Abstract
OBJECTIVE:
Little is known about metabolic factors in cirrhotic patients in China. Therefore, we aimed to quantify the prevalence of both metabolic factors and non-alcoholic steatohepatitis-related liver cirrhosis in China.
METHODS:
The medical records of 1,582 patients diagnosed with liver cirrhosis from June 2003 to July 2013 at Daping Hospital (Chongqing, China) were retrospectively reviewed through a computer-generated search.
RESULTS:
Serum hepatitis B virus surface antigen was present in 1,083 (68.5%) patients, and hepatitis B was found to be the only etiological factor in 938 (59.3%) of all patients. Obesity, diabetes mellitus, and arterial hypertension were observed in 229 (14.5%), 159 (10.1%), and 129 (8.2%) patients, respectively. From 2012-2013, the proportion of non-alcoholic steatohepatitis-related liver cirrhosis increased to 3.2%, whereas the average proportion of non-alcoholic steatohepatitis-related liver cirrhosis in the previous ten years was 1.9%. The incidence of hepatocellular carcinoma was much higher in males than in females (6.3% vs. 3.7%, respectively, p=0.036). Obesity and diabetes mellitus did not significantly increase the incidence of hepatocellular carcinoma in the whole cirrhotic group. The presence of hepatitis B virus was the only risk factor for hepatocellular carcinoma in cirrhotic patients (p<0.001).
CONCLUSIONS:
Although hepatitis B virus remains the main etiology of liver cirrhosis in China, steatohepatitis-related liver cirrhosis is increasing in frequency. Hepatitis B virus was the sole significant risk factor for hepatocellular carcinoma in the whole cirrhotic group in the present study, in contrast to obesity and diabetes mellitus, for which only a trend of increased hepatocellular carcinoma was found.
Keywords: Non-Alcoholic Steatohepatitis, Non-Alcoholic Fatty Liver Disease, Liver Cirrhosis, Obesity, Metabolism
INTRODUCTION
The main etiology of liver cirrhosis (LC) in China is hepatitis B virus (HBV) infection 1. However, the changing proportions of the various potential etiological factors over the last 10 years remain poorly documented, especially the presence of metabolic characteristics such as obesity (or increased body mass index [BMI]), diabetes mellitus (DM), hypertension and alcoholism. In Western countries, non-alcoholic steatohepatitis (NASH)-related cirrhosis is increasingly considered as a major causal factor of cirrhosis. Moreover, hepatocellular carcinoma (HCC) is related to NASH-related cirrhosis in Germany 2. For several possible reasons, few data exist regarding NASH-related LC in China. First, NASH-related cirrhosis is diagnosed by exclusion, and ruling out all other possible causes is difficult. Second, the histological characteristic features of non-alcoholic fatty liver disease (NAFLD) such as fatty infiltration tend to be lost in NASH-related cirrhosis 3. Third, there is a lack of strict criteria for diagnosing NASH-related cirrhosis. According to one nationwide Japanese survey, 2.1% of cirrhosis cases were attributable to NASH-related cirrhosis based on the researchers' own criteria, which mainly diagnosed NASH-related cirrhosis as cryptogenic cirrhosis associated with obesity (BMI over 25), DM or metabolic syndrome (Met-s) 4.
If appropriate criteria are used, NASH-related cirrhosis may account for two thirds of cases currently labeled as cryptogenic cirrhosis 3. Herein, we retrospectively analyzed etiological factors in LC in patients admitted to our hospital over the past 10 years to evaluate the proportion and time trend of NASH-related LC, with special emphasis on the factors favoring HCC development.
PATIENTS AND METHODS
The medical records of patients diagnosed with LC from June 2003 to July 2013 at Daping Hospital (Chongqing, China) were retrospectively reviewed through a computer-generated search. The study included 1,582 patients.
LC was diagnosed based on clinical symptoms, imaging data, and/or histological findings (only 41 patients had a liver biopsy in the present series). Classical criteria for each etiology potentially involved in LC were applied to categorize cirrhotic patients. For NASH-related LC, we adopted the following criteria. First, all NASH-related LC had to be cryptogenic LC and had to be coupled with alcohol consumption of less than 20 grams per day. Second, the BMI of each patient had to be over 25, with or without DM or Met-s; this was slightly different from the Japanese criteria 4, which did not include obesity as a necessary criterion. If the above two criteria were met, then the patient was clinically suspected of having NASH-related LC. Furthermore, if histological findings such as micronodular cirrhosis, perisinusoidal fibrosis or fatty changes were found, then the patient was histologically diagnosed with NASH-related LC. Among the 30 patients diagnosed with NASH-related LC, only one patient was histologically diagnosed with NASH-related LC by liver biopsy.
Clinical information (age, gender, alcohol consumption, virus infection, hepatic complications) was also collected for all patients with LC. Metabolic risk factors such as obesity, DM and arterial hypertension were systematically collected based on the diagnostic history. The Child-Pugh criteria were used to grade cirrhosis.
Statistical analysis
The dichotomous data and continuous data were analyzed by the chi-square test and Student's t test, respectively, using Statistical Package for the Social Sciences (SPSS, version 15.0; Chicago, IL, USA). Logistic regression was used to analyze the selected risk factors for HCC. When examining the differences between males and females, Fisher's exact test and the Mann-Whitney U test were also used. A p-value of <0.05 was considered statistically significant.
RESULTS
Etiology of LC
A total of 1,582 patients diagnosed with LC from 2003-2013 were included in this retrospective study. A total of 1,097 (69.4%) patients were male, 485 (30.6%) were female, and 87 (5.5%) were diagnosed with HCC during hospitalization.
In our study, 14 etiological categories were set for LC (Table 1). The most common etiology was HBV; hepatitis B surface antigen (HBs Ag) was present in the sera of 1,083 (68.5%) patients. HBV was found to be the only etiological factor in 938 (59.3%) patients. Hepatitis C virus (HCV) accounted only for 1.8% of cases (1.0% of patients had HCV alone, 0.5% had HCV combined with HBV, and 0.3% had HCV combined with alcohol abuse). Hepatitis E virus combined with HBV was found in only two patients. In total, 298 (18.7%) patients had a history of alcohol abuse (135 of them also had HBV infection, and 5 patients also had HCV infection; alcohol was the dominant factor in 158 patients). There were 32 patients with autoimmune hepatitis (AIH), 69 patients with primary biliary cirrhosis (PBC), 4 patients with AIH combined with PBC, and 6 patients with Wilson's disease. For NASH-related LC, 30 patients (1.9%) met our criteria. Thirteen patients had other identified etiologies, including intra-hepatic bile-duct stones (9 patients), Budd-Chiari syndrome (2 patients), parasitic infection (1 patient), and veno-occlusive disease (1 patient). The remaining 167 patients had cryptogenic LC, as defined by the absence of identified causes, including NASH-related LC criteria.
Table 1.
Etiologies of liver cirrhosis from 2003-2013.
| Category | No. of patients (%) |
|---|---|
| HBV alone | 938 (59.3) |
| HCV alone | 15 (1.0) |
| HBV/HCV | 8 (0.5) |
| HBV/HEV | 2 (0.1) |
| Alcohol | 158 (9.9) |
| HBV/Alcohol | 135 (8.5) |
| HCV/Alcohol | 5 (0.3) |
| AIH | 32 (2.0) |
| PBC | 69 (4.4) |
| AIH/PBC | 4 (0.3) |
| Wilson's disease | 6 (0.4) |
| NASH-LC | 30 (1.9) |
| Cryptogenic | 167 (10.6) |
| Other | 13 (0.8) |
| Total | 1,582 (100) |
HBV alone: hepatitis B virus was the only etiological factor for cirrhosis; HCV alone: hepatitis C virus was the only etiological factor for cirrhosis; HBV/HCV: hepatitis B virus combined with hepatitis C virus; HBV/HEV: hepatitis B virus combined with hepatitis E virus; HBV/Alcohol: hepatitis B virus combined with alcohol abuse; HCV/Alcohol: hepatitis C virus combined with alcohol abuse; AIH: autoimmune hepatitis; PBC: primary biliary cirrhosis; AIH/PBC: autoimmune hepatitis combined with primary biliary cirrhosis.
Etiological differences between males and females
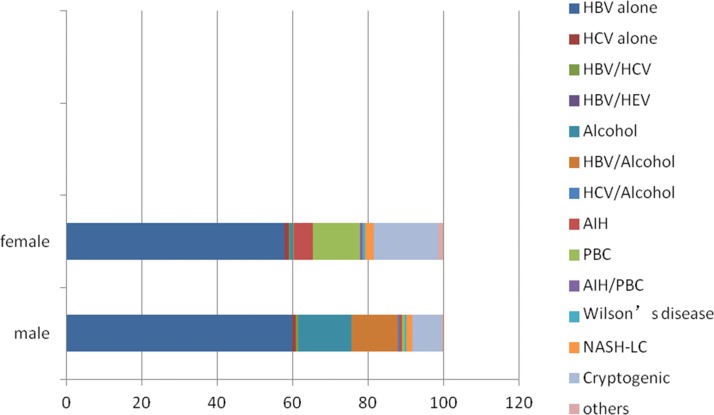
HBV was clearly the main etiology in both males and females (59.8% vs. 57.9%, respectively, p>0.05) (Figure 1). The percentages of patients with alcohol abuse, HBV plus alcohol abuse, and HCV plus alcohol abuse were higher in males vs. females (11.2% vs. 1.0%, p<0.01; 12.2% vs. 0.2%, p<0.01; and 0.5% vs. 0%, p<0.01, respectively). However, the percentages of AIH and PBC were much higher in females vs. males (4.9% vs. 0.7%, p<0.01; 12.6% vs. 0.7%, p<0.01, respectively). No difference between males and females existed for NASH-related LC (1.7% vs. 2.3%, respectively, p=0.47).
Figure 1.
Difference in etiologies between males and females.
Time trend of metabolic factors and HCC in cirrhotic patients
The metabolic information recorded during the periods from 2003-2008 and 2008-2013 was compared (Table 2). Whereas the total number of patients from 2008-2013 was two times higher than the number in the period from 2003-2008 (1,091 and 491, respectively), no significant differences were noted in terms of age, sex ratio, or BMI between the two time periods. Trends of increased obesity, DM, hypertension and HBs Ag positivity were observed in the second five-year period. In contrast, the proportion of HCC significantly decreased in the period from 2008-2013 (4.7% vs. 7.3%, p=0.033) (Table 2).
Table 2.
Clinical characteristics of cirrhotic patients.
| Total | 2003–2008 | 2008–2013 | p-value# | |
|---|---|---|---|---|
| Age (years) | 52.8±12.8 | 52.3±12.8 | 52.7±12.7 | 0.519 |
| Female | 485 | 147 | 338 | 0.678 |
| Male | 1,097 | 344 | 753 | |
| Male/female | 2.26 | 2.34 | 2.23 | |
| BMI (Mean ± SD) | 22.1±3.3 | 22.1±3.5 | 22.1±3.2 | 0.894 |
| Obesity (%) | 229 (14.5) | 71 (14.5) | 158 (14.5) | 0.991 |
| DM (%) | 159 (10.1) | 44 (8.9) | 115 (10.5) | 0.334 |
| Hypertension (%) | 129 (8.2) | 33 (6.7) | 96 (8.8) | 0.162 |
| HBs Ag+ (%) | 1,083 (68.5) | 325 (66.2) | 758 (69.5) | 0.193 |
| HCC (%) | 87 (5.5) | 36 (7.3) | 51 (4.7) | 0.033 |
| Number of patients | 1,582 | 491 | 1,091 |
BMI: body mass index; DM: diabetes mellitus; HBS Ag+: hepatitis B virus surface antigen positive; HCC: hepatocellular carcinoma.
#p-value comparing 2003–2008 with 2008–2013.
NASH-related LC
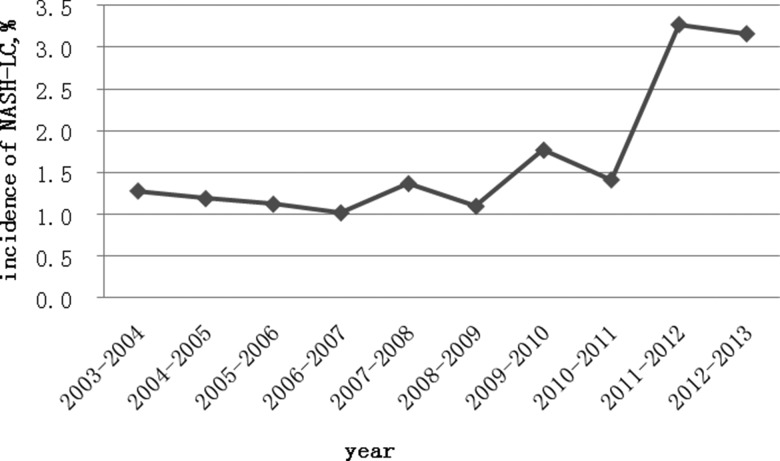
Among the total of 197 patients with cryptogenic LC, 30 patients met our criteria for NASH-related LC. As shown in Figure 2, the proportion of NASH-related LC increased in the last two years of the study to 3.2%, compared with 1.9% for the whole study period.
Figure 2.
Time trend of NASH-related LC over the ten year period from 2003-2013.
Among the 30 patients with NASH-related LC, 19 patients were men, and 11 were women (no statistical significance). Ascites and upper gastrointestinal bleeding (UGB) were the main complications in patients with NASH-related LC, and the complication rate of both ascites and UGB was higher in women, but the difference was not significant. For Child-Pugh grading of NASH-related LC, the rate of Child A was lower in women than in men, but the difference was not significant. HCC was not found in women or men with NASH-related LC. (Table 3).
Table 3.
Clinical characteristics of NASH-related LC.
| Total | Female (F) | Male (M) | p-value (F vs. M) | |
|---|---|---|---|---|
| Number of patients | 30 | 11 | 19 | |
| Age (years) | 57.7±14.1 | 60.6±12.4 | 55.9±15.0 | 0.388a |
| BMI (Kg/m2) | 27.2±2.1 | 27.2±2.2 | 27.2±2.0 | 0.965a |
| Arterial hypertension (%) | 11 (36.7) | 6 (54.6) | 5 (26.3) | 0.238b |
| DM (%) | 8 (26.7) | 1 (9.1) | 7 (36.8) | 0.199b |
| Complications | ||||
| Ascites (%) | 12 (40.0) | 5 (45.5) | 7 (36.8) | 0.712b |
| HCC (%) | 0 (0) | 0 (0) | 0 (0) | |
| UGB (%) | 6 (20.0) | 3 (27.3) | 3 (15.8) | 0.641b |
| HRS (%) | 0 (0) | 0 (0) | 0 (0) | |
| HE (%) | 0 (0) | 0 (0) | 0 (0) | |
| Child-Pugh grading | 0.308c | |||
| A (%) | 16 (53.3) | 5 (45.5) | 11 (57.9) | |
| B (%) | 12 (40.0) | 4 (36.4) | 8 (42.1) | |
| C (%) | 2 (6.7) | 2 (18.2) | 0 (0) |
BMI: body mass index; DM: diabetes mellitus; HCC: hepatocellular carcinoma; UGB: upper gastrointestinal bleeding; HRS: hepatorenal syndrome; HE: hepatic encephalopathy.
p-value determined by a: Student's t test; b: Fisher's exact test; c: the Mann-Whitney U test.
Selected risk factors for HCC
Among the 87 patients with HCC, 69 patients were men, and 18 patients were women. As shown in Table 4, only the sex ratio (male to female) and HBs Ag positivity rate were significantly increased in HCC patients compared with non-HCC patients (p<0.05) by univariate analysis. Metabolic factors, which included obesity, DM, and hypertension, were not significantly different between men and women. HBs Ag positivity was the sole risk factor for HCC in cirrhotic patients (p<0.001) in a multivariate analysis. Obesity and DM did not increase the HCC incidence in the whole cirrhotic group (5.7% vs. 5.5%, respectively, p=0.862; 5.0% vs. 5.6%, respectively, p=0.849). However, among patients with cirrhosis induced only by HBV, a trend of increased rates of HCC in obese and DM patients was found (8.4% vs. 6.5%, respectively, p=0.436; 7.1% vs. 6.7%, respectively, p=0.589).
Table 4.
Selected risk factors for HCC.
| Variable | Control | HCC | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| N=1,495 (%) | N=87 (%) | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | ||||||
| <65 | 1,227 (94.6) | 70 (5.4) | 0.93 (0.52-1.64) | 0.796 | ||
| ≥65 | 268 (94.0) | 17 (5.9) | ||||
| Sex | ||||||
| Female | 468 (96.3) | 18 (3.7) | 1.81 (1.04-3.14) | 0.036 | ||
| Male | 1,027 (93.7) | 69 (6.3) | ||||
| Obesity | ||||||
| Obese | 216 (94.3) | 13 (5.7) | 1.06 (0.58-1.96) | 0.862 | ||
| Non-obese | 1,279 (94.5) | 74 (5.5) | ||||
| Hypertension | ||||||
| Hypertensive | 123 (95.3) | 6 (4.6) | 0.90 (0.37-2.21) | 0.825 | ||
| Non-hypertensive | 1,362 (94.4) | 81 (5.6) | ||||
| DM | ||||||
| Diabetic | 151 (95.0) | 8 (5.0) | 0.93 (0.43-1.99) | 0.849 | ||
| Non-diabetic | 1,344 (94.5) | 79 (5.6) | ||||
| HBs Ag | ||||||
| HBs Ag positive | 1,008 (93.1) | 75 (6.9) | 2.64 (1.01-6.92) | 0.049 | 3.35 (1.76-6.36) | 0.000 |
| HBs Ag negative | 487 (98.6) | 7 (1.4) | ||||
| Hepatitis B* | ||||||
| HBV+obesity | 121 (91.7) | 11 (8.4) | 1.31 (0.66-2.58) | 0.436 | ||
| HBV+non-obesity | 754 (93.6) | 52 (6.5) | ||||
| HBV+DM | 65 (92.8) | 5 (7.1) | 1.27 (0.53-3.07) | 0.589 | ||
| HBV+non-DM | 810 (93.3) | 58 (6.7) | ||||
| Alcohol# | ||||||
| HBV+alcohol abuse | 123 (91.1) | 12 (8.9) | 0.74 (0.39-1.42) | 0.364 | ||
| HBV+no alcohol abuse | 885 (93.4) | 63 (6.7) | ||||
*Cirrhotic patients with chronic liver disease caused only by HBV infection (938 patients).
*Alcohol abuse in cirrhotic patients with HBs Ag positivity (1,083 patients).
DISCUSSION
In our study, HBV remained the main etiological factor (68.5%) for LC in China, whereas HCV was by far the most frequent cause of cirrhosis (60.9%) according to a nationwide survey in Japan 4. HCV accounted for only 1.8% of our cirrhotic patients. Alcoholic liver disease and hepatitis C are the most common causes of cirrhosis in the Western world, whereas hepatitis B is the prevailing cause in most parts of Asia and sub-Saharan Africa 5. For example, in the United Kingdom 6, a total of 3,360 incident cases of cirrhosis in patients aged 25 or older were diagnosed with LC between 1992 and 2001, and 35.9% of these cases were induced by alcohol, whereas only 5.4% were induced by viral hepatitis. In the USA, 7% of cirrhosis cases were attributable to HBV, and 27% were to HCV from 1989-20007. Globally, 57% of cirrhosis cases were attributable to either HBV (30%) or HCV (27%) 7. In China, as reported in our study, 59.3% of cirrhosis cases were only related to HBV infection. In a large community-based study in eastern China 8, a total of 149,175 individuals were investigated in 60 communities in three counties in Jiangsu province from 2011-2012. Of these subjects, 1,175 (0.79%, 95% confidence interval (CI) 0.74-0.83) were anti-HCV antibody positive. The prevalence was even lower in children (0.09%, 95% CI 0.04-0.17). A seroepidemiological survey of HCV conducted in 2007 revealed a lower rate of anti-HCV antibody positivity (0.80%) in Chongqing, Southwest China than in the general population 9. Thus, the prevalence of hepatitis C as a cause of LC is low in Chongqing, China.
The difference between Asia or sub-Saharan Africa and the Western world may be related to the wide implementation of HBV programs, which has not been financially possible in numerous developing countries. In 1992, the World Health Organization (WHO) recommended that all countries include a hepatitis B vaccine in their routine infant immunization programs. As of 2003, the WHO/UNICEF estimated 42% hepatitis B vaccination coverage in the global birth cohort 7. In China, the HBV infection prevalence decreased from 9.7% (in 1992) to 7.2% (in 2006) after the introduction of a universal HBV vaccination program in 1988 10. However, in our study, we did not observe the theoretically expected decrease in the development of HBV-induced LC, possibly because HBV infection was present prior to the start of the vaccination campaign; the development of cirrhosis usually requires a long period of time 11. Nevertheless, the efficacy of the Chinese HBV immunization program is indicated by the decreased prevalence of HBV carrier status to 2.1% among all children and to 1.0% among those born after 1999, together with the presence of anti-HBs antibodies 1,12. Therefore, it is likely that HBV-related etiology of Chinese LC will be significantly reduced in the future.
In Western countries, NAFLD is now considered to be the most common cause of chronic liver disease, with 20–30% of the total population presenting with NAFLD 13,14. In China, NAFLD affects approximately 15% of adults and is present in 68.5% of obese children 15,16. The proportion of NASH-related LC (1.9%) in our study was similar to that in Japan (2.1%) 4. However, our study was retrospective and may not represent the incidence of LC in the general population. In our study, an increasing frequency was noted over the ten years covered by the study; this trend is likely to result in a significant increase in patients with NASH-related LC in the future.
A limitation of the current study, especially in the context of a retrospective study, is that diagnosing LC related to NASH requires a BMI higher than 25. However, our criteria were based on those adopted by the Japanese nationwide study, with the difference that a BMI higher than 25 was necessary but not the sole criterion to consider cryptogenic cirrhosis as NASH-related cirrhosis because abnormal glucose metabolism, arterial hypertension and high triglyceride levels in the patients were also considered. Although NASH can be found in lean patients, the identification of metabolic features or performance of a liver biopsy is required for an accurate diagnosis. Due to the retrospective nature of our study, we could not document the number of lean patients; thus, we adopted a BMI higher than 25 as a necessary criterion. Notably, a liver biopsy may lack relevant metabolic features when cirrhosis is present and therefore may not always contribute to determining the etiology.
Although we considered obesity and DM in our study when diagnosing NASH-related LC, we did not find significant differences in the proportions of obesity, DM and hypertension when comparing the 2003-2008 and 2008-2013 time periods.
Several studies have reported that Met-s (obesity or DM) not only promotes the development of LC but also increases the risk of HCC 17–19. In China, obesity has become an important health problem because the prevalence of obesity in adults, which was 7.1% in 2002, has increased by 97.2% since 1992 20,21. In children, a dramatic increase in obesity has been observed, with a two- to three-fold increase in Beijing and Shanghai between 1985 and 1995. In addition, the prevalence of overweight and obese children approached 29% in 2000 in 7- to 12-year-old boys and 15–17% in girls 21. A 2013 study from Tianjing, China, also found that the prevalence of obesity in children under 7 years old approached 8.2% 22. These data illustrate the rapid development of obesity in both adults and children. In the USA, data from the 2009-2010 National Health and Nutrition Examination Survey reveal that 36% of Americans are obese and that 30% of the general adult population may have NAFLD 23. In the USA, the yearly cumulative HCC incidence in NASH-related LC was 2.6%, compared with 4.0% among patients with HCV-related cirrhosis, over an average follow-up period of 3.2 years 24. Therefore, the rapid development of obesity in China could be contributing to an increase in NAFLD, leading to the increased prevalence of NASH-related LC shown in our study. The importance of obesity in the natural history of NASH-related LC explains why we decided to integrate this risk factor as a necessary criterion for diagnosing NASH-related LC in our study.
Although the increases in NASH-related LC and obesity may lead to a higher incidence of HCC, we unexpectedly found that the HCC incidence was significantly lower in the time period from 2008-2013 than in the period from 2003-2005. Another recent study, which used data from the Cancer Registration Database records in Shanghai, China, also noted a decreasing HCC incidence 25. This study additionally found that the age-standardized incidence rates for each year and the age-standardized mortality rates decreased from 2006-2008 and that the incidence and mortality rates increased with age. The incidence and mortality rates of primary liver cancer were relatively higher in the suburbs than in urban areas and were also higher in males than in females. Additionally, a study from Taiwan found that male gender was a risk factor for HCC, which is in accordance with our results based on univariate analysis. However, DM and obesity were not associated with HCC in areas where either HBV or HCV was endemic 26. In our study, obesity and DM were not associated with HCC in the whole cirrhotic population, in accordance with the Taiwanese study. However, when considering only the HBV-related cirrhotic subgroup, we observed a statistically insignificant trend of increased HCC in patients who were obese or diabetic. The prevalence of NAFLD-related HCC is rising worldwide: 4–22% of HCC cases in Western countries are now attributed to NAFLD 13. Additionally, 10–75% of cases of NAFLD-related HCC occur in patients without cirrhosis 27,28. A higher BMI in childhood increases the risk of primary liver cancer in adults 29. In our study, HCC was not found in patients with NASH-related LC, whereas HCC was present in 31.5% of NASH-related LC cases in the Japanese nationwide study 4. A study from England found that NAFLD accounted for 41 (34.8%) cases of HCC in 2010 30. Another study on HCC from Germany also found an increasing proportion of NASH in HCC patients 2. With the promotion of an HBV vaccination program and the improvement of economic development, this type of time trend may also occur in China. Therefore, we should expand our attention from patients with cirrhosis caused by chronic viral hepatitis to those with cirrhosis induced by NASH in the future. Additionally, patients with NASH-related LC must be followed in the coming decades, paying special attention to the effects in obese children, as the vaccination program has protected them from HBV.
For NASH-related LC, we found that female patients were older than males and that the rates of complications and Child-Pugh grade C tended to be higher in women, which is in accordance with the Japanese nationwide study 4. However, the difference in our study was not significant, possibly due to the small sample size. The epidemiological study in Japan observed a higher NAFLD prevalence in men than in women. However, in contrast to men, NAFLD increased with age in women, showing a higher prevalence among women aged 40-49 years and after menopause 31. Aging is considered as a risk factor for NAFLD in pre-menopausal women 32. Our study showed an average age of 60 years for women who developed NASH-related LC, indicating that they were menopausal women. Because estradiol has been reported to attenuate hepatic stellate cell activation and therefore decrease the fibrotic process 33, estradiol diminution after menopause might contribute to the development of NASH-related cirrhosis.
Our study has several limitations. First, because the study was retrospective, it was not possible to collect certain important metabolic parameters such as blood glucose for all cirrhotic patients, which may have led to underestimation of the rate of DM in LC. Second, histological data were rarely obtained for our patients, which confined the diagnosis of NASH-related cirrhosis to clinical, biochemical, and imaging data. However, it should be recalled that when cirrhosis is histologically present, the etiological features may disappear, so a liver biopsy may not be able to ascertain NASH-related cirrhosis. Third, the relatively small number of NASH-related LC cases might explain the absence of significant differences when considering important risk factors reported in other studies. Finally, the history of NAFLD and the duration of obesity or DM could not be obtained from the database.
In conclusion, HBV remains the main etiology of LC in China. However, NASH-related LC has clearly increased in frequency in recent years. Further studies are warranted to evaluate the exact importance of obesity and diabetes in HCC development in the overall cirrhotic population.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 81170382 and 81200297).
REFERENCES
- 1.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27((47)):6550–7. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 2.Schutte K, Kipper M, Kahl S, Bornschein J, Gotze T, Adolf D, et al. Clinical characteristics and time trends in etiology of hepatocellular cancer in Germany. Digestion. 2013;87((3)):147–59. doi: 10.1159/000346743. [DOI] [PubMed] [Google Scholar]
- 3.Nayak NC, Vasdev N, Saigal S, Soin AS. End-stage nonalcoholic fatty liver disease: evaluation of pathomorphologic features and relationship to cryptogenic cirrhosis from study of explant livers in a living donor liver transplant program. Hum Pathol. 2010;41((3)):425–30. doi: 10.1016/j.humpath.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa Y, Tokumoto Y, Onji M. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol. 2010;45((1)):86–94. doi: 10.1007/s00535-009-0128-5. [DOI] [PubMed] [Google Scholar]
- 5.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371((9615)):838–51. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming KM, Aithal GP, Solaymani-Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the United Kingdom, 1992–2001: A general population-based study. J Hepatol. 2008;49((5)):732–8. doi: 10.1016/j.jhep.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 7.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45((4)):529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Huang P, Zhu LG, Zhai XJ, Zhu YF, Yue M, Su J, et al. Hepatitis C virus infection and risk factors in the general population: a large community-based study in eastern China, 2011-2012. Epidemiol Infect; pp. 1–10. 2015;Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qing Wang, Lianyou Hu, Daihong Zhao, Sun M. Seroepidemiological Survey and Analysis of Virus Hepatitis C in Chongqing. J Prev Med Inf. 2007;23((2)):152–4. [Google Scholar]
- 10.Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, Qiao YL, et al. Hepatitis B and C Virus Infection and Hepatocellular Carcinoma in China: A Review of Epidemiology and Control Measures. Journal of Epidemiology. 2011;21((6)):401–16. doi: 10.2188/jea.JE20100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology; pp. S173–81. 2006;43(2 Suppl 1) [DOI] [PubMed] [Google Scholar]
- 12.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200((1)):39–47. doi: 10.1086/599173. [DOI] [PubMed] [Google Scholar]
- 13.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10((11)):656–65. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 14.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34((3)):274–85. doi: 10.1111/apt.2011.34.issue-3. [DOI] [PubMed] [Google Scholar]
- 15.Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol; pp. 11–7. 2013;28 Suppl 1. [DOI] [PubMed] [Google Scholar]
- 16.Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50((1)):204–10. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AMS. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127((5)):S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Karagozian R, Derdak Z, Baffy G. 2014. Obesity-associated mechanisms of hepatocarcinogenesis. Metabolism. [DOI] [PubMed] [Google Scholar]
- 19.Mena A, Pedreira JD, Castro A, Lopez S, Vazquez P, Poveda E. Metabolic syndrome association with fibrosis development in chronic hepatitis B virus inactive carriers. J Gastroenterol Hepatol. 2014;29((1)):173–8. doi: 10.1111/jgh.2014.29.issue-1. [DOI] [PubMed] [Google Scholar]
- 20.Kristi R DG, Paul KW, Xigui Wu. Prevalence and risk factors of overweight and obesity in China. Brief Epidemiologic Report. 2007;15((1)):10–8. doi: 10.1038/oby.2007.527. [DOI] [PubMed] [Google Scholar]
- 21.CM C. Overview of obesity in Mainland China. Obesity reviews; pp. 14–21. 2008;9(suppl 1) [DOI] [PubMed] [Google Scholar]
- 22.Dongdong Liu WL, Gongshu Liu. Survey on obesity of 24343 children under 7 years old in Tianjing. Zhong Guo Fu You Bao Jian. 2013;28((22)):3646–7. [Google Scholar]
- 23.Corey KE, Kaplan LM. Obesity and liver disease: the epidemic of the twenty-first century. Clin Liver Dis. 2014;18((1)):1–18. doi: 10.1016/j.cld.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51((6)):1972–8. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 25.Jing Gao CW, Li Xie, Pingping Bao. Incidence and mortality of primary liver cancer in Shanghai, 2006-2008. Tumor. 2012;32((7)):526–30. [Google Scholar]
- 26.Chen CT, Chen JY, Wang JH, Chang KC, Tseng PL, Kee KM, et al. Diabetes mellitus, metabolic syndrome and obesity are not significant risk factors for hepatocellular carcinoma in an HBV- and HCV-endemic area of Southern Taiwan. Kaohsiung J Med Sci. 2013;29((8)):451–9. doi: 10.1016/j.kjms.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Kawada N, Imanaka K, Kawaguchi T, Tamai C, Ishihara R, Matsunaga T, et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44((12)):1190–4. doi: 10.1007/s00535-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 28.Yasui K, Hashimoto E, Tokushige K, Koike K, Shima T, Kanbara Y, et al. Clinical and pathological progression of non-alcoholic steatohepatitis to hepatocellular carcinoma. Hepatol Res. 2012;42((8)):767–73. doi: 10.1111/hepr.2012.42.issue-8. [DOI] [PubMed] [Google Scholar]
- 29.Berentzen TL, Gamborg M, Holst C, Sorensen TI, Baker JL. Body mass index in childhood and adult risk of primary liver cancer. J Hepatol. 2014;60((2)):325–30. doi: 10.1016/j.jhep.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Jessica Dyson BJ, Dipankar Chattopadyhay, Rajiv Lochan, Janine Graham, Debasish Das. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. Journal of hepatology. 2014;60:110–7. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigues MH, Bruno AS, Nahas-Neto J, Santos ME, Nahas EA. Nonalcoholic fatty liver disease and metabolic syndrome in postmenopausal women. Gynecol Endocrinol. 2014;30((5)):325–9. doi: 10.3109/09513590.2013.875992. [DOI] [PubMed] [Google Scholar]
- 32.Hamaguchi M, Kojima T, Ohbora A, Takeda N, Fukui M, Kato T. Aging is a risk factor of nonalcoholic fatty liver disease in premenopausal women. World J Gastroenterol. 2012;18((3)):237–43. doi: 10.3748/wjg.v18.i3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu I, Ito S. Protection of estrogens against the progression of chronic liver disease. Hepatol Res. 2007;37((4)):239–47. doi: 10.1111/hep.2007.37.issue-4. [DOI] [PubMed] [Google Scholar]