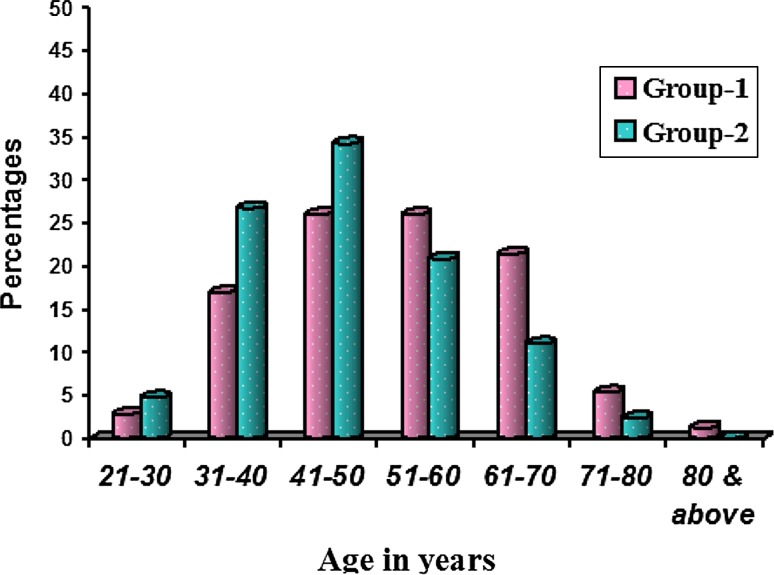Abstract
Objectives
To test the validity of numerous anecdotal claims of poor oral hygiene status being a contributory or etiology for Oral Squamous Cell Carcinoma (OSCC) and to isolate the microorganisms associated with oral cancer, to elucidate their role if any, in oral cancer.
Materials and Methods
A total of 242 OSCC patients and 254 controls were screened. Questionnaires were used to inquire about the past condition of the oral health. Dental caries, oral hygiene status and periodontal disease status were assessed using indices. Microorganisms were identified by bacterial culture methods.
Results
Majority of cases (Group-1) (57.85 %) never visited the dentist compared to controls (Group-2) (46.06 %). Group-1 brushed once in a day (93.4 %) and less often twice a day (6.6 %) compared to Group-2 (81.1 % and 18.9 %). There was no significant difference in caries experience in both groups. Teeth missing due to periodontal reasons were more in Group-1 (40 %) than Group-2 (26 %) (p < 0.002). Poor oral hygiene and increased pocket depth were seen in Group-1 than in Group-2 (p < 0.001). Streptococcus species (α-hemolytic) followed by Staphylococcus species were the predominant microorganisms isolated from Group-1 compared to Group-2 with (62 % vs. 66 %) and without habits (40 % vs. 66 %).
Conclusion
Tobacco consumption, lack of dental visits and infrequent brushing are significantly associated with increased risk of oral cancer. There seems to be no association between dental caries and OSCC. However, periodontal disease experience is directly proportional to OSCC. Increased pathogenic flora may produce carcinogenic metabolites or post-operative infections.
Keywords: Oral cancer, Oral hygiene, Missing teeth, Dental caries, Periodontal disease, Microflora
Introduction
Oral cancer is an important health issue. Globally, it is one of the 10 most common cancers [1]. More than 90 % of cancers in the mouth are squamous cell carcinomas (SCCs) originating from the oral mucosa [2]. Oral Squamous Cell Carcinoma (OSCC) is a multifactorial disease where no single clearly recognizable cause has been found [3]. Tobacco use and alcohol consumption are the major risk factors for the cancer of oral cavity, pharynx, larynx and esophagus [4]. Also, a small percentage of patients develop OSCC in the absence of exposure to tobacco and alcohol and without any obvious predisposing genetic defect, suggesting the existence of other risk factors in oral carcinogenesis, such as the presence of infectious agents [5]. Although viral infections have been associated with carcinogenesis, the evidence for a connection between bacterial infections and carcinogenesis is also convincing [6]. Besides tobacco and alcohol, little is known about the etiology of oral cancer. Oral cancer has been associated with both tooth loss and poor oral hygiene in a number of studies, independent of age, tobacco and alcohol consumption. Infections such as periodontal diseases may play a key role in the etiology of oral cancer. Several studies have reported associations between periodontal disease or tooth loss and risk of oral, upper gastrointestinal, lung, and pancreatic cancer in different populations. The need for the present study was to test the validity of numerous anecdotal claims of poor oral hygiene status being a contributory or etiology for OSCC. Additionally the study also aimed to identify the microorganisms in patients with and without habit and to elucidate their role in oral cancer.
Materials and Methods
Source of Data and Study Groups
A multicentric hospital based study was carried out at The Oxford Dental College Hospital and Research Centre and Mazumdhar Shaw Cancer Hospital, Bangalore, Karnataka Cancer Therapy and Research Institute (KCTRI), Hubli and Shri Dharmasthala Manjunatheswara College of Dental Sciences (SDM), Dharwad.
Study group consisted of oral cancer patients with histopathologically confirmed OSCC prior to definitive treatment. Controls were randomly selected, matched for age, gender and with no previous history of any malignancies. However it was not possible to match habit history and larger part of the control group were non habitués.
Method of Collection of Data
As the screening study would reflect only the present status of the oral health, questionnaires were used to ascertain about the past condition of the oral health.
Oral Health Indicators
We attempted to elicit from study subjects details of their usual lifetime oral health care rather than recent methods. Questionnaires included details consisting of number of dental visits to dentist per year, method of brushing, brushing aids, number of brushes used per year, frequency of brushing, use of mouthwash and other oral hygiene aids and, diet, frequency of consumption of sweets and food habits.
Dental Caries Examination
Dental caries was assessed using decayed missing filled teeth (DMFT) index according to WHO criteria, 1997. Third molars were also included. Teeth missing due to other reasons such as periodontal reasons, trauma and clinically missing teeth were recorded separately.
Oral hygiene and Periodontal Examination
Oral hygiene status and periodontal disease status was assessed by Simplified Oral Hygiene Index (OHI-S) and Community Periodontal Index of Treatment Needs (CPITN) respectively.
Bacterial Culture Methods
Two swabs were collected on sterile cotton swabs from the non-necrotic areas of the tumor. One swab was used for smear preparation and the other swab was used for culture and sensitivity test.
Smear Preparation
Thin smears were prepared on standard glass slides and stained by Gram stain. The smears were observed under oil immersion objective (100×). The morphology of the organisms and presence of inflammatory cells were noted.
Culture
The second swab was used for culture and sensitivity. Cultures were made on sheep blood agar and Maconkey’s media. The media were incubated at 37 °C in CO2 jar. The media were examined after 24 hrs of incubation. The colony morphology was noted. The colonies showing Gram positive cocci were further identified by Gram stain morphology, Catalase, Coagulase and Mannitol fermentation test. The catalase negative Gram positive cocci were identified by growth in 6.5 % NaCl, growth at pH 9.6, bile esculin hydrolysis and sensitivity to Bacitracin. Antibiotic sensitivity was done by Kirby Bauer method as recommended by Clinical Laboratory Standards Institute, USA (CLSI).
The Gram negative bacilli were identified by catalase, oxidase, motility and standard biochemical reactions which included Indol-MR, Vogues Proskeur (VP), citrate, urease, phenyl pyruvic acid deaminase and sugar fermentation reactions. Lysine, arginine, ornithine deamination and oxidative fermentation tests were used when necessary. The antibiotic sensitivity was performed as Kirby Bauer method (CLSI).
Statistical Methods
Statistical analysis was performed using SAS 9.2, SPSS 15.0 (SPSS Inc.) statistical package. The association between dental caries and periodontal disease status was determined by the Chi square test using Fisher’s exact test. Data were considered statistically significant if p value < 0.05.
Results
Totally 242 OSCC cases (Group-1) and 254 controls (Group-2) were screened after obtaining ethical clearance from the concerned institutes and informed consent from the patients. Group-1 comprised 177 male and 65 female patients (Table 1) with a male predominance (M:F = 2.7:1). Age ranged from 3rd to 9th decade (Fig. 1) with mean age of 53.27 ± 12.62 and 47.48 ± 11.05 respectively. Most of the subjects were agriculturists and laborers in both the groups followed by businessmen.
Table 1.
Gender distribution
| Gender | Group-1 | Group-2 | ||
|---|---|---|---|---|
| n = 242 | % | n = 254 | % | |
| Male | 177 | 73.1 | 178 | 70.1 |
| Female | 65 | 26.9 | 76 | 29.9 |
Fig. 1.
Comparison of age distribution of OSCC (Group 1) and controls (Group 2). In Group-1 majority of the subjects were between 4th and 6th decades of life (73.4 %)
In Group-1, majority of the subjects (93.8 %) had the habit of psycho-active substance use, of which paan chewing (48.8 %) was most common, followed by combination of smoked and chewed tobacco (Table 2). In Group-2 areca nut chewing was more common. Only 6.2 % of Group-1 and 71.3 % of the Group-2 had no habits. A statistically significant increased risk of cancer was seen with habit compared to without habit (p < 0.001).
Table 2.
Comparison of Habits
| With/without habits | Group-1 | Group-2 | p value | ||
|---|---|---|---|---|---|
| n = 242 | % | n = 254 | % | ||
| Smoking | 35 | 14.5 | 40 | 15.7 | 0.690 |
| Chewing | 118 | 48.8 | 66 | 25.9 | <0.001** |
| Smoking-chewing | 39 | 16.1 | 11 | 4.3 | <0.001** |
| Betel nut chewing | 81 | 33.5 | 95 | 37.4 | 0.360 |
| Alcohol consumption | 47 | 19.4 | 34 | 13.4 | 0.069+ |
| Without habits | 15 | 6.2 | 73 | 28.7 | <0.001** |
+ Suggestive significance (p value: 0.05 < p < 0.10)
** Strongly significant (p value: p ≤ 0.01)
Oral hygiene practices differed in Group-1 and Group-2 (Table 3). Subjects cleaned their teeth with brush and paste (48.8 % vs. 72.9 %) and fingers (51.2 % vs. 27.2 %) respectively. Subjects in Group-1 changed their brushes less often in a year than that of Group-2 (48.8 % vs. 72.5 %). Majority of the Group-1 subjects brushed once in a day (93.4 %) and a few twice a day (6.6 %) compared to Group-2 subjects (81.1 % and 18.9 %). There was no significant difference between mouth wash use and other oral hygiene aids used in both groups.
Table 3.
Oral hygiene practices
| Oral hygiene practices | Group-1 | Group-2 | ||
|---|---|---|---|---|
| n = 242 | % | n = 254 | % | |
| Brushing aid | ||||
| B/P | 114 | 47.1 | 183 | 72.1 |
| F/PW | 105 | 43.4 | 54 | 21.3 |
| F/P | 18 | 7.4 | 15 | 5.9 |
| B/PW | 4 | 1.7 | 2 | 0.8 |
| F/S | 1 | 0.4 | 0 | 0.0 |
| Brushing method | ||||
| Horizontal | 233 | 96.3 | 232 | 91.3 |
| Vertical | 9 | 3.7 | 22 | 8.7 |
| Brushes changed/year | ||||
| No | 124 | 51.2 | 70 | 27.6 |
| Yes | 118 | 48.8 | 184 | 72.5 |
| 1–2 times | 75 | 30.9 | 53 | 20.9 |
| 3–4 times | 43 | 17.8 | 128 | 50.4 |
| >4 times | 0 | 0.0 | 3 | 1.2 |
| Frequency of brushing | ||||
| One time | 226 | 93.4 | 206 | 81.1 |
| Two times | 16 | 6.6 | 48 | 18.9 |
| Mouth wash use | ||||
| Yes | 1 | 0.4 | 5 | 1.9 |
| No | 241 | 99.6 | 249 | 98.0 |
| Others | ||||
| Tooth pick/Gumtone | 1 | 0.4 | 1 | 0.4 |
| Nil | 241 | 99.6 | 253 | 99.6 |
| Diet | ||||
| Vegetarian | 70 | 28.9 | 109 | 42.9 |
| Non-vegetarian | 172 | 71.1 | 145 | 57.1 |
B/P brush and paste, F/PW finger and powder, F/P finger and paste, B/PW brush and powder, F/S finger and salt
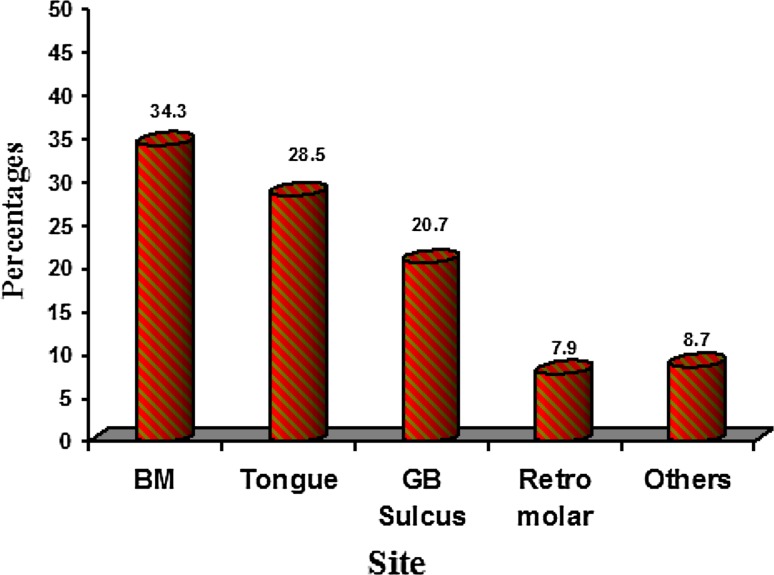
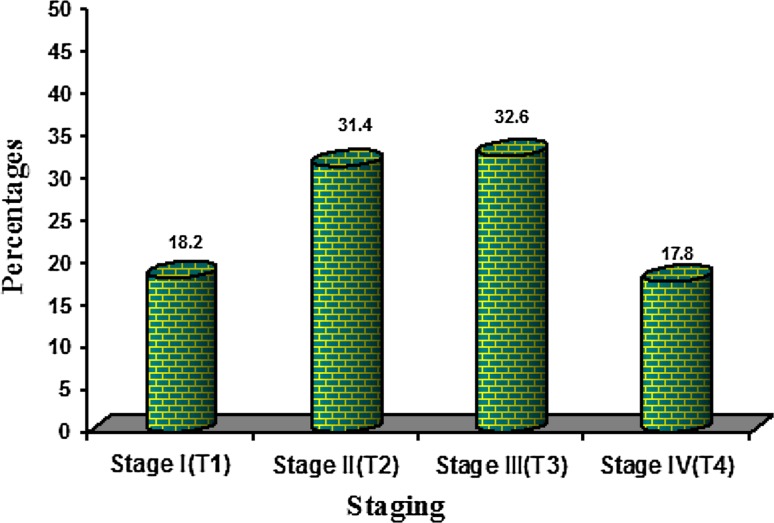
Buccal mucosa (Fig. 2) was the predominantly involved site (34.3 %) by OSCC followed by the tongue (28.5 %). The left mandibular Gingivo-Buccal sulcus (GB sulcus) was involved by 33.5 % of OSCC, correlating with the history of tobacco placement in the left vestibule. Tumor staging according to TNM classification (Fig. 3) showed 44 patients (18.2 %) in Stage I, 76 patients (31.4 %) in Stage II, 79 patients (32.6 %) in Stage III, and 43 patients (17.8 %) in Stage IV.
Fig. 2.
Site distribution of oral cell squamous carcinoma
Fig. 3.
Staging of oral cell squamous carcinoma
Use of prosthesis was not significant in both the groups, although few Group-2 subjects presented with fixed and removal appliances. In Group-1, there were 7 complete upper and lower edentulous patients (2.9 %) and 3 partial edentulous patients (1.2 %) either upper or the lower jaw and none in Group-2 (Table 4).
Table 4.
Edentulous/partial edentulous/missing teeth
| Group-1 | Group-2 | p value | |||
|---|---|---|---|---|---|
| n = 242 | % | n = 254 | % | ||
| Edentulous | 7 | 2.9 | 0 | 0.0 | 0.006** |
| Partial Edentulous | 3 | 1.2 | 0 | 0.0 | 0.075+ |
| At least one tooth missing | 173 | 71.5 | 192 | 75.6 | 0.300 |
| <6 teeth missing | 98 | 40.5 | 139 | 54.7 | 0.002** |
| 6–12 teeth missing | 56 | 23.1 | 41 | 16.1 | 0.049* |
| >12 teeth missing | 19 | 7.9 | 12 | 4.7 | 0.150 |
Majority of the cases in Group-1 were seen in dentulous patients. Missing >6 teeth were significantly associated with risk
+ Suggestive significance (p value: 0.05 < p < 0.10)
* Moderately significant (p value: 0.01 < p ≤ 0.05)
** Strongly significant (p value: p ≤ 0.01)
In Group-2, clinically missing teeth were more compared to Group-1 (Table 5), whereas teeth missing due to periodontal problems were significantly elevated in Group-1.
Table 5.
Teeth missing for different reasons
| Missing teeth | Group-1 (n = 235) | Group-2 (n = 254) | p value | ||
|---|---|---|---|---|---|
| No | % | No | % | ||
| Missing clinically | 49 | 20.9 | 74 | 29.1 | 0.035* |
| Missing due to periodontal problems | 94 | 40.0 | 66 | 25.9 | 0.001** |
| Missing due to trauma | 0 | 0.0 | 5 | 1.9 | 0.031* |
* Moderately significant (p value: 0.01 < p ≤ 0.05)
** Strongly significant (p value: p ≤ 0.01)
There was no significant difference in caries experience (DMFT score) in both the groups. A statistically significant value was observed with respect to filled teeth in Group-2 than in Group-1 (Table 6).
Table 6.
Mean (SD) decayed, missing, filled and DMFT score
| Caries experience | Group-1 (n = 235) | Group-2 (n = 254) | p value |
|---|---|---|---|
| Decayed | 1.57 (1.78) | 1.39 (1.73) | 0.245 |
| Missing | 1.00 (1.53) | 1.26 (1.79) | 0.081+ |
| Filled teeth | 0.04 (0.33) | 0.35 (1.16) | <0.001** |
| Total | 2.59 (2.46) | 3.00 (2.97) | 0.093+ |
+ Suggestive significance (p value: 0.05 < p < 0.10)
** Strongly significant (p value: p ≤ 0.01)
In Group-1, the oral hygiene was poorer compared to Group-2 (Table 7). Bleeding on probing (Code 1) and calculus deposits (Code 2) were seen in Group-2 compared to Group-1 (Table 8). There was no significant difference between Group-1 and Group-2 in having pocket depth of 4 or 5 mm (Code 3). In Group-1, 26.8 % of the subjects had pocket depth of >6 mm (Code 4) and Excluded category (11.1 %) compared to Group-2 (16.1 % and 3.9 %) respectively. In the present study we subdivided Group I into early lesions (Group-1A) comprising Stage I and II lesions and advanced lesions (Group-1B) comprising Stage III and IV lesions and then compared with controls (Group-2). When Group-1A and Group-2 were compared (Table 9), habit and infrequent dental visits were significantly associated with increased risk of cancer when compared to the Group-2 (p < 0.001). Missing more than 12 teeth was not significantly associated with risk of cancer. There was no significant difference in DMFT score between Group-1A and Group-2 (Table 10); however Group 2 had more filled teeth than Group-1A (p < 0.008). Teeth missing due to periodontal problems were significantly increased in Group-1A than Group-2 (p < 0.0039) and there was no difference between numbers of missing teeth among the groups. The oral hygiene measured by OHI-S was significantly poorer even in Group-1A compared to Group-2 (p < 0.001). Majority of the Group-2 had calculus deposits (Code 2) and pocket depth of 4–5 mm, however Group-1A had more than 6 mm pocket depth (Code 4) with p value of 0.061.
Table 7.
Comparison of OHI-S and CPITN scores between Group-1 and Group-2
| Group-1 (n = 235) | Group-2 (n = 254) | p value | |
|---|---|---|---|
| OHI-S score | 2.71 ± 0.64 | 2.34 ± 0.72 | <0.001** |
| CPITN score | 13.85 ± 3.23 | 11.98 ± 3.54 | <0.001** |
** Strongly significant (p value: p ≤ 0.01)
Table 8.
Comparison of CPITN score between Group-1 and Group-2
| CPITN score | Group-1 (n = 235) | Group-2 (n = 254) | p value |
|---|---|---|---|
| CODE 0 | 0 | 0 | – |
| CODE 1 | 0 | 11 (4.3%) | 0.001** |
| CODE 2 | 45 (19.1%) | 95 (37.4%) | <0.001** |
| CODE 3 | 101 (42.9%) | 97 (38.2%) | 0.281 |
| CODE 4 | 63 (26.8%) | 41 (16.1%) | 0.004** |
| EXCLUDED | 26 (11.1%) | 10 (3.9%) | 0.009** |
** Strongly significant (p value: p ≤ 0.01)
Table 9.
Comparison of habits, dental visits, teeth missing, between early lesions (Group-1A) and Group- 2
| Variables | Group-1A (n = 120) | Group 2 (n = 254) | p value |
|---|---|---|---|
| n (%) | n (%) | ||
| Habits | |||
| Smoking | 23 (19.2%) | 40 (15.7%) | 0.410 |
| Chewing | 53 (44.2%) | 66 (26%) | <0.001** |
| Smoking-Chewing | 19 (15.8%) | 11 (4.3%) | <0.001** |
| Betel nut chewing | 32 (26.7%) | 95 (37.4%) | 0.041* |
| Alcohol consumption | 26 (21.7%) | 34 (13.4%) | 0.042* |
| No habits | 9 (7.5%) | 73 (28.7%) | <0.001** |
| Dental visits/year | |||
| 1–2 visits | 43 (35.8%) | 62 (24.4%) | 0.022* |
| 3–5 visits | 13 (10.8%) | 73 (28.7%) | <0.001** |
| >5 visits | 0 (0%) | 2 (0.8%) | 0.330 |
| Total visits | 56 (46.7%) | 137 (53.9%) | 0.189 |
* Moderately significant (p value: 0.01 < p ≤ 0.05)
** Strongly significant (p value: p ≤ 0.01)
Table 10.
Comparison of caries experience, teeth missing due to periodontal problems and OHI-S and CPITN index between early lesions (Group-1A) and Group 2
| Variables | Group-1A (n = 115) | Group 2 (n = 254) | p value |
|---|---|---|---|
| n (%) | n (%) | ||
| Caries experience | |||
| Decayed | 67 (58.3%) | 146 (57.5%) | 0.888 |
| Missing | 45 (39.1%) | 120 (47.2%) | 0.147 |
| Filled | 4 (3.5%) | 31 (12.2%) | 0.008** |
| DMFT | 87 (75.7%) | 189 (74.4%) | 0.799 |
| Missing due to periodontal problems | |||
| <6 teeth | 26 (22.6%) | 43 (16.9%) | 0.195 |
| 6–12 teeth | 9 (7.8%) | 13 (5.1%) | 0.309 |
| >12 teeth | 7 (6.1%) | 10 (3.9%) | 0.362 |
| Total missing | 42 (36.5%) | 66 (26%) | 0.039* |
| OHI-S Index | |||
| OHI-S score | 2.61 ± 0.68 | 2.34 ± 0.72 | 0.001** |
| CPITN score | |||
| CODE 0 | 0 | 0 | – |
| CODE 1 | 0 | 11 (4.3%) | 0.023* |
| CODE 2 | 28 (24.3%) | 95 (37.4%) | 0.014* |
| CODE 3 | 51 (44.3%) | 97 (38.2%) | 0.264 |
| CODE 4 | 28 (24.3%) | 41 (16.1%) | 0.061+ |
| EXCLUDED | 8 (6.9%) | 10 (3.9%) | 0.212 |
+ Suggestive significance (p value: 0.05 < p < 0.10)
* Moderately significant (p value: 0.01 < p ≤ 0.05)
** Strongly significant (p value: p ≤ 0.01)
When Group-1A was compared with Group-1B (Table 11), smoked tobacco was significantly associated with risk of cancer in Group-1A (p < 0.039) whereas betel quid usage was significantly associated with Group-1B (p < 0.026). Infrequent dental visits were again associated with risk of cancer for Group-1B. There was no statistical significant difference in DMFT score (Table 12) and the teeth missing due to periodontal problems and number of missing teeth between Group-1A and Group-1B. However, missing 6–12 teeth due to periodontal problems were more in Group-1B (15.8 %) compared to Group-1A (7.5 %) with a p value of 0.058. Oral hygiene was significantly poorer in Group-1B compared to Group-1A (p < 0.039). In Group-1A, only 6.9 % were excluded from probing compared to Group-1B in which 15 % of the cases were excluded due to missing of index teeth.
Table 11.
Comparison of habits, dental visits, teeth missing in early (Group-1A) and advanced lesions (Group-1B)
| Variables | Group-1A (n = 120) | Group-1B (n = 122) | p value |
|---|---|---|---|
| n (%) | n (%) | ||
| Habits | |||
| Smoking | 23 (19.2%) | 12 (9.8%) | 0.039* |
| Chewing | 53 (44.2%) | 65 (53.3%) | 0.438 |
| Smoking-Chewing | 19 (15.8%) | 20 (16.4%) | 0.906 |
| Betel nut chewing | 32 (26.7%) | 49 (40.2%) | 0.026* |
| Alcohol consumption | 26 (21.7%) | 21 (17.2%) | 0.381 |
| No habits | 9 (7.5%) | 6 (4.9%) | 0.405 |
| Dental visits/year | |||
| 1–2 visits | 43 (35.8%) | 41 (33.6%) | 0.716 |
| 3–5 visits | 13 (10.8%) | 5 (4.1%) | 0.048* |
| >5 visits | 0 (0%) | 0 (0%) | – |
| Total visits | 56 (46.7%) | 46 (37.7%) | 0.158 |
* Moderately significant (p value: 0.01 < p ≤ 0.05)
Table 12.
Comparison of caries experience, teeth missing due lo periodontal problems and OHI-S and CPITN index between early (Group-1A) and advanced lesions (Group-1B)
| Variables | Group-1A (n = l15) | Group-1B (n = 120) | p value |
|---|---|---|---|
| n (%) | n (%) | ||
| Caries experience | |||
| Decayed | 67 (55.8%) | 74 (61.7%) | 0.598 |
| Missing | 45 (37.5%) | 51 (42.5%) | 0.691 |
| Filled | 4 (3.3%) | 1 (0.8%) | 0.160 |
| DMFT | 87 (72.5%) | 85 (70.8%) | 0.404 |
| Missing due to periodontal problems | |||
| <6 teeth | 26 (21.7%) | 24 (20.0%) | 0.625 |
| 6–12 teeth | 9 (7.5%) | 19 (15.8%) | 0.058+ |
| >12 teeth | 7 (5.8%) | 9 (7.5%) | 0.667 |
| Total missing | 42 (36.5%) | 52 (43.3%) | 0.287 |
| OHI-S Index | |||
| OH1-S score | 2.61 ± 0.68 | 2.80 ± 0.58 | 0.039* |
| CPITN score | |||
| CODE 0 | 0 | 0 | – |
| CODE 1 | 0 | 0 | – |
| CODE 2 | 28 (24.3%) | 17 (14.2%) | 0.047* |
| CODE 3 | 51 (44.3%) | 50 (41.7%) | 0.678 |
| CODE 4 | 28 (24.3%) | 35 (29.2%) | 0.404 |
| EXCLUDED | 8 (6.9%) | 18 (15.0%) | 0.049* |
+ Suggestive significance (p value: 0.05 < p < 0.10)
* Moderately significant (p value: 0.01 < p ≤ 0.05)
Eight different types of aerobic microorganisms were isolated individually or in combination with other group of organisms (Table 13). The predominant microorganisms isolated from Group-1 as well as in Group-2 with (62 % vs. 66 %) and without habits (40 % vs. 66 %) were Streptococcus species followed by Staphylococcus species (10 % vs. 4 %) and 13.3 % vs. 6.7 %. Though there were no significant findings between the groups, subjects in Group-1 showed increased number of pathogenic microflora compared to Group-2.
Table 13.
Comparison of microorganisms isolated in Group-1 and Group-2 with and without habits
| Organisms isolated | Group-1 | Group-2 | ||
|---|---|---|---|---|
| With habits (n = 50) | Without habits (n = 15) | With habits (n = 50) | Without habits (n = 15) | |
| No growth | 3 (15.0%) | 1 (6.7%) | 2 (4.0%) | 0 |
| Growth | 47 (94.0%) | 14 (93.3%) | 48 (96.0%) | 15 (100.0%) |
| E. Coli | 2 (4.0%) | 2 (13.3%) | 2 (4.0%) | 0 |
| Enterococcus spp. | 2 (4.0%) | 1 (6.7%) | 1 (2.0%) | 1 (6.7%) |
| Enterococcus and Klebsiella spp. | 0 | 0 | 1 (2.0%) | 0 |
| Klebsiella spp. | 0 | 0 | 1 (2.0%) | 0 |
| Moraxella catarrhalis | 2 (4.0%) | 2 (13.3%) | 2 (4.0%) | 1 (6.7%) |
| Moraxella catarrhalis and Pseudomonas | 1 (2.0%) | 0 | 0 | 0 |
| Multiple organisms or contamination | 1 (2.0%) | 0 | 0 | 0 |
| Pseudomonas aeruginosa | 1 (2.0%) | 1 (6.7%) | 1 (2.0%) | 0 |
| Staphylococcus spp. | 5 (10.0%) | 2 (13.3%) | 2 (4.0%) | 1 (6.7%) |
| Staphylococcus and E. coli | 0 | 0 | 1 (2.0%) | 0 |
| Streptococcus and Enterococcus spp. | 1 (2.0%) | 0 | 1 (2.0%) | 0 |
| Streptococcus and Staphylococcus spp. | 1 (2.0%) | 0 | 0 | 0 |
| Streptococcus spp. | 31 (62.0%) | 6 (40.0%) | 33 (66.0%) | 10 (66.7%) |
| Streptococcus and Klebsiella spp. | 0 | 0 | 0 | 1 (6.7%) |
| Streptococcus spp. and Moraxella catarrhalis | 0 | 0 | 2 (4.0%) | 1 (6.7%) |
| Streptococcus pneumoniae | 0 | 0 | 1 (2.0%) | 0 |
Discussion
There has been substantial scientific dispute, whether and to what extent poor oral hygiene is an independent risk factor for oral cavity cancer. Numerous, both retrospective and case-control studies, have been performed. Several factors of oral condition such as dentition, frequency of tooth brushing, denture wear, and tooth loss, frequency of oral infections, oral sores and mouthwash use have been analyzed. Tooth loss is most commonly caused by dental caries and periodontal disease, but the percentage contribution from each condition depends on age and other factors.
Many studies have reported that tobacco and alcohol consumption are the major risk factors acting separately or synergistically [1, 7–10]. We observed that consumption of tobacco, alcohol and areca nut chewing was significantly associated with increased risk of oral cancer. In our series 93.8 % of Group-1 had habits and 6.2 % of Group-1 had no habits compared to Group-2 with p < 0.001 (Table 2).
A regular dental check-up was found to be associated with a decreased risk of oral cancer in many studies. In our study, subjects who had never attended dental visits had a higher risk of oral cancer than subjects who reported visiting at least once a year and these finding are in agreement with the previous studies [7, 11, 12]. Subjects cleaned their teeth with fingers (51.2 % in Group-1 vs. 27.2 % in Group-2) and less frequently brushed. Use of fingers or sticks for cleaning was a risk factor for oral cancer, and this effect would be modified if the subject consumes alcohol. Fingers and sticks do not adequately clean the teeth, thereby allowing carcinogens to remain longer and to penetrate the oral mucosal surface [13].
Poor oral hygiene and number of missing and defective teeth have been postulated as risk factors for oral cancer in many studies [1, 9, 10, 14, 15]. There was no significant difference in caries experience in both the groups (mean DMFT score 1.57 vs. 1.39) similar to study by Tezal et al. [16] in which besides chronic periodontitis, the effects of dental cavities, fillings, crowns, and root canal treatments were not significantly associated with oral cancer. However, it is in contrast to a Maier et al. study [14] which found a significantly elevated incidence of decayed teeth in the tumor patients (p < 0.001). Tezal et al. [17] also observed that number of teeth with caries was significantly associated with precancerous lesions and the number of filled teeth had no significant effect on any type of oral lesions.
Group-2 subjects had significantly increased clinically missing teeth compared to Group-1 (p < 0.035) whereas teeth missing due to periodontal problems were significantly more in Group-1 (40 % vs. 26 %) with p < 0.002. In Group-2, clinically missing teeth were more (mainly the third molars) than the teeth missing due to periodontal reasons. It implies that tooth loss due to periodontal reasons is significantly associated with elevated risk of cancer.
A strong association of oral cancer with number of missing teeth has been found in our study in accordance with the previous studies [7, 8, 11, 12]. However, when missing teeth were more than 12 in number, no increase in risk was observed. This can be attributed to the absence of a periodontal pathogen with no/minimal teeth remaining [18].
In Group-1, there were seven complete upper and lower edentulous patients (2.9 %) and 3 partial edentulous patients (1.2 %) either the upper or lower jaw and none in Group-2, highlighting the fact that majority of the OSCC occurred in dentulous patients. Possibly the presence of teeth act as habitat for microbial colonization, plaque and calculus deposition and subsequent chronic inflammation may elevate the risk of cancer.
A statistically significant difference was seen related to oral hygiene status (p < 0.001) measured by OHI- S index between the groups. Our findings that average and poor oral hygiene were independent risk factors for OSCC are in agreement with the results of other studies [7, 11–13, 19, 20]. However, in some studies [8, 10] oral hygiene was shown to have little or no importance as a risk factor for oral cancer.
In 26.8 % of Group-1, a pocket depth of >6 mm (Code 4) and Excluded category (11.1 %) was statistically significant compared to Group-2 (16.1 % and 3.9 %). The combined percentage of teeth having pocket depth >6 mm and teeth excluded due to missing teeth were more in Group-1 (37.9 %) compared Group-2 (20 %). These findings are similar to the study by de Rezende et al. [21] in which 76 % of subjects in cancer group showed >6 mm pockets compared to 10 % of control group. The data obtained in our study indicate that the Group-2 subjects had minimal periodontal tissue destruction and subsequent tooth loss compared to Group-1. This indicates that the Group-1 subjects had more periodontal destruction and subsequent tooth loss due to which few teeth were excluded from examination compared to Group-2.
In our study after obtaining complete history and thorough clinical examination we found that individuals with a low level of education tended to have less frequent dental check-ups, smoke more and consume more alcohol, factors which would affect their oral/dental health negatively. These findings are in accordance with those of several other groups [7, 8, 11, 12, 20].
A distinct feature of our study is that we compared early lesions (Group-1A) with advanced lesions (Group-1B) and controls to evaluate the association with respect to caries and periodontal disease. Habits and infrequent dental visits were significantly associated with increased risk of cancer (p < 0.001) when Group-1A was compared with Group-2. There was no significant difference in DMFT score between Group-1A and Group-2. There was significant elevated risk with missing teeth and poor oral hygiene status in Group-1A compared to Group-2 (p < 0.001). Though there were no statistically significant differences in periodontal disease status between Group-1A and Group-2 however, subjects in Group-1A had greater pocket depths than Group-2.
When Group-1A was compared with Group-1B, habits and infrequent dental visits were again associated with increased risk of cancer for Group-1B. There was no statistical significant difference in DMFT score between Group-1A and Group-1B. This highlights the fact that there seems to be no association between dental caries and OSCC. In Group-1A, the oral hygiene was better compared to Group-1B. Group-1A had less amount of periodontal tissue break down as measured by pocket depth and only few were excluded from probing (6.9 %) compared to Group-1B (15 %). This implies that oral hygiene deteriorates with the increase in the stage and does not contribute to etiology of OSCC.
Additionally our study also isolated microorganisms associated with the OSCC and implicated their role in cancer. The predominant microorganisms isolated from Group-1 and Group-2 subjects with (62 % vs. 66 %) and without habits (40 % vs. 66 %) were Streptococcus species (α-hemolytic) followed by Staphylococcus species (10 % vs. 4 %) and 13.3 % versus 6.7 %. These findings are similar to the study by Nagy et al. [22] who demonstrated increased numbers of certain members of the oral microbiota on the surface of tumors in comparison with control sites. It may be an important phenomenon that the rise in the colonization of oral cavity by streptococci, in patients having habits, may be a predisposing factor inducing mucosal changes leading to malignancy, this can be correlated to Homann et al. [23] found increased number of Streptococcus salivarius, alpha-hemolytic Streptococci, Corynebacterium spp. and Stomatococcus spp. in the saliva which is associated with increased production of acetaldehyde which is proved to be carcinogenic. Though there were no significant findings between the groups in relation to habit, Group-1 showed increased number of pathogenic microflora compared to Group-2. Based on the data obtained on microorganisms isolated, it is not possible to consider their role for etiology of OSCC. Bacterial load is more probably related to poor oral hygiene on account of OSCC rather than contributing to OSCC per se given it is more commonly seen in advanced lesions. Considering the role of pathogenic microflora, preoperative tumor culture is still valuable in predicting the bacteriology of a possible post-operative infection, and in selecting the appropriate antibiotic treatment.
Potential limitations of this study should be considered. First, the number of teeth remaining was examined after the occurrence of cancer. Although it is unlikely that the occurrence itself caused tooth loss, this limitation should nevertheless be considered when interpreting the results. In our study we elicited recent history of tooth loss since the appearance of cancer and those teeth were excluded from missing category. Since we inquired about the past oral health conditions the possibility of memory recall bias may affect the results. We used CPITN index to measure the periodontal disease status, the main advantage of the index is its easy use, as well, its world-wide application allows for international comparisons. However, many limitations are associated with the CPITN. First, this index is based on a hierarchical concept of progression of periodontal disease. Thus, a sextant presenting a tooth with a periodontal pocket (Code 3 or 4) should also present calculus (Code 2) and bleeding (Code 1). Another limitation of this index is that it does not measure important signs of periodontal disease, such as dental mobility and attachment loss. It is thus important to keep in mind that the CPITN is not a complete measure of periodontal disease. Partial-mouth exams may underestimate sites with periodontal disease, particularly the more severe conditions.
Conclusion
Lack of dental visits, infrequent brushing and poor periodontal conditions are significantly associated with increased risk of oral cancer. There seems to be no correlation between dental caries experience and OSCC. There is a direct correlation between periodontal disease experience and severity with OSCC. This is possibly on account of the anaerobic nature of both disease processes. Periodontitis is an established anaerobic disease process, the presence of anaerobic environment leads to release of inflammatory cytokines and reduction in oxidation potential all of which trigger the chronic inflammatory response, which has been postulated as one of the mechanism for cancer initiation. In OSCC it is still a matter of conjecture to explain the anaerobic nature though few bacterial species have been identified in saliva of OSCC patients as well within the tumor itself to establish the disease process.
Admittedly poor oral hygiene is an etiologic for gingivitis and periodontitis. This is a confounding finding in our study where we were unable to conclusively find direct relationship between poor oral hygiene and OSCC. In our study we could not find a direct correlation between OSCC and OHI-S however, this study demonstrates a direct correlation between periodontitis and OSCC. All patients in Group-2 who had poor oral hygiene had gingivitis.
OHI status progressively deteriorates with increasing grades of OSCC. Whether this implies that poor OHI is a risk factor for OSCC is still a matter of conjecture. Increase in pathogenic flora may play a role in production of carcinogenic agents or post-operative infections.
Identification of average or poor oral hygiene, defective and missing teeth, poorly fitting dentures and oral mucosal lesions at regular dental check-ups are important factors for preventing OSCC. Patients with periodontal diseases should seek care from their dentists irrespective of the effect on cancer.
Acknowledgments
The authors wish to thank Padmashree Dr. R. B. Patil, Dr. B. R. Patil, Dr. A. C. Deka and Staff KCTRI, Hubli and Dr. Venkatesh, Head of Department, and staff, Department of Oral Medicine and Radiology, SDM College of Dental Sciences, Dharwad for providing an opportunity to conduct the study. The authors also thank Dr. Kulkarni, Professor and Head, Department of Microbiology and staff, SDM Medical College for carrying out the bacterial cultures for this study.
Conflict of interest
None declared.
References
- 1.Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A, Goran-Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population based case—control study in southern Sweden. Acta Otolaryngologica. 2005;125:1327–1336. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 2.Chen AY, Myers JN. Cancer of the oral cavity. Dis Mon. 2001;47:274–361. doi: 10.1016/S0011-5029(01)90005-7. [DOI] [PubMed] [Google Scholar]
- 3.Dimitroulis G, Avery BS. Oral cancer: a synopsis of pathology and management. 1. Oxford: Reed Educational and Professional Publishing; 1998. [Google Scholar]
- 4.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Acay RR, Santos E, de Sousa SO. Correlation between c-Jun and human papillomavirus in oral premalignant and malignant lesions. Oral Oncol. 2008;44:698–702. doi: 10.1016/j.oraloncology.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Lax AJ, Thomas W. How bacteria could cause cancer: one step at a time. Trends Microbiol. 2002;10:293–299. doi: 10.1016/S0966-842X(02)02360-0. [DOI] [PubMed] [Google Scholar]
- 7.Balaram P et al (2002) Cancer in southern India; The influence of smoking, drinking, paan-chewing and oral hygiene. Int J Cancer 98:440–445 [DOI] [PubMed]
- 8.Marshall JR, Graham S, Haughey BP, Shedd D, O’Shea R, Brasure J, et al. Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. Eur J Cancer B Oral Oncol. 1992;28B:9–15. doi: 10.1016/0964-1955(92)90005-L. [DOI] [PubMed] [Google Scholar]
- 9.Garrote LF, Herrero R, Reyes RM, et al. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85:46–54. doi: 10.1054/bjoc.2000.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talamini R, Vaccarella S, Barbone F, Tavani A, La Vecchia C, Herrero R, et al. Oral hygiene, dentition, sexual habits and risk of oral cancer. Br J Cancer. 2000;83:1238–1242. doi: 10.1054/bjoc.2000.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng TZ, Boyle P, Hu HF, Duan J, Jian PJ, Ma DQ, et al. Dentition, oral hygiene, and risk of oral cancer: a case–control study in Beijing, People’s Republic of China. Cancer Causes Control. 1990;1:235–241. doi: 10.1007/BF00117475. [DOI] [PubMed] [Google Scholar]
- 12.Lissowska J, Pilarska A, Pilarski P, Samolczyk-Wanyura D, Piekarczyk J, Bardin-Mikollajczak A, et al. Smoking, alcohol, diet, dentition and sexual practices in the epidemiology of oral cancer in Poland. Eur J Cancer Prev. 2003;12:25–33. doi: 10.1097/00008469-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Velly AM, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34:284–291. doi: 10.1016/S1368-8375(98)80009-2. [DOI] [PubMed] [Google Scholar]
- 14.Maier H, Zoller J, Herrmann A, Kreiss M, Heller WB. Dental status and oral hygiene in patients with head and neck cancer. Otolaryngol Head Neck Surg. 1993;108:655–661. doi: 10.1177/019459989310800606. [DOI] [PubMed] [Google Scholar]
- 15.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9:550–558. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tezal M, Sullivan MA, Reid ME, Marshall JR, Hyland A, Loree T, et al. Chronic periodontitis and the risk for tongue cancer. Arch Otolaryngol Head Neck Surg. 2007;133:450–454. doi: 10.1001/archotol.133.5.450. [DOI] [PubMed] [Google Scholar]
- 17.Tezal M, Grossi SG, Genco RJ. Is periodontitis associated with oral neoplasms? J Periodontol. 2005;76:406–410. doi: 10.1902/jop.2005.76.3.406. [DOI] [PubMed] [Google Scholar]
- 18.Guha N, Boffetta P, Filho VW, Neto JE, Shangina O, Zaridze D, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case–control studies. Am J Epidemiol. 2007;166:1159–1173. doi: 10.1093/aje/kwm193. [DOI] [PubMed] [Google Scholar]
- 19.Lockhart PB, Norris CM, Pulliam C. Dental factors in the genesis of squamous cell carcinoma of the oral cavity. Oral Oncol. 1998;34:133–139. doi: 10.1016/S1368-8375(97)00086-9. [DOI] [PubMed] [Google Scholar]
- 20.Bundgaard T, Wildt J, Frydenberg M, Elbrond O, Nielsen JE. Case–control study of squamous cell cancer of the oral cavity in Denmark. Cancer Causes Control. 1995;6:57–67. doi: 10.1007/BF00051681. [DOI] [PubMed] [Google Scholar]
- 21.de Rezende CP, Ramos MB, Daguila CH, Dedivitis RA, Rapoport A. Oral health changes in patients with oral and oropharyngeal cancer. Braz J Otorhinolaryngol. 2008;74:596–600. doi: 10.1016/S1808-8694(15)30609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagy KN, Sonkodi I, Szoke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34:304–308. doi: 10.1016/S1368-8375(98)80012-2. [DOI] [PubMed] [Google Scholar]
- 23.Homann N, Tillonen J, Meurman J, Rintamaki H, Lindquist C, Jousimies-Somer H, et al. Increased salivary acetaldehyde levels in heavy drinkers and smokers: a microbiological approach to oral cavity cancer. Carcinogenesis. 2000;21:663–668. doi: 10.1093/carcin/21.4.663. [DOI] [PubMed] [Google Scholar]