Abstract
Chemesthetic compounds, responsible for sensations such as burning, cooling, and astringency, are difficult stimuli to work with, especially when the evaluation task requires retasting. Here, we developed a protocol by which chemesthetic compounds can be assessed using sorting. We compared the performance of two cohorts of untrained assessors on this task, one with nose clips and the other without. Similarity matrices were analyzed using multidimensional scaling (MDS) to produce perceptual maps for the two cohorts. Overall, the groupings from the nose open cohort tended to follow a biological basis, consistent with previous findings that suggest compounds that activate a common receptor will elicit similar sensations. The nose-open and nose-pinched cohorts generated significantly different maps. The nose-pinched cohort had a higher variance in the MDS solution than the nose-open group. While the nose-open cohort generated seven clusters, the nose-pinched cohort generated only two clusters, seemingly based on the ready identification of chemesthetic sensations or not. There was less consensus regarding the attributes used to describe the samples in the nose-pinched cohort than in the nose-open cohort as well, as this cohort collectively generated more attributes but fewer were significant in regression.
Keywords: multidimensional scaling, sorting, chemesthesis, untrained assessor, spicy
1. INTRODUCTION
Multidimensional Scaling (MDS), originally developed by Torgerson (1952), is a family of multivariate statistical techniques that are commonly used to visually represent the similarity of items within a data set. These methods can be used to construct perceptual maps that pictorially represent the magnitude of perceptual distances between stimuli and provide information on how panelists group these samples. These techniques, reviewed by Popper and Heymann (1996) have been used for odorants (Chollet and Valentin 2000; Lawless 1989), tastants (Schiffman and Erickson 1971), and foods (Lawless et al. 1995), but have not previously been applied to chemesthetic stimuli. Compared to other descriptive techniques, such as flash profiling and “check-all-that-apply” (CATA) which rely heavily on semantic labels to describe sensation, MDS allows the researcher to explore attributes that are difficult to verbalize and offers the advantage that there is no need to use words to judge similarities (Nestrud and Lawless 2010; Schiffman et al. 1981; Valentin et al. 2012). Participants are free to choose the criteria that they use to make judgments, regardless of whether they have specific words to express these similarities or differences.
Data input for MDS can be any sort of similarity (or dissimilarity) measure such as correlations across rated attributes, interpoint distances, as in napping, direct ratings of pairwise comparisons, or frequency counts (e.g. the number of times that stimuli are placed in a group together), as in sorting (Lawless and Horne 2000; Lawless 1989; Nestrud and Lawless 2010; Rosenberg and Park Kim 1975). One of the advantages of using similarity-based judgments versus multiple attribute scales to collect MDS data is the lack of linguistic contamination. Because MDS can be conducted without expressly relying on words, it is particularly useful when working with sensations, such as those produced by chemesthetic compounds, which may be unfamiliar, semantically unwieldy (i.e., “Is it ‘hot-hot’ or ‘spicy-hot’?”), or otherwise difficult to describe (Bennett and Hayes 2012; Cliff and Heymann 1992; Cliff and Green 1996).
When collecting similarity estimates via pairwise comparisons, participants must judge the level of similarity between all pairs of stimuli. Collecting data in this way quickly becomes fatiguing for participants, as the number of pairs assessed increases rapidly with the number of stimuli ([N*(N-1)]/2 ratings for N stimuli, i.e. 11 stimuli require 55 paired comparisons). Accordingly, pairwise comparisons are not well suited for stimuli in which fatigue, adaptation, or sensitization or desensitization are a concern, as with some tastants, odorants, or chemesthetic stimuli. One alternative technique, sorting, takes much less time and allows for many more stimuli to be tested in a single session. In a sorting task, participants place samples into groups based on perceived similarities and dissimilarities (Rosenberg et al. 1968; Rosenberg and Park Kim 1975). Sorting was first introduced into the chemosensory literature from psychology by Lawless specifically to deal with highly fatiguing stimuli (Lawless 1989; Lawless and Glatter 1990).
One advantage of using free sorting is that the maps are reproducible (Cartier et al. 2006; Chollet et al. 2011; Falahee and MacRae 1997; Lelièvre et al. 2008) and untrained assessors can generate maps comparable to those generated using traditional descriptive analysis techniques (Faye et al. 2004; Saint-Eve et al. 2004) without the substantial time required for descriptive profiling techniques (e.g. Spectrum Descriptive Analysis, QDA). Additionally, it is possible to ask assessors to provide descriptions of individual stimuli or groups once sorting is complete. Asking assessors to provide their own descriptors instead of asking for ratings on attribute scales predetermined by the experimenter provides insight into the perception of and preference for stimuli in the sample set using attributes that are salient to the study participants (Blancher et al. 2012; Cartier et al. 2006; Chollet and Valentin 2000; Faye et al. 2004; Faye et al. 2006; Holliins et al. 1993; Lawless 1989; Lawless et al. 1995; Lelièvre et al. 2008; Lim and Lawless 2005; Saint-Eve et al. 2004; Tang and Heymann 2002). Optionally, cluster analysis can then be used to gain additional information to aid in data interpretation by helping to differentiate groups of products as determined by consumers (Lawless 2013; Nestrud and Lawless 2010).
Given the self- and cross-sensitization and desensitization commonly observed with chemesthetic agents (Cliff and Green 1994; Dessirier et al. 2001; Green 1989; Green 1991; Green and Rentmeister-Bryant 1998; Jancso et al. 1977; Klein et al. 2013; Prescott and Stevenson 1996a; Prescott and Swain-Campbell 2000; Simons et al. 2003), designing an evaluation protocol for these stimuli can be cumbersome. Given the risk of fatigue, potential lapse of participant attention, sensitization, and desensitization that may occur, free sorting is potentially better suited to collecting data when working with chemesthetic compounds, as a pairwise comparison procedure would be extremely lengthy, on the order of 4 or 5 hours. Sorting is also well suited for the present study as it does not require participants to use words to classify sensations while they sample and group the stimuli, thus avoiding the linguistic confusion that surrounds chemesthetic sensations (Bennett and Hayes 2012; Cliff and Heymann 1992; Cliff and Green 1996).
While qualitative differences in pungency had been alluded to previously (Govindarajan 1979; Lawless 1989; Todd et al. 1977), the first formal study was conducted by Cliff and Heymann (1992), who used traditional descriptive analysis techniques to characterize the oral irritancy elicited by various chemesthetic compounds. Stimuli were compared using four attributes (burning, tingling, numbing, and overall irritation) in addition to temporal and spatial characteristics. Importantly, while this seminal work provides the first quantitative characterization of perceptual differences across irritants, use of traditional descriptive analysis potentially limits generalizability to untrained assessors, or to other chemesthetic stimuli that are not described by the attributes generated from the training set. Another key study in exploring oral chemesthesis was conducted by Bertino and Lawless (1993), in which MDS was used to understand mouthfeel attributes of oral healthcare products. In this study, participants sorted 21 cards with terms referring to ‘mouthfeel’ sensations and tastes into groups, generating four groups clustering around sensations elicited by tastes, astringents, local anesthetics, and painful sensations. While the authors hypothesized sorting with actual sampled stimuli would produce similar responses, such a study was not conducted. We build on these prior reports by providing sampled stimuli to participants in a sorting task.
Here, we developed a novel delivery system and testing protocol to allow us to explore the underlying dimensions of oral chemesthesis related to the perception of “spicy”. We presented participants with nine stimuli that were previously shown to evoke a wide range of chemesthetic sensations (Albin and Simons 2010; Bennett and Hayes 2012; Bryant and Mezine 1999; Cliff and Heymann 1992; Cliff and Green 1994; Green 1991; Gwartney and Heymann 1995; Klein et al. 2013; Lawless and Stevens 1988; McDonald et al. 2010; McKemy et al. 2002), but that may all be called “spicy” colloquially; two taste stimuli were also included in the sorting task. Free sorting was conducted with naïve assessors who were split into two groups, a group that completed the task without nose clips and a group that wore nose clips. As a number of these chemesthetic agents have strong aromas (e.g., eucalyptol and cinnamaldehyde), we included a ‘nose-pinched’ condition with nose clips to minimize the possibility that olfaction was used as a panelists’ primary criteria for sorting. Inclusion of a nose-pinched condition allows data to be compared to prior work by Cliff and Heymann (1992) while data collected in the nose open condition is more representative of the experience when these compounds are consumed in food. Utilizing free sorting, MDS, cluster analysis, and regression, we investigated the perceptual similarities of various chemesthetic stimuli in a set of untrained assessors.
2. MATERIALS AND METHODS
2.1. Overview
This study was performed in two separate groups of individuals (n’s =30 and 31). All conditions, stimuli, and instructions were the same in the two groups, with the exception that the second group was required to wear a nose clip for the entirety of the testing session. All data were collected with the approval of the local Institutional Review Board and the informed consent of participants.
2.2. Participants
Participants were recruited from the [REDACTED FOR REVIEW] campus and the surrounding area. To be eligible, individuals needed to be non-smoking, fluent English speakers between 18 and 55 years old, with no known food allergies or defect of taste or smell. Additional exclusion criteria included being pregnant or nursing, taking prescription medication for any chronic pain condition, having known difficulties swallowing, or a history of thyroid irregularities. As sorting procedures have been shown to stabilize with around 25–30 subjects (Faye et al. 2006; Lawless and Horne 2000), we tested ~30 participants in each cohort. Study participants were tested one at a time so they could not observe how other participants completed the sorting task. To avoid any effect of learning on the way that study participants completed the task, individuals were recruited for only one of the cohorts.
2.3. Stimuli
Samples were prepared in ethanol (95%, USP, Koptec, King of Prussia, PA), with the exception of citric acid and quinine, which were prepared in reverse osmosis (RO) water. All samples were Food Grade (FG), Food Chemical Codex (FCC), Kosher, or U.S. Pharmacopeia (USP) grade. Eugenol (12.2mM), menthol (38.4mM), allyl isothiocyanate (0.36M), zingerone (vanillylacetone; 59.7mM), quinine (4.1mM), cinnamaldehyde (0.12M), and carvacrol (0.27M) were obtained from SAFC (St. Louis, MO), citric acid (112mM) from J. T. Baker (Phillipsburg, NJ), capsaicin (100uM) from Sigma, eucalyptol (0.65M) from International Flavors and Fragrances (Union Beach, NJ), and huajiao (red extract; 5% w/w) was a gift from Dr. Christopher Simons (Givaudan, Cincinnati, OH). The stimuli concentrations used in this experiment were identified from previous literature using whole-mouth and filter paper stimulation methods and pilot tested by our research team. The final concentrations of the stimuli were determined to elicit similar levels of sensation intensity when delivered using our tasting protocol.
Solutions were made as stock concentrations and kept up to three weeks. Cotton swabs were saturated in stock solution and dried, cotton end up, with the wooden shaft pressed into blocks of florist’s foam. Swabs impregnated with solutions in ethanol were dried for three hours, an amount of time deemed appropriate for all of the ethanol to evaporate from the swabs, and solutions in water were allowed to dry for 10 hours. All concentrations above are nominal. As in Green and Hayes (2003), we did not control for differing rates of volatility across stimuli; nonethless, the relative concentrations should be roughly stable across participants as swabs were produced strictly following the same protocol each time. Swabs were tagged with three-digit blinding codes and stored in plastic zip-top bags for up to one week.
2.4. Procedure
Stimuli were presented, cotton end down, in glass culture test tubes. Each tube had two swabs in the tube and each stimulus had its own tube (11 test tubes total; see Supplemental materials for photograph). The presentation order was counterbalanced across participants to reduce any systematic influence of cross- or self-sensitization. Participants were instructed to pump 10 mL RO water (held at 35C) into a medicine cup and then hold the swab in the water for three seconds, or until the swab was fully hydrated. They were instructed to roll the swab across their tongue three times, making sure to cross the midline, and then to rub the swab against the roof of their mouth three times. They then breathed in through their mouth three times, allowing air to pass over their tongue, and touched the tip of their tongue to the roof of their mouth three times. Participants were instructed not to rinse with water (RO water at 35C) until after they had placed the sample into the group they deemed appropriate. During the three-minute interstimulus interval, participants rinsed ad libitum (at least twice) until no lingering sensation was perceived. This interval was determined in pilot testing to be sufficient to allow sensations to fully dissipate. Retasting was allowed, however panelists were instructed that any retasting must be done in the same manner as the initial tasting, using a fresh swab, new medicine cup, and new water for each stimulus. They were also instructed that they must rinse with water and allow at least three minutes to pass between tasting samples, moving on to the next sample only when they did not feel that there was any lingering sensation. All samples and rinse water were expectorated.
As placeholders, participants used poker chips labeled with three-digit codes corresponding to the codes labeling the swabs to make their groupings. They were instructed to arrange the samples into groups based on the perceived similarities and dissimilarities such that two samples that were similar were in the same group and two samples that were dissimilar were in two different groups. Participants were told to form as many groups as they felt were appropriate with the only restrictions being that they must form between two and ten groups (number of stimuli – 1), so that there was not one large group nor was each sample in an individual group of one. Participants were instructed to focus only on the sensation elicited by the stimulus and not any physical similarities or differences in the swabs or blinding code tags. Beyond this, the specific criteria on which these groupings were formed were left up to the participants. At the beginning of the protocol participants were told that they did not need to assign names to the groups that they formed during sorting. After they tasted all of the samples and decided on a final configuration, participants entered their groupings into a web-based card-sorting program (Websort, UXPunk, Chicago, IL, USA, subsequently purchased by Optimal Workshop, Wellington, New Zealand and renamed OptimalSort). After the sorting task was complete participants were asked to provide a description of each group to gain insight into how they discriminated the groups that they had formed from one another. In the description task, participants were told that they were able to use whatever attributes they felt necessary to differentiate between groups with the exception that two groups could not have identical descriptions.
In addition to the stimuli, participants were provided a notepad and pen to keep notes if they desired. They also received a sheet with the sampling directions outlined and a list of possible descriptors. Participants were reminded that this list was merely a starting point and was not a comprehensive list. They were instructed to not use the words if they did not feel that the words were appropriate to describe the sensation that they felt. The list of words included anesthetizing, astringent, biting, bitter, burning, buzzing, cooling, drying, hot, irritating, itching, metallic, numbing, pricking, puckering, salty, sharp, sour, spicy, stinging, sweet, swelling, tickling, tingling, umami/savory, and warming. No definitions were provided. These words were chosen as a compilation of words used in previous research working with chemesthetic agents (Albin and Simons 2010; Bennett and Hayes 2012; Cliff and Heymann 1992) with the prototypical tastes added in. The list was presented in alphabetical order.
After the participants completed the sorting task, which took roughly an hour, they completed an online personality questionnaire consisting of Arnett’s Inventory of Sensation Seeking (Arnett 1994) and the Food Involvement Scale (Bell and Marshall 2003); These results are reported elsewhere.
2.5. Data Analysis
Multidimensional scaling (MDS) was performed on dissimilarity matrices using The R Statistics Package (R Foundation for Statistical Computing). In R, we used the smacof library for MDS, the agnes function in the cluster library for cluster analysis, and the FactoMineR (Husson et al. 2007) library to calculate normalized RV coefficients (see below).
For individual participants, data from the free sorting task was put into a binary (0/1) matrix indicating whether two stimuli were grouped together or not. These values were summed across all participants and converted into a triangular similarity matrix showing counts of times that samples were paired together across all participants. By subtracting the triangular similarity matrix from the number of assessors in that group, this similarity matrix was converted to a dissimilarity matrix and submitted to MDS. The MDS procedure created iterations of a perceptual map given the submitted data and at each step a regression was applied to the perceptual map solution using Kruskal’s algorithm (Kruskal 1964). This regression generated a stress value, measuring the quality of fit of the model.
To determine the number of dimensions to be used in the multivariate configurations, we used a Scree plot with Kruskal’s stress values shown as a function of the number of dimensions in the respective MDS solution. As the number of dimensions increases, the Kruskal’s stress values decrease. The appropriate number of dimensions for the MDS solution was chosen as the point when the increase in dimensionality did not provide a meaningful decrease in stress, known as the “elbow” of the Scree plot. Additional dimensions were considered if they provided significant aid in the interpretation of the configuration. Generally, a stress level below 0.1 is considered an acceptable model fit (Krzanowski and Marriott 1994). Using these criteria, two dimensions were determined to be most appropriate for the nose open group (stress = 0.017) and three dimensions were determined to be most appropriate for the nose pinched group (stress = 0.002)
The RV coefficient (Robert and Escoufier 1976), a multivariate generalization of Pearson’s R2, is commonly used as a measure of similarity between the multivariate configurations. This coefficient ranges from 0 to 1, with 1 being more highly correlated than 0. Here, we used the normalized RV coefficient (NRV) because the number of stimuli in a group and dimensions in the perceptual map can influence the RV coefficient (Nestrud and Lawless 2008). The NRV is interpreted similarly to a z-score, with a large score (>2) indicating significant similarity between the maps. The coeffRV function in FactoMineR also computes a p value for the comparison of maps.
In total, 24 distinct attributes were generated for the nose open group, and 35 distinct attributes were generated for the nose pinched cohort. Multiple regression was used to show the relationship between the generated attributes with the stimuli coordinates. The first step in this analysis is to generate a frequency matrix between the stimuli and the attributes that were generated during the descriptive task. The top six attributes for each stimulus were submitted to multiple regression. A linear model was used to predict the coordinates of each stimulus generated by MDS with the attribute frequency matrix. Regressing the stimulus coordinates on the attributes in this manner determines the placement of the attribute vectors in the final perceptual mapping configurations (Schiffman et al. 1981). Attributes with p-values less than 0.1 in regression were considered meaningful and were plotted on the perceptual map.
Agglomerative hierarchical cluster analysis was conducted on the two matrices. This type of cluster analysis begins with each observation in its own cluster and merges the clusters one-by-one based on proximity until only one large cluster containing all observations remains. Agglomerative hierarchical clustering was used as opposed to the k-means nonhierarchical clustering method, as the advantages to using the k-means method manifest in samples with large amounts of data, which we do not have here. In keeping with previously reported studies (e.g. (Faye et al. 2004), we used agglomerative hierarchical clustering methods with Ward’s minimum variance method as the linkage criteria in this study. To determine the appropriate number of clusters, a plot of amalgamation distance versus joining order was used. On this joining distance plot, large jumps in the amalgamation distance (similar to the elbow seen in the Scree plot) indicate items being joined in that step have increased dissimilarity as compared to previously joined items (See (Lawless 2013) for further explanation).
3. RESULTS
3.1. Nose open group
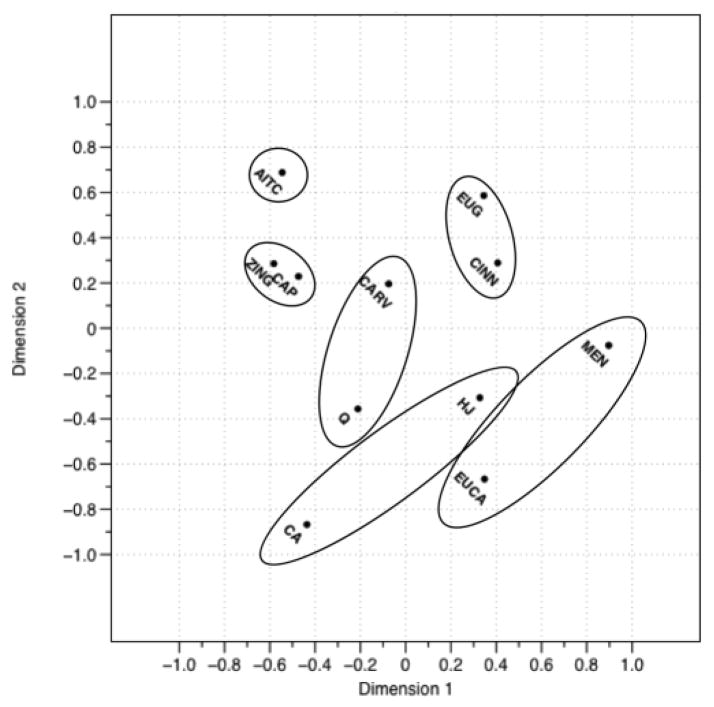
Figure 1 shows the MDS configuration for the participants who conducted the task without nose clips (nose open). Based on the Scree plot, a two-dimensional solution was appropriate for these data, as adding a third dimension did not significantly reduce the stress or add interpretability (2D stress=0.017).
Figure 1.
Perceptual map of 9 chemesthetic compounds and 2 tastants sorted in a free sorting task by participants not wearing nose clips (N=30), with descriptors projected onto the map via regression. Stimuli include allyl isothiocyanate (AITC), capsaicin (CAP), carvacrol (CARV), cinnamaldehyde (CINN), citric acid (CA), eucalyptol (EUCA), eugenol (EUG), huajiao (HJ), menthol (MEN), quinine (Q), and zingerone (ZING).
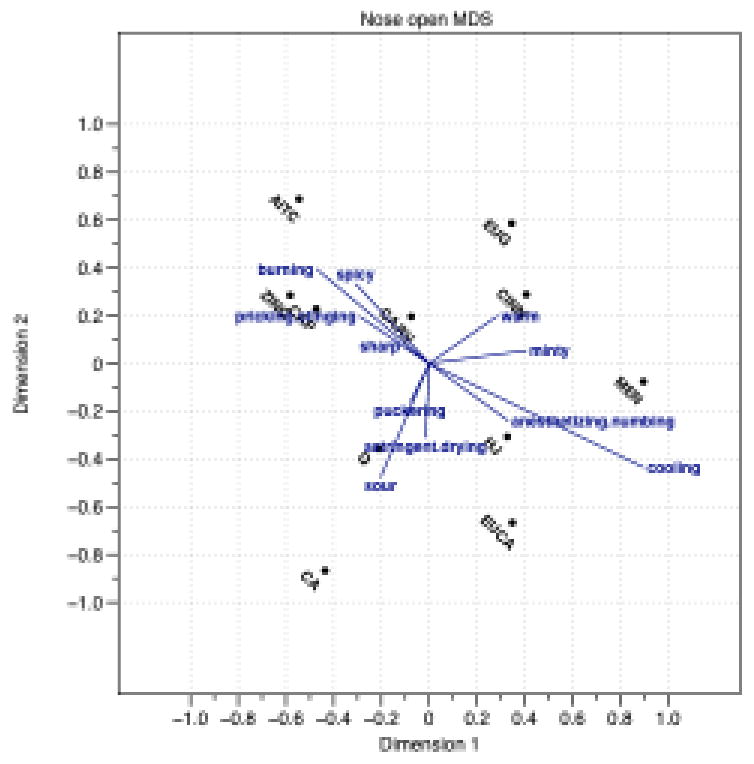
The nose open group of participants generated 24 unique attributes. Significant attributes were anesthetizing/numbing, astringent/drying, burning, cooling, minty, pricking/stinging, puckering, sharp, sour, spicy, and warm. As shown in Figure 1, there are two roughly orthogonal axes on this plot. The first axis opposes burning and cooling, with the attributes burning, spicy, and pricking/stinging at one end, and the attributes cooling and anesthetizing/numbing at the other end. The second is an opposing puckering and warming axis, with the attributes puckering, sour, and astringent/drying at one end and warm at the other end. Minty seems to fall between the warm and the anesthetizing/numbing axes in this configuration.
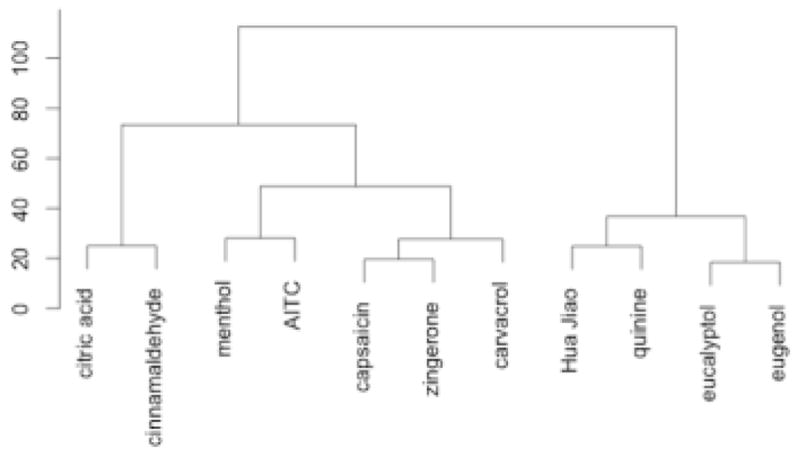
Cluster analysis of sorting data produced six clusters (Figures 2 and 3). In Figure 2, allyl isothiocyanate and the zingerone-capsaicin cluster are along the burning axis and the eucalyptol-menthol group is at the cooling end of this axis. The eugenol-cinnamaldehyde, citric acid-huajiao, and quinine-carvacrol groups fell between these poles. With regard to the second axis, the eugenol-cinnamaldehyde group fell near the warming pole with the citric acid-huajiao group near the puckering pole. The agglomerative coefficient for this configuration is 0.83, indicating a strong clustering structure, as shown in Figure 3.
Figure 2.
Same as Figure 1 (map of 11 stimuli from sorting by 30 participants not wearing nose clips), but with clusters generated via agglomerative hierarchical cluster analysis (agglomerative coefficient = 0.83). Stimuli use the same abbreviations as Figure 1.
Figure 3.
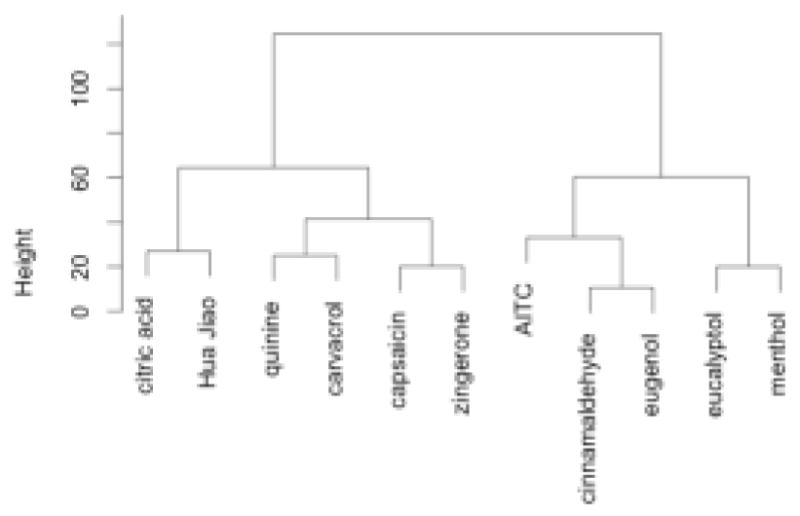
Dendrogram from agglomerative hierarchical clustering of the sorting done by 30 participants not wearing nose clips. Agglomerative coefficient is 0.83.
3.2. Nose pinched group
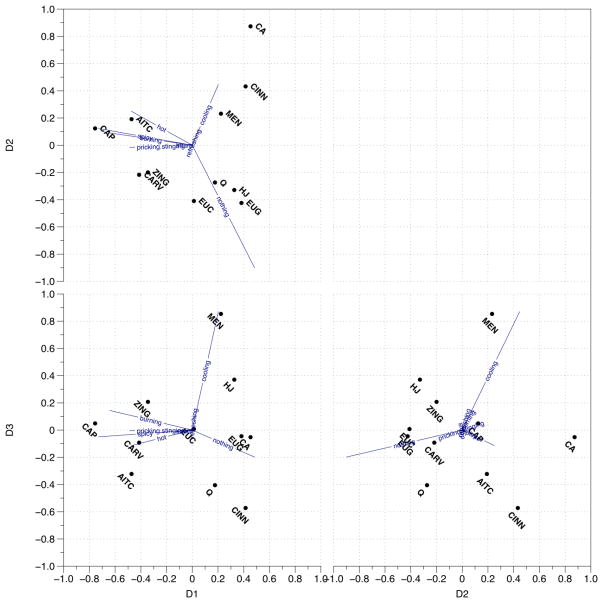
Based on the Scree plot generated from MDS on the data generated by the nose pinched group (N=31), a three-dimensional solution was appropriate for these data. Kruskal’s stress for a two-dimensional solution is 0.018, an acceptable value, however, adding a third dimension added to the interpretability of the data and reduced the stress further (stress = 0.002).
The nose pinched group generated 35 unique attributes for this task. The significant attributes (p<0.1) were burning, cooling, hot, minty, nothing, pricking/stinging, refreshing, sharp, and spicy. In the three-dimensional solution, there are three main attribute vectors. See Figure 4 for a three dimensional representation of the space in Natta notation with clusters indicated over the MDS plot and Figure 5 for an expanded view of each dimension with attribute vectors plotted over the MDS plot. The first attribute vector, burning, points slightly positive on dimensions 2 and 3 and runs parallel to dimension 1. This general vector is made up of the attributes burning, hot, pricking/stinging, and spicy. The second dimension is represented as cooling and is made up of cooling and refreshing. This attribute vector points toward positive values on dimensions 1, 2, and 3. The final vector, ‘nothing’, points towards positive values on dimension 1 and negative values with respect to dimensions 2 and 3.
Figure 4.
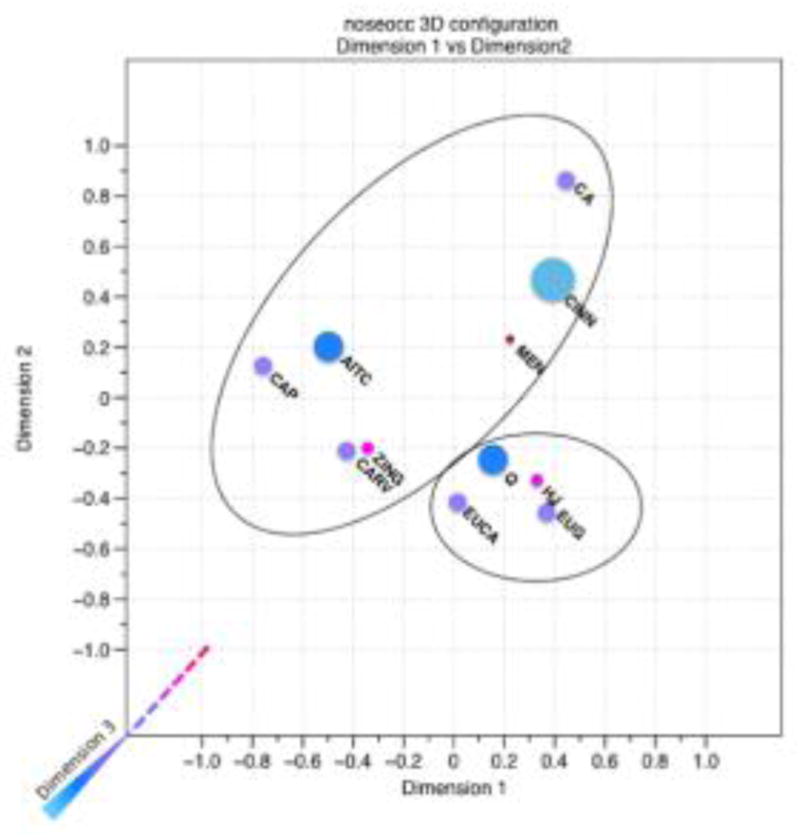
Perceptual map with clusters generated by the participants that completed the free sorting task on 11 compounds with nose clips (N=31). A three-dimensional solution was most appropriate (stress = 0.002) for this group. Notation is in the style of the Natta projection: Dimension 3 in the bottom left of the figure with the dotted line represents values farther away from the viewer (negative values on dimension 3) and the bolded line indicating that the plane is closer to the viewer (positive values on dimension 3). The positions of points with respect to dimension 3 are indicated by the size and color of the point. Larger, lighter blue points, (e.g. CINN), are closest to the viewer, while smaller, redder points, (e.g. MEN), are farthest from the viewer.
Figure 5.
Two-dimensional scatterplot matrix of the perceptual map generated by the nose-pinched cohort with significant attribute vectors plotted over MDS configuration.
Cluster analysis on the group data for the nose pinched condition resulted in two distinct groups. The first of the two groups consisted of carvacrol, zingerone, capsaicin, citric acid, cinnamaldehyde, menthol, and allyl isothiocyanate. The second group was made up of quinine, huajiao, eugenol, and eucalyptol. The agglomerative coefficient for this cluster analysis was 0.79, suggesting that the clustering structure was less well defined than the clusters in the nose open group.
3.3. Normalized RV coefficient
The Normalized RV coefficient (NRV) was used as a measure of similarity between the perceptual maps (MDS plots). The NRV between the two-dimensional “nose open” map and the three-dimensional “nose pinched” maps was 0.933. In this test, a significant p value of less than 0.05 indicates ‘significant similarity’, so the observed p value of 0.172 indicates the two maps are significantly different from one another.
4. DISCUSSION
Chemesthetic stimuli are particularly difficult to work with due to long decay times, the highly fatiguing nature of these compounds, and the possibility of sensitization and desensitization. This work suggests that with appropriate experimental considerations, free sorting can be successfully conducted with a number of chemesthetic stimuli in a reasonable amount of time. The results presented here also show that participants can attend to the task and can reliably differentiate between a number of chemesthetic stimuli.
4.1. Design Considerations for Stimulus Delivery
There are a number of difficulties when working with chemesthetic agents. Cross- and self-sensitization and desensitization (Cliff and Green 1996; Dessirier et al. 2001; Green 1989; Green 1991; Green and Hayes 2003; Green and Hayes 2004; Jancso et al. 1977; Klein et al. 2013; Prescott 1999; Simons et al. 2003) is common, and differing temporal profiles for these compounds (e.g., different onset times and decay rates) make designing a single session study complex. As previous work shows, stimulus concentration and the interstimulus interval can significantly influence whether sensitization or desensitization is observed, so additional precaution in working with chemesthetic compounds is necessary (Green 1989; Green 1991; Prescott and Stevenson 1995; Prescott and Stevenson 1996a; Simons et al. 2003).
To account for these difficulties, we delivered stimuli on swabs to multiple oral regions while limiting the total amount of stimulus in the mouth, and made the tasting protocol sufficiently long to allow for sensation onset prior to assessment. To ensure sufficient decay between samples, we used a minimum interstimulus interval of at least three minutes with rinsing ad libitum, even when retasting. The total sample number (11) was selected to allow participants to complete the entire task in a single one-hour session. In an effort to maximize the number of chemesthetic stimuli that were assessed and ensure that stimuli that spanned a range of various sensations were represented in the sample set, we included two prototypical tastants in the sample set. Based on previous literature suggesting that some individuals report bitter side tastes from capsaicin, we included quinine as one of the prototypical tastants (Green and Hayes 2003; Green and Hayes 2004). Pilot testing was used to choose concentrations that were sufficient to evoke sensations rated as “moderate” on a general Labeled Magnitude Scale. The concentrations used here were high enough to elicit a distinct sensation but low enough that the sensation typically dissipated within three minutes.
In addition to precautions taken to limit the area of stimulation and to maximize time between stimuli, the presentation order of the stimuli was counterbalanced across participants. With the interstimulus interval used here, it is possible that some degree of cross-desensitization was induced in participants, which could potentially alter the qualitative sensations of the stimuli, or more likely, serve to reduce the perceived intensities of the stimuli, which might alter the ability of these participants to determine differences between stimuli. That said, the resultant perceptual maps have face validity, suggesting our participants were have to distinguish between stimuli and describe them. Nonetheless, further research on the effect of desensitization on the outcome of a sorting task is warranted. When comparing the present results with stress values from sorting studies conducted using stimuli that do not sensitize or desensitize – words on cards (Bertino and Lawless 1993), plastic pieces (Faye et al. 2004), odors (Lawless 1989), and grape jellies (Tang and Heymann 2002) – we see lower stress in solutions from both the nose open and nose pinched cohorts (0.017 and 0.002, respectively). Collectively, the stress values and the fact that the stimuli differentiated across several axes indicate that participants in both cohorts were able to distinguish perceptual differences between the stimuli. Our results suggest that with an appropriately designed protocol, it is possible to conduct sorting and mapping with chemesthetic agents.
When participants had all of their senses available to them, they appeared to use chemesthetic qualities as their primary criteria for sorting. These groupings tended to follow a biological basis, in agreement with the suggestion that activation of common receptors should elicit similar perceptual qualities (Bennett and Hayes 2012; Green and Hayes 2004; McNamara et al. 2005; Tominaga et al. 1998). However when comparing the nose open and nose pinched groups, it is obvious that perception in the nasal cavity (either olfaction or nasal chemesthesis) was a significant factor as well, providing important information to participants in the event that the qualities were either reliant on nasal airflow (e.g. cooling in eucalyptol), were difficult to feel (e.g. numbing associated with eugenol), or potentially novel (e.g. buzzing associated with huajiao). The heavy reliance on aroma sensations to distinguish the chemesthetic compounds from one another may reflect the complex nature of these stimuli, or alternatively it may reflect that many people are not practiced in thinking about and describing these stimuli and thus, have a limited lexicon for oral chemesthesis.
4.2. Nose open
In total, the nose open group generated 24 unique attributes for the 11 chemesthetic stimuli. Of these, 11 attributes were significant and there were two opposing attribute vectors that described the sample space, the burning-cooling axis and the drying-numbing axis. For convenience, we refer to the ends of these axes as burning, cooling, drying, and warming, respectively, but in actuality, the perceptual space was described using a number of attributes that regression analysis showed were similar.
As would be expected, naïve assessors in the nose open group used burning, spicy, sharp, and pricking/stinging to describe sensations associated with allyl isothiocyanate, zingerone, and capsaicin, in contrast to eucalyptol and menthol, which were described as anesthetizing/numbing and cooling. The observation that terms like puckering, sour, and drying/astringent fell on similar vectors is not surprising, as organic acids are known to produce astringent sensations (Thomas and Lawless 1995). Notably, the descriptor warm was relatively isolated on the plot (lying closest to minty), but orthogonal to the burning vector. This finding potentially contradicts the assumption that warming is merely a less intense version of hot and burning, indicating instead that warming and hot/burning sensations are perceptually distinct.
In these participants, allyl isothiocyanate, capsaicin, and zingerone all fall in the burning region of the plot as might be expected, given that each of these compounds have previously been described as producing hot, burning, and stinging sensations (Caterina et al. 1997; Dessirier et al. 1998; Green 1991; Green and Shaffer 1993; Karrer and Bartoshuk 1991; Karrer and Bartoshuk 1995; Prescott et al. 1993; Prescott and Stevenson 1996a; Prescott and Stevenson 1996b). However, while these samples fall in the same region of the perceptual map, it is striking to note that in cluster analysis, allyl isothiocyanate only joins the capsaicin/zingerone cluster only at the highest level of the dendrogram. Previously, in both human behavioral and cell culture work, it has been suggested that compounds that elicit perceptually similar sensations would share common receptors, while compounds that elicit perceptually distinct sensations would elicit sensation via different mechanisms (Bennett and Hayes 2012; Green and Hayes 2004; McNamara et al. 2005; Tominaga et al. 1998). Given that capsaicin and zingerone activate the TRPV1 receptor (Caterina et al. 1997; Szallasi and Blumberg 1999; Tominaga et al. 1998; Vriens et al. 2009), it is expected that they might show perceptual similarities and that they would be grouped together. Historically, AITC has been thought to be exclusively a TRPA1 agonist (Bandell et al. 2004; Jordt et al. 2004; Story et al. 2003), though more recent data indicates that AITC may somehow sensitize TRPV1 through TRPA1 activation (Bautista et al. 2006), or that it may act directly on TRPV1 (Alpizar et al. 2013; Everaerts et al. 2011; Ohta et al. 2007). Even though the mechanism has not been definitively identified, a large body of work shows that there is some type of interaction between the TRPA1 and TRPV1 receptors (Alpizar et al. 2013; Bautista et al. 2006; Doerner et al. 2007; Eckert III et al. 2006; Fischer et al. 2014; García-Martínez et al. 2002; García-Sanz et al. 2004; Salas et al. 2009; Simons et al. 2003; Staruschenko et al. 2010; Zurborg et al. 2007). Considering the implicit (but often untested) assumption that activating common receptor leads to a common sensation, dual activation of TRPA1 and TRPV1 by AITC may explain the large distance on the dendrogram from the zingerone-capsaicin cluster and the close proximity on the perceptual map to this cluster of typical TRPV1 agonists.
At the opposite end of this first axis, near cooling and anesthetizing/numbing lays the menthol-eucalyptol cluster. Both menthol and eucalyptol activate TRPM8 and produce predominantly cooling sensations (McKemy et al. 2002; Peier et al. 2002a). While cooling is the sensation commonly associated with menthol, prior work paradoxically describes it as being warming (Green 1985; Hatem et al. 2006). Interestingly, millimolar concentrations of menthol have shown activation of TRPV3 (Karashima et al. 2007; Macpherson et al. 2006; Vogt-Eisele et al. 2007), a receptor that is implicated in the perception of warmth (Chung et al. 2014; Peier et al. 2002b; Xu et al. 2006). Another receptor that reportedly responds to menthol is TRPA1. Interestingly, menthol’s actions on this receptor show species-specific responses (Xiao et al. 2008). In murine TRPA1 receptors menthol activates the receptor at concentrations below 100uM but inhibits the channel above this concentration; conversely, for human TRPA1 menthol acts as an agonist at all concentrations tested (up to 1000uM). Although TRPA1 has been characterized as a cold receptor (Bautista et al. 2006; Karashima et al. 2007; Story et al. 2003), this channel’s role in cold sensing is debated (Bandell et al. 2007). Activation of human TRPM8, TRPA1, and TRPV3 by menthol might account for the placement of menthol at the approximate midpoint between the cooling and warming regions of the perceptual map, as activation of these receptors are presumably responsible for both cooling and warming sensations.
The fourth cluster, eugenol and cinnamaldehyde, associates with the warm region of the plot. Previously, eugenol has been described using a number of descriptors, including numbing, tingling, warming, burning, stinging, and pricking (Cliff and Heymann 1992; Green 2002; Klein et al. 2013; Wise et al. 2012). As with other chemesthetic agents, eugenol activates a number of TRP receptors in vitro in a concentration dependent manner, which may account for its diffuse perceptual nature. Among the receptors that are reportedly activated by eugenol are TRPA1, TRPV1, and TRPV3 (Bandell et al. 2004; Vogt-Eisele et al. 2007; Xu et al. 2006; Yang et al. 2003). In contrast, cinnamaldehyde is thought to be a strict TRPA1 agonist (Bandell et al. 2004; Bautista et al. 2006; Calixto et al. 2005; Karashima et al. 2007; Macpherson et al. 2006; Talavera et al. 2009; Xu et al. 2006). If one assumes the common receptor – common sensation assumption is at least partially correct, we would then expect eugenol and cinnamaldehyde to share perceptual space. Interestingly, other reported TRPA1 agonists like AITC and menthol do not share this space on the perceptual map. Additionally, we should note that cinnamaldehyde and eugenol shared qualitative descriptors referencing their associated spices use in baking applications. It seems likely that learned associations arising from frequent concurrent use of cinnamon and cloves in culinary applications may have influenced the grouping of these stimuli in addition to the sensations that these stimuli elicit.
The fifth and sixth clusters, huajiao and citric acid, and carvacrol and quinine, respectively, are more difficult to interpret. The huajiao/citric acid cluster is diffuse, with huajiao positioned along the anesthetizing/numbing, cooling axis and citric acid along the puckering, sour, astringent/drying axis. The placement of huajiao on the map and association with anesthetizing/numbing descriptions are expected, as this placement is consistent with prior reports that hydroxyl-alpha-sanshool (the compound primarily responsible for the sensation elicited by huajiao) and its derivatives elicit numbing and tingling sensations (Albin and Simons 2010; Bader et al. 2014; Galopin et al. 2004; Yang 2008; Yasuda et al. 1982). Similarly, it is unsurprising that citric acid was described as puckering and sour. On this basis, these two compounds would not seem to be similar enough to be grouped together, at least initially. However, it is possible that citrusy/lemon-like associations of these compounds likely resulted in participants grouping these stimuli. A key component in the aroma of huajiao is limonene, a compound that is also a key component in the aroma of orange juice (Jiang and Kubota 2004; Yang 2008). Indeed, huajiao was described as having a distinct lemon taste by some participants and citric acid is presumably associated with citrus aromas via learned associations. We should also note that our participants had some difficulty describing the sensation elicited by huajiao, generating 17 different descriptors to describe this sample. Additional work with isolated compounds (e.g. hydroxy-alpha-sanshool or synthetic analogues like isobutylalkenyl amide) is warranted.
The final cluster includes stimuli described as bitter: carvacrol and quinine. Both of these compounds were difficult for participants to characterize with any consensus, with 14 distinct attributes generated for carvacrol and 10 attributes for quinine. It is surprising that participants had such difficulty describing quinine, a prototypical bitterant, as participants were aware that they could use taste qualities as descriptors. Examining the dendrogram, this cluster’s closest neighbor is the capsaicin-zingerone cluster, indicating that some individuals may have also experienced bitterness from these two compounds, a finding that would align with previous work (Green and Hayes 2003; Green and Hayes 2004).
4.3. Nose pinched
Both to allow comparison to previous work (Cliff and Heymann 1992), and because a sufficient number of individuals in the nose open cohort used descriptors referencing aromas, we conducted a second experiment where a new cohort of participants completed the sorting task while wearing nose clips. The clips served to occlude the nasal passages and minimize any ortho- or retronasal odors, or nasal chemesthesis that may influence the participants
For the nose pinched map, a three-dimensional solution was found to be most appropriate, as the third dimension greatly enhanced interpretability. Overall, there were 35 distinct attributes generated by this group. Significant attributes in regression included burning, hot, spicy, pricking/stinging, refreshing, cooling, and ‘nothing’. In general, there were three representative dimensions. The first vector was ‘burning’, consistent of the descriptors burning, spicy, hot, and pricking/stinging. The second was ‘cooling’, consisting of cooling and refreshing, and the third vector consisted of the attribute participants denoted as ‘nothing’. It is notable that the nose pinched cohort generated more attributes than the nose open cohort. A possible explanation for this may be that when individuals are less certain about the identity of the origin of the sensation (i.e., mustard or cinnamon), they are more inclined to use more terms to describe what they perceive. Indeed, more individuals in the nose open group attempted to identify the food associated with the compound (data not shown).
Agglomerative hierarchical clustering of the nose pinched cohort’s data indicated two large groups were present in the perceptual space. Crudely, these two clusters appear to map onto the participants ability or inability to readily identify a taste or chemesthetic sensation. The first cluster, with distinct perceptual qualities, contains citric acid, cinnamaldehyde, zingerone, capsaicin, carvacrol, menthol, and allyl isothiocyanate (AITC). This cluster encompasses over half of the plot, covering the regions described by the burning and cooling vectors. The second cluster includes huajiao, eugenol, eucalyptol, and quinine; this cluster falls on the region of the plot characterized by the descriptor ‘nothing’. In contrast to the nose open group, the nose pinched group had far fewer clusters. Also, it is notable that the stimuli put into the ‘nothing’ group by the nose pinched cohort are the same stimuli the participants in the nose open cohort tended to cluster together based on olfaction rather than chemesthesis. It seems possible that ‘nothing’ may not refer to the absence of sensation, as these concentrations were sufficient to elicit sensation in pilot testing, but rather that participants used ‘nothing’ to represent the lack of a clearly identifiable chemesthetic sensation. The dominant sensations associated with eugenol, huajiao, eucalyptol, and menthol are numbing and cooling, although menthol may produce additional, clearly identifiable burning sensation at the concentration used here. As Breslin et al 1993 (Breslin et al. 1993) suggests, there are some oral stimuli that require movement in order to produce an identifiable sensation. The lack of retronasal airflow in the nose pinched cohort may have made the numbing and cooling sensations of eugenol, eucalyptol, and huajiao difficult to identify in the nose pinched cohort, leading to the identification of these sensations as ‘nothing’. Accordingly, future work should carefully consider whether to use nose clips, depending on the question of interest. Additional work to explore the role of retronasal airflow in the perception of chemesthetic stimuli would also be beneficial.
Concerning some of the more volatile components, such as eugenol, cinnamaldehyde, eucalyptol, and AITC, where nasal perception may have played a role in the sorting task (in the nose open cohort), there were differences between descriptors generated by the nose open and nose pinched cohorts. In all cases where attributes describing nasal perception seemed to play a significant role in the sorting decisions of study participants, such as with eugenol, AITC, and cinnamaldehyde, these descriptors were absent in the nose pinched cohort. For stimuli such as capsaicin, where we expect no nasal input, there was no difference between the nose open and nose pinched cohorts. Interestingly, in the nose pinched cohort, cinnamaldehyde was not described as warming by any of the study participants. It may be possible that since there was a general tendency in the nose pinched cohort to use the term “hot” more often (7 individuals used the term in the nose open cohort while 37 individuals used the term in the nose pinched cohort), that the “warming” description was interpreted as “hot” by these individuals, but this finding merits further investigation.
4.4. Summary, Limitations and Conclusions
Overall, the results presented here do not support or refute the common assumption that compounds which activate common receptors will elicit common perceptual sensations (Bennett and Hayes 2012; Green and Hayes 2004; McNamara et al. 2005; Tominaga et al. 1998). While we were not specifically attempting to test this assumption here, it provides a potentially useful framework to consider the perceptual maps generated here. The grouping of stimuli by the nose open cohort generally followed the presumed biological basis, but many participants appeared to use aromas as a factor in some of their groupings. These results may reflect the complexity of chemesthetic stimuli, which often elicit multiple sensations, or the complexity of the sensations themselves and the limited vocabulary that most people have to describe these percepts.
While the idea that common receptors lead to common sensations is intuitively appealing, widespread, and often unchallenged, this assumption, whether implicit or explicit, may be overly reductionist. That is, because neurons may express more than one receptor type, and because neurons converge as they project centrally, a simple labeled line model may not be appropriate for chemesthesis. Moreover, many chemesthetic agents activate diverse receptors in cell culture, so it is also important to keep in mind several caveats when interpreting human behavioral data in the context of cell-based evidence. Foremost is the potential for different levels of expression and differing sensitivity of receptors across species or cell preparations (Bandell et al. 2007; Klein et al. 2011; Riera et al. 2009). Also, receptor proteins may form heteromeric dimers and tetramers in native systems, so data from the cell culture work should be interpreted cautiously to avoid reductionism, as heteromers show altered functionality that cannot be replicated in heterologously expressed systems (Cheng et al. 2012; Fischer et al. 2014). Critically, data on a) species specific differences in TRP channel responses, and b) differences between the peripheral and central nervous systems are lacking, and additional multidisciplinary research is needed to enhance our understanding of chemesthesis. Accordingly, while in vitro work in cultured cells is critical to elucidate molecular mechanisms, behavioral work is also required as it provides additional insight into how perception relates to these molecular mechanisms within the whole organism.
While substantial efforts were taken to minimize the occurrence of desensitization or sensitization within or across stimuli, our protocol did not specifically test for these phenomena. In pilot testing by our team, three minutes was appropriate for all sensation to dissipate, without any obvious influences on the sensation from the next stimulus; however, there are differences in individual sensitivity to these compounds, as well as individual differences in the onset and offset of desensitization or sensitization. Thus, we cannot say with certainty that desensitization did not occur here. More work exploring the extent of desensitization or sensitization in a stimulus delivery protocol such as that presented here is warranted. Nonetheless, even if some degree of desensitization or sensitization was present in our participants, which would presumably, dampen the differences between stimuli, the core goal of this study – to explore perceptual similarities and differences of chemesthetic compounds in naïve assessors – was still successful.
Here, considerable efforts were taken to ensure that the delivery system and sampling method of the stimuli provided adequate stimulation that was consistent across all participants while limiting potential carryover; that said, it is possible that there was variation stimulus concentration that was not accounted for. Given manufacturing tolerances on the amount of cotton on each swab, some swabs may have absorbed different amounts of water, which would influence the concentration of the stimulus being delivered. Also, because we did not control for differing rates of volatility across stimuli, the delivered concentration may not exactly match that of the stock solution. As participants followed a strict sampling protocol under direct observation of the experimenter, and the variation in swab volume was minimal, we do not anticipate these factors would significantly alter the outcome of the experiment.
While we did observe a few instances where olfaction and learned associations appeared to influence the participants’ sorting decisions (i.e. the pairing of eugenol, in cloves, and cinnamaldehyde, in cinnamon, or the pairing of huajiao with citric acid), the perceptual map from the nose open cohort generally aligned with the putative biological mechanisms believed to underlie these sensations. In the nose pinched cohort only two clusters appeared, indicating that the map did not relate as easily to a simple biological interpretation. There are a number of factors which suggest wearing a nose clip made it more difficult for participants to evaluate the stimuli, making the task more difficult to complete. These include the higher variance in the nose pinched cohort, a lower level of consensus regarding attributes, and the presence of only two clusters. It seems likely that with fewer sensory modalities to draw upon for information about the stimuli, it was more difficult for participants to differentiate between them, resulting in fewer groups being formed. It is also possible that due to fewer recognizable differences between stimuli in the nose pinched condition, the memory load of the task was more difficult as compared to the nose open condition. Prior literature suggests a key difference between naïve assessors and trained or expert assessors may be the enhanced memory of trained or expert assessors (Chollet et al. 2005; Nestrud and Lawless 2010; Parr et al. 2004). Here, we provided both cohorts of naïve (non-expert) assessors with a comprehensive list of terms at the onset of the protocol to ensure that all participants were starting from the same point. While we do not anticipate that this had a significant effect, future work comparing the outcome of sorting with chemesthetic stimuli in the absence of such a list may be worthwhile.
Overall, this study suggests free sorting procedures can be successfully completed using chemesthetic agents with naïve participants with all senses available to them. In designing this study, we took into account a number of considerations to reduce the potential for cross- or self-sensitization and desensitization to occur while still evoking sufficiently intense chemesthetic sensations. These design considerations include the delivery method, concentrations, tasting method, and interstimulus interval; nonetheless, we cannot rule out that participants in this study were not in a partially desensitized state given repeated exposure to chemesthetic stimuli. Compared to working with tastants and odorants, the number of stimuli that can be used for a sorting task is significantly lower when working with chemesthetic agents, but it remains feasible. We also demonstrate that naïve participants without prior training can consistently differentiate between the perceptual qualities of different chemesthetic agents.
Supplementary Material
Supplemental Figure 1. Setup used for the sorting task for both cohorts.
Figure 6.
Dendrogram from agglomerative hierarchical clustering of sorting done by 31 participants with nose clips. Agglomerative coefficient is 0.79.
Acknowledgments
This manuscript was prepared in partial fulfillment of a Doctor of Philosophy degree by the first author. The authors of this manuscript would like to thank Dr. Christopher Simons for his donation of a huajiao extract to be used in the experiment, [REDACTED FOR REVIEW] for assistance with data collection, and all of our participants for their time and involvement in the study.
FUNDING
This work was supported by funds from [REDACTED FOR REVIEW] University, United States Department of Agriculture [REDACTED FOR REVIEW] funds and a National Institutes of Health grant from the National Institute National of Deafness and Communication Disorders [REDACTED FOR REVIEW].
Footnotes
CONFLICT OF INTEREST
The lead author declares no conflict of interest. The second author is presently employed by [REDACTED FOR REVIEW], who has no other interest in this work and was not involved in the conception or design of the study or the decision to publish. The corresponding author has previously accepted speaking and/or consulting fees from [REDACTED FOR REVIEW] for unrelated work. The corresponding author has also served on the [REDACTED FOR REVIEW] advisory board. None of these organizations have had any influence over study conception, design or interpretation, or the decision to publish these data.
References
- Albin KC, Simons CT. Psychophysical evaluation of a sanshool derivative (alkylamide) and the elucidation of mechanisms subserving tingle. PLoS One. 2010;5:e9520. doi: 10.1371/journal.pone.0009520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpizar YA, Boonen B, Gees M, Sanchez A, Nilius B, Voets T, Talavera K. Allyl isothiocyanate sensitizes TRPV1 to heat stimulation. Pflügers Archiv-European Journal of Physiology. 2013:1–9. doi: 10.1007/s00424-013-1334-9. [DOI] [PubMed] [Google Scholar]
- Arnett J. Sensation seeking: a new conceptualization and a new scale. Personality and Individual Differences. 1994;16:7. [Google Scholar]
- Bader M, Stark TD, Dawid C, Lösch S, Hofmann T. All-trans-Configuration in Zanthoxylum Alkylamides Swaps the Tingling with a Numbing Sensation and Diminishes Salivation. Journal of agricultural and food chemistry. 2014;62:2479–2488. doi: 10.1021/jf500399w. [DOI] [PubMed] [Google Scholar]
- Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Current opinion in neurobiology. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bell R, Marshall DW. The construct of food involvement in behavioral research: scale development and validation. Appetite. 2003;40:235–244. doi: 10.1016/s0195-6663(03)00009-6. [DOI] [PubMed] [Google Scholar]
- Bennett SM, Hayes JE. Differences in the chemesthetic subqualities of capsaicin, ibuprofen, and olive oil. Chem Senses. 2012;37:471–478. doi: 10.1093/chemse/bjr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertino M, Lawless HT. Understanding mouthfeel attributes: a multidimensional scaling approach. Journal of Sensory Studies. 1993;8:101–114. [Google Scholar]
- Blancher G, Clavier B, Egoroff C, Duineveld K, Parcon J. A method to investigate the stability of a sorting map. Food Quality and Preference. 2012;23:36–43. [Google Scholar]
- Breslin P, Gilmore M, Beauchamp G, Green B. Psychophysical evidence that oral astringency is a tactile sensation. Chem Senses. 1993;18:405–417. [Google Scholar]
- Bryant BP, Mezine I. Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res. 1999;842:452–460. doi: 10.1016/s0006-8993(99)01878-8. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Kassuya CA, André E, Ferreira J. Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacology & therapeutics. 2005;106:179–208. doi: 10.1016/j.pharmthera.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Cartier R, Rytz A, Lecomte A, Poblete F, Krystlik J, Belin E, Martin N. Sorting procedure as an alternative to quantitative descriptive analysis to obtain a product sensory map. Food Quality and Preference. 2006;17:562–571. [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cheng W, et al. Heteromeric heat-sensitive transient receptor potential channels exhibit distinct temperature and chemical response. Journal of Biological Chemistry. 2012;287:7279–7288. doi: 10.1074/jbc.M111.305045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet S, Lelièvre M, Abdi H, Valentin D. Sort and Beer: Everything you wanted to know about the sorting task but did not dare to ask. Food Quality and Preference. 2011;22:507–520. [Google Scholar]
- Chollet S, Valentin D. Le degré d’expertise at-il une influence sur la perception olfactive? Quelques éléments de réponse dans le domaine du vin. L’année psychologique. 2000;100:11–36. [Google Scholar]
- Chollet S, Valentin D, Abdi H. Do trained assessors generalize their knowledge to new stimuli? Food Quality and Preference. 2005;16:13–23. [Google Scholar]
- Chung G, Im S, Kim Y, Jung S, Rhyu M-R, Oh S. Activation of transient receptor potential ankyrin 1 by eugenol. Neuroscience. 2014;261:153–160. doi: 10.1016/j.neuroscience.2013.12.047. [DOI] [PubMed] [Google Scholar]
- Cliff M, Heymann H. Descriptive analysis of oral pungency. Journal of Sensory Studies. 1992;7:12. [Google Scholar]
- Cliff MA, Green BG. Sensory irritation and coolness produced by menthol: evidence for selective desensitization of irritation. Physiol Behav. 1994;56:1021–1029. doi: 10.1016/0031-9384(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Cliff MA, Green BG. Sensitization and desensitization to capsaicin and menthol in the oral cavity: interactions and individual differences. Physiol Behav. 1996;59:487–494. doi: 10.1016/0031-9384(95)02089-6. [DOI] [PubMed] [Google Scholar]
- Dessirier J-M, O’Mahony M, Carstens E. Oral irritant properties of menthol: sensitizing and desensitizing effects of repeated application and cross-desensitization to nicotine. Physiol Behav. 2001;73:25–36. doi: 10.1016/s0031-9384(01)00431-0. [DOI] [PubMed] [Google Scholar]
- Dessirier J-M, O’Mahony M, Sieffermann J-M, Carstens E. Mecamylamine inhibits nicotine but not capsaicin irritation on the tongue: psychophysical evidence that nicotine and capsaicin activate separate molecular receptors. Neuroscience letters. 1998;240:65–68. doi: 10.1016/s0304-3940(97)00930-0. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. Journal of biological chemistry. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Eckert W, III, Julius D, Basbaum A. Differential contribution of TRPV1 to thermal responses and tissue injury-induced sensitization of dorsal horn neurons in laminae I and V in the mouse. Pain. 2006;126:184–197. doi: 10.1016/j.pain.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Everaerts W, et al. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Current Biology. 2011;21:316–321. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Falahee M, MacRae A. Perceptual variation among drinking waters: the reliability of sorting and ranking data for multidimensional scaling. Food Quality and Preference. 1997;8:389–394. [Google Scholar]
- Faye P, Brémaud D, Durand Daubin M, Courcoux P, Giboreau A, Nicod H. Perceptive free sorting and verbalization tasks with naive subjects: an alternative to descriptive mappings. Food Quality and Preference. 2004;15:781–791. [Google Scholar]
- Faye P, Brémaud D, Teillet E, Courcoux P, Giboreau A, Nicod H. An alternative to external preference mapping based on consumer perceptive mapping. Food Quality and Preference. 2006;17:604–614. [Google Scholar]
- Fischer MJ, Balasuriya D, Jeggle P, Goetze TA, McNaughton PA, Reeh PW, Edwardson JM. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflügers Archiv-European Journal of Physiology. 2014:1–13. doi: 10.1007/s00424-014-1497-z. [DOI] [PubMed] [Google Scholar]
- Galopin CC, Furrer SM, Goeke A. Pungent and tingling compounds in Asian cuisine. Challenges in Taste Chemistry and Biology. 2004;867:139–152. [Google Scholar]
- García-Martínez C, et al. Attenuation of thermal nociception and hyperalgesia by VR1 blockers. Proceedings of the National Academy of Sciences. 2002;99:2374–2379. doi: 10.1073/pnas.022285899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sanz N, Fernández-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sánchez E, Fernández-Ballester G, Ferrer-Montiel A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. The Journal of neuroscience. 2004;24:5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan V. Pungency: the stimuli and their evaluation [Food flavour]. ACS Symposium series American Chemical Society; 1979. [Google Scholar]
- Green B. Psychophysical measurement of oral chemesthesis. Methods in chemosensory research. 2002:3–19. [Google Scholar]
- Green BG. Menthol modulates oral sensations of warmth and cold. Physiol Behav. 1985;35:427–434. doi: 10.1016/0031-9384(85)90319-1. [DOI] [PubMed] [Google Scholar]
- Green BG. Capsaicin sensitization and desensitization on the tongue produced by brief exposures to a low concentration. Neuroscience letters. 1989;107:173–178. doi: 10.1016/0304-3940(89)90812-4. [DOI] [PubMed] [Google Scholar]
- Green BG. Capsaicin cross-desensitization on the tongue: psychophysical evidence that oral chemical irritation is mediated by more than one sensory pathway. Chem Senses. 1991;16:675–689. [Google Scholar]
- Green BG, Hayes JE. Capsaicin as a probe of the relationship between bitter taste and chemesthesis. Physiol Behav. 2003;79:811–821. doi: 10.1016/s0031-9384(03)00213-0. [DOI] [PubMed] [Google Scholar]
- Green BG, Hayes JE. Individual differences in perception of bitterness from capsaicin, piperine and zingerone. Chem Senses. 2004;29:53–60. doi: 10.1093/chemse/bjh005. [DOI] [PubMed] [Google Scholar]
- Green BG, Rentmeister-Bryant H. Temporal characteristics of capsaicin desensitization and stimulus-induced recovery in the oral cavity. Physiol Behav. 1998;65:141–149. doi: 10.1016/s0031-9384(98)00162-0. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS. The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain. 1993;53:323–334. doi: 10.1016/0304-3959(93)90228-H. [DOI] [PubMed] [Google Scholar]
- Gwartney E, Heymann H. The Temporal Perception Of Menthol. Journal Of Sensory Studies. 1995;10:393–400. [Google Scholar]
- Hatem S, Attal N, Willer J-C, Bouhassira D. Psychophysical study of the effects of topical application of menthol in healthy volunteers. Pain. 2006;122:190–196. doi: 10.1016/j.pain.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Holliins M, Faldowski R, Rao S, Young F. Perceptual dimensions of tactile surface texture: A multidimensional scaling analysis. Perception & Psychophysics. 1993;54:697–705. doi: 10.3758/bf03211795. [DOI] [PubMed] [Google Scholar]
- Husson F, Lê S, Mazet J. R package version 1.05. 2007. FactoMineR: Factor Analysis and Data Mining with R. [Google Scholar]
- Jancso G, Kiraly E, Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Jiang L, Kubota K. Differences in the volatile components and their odor characteristics of green and ripe fruits and dried pericarp of Japanese pepper (Xanthoxylum piperitum DC.) Journal of agricultural and food chemistry. 2004;52:4197–4203. doi: 10.1021/jf030663a. [DOI] [PubMed] [Google Scholar]
- Jordt S-E, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. The Journal of Neuroscience. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer T, Bartoshuk L. Capsaicin desensitization and recovery on the human tongue. Physiol Behav. 1991;49:757–764. doi: 10.1016/0031-9384(91)90315-f. [DOI] [PubMed] [Google Scholar]
- Karrer T, Bartoshuk L. Effects of capsaicin desensitization on taste in humans. Physiol Behav. 1995;57:421–429. doi: 10.1016/0031-9384(94)e0076-g. [DOI] [PubMed] [Google Scholar]
- Klein AH, Carstens MI, Carstens E. Eugenol and carvacrol induce temporally desensitizing patterns of oral irritation and enhance innocuous warmth and noxious heat sensation on the tongue. Pain. 2013 doi: 10.1016/j.pain.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, et al. A tingling sanshool derivative excites primary sensory neurons and elicits nocifensive behavior in rats. Journal of Neurophysiology. 2011;105:1701–1710. doi: 10.1152/jn.00922.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal JB. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika. 1964;29:1–27. [Google Scholar]
- Krzanowski W, Marriott F. Multivariate Analysis: Part 1: Distributions, Ordination and Inference. 1994. Kendall’s Library of Statistics: No 1. [Google Scholar]
- Lawless H. Quantitative Sensory Analysis: Psychophysics, Models and Intelligent Design. 1. John Wiley & Sons, Ltd; 2013. Segmentation; pp. 323–339. [Google Scholar]
- Lawless H, Horne J. Category Reviews and Multidimensional Scaling. Cornell University; 2000. [Google Scholar]
- Lawless HT. Exploration of fragrance categories and ambiguous odors using multidimensional scaling and cluster analysis. Chem Senses. 1989;14:349–360. doi: 10.1093/chemse/bji064. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Glatter S. Consistency of multidimensional scaling models derived from odor sorting. Journal of Sensory Studies. 1990;5:217–230. [Google Scholar]
- Lawless HT, Sheng N, Knoops SS. Multidimensional scaling of sorting data applied to cheese perception. Food Quality and Preference. 1995;6:91–98. [Google Scholar]
- Lawless HT, Stevens DA. Responses by humans to oral chemical irritants as a function of locus of stimulation. Perception & psychophysics. 1988;43:72–78. doi: 10.3758/bf03208975. [DOI] [PubMed] [Google Scholar]
- Lelièvre M, Chollet S, Abdi H, Valentin D. What is the validity of the sorting task for describing beers? A study using trained and untrained assessors. Food Quality and Preference. 2008;19:697–703. [Google Scholar]
- Lim J, Lawless HT. Qualitative Differences of Divalent Salts: Multidimensional Scaling and Cluster Analysis. Chem Senses. 2005;30:719–726. doi: 10.1093/chemse/bji064. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Molecular and Cellular Neuroscience. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- McDonald S, Barrett P, Bond L. What Kind of Hot Is It? Perfumer & flavorist. 2010;35:32–39. [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- McNamara FN, Randall A, Gunthorpe MJ. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1) British journal of pharmacology. 2005;144:781–790. doi: 10.1038/sj.bjp.0706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestrud MA, Lawless HT. Perceptual mapping of citrus juices using projective mapping and profiling data from culinary professionals and consumers. Food Quality and Preference. 2008;19:431–438. [Google Scholar]
- Nestrud MA, Lawless HT. Perceptual mapping of apples and cheeses using projective mapping and sorting. Journal of Sensory Studies. 2010;25:390–405. [Google Scholar]
- Ohta T, Imagawa T, Ito S. Novel agonistic action of mustard oil on recombinant and endogenous porcine transient receptor potential V1 (pTRPV1) channels. Biochemical pharmacology. 2007;73:1646–1656. doi: 10.1016/j.bcp.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Parr WV, White KG, Heatherbell DA. Exploring the nature of wine expertise: what underlies wine experts’ olfactory recognition memory advantage? Food quality and preference. 2004;15:411–420. [Google Scholar]
- Peier AM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002a;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Peier AM, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002b;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Popper R, Heymann H. Analyzing differences among products and panelists by multidimensional scaling. Data Handling in Science and Technology. 1996;16:159–184. [Google Scholar]
- Prescott J. The generalizability of capsaicin sensitization and desensitization. Physiol Behav. 1999;66:741–749. doi: 10.1016/s0031-9384(99)00012-8. [DOI] [PubMed] [Google Scholar]
- Prescott J, Allen S, Stephens L. Interactions between oral chemical irritation, taste and temperature. Chem Senses. 1993;18:389–404. [Google Scholar]
- Prescott J, Stevenson RJ. Effects of oral chemical irritation on tastes and flavors in frequent and infrequent users of chili. Physiol Behav. 1995;58:1117–1127. doi: 10.1016/0031-9384(95)02052-7. [DOI] [PubMed] [Google Scholar]
- Prescott J, Stevenson RJ. Desensitization to oral zingerone irritation: effects of stimulus parameters. Physiol Behav. 1996a;60:1473–1480. doi: 10.1016/s0031-9384(96)00248-x. [DOI] [PubMed] [Google Scholar]
- Prescott J, Stevenson RJ. Psychophysical responses to single and multiple presentations of the oral irritant zingerone: relationship to frequency of chili consumption. Physiol Behav. 1996b;60:617–624. doi: 10.1016/s0031-9384(96)80039-4. [DOI] [PubMed] [Google Scholar]
- Prescott J, Swain-Campbell N. Responses to Repeated Oral Irritation by Capsaicin, Cinnamaldehyde and Ethanol in PROP Tasters and Non-tasters. Chem Senses. 2000;25:239–246. doi: 10.1093/chemse/25.3.239. [DOI] [PubMed] [Google Scholar]
- Riera C, et al. Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. British journal of pharmacology. 2009;157:1398–1409. doi: 10.1111/j.1476-5381.2009.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert P, Escoufier Y. A unifying tool for linear multivariate statistical methods: the RV-coefficient. Applied statistics. 1976:257–265. [Google Scholar]
- Rosenberg S, Nelson C, Vivekananthan P. A multidimensional approach to the structure of personality impressions. Journal of personality and social psychology. 1968;9:283. doi: 10.1037/h0026086. [DOI] [PubMed] [Google Scholar]
- Rosenberg S, Park Kim M. The method of sorting as a data-gathering procedure in multivariate research. Multivariate Behavioral Research. 1975;10:489–502. doi: 10.1207/s15327906mbr1004_7. [DOI] [PubMed] [Google Scholar]
- Saint-Eve A, Paçi Kora E, Martin N. Impact of the olfactory quality and chemical complexity of the flavouring agent on the texture of low fat stirred yogurts assessed by three different sensory methodologies. Food Quality and Preference. 2004;15:655–668. [Google Scholar]
- Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. European Journal of Neuroscience. 2009;29:1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Erickson RP. A psychophysical model for gustatory quality. Physiol Behav. 1971;7:617–633. doi: 10.1016/0031-9384(71)90117-x. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Reynolds ML, Young FW, Carroll JD. Introduction to multidimensional scaling: Theory, methods, and applications. Academic Press; New York: 1981. [Google Scholar]
- Simons CT, Carstens MI, Carstens E. Oral irritation by mustard oil: self-desensitization and cross-desensitization with capsaicin. Chem Senses. 2003;28:459–465. doi: 10.1093/chemse/28.6.459. [DOI] [PubMed] [Google Scholar]
- Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. Journal of biological chemistry. 2010;285:15167–15177. doi: 10.1074/jbc.M110.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacological reviews. 1999;51:159–212. [PubMed] [Google Scholar]
- Talavera K, et al. Nicotine activates the chemosensory cation channel TRPA1. Nature neuroscience. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- Tang C, Heymann H. Multidimensional Sorting, Similarity Scaling And Free-Choice Profiling Of Grape Jellies. Journal of Sensory Studies. 2002;17:493–509. [Google Scholar]
- Thomas CJC, Lawless HT. Astringent subqualities in acids. Chem Senses. 1995;20:593–600. doi: 10.1093/chemse/20.6.593. [DOI] [PubMed] [Google Scholar]
- Todd P, Bensinger M, Biftu T. Determination of pungency due to capsicum by gas-liquid chromatography. Journal of Food Science. 1977;42:660–665. [Google Scholar]
- Tominaga M, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Torgerson WS. Multidimensional scaling: I. Theory and method. Psychometrika. 1952;17:401–419. doi: 10.1007/BF02289530. [DOI] [PubMed] [Google Scholar]
- Valentin D, Chollet S, Lelievre M, Abdi H. Quick and dirty but still pretty good: a review of new descriptive methods in food science. International Journal of Food Science & Technology. 2012;47:1563–1578. [Google Scholar]
- Vogt-Eisele A, Weber K, Sherkheli M, Vielhaber G, Panten J, Gisselmann G, Hatt H. Monoterpenoid agonists of TRPV3. British journal of pharmacology. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Molecular pharmacology. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- Wise PM, Wysocki CJ, Lundström JN. Stimulus selection for intranasal sensory isolation: eugenol is an irritant. Chem Senses. 2012;37:509–514. doi: 10.1093/chemse/bjs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. The Journal of Neuroscience. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nature neuroscience. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Yang B, et al. Activation of vanilloid receptor 1 (VR1) by eugenol. Journal of dental research. 2003;82:781–785. doi: 10.1177/154405910308201004. [DOI] [PubMed] [Google Scholar]
- Yang X. Aroma constituents and alkylamides of red and green Huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium) Journal of agricultural and food chemistry. 2008;56:1689–1696. doi: 10.1021/jf0728101. [DOI] [PubMed] [Google Scholar]
- Yasuda I, Takeya K, Itokawa H. Distribution of unsaturated aliphatic acid amides in japanese Zanthoxylum species. Phytochemistry. 1982;21:1295–1298. [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nature neuroscience. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Setup used for the sorting task for both cohorts.