Abstract
Purpose
To determine whether enrollees with open-angle glaucoma who switched from brand name to generic prostaglandin analogues (PGAs) exhibited a change in medication adherence compared to those who remained on brand name products when generics became available
Design
Longitudinal cohort analysis
Participants
8427 beneficiaries age >40 years with open-angle glaucoma continuously enrolled in a nationwide managed-care network from 2009–2012 who were taking PGAs prior to generic latanoprost availability
Methods
We calculated the mean adherence rates for topical PGAs during the 18 months prior to generic latanoprost availability (September 2009–February 2011). We then determined the mean adherence rates during the subsequent 18 months after generic latanoprost first became available (July 2011–December 2012) for those enrollees who were exclusively maintained on brand name PGAs and compared these adherence rates to those who switched exclusively to generic latanoprost. Multivariable logistic regression identified factors affecting adherence rates.
Main Outcome Measures
Mean medication adherence rates, odds of experiencing ≥25% improvement or worsening of adherence with 95% confidence intervals
Results
8427 enrollees met the study eligibility criteria. Compared to enrollees who switched to generic latanoprost once it became available, enrollees remaining on brand name PGAs were 28% less likely to experience an improvement of adherence (odds ratio (OR)=0.72, 95% CI 0.55–0.94) and 39% more likely to experience worsening of adherence (OR=1.39, 95% CI 1.04–1.86). Other factors associated with improved adherence during the post-generic period included higher monthly medication copay during the pre-generic period (p=0.02), lower monthly medication copay during the post-generic period (p<0.0001), and black race (OR=1.25, 95% CI 1.04–1.50). A total of 612 patients (7.3%) completely discontinued all interventions for glaucoma at the time generic latanoprost became available.
Conclusions
With cost often being a significant barrier to adherence, switching patients to generic medications may help improve adherence. The ophthalmologic community should be aware of the large number of patients who completely discontinued glaucoma medication use altogether during the transition period when generic latanoprost became available and work with insurers and pharmacists to prevent this from happening when other PGAs become available as generics.
Although various classes of medications are capable of lowering intraocular pressure and preventing glaucomatous progression, these medications are only effective when administered properly. Unfortunately, many patients with glaucoma struggle to maintain adherence.1–7 Prior studies have identified a myriad of reasons why patients struggle with glaucoma medication adherence. Some of the more common reasons for suboptimal adherence include a limited knowledge about glaucoma,8–10 forgetfulness,9–11 side effects,8,10,11 difficulty with drop administration,8–11 complexity of the regimen,8,11 and medication cost.8,9
Prostaglandin analogues (PGAs) have become the most commonly prescribed class of medications for patients with open-angle glaucoma (OAG) and glaucoma suspects, surpassing topical beta blockers in the year 2001.12,13 PGAs are often preferable to other classes due to their greater efficacy at lowering intraocular pressure, once daily dosing regimen, and relatively benign side effect profile. One of the few drawbacks of PGAs relative to other medication classes has been cost, with some patients paying over $100 in out-of-pocket expenses per month for PGAs.
In March 2011 the first generic PGA, latanoprost, became commercially available in the United States. Compared with brand name PGAs, generic latanoprost is considerably less expensive for many patients with differences in cost for a year’s supply of brand name versus generic PGA amounting to over $1300.14 Since some patients continued being prescribed brand name PGAs while others switched from brand name medications to generic latanoprost after it became available, this offers researchers a unique opportunity to study the impact of use of generics on medication adherence rates. Using data from enrollees in a large nationwide managed care network, in this study we assess whether persons with OAG who switched from brand name PGAs (bimatoprost, travaprost, or branded latanoprost) to generic latanoprost in March 2011 exhibited an improvement in medication adherence relative to persons who continued taking branded PGAs after generics became available.
Methods
Data Source
The Clinformatics DataMart database (OptumInsight, Eden Prairie, MN) contains detailed de-identified records of all beneficiaries in a large nationwide managed-care network. We had access to data for all beneficiaries with any form of eye care from January 1, 2001 through December 31, 2012. This subset comprises beneficiaries who had ≥1 International Classification of Diseases (ICD-9-CM) codes for any eye-related diagnosis (360–379.9), Current Procedural Terminology (CPT-4) code for any eye-related visits, diagnostic or therapeutic procedures (65091–68899 or 92002–92499), or any other claim submitted by an ophthalmologist or optometrist during their time in the plan. We had access to all beneficiaries’ medical claims (inpatient, outpatient, skilled nursing facility) for ocular and nonocular conditions, along with sociodemographic information (age, sex, race, education, income) for each enrollee. All enrollees were fully enrolled in the pharmacy plan during their entire time of enrollment in the medical plan. This database has been used previously to study patients with ocular diseases.15–17
Sample Selection
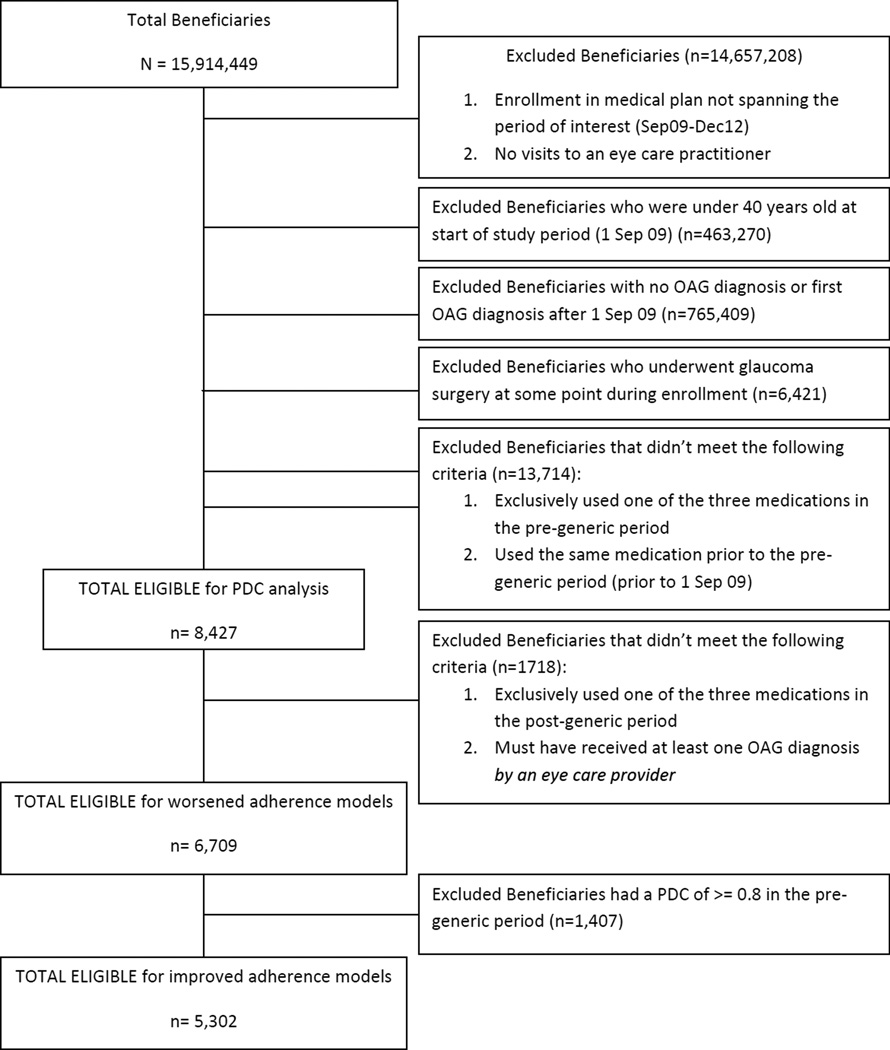
We identified all persons age >40 years who were continuously enrolled in the medical plan from September 1, 2009 through December 31, 2012 and carried at least 1 diagnosis of OAG (ICD-9-CM codes 365.1, 365.10, 365.11, 365.12, or 365.15) before September 2009. Prior work comparing billing records with documentation from actual medical records for the diagnosis of primary OAG has demonstrated that billing records accurately identify most persons with this condition.18 All enrollees with a history of any laser or incisional glaucoma surgical interventions during their time in the plan were excluded since surgery can impact the need for subsequent medications. Figure 1 provides additional details about the inclusion / exclusion criteria for each of the analyses.
Figure 1.
Sample Selection
PDC = proportion of days covered; OAG = open-angle glaucoma
Users of Prostaglandin Analogues
Beneficiaries were identified as users of PGAs if they had one or more outpatient prescriptions of brand name latanoprost (Xalatan), travaprost (Travatan Z), or bimatoprost (Lumigan) during the 18 month period prior to the availability of generic latanoprost in the United States (September 2009 to February 2011). We also required eligible enrollees to have at least one record of use of these medications before September 2009 in order to exclude those who first initiated treatment with PGAs during September 2009 to February 2011. Other classes of glaucoma medications were not considered.
Medication Adherence
PGA adherence was captured for each enrollee by assessing the proportion of days covered (PDC) by the medication, a technique that is frequently used by health services researchers to quantify medication adherence in claims data.19 The PDC is calculated by dividing the total number of days the beneficiary is listed as being prescribed the medication of interest by the number of days from the date of first fill of that medication to the last day of the period of interest.
Analyses
Statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC). Participant characteristics were summarized for the entire sample using means and standard deviations (SD) for continuous variables and frequencies and percentages for categorical variables.
Capturing Prostaglandin Analogue Adherence in the Pre-Generic and Post-Generic Periods
First, we determined for each enrollee the PDC for use of PGAs during the 18 month period prior to the availability of generic latanoprost in the United States (“pre-generic period,” September 2009 to February 2011). Enrollees who had no record of any PGA use prior to September 2009 were excluded to remove persons who had been newly started on these medications during the pre-generic period. Likewise, those with no record of any PGA use during the pre-generic period (e.g. those with a PDC of 0.0) were also excluded. We determined the mean and median PDC for those who were exclusively taking each brand name PGA during the pre-generic period. Generic latanoprost started to become available commercially in March 2011 and we used the time period from March 2011 to June 2011 as an interim period until medication use patterns stabilized. Next, for each enrollee who had been prescribed PGAs during the pre-generic period, we determined the PDC with PGAs during the 18 month period after generic latanoprost became available (“post-generic period,” July 2011 to December 2012). We determined the mean and median PDC for those enrollees who were exclusively taking each brand name PGA during the post-generic period as well as those who were switched from a brand name PGA during the pre-generic period to generic latanoprost during the post-generic period. To simplify the analyses, we excluded enrollees with records of prescriptions for both brand name PGAs and generic latanoprost during the post-generic period. (Figure 1)
Determining Change in Medication Adherence from the Pre-Generic to the Post-Generic Period
For each enrollee, we compared the PDC during the pre-generic period with the PDC during the post-generic period. We computed the mean change in PDC for all enrollees who were taking brand name PGAs during the pre-generic period who remained on the same brand name PGA during the post-generic period. We also computed the mean change in PDC for those enrollees who switched from exclusively taking a brand name PGA during the pre-generic period to generic latanoprost during the post-generic period. (Figure 1) Comparisons of the mean PDCs for those who remained on the brand name PGA during the post-generic period with those who had switched to generic latanoprost were made using the t-test. Similar analyses were performed using medians instead of means and these results are presented in the tables with comparisons performed using the Wilcoxon-Mann-Whitney test.
Factors Associated with Improvement or Worsening of Medication Adherence
Multivariable logistic regression was performed to identify variables associated with an improvement or worsening in medication adherence from the pre-generic to the post-generic period. We empirically defined improvement in medication adherence as a PDC increase of at least 25% from the pre-generic to the post-generic period, and defined worsening of adherence as a reduction in PDC of at least 25%. Variables included in the regression models were age, sex, race, education level, income, type of brand name PGA taken during the pre-generic period (travaprost, bimatoprost, or branded latanoprost), whether the enrollee continued to receive the brand name PGA during the post-generic period or switched to generic latanoprost, average medication copay during the pre-generic period, average medication copay during the post-generic period, and type of eye care provider managing the OAG during the study period (exclusive care by an ophthalmologist, exclusive care by an optometrist, or care by both provider types). For the regression model assessing factors related to improved adherence, we excluded enrollees who exhibited excellent adherence to medications (defined as a PDC during the pre-generic period of ≥0.8) during the pre-generic period since these beneficiaries were already so adherent that they could not achieve improvements in PDC of at least 25%. Checks for multicolinearity were performed and the only variables in the model which were moderately correlated with one another were brand name medication taken in the pre-generic period and whether a brand name or generic medication was taken in the post-generic period, an expected finding.
The regression models generated odds ratios (ORs) with 95% confidence intervals (CI). For all analyses p<0.05 was considered statistically significant. The University of Michigan Institutional Review Board determined that since the data were completely de-identifiable to the researchers, the study received approval as a non-regulated study.
Results
Sociodemographic Characteristics of Eligible Enrollees
There were 8427 enrollees who met the study eligibility criteria. (Figure 1) The mean ± SD age of eligible enrollees was (66.1 ± 12.1 years). There were 4424 females (52.5%) and the racial composition included 6288 whites (74.6%), 1027 blacks (12.2%), 404 Latinos (4.8%), 245 Asians (2.9%), and 66 persons of other races (0.8%). The sample included 8242 persons (97.8%) with at least a high school education and 1471 persons (17.5%) with an income >$125,000. (Table 1) Among the 8427 eligible enrollees, the number who were exclusively taking brand name latanoprost, travaprost, and bimatoprost during the pre-generic period was 3759 (44.6%), 2531 (30.0%), and 2137 (25.4%) respectively. Table 1 shows the sociodemographic characteristics of those enrollees who were exclusively taking each of these classes of brand name PGAs during the 18 month pre-generic period, those who remained on each brand name PGA during the post-generic period, and those who switched to generic latanoprost. Enrollees who switched to generic latanoprost differed from those who were maintained on brand name PGAs with respect to age (p=0.0001), sex (p=0.009), race (p=0.0005), and education (p=0.005) but not income (p=0.38).
Table 1.
Demographic Characteristics of Eligible Enrollees
| Total | Pre-generic period use | Post-generic period use | ||||||
|---|---|---|---|---|---|---|---|---|
| Bimatoprost | Travaprost | Brand name latanoprost |
Generic latanoprost |
Bimatoprost | Travaprost | Brand name latanoprost |
||
| Number of Beneficiaries, n | 8,427 | 2,137 | 2,531 | 3,759 | 2,950 | 1,749 | 1,854 | 156 |
| Age | ||||||||
| Mean, years [Std Dev) | 66.1 [12.1] | 65.8 (11.8) | 64.9 (11.8) | 67.1 (12.4) | 66.9 (12.3) | 66.1 (11.6) | 65.4 (11.9) | 66.3 (12.0) |
| Sex | ||||||||
| Male, n (%) | 4,003 (47.5%) | 1,020 (47.7%) | 1,182 (46.7%) | 1,801 (47.9%) | 1,462 (49.6%) | 854 (48.8%) | 828 (44.7%) | 60 (38.5%) |
| Female, n (%) | 4,424 (52.5%) | 1,117 (52.3%) | 1,349 (53.3%) | 1,958 (52.1%) | 1,488 (50.4%) | 895 (51.2%) | 1,026 (55.3%) | 96 (61.5%) |
| Race | ||||||||
| White, n (%) | 6,288 (74.6%) | 1,571 (73.5%) | 1,830 (72.3%) | 2,887 (76.8%) | 2,273 (77.1%) | 1,300 (74.3%) | 1,349 (72.8%) | 121 (77.6%) |
| Black, n (%) | 1,027 (12.2%) | 275 (12.9%) | 368 (14.5%) | 384 (10.2%) | 298 (10.1%) | 218 (12.5%) | 272 (14.7%) | 14 (9.0%) |
| Latino, n (%) | 404 (4.8%) | 94 (4.4%) | 138 (5.5%) | 172 (4.6%) | 132 (4.5%) | 84 (4.8%) | 87 (4.7%) | 9 (5.8%) |
| Asian American, n (%) | 245 (2.9%) | 62 (2.9%) | 65 (2.6%) | 118 (3.1%) | 93 (3.2%) | 40 (2.3%) | 49 (2.6%) | 4 (2.6%) |
| Other race, n (%) | 66 (0.8%) | 20 (0.9%) | 22 (0.9%) | 24 (0.6%) | 18 (0.6%) | 12 (0.7%) | 21 (1.1%) | 0 (0.0%) |
| Education level | ||||||||
| Less than high school, n (%) | 77 (0.9%) | 19 (0.9%) | 26 (1.0%) | 32 (0.9%) | 24 (0.8%) | 11 (0.6%) | 16 (0.9%) | 2 (1.3%) |
| High school diploma, n (%) | 2,226 (26.4%) | 618 (28.9%) | 748 (29.6%) | 860 (22.9%) | 702 (23.8%) | 477 (27.3%) | 514 (27.7%) | 32 (20.5%) |
| Some college, n (%) | 3,902 (46.3%) | 987 (46.2%) | 1,176 (46.5%) | 1,739 (46.3%) | 1,394 (47.3%) | 804 (46.0%) | 872 (47.0%) | 74 (47.4%) |
| College diploma, n (%) | 2,055 (24.4%) | 474 (22.2%) | 532 (21.0%) | 1,049 (27.9%) | 774 (26.2%) | 423 (24.2%) | 416 (22.4%) | 45 (28.9%) |
| Advanced degree, n (%) | 59 (0.7%) | 8 (0.4%) | 12 (0.5%) | 39 (1.0%) | 24 (0.8%) | 7 (0.4%) | 10 (0.5%) | 2 (1.3%) |
| Household income category | ||||||||
| Less than $30,000, n (%) | 907 (10.8%) | 220 (10.3%) | 308 (12.2%) | 379 (10.1%) | 285 (9.7%) | 171 (9.8%) | 223 (12.0%) | 16 (10.3%) |
| $30,000 –<$60,000, n (%) | 2,993 (35.5%) | 784 (36.7%) | 943 (37.3%) | 1,266 (33.7%) | 1,054 (35.7%) | 622 (35.6%) | 655 (35.3%) | 47 (30.1%) |
| $60,000 –<$100,000, n (%) | 1,700 (20.2%) | 434 (20.3%) | 517 (20.4%) | 749 (19.9%) | 576 (19.5%) | 360 (20.6%) | 384 (20.7%) | 31 (19.9%) |
| $100,000 –<$125,000, n (%) | 853 (10.1%) | 215 (10.1%) | 231 (9.1%) | 407 (10.8%) | 315 (10.7%) | 183 (10.5%) | 183 (9.9%) | 17 (10.9%) |
| ≥$125,000, n (%) | 1,471 (17.5%) | 362 (16.9%) | 379 (15.0%) | 730 (19.4%) | 534 (18.1%) | 323 (18.5%) | 289 (15.6%) | 37 (23.7%) |
Changes in Medication Adherence with the Introduction of Generic Latanoprost
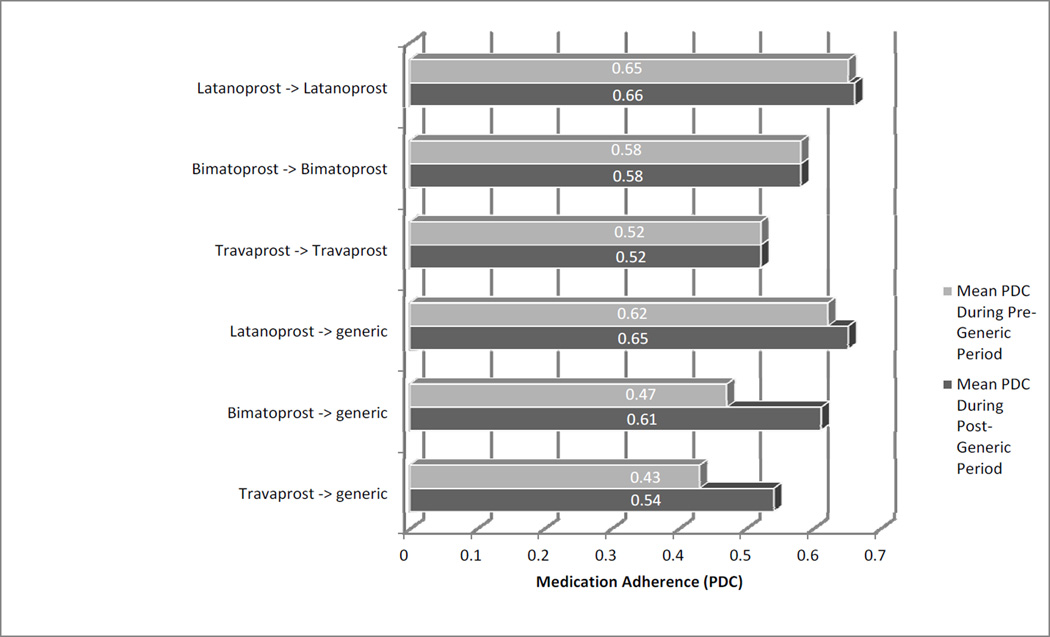
Figure 2 shows the mean glaucoma medication adherence as captured using PDC during the 18 month pre-generic period (the time period before generic latanoprost became available), during the 18 month post-generic period (the time period after generic latanoprost became available). Table 2, available at http://aaojournal.org offers additional details including the mean and median change in adherence from the pre-generic period to the post-generic period for persons who remained on each of the three brand name PGAs as compared to those who switched from a brand name product to generic latanoprost. As the figure depicts, persons who continued taking brand name PGAs after generic latanoprost became available demonstrated little change in adherence. However, the subset of enrollees who switched from travaprost or bimatoprost to generic latanoprost when it became available exhibited lower levels of adherence during the time period before generic latanoprost was available and then exhibited large increases in adherence after they made the switch to generic latanoprost. In fact, once this subset of patients were switched to generic latanoprost, their mean and median adherence levels improved to higher than the levels of those who remained on the brand name products.
Figure 2.
Mean adherence in patients who remained on brand-name drug or were switched to generic
PDC = proportion of days covered
Factors Associated with Improvement in Medication Adherence
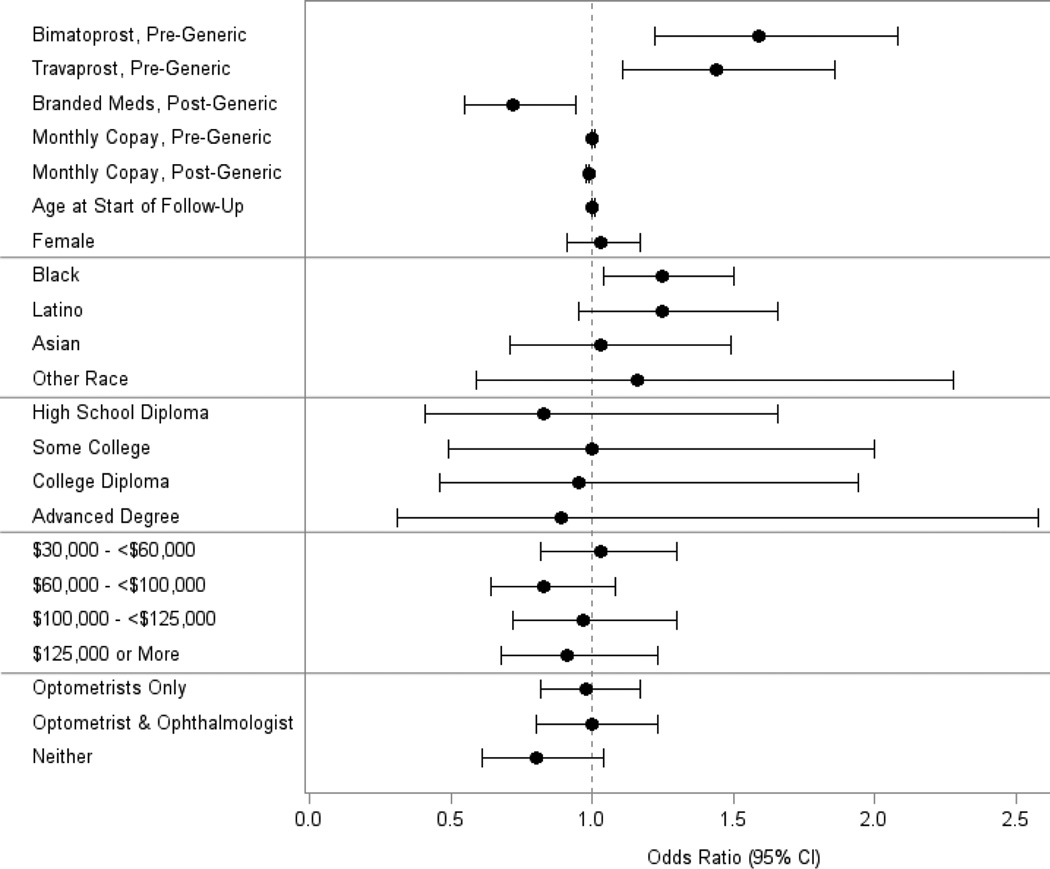
After adjustment for confounding factors, compared to persons who were taking generic latanoprost during the post-generic period, those exclusively receiving any of the brand name PGAs exhibited a 28% decreased odds of experiencing an improvement in adherence during the post-generic period (adjusted OR=0.72, [CI 0.55–0.94]). Users of travaprost exclusively during the pre-generic period had a 44% increased odds of exhibiting an improvement in adherence after generic latanoprost became available (adjusted OR=1.44, [CI 1.11–1.86]) and users of bimatoprost exclusively during the pre-generic period had a 59% increased odds of improved adherence after generic latanoprost became available (adjusted OR=1.59, [CI 1.22–2.08]) compared with exclusive users of brand name latanoprost during the pre-generic period. (Figure 3) In the regression model, for every additional dollar in average monthly copay spent for PGAs during the pre-generic period, enrollees had a 0.5% increased odds of exhibiting improved adherence during the post-generic period (adjusted OR=1.005, [CI 1.001–1.008]).
Figure 3.
Factors Associated with a ≥ 25% Improvement in Medication Adherence After Generic Latanoprost Became Available
For example, enrollees who spent, on average, $15 in monthly copays for PGAs during the pre-generic period had a 5% increased odds of improved adherence during the post-generic period relative to those who spent only $5 on average during the pre-generic period. For every additional dollar in average monthly copay spent on PGAs during the post-generic period, the odds of experiencing improved adherence declined 1% (adjusted OR=0.99, [CI 0.98–0.99]). For example, compared to an enrollee who spent on average $5 in copays during the post-generic period, those spending $15 in copays during this time period had a 11% decreased odds of experiencing improved adherence. Blacks had a 25% increased odds of experiencing an improvement in adherence after generic latanoprost became available relative to whites (adjusted OR=1.25, [CI 1.04–1.50]). There was no difference in the odds of experiencing an improvement in adherence during the post-generic period for any of the other races relative to whites (p>0.05 for all comparisons). Moreover, there was no association between age, sex, education, income, type of eye care provider caring for the patient’s glaucoma, or lack of visits to an eye care provider during the post-generic period and odds of experiencing an improvement in adherence during the post-generic period. (p>0.05 for all comparisons). In an additional analysis (data not shown), we added to the regression model two additional covariates capturing the maximum number of additional pressure-lowering medication classes prescribed during the pre-generic and post-generic periods and the inclusion of these covariates did not materially impact the study findings of the original model.
Factors Associated with Worsening of Adherence
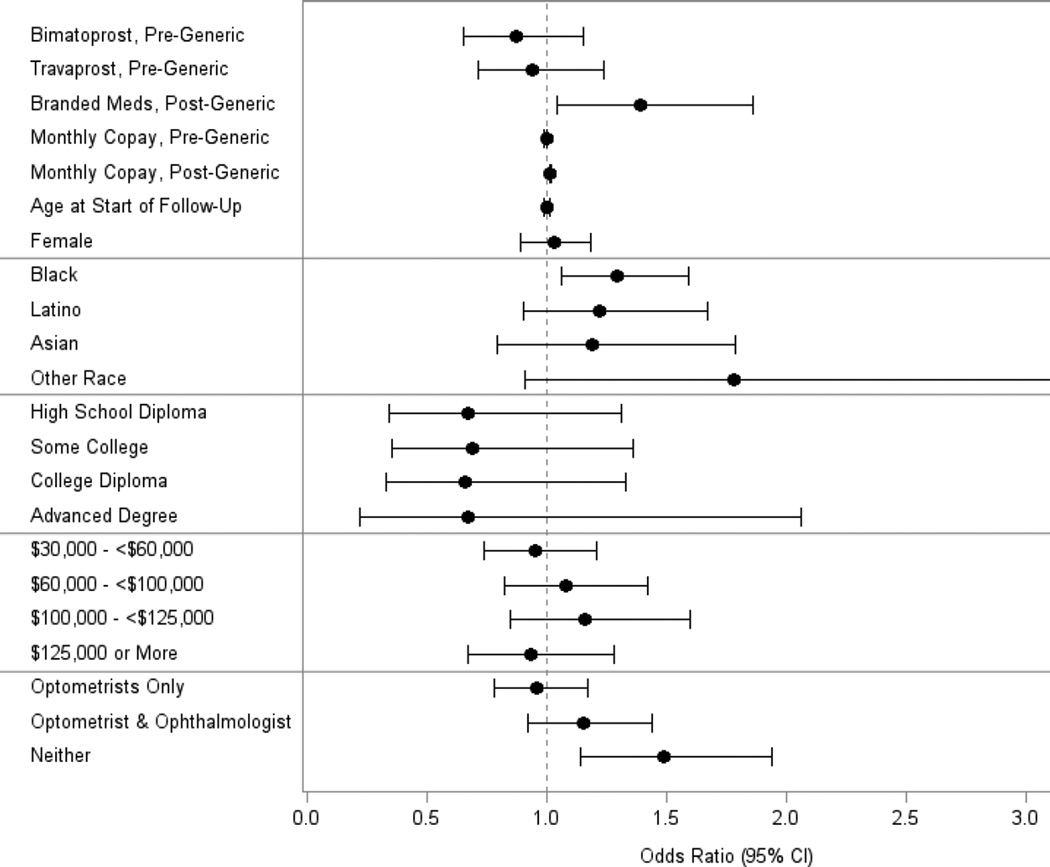
Compared to the patients who switched to generic latanoprost, those who continued to receive one of the brand name products had a 39% increased odds of experiencing a decline in adherence during the post-generic period (adjusted OR=1.39, [CI 1.04–1.86]). (Figure 4) For every additional one dollar increase in average monthly medication copay during the post-generic period, the odds of demonstrating a 25% reduction in adherence increased 1%. In other words, compared to an enrollee whose post-generic period average monthly medication copay was $10, another enrollee whose average medication copay of $30 had a 22% increased odds of experiencing a decline in medication adherence in the post-generic period. Compared with whites, blacks had a 29% increased odds of experiencing a drop in medication during the post-generic period. No differences in the odds of experiencing a decline in medication adherence were seen for other races relative to whites or by age, sex, education level, income, or type of eye care provider caring for them. Persons with no records of any visits to eye care providers during the post-generic period had a 49% increased odds of demonstrating a decline in medication adherence as well (adjusted OR=1.49, [CI 1.14–1.94]). In an additional analysis (data not shown), we added to the regression model two additional covariates capturing the maximum number of additional pressure-lowering medication classes prescribed during the pre-generic and post-generic periods and the inclusion of these covariates did not materially impact the study findings of the original model.
Figure 4.
Factors Associated with a ≥ 25% Worsening of Medication Adherence After Generic Latanoprost Became Available
Given that the regression models showed black race to be associated with both a statistically significant improvement in medication adherence as well as a significant worsening of adherence relative to whites during the post-generic period, we explored the sociodemographic and other characteristics of the subset of blacks who exhibited an improvement, no change, and decline in adherence. Among blacks who achieved improved adherence during the post-generic period, 43.2% had been switched to generic latanoprost while 56.8% remained on a brand name PGA. By comparison, among blacks who experienced declines in adherence, only 29.4% had been switched to generic latanoprost while 70.6% remained on a brand name PGA. (Table 3, available at http://aaojournal.org)
Complete Discontinuation of Glaucoma Medication Use
Among the 8427 users of brand name PGAs during the pre-generic period, a total of 612 patients (7.3%) completely discontinued all glaucoma medications (PGAs and other glaucoma medication classes) after generic latanoprost became available. These persons also had no record of laser or incisional glaucoma surgery. The proportion of users of travaprost, bimatoprost, and brand name latanoprost during the pre-generic period who discontinued all treatment for glaucoma after the availability of generic latanoprost was 8.3%, 6.4%, and 7.0%, respectively. Figure 5, available at http://aaojournal.org shows the month when each enrollee last filled a prescription for a PGA. As the figure depicts, there was a spike in PGA discontinuation at the time generic latanoprost became available.
Discussion
In this large nationwide sample of patients with OAG who were receiving treatment with PGAs, we identified a subset of patients who exhibited reduced medication adherence during the time period when only brand name PGAs were available. When this subset of patients were switched from the brand name PGA to generic latanoprost, their adherence rose considerably, to levels much higher than other patients who had been maintained on brand name PGAs after generic latanoprost became available. After accounting for potential confounding factors, factors found to be associated with an improvement in medication adherence of ≥25% from the time period before generic latanoprost was available to after it became available included exclusive use of travaprost or bimatoprost in the pre-generic period (relative to branded latanoprost), use of generic latanoprost during the post-generic period (relative to brand name agents), higher copays during the pre-generic period, lower copays during the post-generic period, and black race. Likewise, factors associated with a reduction in medication adherence of ≥25% during the post-generic period included persistent use of a branded PGA during the post-generic period, higher monthly copays during the post-generic period, black race, and lack of visits to an eye care provider.
With claims data alone, it is impossible to determine why there was an improvement in adherence for a subset of patients who had been switched from brand name medications to generic PGAs. The observed improvement in adherence may be attributable to lower copays for generic PGAs or due to differences in side effects (i.e less hyperemia with generic latanoprost), greater effectiveness at lowering intraocular pressure with generic latanoprost, or these patients may have received greater attention by the eye care professionals caring for them. Using mass spectrometry, Kahook and colleagues have reported differences in the amount of active ingredients and preservatives between brand name and generic PGAs20, though a head-to-head trial reported that generic and brand name PGAs have similar efficacy and side effects.21
Race
Our finding that black patients had a higher odds than whites to experience a drop in medication adherence is consistent with several prior studies.7,13,22–26 Friedman and colleagues used pharmacy filling records to show that nonwhite race was a risk factor for worse adherence even after adjusting for education level and income, but the authors noted that race may have been confounded by differences in socioeconomic status, geographic access to medications, and the nature of the physician-patient relationship between whites and nonwhites.23 Another study that measured adherence using electronic monitoring of eye drop use reported that blacks had lower adherence independent of education level, income, general health status, health access, and medication cost.24 A study of pharmacy filling records conducted in the Veterans Affairs Health System found that nonwhites, most of whom were black, had a five-fold higher risk of nonadherence than whites.25 “Blacks tend to have more severe disease and a more complex medication regimen which can certainly affect adherence.
Despite the underlying propensity for blacks to become less adherent with glaucoma medications over time, as has been demonstrated both in the present study and others described above, one of the interesting findings from this analysis is that there were a subset of blacks who exhibited a substantial improvement in medication adherence relative to whites after generic latanoprost became available. Among blacks who achieved improved adherence during the post-generic period, 43.2% had been switched to generic latanoprost while 56.8% remained on a brand name PGA. By comparison, among blacks who experienced declines in adherence, only 29.4% had been switched to generic latanoprost while 70.6% remained on a brand name PGA. These findings suggest that prescribing generic latanoprost or switching from brand name PGAs to generic latanoprost can be particularly helpful in combatting difficulties with medication adherence among blacks.
Medication Cost
Cost has been identified as a barrier to medication adherence in several clinical contexts23,27–31 including glaucoma.23,29–31 In a study conducted prior to the availability of generic latanoprost, up to 41% of patients with glaucoma reported difficulty paying for their glaucoma medications.30 Patient concern about cost of glaucoma medications is a significant risk factor for poor adherence independent of several other factors including race.23 Medication cost is often considered a difficult risk factor to modify. To our knowledge, no prior studies have examined impact of a reduction in glaucoma medication copay on adherence, though researchers have demonstrated how changes in copays can affect medication adherence for other diseases.32 Our analysis found improved adherence after patients were switched from the more expense brand name travaprost or bimatoprost to generic latanoprost.
Qualitative studies have identified cost as a major barrier to adherence specifically among blacks with glaucoma.31 Given that blacks tend to have more severe open-angle glaucoma and are at greater risk of blindness from this disease, yet have higher rates of nonadherence, switching patients to a generic PGA whenever possible or considering alternative treatments like laser trabeculoplasty may be an effective means of reducing glaucoma progression in this high risk group.
Medication Discontinuation
Although not the primary aim of our study, an additional finding of importance we identified was that there was a sizable group of patients with OAG (over 7%) who simply stopped taking glaucoma medications altogether at the time generic latanoprost came to market. We looked carefully at this group of commercially insured patients to see whether they had been switched to other classes of glaucoma medications or had records of laser or incisional glaucoma surgery to account for the complete loss of adherence and could not find evidence that they were receiving other interventions for glaucoma. With access to only their claims data, we can only speculate as to why these patients may have stopped undergoing treatment for OAG. One possibility is that the insurance company may have made it unduly difficult or expensive for patients to continue to obtain the more expensive brand name PGA in an attempt to get them to switch to generic latanoprost. If so, for a subset of these enrollees, they either could not be bothered making the switch to the generic product or they may have encountered too many hurdles (e.g. additional visits to eye care providers, completion of paperwork) to make the transition from the brand name product to the generic alternative, resulting in discontinuation of all glaucoma treatment. As travaprost and bimatoprost will soon go off patent and become available as generics, our findings suggest that clinicians need to be particularly mindful to the fact that some patients may be at risk for complete discontinuation of therapy. Moreover, insurers and pharmacy benefit managers need to be cognizant that although bureaucratic and administrative hurdles they employ to motivate patients to switch from brand name products to generics are often effective at getting many patients to switch to the cheaper generic product, there are a subset of patients who are unable to effectively make that switch to the generic product. This subset of patients who end up going untreated are at increased risk for worsening of their OAG and later on may ultimately require costly surgical interventions.
Study Strengths
The large number of patients in this study carrying a diagnosis of open-angle glaucoma enabled us to study patterns of adherence for those exclusively taking each of the common PGAs and to make comparisons among the subset who remained on branded medications versus others who switched to generic products. Second, all of these patients had health insurance including drug coverage. Thus, the observed patterns of medication adherence were not attributable to lack of insurance, a factor known to affect medication adherence.33 Third, medication use in this analysis was captured by using pharmacy claims which has been found to be more reliable than reliance upon patient self-report.34
Study Limitations
Our study has several limitations. When relying upon claims data to study medication adherence, all researchers can tell is the extent to which medication prescriptions are filled, not whether they are taken as directed or whether the patient is successful at getting the medications into her eye. In these analyses we assume that patients exhibiting higher PDCs are more likely to be taking the medications as prescribed. Moreover, claims data lacks information regarding why adherence levels improved or deteriorated over time or the impact of changes in adherence on outcomes such as level of intraocular pressure or progressive structural or functional glaucomatous damage. The database does not contain information on samples dispensed in the office or medications purchased outside of the plan. Finally, the results of these analyses may not be generalizable to persons who are uninsured, a group known to struggle with medication adherence.
Conclusion
In conclusion, this study highlights the impact of medication cost and access to generic PGAs on medication adherence. We identified a subset of patients who clearly exhibited an improvement in adherence when they were switched from a brand name PGA to generic latanoprost. When clinicians know or suspect that a patient is struggling with adherence, attempts should be made, whenever feasible, to switch such patients to generic glaucoma medications. This can be particularly helpful for patients with high copays and racial minorities.
Supplementary Material
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (JDS:1K23EY019511); Research to Prevent Blindness “Physician Scientist” Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no proprietary interest in any material discussed in this manuscript
References
- 1.Kass MA, Gordon M, Morley RE, Jr, Meltzer DW, Goldberg JJ. Compliance with topical timolol treatment. Am J Ophthalmol. 1987;103(2):188–193. doi: 10.1016/s0002-9394(14)74225-4. [DOI] [PubMed] [Google Scholar]
- 2.Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83(5):711–716. doi: 10.2105/ajph.83.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112(6):953–961. doi: 10.1016/j.ophtha.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DS, Quigley HA, Gelg L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS) Invest Ophthalmol Vis Sci. 2007;48(11):5052–5057. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- 6.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically: the Travatan Dosing Aid study. Ophthalmology. 2009;116:191–199. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma. 2012;21:234–240. doi: 10.1097/IJG.0b013e31821dac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stryker JE, Beck AD, Primo SA, et al. An exploratory study of factors influencing glaucoma treatment adherence. J Glaucoma. 2010;19:66–72. doi: 10.1097/IJG.0b013e31819c4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacey J, Cate H, Broadway DC. Barriers to adherence with glaucoma medications: a qualitative research study. Eye (Lond) 2009;23:924–932. doi: 10.1038/eye.2008.103. [DOI] [PubMed] [Google Scholar]
- 10.Tsai JC, McClure CA, Ramos SE, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12:393–398. doi: 10.1097/00061198-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Taylor SA, Galbraith SM, Mills RP. Causes of non-compliance with drug regimens in glaucoma patients: a qualitative study. J Ocul Pharmacol Ther. 2002;18:401–409. doi: 10.1089/10807680260362687. [DOI] [PubMed] [Google Scholar]
- 12.Stein JD, Sloan FA, Lee PP. Rates of glaucoma medication utilization among older adults with suspected glaucoma. Am J Ophthalmol. 2007;143(5):870–872. doi: 10.1016/j.ajo.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein JD, Ayyagari P, Sloan FA, Lee PP. Rates of glaucoma medication utilization among persons with primary open-angle glaucoma, 1992 to 2002. Ophthalmology. 2008 Aug;115(8):1315–1319. doi: 10.1016/j.ophtha.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Engel K, editor. Truven Health Analytics. [Accessed July 2, 2014]. Red Book. Available at http://sites.truvenhealth.com/redbook/. [Google Scholar]
- 15.Stein JD, Blachley TS, Musch DC. Identification of persons with incident ocular diseases using health care claims databases. Am J Ophthalmol. 2013;156(6):1169–1175. doi: 10.1016/j.ajo.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein JD, Pasquale LR, Talwar N, et al. Geographic and climatic factors associated with exfoliation syndrome. Arch Ophthalmol. 2011 Aug;129(8):1053–1060. doi: 10.1001/archophthalmol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein JD, Vanderbeek BL, Talwar N, Nan B, Musch DC, Zacks DN. Rates of nonexudative and exudative age-related macular degeneration among Asian American ethnic groups. Invest Ophthalmol Vis Sci. 2011 Aug;52(9):6842–6848. doi: 10.1167/iovs.11-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muir KW, Gupta C, Gill P, Stein JD. Accuracy of international classification of diseases, ninth revision, clinical modification billing codes for common ophthalmic conditions. JAMA Ophthalmol. 2013 Jan;131(1):119–120. doi: 10.1001/jamaophthalmol.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann PharmacoTher. 2006;40(7):1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 20.Kahook MY, Fechtner RD, Katz LJ, Noecker RJ, Ammar DA. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101–108. doi: 10.3109/02713683.2011.631722. [DOI] [PubMed] [Google Scholar]
- 21.Digiuni M, Manni G, Vetrugno M, et al. An evaluation of therapeutic noninferiority of 0.005% latanoprost ophthalmic solution and xalatan in patients with glaucoma or ocular hypertension. J Glaucoma. 2013;22:707–712. doi: 10.1097/IJG.0b013e318259b47c. [DOI] [PubMed] [Google Scholar]
- 22.Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surgery. 1995;26(3):233–236. [PubMed] [Google Scholar]
- 23.Friedman DS, Hahn SR, Gelb L, et al. Doctor-Patient Communication, Health-Related Beliefs, and Adherence in Glaucoma Results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008;115:1320–1327. doi: 10.1016/j.ophtha.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116(6):1097–1105. doi: 10.1016/j.ophtha.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Sleath B, Ballinger R, Covert D, Robin AL, Byrd JE, Tudor G. Self-reported prevalence and factors associated with nonadherence with glaucoma medications in veteran outpatients. American Journal Geriatric Pharmacotherapy. 2009;7(2):67–73. doi: 10.1016/j.amjopharm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Sleath B, Blalock SJ, Covert D, Skinner AC, Muir KW, Robin AL. Patient race, reported problems in using glaucoma medications, and adherence. ISRN Ophthalmology. 2012:902819. doi: 10.5402/2012/902819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy J, Coyne J, Sclar D. Drug affordability and prescription noncompliance in the United States: 1997–2002. Clin Ther. 2004;26:607–614. doi: 10.1016/s0149-2918(04)90063-x. [DOI] [PubMed] [Google Scholar]
- 28.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 29.Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116:S30–S36. doi: 10.1016/j.ophtha.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Sleath B, Robin AL, Covert D, et al. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113:431–436. doi: 10.1016/j.ophtha.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Dreer LE, Girkin CA, Campbell L, Wood A, Gao L, Owsley C. Glaucoma medication adherence among African Americans: program development. Optom Vis Sci. 2013;90:883–897. doi: 10.1097/OPX.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doshi JA, Zhu J, Lee BY, Kimmel SE, Volpp KG. Impact of a Prescription Copayment Increase on Lipid-Lowering Medication Adherence in Veterans. Circulation. 2009;119:390–397. doi: 10.1161/CIRCULATIONAHA.108.783944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balkrishnan R. Predictors of medication adherence in the elderly. Clinical Therapeutics. 1998;20:764–771. doi: 10.1016/s0149-2918(98)80139-2. [DOI] [PubMed] [Google Scholar]
- 34.Patty L, Wu C, Torres M, Azen S, Varma R. Validity of self-reported eye disease and treatment in a population-based study: the Los Angeles Latino Eye Study. Ophthalmology. 2012;119(9):1725–1730. doi: 10.1016/j.ophtha.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.