Abstract
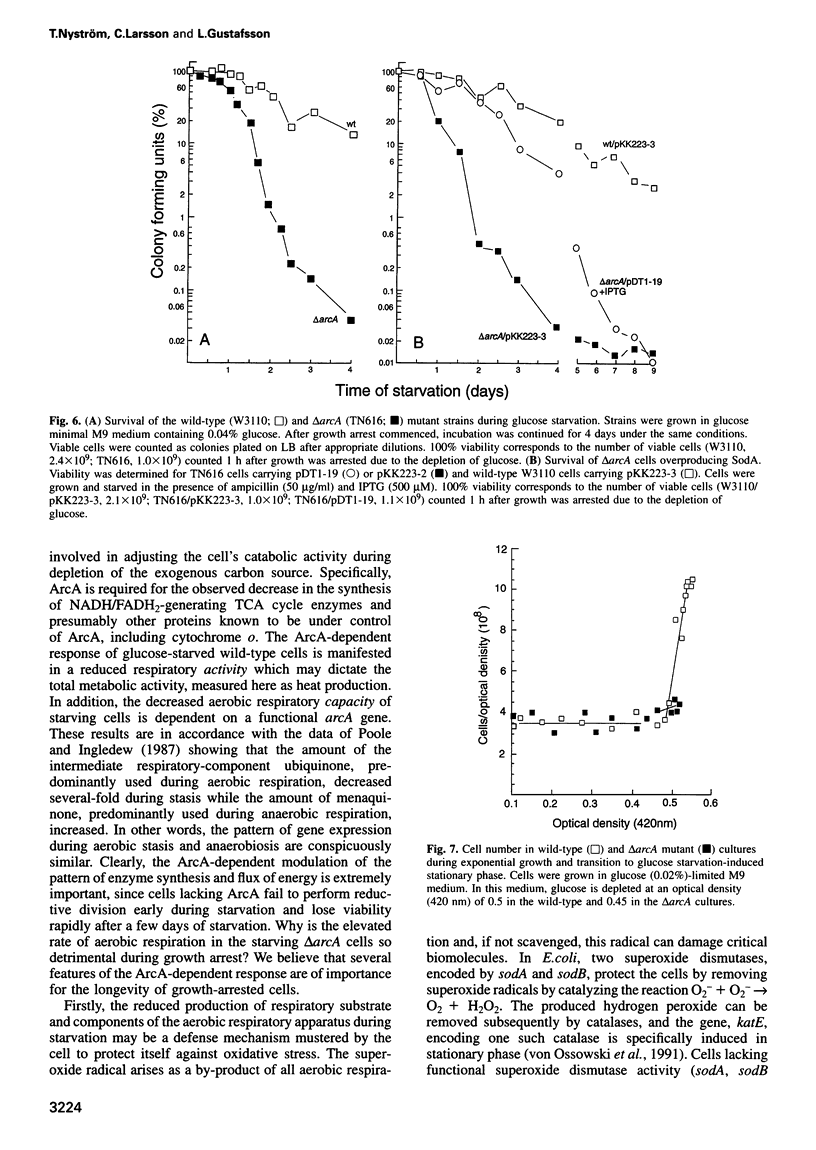
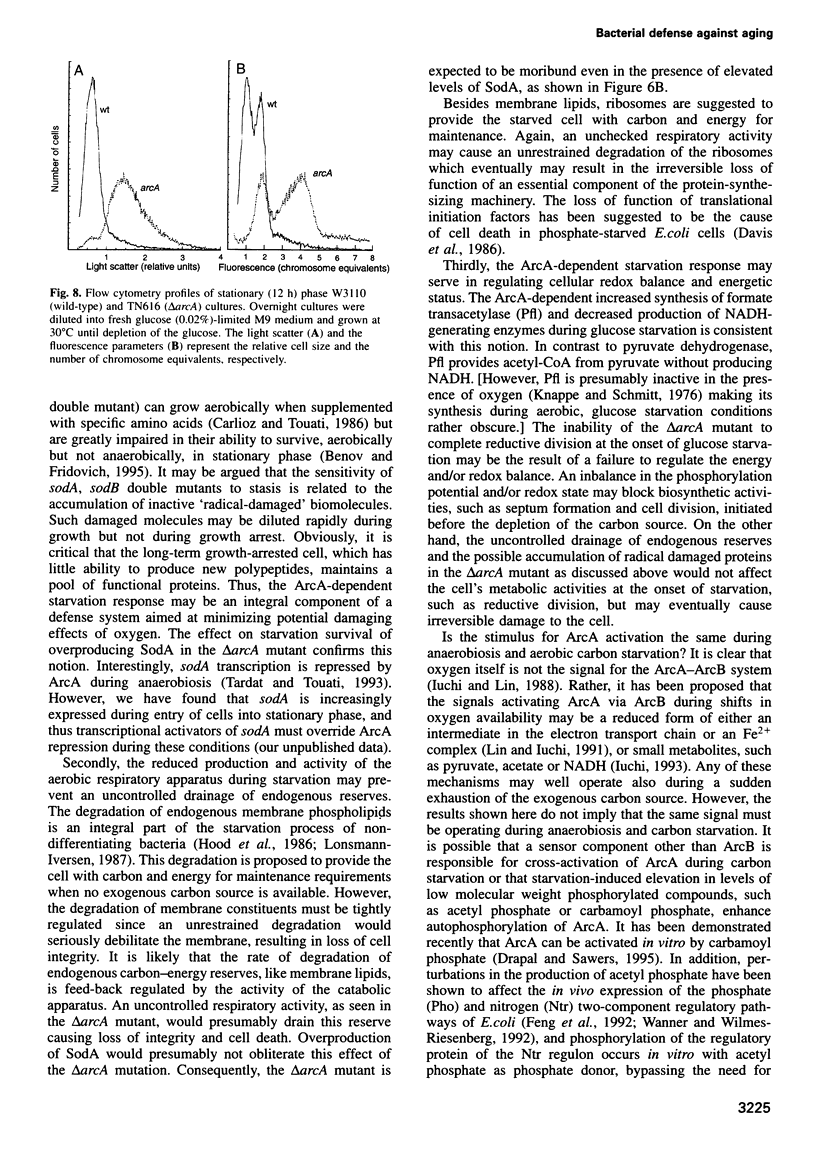
Using two-dimensional gel electrophoresis and N-terminal amino acid sequencing analysis, we demonstrate that a mutant of the global regulatory protein ArcA fails to decrease the synthesis of the TCA cycle enzymes malate dehydrogenase, isocitrate dehydrogenase, lipoamide dehydrogenase E3 and succinate dehydrogenase in response to stasis, while the increased production of the glycolysis enzymes phosphoglycerate mutase and pyruvate kinase is unaffected. Microcalorimetric and respiratory measurements show that the continued production of TCA cycle enzymes in the (delta)arcA mutant is manifested as an elevated rate of respiration and total metabolic activity during starvation. The (delta)arcA mutant is severely impaired in surviving prolonged periods of exogenous carbon starvation, a phenotype that can be alleviated by overproducing the superoxide dismutase SodA. In addition, flow cytometry demonstrates that starving (delta)arcA mutant cells, in contrast to wild-type cells, fail to perform reductive division, remain large and contain multiple chromosomal copies. We suggest that the ArcA-dependent reduced production of electron donors and the decreased level and activity of the aerobic respiratory apparatus during growth arrest is an integral part of a defense system aimed at avoiding the damaging effects of oxygen radicals and controlling the rate of utilization of endogenous reserves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlund T., Nordström K., Bernander R. Analysis of cell size and DNA content in exponentially growing and stationary-phase batch cultures of Escherichia coli. J Bacteriol. 1995 Dec;177(23):6791–6797. doi: 10.1128/jb.177.23.6791-6797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benov L., Fridovich I. A superoxide dismutase mimic protects sodA sodB Escherichia coli against aerobic heating and stationary-phase death. Arch Biochem Biophys. 1995 Sep 10;322(1):291–294. doi: 10.1006/abbi.1995.1465. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. D., Luger S. M., Tai P. C. Role of ribosome degradation in the death of starved Escherichia coli cells. J Bacteriol. 1986 May;166(2):439–445. doi: 10.1128/jb.166.2.439-445.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapal N., Sawers G. Purification of ArcA and analysis of its specific interaction with the pfl promoter-regulatory region. Mol Microbiol. 1995 May;16(3):597–607. doi: 10.1111/j.1365-2958.1995.tb02422.x. [DOI] [PubMed] [Google Scholar]
- Feng J., Atkinson M. R., McCleary W., Stock J. B., Wanner B. L., Ninfa A. J. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J Bacteriol. 1992 Oct;174(19):6061–6070. doi: 10.1128/jb.174.19.6061-6070.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groat R. G., Schultz J. E., Zychlinsky E., Bockman A., Matin A. Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol. 1986 Nov;168(2):486–493. doi: 10.1128/jb.168.2.486-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller T., Ortner M., Gnaiger E. A respirometer for investigating oxidative cell metabolism: toward optimization of respiratory studies. Anal Biochem. 1994 May 1;218(2):338–342. doi: 10.1006/abio.1994.1188. [DOI] [PubMed] [Google Scholar]
- Hood M. A., Guckert J. B., White D. C., Deck F. Effect of nutrient deprivation on lipid, carbohydrate, DNA, RNA, and protein levels in Vibrio cholerae. Appl Environ Microbiol. 1986 Oct;52(4):788–793. doi: 10.1128/aem.52.4.788-793.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. Adaptation of Escherichia coli to redox environments by gene expression. Mol Microbiol. 1993 Jul;9(1):9–15. doi: 10.1111/j.1365-2958.1993.tb01664.x. [DOI] [PubMed] [Google Scholar]
- Iuchi S., Lin E. C. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S. Phosphorylation/dephosphorylation of the receiver module at the conserved aspartate residue controls transphosphorylation activity of histidine kinase in sensor protein ArcB of Escherichia coli. J Biol Chem. 1993 Nov 15;268(32):23972–23980. [PubMed] [Google Scholar]
- Jenkins D. E., Schultz J. E., Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P. From genome to proteome: looking at a cell's proteins. Science. 1995 Oct 20;270(5235):369–370. doi: 10.1126/science.270.5235.369. [DOI] [PubMed] [Google Scholar]
- Knappe J., Schmitt T. A novel reaction of S-adenosyl-L-methionine correlated with the activation of pyruvate formate-lyase. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1110–1117. doi: 10.1016/0006-291x(76)90768-3. [DOI] [PubMed] [Google Scholar]
- Lange R., Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Li C., Clarke S. A protein methyltransferase specific for altered aspartyl residues is important in Escherichia coli stationary-phase survival and heat-shock resistance. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9885–9889. doi: 10.1073/pnas.89.20.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C., Iuchi S. Regulation of gene expression in fermentative and respiratory systems in Escherichia coli and related bacteria. Annu Rev Genet. 1991;25:361–387. doi: 10.1146/annurev.ge.25.120191.002045. [DOI] [PubMed] [Google Scholar]
- McCann M. P., Kidwell J. P., Matin A. The putative sigma factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J Bacteriol. 1991 Jul;173(13):4188–4194. doi: 10.1128/jb.173.13.4188-4194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T., Flärdh K., Kjelleberg S. Responses to multiple-nutrient starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1990 Dec;172(12):7085–7097. doi: 10.1128/jb.172.12.7085-7097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T., Neidhardt F. C. Cloning, mapping and nucleotide sequencing of a gene encoding a universal stress protein in Escherichia coli. Mol Microbiol. 1992 Nov;6(21):3187–3198. doi: 10.1111/j.1365-2958.1992.tb01774.x. [DOI] [PubMed] [Google Scholar]
- Nyström T., Neidhardt F. C. Expression and role of the universal stress protein, UspA, of Escherichia coli during growth arrest. Mol Microbiol. 1994 Feb;11(3):537–544. doi: 10.1111/j.1365-2958.1994.tb00334.x. [DOI] [PubMed] [Google Scholar]
- Nyström T., Olsson R. M., Kjelleberg S. Survival, stress resistance, and alterations in protein expression in the marine vibrio sp. strain S14 during starvation for different individual nutrients. Appl Environ Microbiol. 1992 Jan;58(1):55–65. doi: 10.1128/aem.58.1.55-65.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
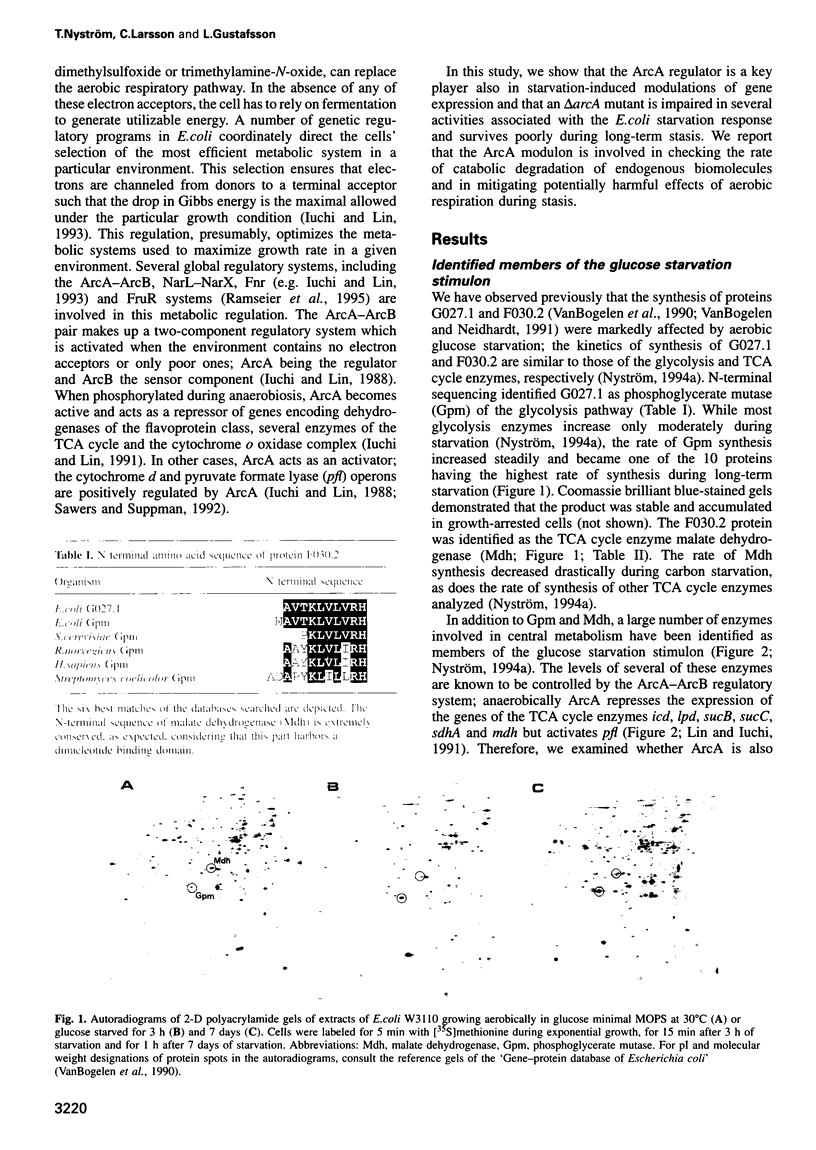
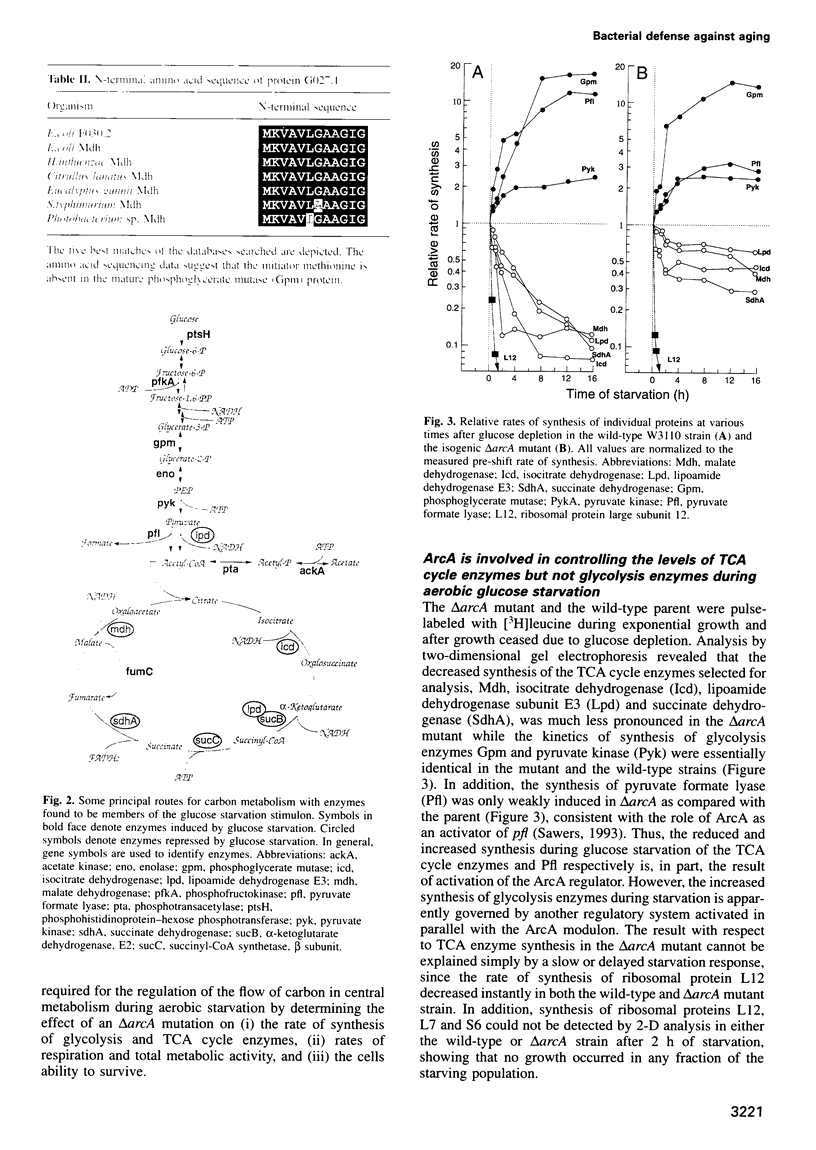
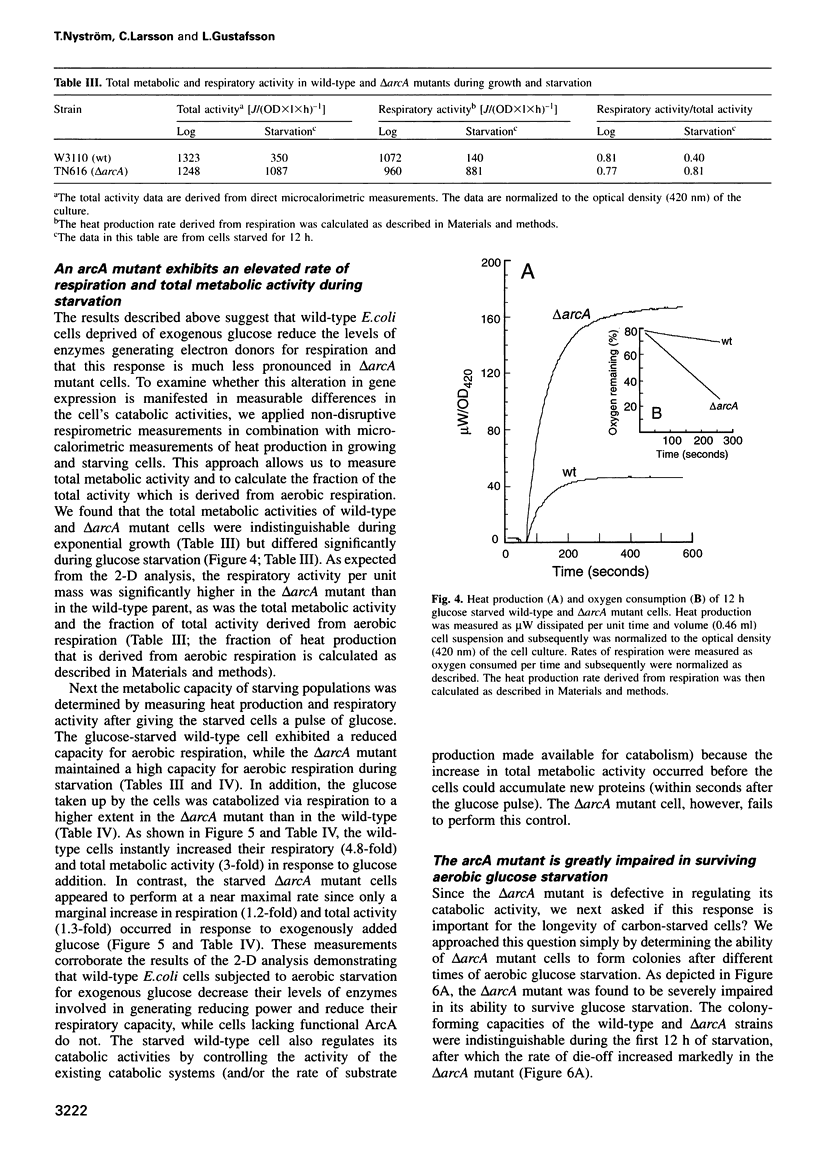
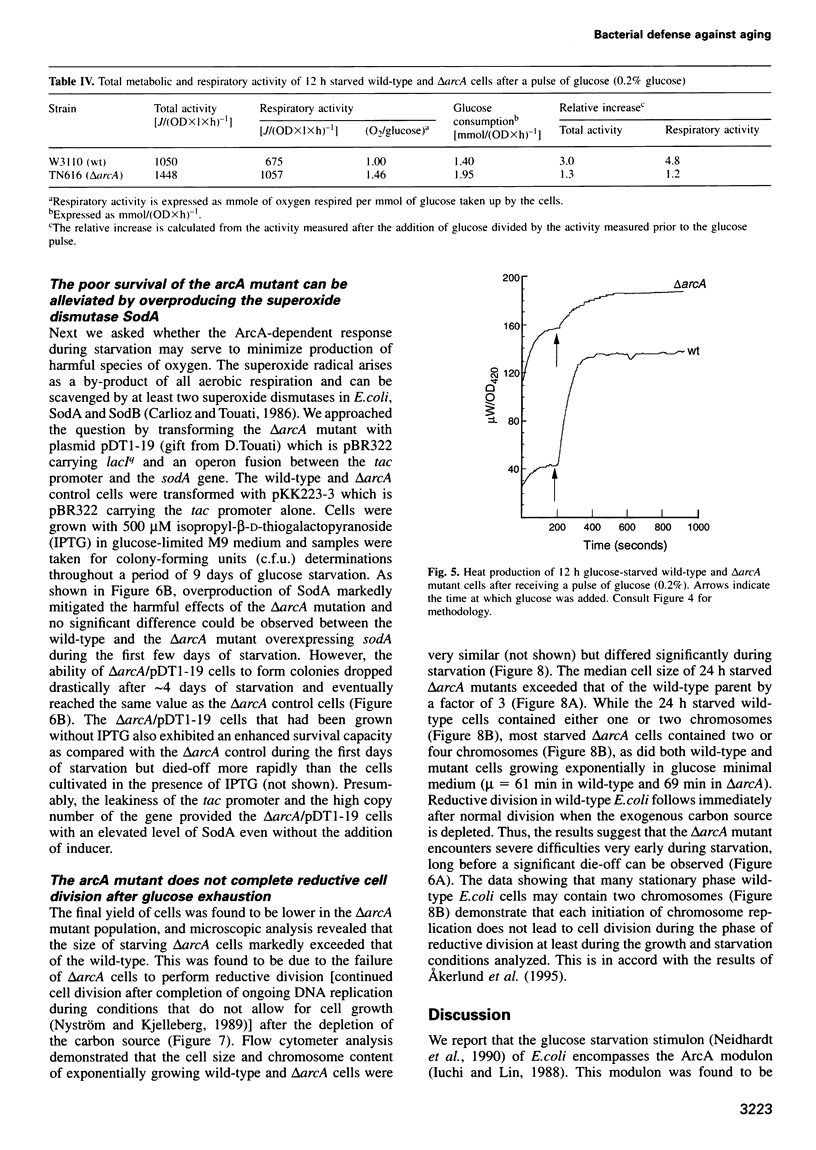
- Nyström T. The glucose-starvation stimulon of Escherichia coli: induced and repressed synthesis of enzymes of central metabolic pathways and role of acetyl phosphate in gene expression and starvation survival. Mol Microbiol. 1994 Jun;12(5):833–843. doi: 10.1111/j.1365-2958.1994.tb01069.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olz R., Larsson K., Adler L., Gustafsson L. Energy flux and osmoregulation of Saccharomyces cerevisiae grown in chemostats under NaCl stress. J Bacteriol. 1993 Apr;175(8):2205–2213. doi: 10.1128/jb.175.8.2205-2213.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Reeh S. V. Analysis of the proteins synthesized in ultraviolet light-irradiated Escherichia coli following infection with the bacteriophages lambdadrifd 18 and lambdadfus-3. Mol Gen Genet. 1976 Mar 30;144(3):339–343. doi: 10.1007/BF00341733. [DOI] [PubMed] [Google Scholar]
- Ramseier T. M., Bledig S., Michotey V., Feghali R., Saier M. H., Jr The global regulatory protein FruR modulates the direction of carbon flow in Escherichia coli. Mol Microbiol. 1995 Jun;16(6):1157–1169. doi: 10.1111/j.1365-2958.1995.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Sawers G. Specific transcriptional requirements for positive regulation of the anaerobically inducible pfl operon by ArcA and FNR. Mol Microbiol. 1993 Nov;10(4):737–747. doi: 10.1111/j.1365-2958.1993.tb00944.x. [DOI] [PubMed] [Google Scholar]
- Sawers G., Suppmann B. Anaerobic induction of pyruvate formate-lyase gene expression is mediated by the ArcA and FNR proteins. J Bacteriol. 1992 Jun;174(11):3474–3478. doi: 10.1128/jb.174.11.3474-3478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by aerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):344–350. doi: 10.1128/jb.154.1.344-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal R. S., Orr W. C. Relationship between antioxidants, prooxidants, and the aging process. Ann N Y Acad Sci. 1992 Nov 21;663:74–84. doi: 10.1111/j.1749-6632.1992.tb38651.x. [DOI] [PubMed] [Google Scholar]
- Spector M. P., Cubitt C. L. Starvation-inducible loci of Salmonella typhimurium: regulation and roles in starvation-survival. Mol Microbiol. 1992 Jun;6(11):1467–1476. doi: 10.1111/j.1365-2958.1992.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Spence J., Cegielska A., Georgopoulos C. Role of Escherichia coli heat shock proteins DnaK and HtpG (C62.5) in response to nutritional deprivation. J Bacteriol. 1990 Dec;172(12):7157–7166. doi: 10.1128/jb.172.12.7157-7166.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardat B., Touati D. Iron and oxygen regulation of Escherichia coli MnSOD expression: competition between the global regulators Fur and ArcA for binding to DNA. Mol Microbiol. 1993 Jul;9(1):53–63. doi: 10.1111/j.1365-2958.1993.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Tormo A., Almirón M., Kolter R. surA, an Escherichia coli gene essential for survival in stationary phase. J Bacteriol. 1990 Aug;172(8):4339–4347. doi: 10.1128/jb.172.8.4339-4347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler R. H., Brar H., Singh M., Latorre A., Graves J. L., Mueller L. D., Rose M. R., Ayala F. J. The effect of superoxide dismutase alleles on aging in Drosophila. Genetica. 1993;91(1-3):143–149. doi: 10.1007/BF01435994. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Hutton M. E., Neidhardt F. C. Gene-protein database of Escherichia coli K-12: edition 3. Electrophoresis. 1990 Dec;11(12):1131–1166. doi: 10.1002/elps.1150111205. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Neidhardt F. C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Neidhardt F. C. The gene-protein database of Escherichia coli: edition 4. Electrophoresis. 1991 Nov;12(11):955–994. doi: 10.1002/elps.1150121114. [DOI] [PubMed] [Google Scholar]
- Volk A., Crémieux A. C., Belmatoug N., Vallois J. M., Pocidalo J. J., Carbon C. Evaluation of a rabbit model for osteomyelitis by high field, high resolution imaging using the chemical-shift-specific-slice-selection technique. Magn Reson Imaging. 1994;12(7):1039–1046. doi: 10.1016/0730-725x(94)91235-o. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Wilmes-Riesenberg M. R. Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli. J Bacteriol. 1992 Apr;174(7):2124–2130. doi: 10.1128/jb.174.7.2124-2130.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R. Superoxide dismutase, aging, and degenerative disease. Free Radic Biol Med. 1994 Sep;17(3):249–258. doi: 10.1016/0891-5849(94)90080-9. [DOI] [PubMed] [Google Scholar]
- von Ossowski I., Mulvey M. R., Leco P. A., Borys A., Loewen P. C. Nucleotide sequence of Escherichia coli katE, which encodes catalase HPII. J Bacteriol. 1991 Jan;173(2):514–520. doi: 10.1128/jb.173.2.514-520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]






