Abstract
The frank loss of a large volume of skeletal muscle (i.e., volumetric muscle loss [VML]) can lead to functional debilitation and presents a significant problem to civilian and military medicine. Current clinical treatment for VML involves the use of free muscle flaps and physical rehabilitation; however, neither are effective in promoting regeneration of skeletal muscle to replace the tissue that was lost. Toward this end, skeletal muscle tissue engineering therapies have recently shown great promise in offering an unprecedented treatment option for VML. In the current study, we further extend our recent progress (Machingal et al., 2011, Tissue Eng; Corona et al., 2012, Tissue Eng) in the development of tissue engineered muscle repair (TEMR) constructs (i.e., muscle-derived cells [MDCs] seeded on a bladder acellular matrix (BAM) preconditioned with uniaxial mechanical strain) for the treatment of VML. TEMR constructs were implanted into a VML defect in a tibialis anterior (TA) muscle of Lewis rats and observed up to 12 weeks postinjury. The salient findings of the study were (1) TEMR constructs exhibited a highly variable capacity to restore in vivo function of injured TA muscles, wherein TEMR-positive responders (n=6) promoted an ≈61% improvement, but negative responders (n=7) resulted in no improvement compared to nonrepaired controls, (2) TEMR-positive and -negative responders exhibited differential immune responses that may underlie these variant responses, (3) BAM scaffolds (n=7) without cells promoted an ≈26% functional improvement compared to uninjured muscles, (4) TEMR-positive responders promoted muscle fiber regeneration within the initial defect area, while BAM scaffolds did so only sparingly. These findings indicate that TEMR constructs can improve the in vivo functional capacity of the injured musculature at least, in part, by promoting generation of functional skeletal muscle fibers. In short, the degree of functional recovery observed following TEMR implantation (BAM+MDCs) was 2.3×-fold greater than that observed following implantation of BAM alone. As such, this finding further underscores the potential benefits of including a cellular component in the tissue engineering strategy for VML injury.
Introduction
Craniofacial and extremity soft tissue trauma resulting in a permanent loss of skeletal muscle mass is a clinical challenge for both military and civilian medicine. This type of injury has been termed volumetric muscle loss (VML) and is defined as “the traumatic or surgical loss of skeletal muscle with resultant functional impairment.”1 The current standard of care for VML injury involves the use of free muscle transfer (i.e., muscle flaps) followed by extensive physical rehabilitation. However, free muscle flaps do not significantly restore muscle function to the injured tissue, and are often primarily used for bony coverage. Functional muscle transfer, in which vasculature and innervation are surgically restored to the graft have been shown to improve strength to an injured muscle group,2 but these procedures require an extraordinary level of surgical expertise that is not likely available at most treatment centers. In either case, the use of large muscle flaps presents an added complication of donor-site morbidity to the patient.3 Lastly, while there is little evidence that physical rehabilitation alone is sufficient to significantly restore strength to muscle with VML injury, physical rehabilitation performed with an energy-storing ankle-foot orthosis has recently proven to be successful in restoring function to patients with extremity trauma.4
In this scenario, skeletal muscle tissue engineering and regenerative medicine therapies offer a promising treatment paradigm for severe soft tissue trauma resulting in the loss of a large volume of musculature. Therapeutic strategies are under development for the repair of VML to craniofacial and extremity muscles, and similar injury conditions (e.g., abdominal wall repair).5–15 Serving as a foundation for many of these strategies, biological extracellular matrices (ECMs) derived from the intestine and bladder (porcine), and skeletal muscle (variety of species) have been studied in in vivo preclinical skeletal muscle injury models previously.7,8,10,12,15,16 Most studies have shown limited regeneration of muscle fibers after transplantation (e.g., Refs.8,9,15,17). The only clinical report currently available describes the use of an acellular matrix for the treatment of quadriceps muscle with VML injury.18 Whereas these initial findings are encouraging for the overall tissue engineering paradigm, only somewhat minor strength gains were observed with continued physical therapy.18
As an extension of this approach, the therapeutic benefit of a combined transplantation of a biological scaffold with stem or progenitor cells has also been characterized thoroughly in preclinical studies in vivo.5,6,8–10,19–21 The rationale for inclusion of a cellular component to skeletal muscle tissue engineering therapies is based on the numerous beneficial effects that have been reported. For example, depending on the cell type used, transplanted cells can improve revascularization, modulate the inflammatory response, reduce fibrosis, or contribute directly to muscle fiber regeneration.22–25 In fact, the combination of bone marrow mesenchymal stem cells with rat muscle ECM,9,10 freshly isolated satellite cells with hyaluronan-based gel,21 and muscle-derived progenitor cells with bladder acellular matrix (BAM)8 have all demonstrated partial restoration of force production following implantation in surgically created VML injuries. In all cases, the observed degree of functional muscle repair and regeneration was superior to the delivery of the respective matrix alone, providing clear evidence for the therapeutic benefit of including a cellular component to tissue engineering strategies designed for the treatment of VML.
In this regard, our recent efforts have exploited this latter strategy and focused on the development of an in vitro method to create tissue engineered muscle repair (TEMR) constructs for the treatment of skeletal muscle tissue following traumatic injury.5,8,26 To date, we have demonstrated that TEMR constructs, which have been created by seeding Lewis rat muscle-derived cells (MDCs) on a BAM, mediate restoration of significant functional capacity (60–70% recovery) to VML injured latissimus dorsi (LD) muscle in nude mice.5,8 This recovery appears to involve, at least to some extent, regeneration of a portion of the muscle fibers that were surgically removed. In support of this supposition, we have observed presumptive formation (regeneration) of new muscle fibers in the area of the defect, where the construct was transplanted, and furthermore, noted that these regenerating fibers express key proteins required for force production and transmission, as well as supporting vascular and neural structures.5,8
In this study, we report our most recent progress in developing TEMR constructs for eventual clinical use. In the current study, the therapeutic potential of TEMR constructs was tested in a Lewis rat tibialis anterior (TA) muscle VML model. The use of the model introduced three important advances relative to our previous studies for the repair of VML injury: (1) the muscle defect is approximately fourfold greater by weight in the female Lewis rat TA muscle than the nude mouse LD muscle, (2) the Lewis rat has a competent immune system, and (3) functional analysis is performed in vivo with neural stimulation nominally requiring regenerating muscle fibers to be innervated to contribute to functional recovery. The results of this study indicate that TEMR constructs as well as BAM scaffolds can improve neural-stimulated in vivo functional recovery following VML injury. However, there was marked variability in the therapeutic response of TEMR constructs that appears to be related to a differential inflammatory response within the TEMR construct population. Discussion is provided to highlight underlying mechanisms for these findings and future directions for the translation of the TEMR technology.
Materials and Methods
Experimental design
This study was designed to perform repeated in vivo functional measurements with the same animal (leg) over a 3-month period using a repeated measures experimental design. Baseline in vivo functional assessments were performed through neural stimulation before VML injury was surgically created in the TA muscle and then every month thereafter for 3 months. After the last in vivo assessment, TA muscles were harvested for morphological and histological analysis. Since the extensor hallicus longus (EHL), extensor digitorum longus (EDL), and TA muscle are innervated by the common peroneal nerve and the EDL and EHL muscles hypertrophy in response to VML injury in the TA muscle, in all experimental VML groups, the EDL and EHL muscles were ablated. To account for any potential strength changes of the TA muscle due to synergist ablation and to provide a theoretical baseline for TA muscle recovery, a group of rats served as a surgical sham, wherein the synergists were ablated, but no VML injury was created in the TA muscle. In total, there were five experimental groups in this study: (1) An unoperated group that served as an age-matched cage control (n=8), a sham/ablation group in which the TA muscle did not have a VML defect, but underwent sham surgery with ablation of the EDL muscle (n=6), a VML injured, but nonrepaired group (n=4), a VML injured with BAM repair group (n=7), and a VML injured TEMR repaired group (n=13). All VML injured groups underwent EDL muscle ablation.
Animals
Male Lewis rats aged 3–4 weeks were used as donors for MDCs, as previously reported.5,8 VML injury studies were performed with female Lewis rats aged 12–14 weeks at the beginning of the study. All rats were purchased from Harlan laboratories. All animal procedures were approved by the Wake Forest University IACUC.
BAM preparation
BAM scaffolds were prepared from the porcine urinary bladder as previously described.5,8 Briefly, the bladder was washed and trimmed to obtain a crude sample of the lamina propria (i.e., some muscle tissue remaining attached), which was placed in 0.05% Trypsin (Hyclone, Logan, UT) for 1 h at 37°C. The bladder was then transferred to the DMEM solution supplemented with 10% FBS and 1% antibiotic/antimycotic and kept overnight at 4°C. The preparation was then washed in a solution containing 1% Triton X (Sigma-Aldrich, St Louis, MO) and 0.1% ammonium hydroxide (Fisher Scientific, Fairlown, NJ) in deionized water for 4 days at 4°C. Finally, the bladder was then washed in deionized water for 3 days at 4°C. The decellularized scaffold was then further dissected by hand to remove any remaining muscle tissue layers to obtain a scaffold of 0.2–0.4 mm thickness. The prepared acellular matrix was then cut into strips of 3×2 cm size and placed onto a custom designed seeding chamber made of silicon (McMaster Carr, Cleveland, OH). Scaffolds and silicon seeding chambers were then individually placed in culture dishes and sterilized by ethylene oxide.
TEMR construct creation
TEMR constructs were generated following previously described cell culture, seeding, and bioreactor preconditioning protocols.5,8 Briefly, BAM scaffolds were seeded with MDCs (≈1 million per cm2) on each side, cultured statically for 10 days allowing for proliferation and differentiation, and then preconditioned in a bioreactor with uniaxial mechanical strain (10% strain, three stretches per minute for the first 5 min of each hour) as described in detail elsewhere.5,8
Surgical procedures
Surgical creation of VML injury was performed in the TA muscle as previously reported.27 Briefly, a longitudinal skin incision was made along the lateral aspect of the left lower leg. The skin was separated bluntly from the underlying fascia covering the anterior compartment. The fascia covering the anterior crural muscles was then separated using blunt dissection. The proximal and distal tendons of the EDL muscle were then isolated and severed. Next, the EHL muscle located underneath the TA muscle was isolated and ablated. Care was taken to avoid disruption of the retinaculum holding the distal tendon of the TA muscle in position. VML injury was created in the TA muscle by excising ∼20% of the TA muscle weight at the middle third of the muscle (Fig. 1C).27 A 20% TA muscle defect was approximated using linear regression determined experimentally (n=16) to estimate TA muscle mass from rat body weight: TA wet weight (mg)=1.553×body weight (g)+83.084 (R2=0.85).
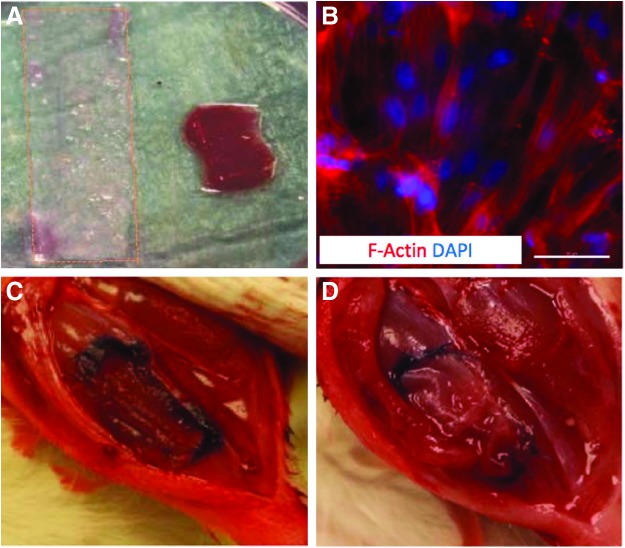
FIG. 1.
TA muscle VML injury repair with BAM scaffold and TEMR constructs. (A) A 3×0.5 cm strip of BAM or TEMR was folded in triplicate before transplantation. (B) TEMR constructs comprised BAM with an aligned cellular content (MDCs were seeded at 1 million cells per cm2). Scale bar=50 μm. (C) VML injury was surgically created by making a ≈1×0.5×0.3 cm defect in the left TA muscle and was (D) immediately treated with BAM or TEMR constructs that were sutured in the fresh wound bed as depicted and described in the Materials and Methods. BAM, bladder acellular matrix; MDCs, muscle-derived cells; TA, tibialis anterior; TEMR, tissue engineered muscle repair; VML, volumetric muscle loss. Color images available online at www.liebertpub.com/tea
Repair of the TA muscle was accomplished by folding TEMR or BAM constructs (≈3×0.5 cm) in triplicate creating an ≈1×0.5 cm construct with three layers (Fig. 1A). The transplant therefore approximated the volume of the defect. The thrice-folded transplant was placed in the defect and the four corners and four margins were sutured (6-0 Vicryl) to the borders of the defect (Fig. 1D). The fascia was closed with 6-0 vicryl and the skin was closed with 5-0 prolene using interrupted sutures. Skin glue was applied between the skin sutures to help prevent the incision from opening. Following surgery, buprenorphine (0.05 mg/kg; sc) was administered every 12 h for 3 days.
In vivo functional analysis
Torque production of the left anterior crural muscles (primarily due to the contraction of the TA and EDL muscles) was measured in vivo using similar methodology as previously described.27–30 After rats were anesthetized (1.5–2.5% isoflurane), the left hindlimb was aseptically prepared. The rat was then placed on a heated platform. The left knee was clamped and the left foot was secured to a custom-made foot plate that is attached to the shaft of an Aurora Scientific 305C-LR-FP servomotor, which in turn was controlled using a PC. Sterilized percutaneous needle electrodes were inserted through the skin for stimulation of the left common peroneal nerve. Electrical stimulus was applied using a Grass S88 stimulator with a constant current SIU (Grass Model PSIU6). Stimulation voltage and needle electrode placement were optimized first with a series of twitch contractions at 1 hz and then with 5 to 10 isometric contractions (400 ms train of 0.05–0.1 ms pulses at 150 Hz). Contractile function of the anterior crural muscles was assessed by measuring peak isometric tetanic torque derived from the maximal response to a range of stimulation frequencies (100–200 Hz). For real-time analysis of torque and length changes, voltage outputs were sampled at 4000 Hz, converted to a digital signal using an A/D board (National Instruments PCI 6221), and recorded using a PC loaded with a custom-made Labview®-based program (provided by the U.S. Army Institute of Surgical Research).
Morphological analysis of VML injured TA muscle
TA muscles were isolated from the leg and laid on a platform with the superior aspect of the muscle facing away from the platform. At the middle of the proximal and distal thirds of the muscle, the width of the muscle was measured using digital calipers.
Histology and immunohistochemistry
TA muscles from all experimental groups were fixed in 10% neutral buffered formalin and stored in 60% ethanol. All samples were processed (ASP300S; Leica Microsystems, Bannockburn, IL) and then embedded in paraffin (EG1160; Leica Microsystems). Seven-μm-thick serial sections were cut from the paraffin embedded blocks and Masson's Trichrome staining and immunohistochemical staining were performed using standard procedures as previously described.5 Immunohistochemical staining was performed using antibodies to detect myosin (MF-20, 1:10; Developmental Studies Hybridoma Bank, Iowa City, IA) and macrophages (CD68, 1:50; Serotec, Raleigh, NC). Images were captured and digitized (DM4000B Leica Upright Microscope; Leica Microsystems) at varying magnifications.
Statistics
Morphological and functional data were analyzed using one- and two-way ANOVAs as indicated in the text. Upon finding a significant ANOVA, post hoc comparison testing of parameters of interest was performed using Fisher's LSD. Statistical significance was achieved at an alpha <0.05. Values presented are mean±SEM. Unless otherwise stated, all mean values are expressed as the arithmetic mean.
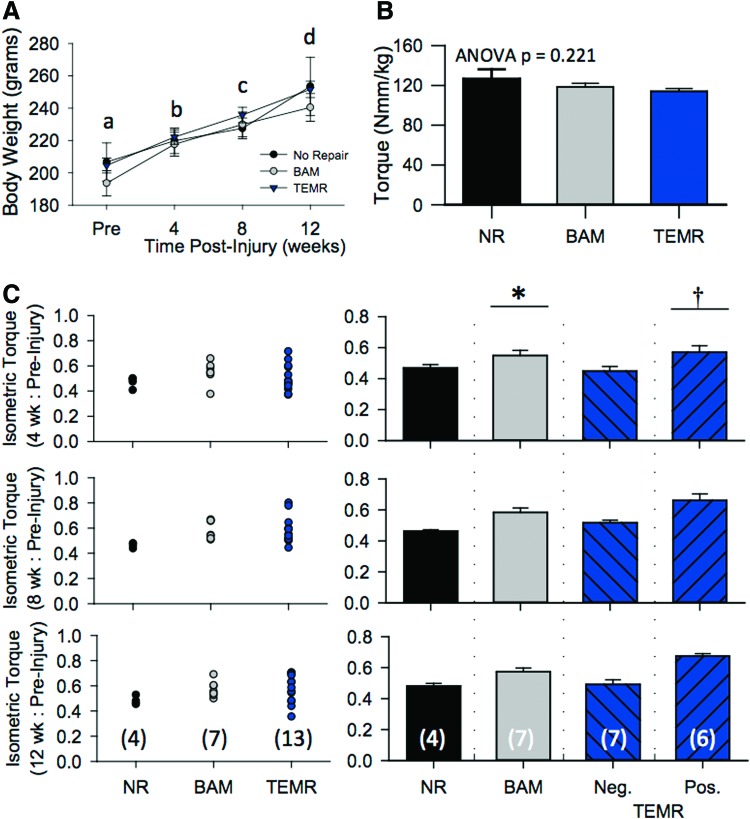
Notably during the course of the study, TEMR responses demonstrated a markedly greater variability than either NR or BAM (Fig. 3). Because of the relatively large variability, the sample size for the TEMR group was increased to 13 observations in an effort to reduce the variability within this group. Ultimately, the coefficient of variation (CV; standard deviation/mean) for the recovery of peak torque 12 weeks postinjury was approximately two times greater for TEMR treated (CV=0.20) than no repair (NR) (CV=0.07) or BAM (CV=0.11) groups. In accordance with this greater variance, the recovery of peak torque (12 weeks postinjury: Pre) ranged from 0.36 to 0.71, indicating a range of functional recoveries from essentially zero (no recovery) to complete TA muscle functional recovery, respectively, across 13 observations (Fig. 3C). In light of this divergent response, the TEMR group was separated into positive and negative responders based on the following criteria. A TA muscle transplanted with a TEMR construct that recovered a peak torque value greater than 1 standard deviation (determined from a combination of BAM and TEMR observation; mean=0.57, sd=0.09) above the mean for the NR group was considered a positive responder.
FIG. 3.
TEMR improves functional recovery of rat TA muscle with VML injury. (A) Group mean rat body weight over the course of 12 weeks. No differences were observed among groups at each time point (see results). All means at each time point demarked with different letters are significantly different (p<0.05). (B) To control for the effect of normal growth over the 3-month study on peak isometric torque production, torque was normalized to body weight (Nmm/kg body weight). Before VML, the average peak isometric torque for each experimental group was similar. (C) Individual responses at each postinjury time point (4, 8, and 12 weeks) were normalized to their respective preinjury group mean. Individual responses and group means at each time point postinjury are presented. Across all time points, BAM significantly restored torque to a greater extent than NR- or TEMR-negative responders (*), while TEMR-positive responders improved torque recovery to a greater extent than all other experimental groups (†). Where applicable, values are listed as mean±SEM. Group sample sizes are listed in parentheses in the 12-week response in panel C. NR, no repair. Color images available online at www.liebertpub.com/tea
Results
Synergist muscle ablation
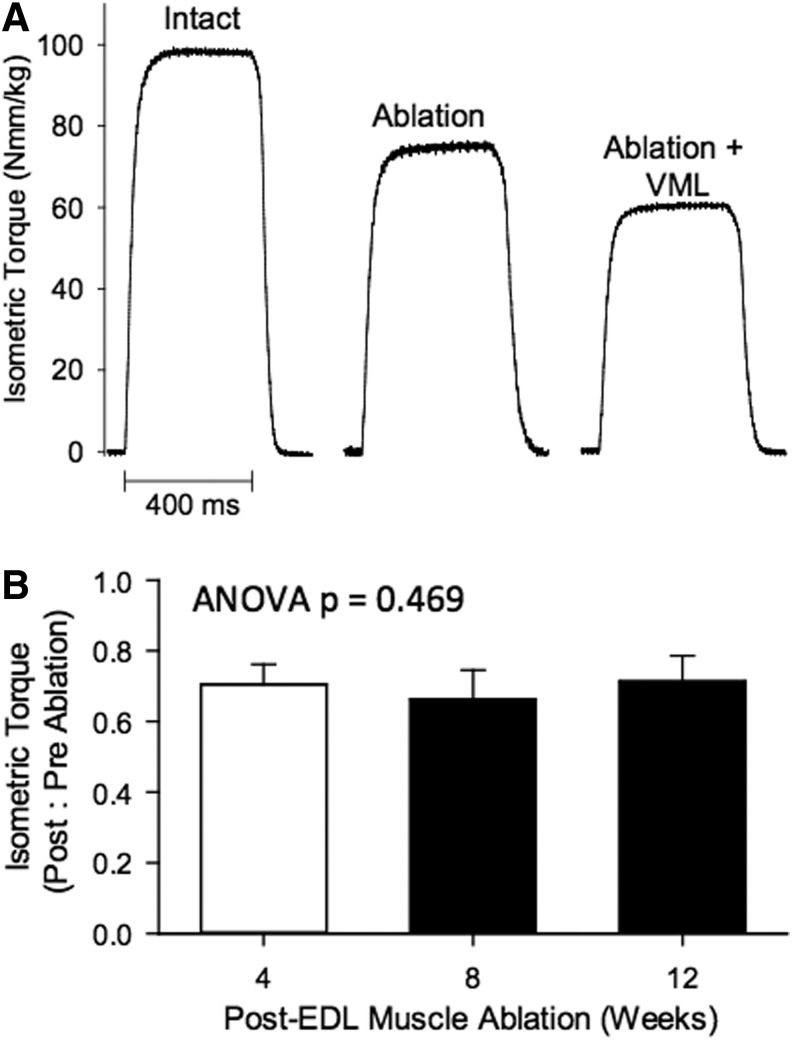
Repeated in vivo functional testing was performed on each animal at a monthly interval over a 3-month time frame to assess the functional capacity of the TA muscle following VML injury and/or repair. To accurately assess the impact of the VML injury as well as recovery of the TA muscle over time in the various treatment groups, the anterior crural muscle synergists (EDL and EHL muscles) to the TA muscle were ablated at the time of VML injury. The EDL and EHL muscles comprised a combined 20.3%±1.3% of the anterior crural muscle wet weight (i.e., Uninjured TA, EDL, & EHL muscle wet weight: 421.0±6.6, 96.7±1.2, 10.9±0.3 mg, respectively), suggesting the potential for TA muscle overload. Functionally, removal of the EDL and EHL muscles resulted in a −29.4%±7.4% loss of peak torque from 4 to 12 weeks postablation when normalized to preinjury values (Fig. 2). Twelve weeks postablation, wet weight was similar among TA muscles from the ablated (446±35 mg) and contralateral control (440±27 mg) legs (p=0.701), indicating that synergist ablation did not provide a significant overload to uninjured muscle similar to that reported for the posterior compartment.31 Importantly, because synergist ablation resulted in an ≈30% reduction of normalized peak tetanic torque, a complete recovery of TA muscle function following VML injury is reflected by the return of ≈70% of preinjury tetanic torque on that same animal using this metric.
FIG. 2.
In vivo isometric torque of the anterior crural muscles before and after TA muscle synergist ablation and VML. (A) Representative digitized torque tracings are presented for uninjured intact anterior crural muscles (TA, EDL, & EHL muscles), and after ablation of the EDL and EHL muscles and subsequent VML. Note: Following ablation (4–12 weeks), the TA muscle produced ≈70% of net anterior crural muscle torque. Subsequent VML of the TA muscle (see Materials and Methods) resulted in a further ≈20% deficit, resulting in a combined ≈50% deficit compared to preinjury (intact) torque. (B) TA muscle peak isometric torque (Nmm/kg body wt) normalized to preinjury values was stable from 4 to 12 weeks postsynergist ablation. EDL, extensor digitorum longus; EHL, extensor hallicus longus.
Creation of VML injury
VML injury was created by surgically excising ≈20% of muscle mass from the middle third of the TA muscle. After surgical excision, animals were divided into 3 treatment groups, no repair (NR), BAM implantation, and TEMR implantation groups. Equivalent injuries were created in all treatment groups, as reflected by the similar weight of muscle removed from the animals in the NR (70.8±6.7 mg), BAM (63.1±3.1 mg), and TEMR (69.3±1.6 mg) treatment groups). For nonrepaired muscles (surgical defect and synergist ablation), peak tetanic torque was reduced compared to preinjury values by 51.5%±2.8%, 51.4%±3.2%, and 49.6%±3.0% at 4, 8, and 12 weeks postinjury, respectively, indicating that, over this time frame, the VML injury did not recover spontaneously (Fig. 3). Twelve weeks postinjury, TA muscle wet weight was ≈17% less for the VML injured NR group than in contralateral control muscles. Together, these results indicate that the TA muscle surgical defect produces an irrecoverable torque deficit of ≈20% of the TA muscle functional capacity up to 12 weeks postinjury (with the remainder of the ≈50% torque deficit attributed to ablation of the EDL and EHL).
Treatment in vivo of VML injury
TA muscle strength assessment
VML injury was repaired by either transplanting BAM scaffolds or TEMR constructs to the defect site immediately after the injury. Whereas the body weight was similar among all experimental groups (i.e., NR, BAM, and TEMR) during the study, over the 12 weeks postinjury, each group gained significant body weight (Fig. 3A; Experimental Group x Time ANOVA p=0.625, Time ANOVA p<0.001). To control for increases in in vivo torque production due to animal growth, peak torque was normalized to body weight (Nmm/kg body wt).32,33 There were no differences in preinjury (anterior crural muscles intact) torque values among the experimental groups (Fig. 3B). As such, the degree of functional recovery observed for NR, BAM, or TEMR groups was determined by calculating the ratio of peak torque at each postinjury time relative to each respective preinjury group mean. The preinjury group mean was used as a conservative approach to minimize any undue bias of a single preinjury measurement (Fig. 3C).
A two-factor ANOVA was used to compare functional outcomes among the different treatment groups over time. Statistical analysis revealed a significant effect of treatment group (p<0.001), a significant effect of time (p<0.049), but no interaction between the time and treatment group (p=0.591). More specifically, all treatment groups displayed improved function from 4–12 weeks postinjury. However, post hoc analysis revealed that at each time point, BAM significantly improved TA muscle functional recovery compared to NR- or TEMR-negative responders (which were not different from each other), while TEMR-positive responders significantly improved functional recovery to a greater extent than NR-, BAM-, and TEMR-negative responders (Fig. 3).
Consistent with the aforementioned observations, comparison of absolute peak torque values among control (unoperated and sham/synergist ablation) and treatment (i.e., no-repair, BAM-, and TEMR-positive and -negative responders) groups at 12 weeks postinjury revealed that TEMR-positive responders had significantly greater peak isometric torque values compared to nonrepaired and BAM-repaired groups (Table 1). Further, when isometric torque was normalized to TA muscle wet weight (an estimate of specific torque), BAM-treated muscles exhibited a significant deficit compared to control groups (e.g., vs. sham, p=0.0278), while TEMR-positive responders were similar to control values (Table 1). Collectively, these findings indicate that TEMR-positive responders, and to a lesser extent BAM, improves the functional capacity of VML injured muscle up to 12 weeks postinjury.
Table 1.
TA Muscle In Vivo Functional Capacity Twelve Weeks Postinjury
| Uninjured | TEMR | |||||
|---|---|---|---|---|---|---|
| Unoperated | Sham/Ablation | No repair | BAM | Negative | Positive | |
| Sample size (n) | 8 | 6 | 4 | 7 | 7 | 6 |
| Body Wt. (g) | 243±7 | 252±13 | 254±18 | 241±9 | 259±7 | 243±7 |
| TA muscle wet Wt. (mg) | 423±16a | 446±36a | 342±28b | 386±14a,b | 356±6b | 390±14a,b |
| Peak tetanic isometric torque | ||||||
| Nmm/kg body wt | 107.9±4.0a | 87.5±8.9b | 60.9±2.1c | 67.9±2.9c | 56.1±3.4c | 77.1±1.7b |
| % Deficit | 19* | 30† | 22† | 36† | 12† | |
| Nmm/g TA wet wt. | 49.9±1.9a,# | 49.4±3.8a | 45.3±2.3a,b | 41.7±2.2b | 40.7±2.3b | 48.2±1.0a |
Values are mean±SEM. Values denoted with the same letter are not significantly different (p>0.05), while values without a like letter denotation are significantly different (p<0.05).
Deficit calculated from unoperated. †Deficit calculated from sham/ablation. #Unoperated values were normalized to the wet weight of all anterior crural muscles.
BAM, bladder acellular matrix; TA, tibialis anterior; TEMR, tissue engineered muscle repair.
Morphological characterization of retrieved TA muscle
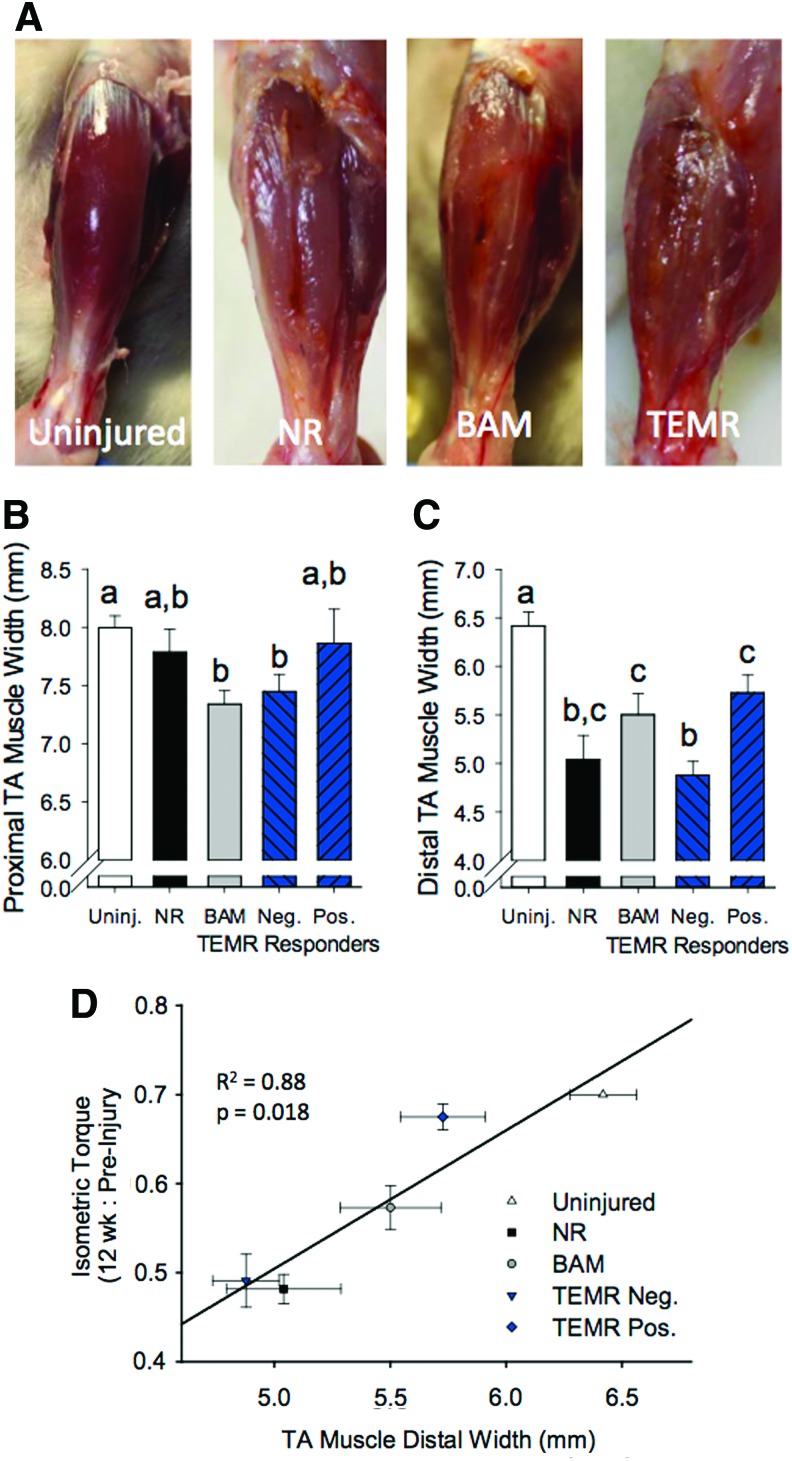
VML injury resulted in significant deformation and gross morphological remodeling of the TA muscle (Fig. 4A). In nonrepaired VML injured muscle, a longitudinal fissure was commonly observed where the defect was originally created. Both the BAM- and TEMR-positive responder groups, defined by their functional response, appeared to partially fill this void, attenuating the atrophic appearance of the muscle. Consistent with this observation, both BAM- and TEMR-positive responders partially improved the distal width of the TA muscle (Fig. 4B, C). Functional recovery observed 12 weeks postinjury was significantly and positively correlated with this improvement in distal TA muscle width (Fig. 4D). Similarly, TA muscle wet weight following VML injury was partially improved by BAM- and TEMR-positive responders 12 weeks postinjury (Table 1).
FIG. 4.
TA muscle gross morphology 12 weeks after VML injury and treatment. (A) The gross appearance of VML injured muscle without (NR) and with (BAM or TEMR) treatment was clearly distinguishable from uninjured muscle. Note: A longitudinal fissure in the middle third of the muscle was visible in NR muscles. Implantation of either BAM or TEMR filled this void. (B, C) TA muscles appeared atrophied, with distinctly smaller widths of the proximal and distal thirds of the muscle. (D) The extent of atrophy in the distal TA width was positively correlated with functional recovery 12 weeks postinjury. Values are mean±SEM. Means at each time point demarked with different letters are significantly different (p<0.05). Color images available online at www.liebertpub.com/tea
Qualitative histological characterization of retrieved TA muscle
Twelve weeks postinjury, VML injured nonrepaired TA muscle presented with fibrotic scarring at the defect site with signs of aberrant muscle fiber generation marked by a few small disorganized muscle fibers (Fig. 5B, G). For treated VML injured muscle, the functional outcomes were generally matched by the qualitative appearance of generated muscle fibers at the site of the defect, that is, TEMR-positive responders and BAM-treated TA muscle presented with signs of muscle fiber regeneration (Figs. 5 and 6), marked by the formation of a band of muscle fibers that regenerated in close proximity to the remaining muscle mass. TEMR-negative responders exhibited little to no signs of muscle regeneration (Figs. 5E, J and 6C). In corroboration with the variable functional recovery with TEMR transplantation, TEMR-positive and -negative responders appeared to have a differential immune response—TEMR-negative responders were marked with a robust macrophage (CD68+ cells) presence taking the form of foreign body giant cells (Fig. 6F), while TEMR-positive responders were marked also by the macrophage presence, but in a manner phenotypically similar, although apparently greater in magnitude, than BAM-treated TA muscles (Fig. 6E, D). A general summary of the performance of BAM scaffolds and TEMR constructs are provided in Table 2.
FIG. 5.
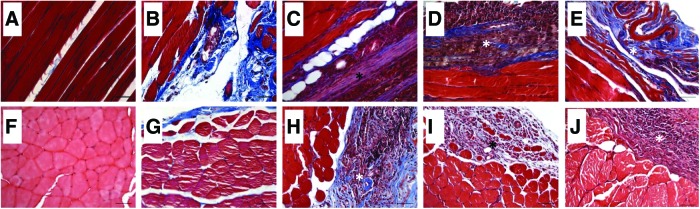
TA muscle tissue morphology following transplantation of BAM and TEMR constructs. TA muscles were harvested 12 weeks after VML injury. Longitudinal (A–E) and cross sections (F–J) from uninjured (A, F), nonrepaired (B, G), BAM repaired (C, H), and TEMR-positive (D, I) and -negative (E, J) responders were stained with Mason's Trichrome (red=tissue; blue=collagen; black=nuclei). *Denotes the area of the defect where the constructs were transplanted. Scale bars=50 μm. Color images available online at www.liebertpub.com/tea
FIG. 6.
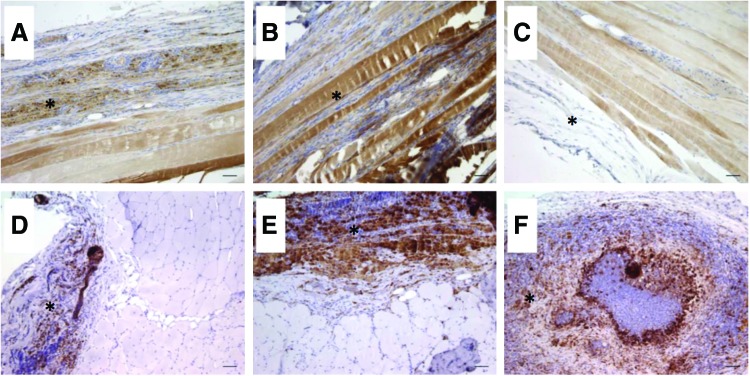
TA muscle fiber generation and inflammation following transplantation of BAM and TEMR constructs. TA muscles were harvested 12 weeks after VML injury. Longitudinal sections were probed for myosin expression (MF20; A–C) and cross sections were probed for macrophage presence (CD68; D–F) in BAM (A, D), TEMR-positive (B, E) and TEMR-negative (C, F) responder muscles. *Denotes the area of the defect where the constructs were transplanted. Scale bars=50 μm. Color images available online at www.liebertpub.com/tea
Table 2.
Summary of BAM Scaffold and TEMR Construct In Vivo Performance
| TEMR | |||
|---|---|---|---|
| BAM | Negative | Positive | |
| Functional recoverya | Moderate | None | High |
| Fiber regenerationb | None—Low | Low—Moderate | Low—Moderate |
| Inflammationb | Low | High | Moderate |
Classifications were set arbitrarily relative to nonrepaired muscles or bamong treated muscles within the area of the repaired defect site.
Discussion
Over the past decade a focused research effort has been placed on developing therapeutic strategies aimed at generating a clinically relevant volume of skeletal muscle for the purpose of ultimately restoring function to traumatized musculoskeletal tissue. Whereas a variety of tissue engineering approaches have been successful in promoting partial regeneration of lost skeletal muscle fibers at the site of VML, ultimately an improvement in force production (i.e., net torque production) of the injured musculature is the most meaningful treatment outcome. The primary finding of this study is that transplantation of the TEMR construct and BAM is associated with significant increases in the magnitude of TA muscle contraction (i.e., torque) following electrical stimulation of the peroneal nerve in vivo, for up to 12 weeks post-VML injury in an immune competent Lewis rat model (Fig. 3; Table 1). Importantly, TEMR transplantation resulted in a highly variable therapeutic response, wherein no (zero)-to-full recovery of function was observed. Twelve weeks postinjury, TEMR-positive responders (n=6 of 13 observations; see Materials and Methods for full details) and BAM transplantation promoted a mean recovery of 26% and 61% of the functional deficit, respectively (Table 1). On the other hand, TEMR-negative responders (n=7 of 13 observations) did not improve the functional recovery of VML injured muscle. Our previous findings with TEMR transplantation in a nude mouse LD muscle VML injury model5,8 and other studies13,16 support the following findings: that TEMR-positive responders and to a lesser extent, BAM transplantation, can improve the functional capacity of skeletal muscle with VML.
Sheet-based acellular matrix tissue engineering approaches with and without the inclusion of a stem or progenitor cell source have previously been shown to promote skeletal muscle fiber regeneration.5,6,8–10,13,15–17,19 The current study confirms and extends our earlier work in the murine LD VML injury model5,8 demonstrating that TEMR transplantation can result in a low to moderate level of muscle fiber regeneration inside the transplanted construct within 3 months after transplantation in an animal model with a competent immune system. Additionally, in the current study, BAM transplantation also promoted muscle fiber regeneration within the same time frame, similar to that reported recently in the mouse quadriceps following transplantation of subintestinal submucosa-ECM material.17 On the other hand, TEMR-negative responders exhibited virtually no evidence of myogenesis in the defect area. This raises the ongoing debate as to whether inclusion of a cellular source with a matrix is necessary to maximize the therapeutic response of ECM-based tissue engineering devices. There is currently ample evidence within the literature to support each approach, for example, Refs.9,16,20,21. Whereas the findings of this study do not settle this question, they do indicate that the inclusion of a cellular component with a biological ECM has the potential (i.e., TEMR-positive responders) to promote a 61% functional recovery, a 2.3-fold increase in recovery compared to that elicited by BAM alone (26% recovery), in this rodent model of VML injury. Clearly, further work is required to increase the reproducibility of the desired functional outcome, and thus, the eventual translational value of the TEMR technology.
The existing literature suggests that the mechanism of functional recovery after VML injury mediated by scaffold-based tissue engineering approaches is multifactorial. Of the VML studies that have reported functional improvements following therapy (e.g., Refs.5,8,9,13), most have demonstrated a partial regeneration of the volume of muscle tissue loss—the magnitude of tissue regeneration in these reports and in the current study, however, are comparable to others that did not measure function. This suggests that functional gains mediated by delivery of acellular scaffolds with and without and stem or progenitor cell source across multiple VML models are due not only to the regeneration of muscle fibers, but also by potentially (1) improving the functional capacity of the remaining muscle mass (e.g., functional hypertrophy34), (2) augmenting the efficiency of force transmission across the defect,35–38 or (3) protecting the remaining muscle mass from continued injury related to mechanical overload that may lead to persistent inflammation and fibrosis.39,40
Highlighting these possibilities, recently, it has been shown that transplantation of a decellularized muscle ECM, which promoted strictly fibrosis within the defect area, still resulted in enhanced functional restoration at intermittent time points (e.g., 2 and 4 months postinjury).41 As an extension of these results, in the current study, we observed a significant atrophy of VML injured muscles that was most severe in the distal third of the tissue (distal to the defect site)—reminiscent of the effect of laceration on skeletal muscle.42–44 Interestingly, the atrophic response of the distal portion of the TA muscle was attenuated with TEMR and BAM transplantation, in a manner directly related to each group's functional recovery (Fig. 4D). The benefit of mechanical loading on tissue regeneration has long been appreciated,45 wherein increased and decreased activity can positively and negatively impact regeneration, respectively. In this context, it is plausible that the transplantation of an acelluar scaffold may improve function by promoting the formation of a mechanical bridge that is capable of improving force transmission throughout the remaining muscle.41 This concept appears to parallel the biological response of skeletal muscle to disruptions in the cytoskeletal architecture (e.g., desmin knockout mice46), with repeated lengthening contraction training,39,40 and clinically following laceration of the biceps brachii muscle.47 Future studies should address the mechanistic basis for the functional effects of various tissue engineering strategies following VML injury, as the mechanisms that drive these distinct aspects of functional recovery will provide important insight into the development of efficient regenerative strategies.
To continue the development of TEMR technology toward clinical applications, it is important to identify why this group as a whole presented such a wide range of functional outcomes in this VML injury model. One possibility is that the additional mechanical manipulation involved with insertion of the TEMR scaffold in the TA VML injury model (relative to the previously reported LD model) results in a decline in the retention of, or damage to, the cellular component at implantation. These disturbances would effectively diminish the presumptive myogenic,5,8 angiogenic,48 or immunomodulatory49 effects of MDCs. This supposition is consistent with the clear delineation in the regeneration of muscle fibers and macrophage infiltration characterizing the positive and negative TEMR responders (Fig. 6). Moreover, delivery of the damaged TEMR construct may have increased inflammation in a manner similar to that observed upon transplantation of grafted whole tissue,50 and may have therefore interfered with improvements in the function mediated through transplantation of BAM alone.
The primary findings of this article highlight that the transplantation of decellularized ECM with (TEMR-positive responders) and without (BAM) the inclusion of MDCs can promote significant functional recovery in skeletal muscle with VML injury. Further, the above findings also highlight the potentially greater regenerative capacity of therapies that include stem or progenitor cells in addition to a scaffold. Specifically, this possibility is evidenced by the fact that TEMR-positive responders promoted a 2.3-fold greater functional recovery than did BAM alone. On the other hand, these findings also point to the additional complexities of cell inclusion, as neither functional recovery nor muscle fiber regeneration was observed in the TEMR-negative responders. The high degree of variance among TEMR responders appears to be related to presurgical handling of the constructs (e.g., physical manipulation), and therefore, we are confident that further optimization of this technology (i.e., reduced surgical handling, improved cellular seeding, layering of scaffold sheets, or utilization of more favorable scaffold geometries) will mitigate these issues. In short, while there is still significant room for functional improvement, these initial studies provide important guidance for the future development of tissue engineering therapies, and furthermore, document the importance of studying these technologies in distinct animal models and VML injuries to develop the strategies that will best accommodate the full spectrum of functional and cosmetic deficits that result from such injuries.
Acknowledgments
We would like to thank Ms. Manasi Vadhavkar for her technical assistance during surgery and functional testing and Mr. Christopher Bergman for his technical assistance with histological procedures. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the United States Government. The authors (btc, clw, and tjw) are employees of the U.S. government and this work was prepared as part of their official duties. This work was supported, in part, by the Armed Forces Institute for Regenerative Medicine (W81XWH-08-2-0032) and by the U.S. Army Medical Research and Medical Command (W81XWH-09-2-0177).
Disclosure Statement
No competing financial interests exist.
References
- 1.Grogan B.F., and Hsu J.R. Volumetric muscle loss. J Am Acad Orthop Surg 19 , S35, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Vekris M.D., Beris A.E., Lykissas M.G., Korompilias A.V., Vekris A.D., and Soucacos P.N. Restoration of elbow function in severe brachial plexus paralysis via muscle transfers. Injury 39 , S15, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Lin C.H., Lin Y.T., Yeh J.T., and Chen C.T. Free functioning muscle transfer for lower extremity posttraumatic composite structure and functional defect. Plast Reconstr Surg 119, 2118, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Owens J.G., Blair J.A., Patzkowski J.C., Blanck R.V., and Hsu J.R. Return to running and sports participation after limb salvage. J Trauma 71, S120, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Corona B.T., Machingal M.A., Criswell T., Vadhavkar M., Dannahower A.C., Bergman C., et al. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng Part A 18, 1213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Coppi P., Bellini S., Conconi M.T., Sabatti M., Simonato E., Gamba P.G., et al. Myoblast-acellular skeletal muscle matrix constructs guarantee a long-term repair of experimental full-thickness abdominal wall defects. Tissue Eng 12, 1929, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Gamba P.G., Conconi M.T., Lo Piccolo R., Zara G., Spinazzi R., and Parnigotto P.P. Experimental abdominal wall defect repaired with acellular matrix. Pediatr Surg Int 18, 327, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Machingal M.A., Corona B.T., Walters T.J., Kesireddy V., Koval C.N., Dannahower A., et al. A Tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A 17, 2291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merritt E.K., Cannon M.V., Hammers D.W., Le L.N., Gokhale R., Sarathy A., et al. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng Part A 16, 2871, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Merritt E.K., Hammers D.W., Tierney M., Suggs L.J., Walters T.J., and Farrar R.P. Functional assessment of skeletal muscle regeneration utilizing homologous extracellular matrix as scaffolding. Tissue Eng Part A 16, 1395, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Perniconi B., Costa A., Aulino P., Teodori L., Adamo S., and Coletti D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 32, 7870, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Turner N.J., Badylak J.S., Weber D.J., and Badylak S.F. Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J Surg Res 176, 490, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Valentin J.E., Turner N.J., Gilbert T.W., and Badylak S.F. Functional skeletal muscle formation with a biologic scaffold. Biomaterials 31, 7475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willett N.J., Li M.T., Uhrig B.A., Boerckel J.D., Huebsch N., Lundgren T.S., et al. Attenuated rhBMP-2 mediated bone regeneration in a rat model of composite bone and muscle injury. Tissue Eng Part C Methods 19, 316, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf M.T., Daly K.A., Reing J.E., and Badylak S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 33, 2916, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner N.J., Yates A.J., Jr., Weber D.J., Qureshi I.R., Stolz D.B., Gilbert T.W., et al. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A 16, 3309, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Sicari B.M., Agrawal V., Siu B.F., Medberry C.J., Dearth C.L., Turner N.J., et al. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng Part A 18, 1941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mase V.J., Jr., Hsu J.R., Wolf S.E., Wenke J.C., Baer D.G., Owens J., et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, 511, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Conconi M.T., De Coppi P., Bellini S., Zara G., Sabatti M., Marzaro M., et al. Homologous muscle acellular matrix seeded with autologous myoblasts as a tissue-engineering approach to abdominal wall-defect repair. Biomaterials 26, 2567, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Page R.L., Malcuit C., Vilner L., Vojtic I., Shaw S., Hedblom E., et al. Restoration of skeletal muscle defects with adult human cells delivered on fibrin microthreads. Tissue Eng Part A 17, 2629, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Rossi C.A., Flaibani M., Blaauw B., Pozzobon M., Figallo E., Reggiani C., et al. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J 25, 2296, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Gharaibeh B., Lavasani M., Cummins J.H., and Huard J. Terminal differentiation is not a major determinant for the success of stem cell therapy—cross-talk between muscle-derived stem cells and host cells. Stem Cell Res Ther 2, 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer N.G., and Caplan A.I. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 6, 457, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Zammit P.S., Partridge T.A., and Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 54, 1177, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Hawke T.J., and Garry D.J. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91, 534, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Moon Du, G., Christ G., Stitzel J.D., Atala A., and Yoo J.J. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng Part A 14, 473, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Wu X., Corona B.T., Chen X., and Walters T.J. A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies. Biores Open Access 1, 280, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corona B.T., Rouviere C., Hamilton S.L., and Ingalls C.P. Eccentric contractions do not induce rhabdomyolysis in malignant hyperthermia susceptible mice. J Appl Physiol 105, 1542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Criswell T.L., Corona B.T., Ward C.L., Miller M., Patel M., Wang Z., et al. Compression-induced muscle injury in rats that mimics compartment syndrome in humans. Am J Pathol 180, 787, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Ingalls C.P., Warren G.L., and Armstrong R.B. Dissociation of force production from MHC and actin contents in muscles injured by eccentric contractions. J Muscle Res Cell Motil 19, 215, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Kandarian S.C., Young J.C., and Gomez E.E. Adaptation in synergistic muscles to soleus and plantaris muscle removal in the rat hindlimb. Life Sci 51, 1691, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Corona B.T., Balog E.M., Doyle J.A., Rupp J.C., Luke R.C., and Ingalls C.P. Junctophilin damage contributes to early strength deficits and EC coupling failure after eccentric contractions. Am J Physiol Cell Physiol 298, C365, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Ingalls C.P., Warren G.L., Lowe D.A., Boorstein D.B., and Armstrong R.B. Differential effects of anesthetics on in vivo skeletal muscle contractile function in the mouse. J Appl Physiol 80, 332, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Gollnick P.D., Timson B.F., Moore R.L., and Riedy M. Muscular enlargement and number of fibers in skeletal muscles of rats. J Appl Physiol 50, 936, 1981 [DOI] [PubMed] [Google Scholar]
- 35.Lehto M., Duance V.C., and Restall D. Collagen and fibronectin in a healing skeletal muscle injury. An immunohistological study of the effects of physical activity on the repair of injured gastrocnemius muscle in the rat. J Bone Joint Surg Br 67, 820, 1985 [DOI] [PubMed] [Google Scholar]
- 36.Purslow P.P. The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Mol Integr Physiol 133, 947, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Purslow P.P. Muscle fascia and force transmission. J Bodyw Mov Ther 14, 411, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Street S.F., and Ramsey R.W. Sarcolemma: transmitter of active tension in frog skeletal muscle. Science 149, 1379, 1965 [DOI] [PubMed] [Google Scholar]
- 39.Stauber W.T., Knack K.K., Miller G.R., and Grimmett J.G. Fibrosis and intercellular collagen connections from four weeks of muscle strains. Muscle Nerve 19, 423, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Stauber W.T., Smith C.A., Miller G.R., and Stauber F.D. Recovery from 6 weeks of repeated strain injury to rat soleus muscles. Muscle Nerve 23, 1819, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Corona B.T., Wu X., Ward C.L., Mcdaniel J.S., Rathbone C.R., and Walters T.J. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials 34, 3324, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Kaariainen M., Kaariainen J., Jarvinen T.L., Sievanen H., Kalimo H., and Jarvinen M. Correlation between biomechanical and structural changes during the regeneration of skeletal muscle after laceration injury. J Orthop Res 16, 197, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Lim A.Y., Lahiri A., Pereira B.P., Tan J.A., Sebastin S.J., Tan B.L., et al. The role of intramuscular nerve repair in the recovery of lacerated skeletal muscles. Muscle Nerve 33, 377, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Pereira B.P., Tan J.A., Zheng L., Tan B.L., Lahiri A., Lim A.Y., et al. The cut intramuscular nerve affects the recovery in the lacerated skeletal muscle. J Orthop Res 24, 102, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Denny-Brown D. The influence of tension and innervation on the regeneration of skeletal muscle. J Neuropathol Exp Neurol 10, 94, 1951 [PubMed] [Google Scholar]
- 46.Meyer G.A., and Lieber R.L. Skeletal muscle fibrosis develops in response to desmin deletion. Am J Physiol Cell Physiol 302, C1609, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kragh J.F., Jr., and Basamania C.J. Surgical repair of acute traumatic closed transection of the biceps brachii. J Bone Joint Surg Am 84-A, 992, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Rhoads R.P., Johnson R.M., Rathbone C.R., Liu X., Temm-Grove C., Sheehan S.M., et al. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol 296, C1321, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lecourt S., Lepelletier Y., Vanneaux V., Jarray R., Domet T., Raynaud F., et al. Human Muscle Progenitor Cells Displayed Immunosuppressive Effect through Galectin-1 and Semaphorin-3A. Stem Cells Int 2012, 412610, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badylak S.F., Valentin J.E., Ravindra A.K., Mccabe G.P., and Stewart-Akers A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14, 1835, 2008 [DOI] [PubMed] [Google Scholar]