Abstract
Objective
The purpose of this retrospective analysis is to document and discuss the features, treatment rendered and result of 25 histologically proven cases of ossifying fibromas of jaw bones operated by a single surgeon over a period of 10 years.
Materials and Methods
The records of ossifying fibroma were obtained from the archives of Oral and Maxillofacial Surgery at Maulana Azad Institute of Dental Sciences (MAIDS) from 2001 to 2011. Only those cases were included in the study where definitive surgery was performed based on clinical, radiological & histopathological features.
Results
Twenty-five patients were analyzed with a final diagnosis of ossifying fibroma comprising of 14 males (56 %) and 11 females (44 %). The age range was 11–45 years with a mean of 24.12 years. Mandible was involved in 72 % and maxilla in 28 % cases with a predominance of mandibular posterior [19 (76 %)] cases. The study showed similar findings in regard to clinical, radiographic & histological features of ossifying fibroma as compared to other studies. It also showed that the treatment rendered in the form of eneucleation, curettage or resection of the lesion depending on its stage and extent were adequate, as no recurrence has been reported till date.
Conclusion
Enucleation is preferred in small and well demarcated lesions. Curettage should be done in relatively large lesions with ill defined borders, not involving basal bone of mandible or cortical perforation. Resection should be reserved for aggressive and extensive cases with involvement of basal bone or perforation of cortices.
Keywords: Fibro-osseous lesions, Benign, Mandible, Enucleation
Introduction
Ossifying fibroma (OF) is a benign and non-odontogenic fibro-osseous lesion, the exact etiology of which is unknown. It tends to arise from the mesenchymal blast cells of periodontal ligament and contains fibrous tissue, bone and cementum like material [1, 2]. The presence of these mineralized tissues designates this group of lesions as ossifying, cemento–ossifying or cementifying fibromas. The common presentation of the lesion is a slow growing, well demarcated or encapsulated intra-bony mass, commonly seen between 3rd and 4th decades of life with a female predilection (5:1) [3–6]. Mandible is affected in 70–90 % of cases [7]. OF mostly presents as a solitary lesion but multiple lesions affecting jaw bone have also been reported which are not associated with hyperthyroidism-jaw tumour syndrome (HPT-JT) [8]. Though histologically proven cases of OF are commonly found in the jaw bone (>90 % of cases), their presence is well documented in other craniofacial and long bones. The lesion affects jaws more commonly because of the maximum amount of mesenchymal cellular induction into the bone and cementum required in odontogenesis, resulting in higher induction error or genetic alteration leading to a neoplasm [9].
They remain clinically asymptomatic until they become large enough to cause facial deformity or hinder function. Other symptoms like pain, mobility of teeth and pus discharge may also be associated with the lesion at a later stage [10]. Radiologically, it shows features of radiolucency, mixed radiolucency and opacity and complete radiopacity depending on the degree of mineralization. Initially the lesion is encapsulated and so it is well demarcated. After reaching a larger size (2–3 cm in diameter), it infiltrates for few millimeters beyond its margins and becomes difficult to be delineated from surrounding bone. Management includes curettage or eneucleation or resection of the lesion depending on the stage and extent of the neoplasm in the involved jaw bone [9].
The aim of this study was to analyze and report the clinical, radiological and histological features of 25 cases of central ossifying fibromas along with their surgical management and outcome over a period of 10 years.
Materials and Methods
The study group consisted of 25 cases of OF of the jaws (operated by same surgeon), the diagnosis of which had been confirmed by histopathology after definitive treatment. The data of these patients were retrieved from the archives of the Department of Oral and Maxillofacial Surgery and Oral and Maxillofacial Pathology at Maulana Azad Institute of Dental Sciences from 2001 to 2011. The initial diagnosis was based on clinical, radiological and histological features following incisional biopsy. Orthopantomogram (OPG) was the standard radiograph for mandibular lesions. Lesions involving midface and paranasal sinuses were evaluated with Water’s view. Computerized tomography (CT) scan was advised for extensive lesions. Histopathologically, the lesions were studied based on the mineralization patterns and the nature of background stroma. Presence of cementicles, ossicles, rounded bony trebaculae and trebaculae of bone were studied. The cellularity and/or fibrous component of the stromal tissue were further evaluated. The lesions were treated either by curettage, eneucleation or resection and reconstruction depending on the clinical and radiological status. Being a retrospective study it did not require review from Institutional Review Board and was in accordance with Helsinki Declaration.
Result
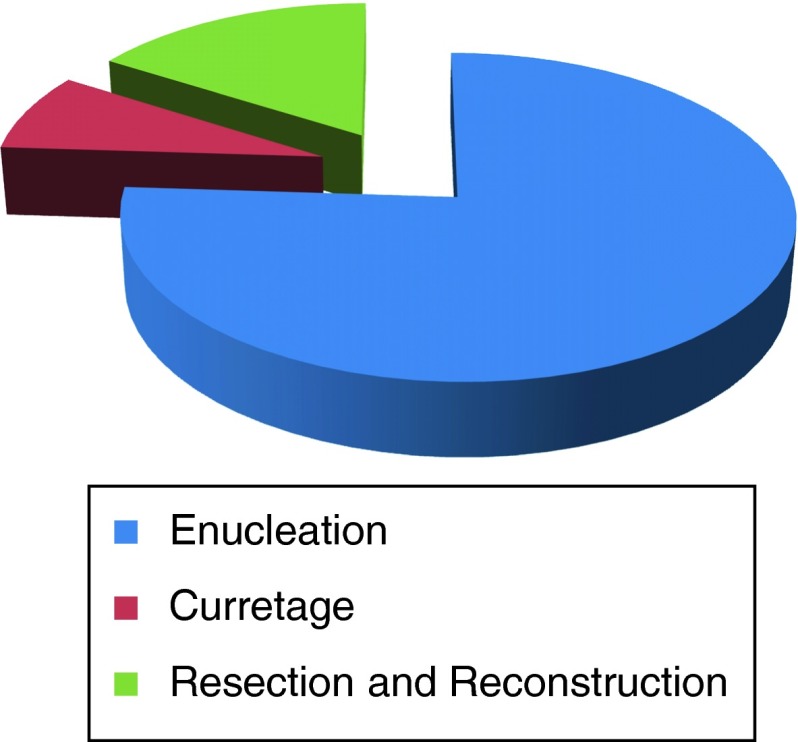
Twenty-five histologically proven case of OF were found. They included 14 males (56 %) and 11 females (44 %) with a ratio of 1:1.27. Age range of the patients was 11–45 years with a mean age of 24.12 years. Mandible was involved predominantly i.e. 19 cases (72 %) followed by 6 cases (28 %) in the maxilla. In mandible, all the lesions were seen in premolar-molar region. In maxilla, 1 case (4 %) involved the molar region and 4 cases (16 %) were found in the anterior maxilla. One lesion was located in the hard palate corresponding to the premolar region. Table 1 depicts the above findings. Most of the patients presented with swelling and others complained of pain, mobility of teeth, pus discharge and paresthesia in the involved area of the bone. The mean size of the lesion was 3.85 cm × 2.6 cm with a range of 1.0–9.5 cm as measured from radiographs. The radiographic features included radiolucent 14 (56 %), mixed 6 (24 %) and radiopaque 5 (20 %) lesions. 18 cases were correctly diagnosed as OF on incisional biopsy. Four cases were diagnosed as fibrous dysplasia and 3 others as odontogenic myxoma, osteoma and odontome respectively on incisional biopsy. After the initial diagnosis, the lesions were treated by enucleation in 19 (76 %), curettage in 2 (8 %), resection and reconstruction with 2.4 mm system plates in 2 (8 %) and resection and reconstruction with rib graft and similar reconstruction plate in 2 cases (8 %) (Fig. 1).Only those cases were included in the study in which final histology revealed features of OF. Microscopically, 24 cases showed the features of true OF with 1 (4 %) case presenting with features of juvenile ossifying fibroma. The above features are discussed in Table 2. Follow-up period ranged from 6 months to 5 years with a review at 1, 3, 6 months and 1 year intervals. OPGs for mandible and PNS views for maxilla were taken at 6 months and 1 year interval. Till date no recurrence has been reported in our series.
Table 1.
Clinical information
| Sl. no. | Age/gender | Clinical finding | Region involved (radiological) | Size (cm) | Provisional diagnosis |
|---|---|---|---|---|---|
| 1 | 35/F | S, P | 35 to asc. ram | 5.7 × 4.2 | FD |
| 2 | 23/M | S, P | 46–48 | 2.8 × 1.5 | OF |
| 3 | 14/M | S, P | 12–15 | 3.5 × 2.8 | OF |
| 4 | 25/F | S, P, Ps | 45 to asc. ram | 6.5 × 2.5 | OF |
| 5 | 20/M | S, P, Ps | 35 to asc. ram | 6.0 × 5.5 | OF |
| 6 | 20/M | S | 45–47 | 3.5 × 3.0 | OF |
| 7 | 14/M | S | 46 to asc. ram | 9.0 × 5.0 | OF |
| 8 | 24/M | S | 22–25 | 3.0 × 2.5 | FD |
| 9 | 11/M | S, Ps | 23–27 | 5.5 × 4.0 | OF |
| 10 | 45/F | S, P, Pus | 33–36 | 1.5 × 1.5 | FD |
| 11 | 12/F | S | 33–36 | 1.5 × 1.3 | OF |
| 12 | 38/F | S | 23–25 | 2.2 × 2.0 | OF |
| 13 | 24/F | S | 46–47 | 1.0 × 0.7 | OF |
| 14 | 22/F | S, Mt | 44–37 | 4.5 × 3.2 | OM |
| 15 | 20/M | S | 22–23 | 1.3 × 0.75 | OF |
| 16 | 37/M | S | Hard palate | 2.0 × 1.0 | FD |
| 17 | 19/M | S, P | 31–36 | 4.0 × 3.5 | OF |
| 18 | 45/F | S, P, Et | 47–31 | 2.0 × 1.5 | OF |
| 19 | 24/F | S | 32–36 | 3.0 × 2.0 | OF |
| 20 | 22/M | S | Zyg bts | 1.0 × 0.75 | Osteoma |
| 21 | 22/M | S | 34–36 | 3.5 × 2.0 | OF |
| 22 | 38/F | S | 41–46 | 7.5 × 2.0 | FD |
| 23 | 28/F | S, P, Ps | 35–38 | 4.2 × 2.9 | OF |
| 24 | 10/M | S | 34–38 | 4.0 × 4.0 | OF |
| 25 | 23/M | S, Pus (extraoral) | 36 to asc ram | 7.5 × 5.0 | Odontome |
S swelling, P pain, Ps paresthesia, Pus pus discharge, Mt mobility of teeth, Et exfoliation of teeth, asc. ram ascending ramus, Zyg bts zygomatic buttress, OF ossifying fibroma, FD fibrous dysplasia, OM odontogenic myxoma
Fig. 1.
Treatment rendered
Table 2.
Radiology, histopathology and treatment
| Sl. no. | Radiological finding | Histopathology (incisional) | Treatment | Follow-up (years) |
|---|---|---|---|---|
| 1 | Diffuse, mixed RL (radiolucent) and RO (radiopaque) | Few cementicles in fibrous background | Resection and reconstruction | 5 |
| 2 | Moderately defined, RL | Few cementicles with ossicles in fibrous background | Curettage | 1.3 |
| 3 | Well defined, RL | Mature bony trebaculae in fibrous stroma | Enucleation | 3.5 |
| 4 | Diffuse, RL | Cementicles in highly cellular stroma | Resection and reconstruction | 6 |
| 5 | Diffuse, RL | Mature bony trebaculae in fibrous stroma | Resection and reconstruction | 3.5 |
| 6 | Diffuse, RL | Few cementicles with ossicles in fibrous background | Enucleation | 2 |
| 7 | Well defined, RO | Few cementicles in highly cellular stroma | Resection and reconstruction with rib graft | 2 |
| 8 | Well defined, RO | Mature bony trebaculae and cementicles in fibrous stroma | Enucleation | 1.8 |
| 9 | Well defined, mixed RL and RO | Small cementicles in highly cellular stroma | Enucleation | 1.6 |
| 10 | Ill defined, RO, impacted teeth | Mature bony trebaculae and few cementicles in cellular stroma | Enucleation | 1.6 |
| 11 | Well defined, mixed RL and RO | Mature bony trebaculae in fibrous stroma | Enucleation | 1.4 |
| 12 | Well defined, RL | Cementicles, bony trebaculae in fibrous stroma with focal cellularity | Enucleation | 1.4 |
| 13 | Well defined, mixed RL and RO, nerve displacement | Cementicles and ossicles in highly cellular stroma | Enucleation | 1.4 |
| 14 | Well defined, RL | Irregular immature bony trebaculae in cellular stroma | Enucleation | 1.2 |
| 15 | Well defined, RL | Rounded ossification (psammoma bodies) in highly cellular stroma | Enucleation | 1.2 |
| 16 | Ill defined, RL | Mature bony trebaculae in cellular stroma | Enucleation | 0.75 |
| 17 | Well defined, RL | Mature bony ossicles in cellular stroma | Enucleation | 1 |
| 18 | Ill defined, mixed RL and RO | Few cementicles in highly cellular stroma | Enucleation | 0.83 |
| 19 | Well defined, RL, displaced teeth, multilocular | Few cementicles and ossicles in cellular stroma | Enucleation | 0.83 |
| 20 | Well defined, RO | Mature bony trebaculae in cellular stroma | Enucleation | 0.83 |
| 21 | Ill defined, RL, root resorption | Cementicles in highly cellular stroma | Enucleation | 0.83 |
| 22 | Moderately defined, mixed RL and RO | Bony trebaculae, ossicles, cementicles in highly cellular stroma | Enucleation | 0.83 |
| 23 | Well defined, RL, root resorption | Immature bony trebaculae in loosely fibrous stroma | Enucleation | 0.75 |
| 24 | Well defined, RL | Mature bony trebaculae in fibrous stroma | Curettage | 0.67 |
| 25 | Well defined, RO | Mature bony trebaculae and ossicles in fibrous stroma | Enucleation | 0.58 |
Discussion
Ossifying fibroma is one of the most common benign fibro-osseous lesions characterized by replacement of normal bony architecture with benign connective tissue matrix having varying amounts of mineralized material that can be bone and/or cementum. Clinically, it may be asymptomatic and accidentally discovered on a radiograph or may lead to a significant aesthetic and functional disturbance. Sometimes, it is difficult to distinguish between OF and fibrous dysplasia due to considerable radiological as well as histological overlap. However, a definitive diagnosis of OF can be reached by correlating all the features [11].
This lesion was known by a variety of names like osteofibrous dysplasia, non osteogenic fibroma, cemento-ossifying fibroma, osteofibroma, fibro-osteoma, benign fibro osseous lesion of periodontal ligament origin. It was also known as osteofibrousdysplasia which was first described by Campanacci [12], where the lesion involved the tibia and fibula [13]. Jaffe and Lichtenstein described the above condition as non-osteogenic fibroma, popularly known as “Jaffe-Campanacci syndrome” [14]. However, the term OF of jaw bones is being used since 1927 [13]. In 1968, OF’s of jaw bones were first classified by ‘Hemner’ and cementum containing tumors were also grouped under them. In 1971, WHO first classified these cementum containing lesions into fibrous dysplasia (FD), ossifying fibroma (OF), cementifying fibroma (CF) and cement-ossifying fibroma (COF) (Pindborg JJ et al. 1971). Second WHO classification in 1992 divided benign fibro-osseous lesions of maxillofacial region into: (1) Neoplasms and other tumours related to odontogenic apparatus and (2) Neoplasms and other tumours related to bone, where COF belonged to the 2nd category. In the latest and third classification of fibro-osseous lesions in 2005, the terminology ‘COF’ has been abolished and replaced by the term “ossifying fibroma”.
New WHO classification (2005)
Fibrous dysplasia (FD):
Monostotic FD
Polyostotic FD
Cement-osseous dysplasia (COD):
Periapical cemental dysplasia
Florid cemento osseous dysplasia
Other cemento osseous dysplasia
Ossifying fibroma (OF):
Juvenile trebacular OF
Juvenile psammomatoid OF
Ossifying fibroma is most commonly seen in the 3rd–4th decades of life with a predilection for the fairer sex (5:1) and mandible being the most favored jaw bone in 70–90 % of cases [15–20]. Usually the tooth bearing areas are affected in the jaws, which show higher rates of bone and cementum induction. It is painless to start with and might be an incidental radiographic finding. As the tumour grows in size, it may present with pain in the affected area, expansion of bone sometimes leading to marked disfigurement and ulceration from occlusion from opposing teeth [9].
In our series, the mean age of the patients was 24.2 years. The discrepancy in age distribution and female to male ratio may be due to differences in demographic factors as compared to the other studies. The involvement of posterior mandible dentate segment is consistent with findings in other studies. Twenty-three of our patients reported with swelling and other related complaints like pain, ulceration, disfigurement, loose teeth, paresthesia and pus discharge varying from case to case. There was no swelling in 2 of the cases but the patients reported with pain and paresthesia respectively. Most of our patients belonged to low socio-economic strata with a low level of awareness and were from far off areas in India which might be the reason why signs and symptoms of advanced disease were found in all the cases. Detail of the data is presented in Table 2.
On radiograph, in their early stages, OF are usually small in size and may be completely radiolucent which should be differentiated from similar periapical pathology, early cemento-osseous dysplasia, ameloblastoma, central giant cell granuloma. As these tumours mature, they will appear as a mixed opaque-lucent lesion on radiographs that should be differentiated from FD, condensing osteitis, calcifying epithelial odontogenic tumor (CEOT) and Florid COD. Longstanding lesions show features of complete radiopaque lesions like odontome, mature cemento-osseous dysplasia, osteoblastoma or osteosarcoma [9].
In our series, 10 cases (40 %) presented as lucent, 8 (32 %) as mixed and 7 (28 %) as radiopaque lesions, with either well or ill defined margins merging with the adjacent healthy bone and/or cortical expansion. The data is presented in Table 2.
Incisional biopsy was performed in all cases before rendering any definitive treatment. In Table 1, patient numbers 1, 8, 10, 16 and 22 were initially diagnosed as FD and numbers 14, 20 and 25 as odontogenic myxoma, osteoma and odontome respectively. Majority of cases (17) were diagnosed as OF on incisional biopsy. Based on the reports, the patients were surgically treated and the specimens were again studied for final histological diagnosis correlating with clinical and radiological features. Specimen no. 15 showed features of psammomatoid JOF.
Ossifying fibroma lack true fibrous capsules but has minimal bone infiltration that distinguishes it from FD. It shows prominent calcified structures i.e. ossicles and cementicles, which appear as eosinophillic or basophilic spherules of osteoid or bone within a moderately cellular dense stroma [21]. Histologically, different types of mineralization patterns such as basophilic rounded cementicles, rounded mineralized trebaculae containing osteocytes i.e. ossicles, and trebaculae of bone of varying sizes and different degrees of mineralization were noted. Overlap between different patterns was observed in many cases. The supporting stroma varied from highly cellular stroma containing plump fibroblasts to more fibrous and collagenous backgrounds. Areas of increased cellularity were noted around zones of mineralization in lesions that were otherwise fibrous. The solitary case of JOF showed basophilic rounded psammoma bodies in a highly cellular background (Fig. 2). Molecular biology is an important aspect to differentiate between the two distinct entities i.e. fibrous dysplasia and OF by the help of important markers. But in our study, we could not analyze the expression of osteogenic markers and GNAS mutations due to lack of facilities. Fibroblastic cells in fibrous dysplasia and OF show strong Runx2 expression in the nucleus. Immunoreactivity for osteocalcin is strong in fibrous dysplasia but weak in OF lesions. PCR analyses for mutations at the Arg201 codon of the alpha subunit of the stimulatory G protein gene (GNAS) are positive in fibrous dysplasia. This is helpful for preoperative differentiation of aggressive OF and fibrous dysplasia and thus deciding the appropriate treatment plan.
Fig. 2.
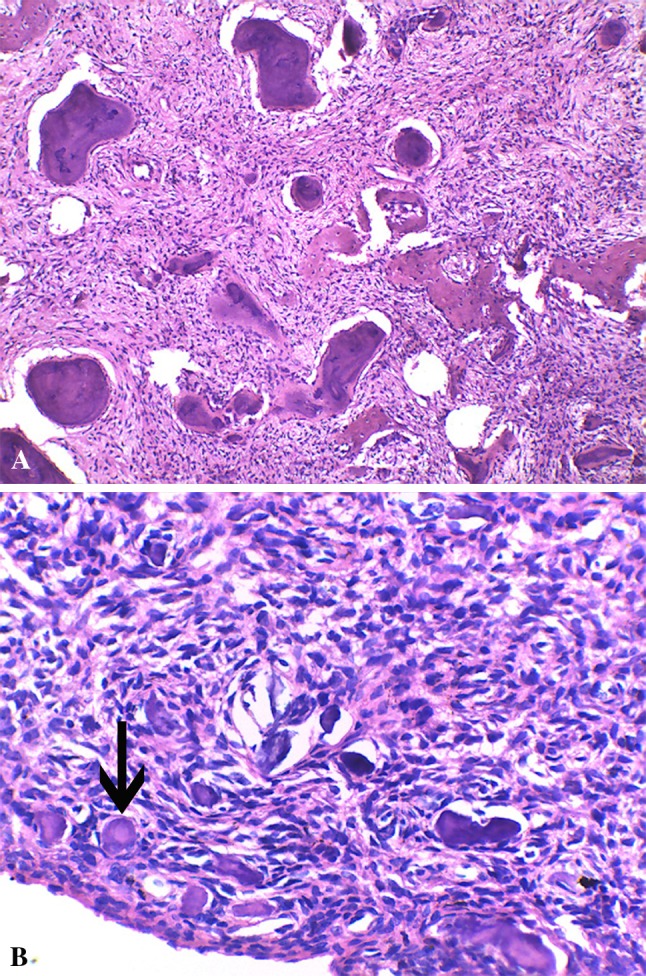
A Microphotograph showing basophilic rounded cementicles and trebaculae of bone in acellular stroma (HE × 100), B microphotograph of Psammomatoid JOF showing concentrically lamellated Psammoma bodies (arrow) in a highly cellular stroma (HE × 100)
The treatment of OF depends on its clinical and radiological picture comprising of one of the following modalities [22–24]:
Curettage
Enucleation
Radical surgery in form of resection and reconstruction
Curettage is done in cases where the lesion comprised of soft bone which merged with the surrounding bone on surgical exploration or there was no clear radiolucency around the pathology or where lesion could not be removed intoto due to its size or lack of access. Enucleation is done if the lesion is well demarcated, encapsulated and had not reached a very large size. Resection with continuity defect is done in cases involving the inferior border of mandible or with close approximation to it. Those extending into the maxillary sinus and nasal cavities having diffuse/ill-defined margins are also subjects for resection. Resection margins need not be more than 5 mm into healthy bone as the tumour does not infiltrate more than 1–2 mm beyond its borders. However, other authors advocate more extensive surgery than the above for more aggressive lesions and for those involving other craniofacial bones in order to prevent any future recurrences [3, 5]. The aim of radical surgery is therefore to eliminate the chances of recurrence as well as of pathological fracture following excision of the lesion.
Most of our cases i.e. 19 (76 %), where the lesion was delineated from the surrounding bone, were treated by eneucleation with or without obliteration of dead space with allogenic graft material. Two cases with ill-defined margins, both clinically and radiographically, underwent curettage. Four cases were treated with resection and reconstruction out of which 2 were reconstructed with rib graft. Radical surgery in form of resection should only be considered when the tumour is of aggressive nature and large size making it difficult or even impossible to treat by more conservative means, there is recurrence of aggressive lesions following curettage/enucleation.
The defect created after eneucleation or curettage is either primarily closed or left open to heal secondarily, which in turn might delay healing. In our series, 19/21 defects were closed primarily without any bone graft, one case was packed with hydroxyapetite and one was reconstructed with medpore. All cases healed uneventfully except the case in which medpore was placed. The patient had wound dehiscence 1 month post-operatively and was managed effectively for the same. Heavy smoking could have been the reason for dehiscence (Figs. 3–6).
Fig. 4.
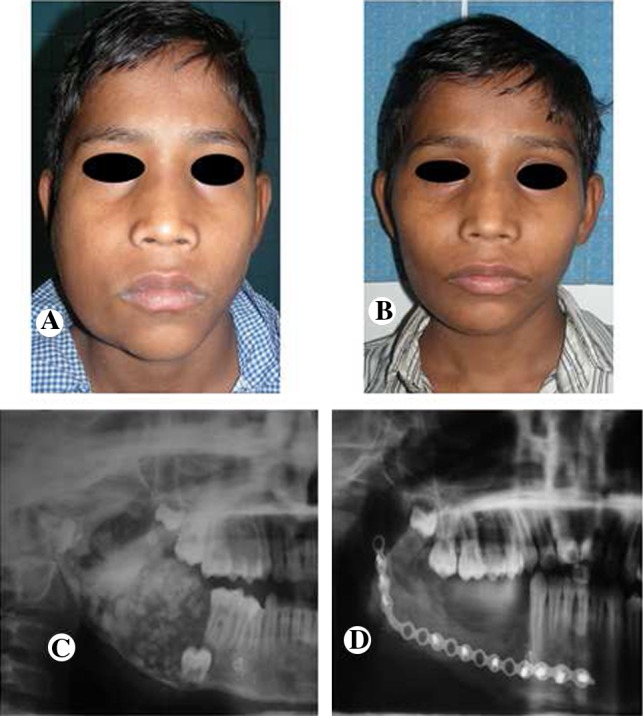
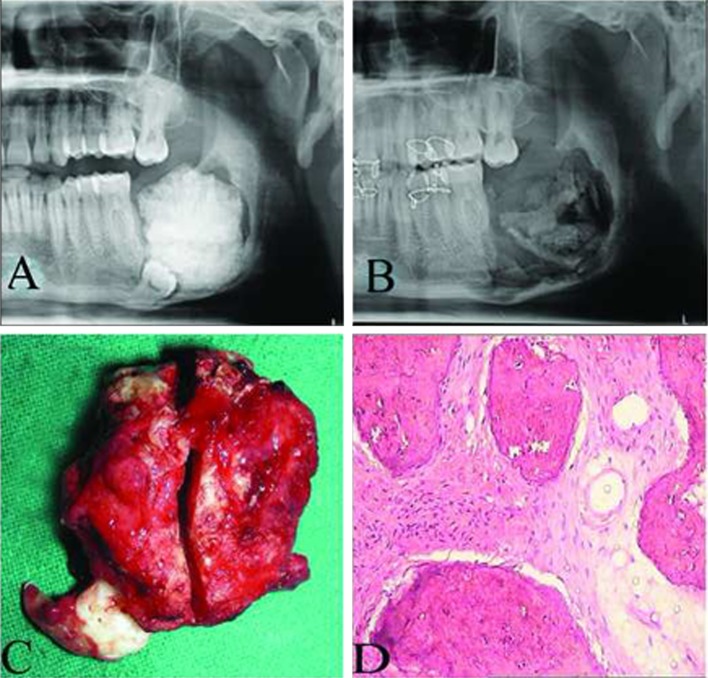
Case 2. A Pre-operative frontal view, B post-operative frontal view with 18 months of follow-up, C pre-operative orthopantomogram view showing a mixed radiological appearance, D post-operative radiograph showing a satisfactory reconstructed right mandible with rib graft
Fig. 5.
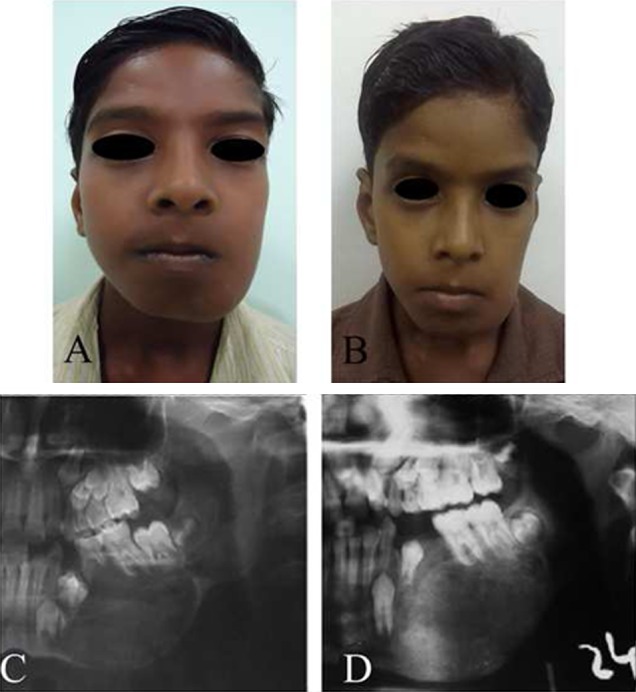
Case 19. A Pre-operative frontal view, B post-operative frontal view after 4 months of follow-up, C pre-operative radiograph view showing a radiolucent appearance, D post-operative radiograph showing good bone formation at the surgical site
Fig. 3.
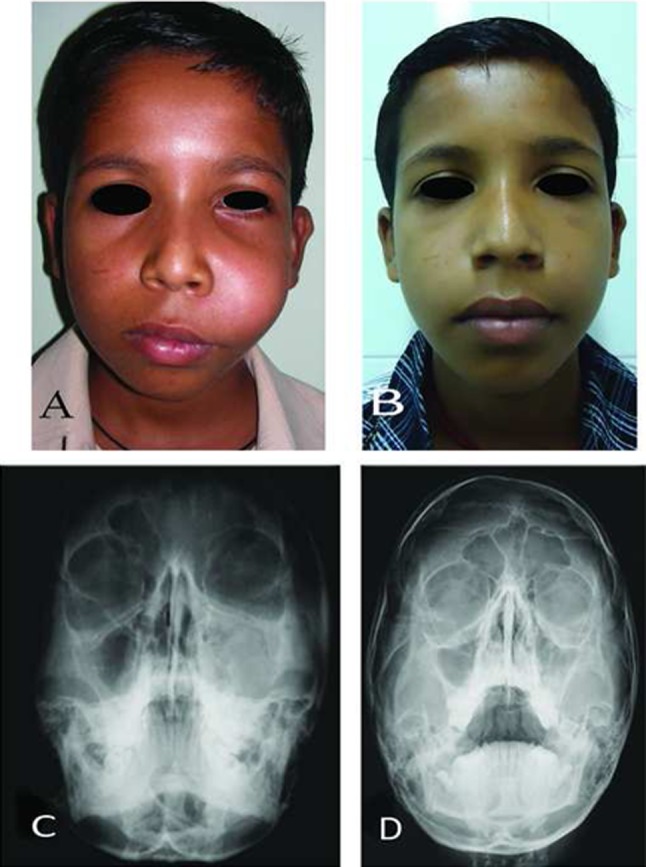
Case 4. A pre-operative frontal views, B post-operative frontal view at 14 months follow-up, C and D pre and post-operative occipitomental views
Fig. 6.
Case 20. A Pre-operative orthopantomogram view showing a radiopaque appearance of the lesion, B post-operative orthopantomogram view after 1 month, with patient on intermaxillary fixation and alloplastic grafting, the patient was lost to follow-up at 3 months, C pathological specimen along with the attached tooth, D histopathological section showing significant osteoid material in fibrous stroma
In our series, the follow-up period ranged between 6 months to 5 years at an interval of 1, 3, and 6 months and then yearly. Radiographs were taken at the first and sixth months and then at yearly intervals. Till date no recurrence has been reported.
Conclusion
Based on our study, it can be summarized that OFs are rare tumours of maxillofacial region. They tend to grow into large size resulting in facial disfigurement, pain and paresthesia if not managed in time. They are often well demarcated and very rarely infiltrate the surrounding bone so it is easier to treat these lesions as compared to other odontogenic or non-odontogenic tumours of similar dimensions. It is justified to conclude that enucleation, curettage and resection and reconstruction are adequate forms of treatment for such kind of lesions as evident from no recurrence of the condition in the present series. Enucleation is preferred in small and well demarcated lesions. Curettage should be done in relatively large lesions with ill defined borders, not involving basal bone of mandible or cortical perforation. Resection should be reserved for aggressive and extensive cases with involvement of basal bone or perforation of cortices. However, there is lack of knowledge in our study regarding the immunohistochemistry (molecular biology) of these lesions. Immunological markers can distinguish between OF and FD which often pose a diagnostic dilemma because of their overlapping radiographic and histological features.
References
- 1.Krammer IRH, Pindborg JJ, Shear M. WHO international classification of tumors. Histological typing of odontogenic tumors. 2. London: Springer-Verlag; 1992. p. 27. [Google Scholar]
- 2.Kolomvos N, Nadia Theologie-lygidakis N, Christopoulos P, Iatrou I (2013) Benign fibro-osseous lesions of the jaws in children: a 12 year retrospective study. J Craniomaxillofac Surg (In Press, corrected proof). Available online Jan 2013. doi: 10.1016/j.jcms.2012.11.029 [DOI] [PubMed]
- 3.Eversole LR, Merrell PW, Strub D. Radiographic characteristics of central ossifying fibroma. Oral Surg Oral Med Oral Pathol. 1985;59(5):522–527. doi: 10.1016/0030-4220(85)90096-9. [DOI] [PubMed] [Google Scholar]
- 4.De Vicente JC, Gonzalez S, Santamaria J, Madrigal B. Non odontogenic tumors of the jaws: classification, behaviour and diagnosis. Med Oral. 1997;2(2):83–93. [PubMed] [Google Scholar]
- 5.Commins DJ, Tolley NS, Milford CA. Fibrous dysplasia and ossifying fibroma of the paranasal sinuses. J Laryngol Otol. 1998;112(10):964–968. doi: 10.1017/S0022215100142203. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Granizo R, Sanchez-Cuellar A, Falahat F. Cemento ossifying fibroma of the upper gingivae. Otolaryngol Head Neck Surg. 2000;122(5):775. doi: 10.1016/S0194-5998(00)70216-6. [DOI] [PubMed] [Google Scholar]
- 7.Vlachou S, Terzakis G, Doundoulakis G, Barbati C, Papazoglou G. Ossifying fibroma of the temporal bone. J Laryngol Otol. 2001;115(8):654–656. doi: 10.1258/0022215011908522. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro AC, Carlos R, Diaz KP, Gouvea AF, Vargas PA. Bilateral central ossifying fibroma affecting the mandible: report of an uncommon case and critical review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e21–e26. doi: 10.1016/j.tripleo.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Marx R, Stern D. Oral and maxillofacial pathology. Chicago: Quintessence; 2003. pp. 789–791. [Google Scholar]
- 10.Godt A, Gulicher D, Kalwitzki M, Krober SM. Dislocation of an upper third molar by an ossifying fibroma—Case report. J Craniomaxillofac Surg. 2008;36(6):360–364. doi: 10.1016/j.jcms.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Voytek TM, Roj Y, Edeiken J. Fibrous dysplasia and cemento ossifying fibroma. A histological spectrum. Am J Surg Pathol. 1995;19(7):775–781. doi: 10.1097/00000478-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Campanacci M. Osteofibrous dysplasia of long bones: a new clinical entity. Ital J Orthop Traumatol. 1976;2(2):221–237. [PubMed] [Google Scholar]
- 13.Hammer JE, Scofied HH, Cornyn J. Benign fibro-osseous jaw lesions of periodontal membrane origin: an analysis of 249 cases. Cancer. 1968;22(4):861–878. doi: 10.1002/1097-0142(196810)22:4<861::AID-CNCR2820220425>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Jaff HL, Lichtenstein Non-osteogenic fibroma of bone. Am J Pathol. 1942;18(2):205–221. [PMC free article] [PubMed] [Google Scholar]
- 15.Pederson CW. Fibro-osseous lesion of the mandible: cementifying fibroma: report of a case. J Oral Surg. 1971;29(4):280–284. [PubMed] [Google Scholar]
- 16.Bradley ES, Leake D. Ossifying fibroma involving the maxilla and mandible. Oral Surg Oral Med Oral Pathol. 1968;26(5):605–614. doi: 10.1016/0030-4220(68)90424-6. [DOI] [PubMed] [Google Scholar]
- 17.Kridel RWH, Miller RH, Greenberg SD. Ossifying fibroma: diagnostic classification. Otolaryngol Head Neck Surg. 1983;91(5):568–573. doi: 10.1177/019459988309100519. [DOI] [PubMed] [Google Scholar]
- 18.Eversole LR, Leider AS, Nelson K. Ossifying fibroma: a clinicopathological study of sixty-four cases. Oral Surg. 1985;60(5):505–511. doi: 10.1016/0030-4220(85)90239-7. [DOI] [PubMed] [Google Scholar]
- 19.Sciubba JJ, Younai F. Ossifying finroma of the mandible and maxilla: review of 18 cases. J Oral Pathol Med. 1989;18(6):315–321. doi: 10.1111/j.1600-0714.1989.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 20.Mintz S, Velez I. Central ossifying fibroma: an analysis of 20 cases and review of literature. Quintessence Int. 2007;38(3):221–227. [PubMed] [Google Scholar]
- 21.Toyosawa S, Yuki M, Kishino M, Ogawa Y, Ueda T, Murakami S, et al. Ossifying fibroma vs fibrous dysplasia of the jaw: molecular and immunological characterization. Mod Pathol. 2007;20(3):389–396. doi: 10.1038/modpathol.3800753. [DOI] [PubMed] [Google Scholar]
- 22.Booth PW, Schendel SA, Hausamen JE. Maxillofacial surgery. 2. Missouri: Churchill Livingstone. St. Louis; 2007. pp. 506–509. [Google Scholar]
- 23.Regezi JA, Sciubba JJ. Oral pathology—clinical pathological correlations. 3. Philadelphia: WB Saunders Co; 1999. pp. 357–360. [Google Scholar]
- 24.Ong AHM, Siar CH. Cemento-ossifying fibroma with mandibular fracture: case report in a young patient. Austr Dent J. 1998;43(4):229–233. doi: 10.1111/j.1834-7819.1998.tb00169.x. [DOI] [PubMed] [Google Scholar]