Abstract
The zebrafish (Danio rerio) represents an important animal model for analyzing genetic contributors to carcinogenesis. To assess the role for mutationally activated Ras in ovarian cancer, we developed a transgenic zebrafish model using the putative promoter for zebrafish insulin-like growth factor 3 (igf3) to drive expression of the human oncogene KRASG12V fused to EGFP. A member of the IGF family, igf3 is unique to teleosts and reportedly exhibits gonad-specific expression in fish species. In contrast to previous studies, we observed igf3 expression in wild-type zebrafish gills in addition to gonads, indicating that igf3 expression is not necessarily gonad specific. In transgenic zebrafish, expression of EGFP-KRASG12V driven by the igf3 promoter occurred only in the gills and resulted in proliferation of a putative progenitor cell population, chondroid hyperplasia, and localized inflammation. KRASG12V-transformed cells in transgenic zebrafish showed activation of the ERK-MAP kinase pathway and expressed the zebrafish homologue for human CXCL8, a cytokine produced by mammalian Ras-transformed cells in tumor-associated inflammatory lesions. These findings indicate that KRASG12V-transformed cells in zebrafish recruit inflammatory cells, but may require additional mutational events for neoplastic transformation. The conserved role for mutationally activated KRAS in leukocyte recruitment indicates that zebrafish can provide a valuable comparative model for Ras-associated inflammation.
Introduction
Ras proteins are GTPases that function in intracellular signaling networks involved in multiple cell functions, including proliferation and differentiation. Mutations in the human oncogene KRAS have been identified in a wide range of human malignancies,1 and activating mutations at codon 12 in human KRAS occur in some types of ovarian cancers.2 In this study, we attempted to develop a transgenic zebrafish model with targeted expression of the human oncogene KRASG12V in the ovaries to provide a platform for assessing collaborating genetic mutations in human ovarian cancer risk.
Although no ovarian-specific promoter has been identified in zebrafish, the insulin-like growth factor-3 (igf3) gene is reportedly expressed exclusively in the gonads of fish species.3–5 igf3 is a novel member of the IGF family that is unique to teleosts.5 In zebrafish, there are two transcript variants for igf3 (igf3 tv1 and tv2) that exhibit different spatiotemporal patterns of expression.3 In zebrafish gonads, igf3 tv1 is expressed in somatic cell populations, including the follicular cells and interstitial cells of ovaries and testes, respectively.3,4
Because the promoter regions for zebrafish igf3 tv1 and tv2 have not been defined, we used a 4.3-kb genomic fragment located 5′ to the ATG start codon for igf3 tv1 to drive expression of EGFP alone or EGFP fused to the human oncogene KRASG12V. However, EGFP was not detectable in gonads from transgenic zebrafish. Instead, EGFP expression occurred exclusively in a putative progenitor cell population in the gills in transgenic zebrafish. Furthermore, targeted expression of EGFP-KRASG12V in zebrafish gills resulted in hyperplasia, activation of the ERK-MAP kinase pathway, and regional inflammation. A subset of EGFP-expressing cells from transgenic zebrafish also expressed the transcription factor sox9, a marker for chondroid precursors, supporting transformation of a progenitor cell population in the gills. Cells expressing EGFP-KRASG12V produced the CXCL8 homologue cxcl8-l1, similar to Ras-transformed mammalian cells,6,7 suggesting that Ras-associated inflammation in zebrafish develops through a conserved signaling mechanism.
Results
Generation of transgenic lines for analyzing igf3 expression in zebrafish
To verify the expression pattern of igf3 in adult zebrafish, we analyzed multiple adult tissues from 3-month-old male and female wild-type zebrafish by reverse transcription polymerase chain reaction (RT-PCR) for expression of igf3 transcript variants 1 and 2. (Fig. 1A and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/zeb). Unexpectedly, RT-PCR analyses indicated that although igf3 transcript variant 1 (igf3 tv1) was highly expressed in ovaries and testes, it was also expressed in nongonadal tissues, including the brain and gills. In contrast, igf3 transcript variant 2 (igf3 tv2) was not reliably detectable in adult tissues, although minimal expression was observed in a few tissues in some experimental replicates.
FIG. 1.
igf3 expression and constructs created to generate igf3 transgenic zebrafish lines. (A) igf3 transcript variant 1 is expressed in multiple adult zebrafish tissues by reverse transcription polymerase chain reaction (RT-PCR), while igf3 variant 2 is not expressed in adult zebrafish tissues. Tissues were derived from 3-month-old wild-type zebrafish. (B) Construct used to generate transgenic zebrafish lines contains the putative igf3 promoter driving EGFP. Flags indicate the positions of primers used to genotype transgenic zebrafish. (C) Construct used to generate transgenic zebrafish lines contains the putative igf3 promoter driving EGFP-KRASG12V. Flags indicate the positions of primers used to genotype transgenic zebrafish. tv1, transcript variant 1; tv2, transcript variant 2; Sk, skin; M, skeletal muscle; F, fin; G, gill; E, eye; B, brain; H, heart; L, liver; Sp, spleen; I, intestine; K, kidney; T, testes; O, ovary; +, 24-h postfertilization wild-type zebrafish embryo; −, negative control; 500, 500 base pair marker. Color images available online at www.liebertpub.com/zeb
Although RT-PCR analyses indicated that igf3 was not exclusively expressed in gonads, expression in nongonadal tissues was limited, and thus we reasoned that the promoter for this gene would drive gene expression predominantly in the gonads. Therefore, we developed two constructs for igf3-driven expression of EGFP and EGFP fused to the human oncogene KRASG12V (Fig. 1B). Because the igf3 promoter has not been characterized in zebrafish, we selected a 4.3-kb genomic fragment proximal to the ATG start codon of zebrafish igf3 (Fig. 1B and Supplementary Table S1) for PCR amplification and cloning. This fragment included a portion of exon 1 from igf3 tv1 and the entire exon 1 from igf3 tv2. We used the I-SceI meganuclease method (Grabher 2004, Soroldoni 2009) to generate four stably transgenic zebrafish lines, two with the EGFP transgene and two with the EGFP- KRASG12V transgene (Supplementary Table S2).
Zebrafish with igf3-driven expression of the human oncogene KRASG12V develop proliferative gill lesions
Transgenic zebrafish from all four transgenic lines exhibited normal embryonic survival and development and were phenotypically normal as adults. EGFP expression was not observed in transgenic embryos examined by fluorescence microscopy, suggesting that this promoter construct does not drive expression of igf3 tv2. As there were no gross abnormalities observed in adult transgenic zebrafish, we performed histologic examinations of adult zebrafish at 3, 6, and 12 months of age (Supplementary Table S3). Equal numbers of male and female zebrafish were examined, and age-matched male and female wild-type zebrafish were included as controls for each time point.
The testes and ovaries were histologically normal in all transgenic zebrafish at each time point examined. However, both male and female tg(igf3:EGFP-KRASG12V)AB zebrafish exhibited proliferative lesions in the gills (Fig. 2). These lesions occurred exclusively in the gill filament, predominantly affecting the proximal third, and did not involve the gill arches or lamellae. In affected filaments, the central cartilage was expanded by irregular nodular clusters of chondrocytes, indicating cartilage hyperplasia. A population of subepithelial cells expanded the interstitium adjacent to the central cartilage. These cells were closely associated with hyperplastic cartilaginous nodules. No other histologic lesions were observed in tg(igf3:EGFP-KRASG12V)AB zebrafish.
FIG. 2.
Progressive gill hyperplasia in adult tg(igf3:EGFP-KRASG12V)AB zebrafish. (A) Histologic sections of gill filaments from tg(igf3:EGFP)AB, tg(igf3:EGFP-KRASG12V)AB, and wild-type zebrafish at 3 months of age. Gills were analyzed by hematoxylin and eosin (upper panels) and Alcian blue staining (lower panels). (B) Histologic sections of gill filaments from tg(igf3:EGFP)AB, tg(igf3:EGFP-KRASG12V)AB, and wild-type zebrafish at 12 months of age. Gills were analyzed by hematoxylin and eosin (upper panels) and Alcian blue staining (lower panels). HE, hematoxylin and eosin. Scale bar, 50 μm. Color images available online at www.liebertpub.com/zeb
Proliferative lesions were more severe in 12-month-old zebrafish (Fig. 2B) than in 3-month-old zebrafish (Fig. 2A). Alcian blue staining confirmed the presence of increased and irregularly shaped cartilage in gills from tg(igf3:EGFP-KRASG12V)AB zebrafish, although the intensity of Alcian blue staining was variable in regions of hyperplasia (Fig. 2). Gill lesions were consistently present after multiple generations in 100% of zebrafish from one line of tg(igf3:EGFP-KRASG12V)AB zebrafish. Gill lesions were observed in 30% of the F1 generation of the second tg(igf3:EGFP-KRASG12V)AB zebrafish line that were confirmed to be transgenic by PCR (Supplementary Fig. S1). The relative presence of gill lesions correlated with expression of the transgene, as described below. In contrast, gills from all tg(igf3:EGFP)AB and wild-type zebrafish were histologically normal at each time point examined (Fig. 2 and Supplementary Fig. S2).
We were unable to verify expression of igf3 in wild-type zebrafish gills by RNA in situ hybridization with a probe expected to detect either igf3 tv1 or igf3 tv2, although the probe did demonstrate igf3 expression in the follicular epithelium of wild-type zebrafish ovaries (Supplementary Fig. S3), as has been previously reported.5 This may reflect a relatively low level of igf3 expression in zebrafish gills, as suggested by RT-PCR analysis (Fig. 1).
The putative igf3 promoter drives gene expression in a specific cell population of zebrafish gills
To determine the tissue-specific gene expression pattern in igf3 transgenic zebrafish, transgenic and wild-type zebrafish were analyzed by immunohistochemistry for EGFP. The tg(igf3:EGFP)AB zebrafish lines showed discrete and exclusive EGFP expression in a subepithelial population of cells in the gill filament, located immediately adjacent to the gill cartilage (Fig. 3 and Supplementary Fig. S2). EGFP expression was not observed in ovaries or testes or in any other tissues from tg(igf3:EGFP)AB zebrafish. Wild-type zebrafish did not express EGFP (Fig. 3).
FIG. 3.
Targeted expression of EGFP and selective targeted expression of phosphorylated p44/42 MAPK in adult transgenic zebrafish. (A) EGFP is expressed in a subepithelial cell population of the gill filament in tg(igf3:EGFP)AB and tg(igf3:EGFP-KRASG12V)AB zebrafish at 3 months of age. (B) Phosho-p44/42 MAPK is expressed in a subepithelial cell population of the gill filament in tg(igf3:EGFP-KRASG12V)AB zebrafish at 3 months of age and is localized in the nucleus (inset). (C) EGFP is expressed in a subepithelial cell population of the gill filament in tg(igf3:EGFP)AB and tg(igf3:EGFP-KRASG12V)AB zebrafish at 12 months of age. (D) Phosho-p44/42 MAPK is expressed in a subepithelial cell population of the gill filament in tg(igf3:EGFP-KRASG12V)AB zebrafish at 12 months of age and is localized in the nucleus (inset). Scale bar, 50 μm. Color images available online at www.liebertpub.com/zeb
EGFP expression in tg(igf3:EGFP-KRASG12V)AB zebrafish was also restricted to the gills and was also present in the above-described subepithelial cell population. EGFP-positive cells were closely associated with regions of cartilage hyperplasia (Fig. 3). Increased numbers of EGFP-positive cells were observed in 12-month-old tg(igf3:EGFP-KRASG12V)AB zebrafish (Fig. 3C) compared with 3-month-old zebrafish (Fig. 3A), correlating with increasing severity of hyperplastic gill lesions over time (Fig. 2). Mature chondrocytes did not express EGFP in either tg(igf3:EGFP)AB or tg(igf3:EGFP-KRASG12V)AB zebrafish (Fig. 3).
To investigate whether proliferative gill lesions were associated with MAPK pathway activation, transgenic and wild-type zebrafish were analyzed by immunohistochemistry for expression of phospho-p44/42-MAPK, a downstream target of activated RAS and a mediator of MAPK pathway signaling.8 Phospho-p44/42-MAPK was highly expressed in gills from tg(igf3:EGFP-KRASG12V)AB zebrafish and closely overlapped EGFP expression in serial sections (Fig. 3). Clear nuclear localization of phospho-p44/42-MAPK was apparent at high magnification (Fig. 3, insets). Increased numbers of cells expressing phospho-p44/42-MAPK expression were observed in 12-month-old zebrafish (Fig. 3D) compared with 3-month-old zebrafish (Fig. 3B). In contrast, phospho-p44/42-MAPK expression was not observed in the gills from tg(igf3:EGFP)AB or wild-type zebrafish (Fig. 3) or in any other tissues.
Both EGFP and phospho-p44/42-MAPK expression were consistently observed in gills after multiple generations from one line of tg(igf3:EGFP-KRASG12V)AB zebrafish (Fig. 3), but were only observed in the F1 generation of the second tg(igf3:EGFP-KRASG12V)AB zebrafish line (Supplementary Fig. S1). Subsequent generations from this second line that were confirmed to be transgenic by PCR had histologically normal gill morphology and did not express EGFP or phospho-p44/42-MAPK (Supplementary Fig. S1).
sox9 expression overlaps with EGFP expression in transgenic zebrafish gills
The above histologic and immunohistochemical studies show that the cells expressing the EGFP or EGFP-KRASG12V transgene are located immediately adjacent to the cartilage cores of the gill filament. Based on this anatomic location, we hypothesized that the cells expressing these transgenes might represent a progenitor cell population associated with the gill cartilage. To further investigate this hypothesis, transgenic and wild-type zebrafish were analyzed by immunohistochemistry for expression of sox9 (Fig. 4A), a transcription factor required for chondrogenesis in zebrafish.9,10 Zebrafish possess two orthologues of sox9 (sox9a and sox9b) with overlapping roles in cartilage development during embryogenesis,10 and the antibody used for this study was predicted to detect expression of both orthologues (Supplementary Fig. S4).
FIG. 4.
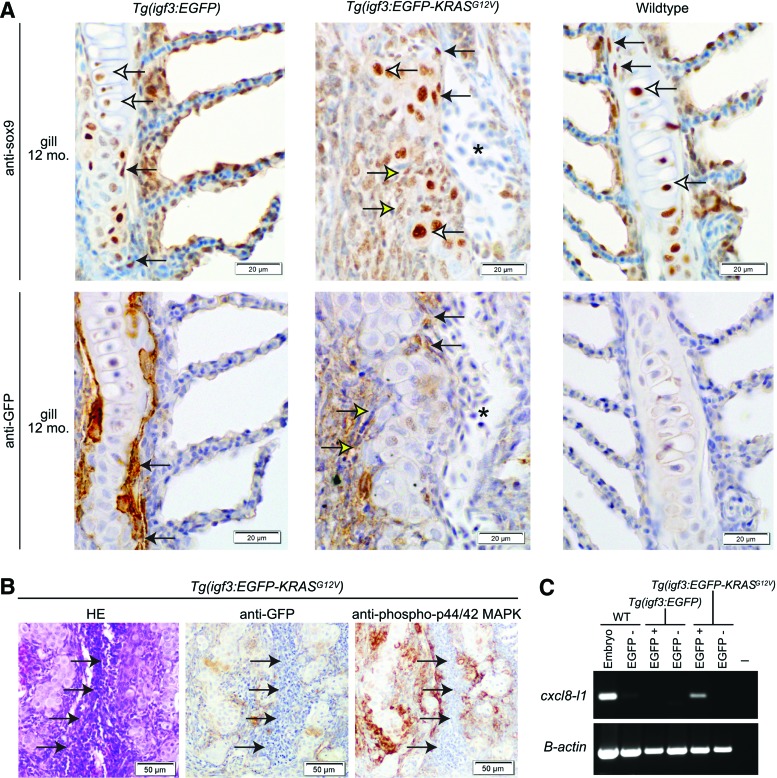
sox9 expression partially overlaps with EGFP expression in transgenic zebrafish gills, while gill hyperplasia is associated with inflammation in adult tg(igf3:EGFP-KRASG12V)AB zebrafish. (A) sox9 is expressed in both mature chondrocytes (white arrows) and a peripherally located putative progenitor cell population (black arrows). EGFP expression in transgenic zebrafish coincides with sox9 expression in some areas. Weak or absent sox9 expression sometimes occurs at the outer margins of hyperplastic nodules in adult tg(igf3:EGFP-KRASG12V)AB gills (yellow arrows). The asterisks in the middle panels indicate a blood vessel containing erythrocytes. (B) In hyperplastic gills from adult tg(igf3:EGFP-KRASG12V)AB zebrafish, numerous inflammatory cells (arrows) in the interstitium of the gill filament do not express EGFP or phosho-p44/42 MAPK. (C) Among cell populations collected by fluorescence-activated cell sorting, only EGFP-expressing cells collected from adult tg(igf3:EGFP-KRASG12V)AB zebrafish express the chemokine, cxcl8-l1, by RT-PCR. WT, wild-type; −, negative control. Color images available online at www.liebertpub.com/zeb
Robust nuclear expression of sox9 was observed in gills from wild-type and tg(igf3:EGFP)AB zebrafish, in both mature chondrocytes (Fig. 4A, white arrows) and in a subset of spindloid cells located along the peripheral margins of mature cartilage (Fig. 4A, black arrows). Robust nuclear expression of sox9 was similarly observed in gills from tg(igf3:EGFP-KRASG12V)AB zebrafish. Sox9 was expressed in the nuclei of mature chondrocytes that form proliferative gill nodules (Fig. 4A, white arrows) and in a subset of spindloid cells located along the peripheral margins of mature cartilage (Fig. 4A, black arrows). In some proliferative nodules, the most peripherally located spindloid cells exhibited weak or absent nuclear sox9 expression (Fig. 4A, yellow arrows). Peripherally located sox9-expressing cells overlapped with EGFP-expressing cells in gills from both tg(EGFP)AB and tg(EGFP-KRASG12V)AB zebrafish, but multiple EGFP-expressing cells did not show nuclear expression of sox9 (Fig. 4A).
Targeted expression of the human oncogene KRASG12V in zebrafish gills induces inflammation and cxcl8-l1 expression
In addition to the histologic changes described above, the gills from tg(igf3:EGFP-KRASG12V)AB zebrafish exhibited numerous, infiltrating, mixed inflammatory cells in the filament (Fig. 4B, left panel). These inflammatory cells formed dense clusters in the gill interstitium peripheral to regions of cartilage hyperplasia. Although the inflammatory cells did not express EGFP or phospho-p44/42-MAPK, they were immediately adjacent to cell populations expressing both of these proteins (Fig. 4B, center and right panels).
Since activating mutations in KRAS have been previously associated with production of proinflammatory cytokines and inflammatory cell recruitment in mouse cancer models,6,7,11 we speculated that the inflammation observed in gills from tg(igf3:EGFP-KRASG12V)AB zebrafish might be associated with chemokine production. Expression of CXCL8 (interleukin-8) or its orthologues has been implicated in Ras-associated inflammation in human and mouse tissues.6,7 Therefore, we analyzed gill tissues from igf3-transgenic zebrafish by RT-PCR for expression of the proinflammatory cytokine, cxcl8-l1, the zebrafish homologue for human CXCL812 (Fig. 4C and Supplementary Table S1). Gill tissues from tg(igf3:EGFP)AB, tg(igf3:EGFP-KRASG12V)AB, and wild-type zebrafish were processed by fluorescence-activated cell sorting (FACS) for collection of EGFP-positive and EGFP-negative cell populations. Analyses of FACS-collected samples by RT-PCR demonstrated cxcl8-l1 expression only in the EGFP-positive cell fraction of gills from tg(igf3:EGFP-KRASG12V)AB zebrafish (Fig. 4C).
Discussion
We developed a novel transgenic zebrafish model with targeted expression of the human oncogene KRASG12V using the putative promoter for zebrafish igf3, a member of the IGF family that is unique to teleost fish.5 Expression of the EGFP-KRASG12V transgene occurred exclusively in the gills and resulted in hyperplasia and localized CXCL8-associated inflammation. These findings indicate that inflammation associated with mutationally activated Ras is instigated in a conserved manner in zebrafish.
Although previously reported to show gonad-specific expression in zebrafish,3–5 this study suggests an unanticipated role for igf3 in zebrafish gills. The four transgenic zebrafish lines generated in this study each exhibited expression of EGFP in a discrete subepithelial cell population in the gills without detectable EGFP expression in the gonads. The consistency of this finding indicates that it is unlikely to be attributable to insertion site bias. Causes for the discrepancy in tissue expression pattern between this report and previous studies of igf3 in zebrafish are not known, but may include differences in assay sensitivity, range of tissues examined, or genetic background of wild-type animals. Interestingly, igf3 expression in another fish species (tilapia) is reported to occur in multiple adult tissues, including gonads, gills, and pituitary,13 similar to the findings in this study. Our results suggest that igf3 also functions in the gills, and other, as yet undetermined, regulatory elements are required to drive igf3 expression in other tissues.
EGFP expression in the gills from tg(EGFP-KRASG12V)AB zebrafish coincided with progressive hyperplasia and ERK-MAP kinase pathway activation. These findings are consistent with the role for KRASG12V in promoting cell growth through transcriptional pathway signaling. In mammalian cells, activating mutations in the Ras proto-oncogene promote cell survival,14 proliferation,15 and invasion/metastasis.16 Cells expressing mutationally activated Ras are stimulated to proliferate through initiation of the ERK-MAP kinase cascade.8,17 No overt evidence of tumor development was observed in gills from tg(igf3:EGFP-KRASG12V)AB zebrafish that were observed beyond 1 year of age, suggesting that additional mutational events are required for progression from hyperplasia to neoplasia in these transgenic lines. Interestingly, hyperplasia without overt neoplastic transformation in association with KRAS mutation has also been described in a human lymphoproliferative syndrome.18
One of the two tg(igf3:EGFP-KRASG12V)AB zebrafish lines lost EGFP expression, phospho-p44/42-MAPK expression, and gill hyperplasia after the F1 generation. We speculate that this zebrafish line underwent transgene silencing, possibly due to the integration site or integration copy number, as has been described in other transgenic animal models.19,20 This transgenic line thus provided an unintended correlative control for EGFP-KRASG12V expression and the associated gill phenotype.
We hypothesized that the gill phenotype observed in tg(igf3:EGFP-KRASG12V)AB zebrafish might reflect transformation of a chondroid progenitor cell population in zebrafish gills. The transcription factor SOX9 is a key mediator of chondrogenesis: SOX9 participates in the chondrogenic differentiation of cranial neural crest cells and is expressed in precursor cell populations that give rise to all osteochodroprogenitors.21,22 While sox9 is known to mediate zebrafish cartilage development during embryogenesis,9,10 it has not been examined in the adult gills. In both wild-type and transgenic zebrafish, immunohistochemistry results support the presence of a sox9-expressing progenitor cell population in zebrafish gills. While overlapping EGFP and sox9 expression occurred in gills from transgenic zebrafish, not all EGFP-expressing cells exhibited nuclear sox9 expression. This may reflect a gradation from uncommitted precursors to chondroid progenitors along the margins of gill filaments. Further characterization of these transgenic lines with additional progenitor cell markers will likely be necessary to refine the origin of the transformed cell population.
Cells expressing mutant forms of Ras can produce proinflammatory cytokines to recruit leukocytes, which may confer a growth advantage to hyperplastic or neoplastic cell populations by modulating the microenvironment.7,11 Human or mouse cells transformed by activating Ras mutations expressed the chemokine CXCL8, (interleukin-8) or its orthologues MIP2 and KC, respectively, and expression of these chemokines was associated with a robust inflammatory response.6,7 Similarly, we observed a substantial inflammatory cell infiltrate in hyperplastic gill lesions from tg(igf3:EGFP-KRASG12V)AB zebrafish. Hyperplastic cells expressing the EGFP-KRASG12V transgene also expressed the CXCL8 homologue, cxcl8-l1, indicating that the link between Ras activation, chemokine production, and inflammation is conserved in zebrafish.
The origin of the EGFP-labeled cell population from igf3 transgenic zebrafish lines is currently undetermined. The histologic and immunohistochemical data presented in this study suggest that transgene expression is targeted to a progenitor cell population in the gill filament (Fig. 5). In the absence of a growth-promoting stimulus, this cell population is quiescent. Targeted expression of the oncogene KRASG12V drives proliferation of the progenitor cell population through ERK-MAP kinase pathway activation and regional inflammation through expression of cxcl8-l1. Associated chondroid hyperplasia in the filament may occur by multiple mechanisms (Fig. 5): (1) directly, through differentiation of Ras-transformed cells; (2) indirectly, through production of stimulators of cartilage production; or (3) indirectly, as a response to regional inflammation. Since EGFP-KRASG12V expression was limited to the putative progenitor cell population and was not expressed in mature chondrocytes, this suggests that the igf3 promoter is suppressed in terminally differentiated cell populations. While further study of the igf3 gene is clearly necessary to more precisely determine its functions in zebrafish tissues, this transgenic model provides a useful tool for analyzing Ras-associated hyperplasia and inflammation in vivo.
FIG. 5.
Model for targeted KRASG12V expression in zebrafish gills. Color images available online at www.liebertpub.com/zeb
Materials and Methods
Zebrafish maintenance
All animal studies were approved by the Intramural Animal Care and Use Committee, National Cancer Institute, National Institutes of Health (Bethesda, MD). Zebrafish selected for histologic examination or tissue collection were euthanized with Tricaine (0.625 g/L) in system water buffered with Sodium Bicarbonate (0.7 g/L).
RNA extraction
Tissue samples were collected by dissection with a Stemi-C stereomicroscope (Zeiss). Samples were either processed for RNA extraction immediately after collection or frozen on dry ice/ethanol. Samples were routinely processed for RNA extraction with TRIZOL (Invitrogen), quantified with the NanoDrop 2000 spectrophotometer (NanoDrop), and treated with DNase I (Fermentas).
Reverse transcription polymerase chain reaction
RT-PCR experiments were performed with the Superscript III RT-PCR System with Platinum Taq (Life Technologies) according to the manufacturer's protocol. Primer sequences are provided in Supplementary Table S1. RT-PCR experiments were performed with 25 ng of RNA, and three technical replicates were performed for each RT-PCR experiment. To confirm primer specificity for igf3 transcript variant 1 and cxcl8-l1, the RT-PCR products generated from 24 hpf wild-type embryo RNA were confirmed by direct DNA sequencing.
Generation of constructs
A 4.3-kb fragment of genomic sequence located 30 base pairs 5′ to the ATG start site of zebrafish igf3 transcript variant 1 (GenBank HQ241070.1),including the first 19 base pairs of exon 1 of igf3 transcript variant 1, was PCR amplified with Accuprime Taq DNA polymerase, High Fidelity (Invitrogen Life Technologies). Primer sequences for the 4.3 igf3 promoter fragment are shown in Supplementary Table S1. The 4.3-kb igf3 promoter fragment was cloned into a pBluescript-SK+ vector containing EGFP and an SV40 polyadenylation signal, flanked by two ISceI sites. The human KRASG12V cDNA was PCR amplified with Accuprime Pfx Taq DNA polymerase (Invitrogen) from pBabe-KRAS-V12 (Addgene) and cloned in-frame at the 3′ end of EGFP. Constructs were confirmed by restriction digest and direct DNA sequencing.
Establishment of transgenic zebrafish lines
Embryos were collected from naturally mated AB wild-type zebrafish for microinjection. One-cell stage embryos were injected with 30 pg of plasmid, 0.5 U/μL ISceI meganuclease (New Englad Biolabs), 0.5X ISceI nuclease buffer (New England Biolabs), and 0.025% Phenol Red. Injected embryos were raised to adulthood and individually outcrossed to wild-type zebrafish. Individual embryos from each cross were collected for DNA extraction and screened by PCR for the presence of the transgene. F0 founders were used to establish the four transgenic lines analyzed in this study.
Maintenance of transgenic lines
Both tg(igf3:EGFP)AB lines were bred to homozygosity and maintained as homozygous lines. Both tg(igf3:EGFP-KRASG12V)AB lines were maintained as hemizygous lines. Adult zebrafish were anesthetized with 0.016% Tricaine in system water buffered with 0.04% Na2HPO4 and fin-clipped, and fin clips were used for DNA extraction as described previously.23 Transgenic zebrafish were identified by PCR amplification of the transgenic construct (Supplementary Table S1 and Fig. 1).
Histologic analyses
For each transgenic line, zebrafish were examined by histology at 3, 6, and 12 months of age. As both tg(igf3:EGFP-KRASG12V)AB lines were maintained as hemizygous lines, wild-type age-matched siblings were collected to serve as controls for each time point. Equal numbers of male and female zebrafish were examined. Zebrafish were fixed and decalcified as previously described24 and were routinely processed to prepare 5-μm hematoxylin and eosin-stained sagittal sections (Histoserv, Inc.). Two sections from each zebrafish were evaluated by histologic examination. See Supplementary Table S3 for additional details.
Immunohistochemistry
Unstained 5-μm serial sections from transgenic and wild-type zebrafish were routinely prepared from paraffin-embedded tissues (Histoserv). Immunohistochemistry was performed with a rabbit anti-GFP antibody (Abcam ab6556) a rabbit anti- phospho-p44/42-MAPK antibody (Cell Signaling Technology 4370), and a rabbit anti-sox9 antibody (Abcam ab3697). For each set of slides processed for immunohistochemistry, one serial section was processed as a negative control (secondary antibody only). Sections were deparaffinized and rehydrated in xylene and ethanol grades. To block endogenous peroxidase activity, sections were incubated in 3% H2O2/70% methanol for 20 min. For sox9 immunohistochemistry, heat-induced epitope retrieval was achieved by incubating slides in DAKO Target Retrieval solution (Dako) in a steamer for 20 min. Protein and avidin/biotin blocking were achieved with Dako protein block (Dako) with 1.5% neutral goat serum and the Avidin/Biotin blocking kit (Vector Laboratories). Sections were incubated in primary antibody solution diluted in Dako antibody diluent for 1 h at room temperature. Sections processed for sox9 expression were blocked for 30 min after primary antibody incubation in Background Buster blocking agent (Innovex Biosciences). Detection was achieved with the Vectastain Elite ABC kit (Vector Laboratories) for GFP and phospho-p44/42-MAPK, and with the ImmPRESS HRP anti-rabbit Ig polymer detection kit (Vector Laboratories) for sox9, and the ImmPact DAB peroxidase substrate (Vector Laboratories) according to the manufacturer's protocol. After detection, sections were lightly counterstained with Mayer's hematoxylin (Sigma-Aldrich), dehydrated, and coverslips mounted with Permount adhesive (Thermo Fisher Scientific).
Alcian blue staining of zebrafish sections
Unstained 5-μm serial sections were routinely prepared from paraffin-embedded tissues (Histoserv). Sections were deparaffinized and rehydrated in xylene and ethanol grades. Sections were incubated in 3% Alcian blue (Electron Microscopy Sciences) and 3% acetic acid for 30 min and counterstained with Nuclear Fast Red solution (Sigma-Aldrich) for 5 min. After staining, sections were dehydrated and coverslips mounted with Permount adhesive (Thermo Fisher Scientific).
In situ hybridization
In situ hybridization for igf3 was performed on unstained sections of the wild-type zebrafish ovary as described previously.23 The igf3 probe contained a 799 bp portion of the cDNA, which included 70 bp from the 3′ end of exon 1; the entirety of exon 2, exon 3, and exon 4; and 221 bp of the 3′ untranslated region.
Image acquisition and processing
Histologic slides were evaluated with an Olympus BX43 microscope (Olympus Corporation), and histologic images were captured with a DP26 digital camera (Olympus Corporation) and cellSens Entry microscope imaging software, version 1.5 (Olympus). Histologic images were minimally processed with Adobe Photoshop CS3, version 10.0.1 (Adobe Systems Incorporated) or with the GNU Image Manipulation Program, version 2.8.6 (www.gimp.org/).
Flow cytometry
The gill arches were dissected from individual wild-type and transgenic zebrafish and pooled, three zebrafish per genotype. Gills were incubated in L-15 medium with 500 U/mL collagenase type I (Life Technologies) at 28°C for 2 h, pipetting gently every 20 min to dissociate cells. Samples were washed thrice with 1X PBS, filtered through a 35-μm filter, counted, and stained with 0.5% DAPI for live/dead cell differentiation. Cells were sorted by EGFP fluorescence with the BD Influx sorter (BD Biosciences) with an 82-μm nozzle at 37 PSI at the rate of 1000 cells/second and collected in 1×PBS. After collection of sorted cell populations, samples were snap-frozen and processed for RNA isolation as described above.
Supplementary Material
Acknowledgments
The authors thank Ms. Veena Kapoor for isolation and collection of zebrafish cells by FACS. This work was supported by the Intramural Research Program of the U.S. National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Author Contributions
H.R.S., R.R.W., L.J.E., and D.D.H. designed the research; H.R.S., R.R.W., L.J.E., and J.M.S. performed the research; H.R.S, R.R.W., L.J.E., and D.D.H. analyzed data; and H.R.S. and D.D.H. wrote the article.
Disclosure Statement
The authors declare that no conflicts of interest exist.
References
- 1.Bos JL. ras oncogenes in human cancer: a review. Cancer Res 1989;49:4682–4689 [PubMed] [Google Scholar]
- 2.Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst 2003;95:484–486 [DOI] [PubMed] [Google Scholar]
- 3.Li J, Liu Z, Wang D, Cheng CH. Insulin-like growth factor 3 is involved in oocyte maturation in zebrafish. Biol Reprod 2011;84:476–486 [DOI] [PubMed] [Google Scholar]
- 4.Li M, Wu F, Gu Y, Wang T, Wang H, Yang S, et al. Insulin-like growth factor 3 regulates expression of genes encoding steroidogenic enzymes and key transcription factors in the Nile tilapia gonad. Biol Reprod 2012;86:163, 1–10 [DOI] [PubMed] [Google Scholar]
- 5.Wang DS, Jiao B, Hu C, Huang X, Liu Z, Cheng CH. Discovery of a gonad-specific IGF subtype in teleost. Biochem Biophys Res Commun 2008;367:336–341 [DOI] [PubMed] [Google Scholar]
- 6.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 2006;25:2105–2112 [DOI] [PubMed] [Google Scholar]
- 7.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 2004;6:447–458 [DOI] [PubMed] [Google Scholar]
- 8.Vojtek AB, Der CJ. Increasing complexity of the Ras signaling pathway. J Biol Chem 1998;273:19925–19928 [DOI] [PubMed] [Google Scholar]
- 9.Yan YL, Miller CT, Nissen RM, Singer A, Liu D, Kirn A, et al. A zebrafish sox9 gene required for cartilage morphogenesis. Development 2002;129:5065–5079 [DOI] [PubMed] [Google Scholar]
- 10.Yan YL, Willoughby J, Liu D, Crump JG, Wilson C, Miller CT, et al. A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development 2005;132:1069–1083 [DOI] [PubMed] [Google Scholar]
- 11.Okumura T, Ericksen RE, Takaishi S, Wang SS, Dubeykovskiy Z, Shibata W, et al. K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res 2010;70:8435–8445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oehlers SH, Flores MV, Hall CJ, O'Toole R, Swift S, Crosier KE, et al. Expression of zebrafish cxcl8 (interleukin-8) and its receptors during development and in response to immune stimulation. Dev Comp Immunol 2010;34:352–359 [DOI] [PubMed] [Google Scholar]
- 13.Berishvili G, Baroiller JF, Eppler E, Reinecke M. Insulin-like growth factor-3 (IGF-3) in male and female gonads of the tilapia: development and regulation of gene expression by growth hormone (GH) and 17alpha-ethinylestradiol (EE2). Gen Comp Endocrinol 2010;167:128–134 [DOI] [PubMed] [Google Scholar]
- 14.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 1997;385:544–548 [DOI] [PubMed] [Google Scholar]
- 15.Downward J. Cell cycle: routine role for Ras. Curr Biol 1997;7:R258–R260 [DOI] [PubMed] [Google Scholar]
- 16.Campbell PM, Der CJ. Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin Cancer Biol 2004;14:105–114 [DOI] [PubMed] [Google Scholar]
- 17.Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene 1998;17:1395–1413 [DOI] [PubMed] [Google Scholar]
- 18.Takagi M, Shinoda K, Piao J, Mitsuiki N, Takagi M, Matsuda K, et al. Autoimmune lymphoproliferative syndrome-like disease with somatic KRAS mutation. Blood 2011;117:2887–2890 [DOI] [PubMed] [Google Scholar]
- 19.Robertson G, Garrick D, Wu W, Kearns M, Martin D, Whitelaw E. Position-dependent variegation of globin transgene expression in mice. Proc Natl Acad Sci U S A 1995;92:5371–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson C, Bellen HJ, Gehring WJ. Position effects on eukaryotic gene expression. Ann Rev Cell Biol 1990;6:679–714 [DOI] [PubMed] [Google Scholar]
- 21.Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A 2005;102:14665–14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci U S A 2003;100:9360–9365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shive HR, West RR, Embree LJ, Azuma M, Sood R, Liu P, et al. brca2 in zebrafish ovarian development, spermatogenesis, and tumorigenesis. Proc Natl Acad Sci U S A 2010;107:19350–19355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shive HR, West RR, Embree LJ, Golden CD, Hickstein DD. BRCA2 and TP53 collaborate in tumorigenesis in zebrafish. PloS One 2014;9:e87177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.