Abstract
Objective
Exercise has widely documented cardioprotective effects, but the mechanisms underlying these effects are not entirely known. Previously, we demonstrated that aerobic but not strength training lowered resting heart rate and increased cardiac vagal regulation, changes that were reversed by sedentary deconditioning. Here, we focus on the sympathetic nervous system and test whether aerobic training lowers levels of cardiovascular sympathetic activity in rest and that deconditioning would reverse this effect.
Methods
We conducted a randomized controlled trial contrasting the effects of aerobic (A) versus strength (S) training on indices of cardiac (preejection period, or PEP) and vascular (low-frequency blood pressure variability, or LF BPV) sympathetic regulation in 149 young, healthy, and sedentary adults. Participants were studied before and after conditioning, as well as after 4 weeks of sedentary deconditioning.
Results
As previously reported, aerobic capacity increased in response to conditioning and decreased after deconditioning in the aerobic, but not the strength, training group. Contrary to prediction, there was no differential effect of training on either PEP (A: mean [SD] −0.83 [7.8] milliseconds versus S: 1.47 [6.69] milliseconds) or LF BPV (A: mean [SD] −0.09 [0.93] ln mm Hg2 versus S: 0.06 [0.79] ln mm Hg 2) (both p values > .05).
Conclusions
These findings, from a large randomized controlled trial using an intent-to-treat design, show that moderate aerobic exercise training has no effect on resting state cardiovascular indices of PEP and LF BPV. These results indicate that in healthy, young adults, the cardioprotective effects of exercise training are unlikely to be mediated by changes in resting sympathetic activity.
Trial Registration
Clinicaltrials.gov identifier: NCT00358137.
Keywords: sympathetic nervous system, preejection period, low-frequency blood pressure variability, aerobic exercise, randomized controlled trial
INTRODUCTION
The role of the sympathetic nervous system (SNS) in heart disease is well established (1). An increase in sympathetic tone is a component of the pathophysiological processes of hypertension (2,3), cardiac arrhythmias (4), and heart failure (5). In contrast, physical activity has widely documented cardioprotective effects (6). Some biological mechanisms responsible for these positive effects are well understood, such as lessened metabolic demands on myocardium and increased electrical stability (7), both of which are influenced by the SNS. This suggests that one mechanism through which exercise exerts its cardioprotective effect might be through diminished SNS activity.
We have demonstrated that a 12-week program of aerobic training, in contrast to strength training, elevated cardiac parasympathetic modulation, measured as high-frequency R-R interval variability, in a sample of healthy young adults (8). Moreover, we showed that 4 weeks of sedentary deconditioning was sufficient to completely reverse these effects. These findings are consistent with many other studies suggesting that aerobic exercise enhances cardiac parasympathetic regulation (9–12). However, evidence also suggests that increased physical activity has effects on SNS activity. For example, Bertagnolli et al. (13) showed that moderate exercise training reduced cardiac norepinephrine (NE) concentrations in rats with hypertension, and DiCarlo et. al. (14) showed that daily spontaneous running decreased low-frequency (LF) blood pressure variability (BPV) in hypertensive rats.
Studies in human subjects have produced conflicting results. Many studies have shown that elevated resting sympathetic tone in cardiovascular disease (2,3) can be reversed by physical activity. For example, exercise training in people with congestive heart failure led to a significant decrease in muscle sympathetic nerve activity (MSNA) during rest (15,16). Similar results have also been observed in patients after acute myocardial infarction (17). However, most studies have shown no effect on MSNA in healthy participants (18). One meta-analysis showed reduced serum-NE concentrations in response to exercise training, suggestive of reduced SNS activity (19). Few large randomized trials of the effect of aerobic exercise training on SNS function in healthy participants have been conducted, raising the question about whether these benefits apply to the general population.
In this study, we examined the effect of aerobic versus strength training on SNS function measured by impedance cardiography (ICG) and LF (0.04–0.15 Hz) BPV. ICG is a noninvasive method of measuring cardiac hemodynamic indices and systolic time intervals. Among these, preejection period (PEP) is often used to assess myocardial β-adrenergic activity (20–22). PEP is defined as the time interval between ventricular depolarization and the opening of the aortic valve. Increases in sympathetically driven myocardial contractility result in decreases in PEP. Numerous studies support PEP as an index of cardiac sympathetic drive. For example, Mezzacappa et al. (23) demonstrated that administration of epinephrine in healthy participants significantly decreased PEP compared with placebo. Blockade of β-adrenergic receptors leads to an increase in PEP (24), further validating it as a noninvasive index of cardiac sympathetic tone. Other studies show similar results (25,26).
Some evidence suggests that LF BPV reflects vascular sympathetic tone (27–30). LF oscillations in blood pressure (BP) exist in the absence of a functional baroreflex loop (31,32), suggesting a central sympathetic origin for LF variability. Cevese et al. (31) showed that in chloralose-anesthesized dogs, BPV less than 0.20 Hz was detectable in the iliac artery even after mechanical isolation from the rest of the circulation. The only source of these oscillations, they concluded, must be the sympathetically innervated vessel itself. Stafford et al. (30) demonstrated that in healthy volunteers, LF systolic BPV increased during nitroprusside-induced hypotension and decreased during NE-induced hypertension, reflecting central SNS output. Also, significant increases in LF BPV amplitude in response to ortho-static challenge have been observed (33,34). In this article, we tested the hypothesis that in contrast to strength training, aerobic training would reduce resting cardiovascular sympathetic indices.
METHODS
Study Design
The study was a randomized controlled trial of aerobic versus strength training on cardiovascular autonomic regulation. All participants provided informed consent. The institutional review boards of Columbia University Medical Center and St. John’s University approved this study.
Previously, we reported findings from this trial on the effects of aerobic training on resting levels of heart rate and cardiac parasympathetic modulation (35). Because that article provided a comprehensive account of the research methods, below we present a less detailed description.
Study Participants
Study participants were healthy, sedentary young adults aged 18 to 45 years. Participants were eligible if they did not exercise regularly or exceed American Heart Association standards for average fitness (VO2max ≤ 43 and 37 ml kg−1 min−1 for men and women, respectively, established by cardio-pulmonary exercise testing). One hundred forty-nine participants met enrollment criteria and were randomized to either the aerobic (n = 74) or strength training (n = 75) group. Participants received a 6-month membership in a fitness facility and $300 for participation in the study. Data collection began in December 1998 and ended in January 2003.
Experimental Protocol
Both training programs were 12 weeks in length. Before training, all participants met individually with a trainer to review their exercise regimens. After that, they exercised on their own, 3 to 4 times per week, in designated facilities. They were permitted to construct individualized exercise programs so long as they met the criteria below. Adherence to training programs was documented by weekly logs and computerized attendance records. Participants were tested before training, after completion of training, and again after 4 weeks of sedentary deconditioning during which they were to abstain completely from any form of exercise. Data were collected during 10-minute seated and supine resting periods as part of a psychophysiology protocol (findings reported elsewhere) (8). Data collection staff were blind to training group assignment.
Conditioning Programs
Aerobic Conditioning
Participants chose from a series of activities, for example, cycling on a stationary ergometer, running on a treadmill, or climbing on a Stairmaster. Participants were instructed to exercise at 70% of their maximal heart rate (220-age for men, 226-age for women). They were given an initial goal of at least 20-minute aerobic exercise per session and increased duration gradually for 2 to 3 weeks, up to 45 to 60 minutes.
Strength Training
At the initial session, participants established a level of effort that permitted them to complete three sets of 10 repetitions for each of the following exercises: bench presses, shoulder presses, quadriceps extensions, biceps curl, lateral pulls, triceps presses, and hamstring curls exercise. Participants were instructed to increase the weight loads for these exercises by 5 lb every 2 weeks.
Measurement of Physiological Signals
Electrocardiogram and ICG
Analog electrocardiogram (ECG) signals were digitized at 500 Hz by a National Instruments 16 bit A/D conversion board and passed to a microcomputer. ICG data were collected using the Minnesota 304B system with no gain. The impedance signal (Z0) and the first derivative of pulsatile impedance acquisition (dZ/dt) were digitized at 250 and 500 Hz, respectively, by the NI A/D board and collected by the microcomputer. MindWare software (MindWare Technologies LTD, Gahanna, OH) was used to analyze ECG and ICG signals in 60-second epochs. PEP was measured as the time interval between the Q wave of the ECG and the B point of the dZ/dt wave. Errors in marking of R waves in the ECG signal and B, Z, and X points in the dZ/dt waveform were corrected by visual inspection.
Low-Frequency BPV
The beat-to-beat BP waveform was obtained using a Finapres noninvasive BP monitor. The analog BP waveform was captured by the NI A/D board and was sampled at 500 samples/s. Systolic and diastolic values for each cardiac cycle were identified using custom-written software resulting in a BP time series. Mean BP and spectral power in the LF (0.04–0.15 Hz) band of the BP power spectrum for both systolic BP and diastolic BP were computed from these time series. Because the servo adjustment of the Finapres monitor was enabled during the last minute of each 300-second period, spectra were calculated on 240-second epochs using an interval method for computing Fourier transforms similar to that described by DeBoer et al. (36). Before computing Fourier transforms, the mean of the BP series was subtracted from each value in the series, and the series was then filtered using a Hanning window (37) and the power, that is, variance (in mm Hg 2), over the LF band was summed. Estimates of spectral power were adjusted to account for attenuation produced by this filter (37).
Statistical Analysis
Models of systolic LF BPV, diastolic LF BPV, and PEP were fitted for the seated and supine baselines separately. A repeated-measures analysis was conducted to examine relationships in the prediction of these outcomes by group assignment and session, while controlling for sex and age. To model the correlation among repeated measures, an unstructured covariance matrix was used. This matrix was selected according to the Akaike criterion (38). A group × session interaction was tested to analyze the effectiveness of the strength or aerobic training group during different sessions.
For all main effects, an α level of less than .05 was considered statistically significant. All analyses were conducted using mixed modeling software (SAS 9.2 Proc Mixed) to generate restricted maximal likelihood estimates, using all available data.
RESULTS
We randomized and tested 149 healthy, nonsmoking adults (58 men and 91 women) before training. Data for analysis of PEP and LF BPV were available for 144 of these 149 participants. Demographic and physical characteristics of these 144 participants before training are presented in Table 1.
TABLE 1.
Pretraining Participant Characteristics
| Variable | Aerobic Training
|
Strength Training
|
||||
|---|---|---|---|---|---|---|
| n | Mean | SE | n | Mean | SE | |
| Age, y | 71 | 30.28 | 0.83 | 73 | 31.29 | 0.92 |
| VO2max, ml kg−1 min−1 | 71 | 34.07 | 0.73 | 73 | 33.10 | 0.65 |
| SBP, mm Hg | 71 | 109.82 | 1.26 | 72 | 110.25 | 1.40 |
| DBP, mm Hg | 71 | 71.11 | 0.93 | 72 | 71.22 | 1.01 |
| HR, beats/min | 71 | 70.97 | 1.07 | 73 | 71.84 | 1.07 |
| BMI, kg/m2 | 70 | 24.59 | 0.45 | 73 | 24.98 | 0.49 |
SE = standard error; SBP = systolic blood pressure; DBP = diastolic blood pressure; HR = heart rate; BMI = body mass index.
Effect of Training on Aerobic Capacity
The group by session interaction was significant (F(2,18) = 3.74, p = .03) indicating a differential effect of group assignment on VO2max. Table 2 presents these data.
TABLE 2.
Changes in Aerobic Capacity (ml kg−1 min−1)
| Group | Session
|
||
|---|---|---|---|
| Baseline | Posttraining | Postdeconditioning | |
| Aerobic | 34.24 (0.60) | 37.50 (0.70) | 34.29 (0.77) |
| Strength | 33.81 (0.60) | 33.43 (0.76) | 32.18 (0.79) |
Data are reported as least-square means (standard error).
Effect of Training on PEP and LF BPV
Results of the analysis are presented in Table 3. As Table 3 indicates, there was a main effect of sex for all but LF diastolic BPV in the seated position. PEP was greater for men compared with women in both positions. LF systolic BPV was greater for men compared with women in both positions. LF diastolic BPV was greater for men compared with women in the supine position only.
TABLE 3.
F Values from Regression Analyses for PEP and LF BPV
| PEP | LF SBPV | LF DBPV | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Seated | Supine | Seated | Supine | Seated | Supine | |
| Age | 7.15a | 1.68 | 0.17 | 0.03 | 1.63 | 3.43 |
| Sex | 4.52a | 9.42a | 4.81a | 14.48a | 1.03 | 11.82a |
| Group | 1.51 | 0.20 | 3.18 | 2.50 | 2.84 | 1.07 |
| Time | 1.14 | 0.55 | 1.94 | 0.31 | 1.17 | 1.64 |
| Group × Time | 1.18 | 0.10 | 0.27 | 0.01 | 2.06 | 0.57 |
PEP = preejection period; LF = left ventricular; BPV = blood pressure variability; SBPV = systolic blood pressure variability; DBPV = diastolic blood pressure variability.
p < .05.
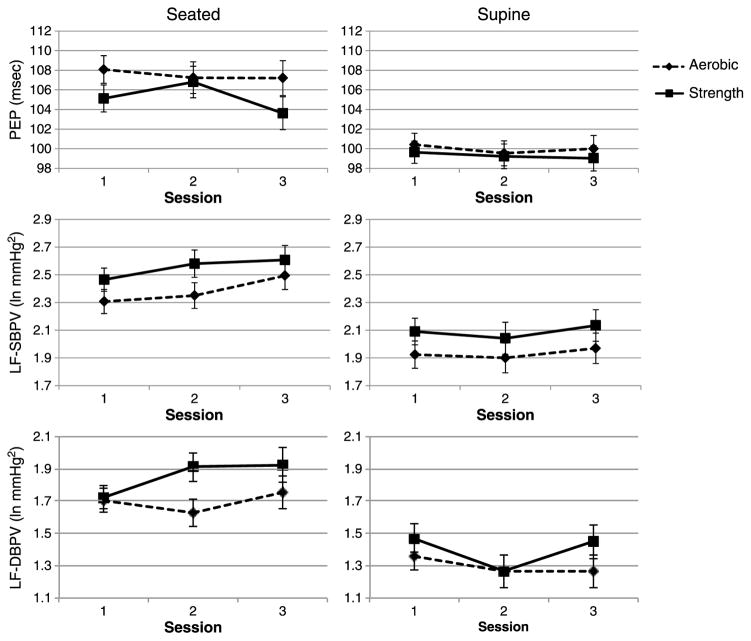
We hypothesized that in contrast to strength training, aerobic training would reduce cardiovascular sympathetic indices. Contrary to expectations, the group × time interaction failed to achieve significance for either PEP or LF BPV in either position. Figure 1 presents these data. Because we previously found a significant group × time × sex interaction for cardiac parasympathetic regulation (39), we tested whether a similar effect was found for our sympathetic indices. However, no three-way interaction was significant. Pearson correlation coefficients between changes from baseline to posttraining in VO2max and either PEP or LF systolic BPV were not significant (0.16 and − 0.13 for the aerobic group and − 0.24 and − 0.17 for the strength group).
Figure 1.
Seated and supine resting levels of pre-ejection period (PEP) and low frequency systolic (LF-SBPV) and diastolic (LF-DBPV) Blood Pressure Variability before training (Session 1), after training (Session 2), and after sedentary deconditioning (Session 3). Data are shown as least-square means. Error bars represent standard errors. PEP = preejection period; LF = low frequency; SBPV = systolic blood pressure variability; DBPV = diastolic blood pressure variability.
DISCUSSION
Engaging in physical activity is arguably the preeminent cardioprotective health behavior, and consensus panels recommend it for both patients and healthy people alike (40,41). Although the benefits of exercise are undisputed, the mechanisms are not completely understood. Because one candidate mechanism is the autonomic nervous system and because numerous studies have demonstrated that aerobic exercise improves parasympathetic function (35,42,43), we reasoned that exercise might also exert a beneficial impact on the sympathetic branch of the ANS. However, we found that a 12-week program of aerobic conditioning had no effect on LF BPV or PEP, suggesting that the intervention had no effect on resting vascular or cardiac inotropic sympathetic regulation.
Evidence suggests that in patient populations, for example, patients with hypertension or congestive heart failure or post–myocardial infarction, exercise training influences SNS activity. These conditions are known to result in autonomic dysregulation in the form of SNS hyperactivity (44,45). Studies suggest that exercise attenuates this dysregulation, lowering sympathetic tone to levels comparable with healthy controls. For example, Izdebska et al. (46) demonstrated that hypertensive participants showed increased baseline levels of LF BPV at rest compared with normotensive participants and that 12 weeks of aerobic conditioning restored levels of LF BPV to the levels of the normotensives. For the normotensive controls in this study, however, no significant change was found. Laterza et al. (47) have shown similar results in a randomized controlled trial of aerobic conditioning for 4 months in a group of never-treated hypertensive patients and an age-matched control group. Also, several studies on patients with heart failure (15,16,48) and myocardial infarction (17) have shown similar reductions in levels of SNS, measured as MSNA, after exercise.
Few studies address the sympathetic consequences of aerobic training in healthy participants. In a small study of sedentary young men, De Geus et al. (49) reported no effect of a 7-week aerobic training program on resting PEP. In a larger and more comprehensive examination, also of young and sedentary men, using both a longer training period and deconditioning, this same group similarly found that aerobic training did not affect PEP or overnight urinary epinephrine excretion (50). More recently, Meredith and colleagues (51) measured total, renal, and cardiac NE spillover to plasma before and after 1 month of regular bicycle exercise and 1 month of sedentary activity. In this small study of young, healthy men, they demonstrated that exercise reduced resting heart rate and BP and total NE spillover. This effect was caused primarily by a reduction in renal NE spillover. Cardiac NE spillover was not changed by training. A more recent study, although not primarily about the effect of aerobic training on SNS function, demonstrated that there was no difference between healthy participants who were either high or only average fitness in MSNA (52). Ray and Carter (53) also found no effect of aerobic training on MSNA in healthy participants. A systematic review of studies concerning aerobic conditioning in healthy individuals showed that for most studies, but not all, there was no effect of exercise training on resting levels of MSNA (18).
In this study, we examined PEP and LF BPV as indices of myocardial and vascular sympathetic function. Some studies have used these indices to examine the effects of exercise training. Goedhart et al. (54) conducted a longitudinal study on healthy participants with ambulatory ICG that showed no difference in resting PEP between exercised and sedentary participants. Similarly, Svedenhag et al. (55) showed no difference in PEP between healthy sedentary and well-trained participants. However, these two studies were small and observational, and few other studies on PEP and exercise are found in the literature.
Several studies have examined the effect of exercise training on LF BPV, reporting negative findings (46,56,57). However, these studies also were small and nonrandomized. One large study of the effect of aerobic conditioning on BPV exists, although it recruited men only. Uusitalo et al. (58) reported that a 5-year aerobic exercise training program, in contrast to a control condition, increased aerobic capacity but had no effect on LF BPV. These studies of exercise and PEP or LF BPV as indices of SNS function support our findings showing that neither of these indices was affected by exercise training.
The consistency of these findings—that exercise training does not alter cardiac NE turnover, MSNA, PEP, or LF BPV—in healthy participants, along with the similarly consistent findings of an effect of training in patients with elevated resting levels of SNS activity, suggests the possibility of a floor effect: because in healthy participants, resting levels of SNS activity are relatively low, to begin with, no further reduction was possible. However, sympathetic activity is greater in the seated compared with the supine position, that is, the SNS-reducing effect of treatment in the seated position should be apparent. As Figure 1 indicates, they were not for either PEP or LF BPV, suggesting that a floor effect is not likely to account for the lack of an impact of aerobic training.
It is also possible that the training regimen we used had insufficient intensity to have a measurable effect on PEP or LF BPV. Using a pharmacologic provocation, Schachinger et al. (24) demonstrated that even in healthy volunteers, resting PEP could be lowered by NE-induced hypertension and that PEP could be increased after nitroprusside infusion. These data indicate that with a powerful enough stimulus, it is possible to affect tonic levels of sympathetic activation. Conceivably, with a more vigorous training program, similar effects might be observed.
Limitations
This study was a randomized controlled trial with a large population using an intent-to-treat design. Nevertheless, several limitations must be considered. Our exercise training program was only moderate in intensity, yielding improvements in aerobic capacity of approximately 15% (39). As previously discussed, it is possible that higher-intensity training programs would lead to greater effects on resting levels of sympathetic activity. However, even moderate improvements in exercise capacity are associated with cardioprotective effects (40,41). If resting sympathetic activity were a mechanism of cardio-protection in healthy people, one could expect that even training of moderate intensity should lead to a measurable effect.
It is possible that our variables, PEP and LF BPV, do not adequately measure SNS. However, there is considerable evidence that PEP is specifically sensitive to β-adrenergic influences on the heart (20–24,59). Moreover, support for the validity of impedance-derived measurements has been documented by comparison with other known measures (22,60), and it is commonly used in psychophysiological research. The evidence supporting LF BPV as an index of vascular sympathetic control is more controversial, with evidence supporting this interpretation (27–30) and other evidence suggesting the LF BPV reflects a resonance phenomenon, product of the delay in the α-adrenergic vasoconstrictor response mediated by the baroreceptors (61–63).
Another potential limitation is that we lack information to determine definitively that participants adhered to the training protocol as intended. Participants were provided with the training protocol and supervised during their first training session, but in subsequent sessions, they exercised on their own. However, the aerobic and strength training groups differed precisely, as expected, on changes in aerobic capacity after training and again after deconditioning. Therefore, participants seem to have exercised as planned.
CONCLUSIONS
Aerobic exercise is widely recognized to confer cardioprotection, although the mechanisms underlying this protection are not completely understood. We previously reported the results that moderate aerobic training, in contrast to strength training, lowered heart rate and increased cardiac parasympathetic regulation. Here, we report that indices of cardiac and vascular SNS regulation were not altered by the aerobic training intervention. These results indicate that if moderate levels of aerobic exercise influence autonomic regulation in such a way as to reduce risk of future cardiovascular disease in healthy individuals, alterations of resting levels of SNS activity are not a likely mechanism.
Acknowledgments
Source of Funding: This study was supported, in part, by Independent Scientist Award K02 MH01491 (Dr. Sloan) from the National Institute of Mental Health and R01 HL61287 (Dr. Sloan) from the National Heart Lung, and Blood Institute, M01-RR00645 from the General Clinical Research Centers Program of the National Institutes of Health, and the Nathaniel Wharton Fund.
Glossary
- PEP
preejection period
- LF BPV
low-frequency blood pressure variability
- SNS
sympathetic nervous system
- NE
norepinephrine
- MSNA
muscle sympathetic nerve activity
- ICG
impedance cardiography
- ECG
electrocardiogram
- A/D
analog to digital
- BP
blood pressure
- ANS
autonomic nervous system
Footnotes
Conflicts of Interest: The other authors report no conflicts of interest.
References
- 1.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Reviews. 2010;90:513–57. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 2.Julius S, Nesbitt S. Sympathetic overactivity in hypertension. A moving target. Am J Hypertens. 1996;9:113S–20S. doi: 10.1016/0895-7061(96)00287-7. [DOI] [PubMed] [Google Scholar]
- 3.Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, Hastings J, Aggarwal A, Esler MD. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension. 2004;43:169–75. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- 4.Zipes DP. Heart-brain interactions in cardiac arrhythmias: role of the autonomic nervous system. Cleve Clinic J Med. 2008;75(Suppl 2):S94–6. doi: 10.3949/ccjm.75.suppl_2.s94. [DOI] [PubMed] [Google Scholar]
- 5.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–23. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 6.Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jonsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyorala K, Raz I, Schernthaner G, Volpe M, Wood D Task Force on D, Cardiovascular Diseases of the European Society of C, European Association for the Study of D. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 7.Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1977;39:221–51. doi: 10.1146/annurev.ph.39.030177.001253. [DOI] [PubMed] [Google Scholar]
- 8.Sloan RP, Shapiro PA, DeMeersman RE, Bagiella E, Brondolo EN, McKinley PS, Crowley O, Zhao Y, Schwartz JE, Myers MM. Impact of aerobic training on cardiovascular reactivity to and recovery from challenge. Psychosom Med. 2011;73:134–41. doi: 10.1097/PSY.0b013e31820a1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter JB, Banister EW, Blaber AP. The effect of age and gender on heart rate variability after endurance training. Med Sci Sports Exerc. 2003;35:1333–40. doi: 10.1249/01.MSS.0000079046.01763.8F. [DOI] [PubMed] [Google Scholar]
- 10.Hautala AJ, Makikallio TH, Kiviniemi A, Laukkanen RT, Nissila S, Huikuri HV, Tulppo MP. Heart rate dynamics after controlled training followed by a home-based exercise program. Eur J Appl Physiol. 2004;92:289–97. doi: 10.1007/s00421-004-1077-6. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R, Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol. 2005;99:1041–9. doi: 10.1152/japplphysiol.00085.2005. [DOI] [PubMed] [Google Scholar]
- 12.Tulppo MP, Hautala AJ, Makikallio TH, Laukkanen RT, Nissila S, Hughson RL, Huikuri HV. Effects of aerobic training on heart rate dynamics in sedentary subjects. J Appl Physiol. 2003;95:364–72. doi: 10.1152/japplphysiol.00751.2002. [DOI] [PubMed] [Google Scholar]
- 13.Bertagnolli M, Schenkel PC, Campos C, Mostarda CT, Casarini DE, Bello-Klein A, Irigoyen MC, Rigatto K. Exercise training reduces sympathetic modulation on cardiovascular system and cardiac oxidative stress in spontaneously hypertensive rats. Am J Hypertens. 2008;21:1188–93. doi: 10.1038/ajh.2008.270. [DOI] [PubMed] [Google Scholar]
- 14.DiCarlo SE, Collins HL, Rodenbaugh DW, Smitha MR, Berger RD, Yeragani VK. Daily exercise reduces measures of heart rate and blood pressure variability in hypertensive rats. Clin Exp Hypertens. 2002;24:221–34. doi: 10.1081/ceh-120003202. [DOI] [PubMed] [Google Scholar]
- 15.Fraga R, Franco FG, Roveda F, de Matos LN, Braga AM, Rondon MU, Rotta DR, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training reduces sympathetic nerve activity in heart failure patients treated with carvedilol. Eur J Heart Fail. 2007;9:630–6. doi: 10.1016/j.ejheart.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol. 2003;42:854–60. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- 17.Mimura J, Yuasa F, Yuyama R, Kawamura A, Iwasaki M, Sugiura T, Iwasaka T. The effect of residential exercise training on baroreflex control of heart rate and sympathetic nerve activity in patients with acute myocardial infarction. Chest. 2005;127:1108–15. doi: 10.1378/chest.127.4.1108. [DOI] [PubMed] [Google Scholar]
- 18.Ray CA, Hume KM. Sympathetic neural adaptations to exercise training in humans: insights from microneurography. Med Sci Sports Exerc. 1998;30:387–91. doi: 10.1097/00005768-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–75. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- 20.Kelsey RM, Alpert BS, Patterson SM, Barnard M. Racial differences in hemodynamic responses to environmental thermal stress among adolescents. Circulation. 2000;101:2284–9. doi: 10.1161/01.cir.101.19.2284. [DOI] [PubMed] [Google Scholar]
- 21.Richter M, Baeriswyl E, Roets A. Personality effects on cardiovascular reactivity: need for closure moderates the impact of task difficulty on engagement-related myocardial beta-adrenergic activity. Psychophysiology. 2012;49:704–7. doi: 10.1111/j.1469-8986.2011.01350.x. [DOI] [PubMed] [Google Scholar]
- 22.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 23.Mezzacappa ES, Kelsey RM, Katkin ES. The effects of epinephrine administration on impedance cardiographic measures of cardiovascular function. Int J Psychophysiol. 1999;31:189–96. doi: 10.1016/s0167-8760(98)00058-0. [DOI] [PubMed] [Google Scholar]
- 24.Schachinger H, Weinbacher M, Kiss A, Ritz R, Langewitz W. Cardiovascular indices of peripheral and central sympathetic activation. Psychosom Med. 2001;63:788–96. doi: 10.1097/00006842-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Benschop RJ, Nieuwenhuis EE, Tromp EA, Godaert GL, Ballieux RE, van Doornen LJ. Effects of beta-adrenergic blockade on immunologic and cardiovascular changes induced by mental stress. Circulation. 1994;89:762–9. doi: 10.1161/01.cir.89.2.762. [DOI] [PubMed] [Google Scholar]
- 26.Obrist PA, Light KC, James SA, Strogatz DS. Cardiovascular responses to stress: I. Measures of myocardial response and relationship to high resting systolic pressure and parental hypertension. Psychophysiology. 1987;24:65–78. doi: 10.1111/j.1469-8986.1987.tb01864.x. [DOI] [PubMed] [Google Scholar]
- 27.Cevese A, Grasso R, Poltronieri R, Schena F. Vascular resistance and arterial pressure low-frequency oscillations in the anesthetized dog. Am J Physiol. 1995;268:H7–16. doi: 10.1152/ajpheart.1995.268.1.H7. [DOI] [PubMed] [Google Scholar]
- 28.Ditor DS, Kamath MV, Macdonald MJ, Bugaresti J, McCartney N, Hicks AL. Reproducibility of heart rate variability and blood pressure variability in individuals with spinal cord injury. Clin Auton Res. 2005;15:387–93. doi: 10.1007/s10286-005-0293-4. [DOI] [PubMed] [Google Scholar]
- 29.Montano N, Lombardi F, Gnecchi Ruscone T, Contini M, Finocchiaro ML, Baselli G, Porta A, Cerutti S, Malliani A. Spectral analysis of sympathetic discharge, R-R interval and systolic arterial pressure in decerebrate cats. J Auton Nerv Syst. 1992;40:21–31. doi: 10.1016/0165-1838(92)90222-3. [DOI] [PubMed] [Google Scholar]
- 30.Stafford RW, Harris WS, Weissler AM. Left ventricular systolic time intervals as indices of postural circulatory stress in man. Circulation. 1970;41:485–92. doi: 10.1161/01.cir.41.3.485. [DOI] [PubMed] [Google Scholar]
- 31.Cevese A, Grasso R, Poltronieri R, Schena F. Vascular resistance and arterial pressure low-frequency oscillations in the anesthetized dog. Am J Physiol. 1995;37:H7–16. doi: 10.1152/ajpheart.1995.268.1.H7. [DOI] [PubMed] [Google Scholar]
- 32.Montano N, Lombardi F, Ruscone TG, Contini M, Finocchiaro ML, Baselli G, Porta A, Cerutti S, Malliani A. Spectral analysis of sympathetic discharge, R-R interval and systolic arterial pressure in decerebrate cats. J Auton Nerv Syst. 1992;40:21–32. doi: 10.1016/0165-1838(92)90222-3. [DOI] [PubMed] [Google Scholar]
- 33.Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. J Physiol. 1999;517(pt 2):617–28. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukai S, Hayano J. Heart rate and blood pressure variabilities during graded head-up tilt. J Appl Physiol. 1995;78:212–6. doi: 10.1152/jappl.1995.78.1.212. [DOI] [PubMed] [Google Scholar]
- 35.Sloan RP, Shapiro PA, DeMeersman RE, Bagiella E, Brondolo EN, McKinley PS, Slavov I, Fang Y, Myers MM. The effect of aerobic training and cardiac autonomic regulation in young adults. Am J Public Health. 2009;99:921–8. doi: 10.2105/AJPH.2007.133165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.deBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events, particularly for heart rate variability spectra. IEEE Trans Biomed Eng. 1984;BME-31:384–7. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- 37.Harris FJ. On the use of windows for harmonic analysis with the discrete Fourier transform. Proc IEEE. 1978;66:51–83. [Google Scholar]
- 38.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;AC19:716–23. [Google Scholar]
- 39.Sloan RP, Shapiro PA, DeMeersman RE, Bagiella E, Brondolo EN, McKinley PS, Slavov I, Fang Y, Myers MM. The effect of aerobic training and cardiac autonomic regulation in young adults. Am J Public Health. 2009;99:921–8. doi: 10.2105/AJPH.2007.133165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearson TA, Bazzarre TL, Daniels SR, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Hong Y, Mensah GA, Sallis JF, Jr, Smith S, Jr, Stone NJ, Taubert KA American Heart Association Expert Panel on P Prevention S. American Heart Association guide for improving cardiovascular health at the community level: a statement for public health practitioners, healthcare providers, and health policy makers from the American Heart Association Expert Panel on Population and Prevention Science. Circulation. 2003;107:645–51. doi: 10.1161/01.cir.0000054482.38437.13. [DOI] [PubMed] [Google Scholar]
- 41.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF, Jr, Smith SC, Jr, Stone NJ, Taubert KA. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–91. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 42.Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz PJ. The autonomic nervous system and sudden death. Eur Heart J. 1998;19(Suppl F):F72–80. [PubMed] [Google Scholar]
- 44.Brook RD, Julius S. Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens. 2000;13:112S–22S. doi: 10.1016/s0895-7061(00)00228-4. [DOI] [PubMed] [Google Scholar]
- 45.Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol. 2012;59:117–22. doi: 10.1016/j.jjcc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Izdebska E, Cybulska I, Izdebskir J, Makowiecka-Ciesla M, Trzebski A. Effects of moderate physical training on blood pressure variability and hemodynamic pattern in mildly hypertensive subjects. J Physiol Pharmacol. 2004;55:713–24. [PubMed] [Google Scholar]
- 47.Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrao CE, Rondon MU. Exercise training restores barore-flex sensitivity in never-treated hypertensive patients. Hypertension. 2007;49:1298–306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 48.de Mello Franco FG, Santos AC, Rondon MU, Trombetta IC, Strunz C, Braga AM, Middlekauff H, Negrao CE, Pereira Barretto AC. Effects of home-based exercise training on neurovascular control in patients with heart failure. Eur J Heart Fail. 2006;8:851–5. doi: 10.1016/j.ejheart.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 49.De Geus EJC, van Doornen LJP, de Visser DC, Orlebeke JF. Existing and training induced differences in aerobic fitness: their relationship to physiological response patterns during different types of stress. Psychophysiology. 1990;27:457–77. doi: 10.1111/j.1469-8986.1990.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 50.de Geus EJ, van Doornen LJ, Orlebeke JF. Regular exercise and aerobic fitness in relation to psychological make-up and physiological stress reactivity. Psychosom Med. 1993;55:347–63. doi: 10.1097/00006842-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Meredith IT, Friberg P, Jennings GL, Dewar EM, Fazio VA, Lambert GW, Esler MD. Exercise training lowers resting renal but not cardiac sympathetic activity in humans. Hypertension. 1991;18:575–82. doi: 10.1161/01.hyp.18.5.575. [DOI] [PubMed] [Google Scholar]
- 52.Young CN, Deo SH, Kim A, Horiuchi M, Mikus CR, Uptergrove GM, Thyfault JP, Fadel PJ. Influence of endurance training on central sympathetic outflow to skeletal muscle in response to a mixed meal. J Appl Physiol. 2010;108:882–90. doi: 10.1152/japplphysiol.01174.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ray CA, Carter JR. Effects of aerobic exercise training on sympathetic and renal responses to mental stress in humans. Am J Physiol Heart Circ Physiol. 2010;298:H229–34. doi: 10.1152/ajpheart.00880.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goedhart AD, de Vries M, Kreft J, Bakker FC, de Geus EJC. No effect of training state on ambulatory measures of cardiac autonomic control. J Psychophysiol. 2008;22:130–40. [Google Scholar]
- 55.Svedenhag J, Martinsson A, Ekblom B, Hjemdahl P. Altered cardiovascular responsiveness to adrenaline in endurance-trained subjects. Acta Physiol Scand. 1986;126:539–50. doi: 10.1111/j.1748-1716.1986.tb07853.x. [DOI] [PubMed] [Google Scholar]
- 56.Perini R, Tironi A, Cautero M, Di Nino A, Tam E, Capelli C. Seasonal training and heart rate and blood pressure variabilities in young swimmers. Eur J Appl Physiol. 2006;97:395–403. doi: 10.1007/s00421-006-0174-0. [DOI] [PubMed] [Google Scholar]
- 57.Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. Autonomic differences between athletes and nonathletes: spectral analysis approach. Med Sci Sports Exerc. 1997;29:1482–90. doi: 10.1097/00005768-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 58.Uusitalo AL, Laitinen T, Vaisanen SB, Lansimies E, Rauramaa R. Physical training and heart rate and blood pressure variability: a 5-yr randomized trial. Am J Physiol Heart Circ Physiol. 2004;286:H1821–6. doi: 10.1152/ajpheart.00600.2003. [DOI] [PubMed] [Google Scholar]
- 59.Newlin DB, Levenson RW. Pre-ejection period: measuring beta-adrenergic influences upon the heart. Psychophysiology. 1979;16:546–53. doi: 10.1111/j.1469-8986.1979.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 60.McFetridge J, Sherwood A. Impedance cardiography for noninvasive measurement of cardiovascular hemodynamics. Nurs Res. 1999;48:109–13. doi: 10.1097/00006199-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Bertram D, Barres C, Cuisinaud G, Julien C. The arterial baroreceptor reflex of the rat exhibits positive feedback properties at the frequency of Mayer waves. J Physiol (Lond) 1998;513:251–61. doi: 10.1111/j.1469-7793.1998.251by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cerutti C, Barres C, Paultre C. Baroreflex modulation of blood pressure and heart rate variabilities in rats: assessment by spectral analysis. Am J Physiol. 1994;266:H1993–2000. doi: 10.1152/ajpheart.1994.266.5.H1993. [DOI] [PubMed] [Google Scholar]
- 63.van de Borne P, Rahnama M, Mezzetti S, Montano N, Porta A, Degaute JP, Somers VK. Contrasting effects of phentolamine and nitroprusside on neural and cardiovascular variability. Am J Physiol. 2001;281:H559–65. doi: 10.1152/ajpheart.2001.281.2.H559. [DOI] [PubMed] [Google Scholar]