Abstract
Purpose
The objectives of this study were to characterise the sexual health of street-connected adolescents in Eldoret, Kenya, analyse gender disparity of risks, estimate the prevalence of sexually transmitted infections (STIs), and identify factors associated with STIs.
Methods
A cross-sectional study of street-connected adolescents ages 12–21 years was conducted in Eldoret, Kenya. Participants were interviewed and screened for Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, herpes simplex virus-2, syphilis and HIV. Descriptive statistics and logistic regression were used to identify factors associated with having any STI.
Results
Of the 200 participants, 81 (41%) were female. 70.4% of females and 60.5% of males reported sexual activity. Of those that participated in at least one STI test, 28% (55/194) had ≥1 positive test, including 56% of females; 14% (28/194) had >1 positive test. Twelve females and zero males (6% overall, 14.8% of females) were HIV positive. Among females, those with HIV infection more frequently reported transactional sex (66.7% vs 26.1%, p=0.01), drug use (91.7% vs 56.5%, p=0.02), and reported a prior STI (50.0% vs 14.7%, p<0.01). Having an adult caregiver was less likely among those with HIV infection (33.3% vs 71.0%, p=0.04). Transactional sex (AOR 3.02, 95% CI (1.05 to 8.73)), a previous STI (AOR 3.46 95% CI (1.05 to 11.46)) and ≥2 sexual partners (AOR 5.62 95% (1.67 to 18.87)) were associated with having any STI.
Conclusions
Street-connected adolescents in Eldoret, Kenya are engaged in high-risk sexual behaviours and females in particular have a substantial burden of STIs and HIV. There is a need for STI interventions targeted to street-connected youth.
Keywords: ADOLESCENT, AFRICA, HIV, SCREENING, EPIDEMIOLOGY (GENERAL)
Introduction
There are estimated to be tens of millions of street-connected children and youth (SCCY) worldwide.1 Urbanisation, diseases such as HIV/AIDS, poverty and civil unrest, contribute to growing numbers. SCCY are a distinctly vulnerable population, often exposed to abuse, neglect, parental abandonment or death, and extreme poverty.1 These experiences drive them to the street, and increase their vulnerability to exploitation and engagement in risky behaviours. In Kenya, up to 300 000 children and adolescents have been reported to be street-connected.2 Most are in the large cities, including Eldoret, the fifth largest city in Kenya.2 3
Adult HIV prevalence in Kenya is estimated to be 6.2% nationally with higher rates in urban areas and near Lake Victoria.4 Women are disproportionately affected; twice the rate of men.4 National HIV prevalence among youth (ages 15–24 years) is lower, 1.7–4.2%.4 5 Kenya is also home to an estimated 1.1 million orphans who have lost one or both parents to HIV/AIDS.4
The burden of sexually transmitted infections (STIs) and HIV among SCCY in sub-Saharan Africa has yet to be well described. Data from North America demonstrate that SCCY are disproportionately affected by HIV and STIs.6–11 Available data regarding STIs and HIV among SCCY in low-income and middle-income countries is more limited, but also suggests a high burden of disease in this population.12 However, data specific to SCCY in sub-Saharan Africa is scarce, with most STI data assessed by self-report.12 Sub-Saharan Africa studies of out-of-school youth have demonstrated more risk behaviours and higher rates of STIs and HIV than those in school.13–15 Data specific to SCCY report engagement in high-risk behaviours including transactional sex and inconsistent condom use, and limited HIV knowledge.16–20 Drug use is common.14 21 22 Yet, in this area of high rates of HIV, data regarding STI and HIV prevalence among this at-risk population remain limited.
The primary objectives of this study were to characterise the sexual risk behaviours of street-connected adolescents in Eldoret, Kenya, analyse gender disparity of these risks, to estimate their prevalence of STIs including HIV, and to identify factors associated with prevalent infections.
Methods
The methods for the survey have been described elsewhere.23 Briefly, 200 SCCY were enrolled between September 2011 and June 2012 in this cross-sectional study. A sample size of 200 participants was selected as an achievable sample size of this hard-to-reach population. Participants were recruited using a combination of convenience and venue-based sampling by approaching youth at several SCCY support organisations, and from known hang-outs around town. Screening and enrolment took place at a dedicated study clinic located at Moi Teaching and Referral Hospital (MTRH), and to address gender disparity at Berur, an Eldoret community organisation working specifically with street girls.
Youth were eligible if they were 12–21 years of age and spent their days only (ie, ‘children on the street’), or nights and days on the streets (ie, ‘children of the street’), and were able to consent or assent. All recruited youth were offered compensation for transportation (50 Kenyan Shilling (KES), US$0.70) and two pens and a workbook whether or not they chose to complete the study. Foot care supplies (soap, water, brush and nail clippers) were available for use at enrolment sites whether or not individuals chose to participate. Females were additionally offered a package of sanitary napkins and a pair of underwear to address gender disparity in enrolment.
Data collection
Study personnel interviewed participants privately and administered a structured questionnaire collecting information regarding sociodemographics, street life, risk behaviours, abuse and exploitation, and access to reproductive healthcare (30–45 min in length).
Following the questionnaire, participants were provided verbal, written and pictorial instruction on self-collection of vaginal and rectal swabs. For males, first-void urine samples were collected. Phlebotomy was performed by trained study personnel. Any participant reporting active STI symptoms was evaluated and treated following WHO syndromic management guidelines (local standard of care).24 Participants received HIV counselling and point-of-care rapid HIV testing by trained counsellors. All participants were counselled about HIV regardless of decision to undergo testing. HIV-positive participants not actively in care were linked to care. Participants reporting prior known HIV diagnosis were not retested unless requested by the participant, but were included in the analysis. STI tests were batched and run on completion of enrolment. Urine samples, vaginal and rectal swabs were tested via nucleic acid amplification tests for Neisseria gonorrhoeae, Chlamydia trachomatis and Trichomonas vaginalis.25 26 Blood was tested with rapid plasma reagin (Immunotrep RPR, Omega Diagnostics, UK) with positive tests confirmed by Treponemal palladium haemagglutination assay (Immunotrep TPHA Omega Diagnostics, UK) and herpes simplex virus-2 (HSV-2) antibodies (HSV Type 2 IgG ELISA, Kalon Biological, UK). Participants with positive tests, not previously treated at the initial visit were sought out using locator information obtained at initial study visit and offered treatment following WHO recommendations.24
Measures
Sociodemographic variables
Sociodemographic data included age, gender, orphan status, adult caregiver in life and frequency of interaction, educational level, length of time the individual has been connected to the street, and whether the individual spends their days only or days and nights on the street. Several youth reported spending days on the street, and nights with other SCCY without adult supervision; these participants are considered ‘of the street’ here. Data regarding daily income, police involvement, and reason for coming to the streets, any drug use and alcohol use are also reported.
Sexual risk behaviors
Sexual risk behaviour variables included sexual activity, age at sexual debut, first sexual encounter involuntary/voluntary, age of first partner, gender of partners, oral/vaginal/anal sex, transactional sex, involuntary sex ever, number of partners, condom use at last sex, condom use overall, access to condoms, and access to free condoms. Prior STI/HIV testing and care variables included prior STI symptoms (genital ulcer or discharge), if yes, sought care, knowledge of STI/ HIV testing locations, comfort going there, previous HIV testing, and HIV test in last 6 months.
Data analysis
Checks for errors and inconsistencies were performed before data were entered into an EpiInfo database. Categorical variables were summarised using proportions while continuous variables were summarised using means and medians together with SDs and IQRs.
Bivariate analysis was used to examine associations between gender and other variables using χ2 test or Fisher's exact test (where cells had an expected value of less than 5). We employed χ2 test to evaluate the relationship between sociodemographic and sexual risk variables with the following two outcomes: (1) HIV positive or (2) any STI. The sociodemographic and sexual risk variables included were age (median), forced sex ever, transactional sex ever, number of sexual partners (median), first sex partner >5 years older, adult caregiver in life, alcohol use, any drug use, police involvement, belonging to a gang, alcoholism/violence at home (versus all other reasons for leaving home), condom use at last sex and prior STI.
Multivariate analysis of factors associated with any STI
All participants who reported sexual activity and participated in testing were included in the multivariable analysis; having any STI was the dependent variable. We tested several sociodemographic and risk behaviour variables in our model based on a priori hypotheses as explanatory variables or potential confounders: gender, age, orphan status, legal guardian presence, having an adult caregiver in their life, ‘on’ versus ‘of’ the street, length of time street-connected, education level, income, sleeps with friends versus other street youth, belonging to a gang and alcohol/violence at home. Based on their association with our outcome on independent analysis, gender, age and education level were ultimately included in our multivariate models. The following exposure variables were tested in individual multivariable logistic regression models adjusted for gender, age and education: any STI test positivity, any alcohol use, first partner >5 years older, transactional sex, forced sex, prior STI, ≥two partners and condom use at last vaginal sex. All analyses used SAS V.9.3.
Human subjects protection
This study received ethical approval from the Kenyan Institutional Research and Ethics Committee of Moi Teaching and Referral Hospital and Moi University College of Health Sciences (Eldoret, Kenya) (IREC FAN 00609, IREC 2010/156), the Indiana University Institutional Review Board (IRB) (Indianapolis, USA) (11020044764), and the Miriam Hospital IRB (Providence, USA) (204011). Approval for the study was also obtained from the District Children's Officer (DCO) in Eldoret.
Results
Sociodemographics
Two hundred and three participants underwent the screening and enrolment process; two failed to meet comprehension assessment criteria, and one failed to meet age criteria. Of 200 adolescents enrolled, 81 (41%) were female (see table 1). Median age was 16 years (IQR 13–19 years), older for females (18 years, IQR 15–20 years) than males (15 years, IQR 13–18 years, p<0.01). Most participants (71%) had at least one parent deceased. The majority of participants reported an adult caregiver in their life, though 52.9% of males and 32.1% of females reported seeing this person less than once a month. Most participants reported some education with more females having attended beyond standard (Grade) 4 (70.3% vs 30.8%, p<0.01). The majority of the participants were classified as ‘of the street,’ 84.9% of males and 51.9% of females. Most participants were street-connected for at least 3 years (55.6% of females, 69.8% of males) and 52.3% reported earning less than 100 KES (US$1.40) daily. Significantly more males than females reported police involvement (74.8% vs 40.7%, p<0.01), and more frequently reported alcoholism and violence in the home as reason for coming to the streets (37.0% vs 14.8%, p<0.01).
Table 1.
Sociodemographic and risk behaviours by gender
| Total (n=200) n (%) | Male (n=119) n (%) | Female (n=81) n (%) | p Value* | |
|---|---|---|---|---|
| Age | ||||
| Median (IQR) | 16 (13–19) | 15 (13–18) | 18 (15–20) | <0.01 |
| Parent/adult in life | ||||
| Double orphan† | 50 (25.0) | 22 (18.5) | 28 (34.6) | |
| Single orphan‡ | 97 (48.5) | 64 (53.8) | 33 (40.7) | |
| Both parents alive | 53 (26.5) | 33 (27.7) | 20 (24.7) | 0.05 |
| Adult caregiver/guardian in life | 157 (78.5) | 100 (84.0) | 57 (70.4) | 0.05 |
| Seen <once a month | 89 (44.5) | 63 (52.9) | 26 (32.1) | 0.01 |
| Schooling | ||||
| Ever in school | 168 (84.0) | 104 (87.4) | 64 (79.0) | 0.11 |
| >Standard 4 | 77 (45.8) | 32 (30.8) | 45 (70.3) | <0.01 |
| Street life | ||||
| ‘Of’ the street | 143 (71.5) | 101 (84.9) | 42 (51.9) | |
| ‘On’ the street | 57 (28.5) | 18 (15.1) | 39 (48.1) | <0.01 |
| ≥3 years on the street | 128 (64.0) | 83 (69.8) | 45 (55.6) | 0.04 |
| Earn less than 100 KES per day | 103 (52.3) | 63 (52.9) | 40 (51.3) | 0.82 |
| Ever had police involvement | 122 (61.0) | 89 (74.8) | 33 (40.7) | <0.01 |
| Reason for coming to the streets—alcoholism/violence at home | 56 (28.0) | 44 (37.0) | 12 (14.8) | <0.01 |
| Substance use | ||||
| Drug use—alcohol | 91 (45.5) | 49 (41.2) | 42 (51.9) | 0.14 |
| Drug use—any | 154 (77.0) | 104 (87.4) | 50 (61.7) | <0.01 |
| Sexual activity | ||||
| Ever had sex | 129 (64.5) | 72 (60.5) | 57 (70.4) | 0.15 |
| Total (n=129) | Male (n=72) | Female (n=57) | ||
| Sexual risk behaviours | ||||
| Median age at sexual debut (IQR) | 13 (11–15) | 12.5 (10–14.5) | 15 (13–16) | 0.05 |
| First sex involuntary | 18 (14.0) | 3 (4.2) | 15 (26.3) | <0.01 |
| First partner >5 years older | 12 (9.3) | 4 (5.6) | 8 (14.0) | 0.13 |
| Same sex partner | None | None | None | Xx |
| Oral Sex | 18 (14.0) | 9 (12.5) | 9 (15.8) | 0.62 |
| Anal Sex | 5 (3.9) | 3 (4.2) | 2 (3.5) | 0.85 |
| Transactional sex | 34 (17.0) | 8 (6.7) | 26 (32.1) | <0.01 |
| Ever forced to have sex | 33 (16.5) | 6 (5.0) | 27 (33.3) | <0.01 |
| Number of partners | ||||
| Median lifetime # partners (IQR) | 4 (1–9) | 4 (1–10) | 4.5 (2–9) | 0.42 |
| Median # partners in 6 months (IQR) | 1 (0–2) | 1 (0–2) | 1 (1–2) | 0.32 |
| Condom use | ||||
| Condom use at last vaginal sex | 23 (17.8) | 8 (11.1) | 15 (26.3) | 0.03 |
| ‘Always’/'most of the time’ use condoms | 23 (17.8) | 7 (9.7) | 16 (28.1) | 0.01 |
| ‘Never’ use condoms | 76 (58.9) | 50 (69.4) | 26 (45.6) | 0.01 |
| Access to condoms | 144 (72.0) | 92 (77.3) | 52 (64.2) | 0.04 |
| Access to free condoms | 78 (39.0) | 47 (39.5) | 31 (38.3) | 0.12 |
| Total (n=200) | Male (n=119) | Female (n=81) | ||
| STI/HIV testing and care | ||||
| Hx of genital ulcer or discharge | 37 (18.5) | 14 (11.8) | 23 (28.4) | 0.01 |
| Sought care for STI§ | 24 (63.2) | 9 (64.3) | 15 (62.5) | 0.91 |
| Knows STI/ HIV testing location | 168 (84.0) | 96 (80.7) | 72 (88.9) | 0.27 |
| Comfortable going there | 153 (76.5) | 85 (71.4) | 68 (84.0) | 0.18 |
| Prior HIV testing | 155 (77.5) | 90 (75.6) | 65 (80.2) | 0.44 |
| Prior HIV testing in last 6 months | 96 (48.0) | 52 (43.7) | 44 (54.3) | 0.14 |
*p Values determined by χ2 test or Fisher's exact test (where cells had expected value of less than 5).
†Both parents deceased or vital status unknown.
‡One parent deceased or vital status unknown.
§Of those who reported history of genital ulcer or discharge.
STI, sexually transmitted infection; KES, Kenyan Shilling; Hx, history.
Risk behaviors and exposures
Most (77%) participants reported drug use and 41.2% of males and 51.9% of females reported alcohol use. Volatile substance misuse (glue or fuel inhalation) was common (84.9% of males, 50.6% of females). More females (70.4%) than males (60.5%) reported sexual activity. Median age of sexual debut was older for females (15) than males (12.5). Females more frequently reported ever having sex involuntarily (33.3% vs 5.0% of males), as well as their first sexual encounter being involuntary (14.0% vs 4.2% males). Transactional sex was more common among females (32.1% vs 6.7% of males). The majority of participants (72.0%) reported access to condoms. Condom use at last sexual encounter was less among males (11.1% vs 26.3% of females, p=0.01). Only 9.7% of males and 28.1% of females reported using condoms ‘always’ or ‘most of the time.’
Prior STI and HIV testing and care
Of the participants 18.5% reported previous STI symptoms, more commonly among females (28.4%) than males (11.8%). Of those, 63.0% sought care. Most participants (84.0%) knew of a location for STI and HIV testing. The majority reported previous HIV testing (77.5%); many within the last 6 months (table 1).
HIV and STI testing results
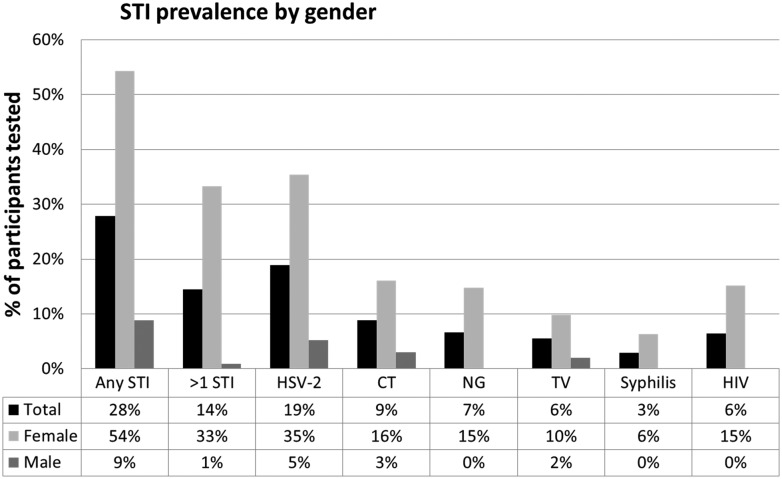
Only three participants reported current STI symptoms. Of those participating in at least one STI test, 28% (55/194) had at least one positive test; 14% (28/194) had more than one positive test. Females were more affected then males. The predominant infection was HSV-2 (5/96 (5%) males, 28/79 (35%) females,), followed by chlamydia (3/100 (3%), 13/81 (16%)), gonorrhoea (0/100 (0%), 12/81 (15%)), HIV (0/113 (0%), 12/79 (15%)), trichomoniasis (3/100 (3%), 12/81 (15%)) and Syphilis (0/96 (0%), 5/79 (6%)) (figure 1). All but six males and two females participated in HIV testing. All 12 HIV-positive participants were previously known positive.
Figure 1.
STI prevalence by gender. CT, Chlamydia trachomatis; HSV-2, herpes simplex virus-2; NG, Neisseria gonorrhoeae; STI, sexually transmitted infection; TV, Trichomonas vaginalis.
Risk factors associated with HIV status among females
All HIV-positive participants were female, thus we restricted our analysis of factors associated with HIV to females (table 2). Those with HIV infection more frequently reported transactional sex (66.7% vs 26.1%, p=0.01), drug use (91.7% vs 56.5%, p=0.02), alcohol use (91.7% vs 44.9%, p=0.01) and a prior STI (50.0% vs 14.7%, p<0.01). Having an adult caregiver in their life was less common among those with HIV infection (33.3% vs 71.0%, p=0.04). Older age, forced sex, multiple partners and older first partner were all more common among those with HIV, though not to the level of statistical significance. Low numbers prevented multivariate analysis.
Table 2.
Bivariate analysis of factors associated with HIV infection among females
| Risk factor | HIV status | ||
|---|---|---|---|
| + n=12 (%) |
− n=69 (%) |
p Value* | |
| Median age, (IQR) | 20 (3.0) | 17 (6.0) | 0.07 |
| Forced sex | 6 (50.0) | 21 (30.4) | 0.18 |
| Transactional sex | 8 (66.7) | 18 (26.1) | 0.01 |
| Multiple partners (≥2) | 10 (90.9) | 34 (75.6) | 0.27 |
| First partner >5 years older | 3 (25.0) | 5 (7.3) | 0.14 |
| Adult caregiver in life | 4 (33.3) | 49 (71.0) | 0.04 |
| Alcohol use | 11 (91.7) | 31 (44.9) | 0.01 |
| Any drug use | 11 (91.7) | 39 (56.5) | 0.02 |
| Police involvement | 5 (41.7) | 28 (40.6) | 0.94 |
| Belonging to a gang | 10 (83.3) | 49 (71.0) | 0.38 |
| Alcoholism/violence at home | 1 (8.3) | 11 (15.9) | 0.68 |
| Prior STI | 6 (50.0) | 10 (14.7) | <0.01 |
| Condom at last vaginal sex | 3 (25.0) | 12 (26.7) | 0.91 |
*Determined by χ2 test.
STI, sexually transmitted infection.
Factors associated with any STI
After adjustment for age, gender and any education, participation in transactional sex (AOR 3.02, 95% CI (1.05 to 8.73)), report of prior STI (AOR 3.46 95% CI (1.05 to 11.46)) and having two or more partners (AOR 5.62 95% (1.67 to 18.87)) were significantly associated with having a STI (tables 3 and 4). Report of condom use at last sex had no association with STI status.
Table 3.
Bivariate analysis of potential sociodemographic risk factors and any sexually transmitted infection among those who tested and reported any sexual activity
| Risk factor | UOR (95% CI) |
|---|---|
| Gender (male vs female*) | 0.07 (0.03 to 0.15) |
| Age | 1.37 (1.21 to 1.54) |
| Ever attended school (yes vs no*) | 0.37 (0.17 to 0.79) |
| Level of education (>SD 4 vs ≤SD 4*) | 1.15 (0.57 to 2.34) |
| Orphan (yes vs no*) | 0.93 (0.46 to 1.87) |
| Has a legal guardian (yes vs no*) | 0.61 (0.28 to 1.31) |
| Adult caregiver in life (yes vs no*) | 0.82 (0.39 to 1.72) |
| Street involvement (‘on’ vs ‘of’ street*) | 1.28 (0.65 to 2.49) |
| Length of time on streets (>2 vs <2 years*) | 1.13 (0.59 to 2.17) |
| Sleeps with friends vs other street youth* | 1.44 (0.77 to 2.68) |
| Police involvement (yes vs no*) | 0.80 (0.43 to 1.50) |
| Belong to a gang (yes vs no*) | 1.55 (0.55 to 4.37) |
| Home violence (yes vs no*) | 1.04 (0.52 to 2.06) |
*Reference variable.
Table 4.
Factors associated with any STI among those who tested and reported any sexual activity
| Exposure | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|
| Alcohol use | 2.78 (1.11 to 6.96) | 1.90 (0.68 to 5.30) |
| Age of first partner >5 years vs ≤5 years | 3.56 (1.03 to 12.31) | 2.01 (0.36 to 11.33) |
| Transactional sex | 6.19 (2.63 to 14.54) | 3.02 (1.05 to 8.73) |
| Forced sex | 3.30 (1.45 to 7.47) | 0.77 (0.26 to 2.28) |
| Prior STI | 2.51 (1.09 to 5.81) | 3.46 (1.05 to 11.46) |
| Number of partners ≥2 vs <2 | 4.58 (1.75 to 12.04) | 5.62 (1.67 to 18.87) |
| Condom use at last vaginal sex | 2.21 (0.89 to 5.51) | 1.33 (0.43 to 4.10) |
*Each factor is an individual model adjusted for gender, age, any education.
STI, sexually transmitted infection.
Discussion
These data are among the first reporting HIV and STI prevalence among SCCY in sub-Saharan Africa. Six per cent of participants were HIV infected, higher than national and regional prevalence data reported in similar age groups: 0.8–1.7% among adolescents (ages 13–19 years) and 4.2–4.8% among young adults (ages 19–24 years)5 27 Among females we found 15% were infected; higher than the national estimates of 2.7% among females ages 15–19 years, and 6.4% of those ages 20–24 years.5 While not yet approaching the rate of female sex workers (23% nationwide) left ignored, this population is at risk for becoming even more affected.4
More than a quarter of adolescents in our study had at least one STI, and females were again disproportionately affected. Compared with reported national data, we found higher rates of STIs in similar age groups; 35% of females with HSV-2 in our study compared with 27.1% in females 20–24 years nationally, and 6% of females with syphilis, compared with <1% in females ages 15–24 years nationally.28 Our data are similar to data from Ethiopia, reporting 13.5% of out-of-school girls had either gonorrhoea or chlamydia, likely a similar population.15 Not surprisingly, engaging in transactional sex, having more than one sexual partner and having a previous STI were associated with having any STI in our study. Only three participants reported symptoms, indicating most infections were asymptomatic.
Our results demonstrate that SCCY in Eldoret, Kenya are engaged in high-risk sexual behaviours, including early age of sexual debut, multiple partners, transactional sex and inconsistent condom use. Females are at particularly high risk, experiencing significantly more forced sex, transactional sex and reported prior STIs, consistent with previous reports.14 16 17 These experiences may contribute to the levels of PTSD reported in this population.29
The number of females with HIV highlights their vulnerability. We found transactional sex, alcohol and drug use, and a prior STI to have significant association with HIV infection, while social factors of orphan status, police involvement and gang involvement did not. Different from orphan status, the report of an adult caregiver in the adolescent's life was found more commonly among those without HIV. Having an adult caregiver in these adolescents’ lives suggests a surrogate parental figure and a source of social support. These relationships may lead to sexual risk behaviour reduction and thus potentially be protective from HIV infection in a similar fashion as has been shown with parental monitoring and parent-adolescent communication.30 Social support from family and peers is widely believed to be a critical determinant of many individual mental and physical health outcomes, even in the presence of adversity.31–34 Those with adult caregivers likely benefit from more social and/or family support with resultant better outcomes than those who do not. However, we did not see a similar finding with any STI as an outcome. Further investigation into this finding is necessary to understand the true impact of this relationship of the health of the youth. In general our research highlights the need to conduct more focused research with street-connected girls and women and better understand gender differences in risks and outcomes among them.
HIV testing uptake was high among our study population. The motivation for frequent testing was not investigated, and may represent a level of understanding of risk among SCCY. Additionally, it is important to note that Eldoret is the home to Academic Model Providing Access to Healthcare, which provides HIV care and multiple community-based testing opportunities.23
Our study has several notable strengths. We were able to recruit a relatively high number of females. To accomplish this, study personnel spent time sensitising SCCY about the study prior to recruitment, building trust with the youth. We employed a self-collected swab technique, not widely used in studies in this region or population. By testing participants regardless of symptoms, we were able to identify asymptomatic infections, and provide a more accurate prevalence than by self-report.
As a cross-sectional, observational study, our study has intrinsic limitations. Our sample size was limited, and did not have the power to evaluate the role of all sociodemographic variables that may contribute to HIV and STI risk. The hard-to-reach nature of this population influenced the sample size for this study and means of recruitment. As recruitment and enrolment were based on convenience and were not random, our sample may not be representative of the entire SCCY population in Eldoret. Our male to female ratio may not reflect that of the SCCY population, as we attempted to oversample the understudied and high-risk female population. There is potential selection bias as willingness to participate may have been based on perceived risk and desire for testing. We are unable to ensure similarity between SCCY who chose to participate and those who did not, thus our results may not be generalisable to all SCCY; it is possible that those who participated are more connected to SCCY support organisations which may impact their risk for STI and HIV. As this study deals with sensitive topics that adolescents are often not comfortable discussing, our results are subject to reporting bias. Participants were informed on multiple occasions that anonymity was being applied and that they were not required to answer questions which caused discomfort.
Our results indicate that street-connected adolescents in Eldoret are at high risk for STIs and HIV, particularly females, demonstrating the clear need for prevention and education programmes specifically targeted to this population. As engaging in risky sexual behaviours such as transactional sex are linked to their survival on the street, integrating sexual health education and HIV/STI prevention and treatment into programmes that address their broader needs is critical. We found that most infections were asymptomatic, thus screening for STIs regardless of symptoms should be considered in programmes addressing care of SCCY, despite constraints of resources and hard-to-reach populations; programmes must include easy access to care and encouragement of health-seeking behaviours.
Our work highlights several questions for future research. Future studies targeting a larger and more representative sample employing different sampling techniques are needed to better understand if this high burden of STIs and high risk is generalisable to the greater SCCY population. Additionally, future work should look at understanding the burden of disease in this population and on how to address the substantial risk and unique challenges that SCCY face. One interesting avenue may be the potential protective role of adult caregivers. Understanding which adult caregiver relationships offer sources of resilience may aid in the development of effective HIV prevention strategies. Further research targeted at tailoring STI and HIV prevention and intervention strategies is needed to better inform the design of specific programmes for this high-risk population.
Key messages.
Street-connected adolescents in western Kenya are engaged in high-risk sexual behaviours.
Street-connected females are particularly affected by HIV and sexually transmitted infections (STIs).
There is a need for STI and HIV interventions targeted to street-connected youth in Kenya.
Acknowledgments
The authors acknowledge Dr Barbara Van Der Pol for her important input to the study design, and for provision of the materials for the self-collection of samples and performing the laboratory tests along with Dr Wilfred Emonyi Injera. The authors also acknowledge Isaac Mwaniki and Dominic Makori for their efforts as their outreach workers. Part of this work was previously presented as posters in San Diego, USA at IDWeek October 2012 and in Istanbul, Turkey at the International Association for Adolescent Health (IAAH) 10th World Congress, June, 2013.
Handling editor Jackie A Cassell
Contributors: SEW, AKC, DA, WN, EJC and PB contributed to the planning, conduct and reporting of this work. LNM and JK contributed to the conduct and reporting of this work.
Funding: This work was supported by a 2011 international developmental grant from the Lifespan/Tufts/Brown Center for AIDS Research (CFAR). The project described was supported by Grant Number P30AI042853 from the National Institute of Allergy And Infectious Diseases and by Award Number R01HD060478 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views, the National Institute of Allergy And Infectious Diseases of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. Additional funding was provided by Framework in Global Health, Brown University Global Health Initiative. SEW received support from the National Institute on Drug Abuse (grant 5T32DA013911).
Competing interests: None.
Ethics approval: IREC, IU IRB, Lifespan/Miriam IRB.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.United Nations International Children's Fund (UNICEF). State of the world's children 2012: children in an urban world. New York, NY: UNICEF, 2012. [Google Scholar]
- 2.United Nations Integrated Regional Informing Network. Youth in crisis: coming of age in the 21st century. Nairobi, Kenya: United Nations Integrated Regional Informing Network, 2007. [Google Scholar]
- 3.Commission on revenue allocation. Kenya: County Fact Sheets, 2011. https://www.opendata.go.ke/Counties/Kenya-County-Fact-Sheets-Dec-2011/zn6m-25cf (accessed 25 Jun 2014). [Google Scholar]
- 4.National AIDS Control Council (NACC) and National AIDS and STIs Control Programme (NASCOP). Kenya AIDS Epidemic update 2011. Nairobi, Kenya: NACC and NASCOP, 2012. http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_KE_Narrative_Report.pdf (accessed 25 Jun 2014). [Google Scholar]
- 5.Kenya National Bureau of Statistics (KNBS) and ICF Macro. Kenya demographic and health survey 2008–09. Calverton, Maryland: KNBS and ICF Macro, 2010. [Google Scholar]
- 6.Pfeifer RW, Oliver J. A study of HIV seroprevalence in a group of homeless youth in hollywood, California. J Adol Health 1997;20:339–42. 10.1016/S1054-139X(97)00038-4 [DOI] [PubMed] [Google Scholar]
- 7.Beech BM, Myers L, Beech DJ, et al. . Human immunodeficiency syndrome and hepatitis B and C infections among homeless adolescents. Semin Pediatr Infect Dis 2003;14:12–19. 10.1053/spid.2003.127212 [DOI] [PubMed] [Google Scholar]
- 8.Boivin J-F, Roy E, Haley N, et al. . The health of street youth: a Canadian perspective. Can J Public Health 2005;96:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Public Health Agency of Canada (PHAC). Sexually Transmitted Infections in Canadian Street Youth: Findings from Enhanced Surveillance of Canadian Street Youth, 1999–2003. 2006. http://librarypdf.catie.ca/PDF/P35/23620e.pdf (accessed 26 Jun 2014).
- 10.Noell J, Rohde P, Ochs L, et al. . Incidence and prevalence of chlamydia, herpes and viral heaptitis in a homeless adolescent population. Sex Transm Dis 2001;28:4–10. 10.1097/00007435-200101000-00003 [DOI] [PubMed] [Google Scholar]
- 11.Shields S, Wong T, Mann J, et al. . Prevalence and correlates of chlamydia infection in Canadian street youth. J Adol Health 2004;34:384–90. 10.1016/j.jadohealth.2003.07.017 [DOI] [PubMed] [Google Scholar]
- 12.Woan J, Lin J, Auerswald C. The health status of street children and youth in low- and middle-income countries: a systematic review of the literature. J Adol Health 2013;53:314–21. 10.1016/j.jadohealth.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 13.Stroeken K, Remes P, De Koker P, et al. . HIV among out-of-school youth in Eastern and Southern Africa: a review. AIDS Care 2012;24:186–94. [DOI] [PubMed] [Google Scholar]
- 14.Khasakhala AA, Mturi AJ. Factors associated with Risky Sexual Behaviour among out-of-school youth in Kenya. J Biosoc Sci 2008;40:641–53. 10.1017/S0021932007002647 [DOI] [PubMed] [Google Scholar]
- 15.Taffa N, Bjune G, Sundby J, et al. . Prevalence of gonococcal and chlamydial infections and sexual risk bheavior among youth in Addis Ababa, Ethiopia. Sex Transm Dis 2002;29:828–33. 10.1097/00007435-200212000-00015 [DOI] [PubMed] [Google Scholar]
- 16.Kayembe PK, Mapatano MA and Fatuma AB. Knowledge of HIV, sexual behaviour and correlates of risky sex among street children in Kinshasa, Democratic Republic of Congo. East Afr J Public Health 2008;5:186–92. [PubMed] [Google Scholar]
- 17.Olley BO. Social and health behaviors in youth of the street in Ibadan, Nigeria. Child Abuse Negl 2006;30:271–82. 10.1016/j.chiabu.2005.10.011 [DOI] [PubMed] [Google Scholar]
- 18.Mudingayi A, Lutala P, Mupenda B. HIV knowledge and sexual risk behavior among street adolescents in rehabilitation centres in Knshasa; DRC: gender differences. Pan Afr Med J 2011;10:23 http://www.panafrican-med-journal.com/content/article/10/23/full/ (accessed 28 Jul 2014). doi:10.4314/pamj.v10i0.72233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swart-Kruger J, Richter LM. AIDS-related knowledge, attitiudes and behaviour among South African street youth: reflections on power, sexuality and the autonomous self. Soc Sci ed 1997;45:957–66. [DOI] [PubMed] [Google Scholar]
- 20.Lockhart C. Kunyenga, “Real Sex”, and survival: assessing the risk of HIV infection among urban street boys in Tanzania. Med Anthropol Q 2002;16:294–311. 10.1525/maq.2002.16.3.294 [DOI] [PubMed] [Google Scholar]
- 21.Embelton L, Ayuku D, Atwoli L, et al. . Knowledge, attitudes and substance use practices among street children in Western Kenya. Subst Use Misuse 2012;47:1234–47. 10.3109/10826084.2012.700678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nada KH, Suliman EDA. Violence, abuse, alcohol and drug use, and sexual beahviors in street children of Greater Cairo and Alexandria, Egypt. AIDS 2010;24(Suppl 2):S39–44. 10.1097/01.aids.0000386732.02425.d1 [DOI] [PubMed] [Google Scholar]
- 23.Sorber R, Winston SE, Koech J, et al. . Social and economic characteristics of street youth by gender and level of street involvement in Eldoret, Kenya. PLoS ONE 2014;9:e97587 10.1371/journal.pone.0097587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO). Integrating STI/RTI care for reproductive health. Sexually transmitted and other reproductive tract infections. A guide to essential practice. Geneva: WHO Press, 2005. [Google Scholar]
- 25.Gaydos CA, Cartwright CP, Colaninno P, et al. . Performance of the abbott RealTime CT/NG for detection of chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 2010;48:3236–43. 10.1128/JCM.01019-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Der Pol B, Craft CS, and Williams JA. Use of an adaptation of a commercially available PCR assay aimed at diagnosis of chlamydia and gonorrhea to detect Trichomonas vaginalis in urogenital specimens. J Clin Microbiol 2006;44:366–73. 10.1128/JCM.44.2.366-373.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wachira J, Ndege S, Koech J, et al. . HIV testing uptake and prevalence among adolescents and adults in a large home-based HIV testing program in Western Kenya. J Aquir Immune Defic Syndr 2014;65:e58–66. 10.1097/QAI.0b013e3182a14f9e [DOI] [PubMed] [Google Scholar]
- 28.National AIDS and STIs Control Programme (NASCOP), CDC Kenya, CDC (U.S.), Joint United Nations Programme on HIV/AIDS, USAID, U.S. PEPFAR, and WHO. Kenyan AIDS indicator survey KAIS 2007 final report. Nairobi, Kenya: NASCOP, 2009. [Google Scholar]
- 29.Atwoli L, Ayuku D, Hogan J, et al. . Impact of domesic care environment on truama and posttraumatic stress disorder among orphans in Western Kenya. PLoS ONE 2014;9:e89937 10.1371/journal.pone.0089937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markham C, Lormand D, Gloppen KM, et al. . Connectedness as a predictor of sexual and reproductive health outcomes for youth: a review. J Adol Health 2010;46:s23–41. 10.1016/j.jadohealth.2009.11.214 [DOI] [PubMed] [Google Scholar]
- 31.D'Abreu RC, Mullis AK, Cook LR. Social support and the ability to adapt to life among Brazilian street children and non-street children. J Soc Psychol 2001;141:127–9. 10.1080/00224540109600531 [DOI] [PubMed] [Google Scholar]
- 32.Moskowitz D, Vittinghoff E, Schmidt L. Reconsidering the effects of poverty and social support on health: A 5-year longitudinal test of the stress-buffering hypothesis. J Urban Health 2013;90:175–84. 10.1007/s11524-012-9757-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleishman JA, Sherbourne CD, Crystal S, et al. . Coping, conflictual social interactions, social support, and mood among HIV-infected persons. HCSUS Consortium. Am J Community Psychol 2000;28:421–53. 10.1023/A:1005132430171 [DOI] [PubMed] [Google Scholar]
- 34.Resnick MD, Peter S, Bearman PS, et al. . Protecting adolescents from harm: findings from the national longitudinal study on adolescent health. JAMA 1997;278:823–32. 10.1001/jama.1997.03550100049038 [DOI] [PubMed] [Google Scholar]