Abstract
Purpose
Closed reduction of mandibular fractures usually entails a relatively long period of immobilization, with the subsequent delay of rehabilitation. Therefore, shorter immobilization period with various approaches to protect or enhance bone healing have been investigated. The aim of this study was to analyze the effects of pulsed electromagnetic field (PEMF) and low intensity laser irradiation (LILI) on the fracture healing process, through radiodensitometric assessment of the bone callus.
Patients and Methods
Eighteen patients with mandibular fractures at the tooth bearing area participated in this prospective study. They were treated by closed reduction using maxillo–mandibular fixation (MMF) and were consecutively assigned into 1 of 3 groups. In group A, the fracture sites were exposed to PEMF for 2 h daily for 12 days. In group B, the fracture sites were exposed to LILI on the tenth and twelfth postoperative days (2 sessions of 6 min per day 2 h apart). The fracture sites in group C acted as controls. MMF was maintained for 2 weeks in group A and 4 weeks in groups B and C. The bone fracture healing was evaluated clinically by investigating the union of the fractured segments and radiographically using computerized densitometry. The union of the fractured segments was tested by manual manipulation and the occlusion was assessed upon removal of MMF. Standardized digital panoramic radiographs were performed for each patient, immediately postoperatively as well as at 2 and 4 weeks. The digital images were manipulated using the IDRISI software. A rectangular area of 10 × 15 mm was drawn along the center of the fracture line. The obtained densitometry values were expressed in gray levels from 0 to 256. The collected data were then tabulated and statistically analyzed.
Results
After releasing the MMF, the bimanual mobility test of the fractured segments in all patients showed stability of the segments. The preinjury occlusion was maintained in all patients. The postoperative radiographs of all patients revealed good bony alignment of the bony segments. In all groups, comparison between the study intervals with respect to both means and changes percentages of the bone density values showed insignificant differences. At 2nd postoperative week, the mean bone density at the fracture sites decreased by 4.74, 6.6 and 27.89 % in groups A, B and C respectively. The period from the 2nd to the 4th postoperative weeks showed increase in the bone density by 1.49, 1.95 and 14.12 % in groups A, B and C respectively. Insignificant difference was found between the means of bone densities of group A and B throughout the study intervals. On the other hand, both groups showed insignificant difference with group C immediately postoperative and significant increase in bone density at the 2nd and 4th postoperative weeks.
Conclusions
Short period immobilization of mandibular fractures for 2 weeks supplemented with PEMF is recommended. Further studies are needed to evaluate the efficacy of LILI as a supplement to reduce the mandibular fracture immobilization period.
Keywords: Mandibular fracture, Low intensity laser irradiation, Pulsed electromagnetic field therapy
Introduction
Mandibular fracture has been managed in several ways. The main objectives to be achieved are to restore proper function by ensuring union of the fractured segments and reestablishing the preinjury occlusion; to correct any contour defect that might arise as a result of the injury; and to prevent infection at the fracture site [1]. The basic treatment principles of orthopedic surgery also apply to mandibular fractures including reduction, fixation, immobilization, and supportive therapies. Reduction of the fracture can be achieved either with an open or closed technique. In closed reduction, the fracture site is not surgically exposed but the reduction is achieved by palpation of the bony fragments and restoration of the dental occlusion. Open reduction entails exposure of the fracture site to allow direct visualization, validation of the procedure and direct application of a fixation device at the fracture site [2].
With the tremendous refinement of plating system, open reduction showed increased popularity among maxillofacial surgeons. However, when considering treatment of mandibular fracture, closed treatment is still a valuable option. Closed reduction techniques preclude the need for hospitalization, surgical morbidity, and the relatively high cost of open ones [3, 4]. However, the need for a relatively long period of immobilization, with the subsequent delay of rehabilitation, has been their main drawback that led to the search for alternative ways to reduce or eliminate the period of immobilization [5, 6]. Therefore, shorter immobilization period with various approaches to protect or enhance bone healing have been investigated [7–10].
Research reports have investigated the use of low-intensity pulsed ultrasound, pulsed electromagnetic field (PEMF) [7, 8] and low intensity laser irradiation (LILI) [11–13] in the biostimulation of bone repair. PEMF was found to promote osteogenesis in vivo, in part through direct action on mesenchymal stem cells and osteoblasts [14, 15]. It was also reported that electromagnetic fields may heal bone fractures [16]. Abdelrahim et al. [17] reported good results when PEMF supplement closed treatment of mandibular fracture with short period of immobilization (2 weeks). Mollon et al. [18] conducted a systematic review and meta-analysis of randomized controlled trials to evaluate the effect of electromagnetic stimulation on long-bone fracture-healing. They found small, methodologically limited trials with wide confidence intervals that leave the impact of electromagnetic stimulation of fracture-healing uncertain and that the current evidence justifies neither enthusiastic dissemination nor confident rejection of this therapeutic modality.
Published reports do recognize that LILI has positive effects on bone [19–21]. These studies reflect the idea that non-differentiated mesenchymal cells could be biomodulated positively to osteoblasts that would more rapidly change to osteocytes [19, 20]. Yamada [22] studied the biological effects of laser irradiation on cloned osteoblastic cells and concluded that laser therapy photoactivates osteoblastic cells, accelerates proliferation of osteoprogenitor cells, enhances osteoblastic calcification, and may promote bone regeneration. The effect of low-level laser on fracture healing was the topic of few studies that indicated that the laser enhanced healing [20, 21, 23].
The necessity for noninvasive and repetitive methods for assessing the process of bone fracture healing has drawn the attention of many researches toward the application of various diagnostic methods. It was reported that image analysis using IDRISI Kilimanjaro software facilitates image restoration, enhancement, and densitometric measurements in comparison to other densitometric measuring software programs [24]. It allows monitoring the changes in bone densities at and around the fracture line [21]. This software offers excellent measurement reproduction and a high level of correlation between the values obtained from the loss or gain in bone minerals during the different phases of bone healing [25–28]. Therefore, it was the aim of the present study to use IDRISI software to quantify bone mineralization following closed treatment of mandibular fracture using two treatment modalities with different bone enhancers and immobilization periods in comparison to the traditional one.
Patients and Methods
Study Design
Eighteen patients participated in this prospective study. They were selected from the outpatient clinic of the oral and maxillofacial surgery department, Faculty of Oral and Dental Medicine, Cairo University. The selection was determined using the following criteria: mandibular fracture at the tooth-bearing area, and sufficient occluding teeth present on either side of the fracture to allow MMF using an arch bar or eyelet wiring. The exclusion criteria included: presence of infection at the fracture site, or any systemic problems that could affect normal bone healing. Surgical procedures and expected complications of treatment modalities were explained to the patients willing to participate in the study. They signed an informed consent form. The Research Ethics Committee at the Faculty of Oral and Dental Medicine, Cairo University approved the study.
On initial presentation, the patients were clinically and radiographically evaluated. The demographic data of the selected patients, as well as the etiology and location of the fractures are listed in Table 1.
Table 1.
Demographic data of the selected patients, as well as the etiology and location of the fractures
| Pt. no. | Age (years) | Gender | Fracture location | Fracture etiology |
|---|---|---|---|---|
| A1 | 23 | Female | Left parasymphyseal/right ramus | Fall |
| A2 | 19 | Male | Right body | Interpersonal violence |
| A3 | 22 | Male | Left body | Interpersonal violence |
| A4 | 19 | Male | Left body | Vehicular accident |
| A5 | 36 | Male | Left body | Vehicular accident |
| A6 | 23 | Male | Left body | Vehicular accident |
| B1 | 40 | Male | Left body | Vehicular accident |
| B2 | 27 | Male | Right body | Fall |
| B3 | 25 | Male | Right body | Interpersonal violence |
| B4 | 20 | Male | Left parasymphyseal | Interpersonal violence |
| B5 | 33 | Male | Right body | Vehicular accident |
| B6 | 37 | Male | Left body | Vehicular accident |
| C1 | 23 | Male | Right body | Interpersonal violence |
| C2 | 30 | Male | Left body | Vehicular accident |
| C3 | 32 | Male | Left body | Vehicular accident |
| C4 | 25 | Male | Left parasymphyseal | Interpersonal violence |
| C5 | 37 | Femal | Right body | Vehicular accident |
| C6 | 28 | Male | Left parasymphyseal | Vehicular accident |
Treatment Phase
Each patient received 75 mg diclofenac sodium intramuscularly immediately preoperatively. Under local anesthesia, the mandibular fractures were manually reduced, fixed and immobilized by MMF using arch bars and 24-gauge circumdental wires or eyelet wiring (according to the condition of the teeth and patient cooperation). The teeth present in the fracture line were not removed, unless they were mobile or interfering with reduction of the fracture. Then the patients were consecutively assigned to three groups. In group A, the fracture sites were exposed to PEMF for 2 h daily for 12 days, using EM-probe Solo device (pulse duration 200 ns, rise time 8 ns; electromagnetic segment at 50 MHz and down to kilohertz range). The pulse was carrier modulated at 72 Hz. In group B, the fracture sites were exposed to LILI on the tenth and twelfth postoperative days (2 sessions of 6 min/day 2 h apart), using semiconductor diode (gallium–aluminium–arsenide) laser system with wave length 904 nm, a frequency of 3,000 Hz and energy output 2 Watt. The applicator tip was applied in a continuous slow circular motion to assure full exposure of the target surface to the laser beam. The fracture sites in group C acted as the controls.
Postoperative Care
All patients received 1.5 g sulbactam intramuscularly every 12 h for 4 days, 75 mg of diclofenac sodium, and chlorhexidine mouth rinse. Patients of groups A and B were exposed to the particular bone enhancer according to the predefined schedule. MMF was maintained for 2 weeks in group A and for 4 weeks in groups B and C. The patients maintained a liquid and pureed diet during the MMF period. They were instructed to continue a soft diet for 3 weeks after MMF removal.
Postoperative Evaluation
All patients were given follow-up appointments on the second and fourth postoperative weeks. At each appointment, the patients were evaluated clinically and radiographically and data were recorded.
Upon removal of MMF, the union of the fractured segments was tested by manual manipulation and the occlusion was assessed. Signs and symptoms of infection such as the presence of erythema, edema, or purulent drainage over the fracture site were also evaluated.
Standardized digital panoramic radiographs at 0, 2 and 4 weeks postoperatively were performed for each patient. These radiographs were made with the same orthopantomograph (OT100 Instrumentarium Imaging, GE, Finland 2003) using the following exposure parameters; 85 kVp, 16 mA, and exposure of panoramic program set at 17.6 s. The exposure parameters were electronically controlled according to preprogrammed procedures and were kept constant for the baseline and follow-up radiographs.
The digital images were manipulated using the IDRISI software (IDRISI is a raster-based image processing program inspired by Clark Labs, Clark University, USA). On each image, an analysis of the changes in the mean gray value was performed using the polygon measurement facility of the used software. The unit of measurement for bone density is pixels (mean gray value). A rectangular area of 10 × 15 mm was drawn along the center of the fracture line. The obtained densitometry values were expressed in gray levels from 0 to 256. Each of these values corresponded to the mean density of the fracture area (Fig. 1). In an attempt to eliminate intra-observer error, these measurements were performed twice by the same investigator who was unaware of the randomization codes. The data of the 2 trials were pooled, and the mean was included in additional statistical analysis. All collected data were then tabulated and statistically analyzed.
Fig. 1.
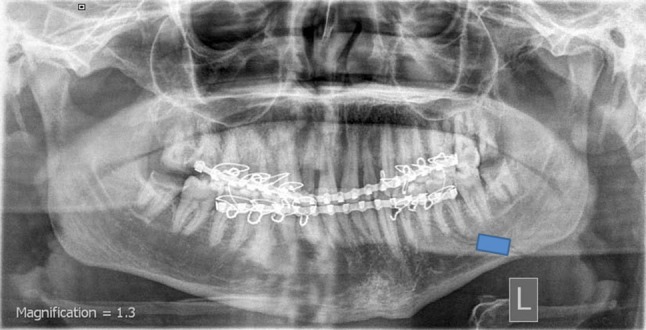
Processing of the digitized image along a rectangular area of 10 × 15 mm centered on the fracture line
The data are presented as mean and standard deviation. Student t test was used to evaluate the change by time and to compare the differences between the three groups. The significance level was set at P ≤ 0.05. Statistical analysis was performed using the Statistical Package for Social Sciences, version 16.0, for Windows (SPSS, Chicago, IL, USA).
Results
All patients passed the 1-month follow-up period for inclusion in the present study. The post-traumatic edema observed at the initial presentation started to resolve by the third postoperative day and completely resolved by the end of the first postoperative week. The associated intraoral and/or extraoral wounds healed by the end of the first postoperative week.
After releasing the MMF (after 2 weeks for group A and 4 weeks for groups B and C), the bimanual mobility test of the fractured segments in all patients showed stability of the segments. The preinjury occlusion was maintained in all patients.
The postoperative radiographs of all patients revealed good bony alignment of the bony segments (Fig. 1). Table 2 summarizes the assessment of the changes percentages of the bone density values in each group throughout the study period. In all groups, comparison between the study intervals with respect to both means and changes percentages of the bone density values showed insignificant differences. However, the changes in bone density within the same period were dependent on the treatment modality used (Fig. 2). This became obvious after the expression of the increase or decrease in the bone densities in percentages. At 2nd postoperative week, the mean bone density at the fracture sites decreased by 4.74, 6.6 and 27.89 % in groups A, B and C respectively. The period from the 2nd to the 4th postoperative weeks showed increase in the bone density by 1.49, 1.95 and 14.12 % in groups A, B and C respectively. The decrease percentages in the mean density at the 4th postoperative week in comparison to the baseline were 3.32, 4.78 and 17.71 in groups A, B and C respectively.
Table 2.
Results of percentage change, mean difference and paired t test for changes in bone densities of each group by time
| Group Period |
A | B | C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % Change | Mean difference | P value | % Change | Mean difference | P value | % Change | Mean difference | P value | |
| Baseline–2 weeks | −4.74 | −7.58 | 0.619 | −6.6 | −10.68 | 0.672 | −27.89 | −45.32 | 0.216 |
| 2–4 weeks | 1.49 | 2.27 | 0.765 | 1.95 | 2.95 | 0.203 | 14.12 | 16.55 | 0.095 |
| Baseline–4 weeks | −3.32 | −5.31 | 0.447 | −4.78 | −7.71 | 0.959 | −17.71 | −28.78 | 0.916 |
Fig. 2.
Changes by time in mean bone density within each group
Insignificant difference was found between the means of bone densities of groups A and B throughout the study intervals. On the other hand, both groups showed insignificant difference with group C immediately postoperative and significant increase in optic density at the 2nd and 4th postoperative weeks (Table 3).
Table 3.
Results of Student’s t test for comparison between bone densities of every two groups at each study intervals including mean and standard deviation (SD) values
| Period | Mean value ± SD | P value | ||||
|---|---|---|---|---|---|---|
| Group A | Group B | Group C | A–B | B–C | A–C | |
| Baseline | 159.90 ± 18.79 | 161.54 ± 14.27 | 162.49 ± 15.91 | 0.88 | 0.92 | 0.82 |
| 2 weeks | 152.32 ± 9.59 | 150.88 ± 6.32 | 117.17 ± 18.02 | 0.92 | 0.02* | 0.034* |
| 4 weeks | 154.59 ± 13.34 | 153.82 ± 8.84 | 133.71 ± 13.59 | 0.79 | 0.007* | 0.005* |
Discussion
The present study demonstrated, through quantitative radiodensitometry using the IDRISI software, that both PEMF and LILI were able to stimulate the bone fracture healing process. However, no significant differences were observed between these two biophysical methods.
In the present study, clinical stability of fracture segments on removal of MMF (2 weeks in group A and for 4 weeks in groups B and C) was found in all patients. The use of relatively long period of immobilization in groups B and C is in general agreement with other studies that reported clinical stability of 75–80 % of mandibular fractures by 4 weeks [29]. Al-Belasy [30] found that the period required for the healing of mandibular fractures in the tooth-bearing area treated by MMF was 4.67 ± 0.72 weeks. On the other hand, the observed clinical stability following short period of immobilization in group A emphasizes once again the positive effect of PEMF stimulation on closed treatment of mandibular fractures reported by Abdelrahim et al. [17]. The current study adopts the concept of enhancing the fracture healing process rather than protecting it as suggested by Amaratunga [31] to apply a short period of immobilization of 2 weeks, followed by splinting the lower jaw with an arch bar or acrylic splint, or a period of a soft diet as options available to the surgeon for the treatment of mandibular fracture. However, both concepts are effective and significantly reduce the potential adverse effects of long-term MMF as evident by the positive result of the current study and that of Al-Belasy [30] who concluded that a short period of MMF followed by an arch bar splint wired to the lower jaw is a suitable alternative to conventional MMF for treatment of fractures of the mandibular tooth bearing area.
In the present study, the dynamics of mandibular fracture healing are similar to those reported by Razukevičius et al. [32]. In all groups, the mean values of bone density tended to decrease during the first 2 weeks after which the degree of the changes in bone densities showed insignificant increase by the end of the 4th postoperative week. These findings could be explained on the basis of contemporary fracture healing concepts that secondary bone healing, healing that occurs using either biologic immobilization alone or medical reduction and incomplete fixation, is characterized by the formation of callus [33]. The sequence of bone healing, according to Yu et al. [34], can be summarized as (1) inflammation and hematoma formation; (2) interfragmentary stabilization by periosteal and endosteal callus formation; (3) restoration of continuity by membranous and endochondral ossification; and (4) haversian remodeling and functional adaptation. A study on experimental fractured animal models published by Yamagiwa and Endo [35] have revealed that fracture healing (secondary healing) is typically characterized by three overlapping stages: the initial inflammatory response, callus formation (soft and hard callus), initial bony union and bone remodeling. It was also reported that fracture healing (secondary healing) in human occurs in four overlapping phases including the hematoma formation phase; early inflammatory phase (2–4 weeks); repair phase (proliferation and differentiation, which is within 1–2 months); and late remodeling phase, which lasts for months or years [36]. Thus the inflammatory phase of fracture healing could attribute to the decrease in the mean values of bone density at the end of the 2nd postoperative week. On the other hand, the increase in the mean values of bone densities by the end of the 4th postoperative week could correspond to the reparative phase of fracture healing.
In the current study, the control group showed that the means of bone densities in the fracture sites insignificantly decreased by 27.89 % at the 2nd postoperative week. On the other hand, the degree of the changes in bone densities within the period from the 2nd to the 4th postoperative weeks showed insignificant increase in the bone density by 14.12 %. These findings are somewhat similar to those reported by Razukevičius et al. [32] who found that following closed fixation methods (wire splint fixation), the means of optic densities in the fracture site decreased by 17.8 % on the 14th day of treatment. While after 4 weeks, the means of optical densities showed insignificant increase by 3.2 %. Also our results are in general agreement with those reported by Abdelrahim et al. [17] who found that 15 days postoperatively, the mean density in the fracture sites decreased by 6 % and that 30 days postoperatively, it increased by 1.9 % compared with the density found at 15 days postoperatively. Despite the similarity of our result with those of Razukevičius et al. [32] and Abdelrahim et al. [17] regarding the dynamics of fracture healing, they differ in the degree of the changes in optical densities. This could be attributed the different software used to assess the optical density.
The finding of the present study indicates speeding up of bone repair in PEMF group as evident by the clinical stability of the segments 2 weeks postoperatively and the significant increase in bone density at the 2nd and 4th postoperative weeks when compared with the control group. This finding is somewhat in accordance with those of Abdelrahim et al. [17] who found clinical stability of the segments 14 days postoperatively and insignificant difference between the mean bone density values of the PEMF and control groups at all study intervals. On the other hand they found that the percentage of changes in bone density in PEMF group showed insignificant decrease at the 15th postoperative day and significant increase 30 days postoperatively compared with control group. Therefore, they concluded that PEMF stimulation might have a beneficial effect on the healing of mandibular fractures treated with closed reduction. The positive effect of the PEMF on the outcome of closed treatment of mandibular fracture confirms the concept of biostimulation of PEMF and could be explained based on the observation of Liu et al. [37] who proved that PEMF stimulation accelerated fracture healing and promoted the maturation of bone trabeculae. They found that PEMF stimulation enhanced alkaline phosphatase activity present in the osteoblast and in the matrix vesicle membrane. This activity elevated significantly at the second week compared to the sham-exposed group. Consequently they determined that PEMF treatment stimulated bone defect healing by increasing the alkaline phosphatase activity level, especially during the first and the second week stimulation period.
The significant increase in bone density at the 2nd and 4th postoperative weeks in laser group when compared with the control group in our study could be explained on the basis of the observations of the experimental studies [38–40]. Bae et al. [39] evaluated bone healing capacity in the fracture of rabbit mandibular bone using LILI and found that in the histological and immunohistological staining, after 6 weeks, fibroblasts, osteogenic cells and collgen fibers were more in the experimental group than in the control group and that in the histochemical analysis, the amount of calcium and phosphorus contents of the experimental group were more than the control group. They suggested that the bone healing is stimulated by LILI in bone fractures. Chen and Zhou [40] reported that LILI using the CO2 laser has the potential of promoting metabolism and mineralization of bone callus, thus accelerating bone healing. The current finding confirms the reported positive effect of LILI on fracture healing [21, 39, 41, 42]. Liu et al. [42] studied the effect of LILI on rabbit tibial fracture that commenced immediately postsurgery and continued once daily for 4 weeks. They found that the bone mineral density (BMD) as ascertained using a gray scale (graded from 0 to 256) showed darker coloration in the LILI group (138) than in the sham-treated control group (125) and suggested that LILI may accelerate the process of fracture repair or cause increases in callus volume and BMD, especially in the early stages of absorbing the hematoma and bone remodeling.
The means of bone densities of PEMF and LILI groups showed insignificant difference throughout the study intervals. Based on this finding, short period immobilization for 2 weeks supplemented with PEMF is recommended. Further studies are needed to evaluate the efficacy of LILI as a supplement to reduce the mandibular fracture immobilization period.
References
- 1.Peled M, Laufer D, Helman J. Treatment of mandibular fractures by means of compression osteosynthesis. Am J Oral Maxillofac Surg. 1989;47:566–569. doi: 10.1016/S0278-2391(89)80068-0. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman WY, Barton RM, Price M. Rigid internal fixation vs. traditional techniques for the treatment of mandible fractures. J Trauma. 1990;30:1032–1035. doi: 10.1097/00005373-199008000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Kromer H. Closed and open reduction of condylar fractures. Dent Rec. 1953;73:569–571. [Google Scholar]
- 4.Hayward JR. Fractures involving the mandibular condyle: a post-treatment survey of 120 cases. J Oral Surg. 1947;5:45–73. [PubMed] [Google Scholar]
- 5.Kahnberg KE. Conservative treatment of uncomplicated mandibular fractures. Swed Dent J. 1981;5:15–20. [PubMed] [Google Scholar]
- 6.Cawood J. Small plate osteosynthesis of mandibular fractures. Br J Oral Maxillofac Surg. 1985;23:77–91. doi: 10.1016/0266-4356(85)90057-9. [DOI] [PubMed] [Google Scholar]
- 7.Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol. 2007;93:384–398. doi: 10.1016/j.pbiomolbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Li JK, Chang WH, Lin JC. Cytokine release from osteoblasts in response to ultrasound stimulation. Biomaterials. 2003;24:2379–2385. doi: 10.1016/S0142-9612(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 9.Luben RA. Effects of low-energy electromagnetic fields (pulsed and DC) on membrane signal transduction processes in biological systems. Health Phys. 1991;61:15–28. doi: 10.1097/00004032-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Gordon GA. Designed electromagnetic pulsed therapy: clinical applications. J Cell Physiol. 2007;212:579–582. doi: 10.1002/jcp.21025. [DOI] [PubMed] [Google Scholar]
- 11.Blaya DS, Gulmaraes MB, Pozza DH. Histologic study of the effect of laser therapy on bone repair. J Contem Dent Pract. 2008;9:41–48. [PubMed] [Google Scholar]
- 12.Sharrard WJ, Sutcliffe ML, Robson MJ. The treatment of fibrous non-union of fractures by pulsing electromagnetic stimulation. J Bone Joint Surg Br. 1982;64:189–193. doi: 10.1302/0301-620X.64B2.6978339. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Shirai Y. The efficacy of ununited tibial fracture treatment using pulsed electromagnetic fields: relation to biological activity on nonunion bone ends. J Nippon Med Sch. 2001;68:149–153. doi: 10.1272/jnms.68.149. [DOI] [PubMed] [Google Scholar]
- 14.Esposito M, Lucariello A, Riccio I, Riccio V, Esposito V, Riccardi G. Differentiation of human osteoprogenitor cells increases after treatment with pulsed electromagnetic fields. In Vivo. 2012;26:299–304. [PubMed] [Google Scholar]
- 15.Schwartz BJ, Simon MA, Duran G, Barabino R, Chaudhri B, Boyan D. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J Orthop Res. 2008;26:1250–1255. doi: 10.1002/jor.20591. [DOI] [PubMed] [Google Scholar]
- 16.Grace KL, Revell WJ, Brookes M. The effects of pulsed electromagnetism on fresh fracture healing: osteochondral repair in the rat femoral groove. Orthopedics. 1998;21:297–302. doi: 10.3928/0147-7447-19980301-12. [DOI] [PubMed] [Google Scholar]
- 17.Abdelrahim A, Hassanein HR, Dahaba M. Effect of pulsed electromagnetic field on healing of mandibular fracture: a preliminary clinical study. J Oral Maxillofac Surg. 2011;69:1708–1717. doi: 10.1016/j.joms.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Mollon B, da Silva V, Jason WB, Thomas AE, Bhandari M. Electrical stimulation for long-bone fracture-healing: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2008;90:2322–2330. doi: 10.2106/JBJS.H.00111. [DOI] [PubMed] [Google Scholar]
- 19.Passarela S, Casamassima E, Molinari S. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated invitro by helium–neon laser. FEBS Lett. 1984;175:95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 20.Yaakobi T, Maltz L, Oron U. Promotion of bone repair in the cortical bone of the tibia in rats by low energy laser (He–Ne) irradiation. Calcif Tissue Int. 1996;59:297–300. doi: 10.1007/s002239900126. [DOI] [PubMed] [Google Scholar]
- 21.Salah AM (2010) Radiodensitometric evaluation of low intensity laser therapy on healing of mandibular fracture in tooth bearing area. Dissertation, University of Cairo, Giza
- 22.Yamada K. The biological effects of low power laser irradiation on clonal osteoblastic cells (MC3T3-E1) J Jpn Orthop Assoc. 1991;65:787–799. [PubMed] [Google Scholar]
- 23.Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S. Effect of low power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med. 1998;22:97–102. doi: 10.1002/(SICI)1096-9101(1998)22:2<97::AID-LSM5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Radwan DA (2005) A clinical study on the effect of diode laser therapy on the osseointegration of delayed vs. immediate implants. Dissertation, University of Cairo, Giza
- 25.Bernd J. Digital image processing: concepts, algorithms, and scientific applications. 2. Berlin: Springer; 1993. p. 429. [Google Scholar]
- 26.Ronald Eastman J. IDRISI Kilimanjaro guide to GIS and image processing, user manual. Worcester: Clark Labs, Clark University; 2003. pp. 39–45. [Google Scholar]
- 27.El Desouky G, Mekky M, Salah El-Din M, Zikry K, Amer W. Radiodensitometric assessment of the effect of low intensity laser irradiation on bone density following loading of endosseous implants. Egypt Dent J. 2007;53:1223–1234. [Google Scholar]
- 28.Salah El-Din M, Selim WA, El-Desouky G. Comparative radiodensitometric study of the effect of passive smoking on the prevalence of mandibular bone loss in different Egyptian individuals. Egypt Dent J. 2010;56:471–479. [Google Scholar]
- 29.Juniper RP, Awty MD. The immobilization period for fractures of the mandibular body. J Oral Surg. 1973;36:157–163. doi: 10.1016/0030-4220(73)90231-4. [DOI] [PubMed] [Google Scholar]
- 30.Al-Belasy FA. Short-term MMF for mandibular fractures. J Oral Maxillofac Surg. 2005;63:953–956. doi: 10.1016/j.joms.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Amaratunga NA. The relation of age to the immobilization period required for healing of mandibular fractures. J Oral Maxillofac Surg. 1987;45:111–113. doi: 10.1016/0278-2391(87)90400-9. [DOI] [PubMed] [Google Scholar]
- 32.Razukevičius D, Sabalys G, Kubilius R. Comparative analysis of the effectiveness of the mandibular angle fracture treatment methods. Baltic Dent Maxillofac J. 2005;7:35–39. [PubMed] [Google Scholar]
- 33.Korotkina AS (1978) Healing of mandibular fractures in patients with parodontosis according to the data of X-ray examination. Proceedings of scientific conference; 25th of June, Moscow, p 26
- 34.Yu J, Dinsmore R, Hebda PA, Gosain AK, Friedman CD. Essential tissue healing of the face and neck. Shelton: People’s Medical Publishing House and BC Decker; 2009. [Google Scholar]
- 35.Yamagiwa H, Endo N. Bone fracture and the healing mechanisms. Histological aspect of fracture healing. Primary and secondary healing. Clin Calcium. 2009;19:627–633. [PubMed] [Google Scholar]
- 36.Harwood PJ, Newman JB, Michael ALR. An update on fracture healing and nonunion. J Orthop Trauma. 2010;24:9–23. doi: 10.1097/BOT.0b013e3181cde5be. [DOI] [Google Scholar]
- 37.Liu H, Sun J, Walter HC, Jimmy KL, Cheng J. Bone defect healing enhanced by pulsed electromagnetic fields stimulation: in vitro bone organ culture model. J Med Biol Eng. 2005;25:27–32. [Google Scholar]
- 38.Lirani AP, Jorgetti V, da Silva OL. Comparative study of how low-level laser therapy and low-intensity pulsed ultrasound affect bone repair in rats. Photomed Laser Surg. 2006;24:735–740. doi: 10.1089/pho.2006.24.735. [DOI] [PubMed] [Google Scholar]
- 39.Bae YH, Han SJ, Kim KW. Bone healing capacity in the fracture of rabbit mandibular bone using low-level laser. J Korean Assoc Oral Maxillofac Surg. 2009;35:120–124. [Google Scholar]
- 40.Chen J, Zhou Y (1989) Effect of low level carbon dioxide laser radiation on biochemical metabolism of rabbit mandibular bone callus. School of Stomatology, West China University of Medical Sciences Chengdu, PROC; March, pp 83–87
- 41.Shakouri SK, Soleimanpour J, Salekzamani Y, Oskuie MR. Effect of low-level laser therapy on the fracture healing process. Lasers Med Sci. 2010;25:61–65. doi: 10.1007/s10103-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Lyon R, Meier HT, Thometz J, Haworth ST. Effect of lower-level laser therapy on rabbit tibial fracture. Photomed Laser Surg. 2007;25:487–494. doi: 10.1089/pho.2006.2075. [DOI] [PubMed] [Google Scholar]



