Abstract
Glioblastoma multiforme (GBM) is recognized as the most common and lethal form of central nervous system cancer. Currently used surgical techniques, chemotherapeutic agents, and radiotherapy strategies have done very little in extending the life expectancies of patients diagnosed with GBM. The difficulty in treating this malignant disease lies both in its inherent complexity and numerous mechanisms of drug resistance. In this review, we summarize several of the primary mechanisms of drug resistance. We reviewed available published literature in the English language regarding drug resistance in glioblastoma. The reasons for drug resistance in glioblastoma include drug efflux, hypoxic areas of tumor cells, cancer stem cells, DNA damage repair, and miRNAs. Many potential therapies target these mechanisms, including a series of investigated alternative and plant-derived agents. Future research and clinical trials in glioblastoma patients should pursue combination of therapies to help combat drug resistance. The emerging new data on the potential of plant-derived therapeutics should also be closely considered and further investigated.
Keywords: glioblastoma multiforme, multidrug resistance, glioblastoma stem cells, p-glycoprotein, hypoxia-inducible factors, miRNAs, alternative therapies
Introduction
Glioblastoma multiforme (GBM), which is a Grade IV brain tumor according to the World Health Organization (WHO) classification, is the most common form of primary brain tumor in the central nervous system (CNS), and its aggressive nature and evasiveness to treatments make it one of the most lethal cancers [1]. Current treatments for GBM, also called glioblastoma, range from common chemotherapeutic agents such as temozolomide (TMZ), paired with radiotherapy, to more recent anti-angiogenic agents and immunotherapeutic treatments [2]. As with many cancers, however, anti-cancer therapeutic agents have not significantly increased the median survival of glioblastoma patients over the past 10 years. The five-year survival rate of glioblastoma patients, after treatment that includes surgical resection, radiation, and chemotherapy, is less than 9.8% - a colossal failure that has partially been attributed to drug resistance [3]. Drug resistance can generally be categorized as either acquired or intrinsic. Acquired drug resistance occurs when a tumor that initially responded to treatment is no longer sensitive to the anti-cancer agent. Intrinsic drug resistance refers to a tumor that shows insignificant or no response to the therapy at the onset of treatment [4]. Recent research indicates that, on a molecular level, acquired and intrinsic resistances share several common foundations [4].
However, the heterogeneous nature of GBM cells and the inherent issues of treating any cancer of the CNS due to its limited repair mechanisms and anatomic complexities, make the treatment of drug resistant GBM a difficult goal. The molecular mechanisms by which GBM displays resistance to apoptosis, a form of programmed cell death, are only partially understood. Understanding the molecular relationship between GBM cells and their environment is critical since these abnormalities can indicate tumor formation and progression, a process that can be reversible via therapeutic targeting for restoration of sensitivity to apoptosis. Thus, the identification of the cellular and molecular mechanisms that confer drug resistance is an important goal for the treatment of glioblastomas. This review will concentrate on the primary chemoresistance mechanisms discussed in the literature, including drug efflux, tumor induced hypoxic barriers, cancer stem cells, DNA damage repair, and microRNAs (miRNAs); and it will show potential alternative therapies that can combat resistance mechanisms (Table 1).
Table 1.
Current resistance prevention targets and discussed array of potential therapeutic strategies
| Reasons for resistance | Therapeutic mechanisms | References |
|---|---|---|
| Drug efflux | Synthetic p-glycoprotein inhibitors | 9, 10 |
| Molecular therapies | 8, 11 | |
| Hypoxic areas | Interruption of HIF cascades | 18, 19 |
| Vascular normalization | 20 | |
| Hypoxia-targeted toxic agents | 16, 17 | |
| miRNAs | Inhibition of miR-21 | 2, 27, 28, 29, 30 |
| Inhibition of miR-195 | 2 | |
| Glioblastoma stem cells | Checkpoint inhibition | 31, 35, 36 |
| Side population inhibition | 37 | |
| Notch signaling inhibition | 38, 39 | |
| Bcl-2 | Bcl-2 siRNA | 40, 41 |
I. Drug Efflux
A. The ABC transporter family
One mechanism by which a living cell can achieve multiple resistances is the active efflux of a broad range of anti-cancer drugs through the cellular membrane by multi-drug resistance proteins. Drugs can be transported across the membrane in ATP-independent manners or by using ATP-dependent proteins to transport across what are often considerable concentration gradients. The ATP-dependent group consists of the ATP-binding cassette (ABC) transporter family, which includes P-glycoprotein (P-gp), multi-drug resistance protein (MRP), and breast cancer resistance protein (BCRP) [5]. All of these proteins are involved in diverse physiological processes and are responsible for the uptake and efflux of a multitude of substances from cancer cells [5].
Of the ABC family, overexpression of P-gp, which is encoded by the MDR1 gene, has been mostly appreciated as a cause of anti-cancer drug resistance. Upregulation of MDR1 can be induced by anti-cancer agents, mutation of the tumor suppressor gene p53, activation of Raf (an upstream component of the MAPK pathway and oncegene transcriptional regulator), and the presence of heat-shock or DNA damaging agents [6]. The over induction of P-gp acts primarily by preventing ATP hydrolysis, disrupting the lipid membrane or blocking binding sites, and thus P-gp has been shown to be involved in both acquired and intrinsic drug resistance [6]. For example, the uptake of erlotinib, an EGFR inhibitor with potential use in glioblastoma treatment, has proven ineffective in clinical trials due to the actions of P-gp in conjunction with Bcrp1 [7]. Chemoresistance to doxurubicin and vincristine, two common anti-cancer agents, has also been directly attributed to P-gp [8]. The inhibition of P-gp, therefore, has been the intensive focus of much of the drug resistance research.
B. Synthetic P-gp inhibitors
The first generation of P-gp inhibitors is composed of drugs that are developed for other indications but they have also been shown to inhibit P-gp. Such drugs include immunosuppressants, calcium channel blockers, anti-hypertensives, and anti-estrogens [9]. However, at levels that would effectively prevent multi-drug resistance, these agents are highly toxic. Second generation inhibitors, such as dexniguldipine and valspodor, have a higher affinity and specificity for P-gp, making them more effective at lower concentrations [9]. However, the second generation drugs are also substrates for cytochrome p450. By interfering with the activity of this enzyme, toxicity of co-administered drugs increases greatly [10]. Hubensack and others investigated the efficacy of two notable third generation P-gp inhibitors, tariquidar and elacridar, when used as adjuvants with paclitaxel, also called taxol [10]. They found that these drugs demonstrate enhanced control at the blood brain barrier, leading to a higher brain to blood ratio of the cytostatic agents [10]. Furthermore, the newer P-gp inhibitors do not change the plasma pharmacokinetics of the anti-cancer drugs, nor do they serve as substrates for cytochrome p450 enzymes [9].
Exciting as these findings are, neither specific nor broadly active P-gp inhibitors have yet shown enhanced chemotherapeutic ability when given with standard of care medications. It is unclear why enhancing known anti-cancer drug concentrations intracellularly has thus far proven ineffective; however, this phenomenon suggests that finding new chemotherapies, versus enhancing the effectiveness of current drugs, should be our primary focus. Wartenberg and colleagues suggested a new avenue of research into P-gp functionality when they correlated increased P-gp expression with elevated glycolysis in tumor spheroids [11]. They discovered that inhibition of the glyocolytic pathway reduced P-gp expression in glioblastoma cells and thus they posited that drug resistant tumors were only able to effectively manage efflux when glycolysis was active. Indeed, this same proposition was brought up five years prior to the Wartenberg study. Swift inactivation of pro-apoptotic Bad by glycolytically regulated dephosphorylation, often found in cancer cells, was reversed by reducing glycolysis-based ATP production [12]. If drug resistance is imparted by defective respiratory activity resulting in enhanced glycolysis and a resultant increase in P-gp expression, then inhibiting glycolysis should: 1) reduce drug resistance by down regulating P-gp, 2) increase the chance of Bad activation and apoptosis, and ultimately 3) enhance chemotherapeutic efficacy [12].
C. Molecular therapies
Other alternative therapies have been studied as well. The use of small interfering RNA (siRNA) designed to target MDR1 was proven to both decrease expression of P-gp due to down regulation of MDR1 and, ultimately, contribute to tumor cell apoptosis [8]. In a similar study, human glioblastoma cells and human endothelial cells were treated with antisense oligodeoxynucleotides that targeted P-gp mRNA post-treatment with doxorubicin. The flow cytometry results indicated that the cells retained up to four times the anti-cancer agent than those untreated with the antisense oligodeoxynucleotides, indicating that this might be an effective inhibitor of P-gp [13]. However, siRNA therapy is currently unproven in animal models due primarily to the difficulty of delivering such a pharmacologically unstable compound to the correct location intact.
II. Hypoxia
A. Hypoxic areas: Hotbeds of resistance?
The rapid proliferation of tumor cells leads to an insufficient supply of blood vessels surrounding the tumor, creating a microenvironment often characterized by areas of hypoxia and acidity. As a result, cancer cells in the interior of the tumor tend to be inactive and do not divide. Although anticancer drugs may kill cells on the exterior portion of the tumor, the hypoxic cells are more likely to survive and develop resistance to anticancer drugs. The most obvious reason for this resistance is the area's distance from vasculature, preventing the therapeutic agent from reaching its target [14]. Another protective factor may be that hypoxic areas are not proliferative, thus being intrinsically immune to the anti-proliferative anticancer agents [15].
While the relationship between hypoxic areas and treatment failure has been studied for decades, the molecular basis for hypoxic interference with anti-cancer agents has only recently been uncovered. The phenomenon is partially due to the dependence of many chemotherapeutics on the creation of oxygen-derived free radicals [16]. More detailed molecular research also suggests that hypoxia-inducible factors (HIFs), a family of hypoxia-induced transcription factors, play a crucial role in tumorigenesis, considering immunohistochemistry findings that several HIFs, particularly HIF-1, are overexpressed by tumor cells. One of the primary actions of HIF-1 is its activation of MDR1, the gene that encodes P-gp expression [17].
B. Hypoxic area targeted drugs
Targeting hypoxic areas has become an increased focus in the fight against drug resistance in solid tumors. Potential treatments include the use of drugs that are toxic only in hypoxic conditions [18]. The drug currently being developed for this purpose, tirapazamine, is a bioreductive agent that acts as a free radical in hypoxic conditions, activating topoisomerase II that then disrupts DNA strands. However, a Phase II clinical study found that there was no significant survival difference in patients treated with both radiation and tirapazamine versus a control population [19].
C. Hypoxia and angiogenesis
The description of hypoxia must be accompanied by a review of angiogenesis treatment. While vascular endothelial growth factor (VEGF) has been implicated in tumorigenesis, the use of anti-angiogenesis agents proves to be complicated. The inhibition of angiogenesis leads to hypoxia, which, in turn, leads to HIF stabilization and, ultimately, the stimulation of further angiogenesis [20]. One potential option to overcome this circular conundrum is the interruption of the HIF signaling cascade [20]. This goal could be achieved by the suppression of HIF-1α expression via the use of antisense HIF-1α-phosphorothioateoligodeoxynucleotide (AS-HIF ODN). This antisense depleted HIF-1α expression by up to 80% and subsequently increased the rate of apoptosis of a human glioblastoma cell line [21]. Another potential treatment may be the normalization of tumor vasculature. The neutralization of tumor vasculature creates a temporary environment for the tumor in which there is increased drug sensitivity. This normalized period is marked by angiopoietin-1 and MMPs, allowing for the associated period of effective treatment [22].
Interestingly, HIF-1 activates numerous glycolysis associated gene transcripts [23], suggesting a connection between HIF-1 and P-gp regulation. Inhibition of HIF-1 would reduce expression of MDR1 (the P-gp gene transcript) and therefore P-gp. Further studies are needed to elucidate these roles, especially given the in vivo limitations of the RNA interference (RNAi) technology.
III. DNA Damage Repair
A. MGMT
Many commonly used chemotherapeutic drugs induce DNA damage (for example, 5-fluoruracial and topoisomerase poisons). The response of the cancer cell to this damage would, ideally, be induction of apoptosis. However, many cancer cells demonstrate DNA repair mechanisms that can reduce chemosensitivity. The current primary treatment for glioblastoma is TMZ, an alkylating agent, in conjunction with ionizing radiation. The mechanism of action of TMZ involves methylation of guanine at O6 position, a change that causes a futile cycle of attempted DNA repair and results in cell apoptosis. This successful eradication of tumor cells, however, can occur only if the tumor is O6-methylguanine methyl transferase (MGMT) negative [24]. MGMT removes the DNA adduct caused by the alkylating agent, resulting in resistance to treatment.
The methylation of the promoter sequence of MGMT has been studied thoroughly as a predictor for treatment and outcome; however, a clearer relationship among mRNA expression, MGMT promoter methylation, and immunohistological assays are required for this biomarker to become commonly used in clinical practice [25]. Thus, it remains clear that high levels of MGMT or, conversely, a deficiency in mismatch repair, will result in TMZ resistance [26].
B. MGMT therapeutic targets
Therapeutic targets for MGMT remain rudimentary. The current preferred strategy is to use a high dose of TMZ in an attempt to overcome the hindering effect of MGMT on DNA alkylation [27]. Patients with recurrent GBM were found to benefit from continuous treatment with TMZ (50 mg/m2/d) [28]. It was also found that the progession-free survival rate after six months in patients with recurrent or progressive GBM was 23.9%, a definitive improvement over a previously found 15% survival rate using cytotoxic agents [28]. In a 2010-case study, it was reported a patient with excessive MGMT who was treated post-surgery and radiotherapy with high doses of fotemustine, a highly lipophilicnitrosurea sometimes used in conjunction with TMZ [29]. The patient experienced a complete response and recovery of three or more years [29]. While this result seems encouraging, the use of high doses of fotemustine has not been tested on a broader clinical scale.
IV. Role of miRNAs in Drug Resistance
All miRNAs are noncoding single-stranded RNA molecules that post-transcriptionally modify the translation of target mRNA and are involved in an array of biological processes. Both down regulation and upregulation of miRNAs have been recently implicated in the development of glioblastomas. Shi et al. investigated the relationship between upregulation of miR-21 and resistance to apoptosis using TMZ [30]. Using a human derived glioblastoma cell line, they found that overexpression of miR-21 significantly inhibited the effect of TMZ on apoptosis. While TMZ upregulates Bax (a pro-apoptotic protein) and caspase-3, both are down regulated in the presence of miR-21. Bcl-2 (an anti-apoptotic protein) is elevated in the presence of miR-21 [30]. The mechanism of miR-21 is potentially related to its upregulation of LRRFIP1, the product of which inhibits NF-κB activation [31]. NF-κB exhibits significant anti-apoptotic tendencies and thus its suppression increases chemosensitivity in cancer cells [32].
Another recent study, using a resistant variant of human derived glioblastoma cell line U251MG, found that three other miRNAs such as miR-195, miR-455-3P, and miR-10a, were the most upregulated miRNAs in the resistant cells. Of the three, knockdown of miR-195 had the greatest effect on initiating tumor cell death, suggesting that it could be a potential therapeutic target for treatment of glioblastoma [2].
V. Stem Cells
A. Glioblastoma stem cells and chemoresistance
Stem cells are well known for their ability to differentiate and propagate. Recent cancer research indicates that there is a population of cancer cells in tumors that, like traditional stem cells, display the ability to both self-renew and maintain proliferation. Despite the fact that many of these cells may be derived from mutated astrocytes post-epigenetic change rather than a normal stem cell line, the term cancer stem cell is often applied to this sub-population of tumor cells [33]. Glioblastoma stem cells (GSCs) exhibit proliferative and self-renewal properties, as well as multi-lineage differentiation into astrocytes, neurons, and oligodendrocytes. However, GSCs are markedly different from normal stem cells in regard to their effect on tumor formation. Due to their unique properties, GSCs have been proven in numerous studies to have significant tumorigenic capabilities when transplanted into the brains of immunocompromised rats [33,34]. GSCs have also been found to express high levels of both VEGF and SDF-1 (stromal-derived factor-1), both of which contribute to tumor growth via angiogenesis [33,35,36]. GSCs not only act as protectors of the tumor; they themselves are highly resistant to traditional therapies. Bao et al. demonstrated that GSCs show a greater resistance to radiation than traditional glioblastoma cells, both in vivo and in vitro [36].
B. Checkpoint inhibition
The mechanisms of GSCs’ chemoresistance remain a subject of speculation. It has been shown that GSCs, when damaged by typically used radiation therapies, activate checkpoint mechanisms, thus allowing more efficient recovery from genotoxicity. Inhibitors of this checkpoint mechanism negate this protective measure, a technique that could be considered for overcoming this aspect of resistance. For example, the inhibition of Chk1 kinase by agents such as debromohymenialdisine (DBH) has been suggested to be a potential mode of therapy [33,37]. The regulation of checkpoint repair may also be managed through L1CAM, a cell surface molecule that heightens the DNA repair capacity [38]. Treatment with an L1CAM inhibitor, when used in conjunction with other existing treatments, could be a future step in the development of new therapeutic agents.
C. Side population inhibition
Other potential mechanisms of resistance and repair include the regulation and control of ABC transporters, which act as efflux pumps for chemotherapeutic agents. A particular population of cancer stem cells, called a side population, express particularly high levels of ABC transporters and demonstrate remarkable drug resistance [39].
D. Notch signaling
Another potential therapeutic target in GSCs is the inhibition of the Notch signaling pathway. Notch is a membrane protein with an intracellular domain which, when activated, can be degraded by γ-secretase and subsequently associate with transcription factors [40]. Both Notch 1 and Notch 2 have been shown to be overexpressed in tumors. While tumors seem to overexpress Notch 2 more than Notch 1, Notch 1 has been implicated in the regulation of EGFR [41]. Notch 1 activates p53, which in turn activates the EGF promoter [41]. The inhibition of the Notch signaling pathway via the inhibition of the enzyme γ-secretase has been shown to increase chemosensitivity of GSCs.
VI. B cell lymphoma- 2 (Bcl-2)
Bcl-2 is a commonly discussed and studied anti-apoptotic protein that is highly expressed in treatment resistant cancer cells. However, recent research has indicated potential manipulation of Bcl-2 as a prime mechanism in tumor survival. Knockdown of Bcl-2 using siRNA during a low dose taxol treatment was found to be helpful for induction of apoptosis [42]. Taxol, a commonly used chemotherapeutic, acts by binding to the B-subunit of tubulin, creating a structural instability so as to promote apoptosis via activation of caspase cascades. The traditionally used high doses of taxol have the potential to induce apoptosis in normal astrocytes. However, a dose (as low as 100 nM) of taxol that is tolerable to normal cells, when complemented by the post-transcriptional gene silencing of Bcl-2, promoted up to 70% apoptosis in glioblastoma cells [42]. Because Bcl-2 inhibits of mitochondrial cytochrome c release, its arrest ultimately allows for one of the caspase cascades necessary for induction of apoptosis. These results were also demonstrated in vivo, with combined treatment using taxol and Bcl-2 siRNA promoting a complete inhibition of tumor angiogenesis and a decrease in tumor growth [43]. Thus, one potential adjuvant therapy to combat drug resistance is a direct attack on the problematic anti-apoptotic protein induced by so many other resistance mechanisms.
VII. Alternative Treatments
A. Flavonoids
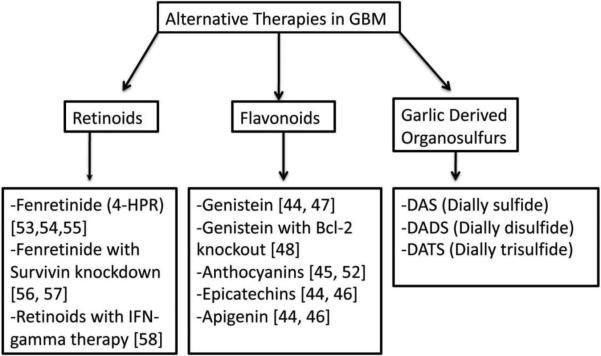
Considering the previous difficulties in finding a synthetic drug that does not interfere with the anti-cancer agent or the metabolic reactions of other medications, recent research has looked for a solution in herbal constituents (see Fig. 1). Current evidence supports the hypothesis that flavonoids can be a natural source of P-gp inhibitors. Bioflavinoids are natural pigments found in a variety of fruits and vegetables common to most diets. The maintenance of a significant effect on P-gp requires an intake of flavonoid concentration of 10 μM; a serving of orange juice alone contains a concentration of 330-740 μM [44]. The conjugated metabolite of flavonoids, however, is believed to have limited interaction with P-gp due to their status as organic anions. High concentration supplementation intravenously may be required to achieve the desired inhibitory effect [45]. Particular flavonoids, including apigenin, epigallocatechin, and genistein, were specifically shown to induce apoptosis of glioblastoma cells [46]. Other flavonoids, including anthocyanins, have also been studied for this purpose [47].
Fig. 1.
Alternative therapeutic aims against drug resistance in GBM.
Apigenin is a flavonoid commonly found in fruits and vegetables that has been studied in depth for its anti-inflammatory and anti-proliferative properties [46]. This compound has been found to increase reactive oxygen species (ROS) and phosphorylate p38 MAPK for inducing apoptosis [48]. In Das et al., apigenin has been demonstrated to selectively target GBM cells, sparing normal human astrocytes [46].
Genistein, an isoflavone estrogen receptor agonist, specifically inhibits protein tyrosine kinase, and it has been proven to inhibit cell growth and proliferation, as well as angiogenesis, when administered at a concentration just above physiological level [46,49]. A combination therapy of Bcl-2 knockout and genistein was examined in vitro and decrease in cell proliferation and increase in apoptosis occurred in the GBM cells [50]. Genistein has been show to act via an increase in Bax-Bcl2 ratio and activation of caspases-9, caspase-12, and caspase-3 [46].
Green tea has also provided a potential polyphenolic compound for treatment of glioblastoma. Chemoresistance has been studied in relation to endoplasmic reticulum stress resistance, and this pro-survival signaling comes partially from a specific stress marker, GRP78 [51]. Epigallocatechin 3-gallate (EGCG), an epicatechin (polyphenol) found in green tea, actually inhibits this stress marker. In vivo studies that administered this compound as an adjuvant with TMZ treatment found that it significantly enhanced TMZ response. Parallel in vitro studies that used siRNA targeting GRP78 also showed a similar affect when used in conjunction with TMZ [52]. Green tea catechins are also believed to inhibit matrix metalloproteinases (MMPs). MMPs are type IV collagenases involved in tumor proliferation by reconstruction of the basement membrane during key tumor growth periods, including tissue repair and angiogenesis [53]. A study by Demeule et al. examined the effects of a host of green tea epicatechins on MMP-2 and MMP-9, and the results appeared in accordance with the hypothesis that these tea-derived polyphenols, particularly EGCG, could have an inhibitory effect on MMPs, inhibiting tumor growth [53].
Similarly, the aglycons of anthocyanins, a flavonoid group that gives red and purple fruits their color, have shown potential as inhibitors of tumor growth [47]. In this initial report of the potential anti-cancer capacity of anthocyanins, researchers found that, while the tested agents did not inhibit cell proliferation, they did slow cell proliferation activity. The aglycons discussed include compounds such as cyanidin (Cy), delphinidin (Dp), and petunidin (Pt), the most potent of which is Dp. These agents essentially are believed to act on plasminogen activation, preventing the cell migration necessary for tumor growth [54].
B. Retinoids
While much of the current GBM treatment is centered on overcoming resistance to commonly used chemotherapies, another alternative is to identify new therapeutic agents that avoid the previously discussed pathways of resistance. One potential therapy is based on the use of retinoids. Most research indicates that retinoids, while successful at inducing differentiation, fail to induce apoptosis. We determined that N-(4-hydroxyphenyl) retinamide (4-HPR), also called fenretinide, a synthetic analog of all-trans retinoic acid (ATRA), can exhibit both anti-proliferative and pro-apoptotic effects [55]. 4-HPR has the potential for greater efficacy than its natural counterpart, ATRA, both because it has fewer side effects and because it has proven to be active even in ATRA-resistant cells [56]. This difference is due to the fact that 4-HPR causes apoptosis via retinoid receptor-independent mechanisms, unlike the naturally occurring compound [57]. In a study, we examined the effects of various concentrations of 4-HPR on two different GBM cell lines and determined that treatment with 4-HPR caused both differentiation and apoptosis in both GBM cell lines [55]. Differentiation occurred earlier during exposure, while apoptosis was a final stage event (72 hours) [55].
Another study from our laboratories confirms that use of 4-HPR in the treatment of GBM, particularly in conjunction with the knockdown of survivin [59]. Survivin is a pro-survival protein that is highly expressed in GBM and has been proven to positively correlate with tumor cell proliferation [58]. In our study of 4-HPR, we first down regulated survivin in two GBM cell lines using a surviving siRNA, and we followed this change with 4-HPR treatment. In vitro studies indicated that over 80% of cells embraced apoptosis with the combined therapy and in vivo angiogenesis studies showed a marked decrease in tumor vascularization [59].
In a related study, retinoids were used to treat GBM cells and it was found that retinoids induced astrocytic differentiation and inhibited telomerase activity, allowing for an increased sensitivity to Interferon-γ therapy [60]. The astrocytic differentiation was directly correlated to an increase in expression glialfibrillary acidic protein (GFAP), which stabilizes the microenvironment for normal astrocytes. Treatment with the retinoids also reduced levels of inflammatory factors, a phenomenon that could increase the efficacy of radiotherapy [61].
C. Ketogenic-restricted diet
The ketogenic-restricted (KR) diet is a low-carbohydrate, high fat diet most frequently used therapeutically in treatment of refractory seizures in children. However, several animal models and a case report suggest that a KR diet may target energy metabolism of tumors. It was found that tumor growth and angiogenesis were inhibited via dietary restrictions in the orthotopic mouse model of brain tumor [62-64]. These results have since been repeated numerous times [62-64]. Another study described the rapid regression of an elderly patient's GBM following treatment with standard therapy and a KR diet [65]. While further studies are needed to confirm the actual efficacy of this KR diet strategy, the animal model results and available case study suggest that this relatively simple dietary medication may, via its effects on tumor metabolism, limit the drug resistance that complicates standard therapies.
D. Garlic-derived organosulfur compounds
Garlic, a common ingredient in food products, has also been indicated as a potential source of anti-cancer therapeutics. The organosulfur garlic compounds diallylsuldife (DAS), diallyl disulfide (DADS), and diallyltrisulfide (DATS) have been shown to induce apoptosis in two different GBM cell lines, T98G and U87MG [66]. The primary mechanism of its success is the production of ROS, which provides a signal for the induction of apoptosis. Garlic compounds also elevate the intracellular concentrations of Ca2+ as well as several other signals for apoptosis (inhibition of BIRC proteins, activation of caspase-3 and caspase-4, increase in Bax-Bcl2 ratio) [66]. The in vivo effects of these compounds in both ectopic and orthotopic models are currently being studied in our laboratories.
VII. Future Directions
Ironically, current treatments may be the cause of drug resistance in GBM tumors. Most current therapies may enhance future drug resistance by destabilizing inherent cellular metabolism in small populations (perhaps even in cancer stem cells) of tumors [67]. Studies indicate that this disregulation takes the form of enhanced glycolytic activity, likely stimulating P-gp mediated resistance. Still, it is likely that intrinsic and extrinsic resistance to common therapies, including chemotherapeutic agents and radiation, come, not from one primary factor, but from a host of interconnected phenomena. Because efficacy of current therapies is so greatly affected by these mechanisms, future therapies must consider alternative and combination therapy, attacking various survival mechanisms that protect from apoptosis to promote growth. Perhaps some of the more promising potential primary and adjuvant therapies being studied are derived from natural sources. Glioblastoma is a heterogeneous and lethal cancer, and attacking its chemoresistance is one of the most important steps in combating its growth.
Acknowledgements
Completion of this project was made possible by funding from the National Institutes of Health (NIH) grants (NS-31622, NS-38146, NS-57811, and NS-41088), the State of South Carolina Spinal Cord Injury Research Fund (SCIRF), and Jerry Zucker Fund for Brain Tumor Research at the MUSC Foundation.
Footnotes
This paper is written in honor of Dr. Robert Ledeen.
References
- 1.Nicholas MK, Lukas RV, Chmura S, Yamini B, Lesniak M, Pytel P. Molecular heterogeneity in glioblastoma: therapeutic opportunities and challenges. Semin Oncol. 2011;38(2):243–253. doi: 10.1053/j.seminoncol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Weller M. Novel diagnostic and therapeutic approaches to malignant glioma. Swiss Med Wkly. 2011;141:w13210. doi: 10.4414/smw.2011.13210. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Goldie JH. Drug resistance in cancer: a perspective. Cancer Metastasis Rev. 2001;20(1-2):63–68. doi: 10.1023/a:1013164609041. [DOI] [PubMed] [Google Scholar]
- 5.Molnar J, Engi H, Hohmann J, Molnar P, Deli J, Wesolowska O, Michalak K, Wang Q. Reversal of multidrug resitance by natural substances from plants. Curr Top Med Chem. 2010;10(17):1757–1768. doi: 10.2174/156802610792928103. [DOI] [PubMed] [Google Scholar]
- 6.Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, Haga N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Sci. 2003;94(1):15–21. doi: 10.1111/j.1349-7006.2003.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9569-1. [Epub ahead of print]. PMID: 20963470. [DOI] [PubMed] [Google Scholar]
- 8.Zhao P, Zhang YZ, Sun MZ. [Regulatory effect of small interfering RNA targeting multidrug resistant protein 1 on chemosensitivity of human multiforme glioblastoma cell line BT325]. Ai Zheng. 2005;24(12):1436–1441. [PubMed] [Google Scholar]
- 9.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 10.Hubensack M, Muller C, Hocherl P, Fellner S, Spruss T, Bernhardt G, Buschauer A. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J Cancer Res Clin Oncol. 2008;134(5):597–607. doi: 10.1007/s00432-007-0323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wartenberg M, Richter M, Datchev A, Gunther S, Milosevic N, Bekhite MM, Figulla HR, Aran JM, Petriz J, Sauer H. Glycolytic pyruvate regulates P Glycoprotein expression in multicellular tumor spheroids via modulation of the intracellular redox state. J Cell Biochem. 2010;109(2):434–446. doi: 10.1002/jcb.22422. [DOI] [PubMed] [Google Scholar]
- 12.Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65(2):613–621. [PubMed] [Google Scholar]
- 13.Rittierodt M, Tschernig T, Harada K. Modulation of multidrug-resistance- associated P-glycoprotein in human U-87 MG and HUV-ECC cells with antisense oligodeoxynucleotides to MDR1 mRNA. Pathobiology. 2004;71(3):123–128. doi: 10.1159/000076466. [DOI] [PubMed] [Google Scholar]
- 14.Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001;28(2 Suppl 8):29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- 15.Oliver L, Olivier C, Marhuenda FB, Campone M, Vallette FM. Hypoxia and the malignant glioma microenvironment: regulation and implications for therapy. Curr Mol Pharmacol. 2009;2(3):263–284. doi: 10.2174/1874467210902030263. [DOI] [PubMed] [Google Scholar]
- 16.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62(12):3387–3394. [PubMed] [Google Scholar]
- 18.Brown JM. Tumor microenvironment and the response to anticancer therapy. Cancer Biol Ther. 2002;1(5):453–458. doi: 10.4161/cbt.1.5.157. [DOI] [PubMed] [Google Scholar]
- 19.Del Rowe J, Scott C, Werner-Wasik M, Bahary JP, Curran WJ, Urtasun RC, Fisher B. Single-arm, open-label phase II study of intravenously administered tirapazamine and radiation therapy for glioblastoma multiforme. J Clin Oncol. 2000;18(6):1254–1259. doi: 10.1200/JCO.2000.18.6.1254. [DOI] [PubMed] [Google Scholar]
- 20.Blagosklonny MV. How Avastin potentiates chemotherapeutic drugs: action and reaction in antiangiogenic therapy. Cancer Biol Ther. 2005;4(12):1307–1310. doi: 10.4161/cbt.4.12.2315. [DOI] [PubMed] [Google Scholar]
- 21.Dai S, Huang ML, Hsu CY, Chao KS. Inhibition of hypoxia inducible factor 1alpha causes oxygen-independent cytotoxicity and induces p53 independent apoptosis in glioblastoma cells. Int J Radiat Oncol Biol Phys. 2003;55(4):1027–1036. doi: 10.1016/s0360-3016(02)04507-8. [DOI] [PubMed] [Google Scholar]
- 22.Lin MI, Sessa WC. Antiangiogenic therapy: creating a unique “window” of opportunity. Cancer Cell. 2004;6(6):529–531. doi: 10.1016/j.ccr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Semenza GL, Artemov D, Bedi A, Bhujwalla Z, Chiles K, Feldser D, Laughner E, Ravi R, Simons J, Taghavi P, Zhong H. 'The metabolism of tumours': 70 years later. Novartis Found Symp. 2001;240:251–260. discussion 260-254. [PubMed] [Google Scholar]
- 24.Zhang J, Stevens MF, Laughton CA, Madhusudan S, Bradshaw TD. Acquired resistance to temozolomide in glioma cell lines: molecular mechanisms and potential translational applications. Oncology. 2010;78(2):103–114. doi: 10.1159/000306139. [DOI] [PubMed] [Google Scholar]
- 25.Suri V, Jha P, Sharma MC, Sarkar C. O6 -methylguanine DNA methyltransferase gene promoter methylation in high-grade gliomas: a review of current status. Neurol India. 2011;59(2):229–235. doi: 10.4103/0028-3886.79128. [DOI] [PubMed] [Google Scholar]
- 26.Cahill DP, Levine KK, Betensky RA, Codd PJ, Romany CA, Reavie LB, Batchelor TT, Futreal PA, Stratton MR, Curry WT, Iafrate AJ, Louis DN. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007;13(7):2038–2045. doi: 10.1158/1078-0432.CCR-06-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26(25):4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 28.Perry JR, Belanger K, Mason WP, Fulton D, Kavan P, Easaw J, Shields C, Kirby S, Macdonald DR, Eisenstat DD, Thiessen B, Forsyth P, Pouliot JF. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(12):2051–2057. doi: 10.1200/JCO.2009.26.5520. [DOI] [PubMed] [Google Scholar]
- 29.Gallo C, Buonerba C, Di Lorenzo G, Romeo V, De Placido S, Marinelli A. Can high-dose fotemustine reverse MGMT resistance in glioblastoma multiforme? J Neurooncol. 2010;100(2):311–319. doi: 10.1007/s11060-010-0168-y. [DOI] [PubMed] [Google Scholar]
- 30.Shi L, Chen J, Yang J, Pan T, Zhang S, Wang Z. MiR-21 protected human glioblastoma U87MG cells from chemotherapeutic drug temozolomide induced apoptosis by decreasing Bax/Bcl-2 ratio and caspase-3 activity. Brain Res. 2010;1352:255–264. doi: 10.1016/j.brainres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Li W, Yang Y, Lu Y, He C, Hu G, Liu H, Chen J, He J, Yu H. MicroRNA-21 targets LRRFIP1 and contributes to VM-26 resistance in glioblastoma multiforme. Brain Res. 2009;1286:13–18. doi: 10.1016/j.brainres.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 32.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3(1):17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 33.Huang Z, Cheng L, Guryanova OA, Wu Q, Bao S. Cancer stem cells in glioblastoma--molecular signaling and therapeutic targeting. Protein Cell. 2010;1(7):638–655. doi: 10.1007/s13238-010-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 35.Folkins C, Shaked Y, Man S, Tang T, Lee CR, Zhu Z, Hoffman RM, Kerbel RS. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69(18):7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 37.Carrassa L, Damia G. Unleashing Chk1 in cancer therapy. Cell Cycle. 2011;10(13):2121–2128. doi: 10.4161/cc.10.13.16398. [DOI] [PubMed] [Google Scholar]
- 38.Cheng L, Wu Q, Huang Z, Guryanova OA, Huang Q, Shou W, Rich JN, Bao S. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. EMBO J. 2011;30(5):800–813. doi: 10.1038/emboj.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sul J, Fine HA. Malignant gliomas: new translational therapies. Mt Sinai J Med. 2010;77(6):655–666. doi: 10.1002/msj.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purow BW, Sundaresan TK, Burdick MJ, Kefas BA, Comeau LD, Hawkinson MP, Su Q, Kotliarov Y, Lee J, Zhang W, Fine HA. Notch-1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis. 2008;29(5):918–925. doi: 10.1093/carcin/bgn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George J, Banik NL, Ray SK. Bcl-2 siRNA augments taxol mediated apoptotic death in human glioblastoma U138MG and U251MG cells. Neurochem Res. 2009;34(1):66–78. doi: 10.1007/s11064-008-9659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George J, Banik NL, Ray SK. Combination of taxol and Bcl-2 siRNA induces apoptosis in human glioblastoma cells and inhibits invasion, angiogenesis and tumour growth. J Cell Mol Med. 2009;13(10):4205–4218. doi: 10.1111/j.1582-4934.2008.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bansal T, Jaggi M, Khar RK, Talegaonkar S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J Pharm Pharm Sci. 2009;12(1):46–78. doi: 10.18433/j3rc77. [DOI] [PubMed] [Google Scholar]
- 45.Morris ME, Zhang S. Flavonoid-drug interactions: effects of flavonoids on ABC transporters. Life Sci. 2006;78(18):2116–2130. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010;116(1):164–176. doi: 10.1002/cncr.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Vareed SK, Nair MG. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005;76(13):1465–1472. doi: 10.1016/j.lfs.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 48.Vargo MA, Voss OH, Poustka F, Cardounel AJ, Grotewold E, Doseff AI. Apigenin-induced-apoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem Pharmacol. 2006;72(6):681–692. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Ravindranath MH, Muthugounder S, Presser N, Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol. 2004;546:121–165. doi: 10.1007/978-1-4757-4820-8_11. [DOI] [PubMed] [Google Scholar]
- 50.George J, Banik NL, Ray SK. Genistein induces receptor and mitochondrial pathways and increases apoptosis during Bcl-2 knockdown in human malignant neuroblastoma SK-N-DZ cells. J Neurosci Res. 2010;88(4):877–886. doi: 10.1002/jnr.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer research. 2007;67(20):9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 52.Chen TC, Wang W, Golden EB, Thomas S, Sivakumar W, Hofman FM, Louie SG, Schonthal AH. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011;302(2):100–108. doi: 10.1016/j.canlet.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Demeule M, Brossard M, Page M, Gingras D, Beliveau R. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 2000;1478(1):51–60. doi: 10.1016/s0167-4838(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 54.Lamy S, Lafleur R, Bedard V, Moghrabi A, Barrette S, Gingras D, Beliveau R. Anthocyanidins inhibit migration of glioblastoma cells: structure-activity relationship and involvement of the plasminolytic system. J Cell Biochem. 2007;100(1):100–111. doi: 10.1002/jcb.21023. [DOI] [PubMed] [Google Scholar]
- 55.Das A, Banik NL, Ray SK. N-(4-Hydroxyphenyl) retinamide induced both differentiation and apoptosis in human glioblastoma T98G and U87MG cells. Brain Res. 2008;1227:207–215. doi: 10.1016/j.brainres.2008.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ulukaya E, Pirianov G, Kurt MA, Wood EJ, Mehmet H. Fenretinide induces cytochrome c release, caspase 9 activation and apoptosis in the absence of mitochondrial membrane depolarisation. Cell Death Differ. 2003;10(7):856–859. doi: 10.1038/sj.cdd.4401242. [DOI] [PubMed] [Google Scholar]
- 57.Lippman SM, Lotan R. Advances in the development of retinoids as chemopreventive agents. J Nutr. 2000;130(2S Suppl):479S–482S. doi: 10.1093/jn/130.2.479S. [DOI] [PubMed] [Google Scholar]
- 58.Mellai M, Caldera V, Patrucco A, Annovazzi L, Schiffer D. Survivin expression in glioblastomas correlates with proliferation, but not with apoptosis. Anticancer Res. 2008;28(1A):109–118. [PubMed] [Google Scholar]
- 59.George J, Banik NL, Ray SK. Survivin knockdown and concurrent 4-HPR treatment controlled human glioblastoma in vitro and in vivo. Neuro Oncol. 2010;12(11):1088–1101. doi: 10.1093/neuonc/noq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das A, Banik NL, Ray SK. Molecular mechanisms of the combination of retinoid and interferon-gamma for inducing differentiation and increasing apoptosis in human glioblastoma T98G and U87MG cells. Neurochemical research. 2009;34(1):87–101. doi: 10.1007/s11064-008-9669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Das A, Banik NL, Ray SK. Retinoids induce differentiation and downregulate telomerase activity and N-Myc to increase sensitivity to flavonoids for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Int J Oncol. 2009;34(3):757–765. doi: 10.3892/ijo_00000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukherjee P, Abate LE, Seyfried TN. Antiangiogenic and proapoptotic effects of dietary restriction on experimental mouse and human brain tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(16):5622–5629. doi: 10.1158/1078-0432.CCR-04-0308. [DOI] [PubMed] [Google Scholar]
- 63.Marsh J, Mukherjee P, Seyfried TN. Akt-dependent proapoptotic effects of dietary restriction on late-stage management of a phosphatase and tensin homologue/tuberous sclerosis complex 2-deficient mouse astrocytoma. Clin Cancer Res. 2008;14(23):7751–7762. doi: 10.1158/1078-0432.CCR-08-0213. [DOI] [PubMed] [Google Scholar]
- 64.Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 2007;4:5. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, Seyfried TN. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metab (Lond) 2010;7:33. doi: 10.1186/1743-7075-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Das A, Banik NL, Ray SK. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer. 2007;110(5):1083–1095. doi: 10.1002/cncr.22888. [DOI] [PubMed] [Google Scholar]
- 67.Seyfried TN, Shelton LM, Mukherjee P. Does the existing standard of care increase glioblastoma energy metabolism? Lancet Oncol. 2010;11(9):811–813. doi: 10.1016/S1470-2045(10)70166-2. [DOI] [PubMed] [Google Scholar]