Abstract
Impulsivity is a pathological hallmark of drug addiction. However, little is known about the neuropsychological underpinnings of this impaired impulsive control network on drug addiction. Twenty two abstinent heroin dependent (HD) subjects and fifteen cognitively normal (CN) subjects participated in this study. Resting-state functional connectivity MRI was employed to measure abnormalities in the intrinsic amygdala functional connectivity (iAFC) network activity and the Barratt Impulsive Scale, 11th version was used to measure impulsivity. Linear regression analysis was applied to detect the neural constructs underlying impulsivity by correlating iAFC network activity with impulsive scores. In the HD group, higher impulsivity scores and significantly enhanced iAFC network activity were found, especially in bilateral thalamus, right insula, and inferior frontal gyrus. Markedly decreased anticorrelated iAFC network activity was seen in the left precuneus, and even switched to positive correlation pattern in right precuneus, relative to the CN group. The iAFC network strengths in the HD group were positively correlated with impulsivity in the right subcallosal gyrus, insula, thalamus and posterior cingulate cortex, and negatively correlated in left fusiform area. In the CN group, the left pre-somamotor area-amygdala connectivity was positively correlated, and right orbital frontal cortex-amygdala and precuneus-amygdala connectivity were negatively correlated with impulsivity scores. Our study demonstrates different constructs of the impulsive network in HD and CN subjects. Altered iAFC network connectivity in HD subjects may contribute to the loss of impulsive control. This further facilitates our understanding of the neural underpinnings of behavior dysfunction in addiction.
Keywords: Heroin dependent, impulsivity, functional connectivity MRI, amygdala network
1. Introduction
Impulsivity is a pathological hallmark of drug addiction and is primarily thought to arise as a result of impaired inhibitory control [17, 18, 32, 34]. Recently, neuroimaging techniques have been widely employed to investigate the neural mechanisms of impulsivity and a set of neural correlates of impulsivity have been identified. These include the ventromedial prefrontal cortex, anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), and subcortical regions, including the amygdala, ventral tegmental area, and nucleus accumbens [2, 14, 19, 23, 37]. Specifically, previous fMRI task-specific studies have demonstrated that significantly lower ability of poor impulse control and impaired response inhibition in performing the response suppressing task in the chronic heroin users and abstinent alcohol-dependent subjects [1, 8]. For example, during a Go/NOGO task, abstinent heroin-dependent patients showed hypoactivity in medial prefrontal cortex and ACC relative to healthy, drug-naïve participants [8, 19], suggesting a sustained negative effect of heroin on the areas involved in executive control function in abstinent addicts. Moreover, it was clearly demonstrated that hypoactivity in the prefrontal cortex, which often operates in control function, and hyperactivity in the impulse system during cue-induced craving in heroin users. These significantly imbalanced neural activities in two interacting, but distinct, neural circuits suggest that the control system was probably hijacked by the impulsive system under the craving state [2, 37]. Although these studies provide important information regarding the brain's structure and functional localization associated with impulsivity, little is known about the behavioral significance of the interconnections among these spatially remote, but functionally localized brain regions using conventional volumetric and functional MRI methods.
It is well-established that the high-order cognitive behavior is determined by the neurocognitive network with a high-degree connectivity pattern among discrete brain regions [25, 28]. Thus, it is necessary to investigate the connectivity pattern of specific regions which can reflect the impulsive system to understand the neural mechanisms of impulsivity. On the one hand, advances in resting-state functional connectivity MRI (R-fMRI) techniques provide a powerful tool to study the construction of the intrinsic, interconnected neural networks and their relation to cognitive or behavioral states in human and non-human primates [4, 35]. Moreover, abnormal pattern in resting-state functional connectivity network in patients with Alzheimer's disease, major depression, generalized anxiety disorder, as well as nicotine addiction have been identified [6, 9, 12, 20]. On the other hand, it is widely accepted that the amygdala, as a hub, is central to the regulation and integration of information processing under cue-elicited drug craving, and is also a crucial component of the impulsivity system, which preferentially responds to small, immediate reward over large, delayed reward [2]. These findings suggest that the detailed, intrinsic amygdala functional connectivity (iAFC) network associated with impulsive systems may characterize the behavioral phenotype of impulsive control and provide additional evidence for the neural mechanisms underlying addiction.
Recently, it was reported that a disrupted iAFC network was found in abstinent heroin users using a R-fMRI approach [21, 22, 33, 38]. These studies suggested that this altered amygdala functional connectivity among brain regions might contribute to the loss of control and impaired inhibitory behaviors, but failed to find direct evidence can reveal the neural substrates of impulsivity. Collectively, these findings lead us to propose the neural network-based hypothesis that the dysfunctional iAFC network is due to chronic exposure to heroin and also should be associated with impaired impulsive control behavior, even under the abstinent state.
In the present study, therefore, we test the hypothesis that 1) The altered iAFC network pattern is associated with impulsive behavior, as measured by the Barratt Impulsiveness Scale (BIS, version 11) [27]; 2) The constructs of the impulsivity-associated network in abstinent heroin dependent (HD) subjects are different in comparison with those of cognitively normal (CN) subjects.
2. Materials and Methods
2.1 Participants
30 abstinent heroin-dependent subjects and 20 healthy volunteers who never used opiates were recruited from Beijing Ankang Hospital (Beijing, China) and the surrounding suburban areas in this study. The experimental procedure was approved by the Beijing Ankang Hospital and the Beijing Institute of Basic Medical Science and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to scan. 8 heroin subjects and 5 healthy subjects were excluded from this study due to motion artifacts (i.e. exceeding more than 1 mm translational movement or more than 1° rotational movement) leaving 22 abstinent heroin subjects and 15 control subjects for the final data analysis.
All participants were male, right-handed and well-matched for age and years of education. Inclusion and exclusion criteria for heroin abusers and control subjects were described previously [8]. Briefly, heroin subjects met DSM-IV criteria for heroin dependence, were abstinent for at least 4 weeks, tested negative for morphine in urine-analysis and human HIV in blood test, had used heroin for at least 2 years, and had normal intelligence. None of the subjects had a history of neurological and psychiatric disease, seizures, head injury and abnormalities demonstrated by an anatomical MRI. Structured interviews by two psychiatrists were conducted with each participant before admission in the study.
2.2 Psychological Instrument
Impulsivity was assessed by self-report instruments of the BIS-11(Chinese revised edition), which contains 30 4-point Likert-type items [27]. Higher scores signify higher impulsivity. All participants completed the questionnaire and the results are presented in Table 1.
Table 1.
Demographic information and behavioral performance
| HD (n=22) | CN (n=15) | P | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (year) | 33.05 | 6.04 | 28.91 | 7.82 | 0.06 |
| Education (years) | 10.86 | 2.40 | 9.83 | 2.94 | 0.26 |
| Duration of heroin use (years) | 6.59 | 3.72 | - | - | - |
| Dosage of heroin use (g/day) | 0.96 | 1.26 | - | - | - |
| Duration of abstinence from heroin (weeks) | 8.05 | 2.51 | - | - | - |
| BIS total score | 66.45 | 10.07 | 59.33 | 6.51 | 0.01 |
Notes: Significant differences were found in BIS total scores between HD group and CN group. Abbreviation: HD: heroin dependent; CN: cognitive normal. SD: standard deviation. p values were obtained by an independent sample two-tailed t test. Unless otherwise indicated, data are presented as mean ± SD.
2.3 MRI Acquisition
Imaging was performed using a whole-body 3T Signa GE scanner with a standard transmits-receive head coil. During the resting-state acquisitions, the study participants were instructed to close their eyes and relax. Axial resting-state fMRI datasets of the whole brain were obtained in 6 minutes with a single-shot gradient echo-echo planar imaging (EPI) pulse sequence. The fMRI imaging parameters were: TE = 25 ms, TR = 2 s, flip angle = 90°, 20 slices, slices thickness = 5 mm, space = 1mm, matrix size 64×64 and field of view 24×24 cm. High resolution spoiled gradient-recalled echo (SPGR) 3D axial images were acquired for anatomical reference. The parameters were: TE/TR of 4.8/10.4ms, flip angle = 15°,140 slices, slice thickness = 1 mm, matrix size of 256×256, and field of view 24×24 cm.
2.4 fMRI data preprocessing
All image data preprocessing and analysis were conducted with Analysis of Functional NeuroImages software (AFNI, http://afni.nimh.nih.gov/afni/), Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/), and Matlab software (Matlab 7.0, MathWorks, Natick, MA, http://www.mathworks.com/). For the preprocessing of fMRI images, the first 5 data points of resting-state datasets were discarded in order to eliminate the magnetization nonequilibrium and obtain a stable state. This was followed by physiological motion correction, volume registration, and motion correction. Possible contamination from the signals existing in white matter, cerebrospinal fluid, and six motion vectors were regressed out. A band-pass filter was then applied to keep only low frequency fluctuations between 0.015 Hz and 0.1 Hz.
2.5 Intrinsic amygdala functional connectivity network
Firstly, the left and right amygdales were drawn manually on T1-weighted 3D SPGR images, according to the literature [11, 31]. The specific procedures used to locate the amygdala region and select the amygdala voxels from the EPI dataset are described below.
The most posterior boundary of the amygdala adjoins the anterior alveus of the hippocampus and the temporal horn of the lateral ventricle in the sagittal plane. The anterior boundary follows the natural boundaries of the gray matter in the anterior direction in the axial plane. The superior and the inferior boundaries were defined coronally as the ventral horn of the subarachnoid space and the most dorsal finger of the white-matter tract under the horn of the subarachnoid space, respectively. The lateral and the mesial boundaries were defined coronally at 2 mm from the surrounding white matter and from the subarachnoid space, respectively. A mouse-controlled cursor traced relevant coronal, sagittal, and axial slices. Boundaries were displayed in real time on these orthogonal MRI slices with AFNI software. The same coordinates in SPGR and functional EPI images were determined, and amygdala masks were applied to the latter. The time course of the amygdala was extracted from the functional EPI images. The spatial resolutions in SPGR (1.0-mm) and EPI images (3.75 mm) were different, therefore, only those voxels in EPI images that contained at least 50% of amygdala voxels masked on the 3D SPGR images were included in the voxel time course analysis [31].
Then, the averaged time course within the amygdala mask, as the seed time course, was correlated to the time courses of all brain voxels using Pearson cross-correlation. Next, we applied the Pearson correlation coefficients (r) to a Fisher's z Transform Analysis, which yielded variants of approximately normal distribution [m = 0.5ln(1+r)/(1-r)] [39]. Finally, the data was spatially normalized to the Talairach template image, resampled to 2mm isotropic voxels, and smoothed with a Gaussian kernel (6mm FWHM) using AFNI software.
2.6 Statistical analysis
2.6.1 Behavioral data
The statistical analyses were conducted with SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). An independent-sample t-test was employed to analyze the difference between demographic data and BIS- 11 scores for group comparison. The results were considered significant at p< 0.05. The data was presented as mean ± standard deviation.
2.6.2 Participant-level analysis for the iAFC network patterns
After obtaining the iAFC map for each individual subject, the individual iAFC maps were grouped together for the CN and heroin groups. The iAFC network for each group was generated by applying a voxelwise one sample t-test within each group of subjects against a null hypothesis of no connectivity (p < 0.01, corrected for multiple comparisons with AlphaSim program).
2.6.3 Group-level analysis for the iAFC network pattern
To find group differences, we selected 30 regions of interest (ROIs) based on our findings and previous neuroimaging studies of patients with heroin addiction that have revealed abnormal functions in regions associated with cue-induced craving processing [21, 22, 37, 38]. These areas include bilateral medial frontal cortex (MeFG), OFC, dorsolateral prefrontal cortex (DLPFC), middle frontal cortex, inferior frontal gyrus (IFG), middle temporal cortex (MTG), posterior cingulate cortex (PCC), inferior parietal cortex (IPC), precuneus, insula, hippocampus, thalamus, caudate, and putamen. Distinctive ROI in our data falling within the iAFC network were comprised of 4mm-radius spheres and centered on the public foci [21, 22, 37]. Then, the averaged m values of all voxels in each ROI were extracted from the individual iAFC map and the strength of changes in the iAFC network was quantitatively analyzed using SPSS16.0. A two-sample t-test and a small volume correction analysis were performed with the statistical threshold p< 0.05.
2.6.4 Behavioral significance of the iAFC network
To determine the behavioral significance of the iAFC network alteration, a linear regression analysis was conducted between the voxelwise iAFC strength (m value) and the BIS total scores in the heroin subjects and the CN subjects separately.
3. Results
3.1 Subject characterization
Demographic information and clinical evaluations are shown in Table 1. No significant difference in age and education was noted between HD and CN groups. BIS-11 total scores in HD group were significantly higher than those in the CN group (p< 0.05).
3.2 The iAFC network patterns for CN group and heroin group
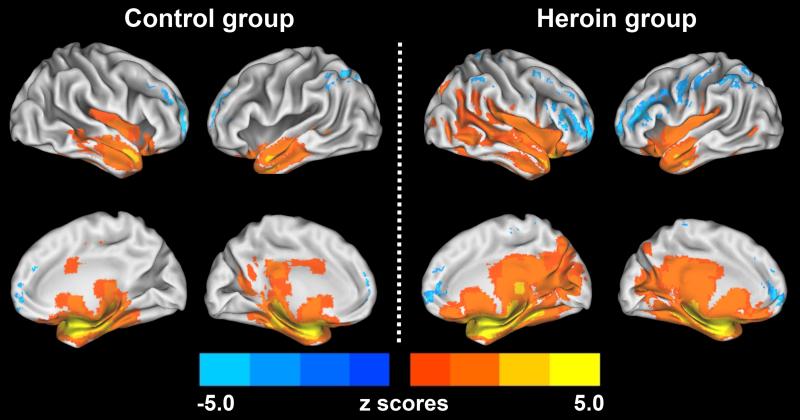
The functional pattern of the iAFC network for CN group was observed in the prefrontal and limbic systems. The positive iAFC network included the regions of bilateral amygdala, MTG (BA21), right OFC (BA25), superior temporal gyrus (STG, BA38), and ACC (BA32). The anticorrelated iAFC network (negative correlation) includes the regions of bilateral precuneus (BA7), IPC (BA40), MeFG (BA10), DLPFC (BA9,46), IFG (BA44), right fusiform area (FFA, BA19), left lingual gyrus (BA19) and cerebellum. These results are described in Figure 1(left) and Appendices A. Similarly, iAFC network pattern for the HD group has increased positive correlation with the amygdala, especially in the regions of the temporal system and subcortical regions, including the bilateral OFC, caudate, lentiform nucleus, thalamus, insula (BA13), STG (BA38), MTG(BA21), FFA(BA37), posterior cingulate cortex (PCC, BA23), and lingual gyrus; and enhanced anticorrelation with the amygdala, especially in the prefrontal system, parietal system, and occipital system, including the regions of bilateral IPC (BA40), DLPFC (BA9,46), MeFG (BA10), IFG(BA45/47), as shown in Figure 1(right) and Appendices B.
Figure 1. The patterns of the iAFC network for CN group and HD group subjects.
The results illustrated that more brain regions are involved in the iAFC network in the heroin group (right) compared to the control group (left), which are mainly located in the prefrontal system, the parietal-occipital system, temporal lobule and subcortical regions. Blue color is anticorrelation (negative), bright color is positive correlation. The pattern was produced by a one sample t-test (p < 0.01, corrected). The color bar is presented with z score. Top image: lateral view; Bottom image: medial view.
3.3 Difference of the iAFC network pattern between HD and CN groups
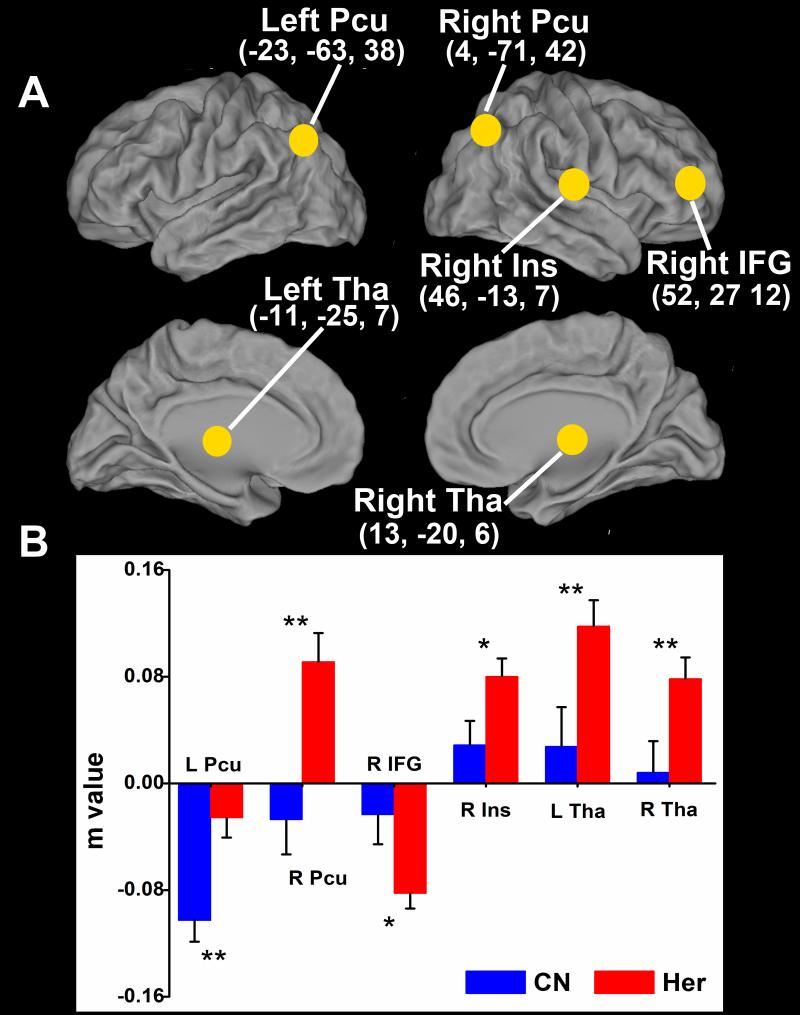
Based on the group t-test comparison between the heroin users and control subjects, a significant alternation of the iAFC network was observed in several brain regions. The positive iAFC network was significantly enhanced in the bilateral thalamus and right insula (BA13). The anticorrelated iAFC network was significantly increased in the right IFG, and significantly decreased in the left precuneus (BA7). In addition the correlation changed from negative to positive in the right precuneus (BA7), as illustrated in Figure 2.
Figure 2. Significantly altered iAFC network in heroin group compared to control group.
The results showed significantly decreased anticorrelation in the left precuneus (close to zero), significantly increased anticorrelation in the right inferior frontal gyrus and significantly increased correlation in the right insula and bilateral thalamus. A reversed correlation pattern in the iAFC network was also found in right precuneus. Significant level was set at: *, p < 0.05; **, p < 0.01. Error bars are presented with standard deviation. Abbreviation: Pcu, precuneus; IFG, inferior frontal gyrus; Ins, insula; Tha, thalamus.
3.4 Correlation of the iAFC network with impulsivity scores
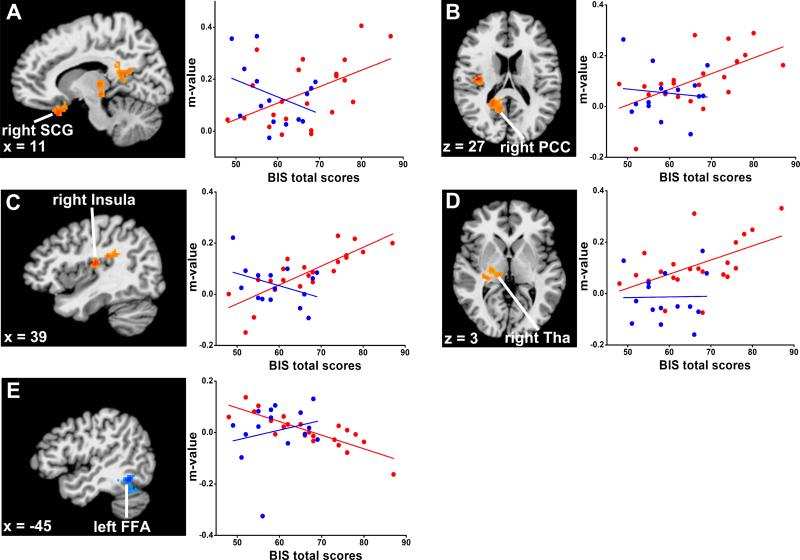
Statistical maps of the linear regression analysis between iAFC network strength (m value) and BIS-11 total scores for impulsivity in the heroin group showed positive correlations in the right subcallosal gyrus (BA25/11), insula (BA13), PCC (BA29) and thalamus. Negative correlations were seen in the left FFA (BA37). No significant correlations in these regions were found in comparison subjects, as illustrated in Figure 3 and Table 2 (whole brain correction with AlphaSim, p<0.01, cluster size > 1180 mm3). The scatter plots in Figure 3 further characterize the quantitative information in the five regions where the iAFC network activity was linearly correlated with impulsiveness scores.
Figure 3. Robust linear regression analysis between the iAFC strength and BIS total scores in distinct regions in HD group.
The results showed a strong positive correlation between the iAFC network strength and the BIS total scores in the right subcallosal gyrus, insula, posterior cingulate cortex (PCC) and thalamus, and robust negative correlation in left fusiform area (FFA) in the HD group (red color), but not in CN group (blue color). A) right subcallosal gyrus: regression coefficient r2 = 0.22, p < 0.02 (HD group), r2 = 0.06, p < 0.19 (CN group); B) right PCC: r2 = 0.26, p < 0.01 (HD group), r2 = 0.003, p < 0.95 (CN group); C) right insula: r2 = 0.64, p < 4.42E-6 (HD group), r2 = 0.10, p < 0.13 (CN group); D) right thalamus (Tha): r2 = 0.33, p < 0.003 (HD group), r2 = 0.06, p < 0.68 (CN group); E) left FFA: r2 = 0.42, p <1.43E-6 (HD group), r2 = 0.06, p < 0.68 (CN group). Each point represents one participant. Images and coordinates are in Talaraich space.
Table 2.
Behavioral significance of intrinsic amygdala functional connectivity (iAFC) network
| Side | Brain region | BA | Cluster Size (mm3) | Talairach coordinates (LPI) |
z score | ||
|---|---|---|---|---|---|---|---|
| x | y | Z | |||||
| Heroin group | |||||||
| Insula | R | 13 | 1992 | 37 | −17 | 16 | 4.61 |
| PCC | R | 29 | 1944 | 13 | −47 | 15 | 3.37 |
| Subcollosal gyrus | R | 25/11 | 1640 | 8 | 14 | −17 | 4.02 |
| Thalamus | R | 1592 | 11 | −22 | 4 | 3.48 | |
| Fusiform area | L | 37 | 2320 | −45 | −51 | −12 | −4.40 |
| Control group | |||||||
| Pre-SMA | L | 6 | 1480 | −21 | −15 | 50 | 5.20 |
| OFC | R | 11 | 1440 | 7 | −69 | 46 | −4.23 |
| Precuneus | R | 7 | 1376 | 19 | 49 | −12 | −5.63 |
Notes: Linear regression analysis between amygdala network strength and BIS total scores across heroin subjects and control subjects, respectively, significant level was set at p < 0.01 with cluster size > 1180 mm3. Abbreviation: BA, Brodmann's area; x,y,z, coordinates of peak locations (highest z-values) in the Talairach space; r, regression coefficient; R, right; L, left; PCC, posterior cingulate cortex; Pre-SMA, pre-somamotor area; OFC, orbital frontal cortex.
Comparison subjects showed a strong positive correlation between iAFC network strength and BIS total scores in the left presomamotor area (pre-SMA, BA6). A negative correlation was seen in the right OFC (BA11) and right precuneus (BA7). No significant correlation in these regions was seen in the heroin subjects, as described in Figure 4 and Table 2 (whole brain correction with AlphaSim, p<0.01, cluster size > 1180 mm3).
Figure 4. Robust linear regression analysis between the iAFC strength and BIS total scores in distinct regions in CN group.
The result showed a strong positive correlation between the iAFC network strength and the BIS total scores in the left pre-SMA, negative correlation in right OFC and right precuneus in the CN group (blue color), but not in the HD group (red color). A) left pre-SMA: regression coefficient r2 = 0.42, p < 0.005 (CN group), r2 = 0.04, p < 0.7(HD group); B) right Precuneus: r2 = 0.66, p < 0.0001 (CN group), r2 = 0.0001, p < 0.33 (HD group); C) right OFC: r2 = 0.57, p < 0.0007 (CN group), r2 = 0.01, p < 0.38 (HD group). Each point represents one participant. Images and coordinates are in the Talaraich space.
4. Discussion
In the current study, we demonstrated the network-based hypothesis that the sustained, hyperactive iAFC network in abstinent HD subjects was associated with high impulsivity. Further, the impulsivity-related regions within the iAFC network were different between abstinent HD subjects and CN subjects. These rewired constructs of iAFC network suggest that the hyperconnection of the iAFC network may play a fundamental role in the high impulsivity in abstinent heroin addicts.
4.1 Altered iAFC network pattern for HD group compared to CN group
In the amygdala connectivity map, the brain regions within the positive iAFC network are largely located in the limbic and striatum systems, and include the bilateral caudate, putamen, nucleus accumbens, and other subcortical regions. Brain regions within the anticorrelated iAFC network are oriented in the prefrontal and parietal-occipital systems, as illustrated in Figure 1. This pattern of the iAFC network during the resting condition is consistent with other studies [6, 30, 31]. Moreover, there is a significant increase in iAFC network strength in both the positive and anticorrelated iAFC networks in HD subjects, especially in the bilateral thalamus, right insula and IFG. Similarly, a remarkably reduced anticorrelated iAFC network in the HD subjects was found in the left precuneus, and a switch from a negative to positive correlation pattern was seen in the right precuneus, as illustrated in Figure 2. These regions with altered connectivities to amygdala in abstinent HD subjects were involved in drug-induced craving, reward, and response inhibition [7, 8, 16, 26]. In addition, dysfunctional connectivity in chronic heroin users was reported in the prefrontal cortex, ACC, ventral striatum, insula, and amygdala by employing a graph theory analysis method. This disconnection may contribute to the reduced self-control, impaired inhibitory function, and deficits in stress regulation in chronic heroin users [21]. More recently, abnormal functional connectivity, including amygdala-OFC connection in chronic heroin users with methadone treatment was identified and supports the view that dissociated functional connectivity is related to weakened cognitive control in the addictive state [22]. Taken together, this altered iAFC network pattern suggests that a functional rewiring of brain networks exists in abstinent heroin users.
4.2 Enhanced iAFC network activity is correlated with impulsivity
Unlike task-dependent fMRI studies where brain responses are dependent on specific cognitive tasks, R-fMRI studies can identify the constructs of brain networks where the changes in distributed network connectivity are associated with behavioral states under task-free conditions. In the current study, we performed a linear regression analysis that combined impulsiveness scores with iAFC network strength and identified the construct of impulsivity at the system level. Specifically, the positively correlated regions included the right subcallosal gyrus, insula, thalamus and PCC in the HD group, and the left pre-SMA in control group subjects. This is illustrated in Figure 3 A-D and Figure 4 A. It indicates that the stronger the connectivity between these regions with the amygdala, the higher the impulsiveness scores, and the lower the impulsive control.
Converging evidence from neuroimaging studies has shown that these regions form part of a system representing internal state-modulated behavior, including fear-memory, self-related mental representations, negative feedback processing, and also are critical for efficient execution of the corresponding actions [5, 13, 36]. In addition, it has also been suggested that these regions are involved in steep time discounting or value discounting regulation, and are preferentially activated by making decisions involved in immediate action over delayed rewards. This can reflect impulsive behavior [10, 24, 29].Moreover, this value discounting reward can be simultaneously reduced by episodic future thinking [29]. Collectively, these studies consistently support the view that the functional integration of the impulsivity-related network constituted by above noted regions is associated with internal state regulation.
Interestingly, we also found the negatively correlated brain regions of the impulsivity-associated iAFC network including the left FFA in the HD group, and right OFC and precuneus in the control group, as illustrated in Figure 3 E and Figure 4 B and C. This indicates that the higher the impulsivity, the weaker the connection strength of these regions to the amygdala. These results suggest the function of these negatively correlated regions may be suppressed when the subject chooses immediate over delayed long-term larger reward underlying the specific goal-directed behavior. In short, these regions represent the subcomponents within the iAFC network correlated with impulsivity and also highlight the critical contribution to the impulsive behavior at the system level that may increase the risk for addiction.
In addition, we have found a different construct of impulsivity-associated networks in abstinent HD subjects and CN subjects. Our finding is consistent with a recent report that the activated BOLD signal was negatively correlated with high impulsivity only in alcoholics but not in control subjects in the ventral striatum and anterior cingulate during gain anticipation elicited by a monetary incentive delay task [3]. This shifting impulsivity-associated network architecture and neurocircuitry in addicts may result from persistent neuronal changes and long-lasting neuroadaptation after exposure to drugs of abuse [15]. Therefore, it is reasonable to propose the neural mechanism of impulsivity in abstinent HD subjects on the network-based level: hyperconnection of iAFC network induced by the altered internal state activates the impulsivity-associated network activity, and leads addicted subjects with high impulsiveness to choose immediate, short reward actions despite a long-term negative consequence. This finding provides new insights into the neural mechanisms of drug addiction.
Our study is not without limitations. First, the self-report questionnaire may not have reported their inner impulsivity honestly. It is necessary to detect the neural correlates of the distinctive behavioral phenotype at the network level by combining the questionnaire with the specific, complex cognitive task associated with impulsive control. Second, we predefined the bilateral amygdala as a seed region and proved the behavioral significance of the iAFC network activity, which may not reflect the changes of the whole brain functional network. Future studies are needed to investigate the functional role of the large-scale, distributed, functional neural network including whole-brain regions in addicts. Third, this is the cross-section of a larger study without drug treatment. Longitudinal studies with detoxification treatment are needed to evaluate the brain functional network alteration and might provide a new strategy for preventing addiction.
5. Conclusion
The findings of this study suggest that the observed hyperconnection of the iAFC network in abstinent heroin users make a critical contribution to higher impulsivity and represent the pathological damage that may underlie the neurobiological mechanism for addictive behavior – loss of impulsive control. This further facilitates our understanding of the neural underpinnings of behavioral dysfunction associated with addiction. It is also suggested that the network-based R-fMRI approach could be applied to evaluate the consequence of heroin abuse and the related alteration of brain circuitry at a system level.
Acknowledgment
Dr. Zhijun Zhang and Dr. Yang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Xie and the other coauthors had full access to all the data in the study. The authors thank Dr. Gao-hong Wu for the data analysis, Mr. Douglas Ward for the statistical analysis.
This work was supported by National Natural Science Foundation of China (30825014, 30971016, ZJZ), Chinese Ministry of Science and Technology grants: 2003CB51540 (ZY), and China Scholarship Council CSC20070320 (CX).
Appendices
A. Intrinsic amygdala functional connectivity (iAFC) network pattern for control subjects
| Brain region | Side | BA | Cluster Size (mm3) | Talairach coordinates |
z score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive iAFC network | |||||||
| Amy/PHG | R | 60368 | 25 | 1 | −14 | 6.55 | |
| Amy/PHG | L | −18 | −4 | −14 | 6.30 | ||
| OFC | R | 25 | 13 | 13 | −17 | 3.97 | |
| STG | R | 38 | 40 | 15 | −23 | 4.46 | |
| ACC | R | 32 | 4 | 32 | −6 | 3.86 | |
| MTG | L | 21 | −47 | −4 | −21 | 4.52 | |
| MTG | R | 21 | 47 | −1 | −19 | 3.09 | |
| Anticorrelated iAFC network | |||||||
| Precuneus | L | 7 | 103360 | −23 | −63 | 38 | −5.06 |
| Precuneus | R | 7 | 4 | −71 | 42 | −3.26 | |
| IPC | L | 40 | −35 | −52 | 39 | −4.08 | |
| MeFG | R | 10 | 15 | 57 | 0 | −3.50 | |
| MeFG | L | 10 | −8 | 61 | 2 | −2.96 | |
| DLPFC | R | 9,46 | 40 | 34 | 26 | −3.98 | |
| DLPFC | L | 9,46 | −44 | 33 | 15 | −3.62 | |
| IFG | L | 44 | −54 | 9 | 20 | −3.92 | |
| IFG | R | 44 | 53 | 9 | 22 | −3.29 | |
| Lingual gyrus | L | 19 | 3416 | −10 | −86 | −14 | −3.41 |
| IPC | R | 40 | 3136 | 47 | −39 | 42 | −3.85 |
| FFA | R | 19 | 1232 | 41 | −75 | −14 | −3.11 |
| Cerebellum | L | 1792 | −35 | −63 | −26 | −3.22 | |
Notes: The results were obtained with one sample t-test, p < 0.01, cluster size > 1180 mm3. Abbreviation: BA, Brodmann's area; x,y,z, coordinates of peak locations (highest z-values) in the Talairach space. R, right; L, left; Amy, amygdala; PHG, parahippocampus gyrus; OFC, orbital frontal cortex; STG, superior temporal gyrus; ACC, anterior cingulate cortex; MTG, middle temporal gyrus; IPC, inferior parietal cortex; MeFG, medial frontal gyrus; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; FFA, fusiform area.
B. Intrinsic amygdala functional connectivity (iAFC) network pattern for heroin subjects
| Brain region | Side | BA | Cluster Size (mm3) | Talairach coordinates (LPI) |
z score | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Positive iAFC network | |||||||
| Amy/PHG | R | 153424 | 25 | −3 | −18 | 7.19 | |
| Amy/PHG | L | −22 | −5 | −15 | 6.99 | ||
| OFC | R | 25 | 13 | 13 | −17 | 5.47 | |
| OFC | L | 11 | −3 | 38 | −13 | 4.68 | |
| Caudate | R | 10 | 14 | −2 | 3.62 | ||
| Caudate | L | −7 | 11 | −2 | 4.11 | ||
| Thalamus | R | 13 | −20 | 6 | 4.48 | ||
| Thalamus | L | −11 | −25 | 7 | 4.30 | ||
| Lentiform nucleus | R | 23 | 0 | −5 | 4.30 | ||
| Lentiform Nucleus | L | −28 | 0 | −2 | 4.62 | ||
| Insula | R | 13 | 46 | −13 | 7 | 4.38 | |
| Insula | L | 13 | −35 | −13 | 10 | 4.18 | |
| STG | R | 38 | 34 | 17 | −27 | 5.82 | |
| STG | L | 38 | −31 | 16 | −23 | 4.99 | |
| MTG | R | 21 | 47 | −1 | −19 | 4.50 | |
| MTG | L | 21 | −47 | −4 | −21 | 5.33 | |
| FFA | R | 37 | 26 | −50 | −9 | 3.82 | |
| FFA | L | 37 | −27 | −41 | −14 | 4.36 | |
| PCC | R | 23 | 6 | −40 | 22 | 4.00 | |
| PCC | L | 23 | −3 | −40 | 23 | 3.49 | |
| Lingual Gyrus | R | 19 | 22 | −61 | 2 | 5.20 | |
| Lingual Gyrus | L | 18 | −19 | −55 | 2 | 4.18 | |
| Anticorrelated iAFC network | |||||||
| IPC | L | 40 | 212824 | −45 | −47 | 42 | −5.15 |
| IPC | R | 40 | 47 | −39 | 42 | −4.88 | |
| DLPFC | R | 9,46 | 26 | 32 | 34 | −4.45 | |
| DLPFC | L | 9.46 | −47 | 21 | 24 | −4.27 | |
| MeFG | R | 10 | 15 | 57 | 0 | −4.88 | |
| MeFG | L | 10 | −8 | 61 | 2 | −4.84 | |
| IFG | R | 47 | 52 | 27 | 12 | −4.17 | |
| IFG | L | 45 | −50 | 20 | 16 | −4.51 | |
Notes: The results were obtained with one sample t-test, p < 0.01, cluster size > 1180 mm3. Abbreviation: BA, Brodmann's area; x,y,z, coordinates of peak locations (highest z-values) in the Talairach space. R, right; L, left; Amy, amygdala; PHG, parahippocampus gyrus; OFC, orbital frontal cortex; STG, superior temporal gyrus; ACC, anterior cingulate cortex; MTG, middle temporal gyrus; FFA, fusiform area; PCC, posterior cingulate cortex; IPC, inferior parietal cortex; DLPFC, dorsolateral prefrontal cortex; MeFG, medial frontal gyrus; IFG, inferior frontal gyrus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Conflict of Interest: No authors of this paper have reported any possible conflict of interests.
Reference
- 1.Adinoff B, Rilling LM, Williams MJ, Schreffler E, Schepis TS, Rosvall T, Rao U. Impulsivity, neural deficits, and the addictions: the “oops” factor in relapse. J Addict Dis. 2007;26(Suppl 1):25–39. doi: 10.1300/J069v26S01_04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 3.Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral Striatal Activation During Reward Anticipation Correlates with Impulsivity in Alcoholics. Biol Psychiat. 2009;66(8):734–42. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 4.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 5.Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62(6):642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- 7.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 8.Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L, Ming F, Yang Z. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett. 2008;438(3):322–6. doi: 10.1016/j.neulet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. Journal of Neuroscience. 2006;26(51):13213–7. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honeycutt NA, Smith PD, Aylward E, Li Q, Chan M, Barta PE, Pearlson GD. Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Res. 1998;83(2):85–94. doi: 10.1016/s0925-4927(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 12.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66(4):431–41. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58(4):334–41. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 14.King JA, Tenney J, Rossi V, Colamussi L, Burdick S. Neural substrates underlying impulsivity. Ann N Y Acad Sci. 2003;1008:160–9. doi: 10.1196/annals.1301.017. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nature Neuroscience. 2005;8(11):1442–4. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 16.Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O'Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165(3):390–4. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology (Berl) 2009;207(1):163–72. doi: 10.1007/s00213-009-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee TM, Pau CW. Impulse control differences between abstinent heroin users and matched controls. Brain Inj. 2002;16(10):885–9. doi: 10.1080/02699050210128915. [DOI] [PubMed] [Google Scholar]
- 19.Lee TM, Zhou WH, Luo XJ, Yuen KS, Ruan XZ, Weng XC. Neural activity associated with cognitive regulation in heroin users: A fMRI study. Neurosci Lett. 2005;382(3):211–6. doi: 10.1016/j.neulet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 20.Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG. Alzheimer Disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225(1):253–9. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, Zhang Y, Wang W, Wang Y, Li Q, Zhao L, Lu L, von Deneen KM, Liu Y, Gold MS. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009;460(1):72–7. doi: 10.1016/j.neulet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 22.Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49(1):738–44. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum Brain Mapp. 2009;30(4):1188–95. doi: 10.1002/hbm.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 25.Mesulam M. Defining neurocognitive networks in the BOLD new world of computed connectivity. Neuron. 2009;62(1):1–3. doi: 10.1016/j.neuron.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9(2):148–58. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 29.Peters J, Buchel C. Episodic Future Thinking Reduces Reward Delay Discounting through an Enhancement of Prefrontal-Mediotemporal Interactions. Neuron. 2010;66(1):138–48. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp. 2010;31(2):173–84. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swann AC, Bjork JM, Moeller FG, Dougherty DM. Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry. 2002;51(12):988–94. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- 33.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(Pt 7):2098–114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32(5):950–66. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 35.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 36.Yalachkov Y, Kaiser J, Naumer MJ. Brain Regions Related to Tool Use and Action Knowledge Reflect Nicotine Dependence. Journal of Neuroscience. 2009;29(15):4922–9. doi: 10.1523/JNEUROSCI.4891-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z, Xie J, Shao YC, Xie CM, Fu LP, Li DJ, Fan M, Ma L, Li SJ. Dynamic neural responses to cue-reactivity paradigms in heroin-dependent users: an fMRI study. Hum Brain Mapp. 2009;30(3):766–75. doi: 10.1002/hbm.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan K, Qin W, Dong M, Liu J, Liu P, Zhang Y, Sun J, Wang W, Wang Y, Li Q, Yang W, Tian J. Combining spatial and temporal information to explore resting-state networks changes in abstinent heroin-dependent individuals. Neurosci Lett. 2010;475(1):20–4. doi: 10.1016/j.neulet.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 39.Zar J. Biostatistical Analysis. 3rd ed. Prentice-Hall, Inc.; Upper Saddle River, NJ: 1996. [Google Scholar]