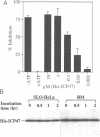
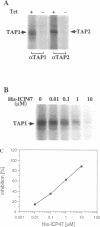
Abstract
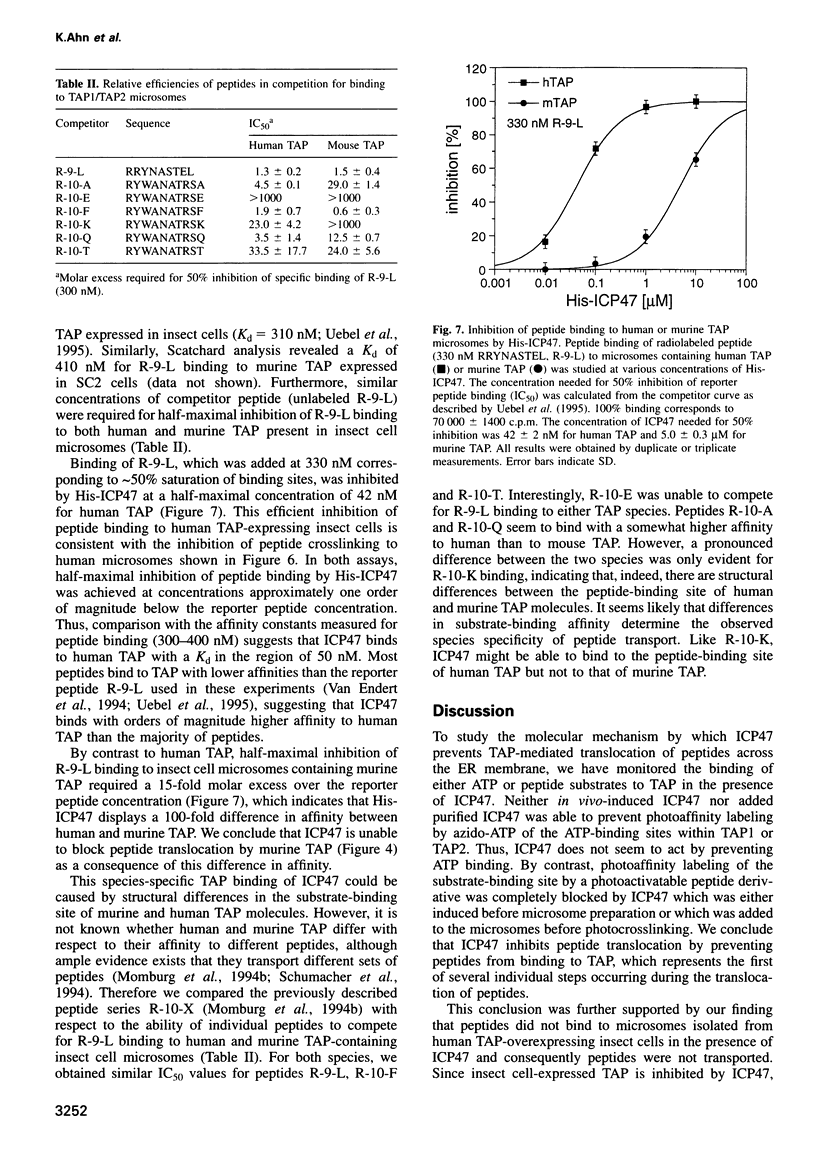
The immediate early protein ICP47 of herpes simplex virus (HSV) inhibits the transporter for antigen processing (TAP)-mediated translocation of antigen-derived peptides across the endoplasmic reticulum (ER) membrane. This interference prevents assembly of peptides with class I MHC molecules in the ER and ultimately recognition of HSV-infected cells by cytotoxic T-lymphocytes, potentially leading to immune evasion of the virus. Here, we demonstrate that recombinant, purified ICP47 containing a hexahistidine tag inhibits peptide import into microsomes of insect cells expressing human TAP, whereas inhibition of peptide transport by murine TAP was much less effective. This finding indicates an intrinsic species-specificity of ICP47 and suggests that no additional proteins interacting specifically with either ICP47 or TAP are required for inhibition of peptide transport. Since neither purified nor induced ICP47 inhibited photocrosslinking of 8-azido-ATP to TAP1 and TAP2 it seems that ICP47 does not prevent ATP from binding to TAP. By contrast, peptide binding was completely blocked by ICP47 as shown both by photoaffinity crosslinking of peptides to TAP and peptide binding to microsomes from TAP-transfected insect cells. Competition experiments indicated that ICP47 binds to human TAP with a higher affinity (50 nM) than peptides whereas the affinity to murine TAP was 100-fold lower. Our data suggest that ICP47 prevents peptides from being translocated by blocking their binding to the substrate-binding site of TAP.
Full text
PDF
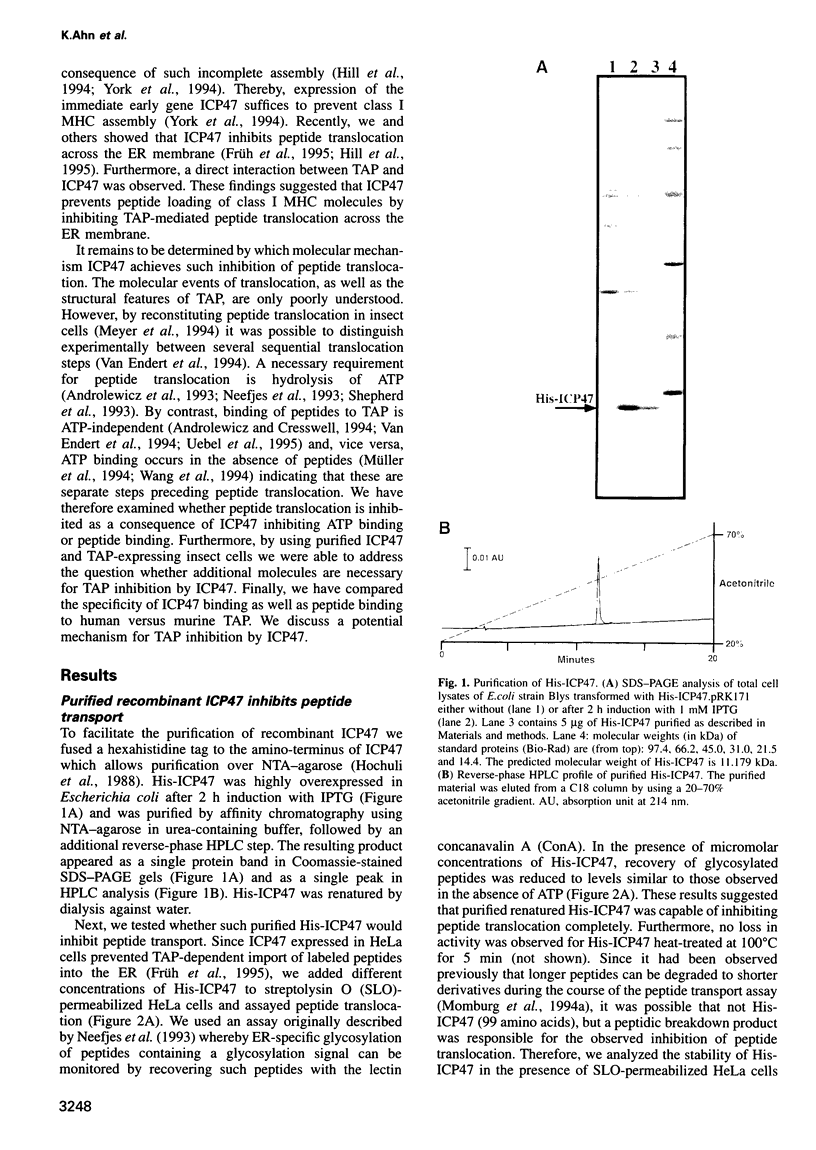





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Androlewicz M. J., Anderson K. S., Cresswell P. Evidence that transporters associated with antigen processing translocate a major histocompatibility complex class I-binding peptide into the endoplasmic reticulum in an ATP-dependent manner. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9130–9134. doi: 10.1073/pnas.90.19.9130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androlewicz M. J., Cresswell P. Human transporters associated with antigen processing possess a promiscuous peptide-binding site. Immunity. 1994 Apr;1(1):7–14. doi: 10.1016/1074-7613(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Androlewicz M. J., Ortmann B., van Endert P. M., Spies T., Cresswell P. Characteristics of peptide and major histocompatibility complex class I/beta 2-microglobulin binding to the transporters associated with antigen processing (TAP1 and TAP2). Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12716–12720. doi: 10.1073/pnas.91.26.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutkiewicz R. R., Welsh R. M. Major histocompatibility complex class I antigens and the control of viral infections by natural killer cells. J Virol. 1995 Jul;69(7):3967–3971. doi: 10.1128/jvi.69.7.3967-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Deverson E. V., Gow I. R., Coadwell W. J., Monaco J. J., Butcher G. W., Howard J. C. MHC class II region encoding proteins related to the multidrug resistance family of transmembrane transporters. Nature. 1990 Dec 20;348(6303):738–741. doi: 10.1038/348738a0. [DOI] [PubMed] [Google Scholar]
- Früh K., Ahn K., Djaballah H., Sempé P., van Endert P. M., Tampé R., Peterson P. A., Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995 Jun 1;375(6530):415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- Früh K., Yang Y., Arnold D., Chambers J., Wu L., Waters J. B., Spies T., Peterson P. A. Alternative exon usage and processing of the major histocompatibility complex-encoded proteasome subunits. J Biol Chem. 1992 Nov 5;267(31):22131–22140. [PubMed] [Google Scholar]
- Heemels M. T., Schumacher T. N., Wonigeit K., Ploegh H. L. Peptide translocation by variants of the transporter associated with antigen processing. Science. 1993 Dec 24;262(5142):2059–2063. doi: 10.1126/science.8266106. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hill A. B., Barnett B. C., McMichael A. J., McGeoch D. J. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J Immunol. 1994 Mar 15;152(6):2736–2741. [PubMed] [Google Scholar]
- Hill A., Jugovic P., York I., Russ G., Bennink J., Yewdell J., Ploegh H., Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995 Jun 1;375(6530):411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- Hill A., Ploegh H. Getting the inside out: the transporter associated with antigen processing (TAP) and the presentation of viral antigen. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):341–343. doi: 10.1073/pnas.92.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. C. Supply and transport of peptides presented by class I MHC molecules. Curr Opin Immunol. 1995 Feb;7(1):69–76. doi: 10.1016/0952-7915(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Peterson P. A. Assembly and intracellular transport of MHC class I molecules. Annu Rev Cell Biol. 1993;9:207–235. doi: 10.1146/annurev.cb.09.110193.001231. [DOI] [PubMed] [Google Scholar]
- Jackson M. R., Song E. S., Yang Y., Peterson P. A. Empty and peptide-containing conformers of class I major histocompatibility complex molecules expressed in Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12117–12121. doi: 10.1073/pnas.89.24.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Kloszewski E. D., Anderson R. W., Thompson D. L., Tevethia S. S. Effect of herpes simplex virus types 1 and 2 on surface expression of class I major histocompatibility complex antigens on infected cells. J Virol. 1985 Dec;56(3):757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup R. A. Virus escape from CTL recognition. J Exp Med. 1994 Sep 1;180(3):779–782. doi: 10.1084/jem.180.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mester J. C., Rouse B. T. The mouse model and understanding immunity to herpes simplex virus. Rev Infect Dis. 1991 Nov-Dec;13 (Suppl 11):S935–S945. doi: 10.1093/clind/13.supplement_11.s935. [DOI] [PubMed] [Google Scholar]
- Michalek M. T., Grant E. P., Gramm C., Goldberg A. L., Rock K. L. A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature. 1993 Jun 10;363(6429):552–554. doi: 10.1038/363552a0. [DOI] [PubMed] [Google Scholar]
- Momburg F., Roelse J., Howard J. C., Butcher G. W., Hämmerling G. J., Neefjes J. J. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature. 1994 Feb 17;367(6464):648–651. doi: 10.1038/367648a0. [DOI] [PubMed] [Google Scholar]
- Momburg F., Roelse J., Hämmerling G. J., Neefjes J. J. Peptide size selection by the major histocompatibility complex-encoded peptide transporter. J Exp Med. 1994 May 1;179(5):1613–1623. doi: 10.1084/jem.179.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco J. J., Cho S., Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990 Dec 21;250(4988):1723–1726. doi: 10.1126/science.2270487. [DOI] [PubMed] [Google Scholar]
- Müller K. M., Ebensperger C., Tampé R. Nucleotide binding to the hydrophilic C-terminal domain of the transporter associated with antigen processing (TAP). J Biol Chem. 1994 May 13;269(19):14032–14037. [PubMed] [Google Scholar]
- Neefjes J. J., Momburg F., Hämmerling G. J. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporter. Science. 1993 Aug 6;261(5122):769–771. doi: 10.1126/science.8342042. [DOI] [PubMed] [Google Scholar]
- Ortmann B., Androlewicz M. J., Cresswell P. MHC class I/beta 2-microglobulin complexes associate with TAP transporters before peptide binding. Nature. 1994 Apr 28;368(6474):864–867. doi: 10.1038/368864a0. [DOI] [PubMed] [Google Scholar]
- Powis S. J., Deverson E. V., Coadwell W. J., Ciruela A., Huskisson N. S., Smith H., Butcher G. W., Howard J. C. Effect of polymorphism of an MHC-linked transporter on the peptides assembled in a class I molecule. Nature. 1992 May 21;357(6375):211–215. doi: 10.1038/357211a0. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Falk K., Rötzschke O. Peptides naturally presented by MHC class I molecules. Annu Rev Immunol. 1993;11:213–244. doi: 10.1146/annurev.iy.11.040193.001241. [DOI] [PubMed] [Google Scholar]
- Rock K. L., Gramm C., Rothstein L., Clark K., Stein R., Dick L., Hwang D., Goldberg A. L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994 Sep 9;78(5):761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Russ G., Esquivel F., Yewdell J. W., Cresswell P., Spies T., Bennink J. R. Assembly, intracellular localization, and nucleotide binding properties of the human peptide transporters TAP1 and TAP2 expressed by recombinant vaccinia viruses. J Biol Chem. 1995 Sep 8;270(36):21312–21318. doi: 10.1074/jbc.270.36.21312. [DOI] [PubMed] [Google Scholar]
- Schumacher T. N., Kantesaria D. V., Heemels M. T., Ashton-Rickardt P. G., Shepherd J. C., Fruh K., Yang Y., Peterson P. A., Tonegawa S., Ploegh H. L. Peptide length and sequence specificity of the mouse TAP1/TAP2 translocator. J Exp Med. 1994 Feb 1;179(2):533–540. doi: 10.1084/jem.179.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. C., Schumacher T. N., Ashton-Rickardt P. G., Imaeda S., Ploegh H. L., Janeway C. A., Jr, Tonegawa S. TAP1-dependent peptide translocation in vitro is ATP dependent and peptide selective. Cell. 1993 Aug 13;74(3):577–584. doi: 10.1016/0092-8674(93)80058-m. [DOI] [PubMed] [Google Scholar]
- Spies T., Bresnahan M., Bahram S., Arnold D., Blanck G., Mellins E., Pious D., DeMars R. A gene in the human major histocompatibility complex class II region controlling the class I antigen presentation pathway. Nature. 1990 Dec 20;348(6303):744–747. doi: 10.1038/348744a0. [DOI] [PubMed] [Google Scholar]
- Suh W. K., Cohen-Doyle M. F., Fruh K., Wang K., Peterson P. A., Williams D. B. Interaction of MHC class I molecules with the transporter associated with antigen processing. Science. 1994 May 27;264(5163):1322–1326. doi: 10.1126/science.8191286. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Trowsdale J., Hanson I., Mockridge I., Beck S., Townsend A., Kelly A. Sequences encoded in the class II region of the MHC related to the 'ABC' superfamily of transporters. Nature. 1990 Dec 20;348(6303):741–744. doi: 10.1038/348741a0. [DOI] [PubMed] [Google Scholar]
- Uebel S., Meyer T. H., Kraas W., Kienle S., Jung G., Wiesmüller K. H., Tampé R. Requirements for peptide binding to the human transporter associated with antigen processing revealed by peptide scans and complex peptide libraries. J Biol Chem. 1995 Aug 4;270(31):18512–18516. doi: 10.1074/jbc.270.31.18512. [DOI] [PubMed] [Google Scholar]
- Wang K., Früh K., Peterson P. A., Yang Y. Nucleotide binding of the C-terminal domains of the major histocompatibility complex-encoded transporter expressed in Drosophila melanogaster cells. FEBS Lett. 1994 Aug 22;350(2-3):337–341. doi: 10.1016/0014-5793(94)00806-x. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Vande Woude G. F. DNA sequence of an immediate-early gene (IEmRNA-5) of herpes simplex virus type I. Nucleic Acids Res. 1982 Feb 11;10(3):979–991. doi: 10.1093/nar/10.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Früh K., Chambers J., Waters J. B., Wu L., Spies T., Peterson P. A. Major histocompatibility complex (MHC)-encoded HAM2 is necessary for antigenic peptide loading onto class I MHC molecules. J Biol Chem. 1992 Jun 15;267(17):11669–11672. [PubMed] [Google Scholar]
- York I. A., Roop C., Andrews D. W., Riddell S. R., Graham F. L., Johnson D. C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994 May 20;77(4):525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- van Endert P. M., Tampé R., Meyer T. H., Tisch R., Bach J. F., McDevitt H. O. A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity. 1994 Sep;1(6):491–500. doi: 10.1016/1074-7613(94)90091-4. [DOI] [PubMed] [Google Scholar]
- van Endert P. M., Tampé R., Meyer T. H., Tisch R., Bach J. F., McDevitt H. O. A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity. 1994 Sep;1(6):491–500. doi: 10.1016/1074-7613(94)90091-4. [DOI] [PubMed] [Google Scholar]