Abstract
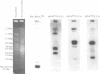
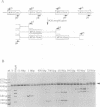
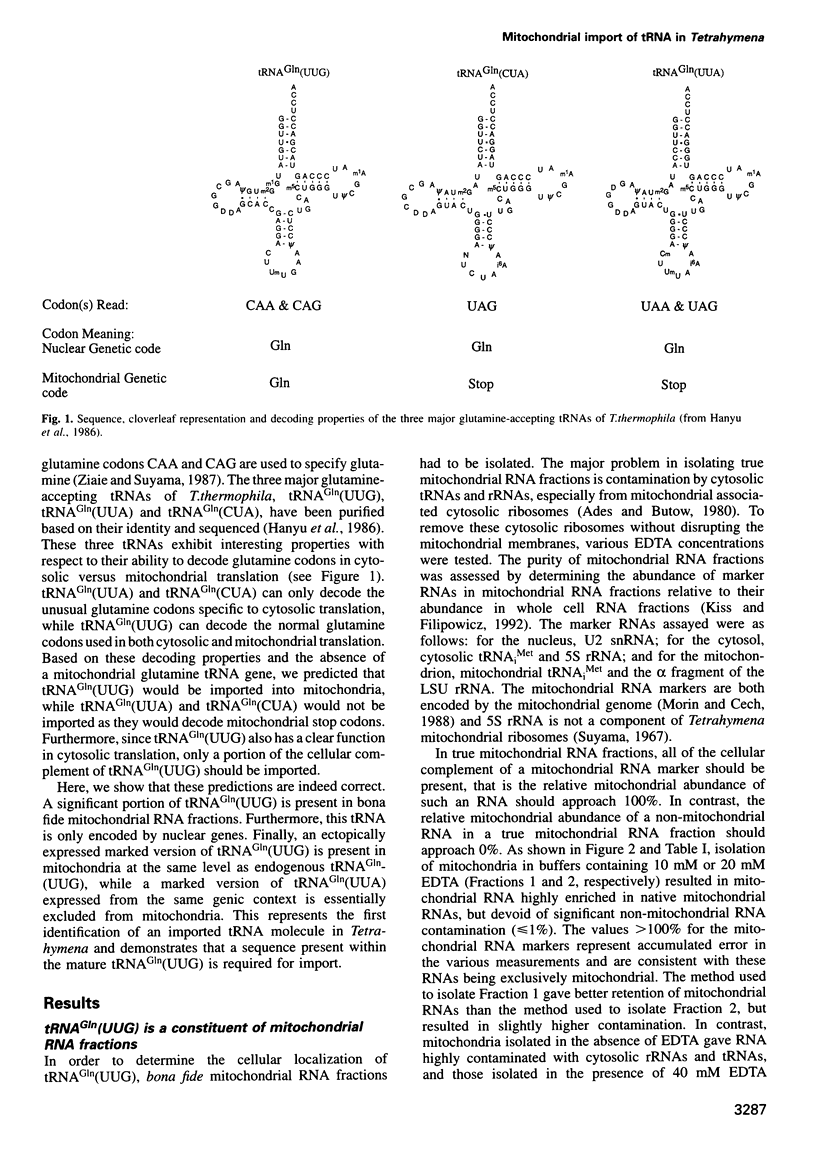
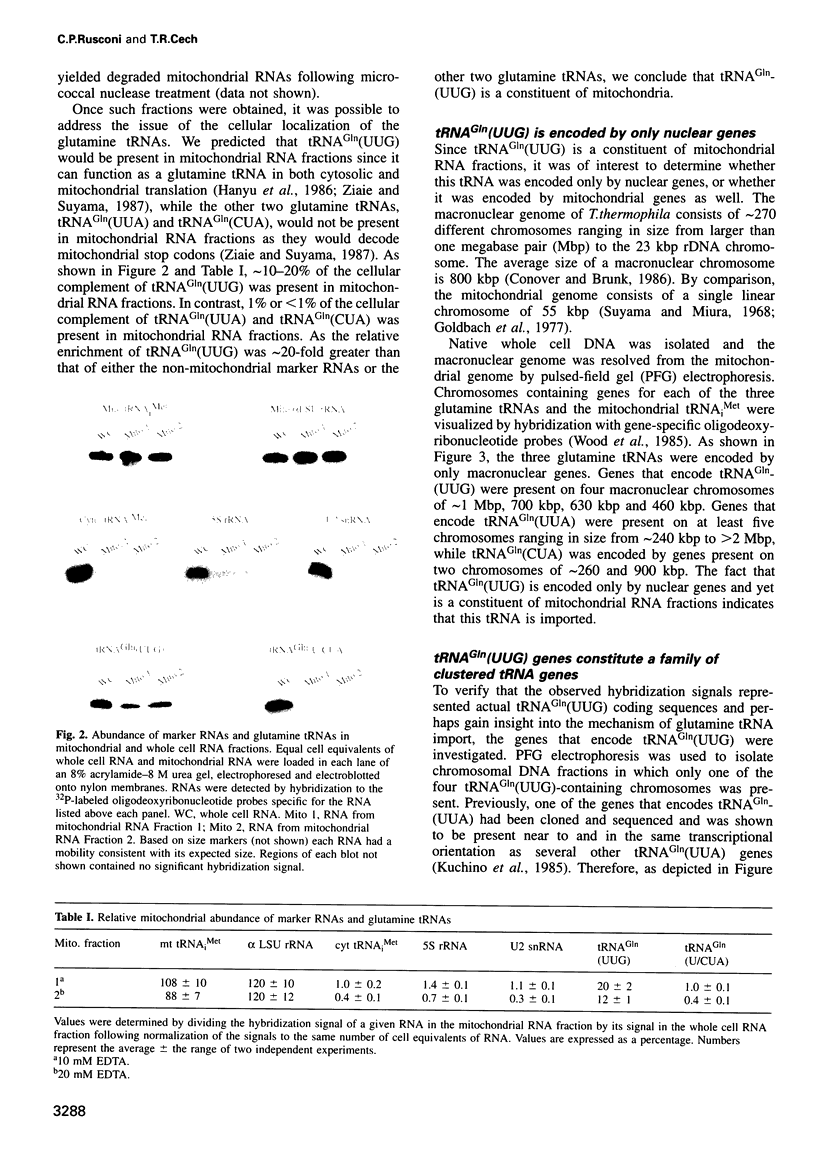
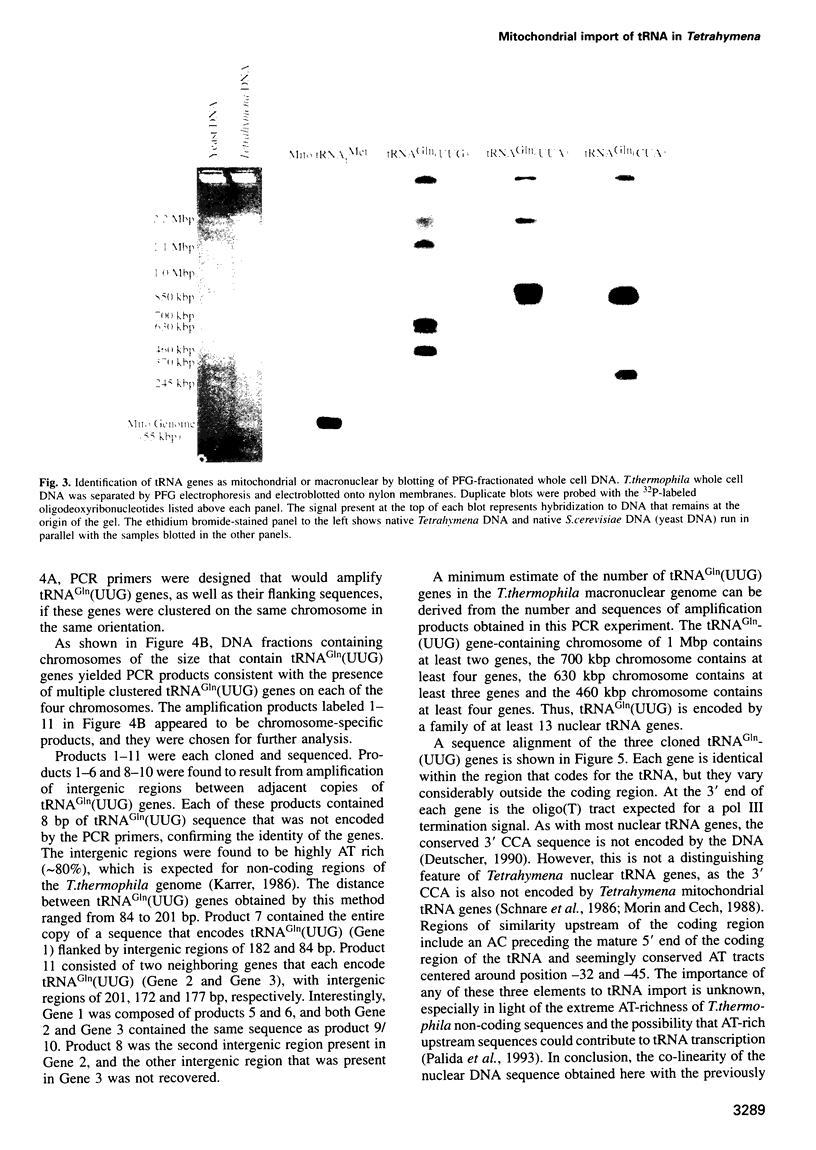
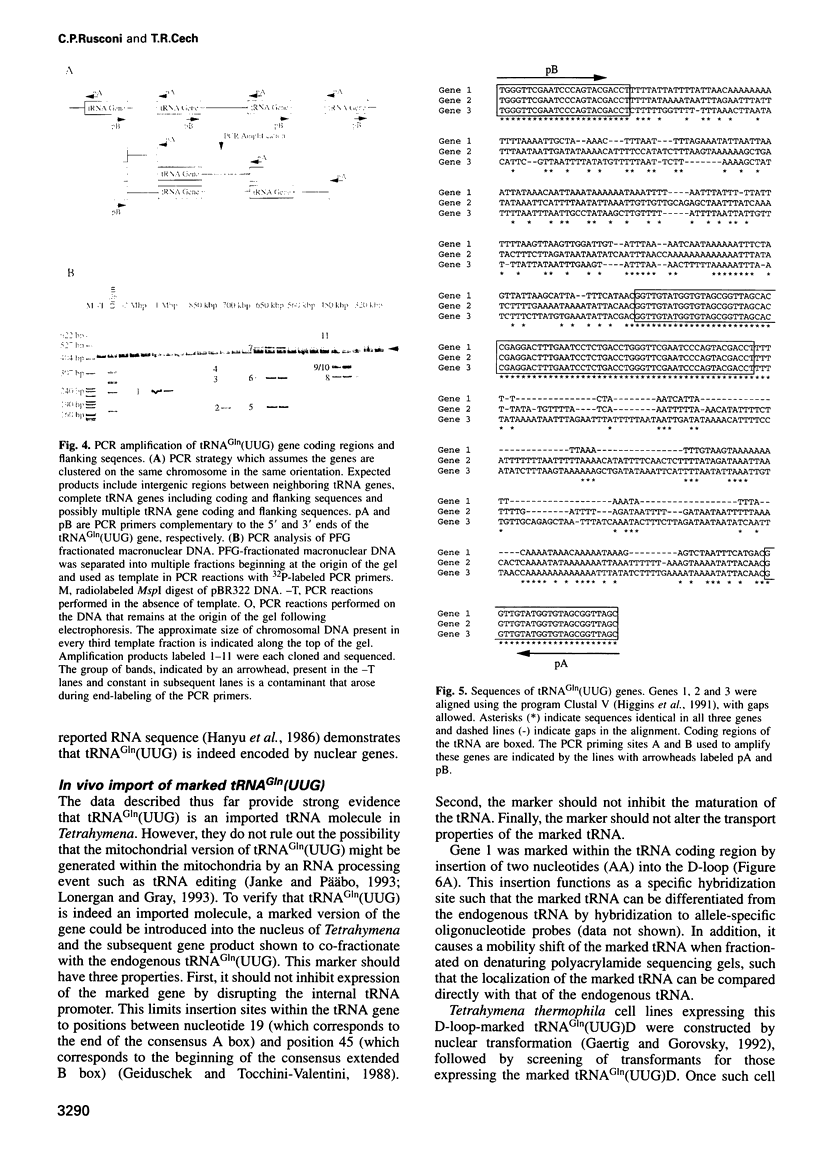
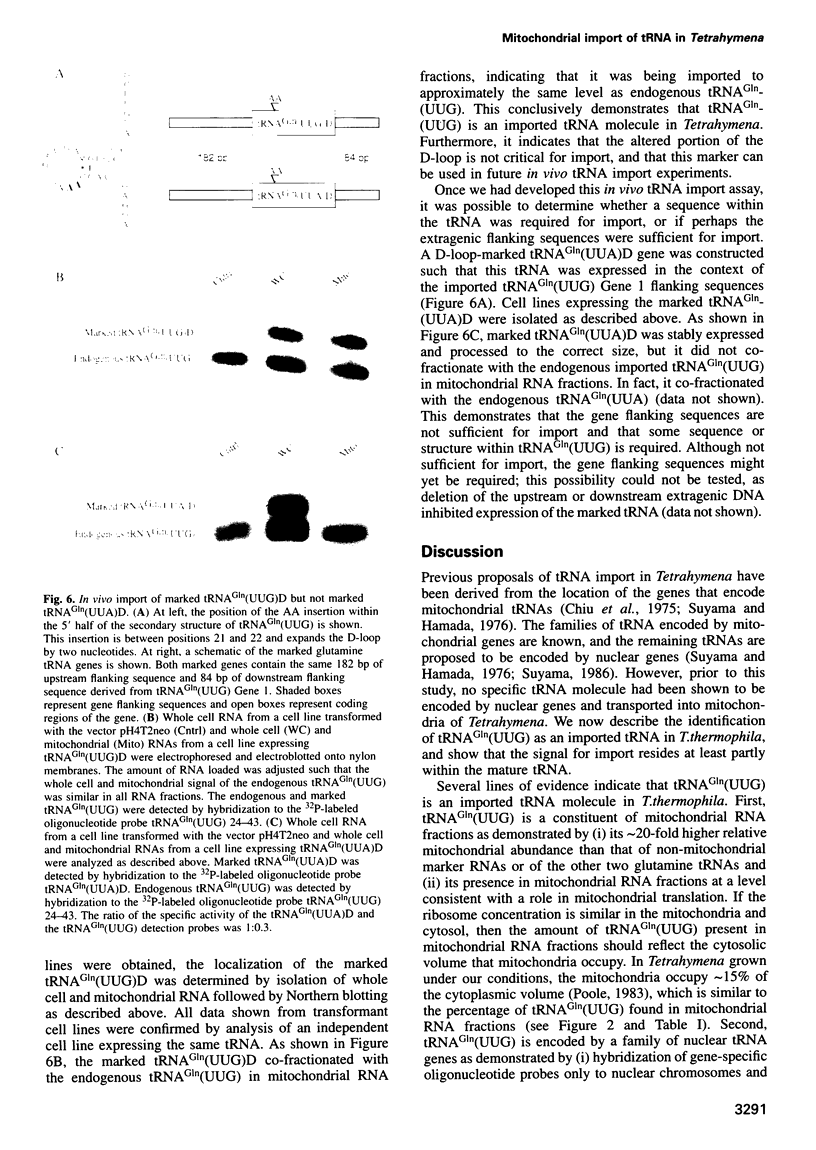
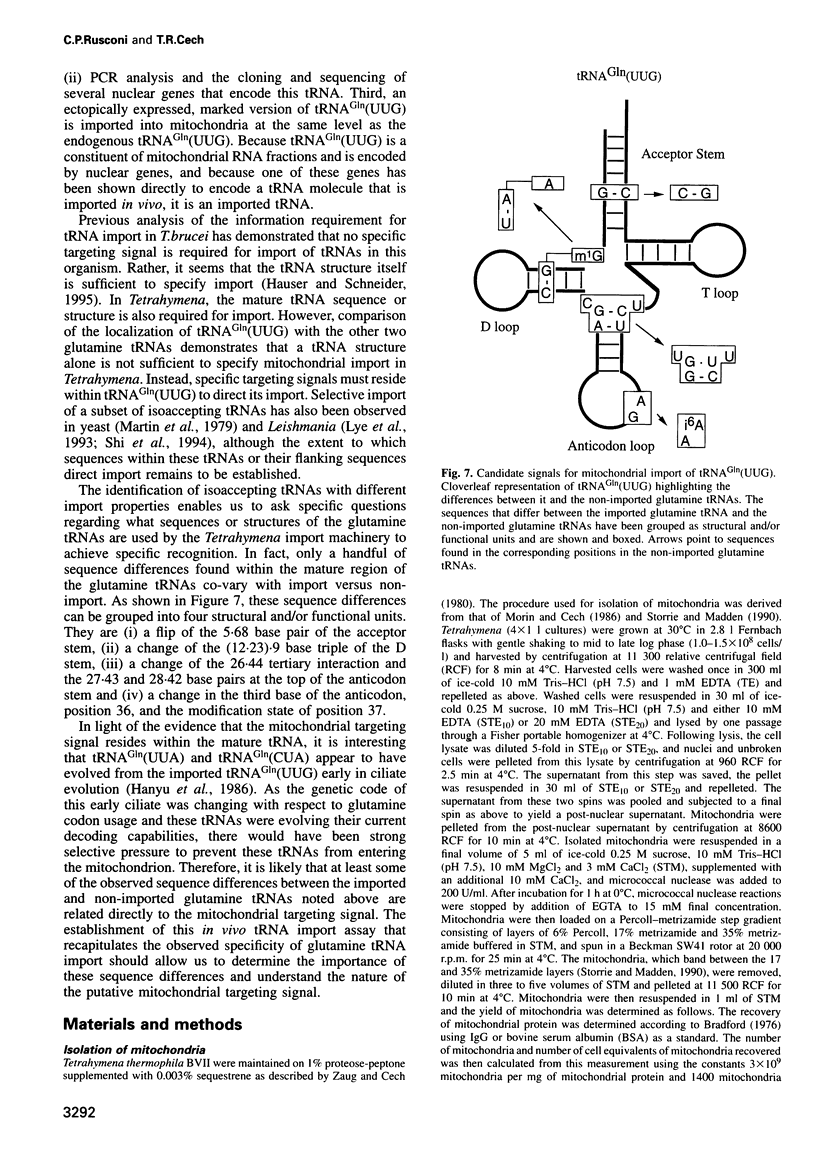
The mitochondrial genome of Tetrahymena does not appear to encode enough tRNAs to perform mitochondrial protein synthesis. It has therefore been proposed that nuclear-encoded tRNAs are imported into the mitochondria. T.thermophila has three major glutamine tRNAs: tRNA(Gln)(UUG), tRNA(Gln)(UUA) and tRNA(Gln)(CUA). Each of these tRNAs functions in cytosolic translation. However, due to differences between the Tetrahymena nuclear and mitochondrial genetic codes, only tRNA(Gln)(UUG) has the capacity to function in mitochondrial translation as well. Here we show that approximately 10-20% of the cellular complement of tRNA(Gln)(UUG) is present in mitochondrial RNA fractions, compared with 1% or less for the other two glutamine tRNAs. Furthermore, this glutamine tRNA is encoded only by a family of nuclear genes, the sequences of several of which are presented. Finally, when marked versions of tRNA(Gln)(UUG) and tRNA(Gln)(UUA) flanked by identical sequences are expressed in the macronucleus, only the former undergoes mitochondrial import; thus sequences within tRNA(Gln)(UUG) direct import. Because tRNA(Gln)(UUG) is a constituent of mitochondrial RNA fractions and is encoded only by nuclear genes, and because ectopically expressed tRNA(Gln)(UUG) fractionates with mitochondria like its endogenous counterpart, we conclude that it is an imported tRNA in T.thermophila.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ades I. Z., Butow R. A. The products of mitochondria-bound cytoplasmic polysomes in yeast. J Biol Chem. 1980 Oct 25;255(20):9918–9924. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiu N., Chiu A., Suyama Y. Native and imported transfer RNA in mitochondria. J Mol Biol. 1975 Nov 25;99(1):37–50. doi: 10.1016/s0022-2836(75)80157-4. [DOI] [PubMed] [Google Scholar]
- Conover R. K., Brunk C. F. Macronuclear DNA molecules of Tetrahymena thermophila. Mol Cell Biol. 1986 Mar;6(3):900–905. doi: 10.1128/mcb.6.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. Ribonucleases, tRNA nucleotidyltransferase, and the 3' processing of tRNA. Prog Nucleic Acid Res Mol Biol. 1990;39:209–240. doi: 10.1016/s0079-6603(08)60628-5. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Weil J. H., Maréchal-Drouard L. Nuclear-encoded transfer RNAs in plant mitochondria. Annu Rev Cell Biol. 1992;8:115–131. doi: 10.1146/annurev.cb.08.110192.000555. [DOI] [PubMed] [Google Scholar]
- Gaertig J., Gorovsky M. A. Efficient mass transformation of Tetrahymena thermophila by electroporation of conjugants. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9196–9200. doi: 10.1073/pnas.89.19.9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig J., Gu L., Hai B., Gorovsky M. A. High frequency vector-mediated transformation and gene replacement in Tetrahymena. Nucleic Acids Res. 1994 Dec 11;22(24):5391–5398. doi: 10.1093/nar/22.24.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Goldbach R. W., Arnberg A. C., van Bruggen E. F., Defize J., Borst P. The structure of Tetrahymena pyriformis mitochondrial DNA. I. Strain differences and occurrence of inverted repetitions. Biochim Biophys Acta. 1977 Jul 5;477(1):37–50. doi: 10.1016/0005-2787(77)90159-9. [DOI] [PubMed] [Google Scholar]
- Hancock K., Hajduk S. L. The mitochondrial tRNAs of Trypanosoma brucei are nuclear encoded. J Biol Chem. 1990 Nov 5;265(31):19208–19215. [PubMed] [Google Scholar]
- Hanyu N., Kuchino Y., Nishimura S., Beier H. Dramatic events in ciliate evolution: alteration of UAA and UAG termination codons to glutamine codons due to anticodon mutations in two Tetrahymena tRNAs. EMBO J. 1986 Jun;5(6):1307–1311. doi: 10.1002/j.1460-2075.1986.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R., Schneider A. tRNAs are imported into mitochondria of Trypanosoma brucei independently of their genomic context and genetic origin. EMBO J. 1995 Sep 1;14(17):4212–4220. doi: 10.1002/j.1460-2075.1995.tb00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen T. Y., Schnare M. N., Young P. G., Gray M. W. Rearranged coding segments, separated by a transfer RNA gene, specify the two parts of a discontinuous large subunit ribosomal RNA in Tetrahymena pyriformis mitochondria. J Biol Chem. 1987 Feb 25;262(6):2879–2887. [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Gorovsky M. A. An unusual genetic code in nuclear genes of Tetrahymena. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2452–2455. doi: 10.1073/pnas.82.8.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke A., Päbo S. Editing of a tRNA anticodon in marsupial mitochondria changes its codon recognition. Nucleic Acids Res. 1993 Apr 11;21(7):1523–1525. doi: 10.1093/nar/21.7.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Filipowicz W. Evidence against a mitochondrial location of the 7-2/MRP RNA in mammalian cells. Cell. 1992 Jul 10;70(1):11–16. doi: 10.1016/0092-8674(92)90528-k. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Hanyu N., Tashiro F., Nishimura S. Tetrahymena thermophila glutamine tRNA and its gene that corresponds to UAA termination codon. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4758–4762. doi: 10.1073/pnas.82.14.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Mita T., Nishimura S. Nucleotide sequence of cytoplasmic initiator tRNA from Tetrahymena thermophila. Nucleic Acids Res. 1981 Sep 25;9(18):4557–4562. doi: 10.1093/nar/9.18.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonergan K. M., Gray M. W. Editing of transfer RNAs in Acanthamoeba castellanii mitochondria. Science. 1993 Feb 5;259(5096):812–816. doi: 10.1126/science.8430334. [DOI] [PubMed] [Google Scholar]
- Lye L. F., Chen D. H., Suyama Y. Selective import of nuclear-encoded tRNAs into mitochondria of the protozoan Leishmania tarentolae. Mol Biochem Parasitol. 1993 Apr;58(2):233–245. doi: 10.1016/0166-6851(93)90045-y. [DOI] [PubMed] [Google Scholar]
- Martin R. P., Schneller J. M., Stahl A. J., Dirheimer G. Import of nuclear deoxyribonucleic acid coded lysine-accepting transfer ribonucleic acid (anticodon C-U-U) into yeast mitochondria. Biochemistry. 1979 Oct 16;18(21):4600–4605. doi: 10.1021/bi00588a021. [DOI] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B., Cech T. R. Phylogenetic relationships and altered genome structures among Tetrahymena mitochondrial DNAs. Nucleic Acids Res. 1988 Jan 11;16(1):327–346. doi: 10.1093/nar/16.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B., Cech T. R. The telomeres of the linear mitochondrial DNA of Tetrahymena thermophila consist of 53 bp tandem repeats. Cell. 1986 Sep 12;46(6):873–883. doi: 10.1016/0092-8674(86)90069-3. [DOI] [PubMed] [Google Scholar]
- Orum H., Nielsen H., Engberg J. Spliceosomal small nuclear RNAs of Tetrahymena thermophila and some possible snRNA-snRNA base-pairing interactions. J Mol Biol. 1991 Nov 20;222(2):219–232. doi: 10.1016/0022-2836(91)90208-n. [DOI] [PubMed] [Google Scholar]
- Palida F. A., Hale C., Sprague K. U. Transcription of a silkworm tRNA(cAla) gene is directed by two AT-rich upstream sequence elements. Nucleic Acids Res. 1993 Dec 25;21(25):5875–5881. doi: 10.1093/nar/21.25.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K. Mitochondria of Tetrahymena pyriformis: enumeration and sizing of isolated organelles using a Coulter Counter and pulse-height analyser. J Cell Sci. 1983 May;61:437–451. doi: 10.1242/jcs.61.1.437. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Heinonen T. Y., Young P. G., Gray M. W. Phenylalanine and tyrosine transfer RNAs encoded by Tetrahymena pyriformis mitochondrial DNA: primary sequence, post-transcriptional modifications, and gene localization. Curr Genet. 1985;9(5):389–393. doi: 10.1007/BF00421610. [DOI] [PubMed] [Google Scholar]
- Schneider A. Import of RNA into mitochondria. Trends Cell Biol. 1994 Aug;4(8):282–286. doi: 10.1016/0962-8924(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Schneider A., Martin J., Agabian N. A nuclear encoded tRNA of Trypanosoma brucei is imported into mitochondria. Mol Cell Biol. 1994 Apr;14(4):2317–2322. doi: 10.1128/mcb.14.4.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Chen D. H., Suyama Y. A nuclear tRNA gene cluster in the protozoan Leishmania tarentolae and differential distribution of nuclear-encoded tRNAs between the cytosol and mitochondria. Mol Biochem Parasitol. 1994 May;65(1):23–37. doi: 10.1016/0166-6851(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Simpson A. M., Suyama Y., Dewes H., Campbell D. A., Simpson L. Kinetoplastid mitochondria contain functional tRNAs which are encoded in nuclear DNA and also contain small minicircle and maxicircle transcripts of unknown function. Nucleic Acids Res. 1989 Jul 25;17(14):5427–5445. doi: 10.1093/nar/17.14.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I., Maréchal-Drouard L., Masson J., Pelletier G., Cosset A., Weil J. H., Dietrich A. In vivo import of a normal or mutagenized heterologous transfer RNA into the mitochondria of transgenic plants: towards novel ways of influencing mitochondrial gene expression? EMBO J. 1992 Apr;11(4):1291–1296. doi: 10.1002/j.1460-2075.1992.tb05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B., Madden E. A. Isolation of subcellular organelles. Methods Enzymol. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Jenney F., Okawa N. Two transfer RNA sequences abut the large ribosomal RNA gene in Tetrahymena mitochondrial DNA: tRNA(leu) (anticodon UAA) and tRNA(met) (anticodon CAU). Curr Genet. 1987;11(4):327–330. doi: 10.1007/BF00355408. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Miura K. Size and structural variations of mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):235–242. doi: 10.1073/pnas.60.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama Y. Nucleotide sequences of three tRNA genes encoded in Tetrahymena mitochondrial DNA. Nucleic Acids Res. 1985 May 10;13(9):3273–3284. doi: 10.1093/nar/13.9.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama Y. The origins of mitochondrial ribonucleic acids in Tetrahymena pyriformis. Biochemistry. 1967 Sep;6(9):2829–2839. doi: 10.1021/bi00861a025. [DOI] [PubMed] [Google Scholar]
- Suyama Y. Two dimensional polyacrylamide gel electrophoresis analysis of Tetrahymena mitochondrial tRNA. Curr Genet. 1986;10(5):411–420. doi: 10.1007/BF00418415. [DOI] [PubMed] [Google Scholar]
- Varshney U., Lee C. P., RajBhandary U. L. Direct analysis of aminoacylation levels of tRNAs in vivo. Application to studying recognition of Escherichia coli initiator tRNA mutants by glutaminyl-tRNA synthetase. J Biol Chem. 1991 Dec 25;266(36):24712–24718. [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. In vitro splicing of the ribosomal RNA precursor in nuclei of Tetrahymena. Cell. 1980 Feb;19(2):331–338. doi: 10.1016/0092-8674(80)90507-3. [DOI] [PubMed] [Google Scholar]
- Ziaie Z., Suyama Y. The cytochrome oxidase subunit I gene of Tetrahymena: a 57 amino acid NH2-terminal extension and a 108 amino acid insert. Curr Genet. 1987;12(5):357–368. doi: 10.1007/BF00405758. [DOI] [PubMed] [Google Scholar]