Abstract
Objectives/Hypothesis
With low-dose and titration protocols, subsequent intratympanic (IT) gentamicin injections are frequently necessary for vertigo control in Ménière’s disease. To date, studies have not provided detailed descriptions of the time course of recurrent vertigo and repeated injections. Our objective is to provide such a description with a Kaplan-Meier survival analysis, which may enable accurate predictions of the probability of recurrent vertigo after a given time or number of injections.
Study Design
Injections of IT gentamicin were administered for unilateral definite Ménière’s disease. One injection (or rarely more) in a 6-week period constituted a “round.” Repeat rounds were given when needed for control of recurrent vertigo.
Methods
We used a Kaplan-Meier method to quantify percentages of patients with control of vertigo over an 8-year period. A separate curve was created for each number of rounds, and failure for each was defined as the need for an additional round.
Results
Of 78 patients, 75 (96%) achieved sufficient vertigo control to avoid ablative surgery, and 42 (54%) required only one round. Thirty-six (46%) required multiple rounds. The probability of needing another round increased with each subsequent one, through four rounds. The median times to the next round after one, two, or three rounds were 148, 118, and 124 days, respectively.
Conclusions
More than half of patients need only one round of IT gentamicin injections. With each additional round through the fourth one, the probability of additional rounds increases. Nevertheless, the majority (96%) of patients do not need ablative surgery after IT gentamicin.
Keywords: Repeated intratympanic gentamicin, Ménière’s disease, timing
INTRODUCTION
Ménière’s disease is a clinical syndrome of episodic vertigo, aural fullness, tinnitus, and sensorineural hearing loss. Medical treatment options for patients with Ménière’s disease include salt restriction, diuretics, and steroids.1,2 Patients who are refractory to medical management have been successfully treated with intratympanic (IT) gentamicin injections, which have been shown to control vertigo attacks in the majority of patients since their first reported use by Lange in 1989.3
Meta-analyses4,5 of gentamicin treatment have documented its success in the management of vertigo, but controversy still exists regarding protocols that use multiple injections from the onset, as opposed to titration protocols that use multiple injections only if and when needed for recurrent vertigo. In addition, previous studies have focused on the success rates of various gentamicin protocols, but have not reported the time course of recurrent vertigo or the success of repeated treatments in great detail.
For example, several investigations have studied vertigo control at arbitrary time points following gentamicin treatment. Using a low-dose protocol consisting of two baseline injections spaced 1 week apart, Quaranta et al.6 (2001) showed that 47% of patients had recurrent vertigo 4–16 weeks after the second injection, and 57% of those had further recurrent vertigo 12–20 weeks after the third injection. Another report by Quaranta et al.7 (1999), using a similar protocol but a higher dosage, documented that 27% of patients had recurrent vertigo 3 to 6 months after the second injection and required one additional dose of gentamicin. Harner et al.8 (2001) employed a single, low-dose technique, and found that 41% of subjects required two to four injections, during at least two years of follow-up.
Using a longer follow-up time, Youssef and Poe9 (1998) found that 53% of subjects who initially had complete vertigo control following a protocol of weekly injections required additional treatment several months to 2 years later. Abou-Halawa and Poe10 (2002) reported that 48% of patients receiving a series of one to four 30 mg/mL injections required a second series of injections after 1 year, and 26% of those receiving a series of one to four 40 mg/mL injections required a second series 1 year later. Our experience has been similar: 21% of subjects had recurrent vertigo 10 to 24 months after an initial course of two to four injections and required two to four subsequent injections for control of vertigo, according to criteria of the American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS).11,12
This study provides a more detailed examination of the time course of recurrence of vertigo in patients receiving a titration protocol of gentamicin injections. We use a Kaplan-Meier survival analysis, where a failure event is defined as the recurrence of vertigo and the subsequent need for an additional gentamicin injection. We also analyze the median time intervals between injections, as well as the probability of vertigofree survival following a given number of gentamicin treatments.
MATERIALS AND METHODS
Patients Included in the Analysis
This study was a review of existing clinical data with patient identifiers removed. It qualified for exemption from an institutional review board protocol based on United States Department of Health and Human Services criteria 45 CFR 46.101(b4). The determination that the study was exempt for a protocol requirement was made by the institutional review board.
The 78 patients included in Section I were drawn from a comprehensive database of patients who were treated at our institution with IT gentamicin between December 1997 and July 2006. The criteria for IT gentamicin treatment were: 1) unilateral Ménière’s disease, probable or definite by the 1995 AAO-HNS criteria,11 and 2) failure of salt restriction and diuretic therapy to control vertigo. A subgroup of 48 patients had sufficiently detailed data available regarding the frequency of vertigo attacks before and after IT gentamicin treatment for quantitative analysis. These patients are included in Section II.
Protocol for Gentamicin Treatments
Gentamicin was administered as we have previously described.12,13 The mid-posterior aspect of the tympanic membrane was anesthetized and punctured, and the middle ear was filled with a buffered gentamicin solution (26.7 mg/mL gentamicin, 0.4 mL typically injected). Patients remained supine with the head angled slightly down and turned to the contralateral side for 30 minutes to continually bathe the round window with gentamicin solution. The solution was then aspirated from the external canal.
A single injection of IT gentamicin was given, and the patients were assessed in clinic approximately 3 weeks later to determine if they had vertigo attacks similar to those experienced pretreatment or if they had symptoms and signs expected from the ablation of unilateral vestibular function. If vertigo persisted, injections were repeated at 3-week intervals until eliminated or controlled to the patient’s satisfaction. All of the injections needed to reach this point were considered to constitute the first “round” of treatment. Only two patients out of 78 received two or more injections during this first round.
Subsequent rounds of treatment were similarly given if patients had recurrent vertigo. For those patients who had partial control of their vertigo after the first injection (analogous to AAO-HNS Class B control), recurrence was defined as an increase in the frequency and/or severity of vertigo, compared to the initial results after treatment. At least 6 weeks elapsed between the first injection of a given treatment round and the first injection of a subsequent treatment round. For the majority of patients (all but 6), each round consisted of a single injection.
Data Analysis
Vertigo Rates
At each visit, patients were asked to provide the number of vertigo attacks that had occurred since the last visit. A raw number of vertigo attacks was considered, independent of the degree of disability caused by each attack. A monthly rate of vertigo attacks was calculated based on this number. Paired, two-tailed t-tests were used to compare preand posttreatment rates of vertigo occurrence.
Kaplan-Meier Curves
We used Kaplan-Meier time-to-event methods to quantify the percentages of patients who would have adequate control of their vertigo using a particular definition of “failure.” In the first case, for all patients, we defined failure as requiring a surgically destructive procedure to control vertigo. In this analysis, all of the gentamicin injections done during this study are considered. In subsequent analyses, we considered failure to be the need for additional rounds of treatment beyond the last one given. For example, need for a second injection is considered failure for those who received one prior injection, need for a third injection is considered failure for those who received two prior injections, etc. Follow-up times were administratively censored for all cases, meaning that follow-up was considered to stop on a common date (August 10, 2006) when the analysis was initiated. Kaplan-Meier methods are suited for this analysis because patients had variable durations of follow-up ranging from 2.5 months to 8 years (median 33 months). Kaplan-Meier calculations were performed using Minitab Versions 11 and 15 (Minitab Inc, State College, PA).
RESULTS
Section I—Kaplan-Meier Analysis for Gentamicin Treatments
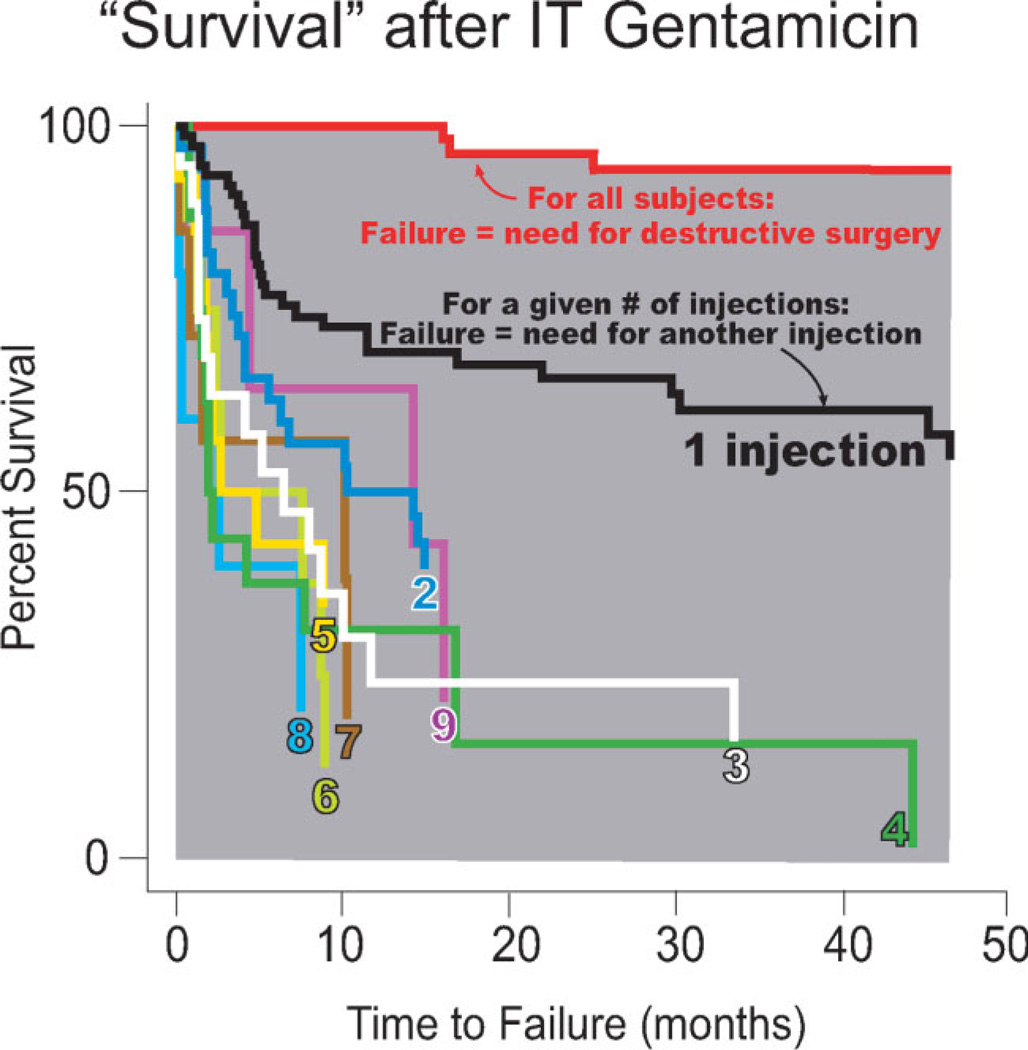
Over the long term, IT gentamicin treatment was effective in controlling vertigo sufficiently to avoid ablative surgery in 75 out of 78 patients (96%) (Fig. 1). The remaining three patients required ablative surgery to treat recurrent vertigo. These data are represented by the uppermost red curve and the underlying gray area in the Kaplan-Meier survival plot (Fig. 1), where a failure event represents initiation of a secondary, surgically destructive procedure to treat recurrent vertigo. Time to failure is measured in units of months, and patients were followed over a period of up to 34.1 ± 23.4 months (range: 2.5–32.7 months).
Fig. 1.
Kaplan-Meier “survival” plot after gentamicin treatment. The uppermost red curve indicates survival for all patients, where failure is defined as the need for destructive surgery. Each remaining curve indicates survival following a given round of gentamicin treatment, for the first through ninth rounds, where failure is instead defined as the need for a subsequent injection. Each separate Kaplan-Meier survival curve shows the percent survival of patients who received a given number of rounds of treatment versus the number of months that they survived following that treatment.
The remaining curves in the Kaplan-Meier plot represent survival analyses specific to each injection, from the first injection through the ninth. For these curves, survival is defined as complete control of vertigo, and a failure event occurs when a patient requires an additional gentamicin injection to manage recurrent vertigo. For instance, all patients are included in the black curve labeled “1 injection.” When a patient “failed” by experiencing recurrent vertigo, he or she received a second injection. Survival data for patients who received a second injection is then plotted on the blue curve labeled “2.” As before, time to failure is measured in months.
Comparison of the survival curves using the log-rank test indicates that statistically significant differences in survival exist for the first through fourth injections (P = .001). The survival curves for the second through fourth injections are located below the 95% confidence interval of the survival curve for the first injection for the first 4 years following treatment. In addition, failure events occur more slowly following the first round of treatment than following any others, suggesting that the first gentamicin round produces the most effective and longest lasting vertigo control. Most failure events happen earlier during the course of treatment, as is shown by the relative steepness of the curves toward the left of the graph.
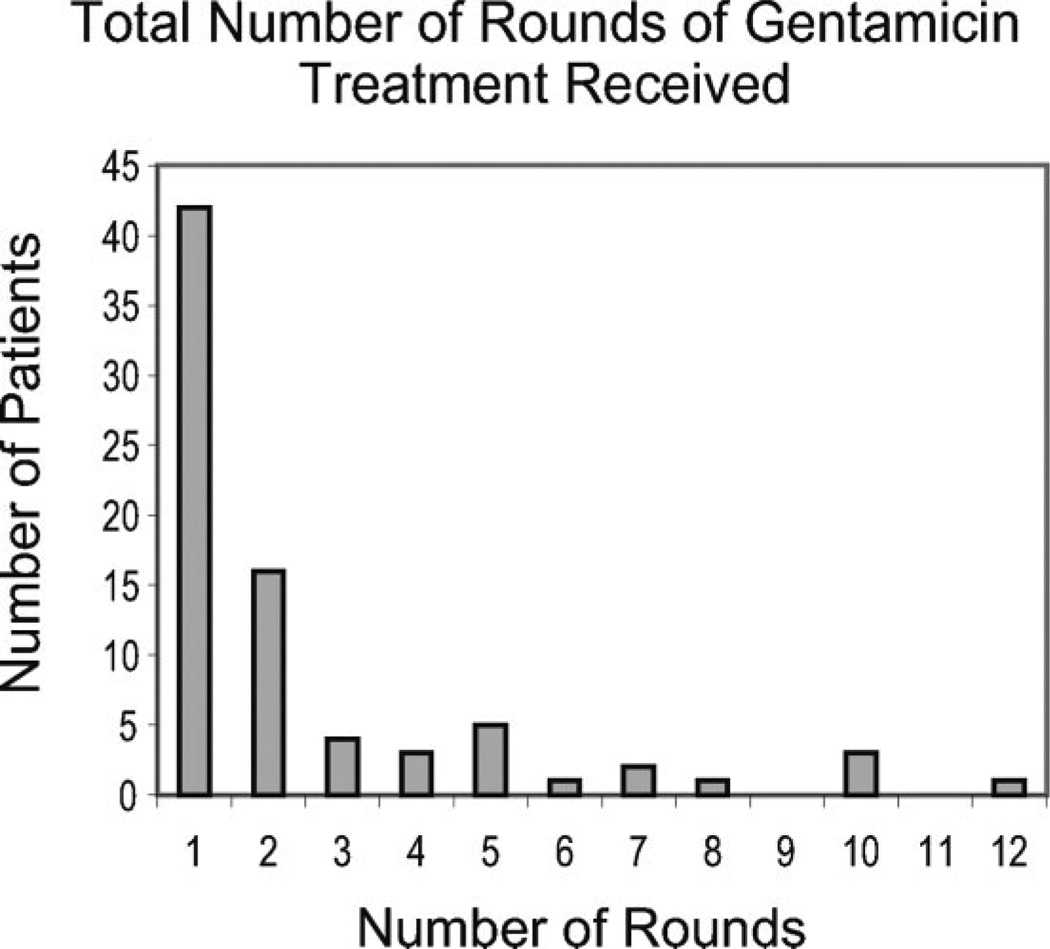
Figure 2 provides a histogram of the total number of rounds of IT gentamicin treatment. Of the 78 patients, 42 (53.8%) received 1 round of treatment, 16 (20.5%) received 2 rounds, 4 (5.1%) received 3 rounds, 3 (3.8%) received 4 rounds, 5 (6.4%) received 5 rounds, 1 (1.3%) received 6 rounds, 2 (2.6%) received 7 rounds, 1 (1.3%) received 8 rounds, 3 (3.8%) received 10 rounds, and 1 subject (1.3%) received 12 rounds of treatment.
Fig. 2.
Total number of rounds of gentamicin treatment received.
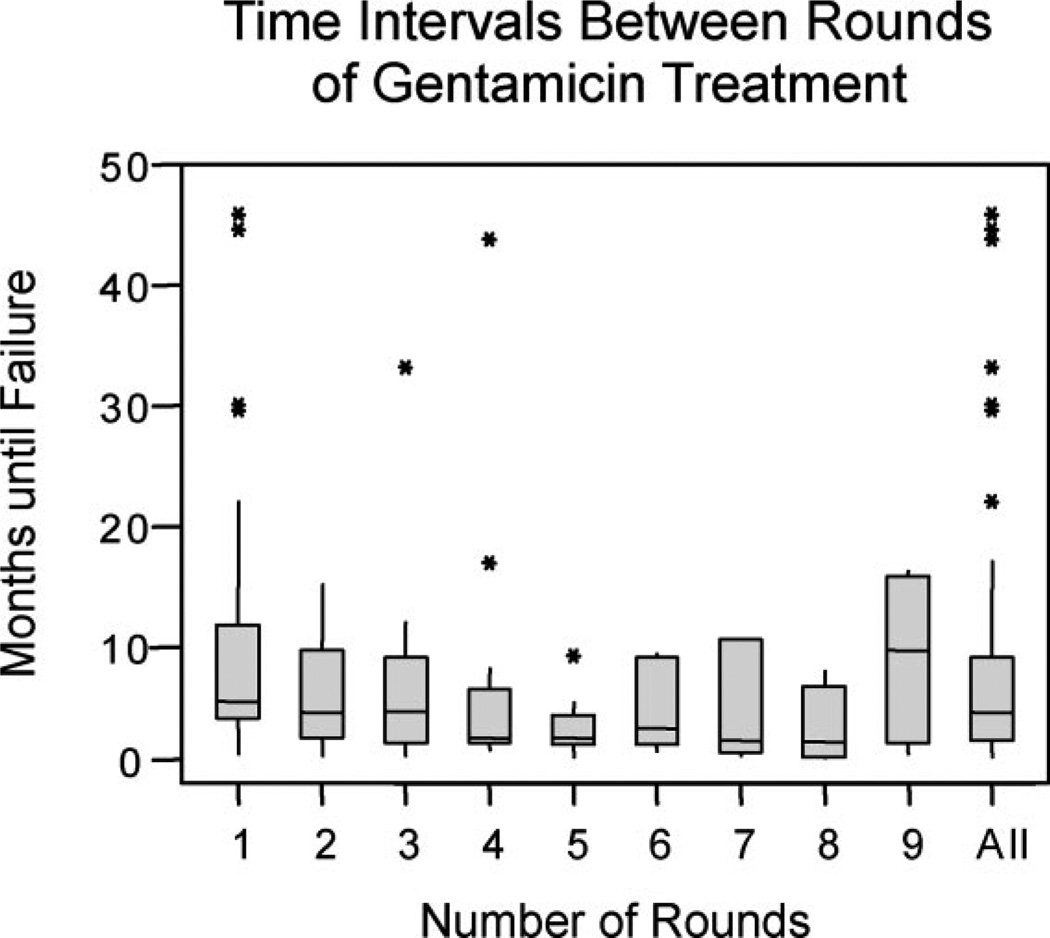
The time intervals between each round of treatment decreased from the second through the fifth rounds of treatment (Fig. 3). The median duration of time between the first and second rounds was 4.9 months, between the second and third rounds was 3.9 months, between the third and fourth rounds was 4.1 months, and between the fourth and fifth rounds was 56 days. An analysis of variance (ANOVA) using a General Linear Model for the first five rounds of treatment yielded P = .34, demonstrating that the decreasing intervals between rounds of treatment for rounds 1 through 5 constitute a trend, but not a statistically significant effect. For subsequent rounds, the median duration of time between rounds was more variable, and, because of small sample sizes, the ranges were large.
Fig. 3.
Duration of time that elapsed between any given round of treatment and the round immediately subsequent to it. (Equivalent to the number of months until failure after a given round of treatment.) The filled boxes span the upper limit of the first quartile (Q1) and the upper limit of the third quartile (Q3); the horizontal line within each box represents the median. The lowermost and uppermost tips of the vertical lines extending from each box represent Q1 − 1.5 * (Q3 − Q1) and Q3 + 1.5 * (Q3 − Q1), respectively, while the stars represent outliers.
The probability of survival without need for further injections was calculated for each number of rounds of treatment (Table I). These data recapitulate the data found in Figure 1, but the table provides a more ready format for prediction of vertigo control at various times after a certain number of rounds of treatment. Again, failure is defined as the receipt of an additional gentamicin injection to treat vertigo, and patients who required surgically destructive procedures to manage their vertigo were excluded from this analysis. Data are categorized according to the treatment number and time elapsed posttreatment. For example, following the first round of treatment, a patient has a 91% chance of vertigo-free survival 100 days after that treatment, and a 70% chance of vertigo-free survival 1 year after that treatment. The chances of vertigo-free survival decrease both with the length of time elapsed posttreatment and the number of rounds of gentamicin treatments received. From this it can be seen that a patient given a single IT gentamicin injection to control vertigo in Ménière’s disease has a two in three chance of vertigo-free survival at 2 years after treatment.
TABLE I.
Probability of Vertigo-Free Survival following × Rounds of Gentamicin Treatment.
| Number of Days Posttreatment | ||||
|---|---|---|---|---|
| Round(s) × of Treatment | 100 | 180 | 365 | 730 |
| After 1 | 91% | 79% | 70% | 63% |
| 2 | 76% | 63% | 50% | 33% |
| 3 | 72% | 67% | 35% | 31% |
| 4 | 50% | 43% | 36% | 9% |
| 5 | 45% | 30% | 20% | 11% |
Rows represent rounds of treatment. Columns represent the number of days after which subjects have completed × rounds of gentamicin treatment.
Section II—Vertigo Control after Initial Injections
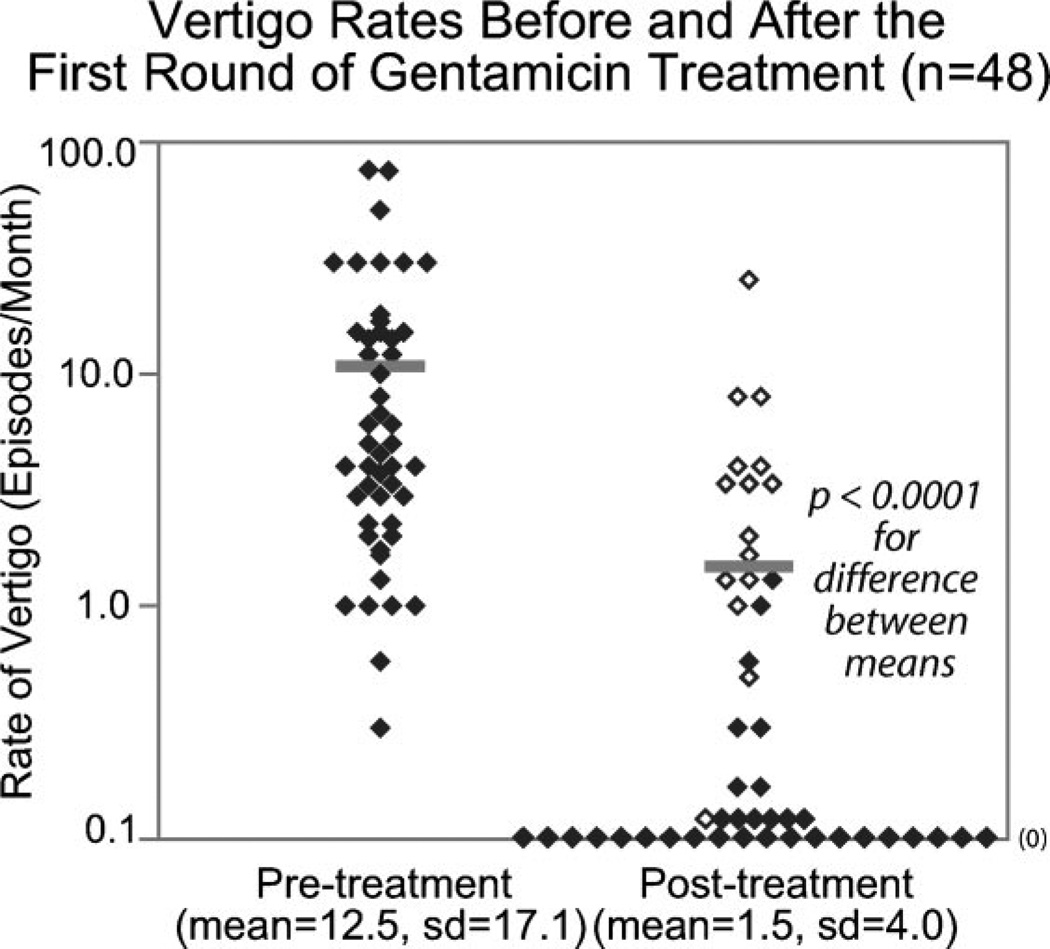
Rates of vertigo occurrence before and after the first round of treatment are reported in Figure 4. Of the 78 patients included in the Kaplan-Meier analysis, estimates of vertigo rates both before and after the first treatment were available for 48 patients. Before treatment, these 48 patients experienced an average of 12.5 ± 17.1 episodes per month (range: 0.3–75 episodes/month, median: 5.0 episodes/month). Following the first injection, the vertigo rate dropped to 1.8 ± 4.3 episodes per month (range: 0–25 episodes/month, median: 0 episodes/month). This represents an 85.6% reduction in vertigo rate (P < .0001, paired, two-tailed t-test). Thirty-five of the 48 patients were seen again within 3 months following treatment, and the mean length of time elapsed between the preinjection and postinjection visits was 4.0 months (range: 14 days to 19.1 months, median: 56 days).
Fig. 4.
Vertigo rates before and after the first round of gentamicin treatment. The filled symbols represent greater than or equal to 60% vertigo control; the open symbols represent less than 60% vertigo control. The bars represent mean values for each group.
As shown in Table II, 75% of patients achieved either complete elimination of vertigo or a 60% or greater reduction in vertigo rate in the time period between the first injection of gentamicin and the subsequent follow-up visit (analogous to AAO-HNS Class A and Class B vertigo control). No patients required a surgically destructive procedure during this time period.
TABLE II.
Control of Vertigo following the First Two Rounds of Gentamicin Treatment.
| Analogous Class (According to AAO-HNS Criteria) |
Definition | First Round of Treatment | Second Round of Treatment | ||
|---|---|---|---|---|---|
| Number of Subjects |
Percentage of Subjects |
Number of Subjects |
Percentage of Subjects |
||
| Class A | Complete elimination | 27 | 56.3 | 5 | 45.5 |
| Class B | Reduction of vertigo frequency to ≤40% of pretreatment frequency | 9 | 18.8 | 4 | 36.4 |
| Class C | Reduction in vertigo frequency to 41%–80% of pretreatment frequency | 7 | 14.6 | 1 | 9.1 |
| Class D | Change in vertigo frequency to 81%–120% of pretreatment frequency | 1 | 2.1 | 0 | 0 |
| Class E | Increase in vertigo frequency to >120% of pretreatment frequency | 4 | 8.3 | 1 | 9.1 |
| Class F | Initiation of secondary treatment because of persistent or recurrent vertigo. | 0 | 0 | 0 | 0 |
Subjects are classified according to the analogous AAO-HNS criteria for vertigo control.
Of the 48 patients, 23 were subsequently treated with a second gentamicin injection to manage recurrent vertigo. These 23 had a mean pretreatment vertigo rate of 8.8 ± 8.6 episodes per month (median: 4.5 episodes/month) and a mean posttreatment vertigo rate of 1.6 ± 2.4 episodes per month (median: 0.6 episodes/month) after their first treatment. Pretreatment and posttreatment data for the second injection were available for 11 of these patients.
Following the second gentamicin injection, 9 of these 11 patients (82%) achieved either complete elimination of vertigo or a 60% or greater reduction in vertigo rate in this time period between the second injection of gentamicin and the follow-up testing session. Again, no subject required a surgically destructive procedure for vertigo control during this time period. Even though these patients still required a second gentamicin injection, comparison of their mean vertigo rates prior to the first injection and prior to the second injection showed a 54% drop in the preinjection monthly vertigo rates. Furthermore, the mean vertigo rate dropped from 4.3 ± 8.9 episodes per month before the second injection (range: 0–30 episodes/month, median: 1.0 episodes/month) to 0.8 ± 1.8 episodes per month (range: 0–6.0 episodes/month, median: 0.3 episodes/month) after that injection. This represents an 81% reduction in vertigo rate (P = 0.24, paired, two-tailed t-test). Postinjection follow-up was obtained within 3 months following treatment for 8 of the 11 patients, and the mean length of time elapsed between the preinjection and postinjection follow-up was 4.5 months (range: 16 days to 10.2 months, median: 4.3 months).
DISCUSSION
Implications of the Findings from the Kaplan-Meier Analysis
In 1995, the AAO-HNS published criteria for the diagnosis and evaluation of therapy in Ménière’s disease.11 Although widely applied in published literature within the past 13 years, the criteria fall short of addressing several issues that have recently arisen. First, these criteria limit the subjects who may be studied by requiring an arbitrary 24-month follow-up period. At any given time, an investigator cannot report on a full cohort, but only on those who meet the criteria for 2-year follow-up. Second, it was not made explicitly clear by the AAO-HNS criteria how to handle repeated treatments, such as IT injection of gentamicin, which are becoming increasingly common. In a meta-analysis of IT gentamicin therapy performed by Chia et al.5 (2004) to evaluate 27 studies, 52% of studies reported using either a titration protocol or a low-dose protocol with repeat injections as needed for recurrent vertigo. Third, the AAO-HNS criteria may bias clinicians to not retreat patients who have recurrent vertigo at time intervals less than 2 years after their initial treatment. As the present data show, one out of three subjects treated with IT gentamicin needed reapplication of this treatment within a 2-year time frame after initial treatment.
The Kaplan-Meier analysis provides an effective and simple means of addressing these concerns. It allows inclusion of all subjects, not just those who meet the criteria for an arbitrary length of follow-up. A typical Kaplan-Meier analysis, such as the one used in this paper, employs a “right-censoring technique,” meaning that subjects with different starting dates are followed until a common termination date for the purpose of the analysis. This allows the investigator to monitor subjects who enter the study at different points in time, and who thus have varying lengths of follow-up. Our analysis allowed us to follow 78 patients who entered the study at varying time points, 56% of whom were followed for over 2 years, with the maximum length of follow-up time being approximately 8 years.
The Kaplan-Meier analysis also provides a fuller representation of the clinical course in treatments that must be repeated over time. Patients in this study received anywhere from 1 to 12 rounds of treatment, but all their data were conveniently plotted on a single chart. Each failure event was clearly shown, and the number of injections and the time at which failure occurred could be read directly from the graph (Fig. 1).
In addition, the Kaplan-Meier analysis enables the investigator to predict the probability of success at any given time after treatment (Table I). Data are continuously available for the entire duration of the study, rather than being limited to specific and predetermined time points.
Our data suggest that recurrent vertigo after a small number of gentamicin injections is not unusual, and does not indicate that further gentamicin treatment would not be successful. We found that recurrent vertigo attacks were significantly less frequent after the first IT gentamicin treatment than they were before this treatment. In most cases, the vertigo control was analogous to AAO-HNS Class A or B control, although 2-year follow-up was not available in all cases, so, strictly speaking, this classification system cannot be applied. No patients required a surgically destructive procedure during this time period, which would be analogous to Class F.
Specifically, upon examination of pre- and posttreatment vertigo rates, we found that 56% of patients achieved complete elimination of vertigo following the first round of treatment, with an additional 19% experiencing a substantial (>60%) reduction in vertigo, measured 120 days after treatment. These results are similar to those obtained by De Beer et al.14 (2007), who assessed vertigo control 6 months after gentamicin treatment and found that 61% of 57 patients had complete vertigo control, while an additional 19% had a substantial reduction in vertigo control. In our series, 70% of subjects who received a single round of IT gentamicin had complete vertigo control at 1 year.
A longer term analysis was performed by Bodmer,15 who analyzed vertigo control 15 years following treatment and reported that a greater percentage, 70% of subjects, experienced no vertigo attacks in the 2-year interval immediately prior to the study. Additionally, Cohen-Kerem et al.4 (2004) conducted a meta-analysis that included 627 patients in 15 trials, showing that 75% of subjects had complete (Class A) vertigo control, with an additional 18% achieving substantial (Class B) control.
The differences between rates of vertigo control reported in these trials may be due to variations in the time intervals between the administration of gentamicin and the posttreatment follow-up of vertigo rates. As our data show, recurrence of vertigo and the probability of needing an additional gentamicin injection are functions of the time elapsed after a given treatment.
Finally, this Kaplan-Meier survival analysis allows clinicians to assess the probability of vertigo-free survival following a given number of rounds of gentamicin treatment, providing quantitative guidance to clinicians and patients as they decide between options for observation, additional injections or ablative surgery. Because of the ease with which IT gentamicin is given, subsequent injections may be recommended by the clinician or sought by the patient for vertigo that is quantitatively and qualitatively less severe than on initial presentation. Furthermore, these subsequent injections may not be needed for long periods of time.
All of the patients treated in this study had unilateral Ménière’s disease, and we cannot extrapolate the same results to cases of bilateral Ménière’s disease. Successful results have been reported for the treatment of both ears in bilateral Ménière’s disease with IT gentamicin.16 However, patients with bilateral Ménière’s disease may have more difficulty after bilateral IT gentamicin treatment because of the difficulties of compensating for bilateral vestibular loss. This would argue even more strongly for a titration approach guided by data such as are provided in this study.
Comparison of Treatment with Intratympanic Gentamicin to Ablative Therapy
Investigations into the mechanism of action of gentamicin are ongoing. Studies of vestibular hair cells in chinchillas have found that intratympanic gentamicin injection caused a 57% decrease in hair cell density, and more specifically, a 99% decrease in Type I hair cell density and a relative preservation of Type II hair cell density.17 Lyford-Pike et al.18 (2007) demonstrated that gentamicin was preferentially accumulated by Type I hair cells of chinchillas, suggesting that the selective damage to Type I hair cells was caused by their increased uptake. Additionally, studies of vestibular nerve afferents have shown that gentamicin treatment in chinchillas produced no effect on the relative ratios of regular, intermediate, and irregular afferents.17 However, gentamicin was shown to selectively decrease the spontaneous firing rate of regular afferents, with minimal effect of the rates of irregular and intermediate afferents. This is in contrast to surgical labyrinthine ablation, which should markedly reduce or completely eliminate vestibular nerve afferent activity.
IT gentamicin treatment is also not without risks. The literature documents that hearing loss can occur at rates ranging from 0%19 to 90%,20 and a meta-analysis by Chia et al.5 found that there was an average rate of hearing loss of 25%. Furthermore, some patients experience prolonged disequilibrium after IT gentamicin treatment.10,20–22 However, this is rare in our experience, and the disequilibrium that occurs in the early period after IT gentamicin treatment responds well to vestibular physical therapy. Of note, only 4% of our patients failed IT gentamicin therapy and required ablative surgery.
A cost analysis performed at our institution showed that IT gentamicin is a much more cost-effective treatment than ablative surgical procedures such as a labyrinthectomy or vestibular nerve section. In our study, patients received an average of 2.5 injections, and the total cost of these 2.5 injections was sixfold lower than that of a labyrinthectomy and 22-fold lower that of a vestibular nerve section.
CONCLUSIONS
Ninety-six percent of patients with Ménière’s disease treated with IT gentamicin therapy achieved sufficient vertigo control to avoid ablative surgery. Furthermore, approximately one-half (54%) of patients required only a single round of injections, with the majority of these being only one injection. For those who needed multiple injections, the Kaplan-Meier survival analysis demonstrated that the probability of requiring more injections increased up to the fourth injection. The Kaplan-Meier survival analysis offers an efficient method for describing treatment success or failure when repeated treatments are given as needed among patients who are followed for variable lengths of time.
Acknowledgments
Source of financial support or funding: NIH R01 DC05040; Doris Duke Charitable Foundation Clinical Research Fellowship.
Footnotes
These results were presented at the 30th Midwinter Meeting of the Association for Research in Otolaryngology, held in Denver, Colorado, U.S.A., on February 10–15, 2007.
BIBLIOGRAPHY
- 1.Schessel D, Minor LB, Nedzelski J. Ménière’s disease and other peripheral vestibular disorders. In: Cummings CW, et al., editors. Otolaryngology—Head and Neck Surgery. 4th ed. Chicago: Mosby Year Book; 2004. pp. 3209–3253. [Google Scholar]
- 2.Boleas-Aguirre MS, Lin FR, la Santina CC, Minor LB, Carey JP. Longitudinal results with intratympanic dexamethasone in the treatment of Ménière’s disease. Otol Neurotol. 2008;29:33–38. doi: 10.1097/mao.0b013e31815dbafc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange G. Gentamicin and other ototoxic antibiotics for the transtympanic treatment of Ménière’s disease. Arch Otorhinolaryngol. 1989;246:269–270. doi: 10.1007/BF00463571. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Kerem R, Kisilevsky V, Einarson TR, Kozer E, Koren G, Rutka JA. Intratympanic gentamicin for Ménière’s disease: a meta-analysis. Laryngoscope. 2004;114:2085–2091. doi: 10.1097/01.mlg.0000149439.43478.24. [DOI] [PubMed] [Google Scholar]
- 5.Chia SH, Gamst AC, Anderson JP, Harris JP. Intratympanic gentamicin therapy for Ménière’s disease: a meta-analysis. Otol Neurotol. 2004;25:544–552. doi: 10.1097/00129492-200407000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Quaranta A, Scaringi A, Aloidi A, Quaranta N, Salonna I. Intratympanic therapy for Ménière’s disease: effect of administration of low concentration of gentamicin. Acta Otolaryngol. 2001;121:387–392. doi: 10.1080/000164801300102879. [DOI] [PubMed] [Google Scholar]
- 7.Quanranta A, Aloisi A, DeBenedittis G, Scaringi A. Intra-tympanic therapy for Ménière’s disease. High-concentration gentamicin with round-window protection. Ann NY Acad Sci. 1999;884:410–424. doi: 10.1111/j.1749-6632.1999.tb08658.x. [DOI] [PubMed] [Google Scholar]
- 8.Harner SG, Driscoll CL, Facer GW, Beatty CW, McDonald TJ. Long-term follow-up of transtympanic gentamicin for Ménière’s syndrome. Otol Neurotol. 2001;22:210–214. doi: 10.1097/00129492-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Youssef TF, Poe DS. Intratympanic gentamicin injection for the treatment of Ménière’s disease. Am J Otol. 1998;19:435–442. [PubMed] [Google Scholar]
- 10.Abou-Halawa AS, Poe DS. Efficacy of increased gentamicin concentration for intratympanic injection therapy in Ménière’s disease. Otol Neurotol. 2002;23:494–502. doi: 10.1097/00129492-200207000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Monsell EM, Balkany TA, Gates GA, Goldenberg RA, Meyerhoff WL, House JW. Committee on hearing and equilibrium guidelines for the diagnosis and evaluation of therapy in Ménière’s disease. Otalaryngol Head Neck Surg. 1995;113:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 12.Minor LB. Intratympanic gentamicin for control of vertigo in Ménière’s disease: vestibular signs that specify completion of therapy. Am J Otol. 1999;20:209–219. [PubMed] [Google Scholar]
- 13.Lin FR, Migliaccio AA, Haslwanter T, Minor LB, Carey JP. Angular vestibulo-ocular reflex gains correlate with vertigo control after intratympanic gentamicin treatment for Ménière’s disease. Ann Otol Rhinol Laryngol. 2005;114:777–785. doi: 10.1177/000348940511401007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Beer L, Stokroos R, Kingma H. Intratympanic gentamicin therapy for intractable Ménière’s disease. Acta Otolaryngol. 2007;127:605–612. doi: 10.1080/00016480600951475. [DOI] [PubMed] [Google Scholar]
- 15.Bodmer D, Morong S, Stewart C, Alexander A, Chen JM, Nedzelski JM. Long-term vertigo control in patients after intratympanic gentamicin instillation for Ménière’s disease. Otol Neurotol. 2007;28:1140–1144. doi: 10.1097/mao.0b013e31815aea05. [DOI] [PubMed] [Google Scholar]
- 16.Pyykko I, Ishizaki H, Kaasinen S, Aalto H. Intratympanic gentamicin in bilateral Ménière’s disease. Otolaryngol Head Neck Surg. 1994;110:162–167. doi: 10.1177/019459989411000204. [DOI] [PubMed] [Google Scholar]
- 17.Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005;93:643–655. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- 18.Lyford-Pike S, Vogelheim C, Chu E, la Santina CC, Carey JP. Gentamicin is primarily localized in vestibular type I hair cells after intratympanic administration. J Assoc Res Otolaryngol. 2007;8:497–508. doi: 10.1007/s10162-007-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnusson M, Padoan S. Delayed onset of ototoxic effects of gentamicin in treatment of Ménière’s disease. Rationale for extremely low dose therapy. Acta Otolaryngol. 1991;111:671–676. doi: 10.3109/00016489109138398. [DOI] [PubMed] [Google Scholar]
- 20.Schoendorf J, Neugebauer P, Michel O. Continuous intratympanic infusion of gentamicin via a microcatheter in Ménière’s disease. Otolaryngol Head Neck Surg. 2001;124:203–207. doi: 10.1067/mhn.2001.112310. [DOI] [PubMed] [Google Scholar]
- 21.Laitakari K. Intratympanic gentamycin in severe Ménière’s disease. Clin Otolaryngol Allied Sci. 1990;15:545–548. doi: 10.1111/j.1365-2273.1990.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 22.Perez N, Martin E, Garcia-Tapia R. Intratympanic gentamicin for intractable Ménière’s disease. Laryngoscope. 2003;113:456–464. doi: 10.1097/00005537-200303000-00013. [DOI] [PubMed] [Google Scholar]