Abstract
Although monoaminergic antidepressants revolutionized the treatment of Major Depressive Disorder (MDD) over a half-century ago, approximately one third of depressed patients experience treatment-resistant depression (TRD). Such patients account for a disproportionately large burden of disease, as evidenced by increased disability, cost, human suffering, and suicide. This review addresses the definition, causes, evaluation, and treatment of unipolar TRD, as well as the major treatment strategies, including optimization, augmentation, combination, and switch therapies. Evidence for these options, as outlined in this review, is mainly focused on large-scale trials or meta-analyses. Finally, we briefly review emerging targets for antidepressant drug discovery and the novel effects of rapidly acting antidepressants, with a focus on ketamine.
Keywords: treatment-resistant depression, optimization, combination, augmentation, switch therapy, treatment strategy, ketamine, scopolamine
Abstract
Aunque hace más de medio siglo los antidepresivos monoaminérgicos revolucionaron el tratamiento del Trastorno Depresivo Mayor (TDM), alrededor de un tercio de los pacientes con este cuadro presentan una depresión resistente al tratamiento (DRT). Tales pacientes representan una parte desproportionadamente alta del costo de la enfermedad, lo que se evidencia en el aumento de la discapacidad, el sufrimiento humano y el suicidio. Esta revisión está orientada a la definición, causas, evaluación y tratamiento del TDM unipolar, así como a las principales estrategias terapéuticas, incluyendo la optimización, aumento, combinación y cambio de tratamientos. La evidencia para estas opciones, como se describe en esta revisión, está focalizada printipalmente en ensayos de gran escala o meta-análisis. Por último, se revisan brevemente los blancos que están emergiendo para el descubrimiento de antidepresivos y los nuevos efectos de los antidepresivos de acción rápida, con el foco en la ketamina.
Abstract
Les antidépresseurs monoaminergiques ont révolutionné le traitement de l'épisode dépressif caractérisé (majeur) (EDM) il y a une cinquantaine d'années, mais environ un tiers des patients déprimés ont une dépression résistante au traitement (DRT). Ces patients représentent un fardeau important et disproportionné de la maladie, comme le prouve l'augmentation du handicap, des coûts, de la souffrance humaine et des suicides. Cet article s'intéresse à la définition, aux causes, à l'évaluation et au traitement de la DRT unipolaire, ainsi qu'aux principales stratégies thérapeutiques, dont les traitements d'optimisation, d'augmentation, d'association et de substitution. L'article souligne que ces solutions se fondent principalement sur des essais à grande échelle ou des métaanalyses. Enfin, nous revoyons brièvement la découverte de nouvelles cibles des médicaments antidépresseurs et les nouveaux effets des antidépresseurs d'action rapide, avec pour objectif principal la kétamine.
Treatment-resistant depression: definition and importance
“Ever tried. Ever failed. No matter. Try again. Fail again. Fail better.” Samuel Beckett
Depression is among the top public health concerns worldwide, causing significant disability and disease burden.1 In the United States alone, a total of 200 billion is spent annually on depression,2 eclipsing the totals spent on cancer and diabetes. In 2012, it was estimated that 16 million people were living with depression in the United States. Individuals with treatmentresistant depression (TRD), often broadly defined as failure to achieve response or remission to at least one proven antidepressant trial with adequate dosing and duration,3,4 account for nearly 64 billion of the total cost of depression.2 Given that approximately one third of depressed patients are considered “treatment-resistant,”5 this group disproportionally accounts for the largest burden of disease, underscoring the importance of innovation and discovery in this area.
The aim of antidepressant therapy is symptom remission or the reinstatement of euthymia—often defined as a score ≤7 on the total Hamilton Depression Rating Scale (HDRS).6 Response, traditionally defined as a ≥50% decrease in the score from baseline on a depression rating scale (commonly, the HDRS), has proven to be an inadequate goal, as many patients meeting the criteria for response will continue to have residual symptoms and functional impairments.6,7 Moreover, unremitted patients report poorer quality of life and are at a higher risk for relapse and recurrence of depression.8 Specifically, in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial—the largest randomized depression study to date—68% of responded relapsed within the first year following treatment, compared with 47% of remitters who relapsed.5 In addition, when more treatment steps are needed, lower acute remission rates and higher relapse rates are to be expected. Indeed, one third of the STAR*D cohort never remitted, even after four consecutive treatment trials.5,9 Furthermore, the remission rate was a mere 27.5% in Phase 1 treatment with citalopram, based on HDRS scores, and 32.9% based on the Quick Inventory of Depressive Symptomology, Self-Report (QIDS-SR) scores.10 These data highlight the importance of aiming for remission at the outset of treatment in patients with depression—and the need for improved treatment strategies for getting there.
Despite the limitations of current antidepressant therapies for the treatment of refractory depression, strides have been made. As such, the purpose of this review is threefold: (i) To describe the definition of and factors associated with TRD; (ii) To outline current approaches to the evaluation and treatment of refractory depression; and (iii) To highlight future directions in the development of novel therapeutics for TRD, with a focus on ketamine and scopolamine.
Definition and associated factors
Multiple definitions of TRD and schemas for staging severity of resistance have been proposed over the years.11,12 As clinical research in this area has not been guided by a single standard, eligibility criteria for studies of TRD have varied quite widely. In addition, some studies (such as STAR*D) have established treatment resistance prospectively through an initial antidepressant trial; others have established resistance retrospectively through review of medical records or patient recall. A notable challenge in studies involving retrospective assessment has been disentangling failure to respond to treatment from treatment intolerance or initial response followed by relapse.
Why is it that some patients with depression will respond to treatment with a single monoaminergic antidepressant, whereas others will require several different trials to reach remission, if remission is, in fact, ever reached? The heterogeneity of depression—coupled with a current lack of reliable biological predictors of response to individual agents—are major factors. Using criteria from The Diagnostic and Statistical Manual of Mental Disorders (DSM),13two individual patients with very different sets of symptoms can both meet diagnostic criteria for major depressive disorder (MDD). Moreover, depression varies in severity (ranging from mild to incapacitating), course (eg, intermittent, episodic, chronic), and comorbidity (eg, anxiety disorders, substance misuse disorders). Grouping these clinically diverse patients into a single diagnostic category for research purposes has the potential to limit an accurate understanding of heterogeneous groups of depressed patients.
Thus, TRD itself is a relative term that is somewhat misleading. Patients with TRD are not so much treatment-“resistant,” but rather, the heterogeneity of the condition “depression” implies that patients are not receiving treatments that are matched to their individual diathesis. More specifically, there is a need for treatment matching, as well as a particular need for novel treatments targeted at “types” of depression not currently addressed by available treatments—hence, for patients with TRD. It is not that patients with TRD cannot respond to antidepressant treatments; TRD represents our inability as clinicians to match depressed patients with a treatment regimen specific for targeting their unique psychopathology.
Though the neurobiology that underpins depression and its subtypes is yet to be elucidated, there are several clinical factors associated with TRD. Specifically, poorer treatment outcomes are associated with minority ethnic/racial status, socioeconomic disadvantage, axis I and II comorbid disorders, lower function and quality of life, and anxious and melancholic features.14 On an important clinical note, approximately 50% of patients with depression have an anxious component,15-17 highlighting anxious depression as a subtype of depression. Furthermore, anxious depression, defined in the literature variably as MDD with anxiety symptoms or MDD with a comorbid anxiety disorder, may have a unique neurobiological signature.18 Even when patients with anxious depression respond to antidepressant therapies, they do not stay as well for as long as their nonanxious counterparts.19 The personality traits of low reward dependence and low cooperativeness, as measured by the Temperament and Character Inventory, have also been suggested as risk factors for TRD.20
Evaluation of patients
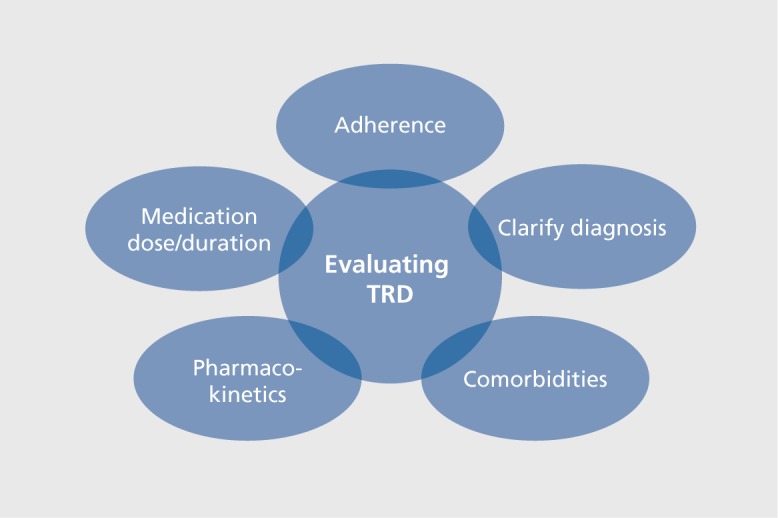
Given the high prevalence of depression, all physicians who treat depression will inevitably encounter patients with TRD. However, a thorough evaluation is critical before concluding that a patient is truly “treatmentresistant.” Table I outlines recommended steps when evaluating patients with TRD.
Table I. Steps in evaluating treatment-resistant depression.
| Principles of evaluation | Specific considerations |
| Diagnosis | Reassess diagnoses; determine if primary psychiatric disorder vs general medical condition (eg, hypothyroidism, anemia) |
| If primary psychiatric disorder, consider treatment based on subtype | |
| Psychiatric comorbidity | Evaluate for comorbid substance abuse, obsessive-compulsive disorder, post-traumatic stress disorder |
| Adherence | Ambivalence towards diagnosis and treatment; cognitive problems; cost; family/significant other influence and biases; side effects |
| Pharmacokinetics | Drug-drug interactions; rapid/fast metabolizers; smokers |
| Dose and duration | Confirm correct dosage and duration of medication trial |
The adage “diagnose before you treat” applies when confronting TRD. Physicians should use careful consideration to critically examine the diagnosis, even if the patient has carried the diagnosis for years. For example, identifying that a patient has MDD with psychotic features, as opposed to melancholic depression, is essential for guiding the next steps in treatment. Ruling out medical correlates of depression, such as hypothyroidism or anemia, is similarly valuable. Assessing for comorbid psychiatric diagnoses (eg, substance use disorders, obsessive-compulsive disorder [OCD] and post-traumatic stress disorder [PTSD]) is also important for evaluating patients with TRD.
Assessing adherence at each visit is also crucial, as TRD may result from nonadherence to medication regimens. Patients, as well as their friends and family members, can be ambivalent about diagnosis and treatment. Cost may also be a barrier, though this issue may be embarrassing or uncomfortable for patients to talk about with their provider. Hence, the provider should create a secure environment for such discussions to take place. Cognitive deficits and difficulty with instructions can also be a barrier to treatment. In these instances, it is especially important for the provider to educate and advocate for his or her patients, as well as to involve family members and caretakers, when possible. Additionally, side effects can influence nonadherence. In this instance, providers can consider adjusting the medication dose, or adding an agent for addressing side effects.21 Improving adherence measures alone may save the patient from unnecessary suffering.
Differences in pharmacokinetics are important to consider when evaluating patients with TRD, as certain genetic factors may predispose patients to treatment resistance and/or side effects. For example, several single nucleotide polymorphisms (SNPs) in the CYP1A2 gene, which codes for the hepatic enzyme CYP1A2, are indicators for rapid escitalopram metabolism; furthermore, fast metabolizers may experience more severe adverse events (especially in early treatment stages), which are thought to be related to higher ratios of S-didesmethylcitalopram to S-desmethylcitalopram.22 Cigarette smoking induces activity of the CYP1A223 and CYP2B624 enzymes, as well as the metabolism of clozapine and olanzapine (antipsychotic medications).25 Effective concentrations of several antidepressants (eg, duloxetine, amitriptyline, bupropion, and fluvoxamine) may be negatively affected by smoking status. Conversely, smoking cessation can decrease antidepressant metabolism, potentially leading to increased levels and side-effect burden. Additionally, it is important to consider drug-drug interactions in light of the frequency of polypharmacy, as induction or inhibition of hepatic enzymes by one medication can induce serious side effects and/or impair functioning of other medications.26,27 When evaluating regimens of two or more medications, computing interactions through a technology-based application should be considered.
Though all physicians will encounter patients with TRD to varying degrees depending on medical specialty, certain providers may feel more comfortable with treating TRD patients than others. In general, primary care physicians should refer patients to a psychiatrist (for consultation and/or long-term care) following the failure of two or more standard antidepressant therapies at adequate dosing and duration, or at any point during treatment when they desire a second opinion. Once in the care of a psychiatrist, TRD patients can benefit from trying other classes of antidepressants—such as tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs)—which are not often prescribed by primary care doctors for depression. Referral to a psychopharmacologist is typically good practice for patients requiring augmentation strategies.
Perhaps the best practice approach to treating depressed patients in the primary care setting is a collaborative one, as 67% of patients treated by a psychiatrist in collaboration with a primary care physician have been found to have an adequate antidepressant treatment regimen (compared with 55% of patients treated by a psychiatrist alone, and 29% of patients treated by a primary care physician alone).28 For patients who do not achieve remission in this approach, one could consider referral to an experimental therapeutic environment, such as a psychopharmacological research study or medical device trial.
Preparing the patient for treatment
Recognizing that only one in three depressed individuals will achieve remission on their first antidepressant,5 the possibility of requiring additional steps should be anticipated with a patient whenever initiating an antidepressant. If initial treatment has not been successful, it is helpful to revisit this discussion and prepare the patient for the journey that lies ahead. While depressed, many patients need to be disabused of the notion that failure to respond to an antidepressant is their failure or evidence that they are beyond help. Patients need to know that lack of response or remission on a prior agent does not preclude positive outcomes on subsequent trials. Indeed, most individuals who eventually remit require two or more antidepressant trials. Although only 33% of patients in STAR*D remitted in the first level, 67% remitted overall by the end of the fourth level.5 Furthermore, although side effects are possible, they may dissipate. If side effects persist, changes in dose or medication may be required. Although providers often select treatment regimens through evidence-based procedures and experience, there still exists a level of “trial and error” that may take the therapeutic relationship through multiple treatment trials. It is important to convey the message that the clinician will stick with the patient on the road to recovery, whether it involves a single next-step treatment or many steps.
Treatment strategies
There are no straightforward algorithms or charts for treating resistant depression. Instead, several options must be considered and tailored for each patient. Specifically, current options consist of Switching therapies, Augmentation, Combination, and Optimization. We suggest the mnemonic “SACO” to aid providers in choosing the next option. As saco can mean bag in Spanish, these options are a “bag of tricks” for the psychiatrist to use when confronting the treatment of refractory depression. Here, we will briefly review each strategy. Of note, we will focus on psychopharmacological treatment strategies. Thus, more comprehensive reviews are discussed elsewhere for strategies pertaining to psychotherapies,29 electroconvulsive therapy (ECT),30 transcranial magnetic stimulation (TMS),30 deep-brain stimulation (DBS),30 vagal nerve stimulation,30 light based-therapies,31 exercise,31 acupuncture,31 and yoga.31
Optimization
Though there is no evidence-based order to adhere to when considering SACO, optimization of current medication dose is typically the most parsimonious next step in treatment. Indeed, optimization should be considered before a referral to a higher level of care is made. Optimization generally consists of increasing the medication dose, as tolerated, at least to standard maximal doses for 6 to 12 weeks,32,33 though potentially involving doses generally considered supratherapeutic (eg, sertraline 250 mg to 350 mg), particularly for individuals who have required unusually high medication doses in the past or have demonstrated good tolerability and partial response.
Augmentation
The addition of a non-antidepressant medication, or augmentation, to an adequate dose and duration of a tolerated antidepressant is a logical next strategy for treatment resistance. This strategy is typically employed when a patient experiences at least a partial response to the initial agent. Commonly used augmentation strategies are listed in Table II.
Table II. Augmentation strategies.
| First-line | Atypical antipsychotics |
| Lithium | |
| Thyroid hormone (T3) | |
| Second-line | Celecoxib |
| L-methylfolate | |
| Modafinil | |
| Benzodiazepines | |
| S-adenosyl-methionine | |
| Mixed/little/no evidence | Folate |
| Omega-3 fatty acids | |
| Buspirone | |
| Lamotrigine | |
| Methylphenadate/amphetamines | |
| Estrogen/testosterone | |
| Pindolol | |
| Pramipexole | |
| Nonbenzodiazepine hypnotics |
Atypical antipsychotics are the most studied class of augmenting agents to selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for depression.34 Specifically, the FDA has approved both aripiprazole and quetiapine for augmentation, and combination olanzapine-fluoxetine (OFC). Other atypical antidepressants which have been shown to be effective in randomized controlled trials include risperidone35 and ziprasidone.36 Patients on atypical antipsychotics as augmentation agents have approximately twofold higher odds for reaching remission compared with placebo, as highlighted in several meta-analyses.37-39 Target dosing recommendations for antipsychotic augmentation differ from those recommended for antipsychotic dosing, and range from aripiprazole 5 mg to 20 mg/day, quetiapine 150 mg to 300 mg/day, and OFC 3 mg/25 mg to 12 mg/50 mg per day.34 The use of atypical antipsychotics involves a careful risk-benefit assessment as these agents possess serious short- and long-term treatment-emergent side effect burdens (eg, sedation, metabolic syndrome and central obesity, extrapyramidal side effects),40 increasing the related health risks and risk of discontinuation.38
A recent review of the literature regarding lithium augmentation in 30 open-label studies and 10 placebocontrolled trials highlights its favorable efficacy for the treatment of resistant depression.41 Lithium augmentation has significantly better antidepressant effects compared with placebo, with a mean response rate of 41.2% (versus 14.4%). However, much of the previous literature studied lithium augmentation of TCAs. The paucity of data on augmentation of contemporary agents, together with the need for therapeutic drug monitoring and risk of side effects and toxicity, has limited the use of lithium.
Though less studied than lithium augmentation, thyroid augmentation can also be considered as a treatment strategy. Specifically, triiodothyronine (T3) is preferred to thyroxine (T4) as antidepressant augmentation, due to its bioactivity in the CNS.42-44 In a meta-analysis of T3 augmentation (25-50 (μg/day) in patients who failed to respond to a TCA, Aronson and colleagues found that patients were twice as likely to achieve response compared with placebo.45 More recently in STAR*D, T3 augmentation resulted in a 24.7% remission rate, compared with 15.9% with lithium augmentation, in treatment-resistant patients who failed two previous antidepressant trials.46 Though these rates were not significantly different, thyroid augmentation is safer and better tolerated than lithium46 and generally has high patient acceptability compared with many psychotropics. However, randomized, double-blind studies involving current agents are lacking.45
Though the most data exists for augmentation with atypical antipsychotics, lithium, and T3, several other augmentation strategies are popularly employed. Briefly, inflammation has been suggested to play a role in the pathophysiology of depression.47,48 As such, there has been an increasing interest in studying anti-inflammatory drugs as augmenting treatments for depression. For example, a meta-analysis of 4 trials (n=150) of celecoxib, a nonsteroidal anti-inflammatory drug, showed significant antidepressant efficacy compared with placebo.49 Similarly, the monoclonal antibody infliximab (an inhibitor of tumor necrosis factor-alpha [TNF-α], an inflammatory cytokine) was found to improve depressive symptoms among patients with elevated inflammatory biomarkers (eg, C-reactive protein) in a single study.50 Omega-3-fatty acids (eg, eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]), which also have anti-inflammatory properties, have been studied for treatment of MDD. A meta-analysis showed a small but insignificant benefit of omega-3-fatty acids for the treatment of MDD, with no difference in efficacy between monotherapy and augmentation, although publication bias cannot be ruled out.51
Folate, L-methylfolate (also known as 5-methyltetrahydrofolate or 5-MTIIF), and S-adenosyl-methionine (SAMe) have also been evaluated as potential antidepressant augmentation agents. Folate, an essential amino acid, is metabolized to SAMe through the one-carbon cycle—an essential metabolic pathway which also plays a role in neurotransmitter synthesis. L-methylfolate is a biologically active intermediary molecule in this pathway, which readily crosses the blood-brain barrier. Folate (folic acid, 5 mg/day) has not been shown to add benefit to active antidepressant treatment,52-53 though the use of L-methylfolate and SAMe appears to be more promising.54 In one study, outpatients with TRD (n=148) were randomized to placebo for 60 days, L-methylfolate (7.5 mg/day for 30 days followed by 15 mg/day for 30 days), or a combination of placebo for 30 days followed by Lmethylfolate (7.5 mg/day) for 30 days, all while being maintained on their SSRI antidepressant regimen.55 The results yielded no significant differences in outcomes between the treatment groups. However, in a second trial of the same design, patients (n=75) randomized to L-methylfolate received 15 mg/day.55 At this dose, adjunctive L-methylfolate was found to have significantly greater antidepressant efficacy compared with placebo. L-methylfolate was well tolerated overall, with no differences to placebo in adverse events. Further post-hoc testing revealed that biomarkers associated with inflammation, as well as genetic markers associated with L-methylfolate synthesis and metabolism, may be useful for identifying responders to adjunctive therapy.56 Along those lines, SAMe (800 mg to 1600 mg/day) has been shown to be a safe and effective augmentation agent to serotonin reuptake inhibitors.57 A placebo-controlled trial found that patients with TRD (n=73) had significantly greater response and remission rates with SAMe augmentation (800 mg twice daily) compared with placebo.58 Of note, SAMe monotherapy failed to show an efficacy advantage over escitalopram and placebo in a recent 12-week placebo-controlled, randomized, double-blind clinical trial,59 suggesting that it may be more effectively used for augmentation than as monotherapy.
As discussed, anxious depression represents a subtype of depression that is more difficult to treat19,60; therefore, benzodiazepines are frequently used as augmenting agents in clinical practice.61 One doubleblind, placebo-controlled study found that lormetazepam augmentation with TCAs resulted in significant improvements in depression scores after 4 weeks of treatment.62 Clonazepam augmentation of fluoxetine was superior to fluoxetine monotherapy for treating depressive symptoms in the first 3 weeks of treatment, though patients were not recruited based on treatment resistance.63 Furthermore, a review of the literature found that benzodiazepine augmentation of SSRIs can result in rapid control of baseline anxiety, as well as SSRI-induced anxiety (at the beginning of treatment), and improved adherence to antidepressant therapy.61 However, as always, the benefits of treatment must be weighed against the risks of side effects (eg, cognitive slowing in elders, transient sedation), abuse potential in prior abusers and withdrawal symptoms with abrupt discontinuation, and possible reports of worsening of mood.61 Of note, augmentation of fluoxetine with the non-benzodiazepine hypnotic eszopiclone (3 mg/night) resulted in rapid, substantial, and sustained sleep improvement, as well as faster and greater antidepressant effects, compared with placebo in one 8-week trial.64
For patients with depression, fatigue is one of the most common residual symptoms and inhibits functionality.65 Although its specific mechanism of action is unclear, modafinil (racemic mix of R- and S-enantiomers) and armodafinil (R-enantiomer only) are novel stimulant-like drugs that have been hypothesized as useful as augmentation for depression in patients with fatigue. Indeed, a meta-analysis of six double-blind, randomized, placebo-controlled trials of modafinil or armodafinil augmentation for depression (unipolar and bipolar) found significant effect for improvements in depressive symptoms, remission rates, and fatigue symptoms, with no increased adverse events, compared with placebo.66
Augmentation strategies with little-to-no evidence
Despite having little-to-no evidence base for their use, several medications are frequently used in clinical practice.
As reviewed, given the relatively positive evidence for the use of lithium as an antidepressant augmentation agent, it is logical to consider other mood stabilizers as potential augmentation agents. For instance, lamotrigine has shown positive antidepressant effects over placebo in patients with bipolar depression—especially those with more severe depression.67 However, a recent review of the literature on lamotrigine's use as an augmentation agent for patients with treatmentresistant depression revealed disappointing results.68 Specifically, only one randomized, double-blind studywas published that showed lamotrigine's significantly superior antidepressant effects compared with placebo as measured on the Clinical Global Impressions (CGI) scale; however, significance was not seen on the HDRS (primary end point).
Though its exact mechanism of action is unknown, buspirone is thought to exert its anxiolytic mechanism via partial agonism at 5-HT1a receptors; when added to other serotonergic agents, this enhanced serotonergic activity has hypothetical efficacy as an augmentation agent for depression. Unfortunately, buspirone did not outperform placebo augmentation in one randomized, double-blind trial of 119 patients with depression unresponsive to an SSRI.69 In another similar study, although buspirone initially outperformed placebo as an augmentation agent, by study end point, there were no significant differences between the groups as a whole.70 In STAR*D, augmentation buspirone to citalopram did notshow a response or remission advantage over augmentation bupropion (though both medications resulted in remission rates around 30%, as measured by the HDRS); furthermore, bupropion was better tolerated with fewer adverse events associated with its use.71
Pindolol is an antagonist at the 5-HT1A and β-adrenergic receptors; its serotonergic effects have been suggested to have a potential effect on depressive symptoms. Indeed, a meta-analysis of pindolol augmentation found that it shortened the latency to respond to serotonergic agents (ie, SSRIs) compared with placebo, though pindolol did not offer an outcome advantage beyond the first 2 weeks of treatment.72 Another meta-analysis found significant heterogeneity between studies of pindolol augmentation.73 Though the literature on pindolol as an augmentation agent has been widely mixed, a recent double-blind, randomized, placebocontrolled study of pindolol augmentation to venlafaxine found that patients who were poor metabolizers of venlafaxine might be a specific group that can benefit from pindolol augmentation.74 Although the latter study involved a small sample, genetic polymorphisms may be one source of variability seen in other studies.
Though pramipexole is FDA-approved for the treatment of Parkinson's disease (PD) and Restless Leg Syndrome, it also is thought to positively influence mood in patients with PD via agonism at the D3-receptors (which are widely distributed in the mesolimbic system).75 As such, it has been suggested as a possible augmentation agent in the treatment of depression in patients with MDD. However, initial modest antidepressant benefits with flexible-dose pramipexole in a small 8-week trial did not remain statistically significant at end point in the last-observation-carried-forward analysis.76 Another study failed to demonstrate an antidepressant advantage of pramipexole added to an SSRI over either agent alone in 39 patients with treatment resistance; furthermore, only 15% of the patients receiving the combination were able to tolerate increases in dose, suggesting that pramipexole was poorly tolerated.77 Similarly, although psychostimulants have a long history of clinical use in MDD, methylphenidate (another dopaminergic enhancer, used in the treatment of ADHD) augmentation has failed to demonstrate significant efficacy in patients with TRD.78,79 Together, these findings suggest the limited utility of antidepressant augmentation with dopaminergics for refractory depression.
As reviewed, modest evidence exists for the use of low-dose thyroid hormone augmentation for TRD, particularly on non-TCA antidepressants. Similarly, there has been an interest in the use of other hormones—namely testosterone and/or estrogens—in the treatment of resistant depression. A recent review of the literature regarding testosterone use for depression revealed that testosterone augmentation has the largest effect in middle-aged (<60 years old) hypogonadal males with MDD; in contrast, testosterone monotherapy was found to be most useful as monotherapy in patients with dysthymia or minor depression.80 There were no significant differences found between oral testosterone, oral dehydroepiandrosterone, testosterone gel, or intramuscular testosterone use. However, the use of testosterone augmentation beyond middle-aged hypogonadal men remains unclear. Further studies are ongoing, including a testosterone gel augmentation study for women with treatment-resistant depression/partial responded (ClinicalTrials.gov Identifier: NCT01783574). Regarding estrogens, a recent review found that exogenous estrogen monotherapy is not effective for major depression, though it may help to improve mild mood symptoms in premenopausal women.81 Certainly, there is a need for more research in this area, as well as an imperative to carefully weigh the risks and benefits of hormone replacement therapy in peri- and postmenopausal women.
Special section: mitochondrial modulators
Though the pathophysiology of depression and its subtypes remains an active focus of investigation, a role for dysregulated mitochondrial function has been postulated in both unipolar82 and bipolar 83 depression via several putative mechanisms (eg, genetics, oxidative stress, alterations in neuroplasticity, and inflammatory mechanisms).82,83 As such, mitochondria modulators have been suggested as potentially useful agents in the treatment of depression. These modulators include, but are not limited to: melatonin, acetyl-L-carnitine, creatine monohydrate, and (as previously discussed) SAMe.
Though popularly thought of as a natural sleep aid, melatonin is also a mitochondrial modulator. As such, it is an antioxidant that acts as a free radical scavenger. Melatonin also increases oxidative phosphorylation (thereby improving mitochondrial functioning) and prevents the degradation of mitochondria DNA.82 Guided by their preclinical findings, Fava and colleagues84 conducted a 6-week, multicenter, randomized, double-blind, placebo- and active comparator-controlled study of combination melatonin plus buspirone versus either placebo or buspirone monotherapy for the treatment of acute MDD. The antidepressant response with the combination therapy was significantly greater than the other arms of the study, showing promise for the use of melatonin in the treatment of depression.
Unfortunately, other explorations into the use of mitochondrial modulators for treating depression have been less positive overall. Acetyl-L-carnitine (ALCAR) is an important mitochondria modulator that facilitates the uptake of acetyl-CoA into the mitochondria during fatty-acid oxidation. Tills, in turn, stimulated acetylcholine production, helps with protein and membrane phosoholipid production, and prevents excessive neuronal cell death.85 Two very small studies in geriatric depression showed ALCAR's promising use for treating symptoms of depression 86,87 However, there remains a dearth in the literature on more generalizable ALCAR studies in depression. Recently, one study examined the use of ALCAR plus oc-lipoic acid (ALA; another mitochondrial modulator) compared with placebo in patients with bipolar depression. However, no significant differences in antidepressant measures were seen with ALCAR/ ALA versus placebo; furthermore, mitochondrial functioning was not enhanced by treatment, as evidenced by magnetic resonance spectroscopy measurements of cerebral energy metabolism.88
Among hypothetical mechanisms, creatine monohydrate is thought to play a role in mitochondrial energy metabolism through conversion to phosphocreatine to adenosine triphosphate (ATP). In one study, creatine monohydrate augmentation had significant antidepressant efficacy compared with placebo in one double-blind clinical trial of women treated with SSRIs (n=52).89 However, a more recent 4-week pilot study of 18 patients (14 women) failed to demonstrate an antidepressant advantage over placebo with creatine augmentation to standard ongoing antidepressant therapy.90
Mitochondrial modulators remain a topic of interest for the discovery and development of novel therapeutics—especially as augmentation agents—in the treatment of resistant depression. However, given the small/ mixed results, further studies are necessary to elucidate their place in the treatment of depression.
Switching
Following monotherapy failure, the efficacy of switching medications versus augmentation appears comparable.91 However, switching is particularly appealing in the setting of poor tolerability of an initial antidepressant. It is also the more common clinical choice in the setting of complete non-response as opposed to partial response. Little evidence exists to guide the clinician's decision to switch to a medication within the same class or mechanism of action (eg, switching from fluoxetine to sertraline within the SSRI class), or to try a medication from a different class (eg, switching from the SSRI fluoxetine to the norepinephrine-dopamine reuptake inhibitor (NDRI) bupropion). Furthermore, there is a dearth of literature comparing head-to-head trials between switch techniques, though efficacy is generally considered comparable across switches.5,92 However, one meta-analysis of four clinical trials (n=1496) showed a modest, but statistically significant remission advantage in patients switched to a non-SSRI antidepressant (bupropion, mirtazapine, venlafaxine) versus a second SSRI trial (28% vs 23.5%, respectively; P=0.007).93 Patients who fail two trials of SSRIs should be switched to another class on the third switch attempt. Regardless of which switch class is chosen, it is important to check drug-drug interactions when cross-titrating (ie, when tapering off the ineffective medication and starting a new agent at the same time). Certain types of medication classes, such as MAO Is, require a washout of other monoaminergic medications prior to starting or stopping, in order to avoid serotonin syndrome and serious autonomic dysfunction. In addition, comorbidities may help guide which switch therapy is chosen (eg, less appeal for mirtazapine in obese patients).
The SNRI venlafaxine is a plausible first switch after SSRI failure, as two large meta-analyses found venlafaxine to have superior antidepressant efficacy for remission compared with switch to a second SSRI.92,93 However, compared with switches with sertraline or bupropion, venlafaxine did not yield significantly different end points in STAR*D.94 Other SNRIs, such as duloxetine, desvenlafaxine, and levomilnacipran can also be considered, though little data exists to support their use in switching. The SNRIs share a greater efficacy than SSRIs for comorbid pain syndromes, which may be an additional advantage, though are characterized by a generally high prevalence of discontinuation emergent effects and the small possibility of elevated blood pressure.
Mirtazapine is a unique antidepressant that acts as an antagonist at α2, 5-HT2, and 5-HT3, receptors and as an agonist at postsynaptic 5-HT1Areceptors. Several studies showed statistically comparable remission rates between mirtazapine and SSRIs.95,96 STAR*D found no statistically significant differences in remission rates between mirtazapine and the TCA nortriptyline (12.3% vs 19.8%, respectively) in patients with TRD.97 Similarly, bupropion did not separate from venlafaxine in STAR*D as a switching tactic94; however, bupropion may be a good choice for patients with sexual side effects on initial serotonergic monotherapy.98 Of note, vortioxetine (10 mg to 20 mg/day), a novel serotonin receptor modulator and serotonin transporter inhibitor, is safe, well- tolerated, and superior to agomelantine (melatonergic antidepressant) in patients with a previous SSRI/SNRI monotherapy failure, though this was demonstrated in only one double -blind, randomized trial.99
Although TCAs were once considered first-line treatments, they have largely been replaced by newer antidepressants due to improved safety and tolerability profiles. Because of their increased danger in overdose and adverse events, they should be reserved for patients who have failed with other types of switch therapies. STAR*D found no statistically significant efficacy differences between nortriptyline (up to 200 mg/day) and mirtazapine (up to 60 mg/day) as switch therapies for TRD (remission rates 20% vs 12%, respectively).97 Similarly, sertraline switch to imipramine, or vice versa, did not yield significantly superior outcomes in chronically depressed patients with TRD.100
For patients with severe TRD, MAOIs should be considered, though these agents are often reserved for later in the sequence of switches given their challenging and potentially fatal drug and food interactions.101 One double-blind crossover study of imipramine to phenelzine (and vice versa) in patients with TRD found statistically higher response rates to phenelzine (67%) versus imipramine (41 %).102 However, the MAOI tranylcypromine was compared with combination venlafaxine and mirtazapine in patients with three prior medication trials as part of STAR*D; remission rates were low, and statistically comparable, between the treatment groups (6.9% vs 13.7%, respectively).103 Of note, MAOIs may be particularly helpful to patients with atypical features of depression.102 However, this impression is based on the lesser efficacy of TCAs vs. MAOIs in atypical depression. It is not known whether MAOIs are superior to SSRIs or SNRIs for this depressive subtype.
Combination
As opposed to augmentation of an existing antidepressant monotherapy with a drug that is not traditionally used as an antidepressant, combination therapy refers to use of two or more antidepressants, typically from two different mechanistic classes, to form a broader spectrum antidepressant regimen. Combination strategies offer less risk of discontinuation symptoms compared with cross-titration strategies used during switch, and increase the probability of positive antidepressant effects through drug synergism.44 As with polypharmacy of any type, it is important to check for interactions and contraindications when prescribing two antidepressants simultaneously, as some combinations can results in devastating and harmful consequences (eg, combining serotonergic agents with MAOIs).
The evidence base for combination strategies is quite limited. One positive meta-analysis found that patients (n=250) were three times more likely to experience remission if started initially on combination therapy (mirtazapine/SSRI, mirtazapine/bupropion, mirtazapine/venlafaxine, SSRI/TCA), versus monotherapy.104 The practice of combination therapy is generally not supported in the literature, as highlighted by large trials105,106 and a recent meta-analysis.107 Nevertheless, this small literature is largely based on studies assessing initial combination rather than combination in the setting of treatment resistance.
Special case: tachyphylaxis
Tachyphylaxis, or medication “poop out,” is generally defined as a rapid and progressive decrease in response to a given dose after repetitive administration of a pharmacologically or physiologically active substance.108 Though this phenomenon is not widely acknowledged in the antidepressant literature, nor are there accurate estimates of its prevalence, loss of response to an ongoing antidepressant appears to be a common clinical occurrence. Depressions that respond to antidepressants—but fail to maintain the response over time—may represent a specific subtype of TRD. Several factors can lead to apparent tachyphylaxis, including nonadherence, receptor desensitization, pharmacokinetic changes, and loss of placebo effect, to name a few.109 Further studies of failure to sustain an initial response are clearly needed.
Future directions
Antiglutamatergics
Despite the most resourceful therapeutic efforts, some patients will experience persistent marked depression. For this group of patients, agents with novel mechanisms of action that depart from the traditional monoaminergic antidepressants offer particular promise. Here, we will focus on two examples of such promising agents: ketamine and scopolamine.
Ketamine hydrochloride
Ketamine is perhaps the most well-known and most studied non-monoaminergic antidepressant compound in recent years. Although its exact antidepressant mechanism of action remains unknown, ketamine is classified as an N-methyl-D-aspartate (NMDA) receptor antagonist, and had been used for decades as an anesthetic prior to its serendipitous discovery as an antidepressant. The investigational use of ketamine for depression was first reported by Berman and colleagues110 over a decade ago; additional research has yielded interesting clinical evidence and provides important insights into the treatment of TRD. Ketamine's rapid (within 1 day), robust (across a variety of symptoms), and relatively sustained (approximately 7 days) antidepressant efficacy at subanesthetic intravenous doses (only 0.5 mg/kg over 40 minutes, compared with the 2 mg to 3 mg/kg dosage used in anesthesia over much shorter time periods) has been demonstrated in several randomized, double-blind, placebo-controlled trials.111,112 Recently, Murrough and colleagues113 further confirmed ketamine's antidepressant effects by using midazolam as an active comparator, thereby minimizing the risk of unblinding due to the treatment-emergent side effects associated with ketamine (eg, dissociative, psychotomimetic, and sympathomimetic side effects). In this trial of 73 patients with TRD, ketamine had a significant effect in reducing scores on the Montgomery-Asberg Depression Rating Scale (MADRS) compared with midazolam (Cohen's d=0.81). These results were significant 24 hours following infusion, and were sustained for several days.
Several clinical characteristics appear to be useful for predicting ketamine's antidepressant effects. Specifically, ketamine has been shown to decrease symptoms of depression in patients with anxious depression to a significantly greater degree than patients with nonanxious depression114 —an exciting finding, given the anxious depression typically represents a difficult clinical treatment challenge.19 Similarly, ketamine appears to have a superior antidepressant action in patients with a family history of alcoholism (as opposed to patients with a negative family history for alcoholism).115 Furthermore, ketamine also rapidly decreases symptoms of suicidal ideation116,117 and anhedonia118; interestingly, decreases in suicidal ideation have been found to be related to, but not dependent on, decreases in depression and anxiety symptoms.119 Together, these clinical predictors may help researchers learn more about how ketamine's antidepressant effects are realized.
Most of the data on ketamine's antidepressant effects have come from intravenous studies (which typically use the subanesthetic slow-infusion dose of 0.5 mg/kg over 40 minutes). However, the extent to which the dosing of ketamine effects its antidepressant action remains unknown. As such, a nationwide dose-finding study is currently under way (ClinicalTrials.gov Identifier: NCT01920555).This study, funded by the National Institute of Mental Health (NIMH), is being conducted by the Rapidly Acting Treatments for Treatment-Resistant Depression (RAPID) network of sites. From this clinical research, insights gained from knowing the optimal dose for ketamine's antidepressant response will aim to set the stage for future explorations into ketamine's mechanism of action. Of note, in a departure from the intravenous ketamine infusions, a recent trial of intranasal ketamine (50 mg) also significantly reduced depressive symptoms within 24 hours of administration compared with placebo in 20 patients with TRD, though its antidepressant effects were no longer significant at 72 hours post-administration.120 These results are particularly promising, due to the ease of use of intranasal (versus intravenous) administration.
Despite the great promise of ketamine, its antidepressant effects are generally not sustained beyond a week110,112 (though its effects in anxious depression are sustained over the course of 28 days).114 This has launched several attempts at extending its effects. For example, one recent study examined the effects of six repeated ketamine infusions (0.5 mg/kg over 40 minutes) over the course of 2 weeks in 10 patients with TRD who previously responded to a single infusion of ketamine. Ketamine appeared to be safe and efficacious when given in this study,121 with a mean time-to-relapse of 19 days. This study was later expanded to include 24 patients; the median time-to-relapse in the responders was 18 days.122 Certainly, repeated doses of ketamine appear to extend its antidepressant response, but it remains unknown as to how long ketamine needs to be given for, and at what dose. Furthermore, another study attempted to extend the antidepressant effects of a single infusion of ketamine by giving oral riluzole, a glutamatergic modulator, for 28 days.123 However, riluzole did not outperform placebo over the course of the trial. Together, these findings underscore the need for agents that extend ketamine's antidepressant effects beyond a few weeks.
What about the safety and tolerability of ketamine? Overall, when given in slow, subanesthetic doses for depression research, ketamine appears to be safe and well tolerated. Specifically, a recent report analyzed the safety and tolerability data from a total of 205 ketamine infusions in patients with depression; only four infusions were stopped because of adverse events.124 In this dataset, despite transient increases in sympathomimetic, dissociative, and psychotomimetic side effects during the infusions, no side effects were found to be persistent, and no adverse medical events occurred. Furthermore, there was no evidence for increased substance use in a subgroup patients followed long-term. Interestingly, the dissociative side effects seen with acute ketamine administration may mediate—and may actually be necessary for—the antidepressant effects seen in ketamine responders.125 Further research is necessary on this topic.
As mentioned, ketamine is generally classified as a noncompetitive NMDA glutamatergic receptor antagonist, which may contribute to its antidepressant effects.126,127 This action is thought to disinhibit glutamate transmission, which leads to a rapid (albeit transient) glutamate burst—resulting in enhanced a-amino-3hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor throughput. These actions lead to increased brain-derived neurotrophic factor (BDNF) release and the activation of signaling cascades (eg, mammalian target of rapamycin (mTOR), an essential kinase in regulating proteins involved in synaptic plasticity). In animal experiments, these actions have been shown to stimulate synaptic spine formations in the prefrontal cortex and reverse the deficits of chronic stress in models of depression.127 Ketamine may also have antiinflammatory properties, also contributing to its antidepressant effects.126 From these findings, it is unlikely that one single mechanism explains the antidepressant actions of ketamine. Further elucidation of these mechanisms is the goal of several ongoing trials (ClinicalTrials.gov identifiers: NCT02122562, NCT00088699).
Based on ketamine's efficacy, a variety of antiglutamatergic agents have now been studied in MDD. More comprehensive reviews128,129 on ketamine and related compounds127 are currently available.
Scopolamine
Similarly to ketamine, intravenous scopolamine (a muscarinic acetylcholine receptor antagonist) rapidly and robustly decreases symptoms of depression in wellcharacterized, medication-free patients with TRD, as demonstrated by two randomized, placebo-controlled trials.130,131 Like ketamine, the mechanism of action for scopolamine's antidepressant effects is not known. Both compounds may increase synaptogenesis through intracellular signaling pathways.127 Although rapidacting antidepressants (RAADs)—such as ketamine and scopolamine—offer the prospect of catalyzing response in acute settings (eg, emergency departments and inpatient units), they may also provide critical insights for the development of other novel therapeutics for depression. Similarly to ketamine, explorations into scopolamine's utility as a RAAD holds promise for the discovery of clinically relevant biomarkers of treatment response, which can be used as targets for the a priori investigation into new medications for TRD.
Conclusions
The serendipitous discovery of monoaminergic antidepressants revolutionized the field of psychiatry. However, as many as two thirds of depressed patients will not remit after one antidepressant trial; up to one third will not remit after sequential trials. In the setting of nonremission, it is important to re-evaluate diagnosis, adherence and comorbid conditions, and to consider dose optimization, as well as switching, augmenting, or combining antidepressants (Figure 1).The evidence base, though expanding, is still limited and based on studies with widely varying methodologies. In all cases, nonpharmacological approaches, including psychotherapies and neuromodulation (eg, ECT or TMS) should be considered.
Figure 1. Steps in the evaluation of treatment-resistant depression (TRD).
For those with exceptionally resistant depression, physicians should consider referring their patients to clinical trials—whether a medication trial, a devicebased exploration, a complementary and integrative medicine trial, or a trial involving psychotherapy. A list of trials can be accessed by patients and clinicians at ClinicalTrials.gov. From this Web site, searches can be performed to find studies open for enrollment based on location, diagnosis, and/or keywords. In other fields of medicine—such as oncology, infectious disease, and neurology—physicians often refer their most severely ill patients to clinical trials. Psychiatrists should begin taking a similar route with their sickest patients. Indeed, it is only through vigorous clinical research involving participants with resistant depression that our field will be able to advance treatment and to better understand this challenging and often devastating condition.
Traditionally, antidepressant treatment therapies have been selected largely based on tolerability and safety.132 The identification of clinically relevant biomarkers that predict antidepressant response, still an elusive but major goal, is likely to transform the care of depressed patients. In addition, further clues to the mechanism of action of novel/rapidly acting antidepressants, as well as the neurobiology of depressive subtypes, are important steps for the development of targeted therapies. While awaiting these key advances, patients with resistant depression require the expert persistence of determined and compassionate clinicians.
Disclosures: Ionescu: None; Alpert: Belvoir Publishing (honoraria) and NIMH; Rosenbaum: Medavante and Psybrain (equity).
Contributor Information
Dawn F. Ionescu, Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts, USA.
Jerrold F. Rosenbaum, Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts, USA.
Jonathan E. Alpert, Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts, USA.
REFERENCES
- 1.Whiteford HA., Degenhardt L., Rehm J., et al Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 2.Mrazek DA., Hornberger JC., Altar CA., Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65:977–987. doi: 10.1176/appi.ps.201300059. [DOI] [PubMed] [Google Scholar]
- 3.Thase ME, Rush AJ. When at first you don't succeed: sequential strategies for antidepressant nonresponders. J Clin Pyschiatry. 1997;58(suppl 13):23–29. [PubMed] [Google Scholar]
- 4.Nierenberg AA., DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Pyschiatry. 2001;62(suppl 16):5–9. [PubMed] [Google Scholar]
- 5.Rush AJ., Trivedi MH., Wisniewski SR., et al Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 6.Thase ME. Evaluating antidepressant therapies: remission as the optimal outcome. J Clin Pyschiatry. 2003;64(suppl 13):18–25. [PubMed] [Google Scholar]
- 7.Boulenger JP. Residual symptoms of depression: clinical and theoretical implications. Eur Psychiatry. 2004;19:209–213. doi: 10.1016/j.eurpsy.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Paykel ES., Ramana R., Cooper Z., Hayhurst H., Kerr J., Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- 9.Gaynes BN., Warden D., Trivedi MH., Wisniewski SR., Fava M., Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Sen. 2009;60:1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi MH., Rush AJ., Wisniewski SR., et al Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 11.Trevino K., McClintock SM., McDonald Fischer N., Vora A., Husain MM. Defining treatment-resistant depression: A comprehensive review of the literature. Ann Clin Psychiatry. 2014;26:222–232. [PubMed] [Google Scholar]
- 12.Ruhe HG., van Rooijen G., Spijker J., Peeters FP., Schene AH. Staging methods for treatment resistant depression. A systematic review. J Affect Disord. 2012;137:35–45. doi: 10.1016/j.jad.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual Of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing. 2013 [Google Scholar]
- 14.Rush AJ., Warden D., Wisniewski SR., et al STAR*D: revising conventional wisdom. CNS Drugs. 2009;23:627–647. doi: 10.2165/00023210-200923080-00001. [DOI] [PubMed] [Google Scholar]
- 15.Fava M., Alpert JE., Carmin CN., et al Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34:1299–1308. doi: 10.1017/s0033291704002612. [DOI] [PubMed] [Google Scholar]
- 16.Fava M., Rankin MA., Wright EC., et al Anxiety disorders in major depression. Compr Psychiatry. 2000;41:97–102. doi: 10.1016/s0010-440x(00)90140-8. [DOI] [PubMed] [Google Scholar]
- 17.Sanderson WC., Beck AT., Beck J. Syndrome comorbidity in patients with major depression or dysthymia: prevalence and temporal relationships. Am J Psychiatry. 1990;147:1025–1028. doi: 10.1176/ajp.147.8.1025. [DOI] [PubMed] [Google Scholar]
- 18.Ionescu DF., Niciu MJ., Mathews DC., Richards EM., Zarate CA., Jr. Neurobiology of anxious depression: a review. Depress Anxiety. 2013;30(4):374–385. doi: 10.1002/da.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ionescu DF., Niciu M., Richards EM., Zarate CA. Pharmacologic treatment of dimensional anxious depression: a review. Prim Care Companion CNS Disord. 2014;16 [Epub ahead of print](3) doi: 10.4088/PCC.13r01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi M., Shirayama Y., Muneoka K., Suzuki M., Sato K., Hashimoto K. Personality traits as risk factors for treatment-resistant depression. PloS One. 2013;8:e63756. doi: 10.1371/journal.pone.0063756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly K., Posternak M., Alpert JE. Toward achieving optimal response: understanding and managing antidepressant side effects. Dialogues Clin Neurosci. 2008;10:409–418. doi: 10.31887/DCNS.2008.10.4/kkelly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo HW., Liu SC., Tsou HH., et al CYP1A2 genetic polymorphisms are associated with early antidepressant escitalopram metabolism and adverse reactions. Pharmacogenomics. 2013;14:1191–1201. doi: 10.2217/pgs.13.105. [DOI] [PubMed] [Google Scholar]
- 23.Zhou SF., Yang LP., Zhou SF., Liu YH., Chan E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1 A2. AA.PSJ. 2009;11:481–494. doi: 10.1208/s12248-009-9127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washio I., Maeda M., Sugiura C., et al Cigarette smoke extract induces CYP2B6 through constitutive androstane receptor in hepatocytes. Drug MetabDispos. 2011;39(1):1–3. doi: 10.1124/dmd.110.034504. [DOI] [PubMed] [Google Scholar]
- 25.Haslemo T., Eikeseth PH., Tanum L., Molden E., Refsum H. The effect of variable cigarette consumption on the interaction with clozapine and olanzapine. Eur J Clin Pharmacol. 2006;62:1049–1053. doi: 10.1007/s00228-006-0209-9. [DOI] [PubMed] [Google Scholar]
- 26.Howell T. Managing medical and psychiatric comorbidities. WMJ. 2004;103(6):23–27. [PubMed] [Google Scholar]
- 27.Alpert J. Drug-Drug Interactions in Psychopharmacology.New York, NY: McGraw-Hill; 2012 [Google Scholar]
- 28.Weilburg JB., O'Leary KM., Meigs JB., Hennen J., Stafford RS. Evaluation of the adequacy of outpatient antidepressant treatment. Psychiatric Serv. 2003;54:1233–1239. doi: 10.1176/appi.ps.54.9.1233. [DOI] [PubMed] [Google Scholar]
- 29.Trivedi RB., Nieuwsma JA., Williams JW., Jr. Examination of the utility of psychotherapy for patients with treatment resistant depression: a systematic review. J Gen Int Med. 2011;26:643–650. doi: 10.1007/s11606-010-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cusin C., Dougherty DD. Somatic therapies for treatment-resistant depression: ECT, TMS, VNS, DBS. Biol Mood Anxiety Disord. 2012;2:14. doi: 10.1186/2045-5380-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qureshi NA., Al-Bedah AM. Mood disorders and complementary and alternative medicine: a literature review. Neuropsychiatr Dis Treat. 2013;9:639–658. doi: 10.2147/NDT.S43419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 33.Rush AJ. STAR*D: what have we leamed 4m7Psyc/i/3&y. 2007;164:201–204. doi: 10.1176/ajp.2007.164.2.201. [DOI] [PubMed] [Google Scholar]
- 34.McIntyre RS., Filteau MJ., Martin L., et al Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7. doi: 10.1016/j.jad.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 35.Mahmoud RA., Pandina GJ., Turkoz I., et al Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann int Med. 2007;147:593–602. doi: 10.7326/0003-4819-147-9-200711060-00003. [DOI] [PubMed] [Google Scholar]
- 36.Papakostas Gl., Petersen TJ., Nierenberg AA., et al Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Pyschiatry. 2004;65:217–221. doi: 10.4088/jcp.v65n0212. [DOI] [PubMed] [Google Scholar]
- 37.Spielmans Gl., Berman Ml., Linardatos E., Rosenlicht NZ., Perry A., Tsai AC. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10:e1001403. doi: 10.1371/journal.pmed.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson JC., Papakostas Gl. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166:980–991. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- 39.Papakostas Gl., Shelton RC., Smith J., Fava M. Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Pyschiatry. 2007;68:826–831. doi: 10.4088/jcp.v68n0602. [DOI] [PubMed] [Google Scholar]
- 40.Cha DS., Mclntyre RS. Treatment-emergent adverse events associated with atypical antipsychotics. Exp Opin Pharmacother. 2012;13:1587–1598. doi: 10.1517/14656566.2012.656590. [DOI] [PubMed] [Google Scholar]
- 41.Bauer M., Adli M., Ricken R., Severus E., Pilhatsch M. Role of lithium augmentation in the management of major depressive disorder. CNS Drugs. 2014;28:331–342. doi: 10.1007/s40263-014-0152-8. [DOI] [PubMed] [Google Scholar]
- 42.Iosifescu DV., Bolo NR., Nierenberg AA., Jensen JE., Fava M., Renshaw PF. Brain bioenergeticsand response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–1134. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Lifschytz T., Segman R., Shalom G., et al Basic mechanisms of augmentation of antidepressant effects with thyroid hormone. Curr Drug Targets. 2006;7:203–210. doi: 10.2174/138945006775515482. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho AF., Berk M., Hyphantis TN., Mclntyre RS. The integrative management of treatment-resistant depression: a comprehensive review and perspectives. Psychother Psychosomat. 2014;83:70–88. doi: 10.1159/000357500. [DOI] [PubMed] [Google Scholar]
- 45.Aronson R., Offman HJ., Joffe RT., Naylor CD. Triiodothyronine augmentation in the treatment of refractory depression. A meta-analysis. Arch Gen Psychiatry. 1996;53:842–848. doi: 10.1001/archpsyc.1996.01830090090013. [DOI] [PubMed] [Google Scholar]
- 46.Nierenberg AA., Fava M., Trivedi MH., et al A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psychiatry. 2006;163:1519–30; quiz 665. doi: 10.1176/ajp.2006.163.9.1519. [DOI] [PubMed] [Google Scholar]
- 47.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- 48.Lotrich FE. Inflammatory cytokine-associated depression. Brain Res. 2014. [Epub ahead of print] doi: 10.1016/j.brainres.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Na KS., Lee KJ., Lee JS., Cho YS., Jung HY. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:79–85. doi: 10.1016/j.pnpbp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Raison CL., Rutherford RE., Woolwine BJ., et al A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bloch MH., Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry. 2012;17:1272–1282. doi: 10.1038/mp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bedson E., Bell D., Carr D., et al Folate Augmentation of Treatment-Evaluation for Depression (FolATED): randomised trial and economic evaluation. Health TechnoI Assess. 2014;18(48):vii–viii, 1-159. doi: 10.3310/hta18480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts SH., Bedson E., Hughes D., et al Folate augmentation of treatment - evaluation for depression (FolATED): protocol of a randomised controlled trial. BMCP Psychiatry. 2007;7:65. doi: 10.1186/1471-244X-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papakostas Gl., Cassiello CF., lovieno N. Folates and S-adenosylmethionine for major depressive disorder. Can J Psychiatry. 2012;57:406–413. doi: 10.1177/070674371205700703. [DOI] [PubMed] [Google Scholar]
- 55.Papakostas Gl., Shelton RC., Zajecka JM., et al L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169:1267–1274. doi: 10.1176/appi.ajp.2012.11071114. [DOI] [PubMed] [Google Scholar]
- 56.Papakostas Gl., Shelton RC., Zajecka JM., et al Effect of adjunctive Lmethylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Pyschiatry. 2014;75:855–863. doi: 10.4088/JCP.13m08947. [DOI] [PubMed] [Google Scholar]
- 57.Alpert JE., Papakostas G., Mischoulon D., et al S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24:661–664. doi: 10.1097/01.jcp.0000145339.45794.cd. [DOI] [PubMed] [Google Scholar]
- 58.Papakostas Gl., Mischoulon D., Shyu I., Alpert JE., Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a doubleblind, randomized clinical trial. Am J Psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 59.Mischoulon D., Price LH., Carpenter LL., et al A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder. J Clin Pyschiatry. 2014;75:370–376. doi: 10.4088/JCP.13m08591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ionescu DF., Niciu MJ., Henter ID., Zarate CA. Defining anxious depression: a review of the literature. CNS Spectr. 2013:1–9. doi: 10.1017/S1092852913000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dunlop BW., Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Pyschiatry. 2008;10:222–228. doi: 10.4088/pcc.v10n0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nolen WA., Haffmans PM., Bouvy PF., Duivenvoorden HJ. Hypnotics as concurrent medication in depression. A placebo-controlled, doubleblind comparison of flunitrazepam and lormetazepam in patients with major depression, treated with a (tri)cyclic antidepressant. J Affect Disord. 1993;28:179–188. doi: 10.1016/0165-0327(93)90103-q. [DOI] [PubMed] [Google Scholar]
- 63.Smith WT., Londborg PD., Glaudin V., Painter JR. Short-term augmentation of fluoxetine with clonazepam in the treatment of depression: a double-blind study. Am J Psychiatry. 1998;155:1339–45. doi: 10.1176/ajp.155.10.1339. [DOI] [PubMed] [Google Scholar]
- 64.Fava M., McCall WV., Krystal A., et al Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–1060. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 65.Zajecka JM. Residual symptoms and relapse: mood, cognitive symptoms, and sleep disturbances. J Clin Pyschiatry. 2013;74(suppl 2):9–13. doi: 10.4088/JCP.12084su1c.02. [DOI] [PubMed] [Google Scholar]
- 66.Goss AJ., Kaser M., Costafreda SG., Sahakian BJ., Fu CH. Modafinil augmentation therapy in unipolar and bipolar depression: a systematic review and meta-analysis of randomized controlled trials. J Clin Pyschiatry. 2013;74:1101–1107. doi: 10.4088/JCP.13r08560. [DOI] [PubMed] [Google Scholar]
- 67.Geddes JR., Calabrese JR., Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194:4–9. doi: 10.1192/bjp.bp.107.048504. [DOI] [PubMed] [Google Scholar]
- 68.Thomas SP., Nandhra HS., Jayaraman A. Systematic review of lamotrigine augmentation of treatment resistant unipolar depression (TRD). J Ment Health. 2010;19:168–175. doi: 10.3109/09638230903469269. [DOI] [PubMed] [Google Scholar]
- 69.Landen M., Bjorling G., Agren H., Fahlen T. A randomized, doubleblind, placebo-controlled trial of buspirone in combination with an SSRI in patients with treatment-refractory depression. J Clin Pyschiatry. 1998;59:664–668. [PubMed] [Google Scholar]
- 70.Appelberg BG., Syvalahti EK., Koskinen TE., Mehtonen OP., Muhonen TT., Naukkarinen HH. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Pyschiatry. 2001;62:448–452. doi: 10.4088/jcp.v62n0608. [DOI] [PubMed] [Google Scholar]
- 71.Trivedi MH., Fava M., Wisniewski SR., et al Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–52. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 72.Ballesteros J., Callado LF. Effectiveness of pindolol plus serotonin uptake inhibitors in depression: a meta-analysis of early and late outcomes from randomised controlled trials. J Affect Disord. 2004;79:137–147. doi: 10.1016/S0165-0327(02)00404-4. [DOI] [PubMed] [Google Scholar]
- 73.Whale R., Terao T., Cowen P., Freemantle N., Geddes J. Pindolol augmentation of serotonin reuptake inhibitors for the treatment of depressive disorder: a systematic review. J Psychopharmacol. 2010;24:513–520. doi: 10.1177/0269881108097714. [DOI] [PubMed] [Google Scholar]
- 74.Martiny K., Lunde M., Bech P., Plenge P. A short-term double-blind randomized controlled pilot trial with active or placebo pindolol in patients treated with venlafaxine for major depression. Nordic J Psychiatry. 2012;66:147–154. doi: 10.3109/08039488.2012.674553. [DOI] [PubMed] [Google Scholar]
- 75.Piercey MF. Pharmacology of pramipexole, a dopamine OS-preferring agonist useful in treating Parkinson's disease. Clin Neuropharrnacol. 1998;21:141–51. [PubMed] [Google Scholar]
- 76.Cusin C., lovieno N., Iosifescu DV., et al A randomized, doubleblind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J Clin Pyschiatry. 2013;74:e636–e641. doi: 10.4088/JCP.12m08093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Franco-Chaves JA., Mateus CF., Luckenbaugh DA., Martinez PE., Mallinger AG., Zarate CA, Jr. Combining a dopamine agonist and selective serotonin reuptake inhibitor for the treatment of depression: a doubleblind, randomized pilot study. J Affect Disord. 2013;149:319–25. doi: 10.1016/j.jad.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patkar AA., Masand PS., Pae CU., et al A randomized, double-blind, placebo-controlled trial of augmentation with an extended release formulation of methylphenidate in outpatients with treatment-resistant depression. J Clin Psychopharmacol. 2006;26:653–656. doi: 10.1097/01.jcp.0000246212.03530.fd. [DOI] [PubMed] [Google Scholar]
- 79.Ravindran AV., Kennedy SH., O'Donovan MC., Fallu A., Camacho F., Binder CE. Osmotic-release oral system methylphenidate augmentation of antidepressant monotherapy in major depressive disorder: results of a double-blind, randomized, placebo-controlled trial. J Clin Pyschiatry. 2008;69:87–94. doi: 10.4088/jcp.v69n0112. [DOI] [PubMed] [Google Scholar]
- 80.Amanatkar HR., Chibnall JT., Seo BW., Manepalli JN., Grossberg GT. Impact of exogenous testosterone on mood: a systematic review and meta-analysis of randomized placebo-controlled trials. Ann Clin Psychiatry. 2014;26:19–32. [PubMed] [Google Scholar]
- 81.Howland RH. Use of endocrine hormones for treating depression. J Psychosoc Nurs Ment Health Serv. 2010;48:13–16. doi: 10.3928/02793695-20101105-01. [DOI] [PubMed] [Google Scholar]
- 82.Klinedinst NJ., Regenold WT. A mitochondrial bioenergetic basis of depression. J Bioenerget Biomembr. 2015;47(1-2):155–171. doi: 10.1007/s10863-014-9584-6. [DOI] [PubMed] [Google Scholar]
- 83.Nierenberg AA., Kansky C., Brennan BP., Shelton RC., Perlis R., Iosifescu DV. Mitochondrial modulators for bipolar disorder: a pathophysiologically informed paradigm for new drug development. Aust N Z J Psychiatry. 2013;47:26–42. doi: 10.1177/0004867412449303. [DOI] [PubMed] [Google Scholar]
- 84.Fava M., Targurn SD., Nierenberg AA., et al An exploratory study of combination buspirone and melatonin SR in major depressive disorder (MDD): a possible role for neurogenesis in drug discovery. J Psychiatr Res. 2012;46:1553–1563. doi: 10.1016/j.jpsychires.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 85.Wang SM., Han C., Lee SJ., Patkar AA., Masand PS., Pae CU. A review of current evidence for acetyl-l-carnitine in the treatment of depression. J Psychiatr Res. 2014;53:30–37. doi: 10.1016/j.jpsychires.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Garzya G., Corallo D., Fiore A., Lecciso G., Petrelli G., Zotti C. Evaluation of the effects of L-acetylcarnitine on senile patients suffering from depression. Drugs. under experimental and clinical research. 1990;16:101–106. [PubMed] [Google Scholar]
- 87.Pettegrew JW., Levine J., Gershon S., et al 31P-MRS study of acetylL-carnitine treatment in geriatric depression: preliminary results. Bipolar Disord. 2002;4:61–66. doi: 10.1034/j.1399-5618.2002.01180.x. [DOI] [PubMed] [Google Scholar]
- 88.Brennan BP., Jensen JE., Hudson Jl., et al A placebo-controlled trial of acetyl-L-carnitine and alpha-lipoic acid in the treatment of bipolar depression. J Clin Psychopharmacol. 2013;33:627–635. doi: 10.1097/JCP.0b013e31829a83f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lyoo IK., Yoon S., Kim TS., et al A randomized, double-blind placebocontrolled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am J Psychiatry. 2012;169:937–945. doi: 10.1176/appi.ajp.2012.12010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nemets B., Levine J. A pilot dose-finding clinical trial of creatine monohydrate augmentation to SSRIs/SNRIs/NASA antidepressant treatment in major depression. Int Clin Psychopharmacol. 2013;28:127–133. doi: 10.1097/YIC.0b013e32835ff20f. [DOI] [PubMed] [Google Scholar]
- 91.Gaynes BN., Dusetzina SB., Ellis AR., et al Treating depression after initial treatment failure: directly comparing switch and augmenting strategies in STAR*D. J Clin Psychopharmacol. 2012;32:114–119. doi: 10.1097/JCP.0b013e31823f705d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruhe HG., Huyser J., Swinkels JA., Schene AH. Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Pyschiatry. 2006;67:1836–1855. doi: 10.4088/jcp.v67n1203. [DOI] [PubMed] [Google Scholar]
- 93.Papakostas Gl., Fava M., Thase ME. Treatment of SSRI-resistant depression: a meta-analysis comparing within- versus across-class switches. Biol Psychiatry. 2008;63:699–704. doi: 10.1016/j.biopsych.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 94.Rush AJ., Trivedi MH., Wisniewski SR., et al Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 95.Fang Y., Yuan C., Xu Y., et al Comparisons of the efficacy and tolerability of extended-release venlafaxine, mirtazapine, and paroxetine in treatment-resistant depression: a double-blind, randomized pilot study in a Chinese population. J Clin Psychopharmacol. 2010;30:357–364. doi: 10.1097/JCP.0b013e3181e7784f. [DOI] [PubMed] [Google Scholar]
- 96.Connolly KR., Thase ME. If at first you don't succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs. 2011;71:43–64. doi: 10.2165/11587620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 97.Fava M., Rush AJ., Wisniewski SR., et al A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: a STAR*D report. Am J Psychiatry. 2006;163:1161–1172. doi: 10.1176/ajp.2006.163.7.1161. [DOI] [PubMed] [Google Scholar]
- 98.Pereira VM., Arias-Carrion O., Machado S., Nardi AE., Silva AC. Bupropion in the depression-related sexual dysfunction: a systematic review. CNS Neurol Disord Drug Targets. 2014;13:1079–1088. doi: 10.2174/1871527313666140612112630. [DOI] [PubMed] [Google Scholar]
- 99.Montgomery SA., Nielsen RZ., Poulsen LH., Haggstrom L. A randomised, double-blind study in adults with major depressive disorder with an inadequate response to a single course of selective serotonin reuptake inhibitor or serotonin-noradrenaline reuptake inhibitor treatment switched to vortioxetine or agomelatine. Hum Psychopharmacol. 2014;29(5):470–482. doi: 10.1002/hup.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thase ME., Rush AJ., Howland RH., et al Double-blind switch study of imipramine or sertraline treatment of antidepressant-resistant chronic depression. Arch Gen Psychiatry. 2002;59:233–239. doi: 10.1001/archpsyc.59.3.233. [DOI] [PubMed] [Google Scholar]
- 101.Thase ME. The role of monoamine oxidase inhibitors in depression treatment guidelines. J Clin Pyschiatry. 2012;73(suppl 1):10–16. doi: 10.4088/JCP.11096su1c.02. [DOI] [PubMed] [Google Scholar]
- 102.McGrath PJ., Stewart JW., Nunes EV., et al A double-blind crossover trial of imipramine and phenelzine for outpatients with treatment-refractory depression. Am J Psychiatry. 1993;150:118–123. doi: 10.1176/ajp.150.1.118. [DOI] [PubMed] [Google Scholar]
- 103.McGrath PJ., Stewart JW., Fava M., et al Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163:1531–1541; quiz 666. doi: 10.1176/ajp.2006.163.9.1531. [DOI] [PubMed] [Google Scholar]
- 104.Rocha FL., Fuzikawa C., Riera R., Hara C. Combination of antidepressants in the treatment of major depressive disorder: a systematic review and meta-analysis. J Clin Psychopharmacol. 2012;32:278–281. doi: 10.1097/JCP.0b013e318248581b. [DOI] [PubMed] [Google Scholar]
- 105.Stewart JW., McGrath PJ., Blondeau C., et al Combination antidepressant therapy for major depressive disorder: speed and probability of remission. J Psychiatr Res. 2014;52:7–14. doi: 10.1016/j.jpsychires.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 106.Rush AJ., Trivedi MH., Stewart JW., et al Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168:689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 107.Lopes Rocha F., Fuzikawa C., Riera R., Ramos MG., Hara C. Antidepressant combination for major depression in incomplete responders-a systematic. review. J Affect Disord. 2013;144:1–6. doi: 10.1016/j.jad.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 108.Stedman's Medical Dictionary. Philadelphia, PA: Lippincott, Williams, and Wilkins. 2000 [Google Scholar]
- 109.Katz G. Tachyphylaxis/tolerance to antidepressive medications: a review. Isr J Psychiatry Relat Sci. 2011;48:129–135. [PubMed] [Google Scholar]
- 110.Berman RM., Cappiello A., Anand A., et al Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 111.Sos P., Klirova M., Novak T., Kohutova B., Horacek J., Palenicek T. Relationship of ketamine's antidepressant and psychotomimetic effects in unipolar depression. Neuroendocrinol Lett. 2013;34:287–293. [PubMed] [Google Scholar]
- 112.Zarate CA., Jr., Singh JB., Carlson PJ., et al A randomized trial of an Nmethyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 113.Murrough JW., Iosifescu DV., Chang LC., et al Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ionescu DF., Luckenbaugh DA., Niciu MJ., et al Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. J Clin Pyschiatry. 2014;75:e932–e938. doi: 10.4088/JCP.14m09049. [DOI] [PubMed] [Google Scholar]
- 115.Phelps LE., Brutsche N., Moral JR., Luckenbaugh DA., Manji HK., Zarate CA., Jr. Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DiazGranados N., Ibrahim LA., Brutsche NE., et al Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Pyschiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Price RB., Nock MK., Charney DS., Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatmentresistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lally N., Nugent AC., Luckenbaugh DA., Ameli R., Roiser JP., Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatmentresistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ballard ED., Ionescu DF., Vande Voort JL., et al Increased fear-potentiated startle in major depressive disorder patients with lifetime history of suicide attempt. J Affect Disord. 2014;162:34334–8. doi: 10.1016/j.jad.2014.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lapidus KA., Levitch CF., Perez AM., et al A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76(12):970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.aan het Rot M., Collins KA., Murrough JW., et al Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 122.Murrough JW., Perez AM., Pillemer S., et al Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ibrahim L., Diazgranados N., Franco-Chaves J., et al Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebocontrolled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wan LB., Levitch CF., Perez AM., et al Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Pyschiatry. 2014 [Epub ahead of print] doi: 10.4088/JCP.13m08852. [DOI] [PubMed] [Google Scholar]
- 125.Luckenbaugh DA., Niciu MJ., Ionescu DF., et al Do the dissociative side effects of ketamine mediate its antidepressant effects? J Affect Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zunszain PA., Horowitz MA., Cattaneo A., Lupi MM., Pariante CM. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol Psychiatry. 2013;18:1236–1241. doi: 10.1038/mp.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duman RS. Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci. 2014;16:11–27. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mathews DC., Zarate CA., Jr. Current status of ketamine and related compounds for depression. J Clin Pyschiatry. 2013;74:516–517. doi: 10.4088/JCP.13ac08382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McGirr A., Berlim MT., Bond DJ., Fleck MP., Yatham LN., Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebocontrolled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2014;45(4):693–704. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- 130.Furey ML., Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Furey ML., Drevets WC., Hoffman EM., Frankel E., Speer AM., Zarate CA., Jr. Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry. 2013;70:280–290. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zimmerman M., Posternak M., Friedman M., et al Which factors influence psychiatrists' selection of antidepressants? Am J Psychiatry. 2004;161:1285–1289. doi: 10.1176/appi.ajp.161.7.1285. [DOI] [PubMed] [Google Scholar]


