Abstract
Introduction
Anorganic bovine xenogenous grafts show the best performance as bone substitutes in implantodontics. Bio-Oss is the world’s most widely used and investigated anorganic bone substitute. This article compares two anorganic bovine bone substitutes (Bone-Fill and Gen-Ox anorganic) with Bio-Oss.
Materials and Methods
Eight New Zealand rabbits were implanted with 4 titanium cylinders randomly filled with Bio-Oss, Bone-Fill, Gen-Ox anorganic or a blood clot. Four animals were sacrificed after 8 weeks; 12 weeks later, the remaining four were sacrificed. The contents of the cylinders were removed, cut and stained with HE before they were evaluated with an optical microscope. The samples were submitted to histomorphometry for analysis.
Results
The bone formation with Bio-Oss at 8 weeks was 8.43 mm2; at 12 weeks, it was 9.32 mm2. The bone formation with Bone-Fill at 8 weeks was 7.24 mm2; at 12 weeks, it was 9.01 mm2. The bone formation with Gen-Ox anorganic at 8 weeks was 2.78 mm2; at 12 weeks, it was 3.02 mm2. The bone formation with the blood clot at 8 weeks was 0.65 mm2; at 12 weeks, it was 0.63 mm2.
Conclusion
Following this model, Bone-Fill was comparable to Bio-Oss and superior to Gen-Ox and blood clot.
Keywords: Osseointegrated implants, Bone reconstruction, Anorganic bovine xenogenous graft
Introduction
Bone resorption following the loss of tooth elements demands from surgeons the search for techniques and materials for the regeneration and recovery of that tissue, allowing the installation of osseointegrated implants. There are frequent reports of research on several bone substitutes in search of materials with higher capacity of bone formation and smaller morbidity.
Autogenous bones are regarded as the state-of-the-art in bone reconstruction in implantodontics [1]; they have been widely discussed in literature and ensure better predictability in terms of amount and quality of bone obtained [1, 2], because it is the only one presenting osteogenesis, osteoinduction and osteoconduction during all repair phases [1, 3]. The disadvantages of using autogenous bones, such as the increase of morbidity and surgical time, the limited amount of available bones and greater blood loss have motivated the search for biological or aloplastic materials that could be used as bone substitutes [4].
Comparative studies with bone substitutes indicate anorganic xenogenous bovine bone as the bone substitute with the best performance for purposes of bone reconstructions for the installation of osseointegratable implants [2–4]; in several studies published in literature, anorganic xenogenous bovine bone presented similar results to autogenous bone, whether alone or associated to the autogenous bone itself [4–12].
One of the main advantages of using anorganic xenogenous bovine bone is the fact that it is natural and can supply structural components similar to human bone [13]. Another advantage is the increased rate of bone formation through a more predictable regeneration in the early stages of repair, the facilitation of precocious migration of osteoblast line cells in the surgical lesion and the osteoconductor nature of the graft, increases the surface available for bone deposition [14].
More recent studies have shown that bovine anorganic bone interferes genetically upon the osteoblast function, which could be the reason for its clinical success [15, 16].
The Bio-oss is a gold standard in anorganic xenogenous bovine bones. In Brazil there are some lower-cost bovine xenogenous products that are also easier to find. Despite the similarities of production between Bio-Oss and brazilian products, these products do not present clinical research relevant. The aim of this article is to evaluate the osteoconductor power of two Brazilian anorganic bovine bone products and compare them histologically and histomorphometrically to Bio-Oss, which is the world’s most studied and commercialized xenogenous bone.
Materials and Methods
Eight male New Zealand rabbits were selected, with ages ranging from 8 to 10 months and approximately 4 kg weight, provided by the bioterium of the University of Santo Amaro (UNISA).
The rabbits were separated into two groups with four rabbits each. Four titanium cylinders (Fig. 1) were installed in the cranium. Three of them were filled with anorganic xenogenous bovine bone (Bio-Oss,1 Bone-Fill2 and Gen-Ox3) and the other one with blood clot. The first group was sacrificed 8 weeks after grafting and the second was sacrificed 12 weeks after grafting.
Fig. 1.
Cylinders of titanium and cap screws
The animals were weighted so that the ideal amount of anesthesia could be used for each. Anesthetic induction was made with Ketamine 10 mg/kg, Midazolan 0.5 mg/kg and Atropine 10 mg/kg intramuscular (IM). Maintenance of anesthesia was made through Ketamine and local infiltration with Mepivacaine 3 %.
Surgical access was made through sagittal incision including skin, subcutaneous tissue and pericranium followed by subperiosteal displacement exposing the whole cranium. Four 6 mm diameter and 4 mm high cylinders manufactured specially for this study were installed into the rabbits’ craniums, fixed using two 1.5 mm diameter and 3 mm deep screws. Decortication of the internal region of the cylinder was made with 1.1 mm diameter drill with constant irrigation of 0.9 % saline solution. This procedure was made in order to facilitate the revascularization of the grafts to be installed.
The cylinders were then filled with Bio-Oss, Bone-Fill, Gen-Ox anorganic and blood clot chosen randomly (Fig. 2). The flap was divided in order to facilitate primary closing. The suture was made with mononylon 5–0 (Ethicon®).
Fig. 2.
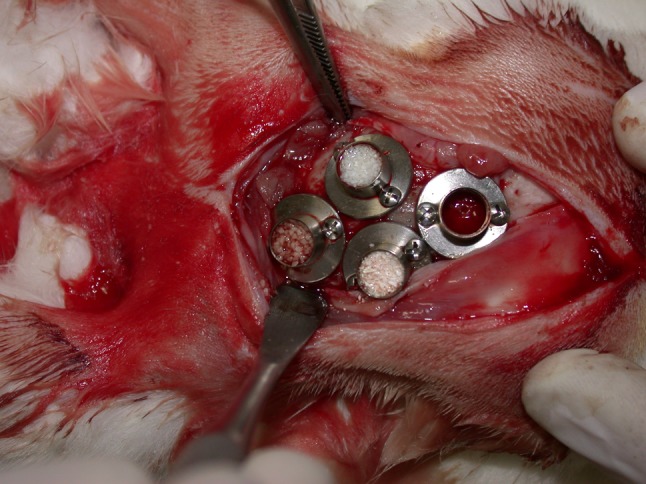
Cylinders with biomaterials
The animals were maintained with Benzatine Penicillin 40,000 UI/kg IM every 48 h for 15 days and Dipirone Sodium 0.25 mg/kg subcutaneous.
After the time indicated for each group—group I, 8 weeks and group II, 12 weeks—the rabbits were sacrificed with an over dose of sedative. Then the surgical access of the first surgery was repeated. The lids of the cylinders were removed and the consistency of the internal content of the cylinder was tested with the tip of a 2/4 Molt curette. With the aid of a 4.3 mm diameter trephine drill installed in straight piece at low rotation, the internal sample of the cylinder was removed along with the bone of the adjacent cranial vault (Fig. 3). This sample was put in a bottle with 10 % formol.
Fig. 3.
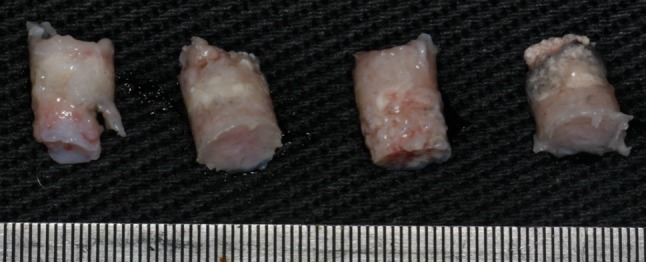
Material removed from the cylinder after 8 weeks
Slides were prepared at UNISA histology laboratory as follows: the samples were decalcificated with nitric acid at 20 % for an average period of 1 week. They were washed, dehydrated and fixed in paraffin. Then they were cut at width of 5 micrometers. The cuts were made at the upper and lower regions of the cylinder. They were then stained with hematoxiline and eosine viewed under an optical microscope, when the following were observed:
presence of inflammatory reaction;
presence of cells capable of forming bones (mesenchymal undifferientiated cells, osteoblasts, macrophages);
proximity of bone formation with the bone of the cranial vault;
presence of neovases;
bone neoformation;
formation of fibrous tissue;
presence of xenogenous reminiscence.
One sample from each material of each species was submitted to histomorphometry at the UNICAMP Histology laboratory (Campinas/SP). The amount of bone tissue formed, the amount of residual particles and the amount of non-mineralized tissue were observed in that exam. The average samples from each material were calculated and the results were submitted to statistical analysis with z-test for two average values.
Results
The following results were observed in the analysis made under optical microscopy:
8 Week Samples
Blood Clot
The samples filled with blood clot showed a large amount of collagenous fibers and fibroblasts, and little presence of osteoid tissue (Fig. 4).
Fig. 4.
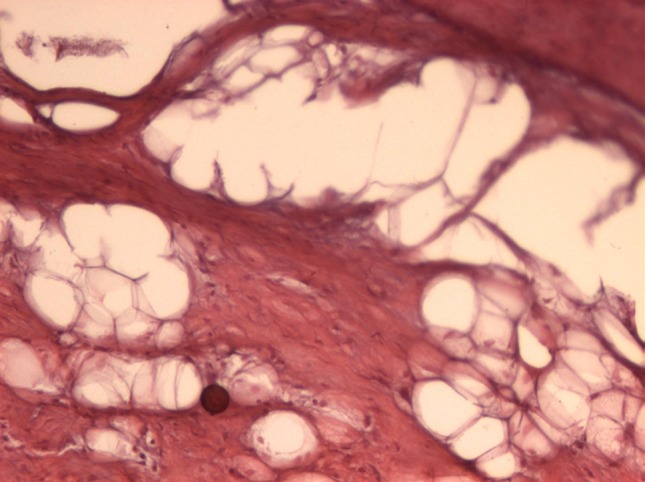
Blood clot 8 weeks (original magnification 40×): intense formation of collagen fibers and little osteoid tissue
Gen-Ox
The Gen-Ox samples showed large amounts of the present material, little sign of material resorption, presence of collagenous fibers and fibroblasts and some areas of bone neoformation. The neoformed bone was irregular with little density (Fig. 5).
Fig. 5.
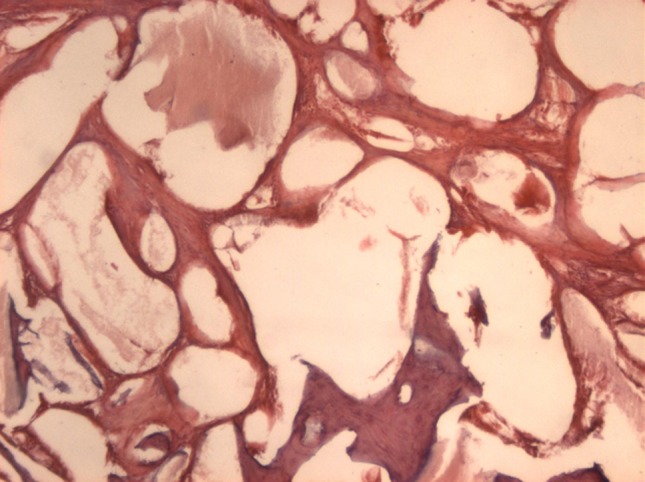
Gen-Ox 8 weeks (original magnification 40×): there is large amount of waste material, little bone formation and presence of much collagen fiber
Bone-Fill
The Bone-Fill samples showed smaller amounts of residual material than Gen-Ox, a larger amount of neoformed bone and denser, more organized bone. The amount of collagenous fibers was little noticed (Fig. 6). There were clear signs of material resorption mainly inside, giving the graft bone an aspect of porosity.
Fig. 6.
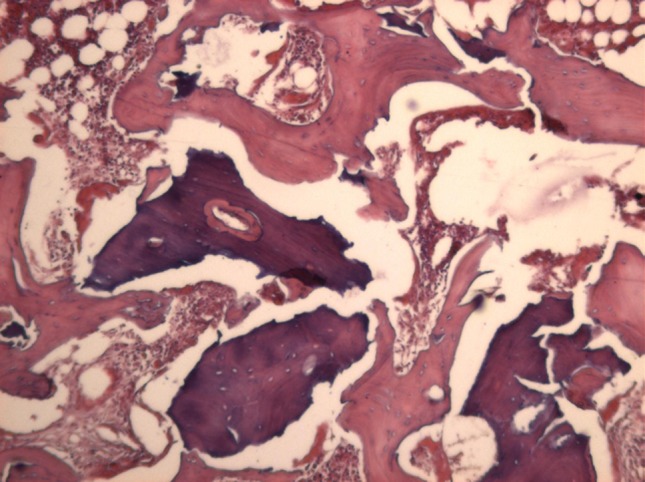
Bone-Fill 8 weeks (original magnification 40×: FC: collagen fibers). There is waste material, observe the porosity, new bone already in the organization with presence of cell arrangements
Bio-Oss
Bio-Oss samples showed presence of residual material on resorption, followed by a large quantity of macrophages, presence of neoformed bone, primary bone with few Havers systems and neovases. The presence of collagenous fibers was observed in small amounts (Fig. 7).
Fig. 7.
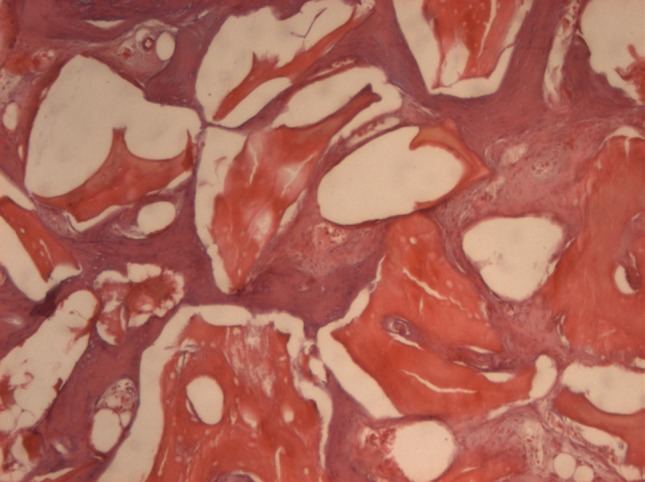
Bio-Oss 8 weeks (original magnification 40×): presence of residual material, observe the porosity of this material, presence of immature bone formation and osteoid tissue
12 Week Samples
Blood Clot
The samples filled with blood clot showed a large amount of collagenous fibers and fibroblasts, presence of osteoid tissue and formation of primary bone (Fig. 8).
Fig. 8.
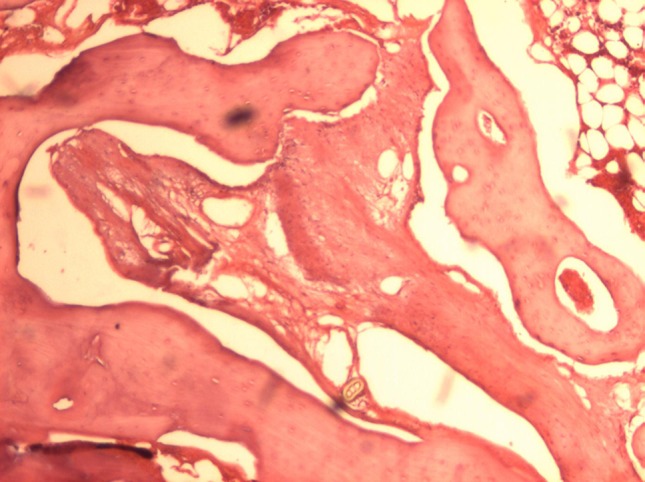
Blood clot 12 weeks (original magnification 40×) Observe the presence of immature bone associated with osteoid tissue and collagen fibers
Gen-Ox
The Gen-Ox samples showed presence of secondary bone with smaller amounts of residual material. The presence of residual material was noticed with higher frequency in the upper part of the cylinder rather than in the base. Signs of resorption of the filling material were noticed in the entire cylinder. Collagenous fibers were also noticed in the entire cylinder (Fig. 9).
Fig. 9.
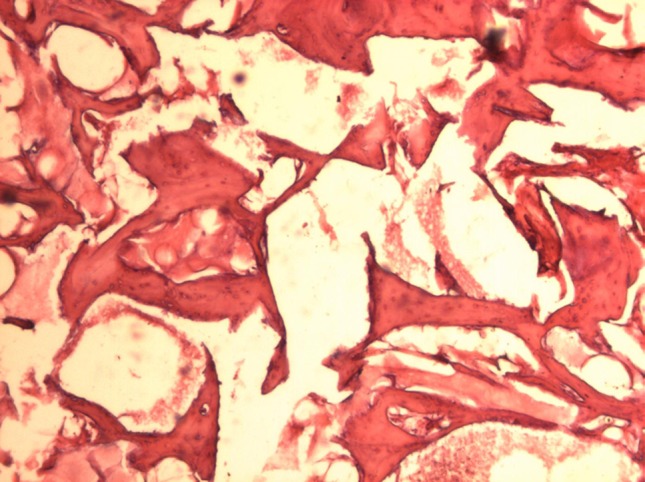
Gen-Ox 12 weeks (original magnification 40×). Little presence of newly formed bone, lots of waste material
Bone-Fill
The Bone-Fill samples showed formation of secondary bone in the entire cylinder. The amount of residual material decreased considerably and the presence of secondary bone increased as compared to the 8 week sample (Fig. 10).
Fig. 10.
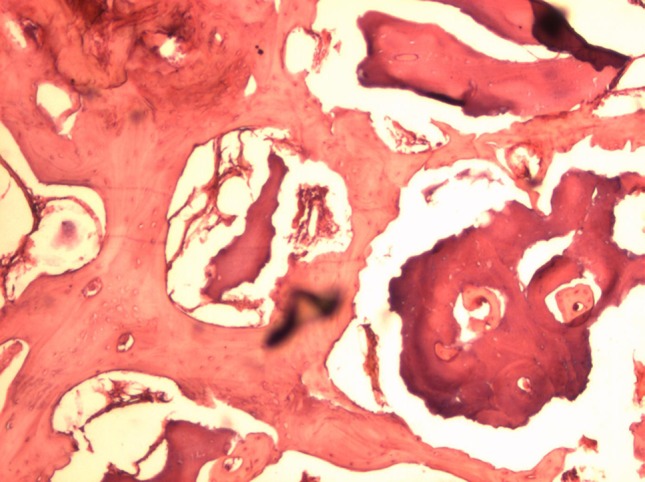
Bone-Fill 12 weeks (original magnification 40×) observe the amount of secondary bone formed, the presence of small residual material and appearance of reabsorption thereof
Bio-Oss
Bio-Oss samples showed formation of secondary bone in the entire cylinder in larger amounts than the 8 week samples. Resorption of residual material was also noticed in larger amounts. The presence of collagenous fibers was noticed in small amounts (Fig. 11).
Fig. 11.
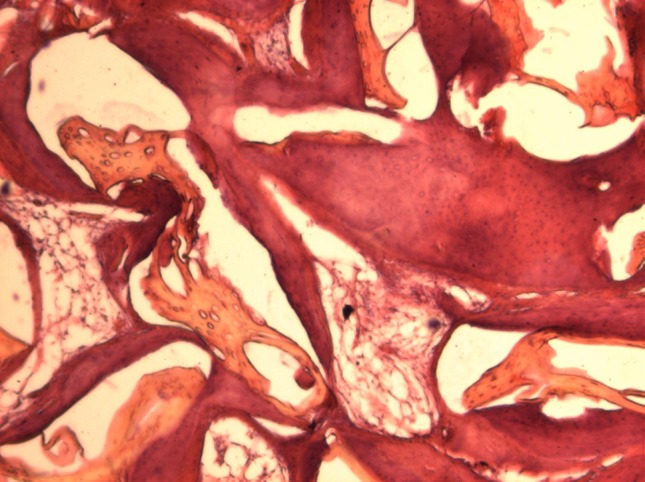
Bio-Oss: 12 weeks (part proximal cylinder; original magnification 40×) observe the amount of bone formed and the presence of secondary small residual material
The histomorphometric analysis is shown in Tables 1 and 2.
Table 1.
Histomorphometric analysis at 8 weeks
| Bio-Oss | Bone-Fill | Gen-Ox | Blood clot | |
|---|---|---|---|---|
| New bone (mm2) | 8.43 | 7.24 | 2.78†‡ | 0.65†‡ |
| Residual particles (mm2) | 8.37 | 7.31 | 3.76†‡ | – |
| Non-mineralized tissue (mm2) | 4.99 | 6.01 | 3.87‡ | 3.84‡ |
†Significant difference compared to Bio-Oss
‡Significant difference compared to Bone-Fill
Table 2.
Histomorphometric analysis at 12 weeks
| Bio-Oss | Bone-Fill | Gen-Ox | Blood clot | |
|---|---|---|---|---|
| New bone (mm2) | 9.32 | 9.01 | 3.02†‡ | 0.63†‡ |
| Residual particles (mm2) | 5.81 | 4.99 | 3.62 | – |
| Non-mineralized tissue (mm2) | 3.87 | 4.59 | 3.03 | 3.29 |
†Significant difference compared to Bio-Oss
‡Significant difference compared to Bone-Fill
Discussion
Bio-Oss is the world’s most widely used and studied trademark of that product. Similar products are available in Brazil, but with little scientific research. This is the main objetvo this article compare these materials with the gold standard, Bio-Oss.
The use of anorganic xenogenous bovine bone has proven to be the best choice. Articles comparing the many bone substitutes used in implantodontics have shown that such material presents the best clinical as well as histological and histomorphometric results [4–7].
Particles of anorganic xenogenous bovine graft are gradually resorpted and replaced by a new bone with structural characteristics very similar to those of a human bone [4, 5, 13, 17].
One of the factors can be the release of type I collagenous fibers during Bio-Oss resorption, as verified by Taylor et al. [18] under electronic microscope, as such release would allow larger aggregation of hydroxyapatite crystals. In the same work the authors noticed that the degradation of Bio-Oss particles increases the concentration of nitrogen to levels equivalent to those released during the process of resorption and neoformation of human bones and considered that it may be another key to success.
The genetic alteration caused by the biomaterial in function of the osteoblasts genes becomes the most significant factor among those cited [15, 16]. The need to repeat the same with other biomaterials, as proposed by the authors, would help define how important this genetic potential is for the repair conducted by Bio-Oss [15].
The need of such studies has motivated the use of Bio-Oss as a comparative in this study, for there are no doubts of its efficiency as bone substitute in implantodontics [4].
Despite a number of studies carried out in rabbits, there is no pattern with regard to the part of the body to be used, the kind of defect to be treated and the evaluation time [4, 7, 19–25]. The option for the defect to be used was based on Frame’s conclusions in 1980 [26]; he studied defects with trephines of several different diameters in rabbit cranial vault in order to determine the size of the defect necessary to study the materials. Frame concludes that the minimum defect for the result of repair to be considered reliable in research using rabbit craniums is 15 mm diameter.
The used titanium cylinders installed in rabbits’ cranial vault has been frequently used to compare bone substitutes [7, 19–24]. The use of cylinders allows the comparison of four samples in the same animal and also represents a defect of greater complexity than those defects treated in implantodontics [7, 21, 23].
The cylinder, which was manufactured specifically for this study, allowed fine tuning with the rabbits’ cranial vault; this, associated to the perfect adaptation of the lid, which was screwed to the cylinder, prevented penetration of tissue coming from the periosteum and the subcutaneous, thus not interfering in the bone neoformation process conducted by the biomaterials tested.
The evaluation times of 8 and 12 weeks was based on several books referred in literature in which the same evaluation periods were used. Frost [27] concluded that 6 weeks of evaluation corresponds to 17 weeks in human beings, and so 8 weeks in rabbits corresponds to 24 weeks in human beings; therefore, the results evaluated in this study correspond to the time recommended for the installation of implants in human beings after reconstruction procedures.
Filling the cylinders randomly prevented the difference in density and vascularization of bones at the animal’s cranial vault from interfering with the results.
The methodology allowed us to establish a reliable model to be applied in human beings.
The macroscopic results found in the 8 week samples were similar to the microscopic results. Macroscopically, Bio-Oss showed a mineralized aspect with good particle incorporation. The macroscopic impression was confirmed histologically. Under optical microscope, Bio-Oss samples showed incorporation of particles in neoformed bone. Although that bone was still immature, it was already present in large amounts. The presence of resorption of the material could also be noticed. Some granules showed resorption or bone formation inside. This is because the Bio-Oss granules were porous.
Bone-Fill was macroscopically similar to Bio-Oss. The formation of new bone in some pairs resembling secondary bone was noticed microscopically. The presence of particles of the material was also noticed, as well as a small amount of collagenous fibers. As compared to Bio-Oss, Bone-Fill showed bone formation in a smaller quantity, though with more advanced structural stage. The resorption process seen was very similar to that of Bio-Oss with aspect of resorption and internal neoformation, also giving the Bone-Fill granule an aspect of porosity.
Such porosity shown by Bio-Oss, which seems to be also present in Bone-Fill, is quoted as one of the main factors for the great osteoconductor potential of such materials [4, 5, 18, 27].
During the removal of samples, Gen-Ox anorganic showed little incorporation of its particles, particularly those located at the upper part of the cylinder, and the there were small amount of mineralization tissue. The macroscopic impression was confirmed during evaluation under light microscope. Large amounts of residual material and collagenous fibers were noticed. Significant amounts of neoformed bone were noticed only in one of the four slides, and small amounts of neoformed bone were found in the other slides. In all samples in which bone tissue was found, it was primary, slightly mineralized and without a defined structure. The resorption pattern observed in Gen-Ox anorganic samples differed from the other materials. No slides showed internal resorption of particles in the periphery. This suggests that the material does not show porosity, which might explain slower resorption and less bone formation than the other materials.
The macroscopic and microscopic findings were confirmed through histomorphometric analysis, which put into numbers the results observed in previous tests.
Conclusions
The model proposed in this study has allowed us to conclude that, although the three materials showed osteoconductor properties, histologically Bio-Oss and Bone-Fill showed similar and better performance than Gen-Ox anorganic, which showed smaller formation of bone tissue, thus showing the worst performance among the three anorganic xenogenous bovine grafts.
Acknowledgments
The authors are grateful to Sistema de Implantes Nacional represented by Ms Neide Lenharo and Dr. Ariel Lenharo for donating the titanium cylinders and screws used to conduct the experiments. The authors also thank Dr. Nelson Villa (Department of Histology, School of Dentistry, University of Santo Amaro/SP) and Dr. Andre Pelegrine for surgical assistance. The authors report no conflicts of interest related to this study.
Footnotes
Bio-Oss—Switzerland Re-Order. No 03-0502.
Bone-Fill—Bionnovations. São Paulo—Brasil Lot. 1096/03.
Gen-Ox—Baumer São Paulo—Brasil Lot. 009733.
References
- 1.Rokn AR, Khodadoostan MA, Reza Rasouli Ghahroudi AA, Motahhary P, Kharrazi Fard MJ, Bruyn HD, Afzalifar R, Soolar E, Soolari A. Bone formation with two types of grafting materials: a histologic and histomorphometric study. Open Dent J. 2011;5:96–104. doi: 10.2174/1874210601105010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fugazzotto PA, Vlassis J. Long-term success of sinus augmentation using various surgical approaches and grafting materials. Int J Oral Maxillofac Implants. 1998;13:52–58. [PubMed] [Google Scholar]
- 3.Al Ruhaimi KA. Bone graft substitutes: a comparative qualitative histologic review of current osteoconductive grafting materials. Int J Oral Maxillofac Implants. 2001;16:105–114. [PubMed] [Google Scholar]
- 4.Jensen T, Schou S, Stavropoulos A, Terheyden H, Holmstrup P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft: a systematic review. Clin Oral Implants Res. 2012;23:263–273. doi: 10.1111/j.1600-0501.2011.02168.x. [DOI] [PubMed] [Google Scholar]
- 5.McAllister BS, Margolin MD, Cogan AG, et al. Eighteen-month radiographic and histologic evaluation of sinus grafting with anorganic bovine bone in the chimpanzee. Int J Oral Maxillofac Implants. 1999;14:361–368. [PubMed] [Google Scholar]
- 6.Schlegel KA, Fichtner G, Shultze-Mosgau S. Histologic Findings in sinus augmentation with autogenous bone chips versus a bovine bone substitute. Int J Oral Maxillofac Implants. 2003;18:53–58. [PubMed] [Google Scholar]
- 7.Slott EC, Lundgren D, Burgos PM. Placement of autogeneic bone chips or bovine bone mineral in guided bone augmentation: a rabbit skull study. Int J Oral Maxillofac Implants. 2003;18:795–805. [PubMed] [Google Scholar]
- 8.Ferreira CEA, Novaes AB, Jr, Haraszthy VI, Bittencourt M, Martinelli CB, Luczyszyn SM. A clinical study of 406 sinus augmentation with 100 % anorganic bovine bone. J Periodontol. 2009;80:1920–1927. doi: 10.1902/jop.2009.090263. [DOI] [PubMed] [Google Scholar]
- 9.Urban IA, Nagursky H, Lozada JL. Horizontal ridge augmentation with a resorbable membrane and particulated autogenous bone with or without anorganic bovine bone-derived mineral: a prospective case series in 22 patients. Int J Oral Implants. 2011;26:404–414. [PubMed] [Google Scholar]
- 10.Aghazadeh A, Rutger Persson G, Renvert S. A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: results after 12 months. J Clin Periodontol. 2012;39:666–673. doi: 10.1111/j.1600-051X.2012.01880.x. [DOI] [PubMed] [Google Scholar]
- 11.Jensen T, Schou S, Stavropoulos A, Terheyden H, Holmstrup P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft in animals: a systematic review. Int J Oral Maxillofac Surg. 2012;41:114–120. doi: 10.1016/j.ijom.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Jensen T, Schou S, Gundersen HJ, Forman JL, Terheyden H, Holmstrup P. Bone-to-implant contact after maxillary sinus floor augmentation with Bio-Oss and autogenous bone in different ratios in mini pigs. Clin Oral Implants Res. 2013;24:635–644. doi: 10.1111/j.1600-0501.2012.02438.x. [DOI] [PubMed] [Google Scholar]
- 13.Cohen RE, Mullary RH, Noble B, Comeau RL, Neiders ME. Phenotypic characterization of mononuclear cells following anorganic bovine bone implantation in rats. J Periodontol. 1994;65:1008–1015. doi: 10.1902/jop.1994.65.11.1008. [DOI] [PubMed] [Google Scholar]
- 14.Hämmerle CH, Lang NP. Single stage surgery combining transmucosal implant placement with guide bone regeneration and bioresorbable materials. Clin Oral Implants Res. 2001;12:9–18. doi: 10.1034/j.1600-0501.2001.012001009.x. [DOI] [PubMed] [Google Scholar]
- 15.Carinci F, Piatelli A, Degidi M, Palmieri A, Perrotto V, Scapoli L, Martinelli M, Laino G, Pezzetti F. Genetic effects of anorganic bovine bone (Bio-Oss) on osteoblast-like MG63 cells. Arch Oral Biol. 2006;51:154–163. doi: 10.1016/j.archoralbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Palmieri A, Pezzetti F, Brunelli G, Martinelli M, Muzio L, Scarano A, Scapoli L, Arlotti M, Guerzoni L, Carinci F. Anorganic bovine bone (Bio-Oss) regulates mi RNA of osteoblast-like cells. Int J Periodontics Restor Dent. 2010;30:83–87. [PubMed] [Google Scholar]
- 17.Piatelli M, Favero GA, Scarano A, Orsini G, Piatelli A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: a histologic long-term report of 20 cases in humans. Int J. Oral Maxillofac Implants. 1999;14:835–840. [PubMed] [Google Scholar]
- 18.Taylor JC, Cuff SE, Leger JPL, et al. In vitro osteoclast resorption of bone substitute biomaterials used for implant site augmentation: a pilot study. Int J Oral Maxillofac Implants. 2002;17:321–330. [PubMed] [Google Scholar]
- 19.Aghaloo TL, Moy PK, Frymiller EG. Evaluation of platelet-rich plasma in combination with anorganic bovine bone in the rabbit cranium: a pilot study. Int J Oral Maxillofac Implants. 2004;19:59–65. [PubMed] [Google Scholar]
- 20.Schmidlin PR, Nicholls F, Kruse A, Zwahlen RA, Weber FE. Evaluation of moldable, in situ hardening calcium phosphate bone graft substitutes. Clin Oral Impl Res. 2013;24:149–157. doi: 10.1111/j.1600-0501.2011.02315.x. [DOI] [PubMed] [Google Scholar]
- 21.Ezirganlı S, Polat S, Barıs E, Tatar I, Celik HH. Comparative investigation of the effects of different materials used with a titanium barrier on new bone formation. Clin Oral Impl Res. 2013;24(312):319. doi: 10.1111/j.1600-0501.2011.02323.x. [DOI] [PubMed] [Google Scholar]
- 22.Polo CI, Lima JLO, De Lucca L, Piacezzi CB, Homem MGN, Chavez VEA, Sendyk WR. Effect of recombinant human bone morphogenetic protein 2 associated with a variety of bone substitutes on vertical guided bone regeneration in rabbit calvarium. J Periodontol. 2013;84:360–370. doi: 10.1902/jop.2012.110674. [DOI] [PubMed] [Google Scholar]
- 23.Dung SZ, Tu YK. Effect of different alloplast materials on the stability of vertically augmented new tissue. Int J Oral Maxillofac Implants. 2012;27:1375–1381. [PubMed] [Google Scholar]
- 24.Lundgren AK, Lundgren D, Hämmerle CH, Nyman S, Sennerby L. Influence of decortication of the donor bone on guided bone augmentation. An experimental study in the rabbit skull bone. Clin Oral Implants Res. 2000;11:99–106. doi: 10.1034/j.1600-0501.2000.00002.x. [DOI] [PubMed] [Google Scholar]
- 25.Guirado JLG, Fernández MPR, Negri B, Ruiz RAD, de-Val JEMS, Moreno JG. Experimental model of bone response to collagenized xenografts of porcine origin (OsteoBiol® mp3): a radiological and histomorphometric study. Clin Implant Dent Relat Res. 2013;15:143–151. doi: 10.1111/j.1708-8208.2011.00337.x. [DOI] [PubMed] [Google Scholar]
- 26.Frame JW. A convenient animal model for testing bone substitute materials. J Oral Surg. 1980;38:176–187. [PubMed] [Google Scholar]
- 27.Frost HM. Bone remodeling dynamics. Springfield: Charles C Thomas Publisher; 1963. [Google Scholar]


