Abstract
Objective
Persistent fibroblast activation underlies skin fibrosis in systemic sclerosis (SSc), but the transcriptional and epigenetic mechanisms controlling this process are not well understood. In view of the potent influence of acetylation status governing tissue fibrosis, we undertook this study to investigate the expression of the antiaging deacetylase enzyme sirtuin 1 (SIRT1) in SSc and its effects on fibrotic responses in vitro and in vivo.
Methods
Tissue expression of SIRTs was interrogated from publicly available genome-wide expression data sets and by immunohistochemistry. The effects of SIRT1 on modulating fibrotic responses, as well as the underlying mechanisms, were examined in human and mouse fibroblasts in culture and in an experimental fibrosis model in the mouse.
Results
Analysis of transcriptome data revealed a selective reduction of SIRT1 messenger RNA (mRNA) levels in SSc skin biopsy samples as well as a negative correlation of SIRT1 mRNA with the skin score. Cellular SIRT1 levels were suppressed in normal fibroblasts exposed to hypoxia or platelet-derived growth factor and were constitutively down-regulated in SSc fibroblasts. Activation of SIRT1 attenuated fibrotic responses in skin fibroblasts and skin organ cultures, while genetic or pharmacologic inhibition of SIRT1 had profibrotic effects. The antifibrotic effects of SIRT1 were due in part to decreased expression and function of the acetyltransferase p300. In mice, experimentally induced skin fibrosis was accompanied by reduced SIRT1 expression in lesional tissue fibroblasts, and both fibrosis and loss of SIRT1 in these mice were mitigated by treatment with a SIRT1 activator.
Conclusion
SIRT1 has antifibrotic effects, and its reduced tissue expression in patients with SSc might have a direct causal role in progression of fibrosis. Pharmacologic modulation of SIRT1 in these patients therefore might represent a potential treatment strategy.
Excessive extracellular matrix synthesis and accumulation in systemic sclerosis (SSc) is due to persistent activation of myofibroblasts triggered by transforming growth factor β (TGFβ) and platelet-derived growth factor (PDGF), as well as reactive oxygen species (ROS) and tissue hypoxia (1). TGFβ-induced transcription of fibrotic genes is critically dependent on histone acetylation catalyzed by acetyltransferases such as p300 (2–4). Several observations implicate p300-dependent histone and nonhistone protein acetylation in fibrosis (5). We had demonstrated significantly elevated lesional tissue p300 levels in experimental models of skin and pulmonary fibrosis in mice as well as in SSc skin biopsy samples and explanted fibroblasts (4,6). In cell-based assays, augmenting p300 expression or activity promotes histone H4 hyperacetylation and collagen transcription, whereas pharmacologic inhibition of p300 activity abrogates TGFβ-induced responses (4). These observations implicate p300 as a critical mediator of fibrosis, but the mechanisms governing p300 expression and activity are not well understood (5).
Sirtuins (SIRTs) are mammalian orthologs of yeast silent information regulator 2 (Sir2) linked to regulation of lifespan. The SIRTs function as class III histone deacetylases with pleiotropic effects on cell survival, cell cycle, metabolism, and processes of aging (7). While SIRT-mediated histone deacetylation is generally associated with transcriptional repression, SIRTs also target nonhistone proteins, including transcription factors and signaling molecules (8). In particular, SIRT1 has been shown to suppress both the expression and acetyltransferase function of p300 (9–11).
In view of the fundamental role that epigenetic and posttranslational mechanisms play in regulating the process of fibrosis, in the present studies we sought to investigate the expression and function of SIRT1 and its links with p300 in the context of SSc and fibrosis. The results show that SIRT1 expression was significantly reduced in SSc skin biopsy samples and explanted skin fibroblasts, as well as in lesional skin from mice with bleomycin-induced scleroderma. Pharmacologic activation of SIRT1, or its ectopic expression in normal fibroSblasts, abrogated TGFβ-induced stimulation of collagen synthesis and myofibroblast differentiation through disruption of canonical TGFβ/Smad signaling. Treatment of mice with a SIRT1 activator ameliorated bleomycin-induced skin fibrosis and reversed the suppression of SIRT1. Taken together, our results identify SIRT1 as a cell-autonomous negative regulator of the fibrotic process in skin fibroblasts, and they suggest that its deficiency in SSc may contribute to progression of skin fibrosis.
SUBJECTS AND METHODS
Human subjects
Skin biopsy samples were obtained from the dorsal forearm of 5 healthy adults and 10 patients with SSc. All patients fulfilled the American College of Rheumatology/European League Against Rheumatism 2013 classification criteria for SSc (12). Biopsies were performed after obtaining written informed consent and in compliance with the policies of the Institutional Review Boards for Human Studies (Boston University and Northwestern University).
Analysis of complementary DNA (cDNA) microarray data sets
To analyze levels of SIRT messenger RNA (mRNA) in SSc skin biopsy samples, we queried publicly available genome-wide expression microarray data sets (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE9285 and http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE45485) (13,14). Levels of mRNA in each biopsy sample were centered on their median values across all arrays.
Bleomycin-induced scleroderma in the mouse
Animal protocols were institutionally approved by the Animal Care and Use Committees of Northwestern University or Shanghai Fudan University. Six-to-eight week-old female C57BL/6J mice (The Jackson Laboratory or Sino-British Sippr/BK Lab Animal Ltd) were randomized to receive vehicle, bleomycin, or a combination of resveratrol and bleomycin. Mice were given daily subcutaneous injections of phosphate buffered saline (PBS) or 10 mg/kg bleomycin (Hospira) for 14 days (15). Resveratrol (Sigma) was dissolved in 80% PBS, 10% DMSO, and 10% Cremophor EL (Sigma) and administered by daily intraperitoneal (IP) injection (20 mg/kg/day) for 28 days. Mice were killed on day 28, and lesional skin was harvested.
Collagen accumulation in lesional skin was determined by measuring the hydroxyproline content in 6-mm skin biopsy samples (16). Results are expressed as total hydroxyproline content per mg tissue.
Fibroblast and skin organ cultures
Primary cultures of dermal fibroblasts were established by explantation from foreskins from healthy newborns or from skin biopsy samples from SSc patients or age-matched healthy controls (15). SIRT1−/− and wild-type mouse embryonic fibroblasts (MEFs) were a gift from Dr. Deyu Fang (Northwestern University). Cells were maintained at 37°C in an atmosphere of 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 1% vitamins, 1% penicillin/streptomycin, and 2 mM l-glutamine (all from BioWhittaker) and studied between passages 4 and 8. At early confluence, fibroblasts were placed in serum-free medium overnight and then incubated with resveratrol or the selective agonists SIRT1 activator 3 (SA3; Santa Cruz Biotechnology) and SRT1720 (Selleckchem) in the presence or absence of TGFβ2 (Pepro-Tech). In selected experiments, cultures were pretreated with the SIRT1 antagonist nicotinamide (Sigma) for 30 minutes prior to incubation with resveratrol. Cell toxicity was evaluated using lactate dehydrogenase cytotoxicity assay kits (BioVision), viability was evaluated by trypan blue dye exclusion, and cell proliferation was evaluated by bromodeoxyuridine assays (Bio-Vision). At the end of the incubation periods, culture media were harvested and levels of secreted collagen were determined by Sircol assays (Biocolor).
For experiments with skin organ cultures, fresh foreskin biopsy samples were cut into 0.5 × 0.5-cm pieces that were placed in 6-well plates and maintained in an air-medium interface with the epidermal layer exposed to air. Explants were then incubated in medium in the presence or absence of 10 ng/ml TGFβ2 and resveratrol (100 µM) for 7 days.
Gel contraction and cell migration assays
To assess the effects of SIRT1 on cell contraction, fibroblasts were seeded in type I collagen gels (BD Biosciences) that were then incubated in medium with or without TGFβ2 (10 ng/ml) and resveratrol for up to 48 hours (15). At the end of the incubation periods, gel diameters were determined. Modulation of cell migration by SIRT1 was evaluated by in vitro wounding assays (15). Briefly, confluent monolayers of fibroblasts were incubated in serum-free medium with resveratrol for 12 hours in the presence of 10 µg/ml mitomycin C (Sigma), and scratch wounds were inflicted using standard p1000 pipette tips. Cell migration was then monitored by phase-contrast microscopy for up to 48 hours. Gap width was determined at 3 different sites per sample at indicated intervals.
Plasmids, small interfering RNA (siRNA), and transient transfection assays
The SIRT1 expression vector was a gift from Dr. Deyu Fang (17). Small interfering RNAs coding for SIRT1 and scrambled control siRNA were from Dharmacon. The plasmid SBE4-TK-Luc contains 4 copies of the consensus Smad-binding element linked to thymidine kinase and luciferase genes (18). Fibroblasts at early confluence were transiently transfected with reporter or expression constructs or appropriate empty vectors using Lipofectamine LTX (Invitrogen) (15). Following incubation in medium with or without TGFβ or resveratrol for 24 hours, cultures were harvested and whole-cell lysates were assayed for their luciferase activities using the Dual-Luciferase Reporter Assay system (Promega) (15). The reporter vector pRL-TK Renilla luciferase (pRL-TK-Luc) was used in each experiment as an internal control, and experiments were performed in triplicate.
RNA isolation and real-time quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from explanted fibroblasts or skin biopsy samples using Quick RNA Miniprep (Zymo Research) and reverse-transcribed for real-time qPCR using qScript cDNA SuperMix (Quanta Bio-Sciences) (15). Real-time qPCR was performed on an ABI Prism 7300 PCR machine (Applied Biosystems). Levels of mRNA normalized to GAPDH levels in each sample were determined by calculating ΔΔCt.
Western blot analysis
At the end of the incubation periods, cultures were harvested, and equal amounts of whole-cell lysates (5–15 µg) were subjected to electrophoresis in Tris–glycine 4–15% gradient gels and transferred to PVDF membranes (15). Membranes were incubated with the primary antibodies anti–type I collagen (1:400; SouthernBiotech), anti–phospho-Smad2 (1:1,000; Cell Signaling Technology), anti-SIRT1, anti–histone H4, anti–β-actin, and anti-p300 (all 1:200; all from Santa Cruz Biotechnology), anti–α-smooth muscle actin (anti–α-SMA) and anti–α-tubulin (both 1:3,000; both from Sigma), and anti-GAPDH (1:3,000; Invitrogen), followed by appropriate secondary antibodies. Antigen–antibody complexes were visualized by enhanced chemiluminescence (Pierce). Protein levels were quantitated by determining band intensities normalized to loading controls in each lane using ImageJ software (http://rsb.info.nih.gov/ij/).
Immunoprecipitation and chromatin immunoprecipitation (ChIP) assays
Confluent fibroblasts were preincubated in serum-free DMEM with 100 µM resveratrol for 24 hours prior to addition of TGFβ (10 ng/ml) for 60 minutes. Whole-cell lysates were prepared and immunoprecipitated with anti-Smad1/2/3 antibodies (Santa Cruz Biotechnology) and subjected to immunoblot analysis.
ChIP assays were performed using antibodies against p300 (Santa Cruz Biotechnology) and acetyl histone H4 (Cell Signaling Technology) with EZ Magna ChIP Assay Kits (Upstate/Millipore) (19). Real-time qPCR amplification of the captured DNA sequences was performed using primers complementary to human COL1A2 DNA sequences flanking the Smad-binding element (19). The results were normalized to input DNA.
Immunohistochemistry and immunofluorescence
To assess tissue levels of SIRT1 by immunohistochemistry and immunofluorescence, 4-µm sections of skin biopsy samples from SSc patients and healthy controls, or from mice, were incubated overnight with primary antibodies to SIRT1 (Santa Cruz Biotechnology), F4/80 (BD Biosciences), or α-SMA (Sigma), followed by incubation with biotinylated donkey anti-rabbit secondary antibodies and streptavidin-linked alkaline phosphatase (AP) (Jackson ImmunoResearch) or by incubation with AP-conjugated anti-mouse/anti-rabbit antibodies (DakoCytomation) (4). The SIRT1 score was calculated by taking the means of immunostaining intensities in 5 control and 10 diffuse cutaneous SSc (dcSSc) skin biopsy samples assessed by 2 independent blinded observers (JW, RGM) scoring ≥60 individual fibroblastic cells in 3 high-power fields per slide throughout the dermis as follows: 0 = no visible staining; 1 = faint staining; 2 = moderate staining; 3 = strong staining. For immunofluorescence, tissues were incubated with Alexa Fluor–conjugated secondary antibodies (Invitrogen), followed by DAPI staining. Images were obtained using a Nikon A1 confocal microscope. The proportion of cells with double staining (protein of interest plus marker protein) was determined by calculating the ratio of double-positive cells to single-positive cells from at least 4 representative images.
To evaluate cellular levels of SIRT1 and α-SMA by immunocytochemistry, confluent fibroblasts were incubated in serum-free medium with 0.1% bovine serum albumin in the presence or absence of resveratrol (100 µM) for 60 minutes prior to incubation with TGFβ2 (10 ng/ml). Following a further 24-hour incubation, cells were fixed in 4% paraformaldehyde, washed in PBS, and incubated with primary antibodies against α-SMA (1:400) or SIRT1 (1:100) for 120 minutes, followed by incubation with Alexa Fluor 488–conjugated chicken anti-mouse or Alexa Fluor 594–conjugated donkey anti-rabbit (Invitrogen) for 60 minutes. Nuclei were identified by DAPI staining. Nonimmune IgG was used as a negative control in each experiment. Following stringent washing, slides were examined under a Zeiss UV Meta 510 confocal microscope. Each experiment was repeated at least 3 times with consistent results.
Statistical analysis
Data are presented as the mean±SD. The significance of differences between groups was determined by Student’s t-test. In the human and animal studies, differences between the groups were evaluated using the nonparametric Mann-Whitney U test. Correlations between SIRT1 expression and the modified Rodnan skin thickness score (MRSS) (20) or local (forearm) skin score were analyzed by Spearman’s rank correlation test. P values less than 0.05 were considered significant.
RESULTS
Reduced SIRT1 expression in SSc skin biopsy samples
To examine SIRT expression in SSc skin, we first queried a publicly available data set comprising genome-wide expression data from SSc (n = 24), morphea (n = 3), and healthy control (n = 6) skin biopsy samples (13). Based upon their gene expression patterns, biopsy samples clustered into 5 previously defined intrinsic subsets (13). Levels of SIRT1 mRNA were found to be significantly reduced (P<0.001) in skin biopsy samples mapping to the diffuse-proliferative intrinsic subset (Figure 1A). Reduced SIRT1 mRNA expression in SSc skin biopsy samples (n = 3) was confirmed by real-time qPCR (P<0.05) (Figure 1B). Microarray data analysis showed that reduced SIRT1 mRNA expression was accompanied by a parallel decrease in nova2, a SIRT1 target gene (21), in the same biopsy samples (r = 0.465, P < 0.00005). Sirtuin deficiency in SSc biopsy samples was selective for SIRT1, since mRNA levels for SIRT2 through SIRT6 showed no significant difference among the 5 intrinsic subsets; of note, levels of SIRT7, a sirtuin that regulates ribosomal gene transcription (22), were significantly elevated in biopsy samples mapping to diffuse-proliferative intrinsic subsets compared to the normal-like subset (data not shown). Expression levels of SIRT1 mRNA were negatively correlated with the total skin score (MRSS) (P = 0.004) (further information is available at https://www.researchgate.net/publication/273941227_Supplemental_Figures_AR_14–0700?fulltextDialog=true).
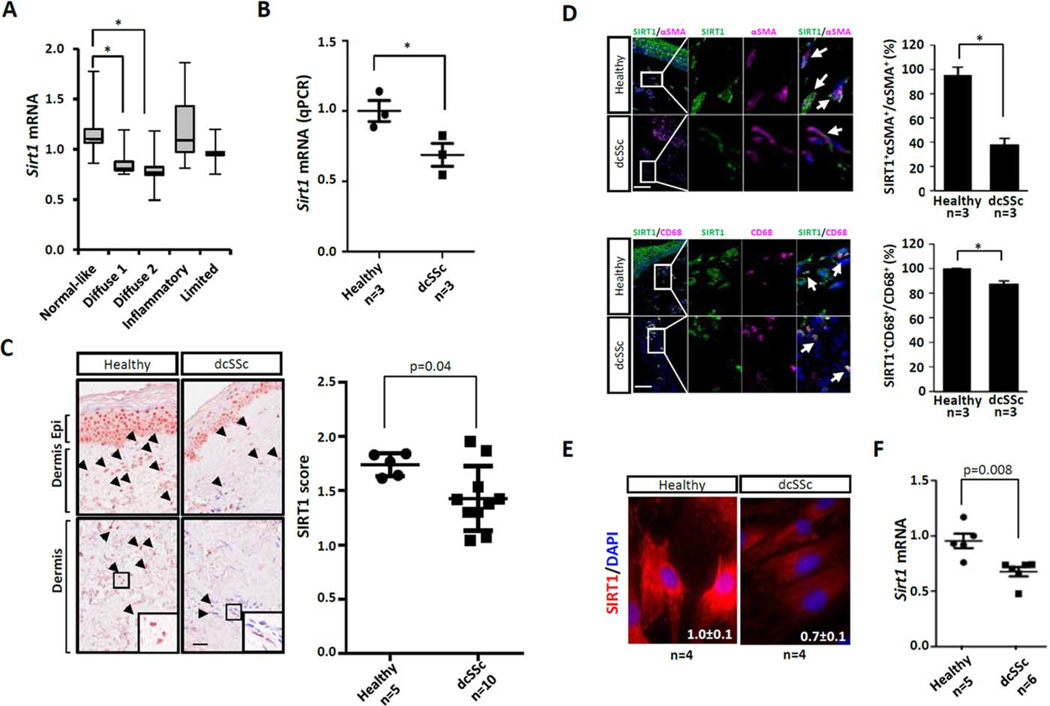
Figure 1. Reduced sirtuin 1 (SIRT1) expression in systemic sclerosis (SSc).
A, Reduced SIRT1 mRNA in SSc skin biopsy–derived microarray data sets. Data are shown as box plots. Each box represents the 25th to 75th percentiles. Lines inside the boxes represent the median. Lines outside the boxes represent the minimum and maximum values. * = P<0.001. B, Determination of SIRT1 mRNA levels using real-time quantitative polymerase chain reaction (qPCR), following isolation of RNA from forearm lesional skin from patients with diffuse cutaneous SSc (dcSSc) and healthy controls. Levels of mRNA were normalized to GAPDH levels. * = P <0.05. C, Immunohistochemistry. Healthy control and dcSSc skin biopsy samples were examined using antibodies to SIRT1. Representative images are shown at the left. Arrowheads indicate immunopositive cells within the dermis. Boxed areas in bottom panels are shown at higher magnification in bottom right of panels. Epi = epidermis. Original magnification × 400. Bar = 50 µm. SIRT1 scores are shown at the right. Immunostaining was quantified as described in Subjects and Methods. D, SIRT1 immunofluorescence in skin biopsy samples. Healthy control and dcSSc skin biopsy samples were immunostained with antibodies to α-smooth muscle actin (α-SMA) or CD68 (both pink), SIRT1 (green), and DAPI (blue). Representative images are shown at the left. Arrows indicate double-immunopositive cells. Bars = 50 µm. The proportion of double-positive cells is shown at the right and was calculated as described in Subjects and Methods. Values are the mean±SEM. * = P <0.05. E, Representative images of SIRT1 immunofluorescence in healthy control and dcSSc fibroblasts in culture. Red indicates SIRT1; blue indicates DAPI. Original magnification × 400. F, Determination of SIRT1 mRNA levels using real-time qPCR, following isolation of RNA from healthy control (n = 5) or dcSSc (n = 6) skin fibroblasts. Levels of mRNA were normalized to GAPDH levels. In B, C, and F, symbols represent individual subjects; bars show the mean ± SEM.
These findings were replicated in a second skin biopsy sample data set representing an independent cohort of 22 SSc patients (20 with dcSSc, 2 with limited cutaneous SSc) (P = 0.002) (14) (further information is available at https://www.researchgate.net/publication/273941227_Supplemental_Figures_AR_14–0700?fulltextDialog=true). In that cohort, SIRT1 mRNA levels were also negatively correlated with the local (forearm) skin score (P = 0.004) (further information is available at https://www.researchgate.net/publication/273941227_Supplemental_Figures_AR_14–0700?fulltextDialog=true).
To assess tissue levels of SIRT1 protein, dorsal forearm skin biopsy samples from 10 patients with dcSSc and from 5 age-matched healthy adults were examined by immunohistochemistry. The results showed substantially lower SIRT1 levels in SSc skin biopsy samples compared to healthy control skin biopsy samples (P = 0.04) (Figure 1C). SIRT1 expression in the normal dermis was evident in spindle-shaped fibroblastic cells, as well as in round cells in both the papillary and reticular dermis, and was particularly prominent in keratinocytes of the epidermis. Dual-color immunofluorescence indicated that in normal skin biopsy samples, virtually all dermal cells were positive for SIRT1, while in SSc skin biopsy samples, a majority of α-SMA–positive fibroblasts in the fibrotic dermis showed absence of colocalization with SIRT1 (Figure 1D). In contrast, only a modest reduction was found in SIRT1/CD68 double-positive cells in SSc skin biopsy samples. Isotype control antibodies established the specificity of immunostaining.
To examine the cell-autonomous expression of SIRT1, skin fibroblasts explanted from biopsy samples from dcSSc patients (n = 4) and matched healthy controls (n = 4) were examined in parallel by immunohistochemistry. The results indicated significantly lower SIRT1 levels in SSc fibroblasts (Figure 1E). Strikingly, while in healthy fibroblasts SIRT1 was detected in both the cytoplasm and nucleus, in SSc fibroblasts nuclear staining was largely absent. These findings were consistent across each SSc fibroblast line examined. In other studies, SSc fibroblasts in culture (n = 6) showed significantly reduced levels (P = 0.008) of SIRT1 mRNA (Figure 1F). Taken together, these results indicate consistent and cell-autonomous impairment of SIRT1 expression in SSc.
To explore mechanisms that might account for low SIRT1 expression in SSc, we examined SIRT1 regulation by factors prominently implicated in skin fibrosis (1). Incubation of explanted normal dermal fibroblasts for 24–96 hours with TGFβ or PDGF caused a 33% reduction in SIRT1 mRNA (P <0.05) (further information is available at https://www.researchgate.net/publication/273941227_Supplemental_Figures_AR_14–0700?fulltextDialog=true). Exposure of fibroblasts to prolonged hypoxia (1.5% O2 for 24 hours) or H2O2 resulted in a highly significant down-regulation of SIRT1 protein (P <0.005). In contrast, no consistent decrease of SIRT1 protein was observed in fibroblasts incubated with TGFβ (further information is available at https://www.researchgate.net/publication/273941227_Supplemental_Figures_AR_14–0700?fulltextDialog=true). These results indicate that selected mediators of fibrosis involved in the pathogenesis of SSc negatively regulate SIRT1 expression, suggesting that they might contribute to down-regulation of SIRT1 in SSc skin.
Inhibition of fibrotic responses in vitro by resveratrol
In light of its reduced expression in SSc skin biopsy samples and explanted fibroblasts, we speculated that SIRT1 may have a negative regulatory function in maintaining skin homeostasis and that failure of this regulatory homeostatic function contributes to pathologic fibrosis. To begin to explore this possibility, we used resveratrol, a naturally occurring SIRT1 activator (7). As has been shown previously (23), resveratrol induced a dose-dependent stimulation of SIRT1 mRNA and protein expression in the absence of cell death or significant cell proliferation (Figure 2A and data not shown). Moreover, resveratrol stimulated nuclear translocation of SIRT1 in dermal fibroblasts (Figure 2B).
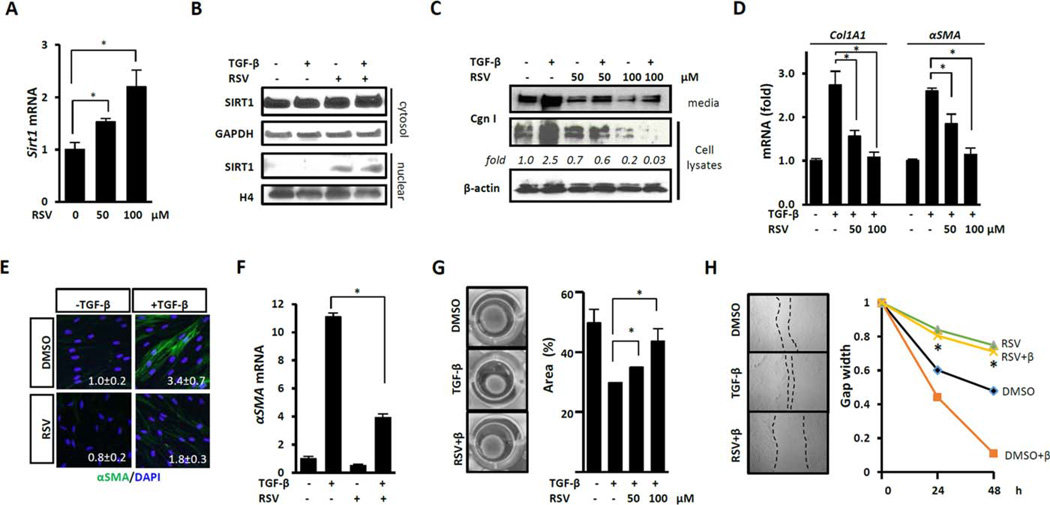
Figure 2. Sirtuin 1 (SIRT1) activation abrogates fibrotic responses.
Confluent foreskin (A–E, G, and H) or adult human skin (F) fibroblasts were preincubated for 60 minutes with resveratrol (RSV; 100 µM unless indicated otherwise), followed by incubation with transforming growth factor β (TGFβ; 10 ng/ml) (B–H). A, D, and F, Messenger RNA was analyzed by real-time quantitative polymerase chain reaction. Levels of mRNA were normalized to GAPDH levels. Values are the mean±SD of triplicate determinations from 1 representative experiment * = P <0.05. B and C, Nuclear and cytoplasmic (cytosol) protein fractions (B) or whole-cell lysates and supernatants (C) were examined by Western blot analysis. Band intensities normalized to GAPDH (fold) are shown. E, Shown are representative images of immunofluorescence. Green indicates α-smooth muscle actin (α-SMA); blue indicates DAPI. Original magnification × 400. Mean fluorescence intensity normalized to nuclear staining (mean ±SD of 5 independent areas per microscopic field) is shown in each panel. G, Shown are gel contraction assays. Representative images are shown at the left. Results shown at the right are expressed as the percent of gel area compared to time zero. Values are the mean ± SD of triplicate determinations from 1 experiment representative of 3. * = P <0.05. H, Shown are in vitro wound healing assays. Representative microscopic images are shown at the left. Fibroblast migration was monitored for 48 hours and results are shown at the right. Values are the mean±SD of triplicate determinations from 3 randomly chosen sites in each sample from 1 experiment representative of 3. P <0.05 versus fibroblasts treated with TGFβ alone. H4=histone H4; Cgn I=type I collagen; RSV+β=RSV+TGFβ; DMSO+β=DMSO+TGFβ.
To investigate the modulation of fibroblast responses by SIRT1, confluent fibroblasts were preincubated with resveratrol for 30 minutes and then stimulated with TGFβ. While TGFβ induced a significant increase in collagen gene expression and myofibroblast differentiation, as expected, resveratrol pretreatment resulted in dose-dependent suppression of these TGFβ-induced responses (Figures 2C – E). Importantly, resveratrol not only prevented but also reversed these fibrotic responses (data not shown). Comparable antifibrotic effects were elicited by resveratrol in multiple experiments using neonatal as well as adult skin fibroblasts (Figure 2F). Migration and collagen lattice contraction are fibroblast activities critical for executing both physiologic wound repair and pathologic fibrogenesis. A series of in vitro assays indicated that resveratrol significantly attenuated TGFβ-induced stimulation of both fibroblast contractility (Figure 2G) and fibroblast migration (Figure 2H).
To evaluate the effects of resveratrol on fibroblast activity and function in a more physiologically relevant context, we used organ cultures derived from normal skin (15). Incubation of organ cultures with TGFβ induced an ~4-fold increase of α-SMA expression, which was markedly attenuated in organ cultures preincubated with resveratrol (4.22 ± 0.45 versus 1.05±0.03; P <0.001). Moreover, time-dependent out-migration of dermal fibroblasts from the skin explants onto the dish observed in TGFβ-treated cultures was substantially inhibited in the presence of resveratrol (data not shown).
Inhibitory effects of resveratrol mediated by SIRT1
While resveratrol has been shown to be an activator of SIRT1 (24), it can also activate AMP kinase and other enzymes in a cell type–specific manner (25). To examine the role of SIRT1 in mediating the potent antifibrotic effects of resveratrol, we performed a series of gain-of-function and loss-of-function studies. Transient ectopic expression of SIRT1 in normal fibroblasts was sufficient to suppress TGFβ-induced stimulation of collagen synthesis and α-SMA expression, recapitulating the antifibrotic effects of resveratrol that we observed in these cells (Figures 3A and B). Pharmacologic activation of cellular SIRT1 using the selective SIRT1 activators SA3 and SRT1720 elicited comparable inhibitory effects (Figure 3C). To address whether SIRT1 was necessary for the antifibrotic effects elicited by resveratrol, we used a combination of genetic and pharmacologic approaches. Partial siRNA-mediated knockdown of cellular SIRT1 in normal fibroblasts substantially rescued TGFβ stimulation of type I collagen, α-SMA, and fibronectin extra domain A (FnEDA) gene expression in the presence of inhibitory resveratrol (Figure 3D and data not shown). Moreover, nicotinamide, a selective pharmacologic antagonist of SIRT1, similarly overcame the inhibitory effects of resveratrol on TGFβ-stimulated fibroblast gene expression (Figure 3E).
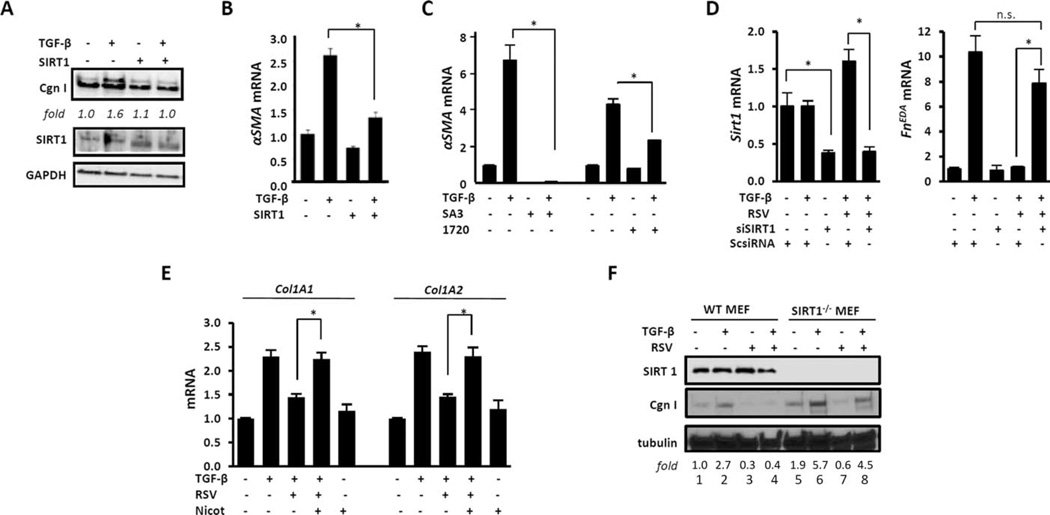
Figure 3. Sirtuin 1 (SIRT1) mediates the antifibrotic effects of resveratrol (RSV).
Confluent human skin fibroblasts (A–E) were transiently transfected with SIRT1 expression vectors (A and B), preincubated for 30 minutes with SIRT1 activator 3 (SA3) or SRT1720 (1720) (C) or with nicotinamide (Nicot; 10 mM) (E), or were transiently transfected with small interfering RNA (siRNA) coding for SIRT1 (siSIRT1) or scrambled control siRNA (ScsiRNA) for 48 hours (D). Transforming growth factor β (TGFβ; 10 ng/ml) was added for 24 hours. A, Whole-cell lysates were examined by Western blot analysis. Band intensities normalized to GAPDH (fold) are shown. B–E, Messenger RNA was analyzed by real-time quantitative polymerase chain reaction. Levels of mRNA were normalized to GAPDH levels. Values are the mean±SD of triplicate determinations from 1 representative experiment. * = P <0.05. F, Confluent SIRT1−/− and wild-type (WT) mouse embryonic fibroblasts (MEFs) in parallel were incubated with resveratrol (50 µM) for 30 minutes, followed by incubation with TGFβ (10 ng/ml) for 24 hours. Whole-cell lysates were examined by Western blot analysis. Band intensities normalized to tubulin (fold) are shown. Cgn I = type I collagen; α-SMA = α-smooth muscle actin; FnEDA = fibronectin extra domain A; NS = not significant.
Next, SIRT1−/− MEFs were used to further examine the role of cellular SIRT1 in regulating fibrotic responses and mediating the antifibrotic effects of resveratrol. In the absence of SIRT1, both basal and TGFβ-stimulated levels of type I collagen were elevated (Figure 3F, compare lanes 1 and 2 with lanes 5 and 6), and SIRT1−/− MEFs showed relative resistance to the inhibitory effects of resveratrol (Figure 3F, compare lane 4 with lane 8). Taken together, using combined genetic and pharmacologic approaches, these results demonstrate that SIRT1 activation is by itself sufficient to attenuate TGFβ-mediated fibroblast activation, and that cellular SIRT1 negatively regulates collagen gene expression and is required for mediating the inhibitory effects elicited by resveratrol.
Resveratrol disrupts Smad signaling and inhibits p300 expression and function
In light of the antifibrotic effects of SIRT1, it was important to delineate the targeted profibrotic intracellular pathways, focusing on canonical TGFβ signaling. Transient transfection assays indicated that TGFβ stimulation of SBE4-Luc activity was significantly reduced by SIRT1 as well as by resveratrol (Figure 4A). Of note, rapid Smad2 phosphorylation and nuclear translocation were unaffected, indicating that SIRT1 disrupted Smad-dependent TGFβ signaling at a downstream level.
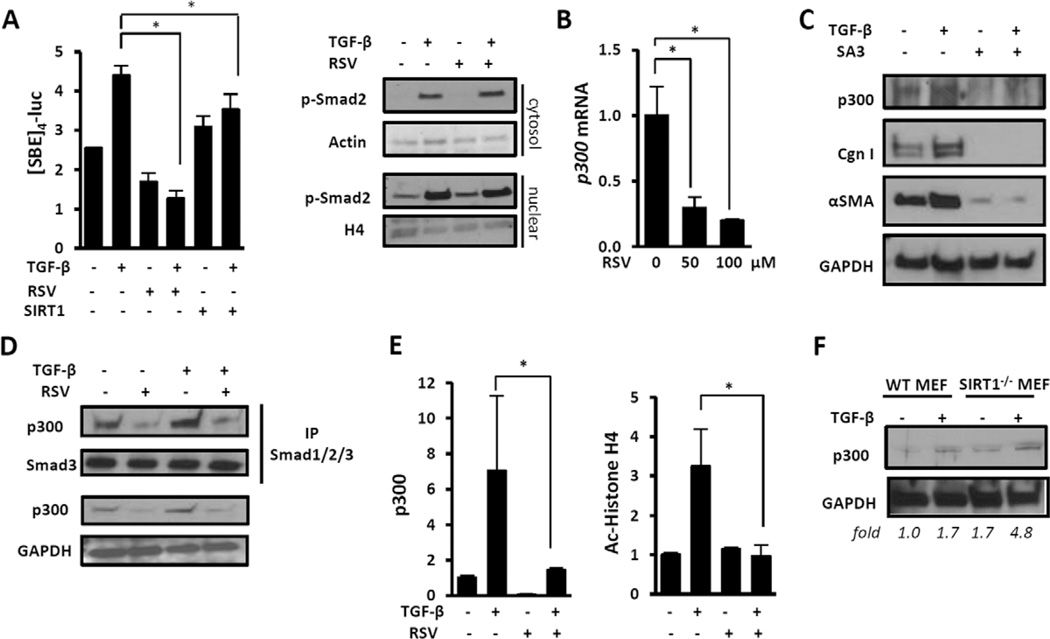
Figure 4. Resveratrol blocks canonical TGFβ signaling by disrupting p300 function.
A, Shown is modulation of Smad signaling. Left, Fibroblasts transiently transfected with SBE4-Luc along with SIRT1 or empty vector were preincubated with resveratrol, followed by incubation with TGFβ (10 ng/ml) for 24 hours. Whole-cell lysates were assayed for their luciferase activities. Results were normalized to Renilla luciferase levels. Values are the mean ± SD of triplicate experiments. * = P <0.05. Right, Fibroblasts preincubated with resveratrol were incubated with TGFβ for 120 minutes. Cytosolic and nuclear fractions were examined by Western blot analysis. B and C, Fibroblasts were preincubated with SIRT1 activator 3 (20 µM) for 30 minutes, followed by incubation with TGFβ for 24 hours. B, Messenger RNA was analyzed by real-time quantitative polymerase chain reaction. Levels of mRNA were normalized to GAPDH levels. Values are the mean±SD of triplicate determinations from 1 representative experiment. * = P<0.05. C, Whole-cell lysates were examined by Western blot analysis. D and E, Fibroblasts were preincubated with resveratrol for 24 hours, followed by incubation with TGFβ (10 ng/ml) for 60 minutes. D, Whole-cell lysates were immunoprecipitated (IP) with antibodies to Smad1/2/3 and subjected to Western blot analysis. E, Shown are chromatin immunoprecipitation assays. DNA was immunoprecipitated using the indicated antibodies to p300 or acetylated histone H4 (Ac-histone H4) or IgG and then amplified using primers spanning the human COL1A2 promoter TGFβ response element. Results are expressed as the fold change in precipitated DNA. Values are the mean±SD of triplicate determinations. * = P <0.05. F, SIRT1−/− and WT MEFs in parallel were incubated with TGFβ (10 ng/ml) for 24 hours. Whole-cell lysates were examined by Western blot analysis. Band intensities normalized to GAPDH (fold) are shown. p-Smad2 = phosphorylated Smad2; H4 = histone H4 (see Figure 3 for other definitions).
Protein acetylation catalyzed by the acetyltransferase p300 is a critical event in fibrotic Smad signaling downstream of TGFβ (26). We noted that in normal fibroblasts, the antifibrotic effects of resveratrol were accompanied by a dose-dependent reduction in cellular p300 (Figures 4B and C). The SIRT1-selective activator SA3 elicited comparable suppression of p300. Moreover, coimmunoprecipitation experiments showed that in fibroblasts treated with TGFβ in the presence of resveratrol, p300 interaction with activated Smad2/3 was attenuated (Figure 4D). Furthermore, ChIP assays showed a marked increase in the accumulation of p300 on the COL1A2 promoter and consequent histone H4 acetylation in TGFβ-treated fibroblasts, both of which are required for profibrotic outputs in response to TGFβ (5). These inducible epigenetic modifications were completely abrogated in the presence of resveratrol (Figure 4E). Studies with SIRT1−/− MEFs showed a constitutive 1.7-fold up-regulation of p300 that was further enhanced by TGFβ, providing support for a role of SIRT1 in regulating cellular p300 abundance (Figure 4F).
Resveratrol mitigation of the activated phenotype of SSc fibroblasts
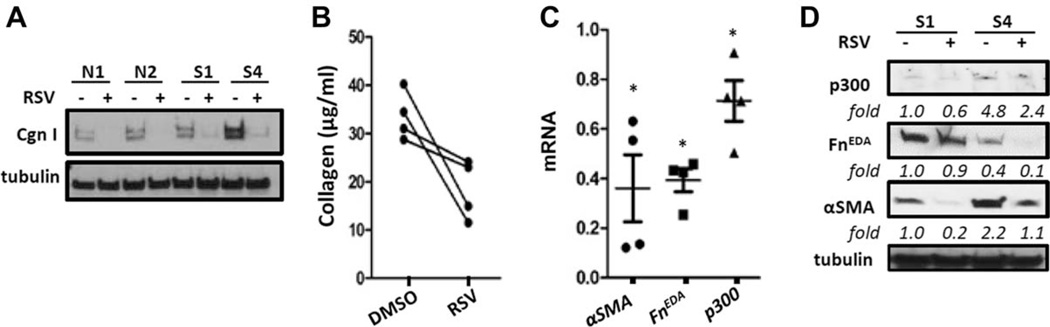
Explanted SSc fibroblasts maintain their activated profibrotic phenotype in culture even in the absence of exogenous ligand (27,28). The cell-autonomous activated phenotype is due in part to up-regulation of p300 expression and activity in these cells. Our results (Figures 1E and F) indicated down-regulated SIRT1 expression in SSc fibroblasts, suggesting the possibility that the activated fibroblast phenotype and elevated p300 might result from impaired SIRT1 signaling. We therefore sought to determine whether rescuing SIRT1 in SSc fibroblasts could normalize fibrotic gene expression and p300 levels. Incubation of SSc fibroblasts (n = 4) with resveratrol resulted in reduced expression of collagen, α-SMA, and FnEDA genes that was accompanied by a modest decrease in p300 levels (Figure 5).
Figure 5. Resveratrol attenuates collagen overproduction in systemic sclerosis (SSc) fibroblasts in culture.
Confluent healthy control (n=4) or diffuse cutaneous SSc (dcSSc) (n = 4) skin fibroblasts were incubated in medium with or without resveratrol (100 µM) for 72 hours. A, Whole cell lysates were examined by Western blot analysis. Shown are representative blots of 2 healthy control (N1 and N2) and 2 dcSSc (S1 and S4) fibroblasts. B, Secreted collagen in medium was quantified by Sircol assays in dcSSc fibroblasts. Lines indicate results from the same fibroblast cell lines. C, Messenger RNA levels in dcSSc fibroblasts were analyzed by real-time quantitative polymerase chain reaction. Results are expressed relative to untreated cultures and are normalized to 18S ribosomal RNA levels. Values are the mean ± SD. * = P <0.05. D, Whole-cell lysates from 2 dcSSc fibroblasts (S1 and S4) were examined by Western blot analysis. See Figure 3 for other definitions.
Amelioration of skin fibrosis in the mouse by resveratrol
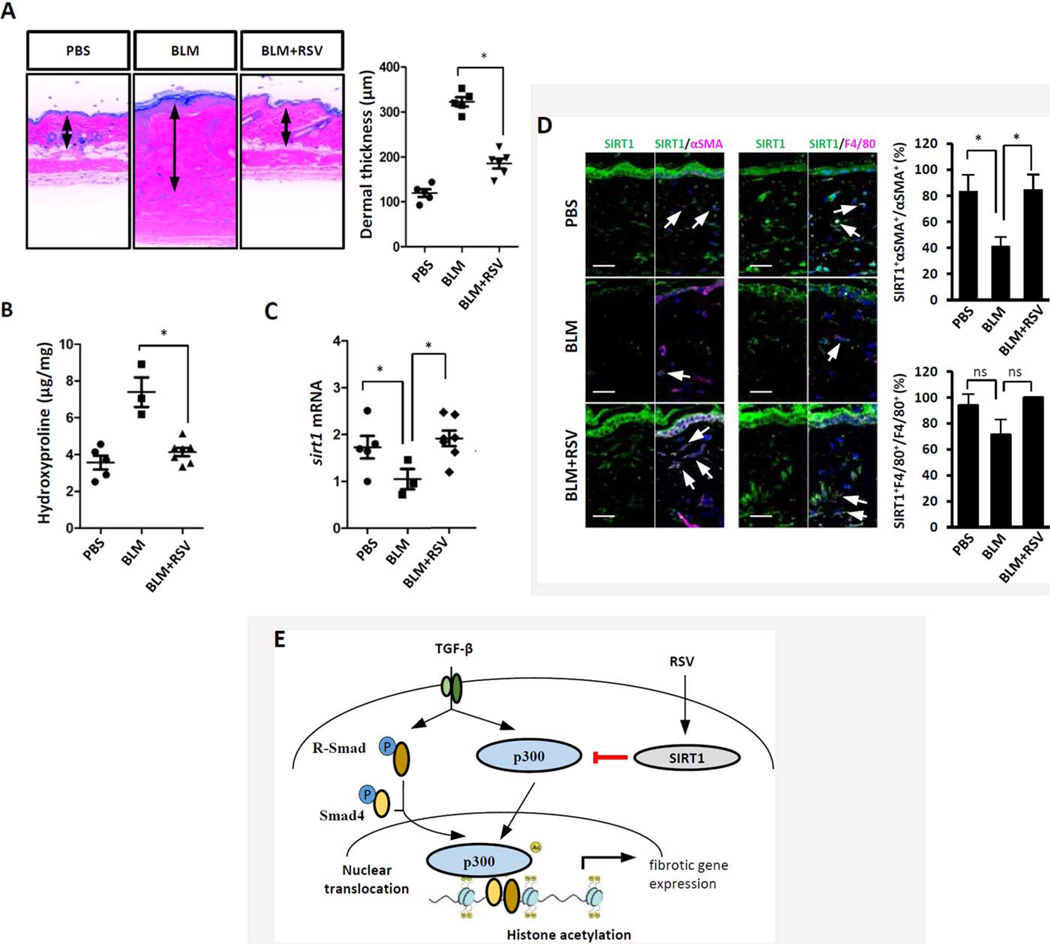
To assess the regulation of SIRT1 and its effect on fibrosis in vivo, we used a mouse model of bleomycin-induced scleroderma. Female C57BL/6J mice were treated with vehicle, bleomycin, or a combination of resveratrol and bleomycin (20 mg/kg/day IP). In pilot experiments (data not shown), resveratrol in doses up to 50 mg/kg/day was well tolerated with no significant weight loss or other signs of toxicity. As shown in Figure 6A, the increase in dermal thickness was markedly ameliorated in mice treated with resveratrol (323 ± 21 µm versus 177 ± 27 µm; P<0.01). Moreover, the increase in collagen accumulation in the lesional skin was significantly attenuated after treatment with resveratrol (Figure 6B). Real-time qPCR showed that levels of SIRT1 mRNA were significantly decreased in the skin from mice treated with bleomycin, and this decrease was reversed by treatment with resveratrol (Figure 6C). Immunofluorescence showed reduced numbers of SIRT1 and α-SMA double-positive myofibroblasts in the lesional dermis, while SIRT1 expression was unaltered in F4/80-positive dermal macrophages (Figure 6D).
Figure 6. Pharmacologic SIRT1 activation ameliorates skin fibrosis.
Mice were given daily subcutaneous injections of bleomycin (BLM) alone for 14 days or daily intraperitoneal injections of bleomycin combined with resveratrol for 21 days. At the end of the experiments, lesional skin was harvested. A, Left, Hematoxylin and eosin staining. Original magnification × 200. Right, Quantification of dermal thickness (3–7 mice per group). * = P <0.001. B, Hydroxyproline assays (3–7 mice per group). C, Determination of SIRT1 mRNA levels by real-time quantitative polymerase chain reaction, following isolation of RNA from lesional skin. Results were normalized to 36b4 mRNA levels. In A–C, symbols represent individual mice; bars show the mean ±SD of triplicate determinations (3–7 mice per group). D, Immunofluorescence with antibodies to SIRT1, α-SMA, or F4/80. Left, Representative images. Arrows indicate double-immunopositive dermal cells. Original magnification × 200. Bars = 50 µm. Right, Immunopositive cells were quantified. Values are the mean± SD of 5 determinations per high-power field (3 mice per group). In B–D, * = P <0.05. E, Working model for the antifibrotic effects of SIRT1. TGFβ up-regulates p300 transcription and activity in fibroblasts, resulting in COL1A2 promoter histone acetylation and increased gene transcription. In systemic sclerosis fibroblasts, constitutive p300 up-regulation enhances Smad2/3-dependent transcription. By suppressing p300/Smad-dependent transcription, SIRT1 attenuates TGFβ-induced pro-fibrotic responses. PBS = phosphate buffered saline; R-Smad=receptor-activated Smad; p = phosphorylated (see Figure 3 for other definitions).
DISCUSSION
Acetylation and deacetylation of histones and nonhistone proteins play important roles in the pathogenesis of skin fibrosis (29). Levels of the acetyltransferase p300 are elevated in SSc skin biopsy samples and explanted fibroblasts, as well as in lesional skin from mice with experimentally induced scleroderma (5). However, little is known about the factors governing the expression and function of histone acetyltransferases and deacetylases and their dynamic balance in tissue remodeling (4,6,28,30). The present studies focused on the acetylase SIRT1, which has been linked to regulation of p300 expression and function. Our results show that SIRT1 expression is reduced in SSc, and they reveal an important negative regulatory role of SIRT1 in skin fibrosis.
Sirtuins regulate expression of genes involved in a broad range of biologic responses (for review, see refs. 31 and 32). SIRT1, the most extensively studied sirtuin, is implicated in aging, metabolism, circadian regulation, and autoimmunity (33). Calorie restriction and exercise are known to be potent stimuli for increased SIRT1 expression and activity (34). We now show that SIRT1 is reduced in SSc lesional skin and explanted fibroblasts, consistent with a recent report (35). Mechanisms that might account for impaired SIRT1 expression in SSc include transcriptional suppression by PDGF, tissue hypoxia, and ROS. Furthermore, oxidative stress, a hallmark of SSc, depletes cellular nicotinamide, the obligatory substrate for SIRT1 activity, resulting in impaired function (36).
Deregulated SIRT1 expression or function are prominent features of aging and are implicated in the pathogenesis of metabolic, neurodegenerative, and malignant diseases (37,38). SIRT1 has potent effects on endothelial cell function, and mice with loss of endothelial SIRT1 display abnormal angiogenesis (39). Pharmacologic SIRT1 activation in mice results in attenuated hepatic (40), cardiac (41), kidney (42), and lung (43) fibrosis. While recent studies show a role of SIRT1 in inhibiting epithelial-to-mesenchymal transition (EMT) in cancer and organ fibrosis (44,45), the effects of SIRT1 in fibrosis appear to be cell type–specific and context-dependent. As an example, in prostate cancer cells and renal fibroblasts, SIRT1 and SIRT2 were shown to induce, rather than inhibit, EMT and fibrotic responses (46,47). In transgenic mice overexpressing TGFβ receptor type I, selective SIRT1 deletion in fibroblasts was reported to cause reduced skin fibrosis (35). In contrast to these findings, our results with normal fibroblasts in culture indicated that while activation of SIRT1 attenuated TGFβ signaling, disruption of SIRT1 in these cells resulted in increased basal and TGFβ-stimulated collagen gene expression, indicating an antifibrotic role of SIRT1.
To explore the mechanistic basis for the antifibrotic effects of SIRT1, we performed a series of experiments. In explanted dermal fibroblasts SIRT1 blocked Smad-dependent responses, in part by suppression of the p300 coactivator (Figure 6E). Counter-regulation of histone acetylation by SIRT1 occurs in a variety of cell types (9–11). Moreover, p300 is itself a direct target of SIRT1, and these 2 factors regulate each other in a reciprocally antagonistic manner (48). The acetyltransferase p300 showed constitutive up-regulation and hyperacetylation in SIRT1−/− MEFs (49), while SIRT1 activation resulted in p300 deacetylation, ubiquitination, and degradation (50). Additional mechanisms that might contribute to the antifibrotic effect of SIRT1 include reduced Smad signaling due to Smad3/4 deacetylation (45,51), and stimulation of the antifibrotic mediator adiponectin, which has been shown to be reduced in patients with SSc and to be inversely correlated with disease activity (52).
Our results show that SIRT1 expression is significantly reduced in a subset of patients with SSc, and SIRT1 exerts potent antifibrotic effects by blocking Smad-dependent transcription. Furthermore, pharmacologic activation of SIRT1 ameliorated experimental skin fibrosis in mice. Taken together, these results provide novel insight into the mechanisms of skin fibrosis and the dynamic balance between acetyltransferases and deacetylases that affects fibrotic gene expression. Pharmacologic compounds that modulate SIRT1 activity are currently being evaluated in the clinic for the treatment of cancer, diabetes, and chronic inflammatory and neurodegenerative diseases. In view of its antifibrotic, antiinflammatory, and antioxidative effects, we propose that SIRT1 merits further investigation as a potential therapeutic target in SSc.
Supplementary Material
ACKNOWLEDGMENTS
We thank Warren G. Tourtellotte, Deyu Fang, and David Gius (Northwestern University) for helpful discussions, and Xiaoxia Zhu and Hejian Zou (Fudan University) and the staffs of the Cell Imaging Facility and Mouse Histology and Phenotyping Laboratory at Northwestern University and the Skin Pathology Laboratory at Boston University for technical assistance.
Supported by the NIH (grants AR-059763 to Dr. Hinchcliff and AR-49025 to Dr. Varga), the Scleroderma Research Foundation (grant to Dr. Hinchcliff), the National Science Foundation of China (grant 81270120 to Dr. Wang), the Ministry of Education of China (111 Project grant B13016 to Dr. Wang), and the National Scleroderma Foundation (grant to Dr. Varga).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Wei and Varga had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Wei, Hinchcliff, Wang, Varga.
Acquisition of data. Wei, Ghosh, Chu, Fang, Hinchcliff, Wang, Marangoni.
Analysis and interpretation of data. Wei, Ghosh, Wang, Marangoni, Varga.
REFERENCES
- 1.Bhattacharyya S, Wei J, Varga J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol. 2012;8:42–54. doi: 10.1038/nrrheum.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghosh AK, Varga J. The transcriptional coactivator and acetyl-transferase p300 in fibroblast biology and fibrosis. J Cell Physiol. 2007;213:663–671. doi: 10.1002/jcp.21162. [DOI] [PubMed] [Google Scholar]
- 3.Mann J, Mann DA. Epigenetic regulation of wound healing and fibrosis. Curr Opin Rheumatol. 2013;25:101–107. doi: 10.1097/BOR.0b013e32835b13e1. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh AK, Bhattacharyya S, Lafyatis R, Farina G, Yu J, Thimmapaya B, et al. p300 is elevated in systemic sclerosis and its expression is positively regulated by TGF-β epigenetic feedforward amplification of fibrosis. J Invest Dermatol. 2013;133:1302–1310. doi: 10.1038/jid.2012.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh AK. FAT-free p300 is good for scar-free tissue repair. J Cell Biochem. 2014;115:1486–1489. doi: 10.1002/jcb.24820. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya S, Ghosh AK, Pannu J, Mori Y, Takagawa S, Chen G, et al. Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to transforming growth factor β. Arthritis Rheum. 2005;52:1248–1258. doi: 10.1002/art.20996. [DOI] [PubMed] [Google Scholar]
- 7.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 9.Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113–117. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong S, Kim SJ, Sandal B, Lee SM, Gao B, Zhang DD, et al. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J Biol Chem. 2011;286:16967–16975. doi: 10.1074/jbc.M111.218206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 12.Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milano A, Pendergrass SA, Sargent JL, George LK, McCalmont TH, Connolly MK, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PloS One. 2008;3:2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinchcliff M, Huang CC, Wood TA, Mahoney JM, Martyanov V, Bhattacharyya S, et al. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J Invest Dermatol. 2013;133:1979–1989. doi: 10.1038/jid.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei J, Zhu H, Komura K, Lord G, Tomcik M, Wang W, et al. A synthetic PPAR-γ agonist triterpenoid ameliorates experimental fibrosis: PPAR-γ-independent suppression of fibrotic responses. Ann Rheum Dis. 2014;73:446–454. doi: 10.1136/annrheumdis-2012-202716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakos G, Takagawa S, Chen SJ, Ferreira AM, Han G, Masuda K, et al. Targeted disruption of TGF-β/Smad3 signaling modulates skin fibrosis in a mouse model of scleroderma. Am J Pathol. 2004;65:203–217. doi: 10.1016/s0002-9440(10)63289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh AK, Bhattacharyya S, Wei J, Kim S, Barak Y, Mori Y, et al. Peroxisome proliferator-activated receptor-γ abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivator. FASEB J. 2009;23:2968–2977. doi: 10.1096/fj.08-128736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements P, Lachenbruch P, Seibold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–1285. [PubMed] [Google Scholar]
- 21.Torres G, Frisella PD, Yousuf SJ, Sarwar S, Baldinger L, Zakhary SM, et al. A ChIP-cloning approach linking SIRT1 to transcriptional modification of DNA targets. Biotechniques. 2008;44:Pxii–Pxiv. doi: 10.2144/000112748. [DOI] [PubMed] [Google Scholar]
- 22.Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20:1075–1080. doi: 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh AK, Yuan W, Mori Y, Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene. 2000;19:3546–3555. doi: 10.1038/sj.onc.1203693. [DOI] [PubMed] [Google Scholar]
- 27.Mori Y, Chen SJ, Varga J. Expression and regulation of intra-cellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48:1964–1978. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- 28.Jinnin M, Ihn H, Mimura Y, Asano Y, Tamaki K. Involvement of the constitutive complex formation of c-Ski/SnoN with Smads in the impaired negative feedback regulation of transforming growth factor β signaling in scleroderma fibroblasts. Arthritis Rheum. 2007;56:1694–1705. doi: 10.1002/art.22588. [DOI] [PubMed] [Google Scholar]
- 29.Altorok N, Almeshal N, Wang Y, Kahaleh B. Epigenetics, the holy grail in the pathogenesis of systemic sclerosis. Rheumatology (Oxford) 2014 doi: 10.1093/rheumatology/keu155. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 31.Hall JA, Dominy JE, Lee Y, Puigserver P. The sirtuin family’s role in aging and age-associated pathologies. J Clin Invest. 2013;123:973–979. doi: 10.1172/JCI64094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:81–90. doi: 10.1101/sqb.2011.76.010629. [DOI] [PubMed] [Google Scholar]
- 33.Sequeira J, Boily G, Bazinet S, Saliba S, He X, Jardine K, et al. sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314:3069–3074. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor γ coactivator-1αprotein expressions in rat skeletal muscle. Metabolism. 2008;57:986–998. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Zerr P, Palumbo-Zerr K, Huang J, Tomcik M, Sumova B, Distler O, et al. Sirt1 regulates canonical TGF-β signalling to control fibroblast activation and tissue fibrosis. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2014-205740. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD1 metabolism in human tissue. PloS One. 2012;7:42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasko R, Xavier S, Chen J, Lin CH, Ratliff B, Rabadi M, et al. Endothelial sirtuin 1 deficiency perpetrates nephrosclerosis through downregulation of matrix metalloproteinase-14: relevance to fibrosis of vascular senescence. J Am Soc Nephrol. 2014;25:276–291. doi: 10.1681/ASN.2013010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Pascoli M, Divi M, Rodriguez-Vilarrupla A, Rosado E, Gracia-Sancho J, Vilaseca M, et al. Resveratrol improves intra-hepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2013;58:904–910. doi: 10.1016/j.jhep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2010;298:833–843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177:1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akgedik R, Akgedik S, Karamanli H, Uysal S, Bozkurt B, Ozol D, et al. Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation. 2012;35:1732–1741. doi: 10.1007/s10753-012-9491-0. [DOI] [PubMed] [Google Scholar]
- 44.Sun L, Li H, Chen J, Iwasaki Y, Kubota T, Matsuoka M, et al. PIASy mediates hypoxia-induced SIRT1 transcriptional repression and epithelial-to-mesenchymal transition in ovarian cancer cells. J Cell Sci. 2013;126:3939–3947. doi: 10.1242/jcs.127381. [DOI] [PubMed] [Google Scholar]
- 45.Simic P, Williams EO, Bell EL, Gong JJ, Bonkowski M, Guarente L. SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep. 2013;3:1175–1186. doi: 10.1016/j.celrep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byles V, Zhu L, Lovaas JD, Chmilewski LK, Wang J, Faller DV, et al. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012;31:4619–4629. doi: 10.1038/onc.2011.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponnusamy M, Zhou X, Yan Y, Tang J, Tolbert E, Zhao TC, et al. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. J Pharmacol Exp Ther. 2014;350:243–256. doi: 10.1124/jpet.113.212076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8:54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Zhao W, Yang JS, Cheng Z, Luo H, Lu Z, et al. Quantitative acetylome analysis reveals the roles of SIRT1 in regulating diverse substrates and cellular pathways. Mol Cell Proteomics. 2012;11:1048–1062. doi: 10.1074/mcp.M112.019547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuno A, Hori YS, Hosoda R, Tanno M, Miura T, Shimamoto K, et al. Resveratrol improves cardiomyopathy in dystrophin-deficient mice through SIRT1 protein-mediated modulation of p300 protein. J Biol Chem. 2013;288:5963–5972. doi: 10.1074/jbc.M112.392050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang XZ, Wen D, Zhang M, Xie Q, Ma L, Guan Y, et al. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-b/Smad3 pathway. J Cell Biochem. 2014;115:996–1005. doi: 10.1002/jcb.24748. [DOI] [PubMed] [Google Scholar]
- 52.Lakota K, Wei J, Carns M, Hinchcliff M, Lee J, Whitfield ML, et al. Levels of adiponectin, a marker for PPAR-γ activity, correlate with skin fibrosis in systemic sclerosis: potential utility as biomarker? Arthritis Res Ther. 2012;14:102. doi: 10.1186/ar3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.