-training days, the participants were instructed to consume one serving with each meal throughout the day. The consumed HMB dose was equivalent to the most commonly recommended intake of 3 g HMB per day [2, 3, 5, 19, 26].
Among all participants, the efficacy of HMB supplementation was assessed using four series of research (each included: evaluation of body composition, aerobic and anaerobic capacity, as well as blood sampling and biochemical analyses), consisting of identical procedures in two cycles separated by a washout period: the tests took place prior to the onset of the intervention (PreHMB and PrePLA), following the 12 weeks of supplementation with the HMB preparation (PostHMB) and the placebo (PostPLA). As mentioned in the methods section, all athletes declared that they had not undergone changes in their lifestyle, characteristics of training, or nutrition.
Evaluation of body mass and composition
Body weight and height were measured using a WPT 60/150 OW medical anthropometer by RADWAG® (Poland). Body composition was analysed by determining the values of electrical resistance and reactance through bioelectric impedance with the use of a BIA 101S analyser by AKERN-RJL (Italy) and the Bodygram 1.31 computer software by AKERN-RJL (Italy). Body composition was measured strictly following the recommended measurement conditions, i.e. in the morning hours, following overnight fasting, in subjects lying in a supine position, and with the recommended application of measuring electrodes [32]. Athletes were also instructed to abstain from drinking coffee, strong tea, caffeine-containing products, and alcohol for at least 24 h before the test, as well as to refrain from physical exercise for a minimum of 18 h before measurements.
Assessment of aerobic capacity
The exercise tests to assess the selected capacity parameters in the athletes were conducted in the morning hours (between 7:00 and 10:30 a.m.), always in the same conditions (temperature of 20–22 °C, relative humidity of 50–60 %) at the Exercise Tests Laboratory at the Department of Human Nutrition and Hygiene, Poznań University of Life Sciences. Prior to each test, the athletes were informed, in detail, of the objective, procedure and methods of the exercise tests. The level of aerobic capacity in athletes was assessed based on the recorded maximal oxygen uptake () and the VT during a test that involved performing exercise with increasing intensity (+50 W each 3 min) on a Kettler X1 cycloergometer (Kettler, Germany), following recommendations for such tests [33]. During the tests, the respiration indices were recorded using a portable K4b2 ergospirometer (Cosmed, Italy) and the COSMED CPET Software Suite (ver. 9.1b, 2010). Moreover, the Cosmed K4b2 system was calibrated prior to each individual test according to the manufacturer’s guidelines.
In this study, maximal exercise was assumed to occur when an increase in load failed to produce an increase in oxygen uptake () and heart rate (HR). In order to determine the VT, the V-slope method was applied based on an analysis of linear regression for the curve of increasing CO2 exhalation in relation to the curve of increasing O2 uptake [34].
Blood sampling and biochemical analyses
The most widely used markers of adaptation and homeostasis in studies involving athletes were applied in this investigation. The activity of the CK and LDH enzymes, and the concentration of testosterone and cortisol (for calculating the T/C ratio) were assessed based on a quantitative analysis of the blood plasma of the athletes using commercial diagnostic tests. Twenty to twenty-five minutes after the exercise test, blood samples were collected from the athletes from the ulnar vein to two closed-system evacuated test tubes of 2.7 mL, using lithium heparine and sodium fluoride as anticoagulants (Sarstedt Monovette®, Germany). The collected plasma was subjected to further laboratory analyses on the same day. CK and LDH activity were assayed using a standardised colorimetric enzymatic method with a COBAS® 6000 analyser (module c 501, Roche/Hitachi, USA). The concentrations of testosterone and cortisol in blood plasma were assayed by ECLIA electrochemiluminescence using a COBAS® 6000 analyser (module e 601, Roche/Hitachi, USA).
Assessment of anaerobic capacity
Anaerobic capacity was assessed using the classical Wingate test on a cycloergometer (Monark 894E, Sweden) following recommendations for such tests proposed by Bar-Or [35]. Seat height was adjusted to each participant’s satisfaction, and toe clips with straps were used to prevent the feet from slipping off the pedals. The primary test was preceded by a 5-min warm-up period of approximately 50 W power, followed by a 5-min break. This was followed by two run-up practices of 3 s, during which the actual test load was imposed to accustom the participants to the resistance. The test lasted for 30 s. External loading was estimated individually at 7.5 % body weight. During the test, the athletes were encouraged to maintain maximum effort. Recorded results included the peak power output (PP), the average power output (AP), and the mean power output (MP), which were analysed using Monark Anaerobic Test Software (ver. 3.0.1, 2009, Sweden). The familiarization session for subjects was not needed, because the rowers were quite familiar the Wingate test, due to previous studies and training procedure.
Statistical analysis
All statistical calculations were performed using the Statistica 10.0 package (StatSoft, Poland, 2011). Sample size was determined using previously published data [25, 26]. Basic descriptive statistics were calculated for the tested parameters, and the results are presented as means and standard deviations (± SD) for at least four independent series of measurements. The Shapiro-Wilk test was applied in order to determine whether the random sample came from a population with a normal distribution. The statistical analysis was performed based on the research hypothesis that the HMB supplementation support the physical capacity and body composition regulation in trained athletes. Therefore statistical tests were selected in order to compare the significance of the changes resulting from the HMB supplementation or placebo. Since a crossover design was used in this study and all subjects received both HMB and placebo. The significance of the differences in the mean value of parameters between HMB and placebo were tested using independent samples t-tests in the case of normally distributed variables, or by Mann–Whitney U tests in the case of non-normally distributed variables. Differences in mean values of parameters between baseline (Pre) and post-intervention (Post) were tested by dependent samples t-tests (normally distributed variables) or Wilcoxon-signed rank tests (non-normally distributed variables).
Results
Body composition
Following the 12-week HMB supplementation, a significant decrease (p = 0.03) was recorded in fat mass (−0.9 kgHMB), while after the placebo treatment this tissue component increase (+0.8 kgPLA) (Fig. 2). Moreover, in both groups, weight loss was observed, although there were no differences between the HMB and placebo. In relation to the pre-investigation value, after HMB supplementation, the changes in body mass were significant (p = 0.016), but were associated with a slight (p = 0.12) reduction in lean body mass, which significantly decreased by 2.1 kg (p = 0.001) after the placebo treatment (Table 2).
Fig. 2.
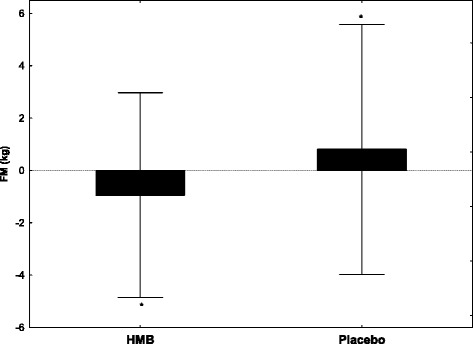
Changes in fat mass after 12-week supplementation of HMB. Values are expressed as mean ± SD. Significant differences compared with placebo (independent samples t-tests) at: * p = 0.03
Table 2.
Levels of the monitored indices before and after the 12-week supplementation with HMB preparation and placebo
| Research period | PREHMB vs. PLA | HMB | Difference | PLACEBO | Difference | |
|---|---|---|---|---|---|---|
| p valuea | p valueb | p valueb | ||||
| BM (kg) | Pre | 0.925 | 86.5 ± 9.3 | 0.016 | 86.2 ± 9.0 | 0.063 |
| Post | 84.6 ± 8.1 | 84.8 ± 8.5 | ||||
| FFM (kg) | Pre | 0.921 | 72.7 ± 6.3 | 0.117 | 72.9 ± 6.1 | 0.001 |
| Post | 71.7 ± 5.6 | 70.9 ± 5.7 | ||||
| FM (kg) | Pre | 0.755 | 13.8 ± 4.4 | 0.073 | 13.3 ± 4.7 | 0.197 |
| Post | 12.8 ± 4.0 | 14.1 ± 4.3 | ||||
| (mL · min−1) | Pre | 0.681 | 5794 ± 676 | 0.240 | 5897 ± 735 | 0.067 |
| Post | 5905 ± 709 | 5723 ± 745 | ||||
| (mL · min−1 · kg−1) | Pre | 0.572 | 67.3 ± 6.9 | 0.033 | 68.8 ± 8.0 | 0.438 |
| Post | 70.0 ± 6.9 | 67.8 ± 8.5 | ||||
| Tref (min) | Pre | 0.947 | 17.6 ± 3.1 | 0.043 | 17.7 ± 3.1 | 0.233 |
| Post | 19.0 ± 3.6 | 18.5 ± 3.7 | ||||
| Wmax (W) | Pre | 0.777 | 372 ± 52 | 0.052 | 378 ± 52 | 0.625 |
| Post | 397 ± 56 | 384 ± 60 | ||||
| Wmax (W · kg−1) | Pre | 0.621 | 4.31 ± 0.50 | 0.039 | 4.39 ± 0.41 | 0.234 |
| Post | 4.70 ± 0.55 | 4.55 ± 0.65 | ||||
| HRmax (bpm) | Pre | 0.962 | 185 ± 13 | 0.258 | 185 ± 9 | 0.136 |
| Post | 188 ± 9 | 188 ± 10 | ||||
| TVT (min) | Pre | 0.440 | 12.5 ± 2.1 | 0.003 | 12.8 ± 1.9 | 0.619 |
| Post | 13.7 ± 2.2 | 12.6 ± 2.6 | ||||
| WVT (W) | Pre | 0.187 | 278 ± 41 | 0.012 | 294 ± 36 | 0.310 |
| Post | 309 ± 42 | 284 ± 47 | ||||
| WVT (W · kg−1) | Pre | 0.248 | 3.24 ± 0.51 | 0.001 | 3.42 ± 0.32 | 0.554 |
| Post | 3.66 ± 0.36 | 3.36 ± 0.49 | ||||
| HRVT (bpm) | Pre | 0.399 | 157 ± 9 | 0.001 | 160 ± 10 | 0.598 |
| Post | 166 ± 11 | 161 ± 9 | ||||
| CK (U · L−1) | Pre | 0.865 | 328 ± 160 | 0.004 | 348 ± 169 | 0.008 |
| Post | 241 ± 103 | 265 ± 156 | ||||
| LDH (U · L−1) | Pre | 0.573 | 318 ± 36 | 0.033 | 326 ± 45 | 0.002 |
| Post | 302 ± 35 | 302 ± 39 | ||||
| Testosterone (mg · dL−1) | Pre | 0.895 | 510 ± 202 | 0.352 | 510 ± 154 | 0.569 |
| Post | 552 ± 160 | 487 ± 160 | ||||
| Cortisol (mg · dL−1) | Pre | 0.585 | 19.9 ± 5.3 | 0.331 | 20.0 ± 5.9 | 0.516 |
| Post | 20.6 ± 5.8 | 20.8 ± 4.1 | ||||
| T/C ratio (T · C−1*10−2) | Pre | 0.51 | 3.39 ± 1.77 | 0.53 | 3.36 ± 0.99 | 0.30 |
| Post | 3.41 ± 1.08 | 3.03 ± 1.11 | ||||
| Peak power (W) | Pre | 0.615 | 1003 ± 125 | 0.027 | 1028 ± 147 | 0.853 |
| Post | 1054 ± 116 | 1024 ± 122 | ||||
| Peak power (W · kg−1) | Pre | 0.488 | 11.65 ± 1.01 | 0.020 | 11.92 ± 1.16 | 0.960 |
| Post | 12.26 ± 0.83 | 11.91 ± 1.15 | ||||
| Average power (W) | Pre | 0.924 | 725 ± 87 | 0.648 | 722 ± 87 | 0.660 |
| Post | 720 ± 83 | 718 ± 82 | ||||
| Average power (W · kg−1) | Pre | 0.854 | 8.36 ± 0.60 | 0.688 | 8.32 ± 0.55 | 0.736 |
| Post | 8.30 ± 0.59 | 8.28 ± 0.65 | ||||
| Minimal power (W) | Pre | 0.478 | 503 ± 75 | 0.095 | 486 ± 52 | 0.763 |
| Post | 475 ± 54 | 491 ± 78 | ||||
| Minimal power (W · kg−1) | Pre | 0.465 | 5.80 ± 0.78 | 0.119 | 5.63 ± 0.55 | 0.821 |
| Post | 5.49 ± 0.53 | 5.67 ± 0.76 |
Values are expressed as mean ± SD
BM body mass, FFM fat-free mass, FM fat mass, maximal oxygen uptake, Tref exercise time before athlete’s refusal to continue exercising, Wmax maximum load, HRmax maximum heart rate, VT ventilatory threshold, T VT time to VT, W VT load at VT, HR VT heart rate at VT, CK creatine kinase, LDH lactate dehydrogenase, T/C ratio testosterone/cortisol ratio
aDepending on the data distribution: independent samples t-tests or Mann–Whitney U tests
bDepending on the data distribution: dependent samples t-tests or Wilcoxon-signed rank tests
Maximal oxygen uptake ()
The analysis of the aerobic capacity indices showed an increase (p = 0.03) in following the 12 weeks of supplementation with the HMB preparation (+2.7 mL min−1 · kg−1) in comparison to the reduction of aerobic capacity following the placebo treatment (−1.0 mL · min−1 · kg−1) (Fig. 3). Moreover, in relation to the pre-investigation value, after the HMB supplementation, a significant increase was recorded in (p = 0.03) and exercise time (+1.4 minHMB, p = 0.04) and maximal load of cycloergometer (+0.38 W · kg−1HMB, p = 0.04), before the athlete refused to continue the test.
Fig. 3.
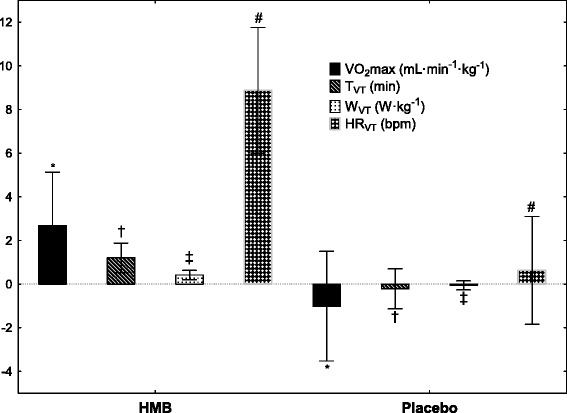
Changes in maximal oxygen uptake and rates at ventilatory threshold after 12-week supplementation of HMB. Values are expressed as mean ± SD. Significant differences compared with placebo (independent samples t-tests) at: *- p = 0.03; † - p = 0.012, ‡ - p = 0.002; # - p < 0.001. : maximal oxygen uptake; TVT: time to VT; WVT: load at VT; HRVT: HR at VT
Ventilatory threshold
Analysis of the changes in the recorded markers shows that, following HMB supplementation, time to reach VT (TVT: +1.2 minHMB vs. −0.2 minPLA, p = 0.012), relative threshold load at VT (WVT: +0.42 W · kg−1HMB vs. −0.06 W · kg−1PLA, p = 0.002), and the threshold HR at VT (HRVT: +9 bpmHMB vs. +1 bpmPLA, p < 0.001) (Fig. 3) increased significantly. In addition, in comparison to the pre-investigation value after the HMB treatment, a significant increase was recorded in TVT (p = 0.003), WVT (p < 0.001) and HRVT (p < 0.001) (Table 2).
Biochemical blood markers
Thus was no statistically significant differences between the HMB and placebo groups in the tested biochemical markers in the blood. In relation to the initial concentration of the markers assessed, in both groups, significant decreases were recorded in CK activity (CKPOST-HMB-CKPRE-HMB: −87 U · L−1, p = 0.004; CKPOST-PLA-CKPRE-PLA: −83 U · L−1, p = 0.008) and LDH activity (LDHPOST-HMB-LDHPRE-HMB: −15 U · L−1, p = 0.033; LDHPOST-PLA-LDHPRE-PLA: −24 U · L−1, p = 0.002) after the exercise test with increasing intensity (Table 2).
Anaerobic capacity
Comparing HMB supplementation to the placebo, no significant differences were observed in the changes in the anaerobic power indices during the Wingate test. However, the analysis of the peak power output showed an increase (p = 0.02) following the 12 weeks of supplementation with the HMB preparation (+0.61 W · kg−1) in comparison to the pre-HMB period (Table 2).
Discussion
The findings in this study indicate that a 12-week HMB supplementation in athletes practicing endurance sports influences a reduction of fat mass (p = 0.03). Despite the observed lack of differences in the diets of rowers in the course of the experimental procedure, changes in body weight found in both periods were probably caused by a low energy intake, witch concern only some athletes. It may have resulted from the insufficient coverage of high-energy expenditure connected with rowing training. As mentioned in the methodological part, dietary recordings were performed every second week, during the whole study, which proved that athletes did not change their dietary habits during the HMB supplementation and placebo period. Furthermore the potential impact energy intake is significantly reduced by randomised crossover design of the study. Thus, the observed effect of HMB seems considerable, since a reduction of body weight in supplemented athletes was connected with not significant reduction of FM and FFM, while in placebo group FFM reduction was statistically significant (p = 0.001). Additionally, despite the supreme importance of aerobic capacity in endurance sports, a significant role may also be played by a high level of anaerobic adaptation, particularly at the start or finish of the race [36, 37]. In this study, in relation to the initial values, only after HMB supplementation was an increase observed in peak power (p = 0.02). In turn, in relation to placebo administration after HMB intake, advantageous trends were observed indicating an increase in PP (p = 0.06). No differences were found in the case of the other indices of anaerobic capacity of rowers.
However, we would like to highlight here that the aim of the training regime in the investigated group of athletes was not to change body composition or anaerobic adaptation, but to increase their training potential. Probably for this reason, changes in fat-free body mass and power were less important, connected not only with the assumed negative energy balance (suggested by the reduced body weight of athletes), but particularly with the lack of an adequate exercise stimulus for the synthesis of muscle proteins. This may suggest a limited role of HMB, as postulated in the literature, in the activation of, for example, mTOR kinase pathways [6, 8, 12, 13], expression of insulin-like growth factor-1 (IGF-1) [11, 12], or the reduction of activity of the ubiquitin-proteasome system and caspase activity [9, 14–16]. This thesis may be confirmed by the studies, in which individuals supplemented with HMB performed only resistance exercise, stimulating (to a greater extent) an increase in fat-free body mass, which was reported, for example, by Nissen et al. [2] (FFM: +1.21 kg3.0gHMB vs. +0.8 kg1.5gHMB vs. +0.4 kgPLA) and Gallagher et al. [19] (FFM: +1.9 kg). In turn, upon HMB supplementation in experiments on volleyball players, in which a key role was played by muscle power, an increase in FFM (+2.3 kg) was recorded, as well as reduction of fat mass by ~0.6 kgFM - comparable to that found our this study [3]. Moreover, in athletes administered the placebo in Portal et al. [3], a slight decrease in FFM (0.1 kg) and an increase of fat mass (3.6 % FM) was found. Furthermore, with an increase in FFM in volleyball players, an increase was recorded in peak (1.7 W · kg−1HMB vs. 0.4 W · kg−1PLA) and average power (0.9 W · kg−1HMB vs. 0.1 W · kg−1PLA). These observations may be confirmed by a study by Molinari et al. [38], who recorded increased muscle power (9.2 % HMB vs. 1.7 % PLA) in volleyball players using HMB. In turn, in a study on judoists subjected to a 3-day limitation of energy intake (20 kcal · kgbm−1 day−1) a reduction (p < 0.05) was recorded for fat mass (−0.85 % HMB vs. 0.2 % PLA) only in the group of athletes supplemented with HMB, although no differences were found in indices of anaerobic power between athletes using HMB and placebos [23]. Apart from the negative energy balance, this may have resulted from the fact that HMB supplementation lasted only 3 days, which seems too short to cause significant changes in systemic anaerobic potential. HMB impact on body composition observed also Caperuto et al. [39]. In rats supplemented with 320 mg · kg−1 body weight of HMB, carcass fat significantly decreased. Such a large effect was not observed in our study, which we may assume could be related to the dose/response concept. Furthermore, no significant results from the supplemented group when compared to placebo might explain the fact, that HMB supplementation can increase adaptation and promotes metabolic changes in a time-dependent manner [39]. In turn, in the case of endurance sports, changes in FM described in this study seem to be explained by the increase in fatty acid oxidation, as well as lipolysis and insulin sensitivity (e.g., due to the stimulation of activation of AMPK kinase, Sirt1 and the dependent metabolic pathways) [18].
We need to stress here that there is a limited body of literature assessing the efficacy of HMB intake in endurance sports. In terms of the effect of HMB on body composition in runners, Knitter et al. [20], Lamboley et al. [26] and Robinson et al. [25] observed no differences in body composition in athletes who were administered HMB or a placebo. Does that mean that HMB has a definite effect on body composition and anaerobic adaptation only in individuals involved in resistance training? This study was conducted on trained rowers, practicing (first of all) to increase endurance, although their training procedure also included periodical resistance exercise. The recorded results seem to indicate efficacy of HMB supplementation in relation to the advantageous reduction of FM and a tendency to increase peak power. However, it seems obvious that the efficacy of HMB in stimulating an increase in FFM and anaerobic capacity may be observed particularly in the case of incorporation of resistance exercise and/or high-intensity exercise to the training regime, stimulating, to a greater extent, muscle protein synthesis pathways, which would enhance the potential anticatabolic role of HMB.
When assessing the effect of HMB supplementation in endurance sports, the effect of this preparation on aerobic adaptation seems to play a key role. Vukovich and Dreifort [24], after a 2-week HMB supplementation in cyclists, recorded an increase (p < 0.05) in peak oxygen uptake () by 4 % and an extension of the time required to reach by 3.6 %. Moreover, values of these indices increased (p < 0.05) also in comparison to the results recorded in groups administered leucine or a placebo. Similar results were observed in this study, in which of rowers increased after 12-week HMB supplementation, both in relation to the placebo treatment and values before its supplementation (PreHMB). In the study by Vukovich and Dreifort cited above, the administration of HMB also led to an advantageous increase in the lactate threshold, which, when expressed in percent , increased by 8.6 %. Also found was a delayed OBLA observed at the oxygen uptake, which increased by 9.1 %. Thus, these results seem to confirm the effect of HMB supplementation observed in this study on the increase in aerobic adaptation of athletes. Furthermore, these observations correspond also to the latest results reported by Robinson et al. [25], who, in a group of males and females after a 4-week HMB supplementation, combined with high-intensity interval training, found levels of higher by almost 5.9 % (p = 0.032) and 9.8 % (p < 0.001), respectively, in comparison to the placebo and the control groups. The authors also found VT to be higher by almost 9.3 % (p = 0.017) and 16.5 % (p = 0.012), respectively. Another important point showed Lamboley et al. [26], in the previously described study, also showed an advantageous effect of HMB supplementation resulting from the considerable increase in by as much as 7.7 ml · kg−1 · min−1. In both groups, a significant improvement was also found in VT (+11.1 % HMB vs. +9.0 % PLA). Despite the increase in values recorded in this study in the group supplemented with HMB, the differences were not high, which suggests that they may have resulted, to a considerable degree, from the fact that participants in this study practiced sports as recreation and, prior to the onset of the experimental procedure, had no aerobic training. In contrast, this study involved elite rowers and, even a slight increase in aerobic capacity in their case, may be considered to be particularly advantageous.
Thus when considering the results of this study, literature data as well as the above mentioned potential mechanisms of HMB action, connected (for example) with the regulation of muscle protein expression, maintenance of cell wall integrity or stimulation of activity of AMPK kinase and Sirt 1, which promotes stimulation of mitochondrial biogenesis, higher oxygen consumption and increased efficiency of carbohydrate, glycogen and fat metabolism [17, 18, 39, 40], it may be inferred that HMB supplementation under specific conditions seems to also enhance the increase in aerobic capacity. Increasing the usability and availability of energy substrates thus appears to explain the growth of aerobic adaptation (VO2max, VT) of rowers. Observed after HMB supplementation physical capacity increase, could be in practice due to the more efficient use of exercise stimulus, as well as increase the efficiency of post-exercise recovery period, which are necessary to achieve super compensation.
It turns out that HMB supplementation applied in this study had no effect on the levels of blood biochemical markers. Literature data also did not clearly show the effect of HMB on changes in their concentrations. Nissen et al. [2] and van Someren et al. [22], following HMB supplementation, found a lower activity of CK and/or LDH in the blood of examined individuals. The above observations seem to suggest that HMB supplementation may play a significant role in the reduced rate of muscle damage. However, long-term HMB supplementation in trained individuals, e.g. as a result of homeostatic mechanisms in the organism, may reduce the effects of this substance on the level of adaptation of the organism verified by the analyses of levels of standard biochemical markers in the blood. To confirm this thesis, Gallagher et al. [19], in a group receiving HMB, showed a lower CK activity (by approximately 200 U · kg−1) 48 h after a series of resistance exercises; however, this effect disappeared after a longer supplementation period. In turn, in runners, Knitter et al. [20] observed lower concentrations of CK and LDH in a group supplemented with HMB immediately after they completed a 20 km race, as well as during the three successive days after this exercise. Thus, the cited studies seem to confirm the hypothesis on the effect of HMB on the stimulation of sarcolemma integrity and inhibition of proteolytic activity of the ubiquitin-proteasome system. This may indicate the advisability of HMB supplementation in sports due to the reduced rate of muscle damage as a consequence of intensive exercise loads.
It’s important to observe that a limited number of studies analysed the effect of HMB uptake on the systemic hormone metabolism. In comparison to the resting-state hormone concentrations recorded prior to the tests and following 12 weeks of HMB administration combined with power training, Kreamer et al. [41] showed a significant increase in the pre-exercise concentration of testosterone and a reduction of cortisol levels, which were not observed in the control. In supplemented group 15 min after the completion of exercise, blood testosterone concentration increased considerably, but after 30 min, the level of this hormone was similar to that recorded in the control group. No significant differences were observed in the blood concentrations of cortisol, although in this supplemented group a reduced level 30 min after exercise was found. In a recent paper of Townsend et al. [42] testosterone levels significant increased immediately after exercise in comparison to baseline, but also returned after 30 min, in resistance trained men, supplemented with HMB. This finding might explain no significant results observed in this study. Seems to be possible that HMB supplementation can promotes hormonal changes observed in a time-dependent manner.
We would like to highlight here that numerous studies are consistent with the results of this study and do not confirm the effect of HMB, in comparison to placebo, on activity of CK and LDH [19, 27–29, 43] or blood testosterone concentration [3, 27, 28]. We need to mention here that when assessing levels of biochemical markers following supplementation, it is difficult to reliably compare the presented studies. The final results may have been affected not only by the dose of the preparation, but also by the duration of supplementation, the training standard of athletes involved in the experiment and applied training loads.
Conclusions
This study indicates that HMB supplementation in athletes training for endurance sports promotes the advantageous increase in aerobic capacity of the organism, mainly due to the increased values of maximum oxygen uptake and indices of the VT, as well as the reduction of fat mass. It may also enhance peak anaerobic power. Long-term HMB supplementation seems not only to have a significant effect on changes in activity of selected intramuscular enzymes testosterone and cortisol concentration, but also on values of the T/C ratio in blood.
Acknowledgments
The authors wish to thank the coaches and athletes for their help and participation in the research project. We gratefully acknowledge financial support for this work provided by the Polish National Science Centre, grant number N N312 262340.
Abbreviations
- AMPK kinase
Adenosine monophosphate-activated protein kinase
- AP
Average power
- BM
Body mass
- CK
Creatine kinase
- FFM
Fat-free mass
- FM
Fat mass
- HMB
β-hydroxy-β-methylbutyric acid
- HRmax
Maximum HR
- HRVT
HR at VT
- LDH
Lactate dehydrogenase
- MP
Minimal power
- mTOR kinase
Mammalian target of rapamycin kinase
Maximal oxygen uptake
Peak oxygen uptake
- PLA
Placebo
- PP
Peak power
- Sirt 1
Sirtuin 1
- T/C ratio
Testosterone/cortisol ratio
- Tref
Exercise time to athlete’s refusal to continue exercising
- Wmax
Maximum load
- TVT
Time to VT
- VT
Ventilatory threshold
- WVT
Load at VT
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The study was designed and funded by KDM and JJ; data were collected and analysed by KDM; data interpretation and manuscript preparation were undertaken by KDM and JJ. All authors drafted the manuscript and approved the final version.
Contributor Information
Krzysztof Durkalec-Michalski, Phone: +48 61 848 73 32, Email: durkmich@up.poznan.pl.
Jan Jeszka, Email: jeszkaj@up.poznan.pl.
References
- 1.Froiland K, Koszewski W, Hingst J, Kopecky L. Nutritional supplement use among college athletes and their sources of information. Int J Sport Nutr Exerc Metab. 2004;14:104–20. doi: 10.1123/ijsnem.14.1.104. [DOI] [PubMed] [Google Scholar]
- 2.Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC, Jr, et al. Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol. 1996;81:2095–104. doi: 10.1152/jappl.1996.81.5.2095. [DOI] [PubMed] [Google Scholar]
- 3.Portal S, Eliakim A, Nemet D, Halevy O, Zadik Z. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: a prospective randomized, double-blind, placebo-controlled study. Eur J Appl Physiol. 2011;111:2261–9. doi: 10.1007/s00421-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 4.Van Koevering M, Nissen S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am J Physiol. 1992;262:E27–31. doi: 10.1152/ajpendo.1992.262.1.E27. [DOI] [PubMed] [Google Scholar]
- 5.Zanchi NE, Gerlinger-Romero F, Guimarães-Ferreira L, de Siqueira Filho MA, Felitti V, Lira FS, et al. HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids. 2011;40:1015–25. doi: 10.1007/s00726-010-0678-0. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel GD, Rosa JC, Lira FS, Zanchi NE, Ropelle ER, Oyama LM, et al. β-Hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr Metab (Lond) 2011;8(1):11. doi: 10.1186/1743-7075-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson JM, Grant SC, Lee SR, Masad I, Park YM, Henning PC, et al. Beta-hydroxy-beta-methyl-butyrate blunts negative age-related changes in body composition, functionality and myofiber dimensions in rats. J Int Soc Sports Nutr. 2012;9(1):18. doi: 10.1186/1550-2783-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aversa Z, Bonetto A, Costelli P, Minero VG, Penna F, Baccino FM, et al. β-hydroxy-β-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int J Oncol. 2011;38:713–20. doi: 10.3892/ijo.2010.885. [DOI] [PubMed] [Google Scholar]
- 9.Smith HJ, Mukerji P, Tisdale MJ. Attenuation of proteasome-induced proteolysis in skeletal muscle by beta-hydroxy-beta-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005;65:277–83. doi: 10.1158/0008-5472.CAN-05-0169. [DOI] [PubMed] [Google Scholar]
- 10.Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC., Jr Beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr. 2000;130:1937–45. doi: 10.1093/jn/130.8.1937. [DOI] [PubMed] [Google Scholar]
- 11.Gerlinger-Romero F, Guimarães-Ferreira L, Giannocco G, Nunes MT. Chronic supplementation of beta-hydroxy-beta methylbutyrate (HMβ) increases the activityof the GH/IGF-I axis and induces hyperinsulinemia in rats. Growth Hormon IGF Res. 2011;21:57–62. doi: 10.1016/j.ghir.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O. Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2009;1793:755–63. doi: 10.1016/j.bbamcr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Eley HL, Russell ST, Tisdale MJ. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin IIby beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295:E1409–16. doi: 10.1152/ajpendo.90530.2008. [DOI] [PubMed] [Google Scholar]
- 14.Holecek M, Muthny T, Kovarik M, Sispera L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem Toxicol. 2009;47:255–9. doi: 10.1016/j.fct.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Kovarik M, Muthny T, Sispera L, Holecek M. Effects of β-hydroxy-β-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J Physiol Biochem. 2010;66:311–9. doi: 10.1007/s13105-010-0037-3. [DOI] [PubMed] [Google Scholar]
- 16.Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R701–15. doi: 10.1152/ajpregu.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Zemel MB. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr Metab (Lond) 2009;6:26. doi: 10.1186/1743-7075-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruckbauer A, Zemel MB, Thorpe T, Akula MR, Stuckey AC, Osborne D, et al. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr Metab (Lond) 2012;9(1):77. doi: 10.1186/1743-7075-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher PM, Carrithers JA, Godard MP, Schulze KE, Trappe SW. Beta-hydroxy-beta-methylbutyrate ingestion, part I: effects on strength and fat free mass. Med Sci Sports Exerc. 2000;32:2109–15. doi: 10.1097/00005768-200012000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Knitter AE, Panton L, Rathmacher JA, Petersen A, Sharp R. Effects of beta-hydroxy-beta-methylbutyrate on muscle damage after a prolonged run. J Appl Physiol. 2000;89:1340–4. doi: 10.1152/jappl.2000.89.4.1340. [DOI] [PubMed] [Google Scholar]
- 21.Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol. 2003;94:651–9. doi: 10.1152/japplphysiol.00755.2002. [DOI] [PubMed] [Google Scholar]
- 22.Van Someren KA, Edwards AJ, Howatson G. Supplementation with beta-hydroxy-beta-methylbutyrate (HMB) and alpha-ketoisocaproic acid (KIC) reduces signs and symptoms of exercise-induced muscle damage in man. Int J Sport Nutr Exerc Metab. 2005;15:413–24. doi: 10.1123/ijsnem.15.4.413. [DOI] [PubMed] [Google Scholar]
- 23.Hung W, Lui TH, Chen CY, Chang CK. Effect of β-hydroxy-β-methylbutyrate supplementation during energy restriction in female judo athletes. J Exerc Sci Fit. 2010;8:50–3. doi: 10.1016/S1728-869X(10)60007-X. [DOI] [Google Scholar]
- 24.Vukovich MD, Dreifort GD. Effect of beta-hydroxy beta-methylbutyrate on the onset of blood lactate accumulation and V(O)(2) peak in endurance-trained cyclists. J Strength Cond Res. 2001;15:491–7. [PubMed] [Google Scholar]
- 25.Robinson EH, Stout JR, Miramonti AA, Fukuda DH, Wang R, Townsend JR, et al. High-intensity interval training and β-hydroxy-β-methylbutyric free acid improves aerobic power and metabolic thresholds. J Int Soc Sports Nutr. 2014;11:16. doi: 10.1186/1550-2783-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamboley CR, Royer D, Dionne IJ. Effects of beta-hydroxy-beta-methylbutyrate on aerobic-performance components and body composition in college students. Int J Sport Nutr Exerc Metab. 2007;17:56–69. doi: 10.1123/ijsnem.17.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Crowe MJ, O’Connor DM, Lukins JE. The effects of beta-hydroxy-beta-methylbutyrate (HMB) and HMB/creatine supplementation on indices of health in highly trained athletes. Int J Sport Nutr Exerc Metab. 2003;13:184–97. doi: 10.1123/ijsnem.13.2.184. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman JR, Cooper J, Wendell M, Im J, Kang J. Effects of beta-hydroxy beta-methylbutyrate on power performance and indices of muscle damage and stress during high-intensity training. J Strength Cond Res. 2004;18:747–52. doi: 10.1519/13973.1. [DOI] [PubMed] [Google Scholar]
- 29.Kreider RB, Ferreira M, Greenwood M, Wilson M, Grindstaff P, Plisk S, et al. Effects of calcium (beta)-HMB supplementation during training on markers of catabolism, body composition, strength and sprint performance. J Exerc Physiol Online. 2000;3:48–59. [Google Scholar]
- 30.Vukovich MD, Slater G, Macchi MB, Turner MJ, Fallon K, Boston T, et al. Beta-hydroxy-beta-methylbutyrate (HMB) kinetics and the influence of glucose ingestion in humans. J Nutr Biochem. 2001;12(11):631–9. doi: 10.1016/S0955-2863(01)00182-6. [DOI] [PubMed] [Google Scholar]
- 31.Chang CK, Chang Chien KM, Chang JH, Huang MH, Liang YC, Liu TH. Branched-chain amino acids and arginine improve performance in two consecutive days of simulated handball games in male and female athletes: a randomized trial. PLoS One. 2015;10:e0121866. doi: 10.1371/journal.pone.0121866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis-part I: review of principles and methods. Clin Nutr. 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Winter EM, Jones AM, Davison RCR, Bromley PD, Mercer T. Sport and exercise physiology testing guidelines: vol. 1 sport testing. The British Association of Sport and Exercise Sciences. Abingdon: Taylor and Francis e-Library; 2009. [Google Scholar]
- 34.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting the anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–7. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 35.Bar-Or O. The Wingate anaerobic test: an update on methodology, reliability and validity. Sports Med. 1987;4(6):381–94. doi: 10.2165/00007256-198704060-00001. [DOI] [PubMed] [Google Scholar]
- 36.Mikulic P. Development of aerobic and anaerobic power in adolescent rowers: a 5-year follow-up study. Scand J Med Sci Sports. 2011;21(6):e143–9. doi: 10.1111/j.1600-0838.2010.01200.x. [DOI] [PubMed] [Google Scholar]
- 37.Secher NH. Physiological and biomechanical aspects of rowing. Implications for training Sports Med. 1993;15(1):24–42. doi: 10.2165/00007256-199315010-00004. [DOI] [PubMed] [Google Scholar]
- 38.Molinari JM, Molinari SA, Braz AG, Bulgarelli D, Sauro EE, de Moraes FB, et al. Estudo dos efeitos da eletroestimulação neuromuscular associadoa a hidróxi b-metilbutirato em voleibolistas. 2007:2054–59. http://www.inicepg.univap.br/cd/INIC_2007/trabalhos/saude/epg/EPG00409_01O.pdf. Accessed 22 Feb 2014.
- 39.Caperuto EC, Tomatieli RV, Colquhoun A, Seelaender MC, Costa Rosa LF. Beta-hydoxy-beta-methylbutyrate supplementation affects Walker 256 tumor-bearing rats in a time-dependent manner. Clin Nutr. 2007;26:117–22. doi: 10.1016/j.clnu.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Pinheiro CH, Gerlinger-Romero F, Guimarães-Ferreira L, de Souza-Jr AL, Vitzel KF, Nachbar RT, Nunes MT, et al. Metabolic and functional effects of beta-hydroxy-beta-methylbutyrate (HMB) supplementation in skeletal muscle. Eur J Appl Physiol. 2012;112:2531–7. doi: 10.1007/s00421-011-2224-5. [DOI] [PubMed] [Google Scholar]
- 41.Kraemer WJ, Hatfield DL, Volek JS, Fragala MS, Vingren JL, Anderson JM, et al. Effects of amino acids supplement on physiological adaptations to resistance training. Med Sci Sports Exerc. 2009;41:1111–21. doi: 10.1249/MSS.0b013e318194cc75. [DOI] [PubMed] [Google Scholar]
- 42.Townsend JR, Hoffman JR, Gonzalez AM, Jajtner AR, Boone CH, Robinson EH, et al. Effects of β-Hydroxy-β-methylbutyrate Free Acid Ingestion and Resistance Exercise on the Acute Endocrine Response. Int J Endocrinol. 2015;2015:856708. doi: 10.1155/2015/856708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunan D, Howatson G, van Someren KA. Exercise-induced muscle damage is not attenuated by beta-hydroxy-beta-methylbutyrate and alpha-ketoisocaproic acid supplementation. J Strength Cond Res. 2010;24:531–7. doi: 10.1519/JSC.0b013e3181c4d370. [DOI] [PubMed] [Google Scholar]


