Abstract
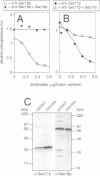
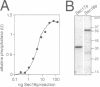
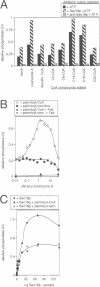
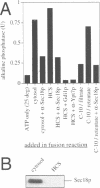
In Saccharomyces cerevisiae, vacuoles are inherited by the formation of tubular and vesicular structures from the mother vacuole, the directed projection of these structures into the bud and the homotypic fusion of these vesicles. We have previously exploited a cell-free inheritance assay to show that the fusion step of vacuole inheritance requires cytosol, ATP and the GTPase Ypt7p. Here we demonstrate, using affinity-purified antibodies and purified recombinant proteins, a requirement for Sec17p (yeast alpha-SNAP) and Sec18p (yeast NSF) in homotypic vacuole fusion in vitro. Thus, Sec17p and Sec18p, which are typically involved in heterotypic transport steps, can also be involved in homotypic organelle fusion. We further show that vacuole-to-vacuole fusion is stimulated by certain fatty acyl-coenzyme A compounds in a Sec18p-dependent fashion. Finally, our data suggest the presence of a cytosolic factor which activates vacuole membrane-bound Sec18p.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya U., Jacobs R., Peters J. M., Watson N., Farquhar M. G., Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995 Sep 22;82(6):895–904. doi: 10.1016/0092-8674(95)90269-4. [DOI] [PubMed] [Google Scholar]
- Acharya U., McCaffery J. M., Jacobs R., Malhotra V. Reconstitution of vesiculated Golgi membranes into stacks of cisternae: requirement of NSF in stack formation. J Cell Biol. 1995 May;129(3):577–589. doi: 10.1083/jcb.129.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Dunphy W. G., Braell W. A., Rothman J. E. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984 Dec;39(2 Pt 1):405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Bankaitis V. A., Johnson L. M., Emr S. D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers C. J., Block M. R., Glick B. S., Rothman J. E., Balch W. E. Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature. 1989 Jun 1;339(6223):397–398. doi: 10.1038/339397a0. [DOI] [PubMed] [Google Scholar]
- Block M. R., Glick B. S., Wilcox C. A., Wieland F. T., Rothman J. E. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary D. O., Griff I. C., Rothman J. E. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990 May 18;61(4):709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Conradt B., Haas A., Wickner W. Determination of four biochemically distinct, sequential stages during vacuole inheritance in vitro. J Cell Biol. 1994 Jul;126(1):99–110. doi: 10.1083/jcb.126.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt B., Shaw J., Vida T., Emr S., Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1992 Dec;119(6):1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couve A., Protopopov V., Gerst J. E. Yeast synaptobrevin homologs are modified posttranslationally by the addition of palmitate. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):5987–5991. doi: 10.1073/pnas.92.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Mayorga L. S., Weidman P. J., Rothman J. E., Stahl P. D. Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature. 1989 Jun 1;339(6223):398–400. doi: 10.1038/339398a0. [DOI] [PubMed] [Google Scholar]
- Eakle K. A., Bernstein M., Emr S. D. Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol Cell Biol. 1988 Oct;8(10):4098–4109. doi: 10.1128/mcb.8.10.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev. 1989 Jul;69(3):765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- Fröhlich K. U., Fries H. W., Rüdiger M., Erdmann R., Botstein D., Mecke D. Yeast cell cycle protein CDC48p shows full-length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation, and gene expression. J Cell Biol. 1991 Aug;114(3):443–453. doi: 10.1083/jcb.114.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. S., Rothman J. E. Possible role for fatty acyl-coenzyme A in intracellular protein transport. Nature. 1987 Mar 19;326(6110):309–312. doi: 10.1038/326309a0. [DOI] [PubMed] [Google Scholar]
- Goda Y., Pfeffer S. R. Identification of a novel, N-ethylmaleimide-sensitive cytosolic factor required for vesicular transport from endosomes to the trans-Golgi network in vitro. J Cell Biol. 1991 Mar;112(5):823–831. doi: 10.1083/jcb.112.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes de Mesquita D. S., ten Hoopen R., Woldringh C. L. Vacuolar segregation to the bud of Saccharomyces cerevisiae: an analysis of morphology and timing in the cell cycle. J Gen Microbiol. 1991 Oct;137(10):2447–2454. doi: 10.1099/00221287-137-10-2447. [DOI] [PubMed] [Google Scholar]
- Graham T. R., Emr S. D. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991 Jul;114(2):207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griff I. C., Schekman R., Rothman J. E., Kaiser C. A. The yeast SEC17 gene product is functionally equivalent to mammalian alpha-SNAP protein. J Biol Chem. 1992 Jun 15;267(17):12106–12115. [PubMed] [Google Scholar]
- Haas A., Conradt B., Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994 Jul;126(1):87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A., Scheglmann D., Lazar T., Gallwitz D., Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995 Nov 1;14(21):5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D. T., Slater T. M., Wilson M. C., Skene J. H. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992 Dec;12(12):4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E., Tagaya M., Ullrich O., Montecucco C., Simons K. Different requirements for NSF, SNAP, and Rab proteins in apical and basolateral transport in MDCK cells. Cell. 1995 May 19;81(4):571–580. doi: 10.1016/0092-8674(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Jones H. D., Schliwa M., Drubin D. G. Video microscopy of organelle inheritance and motility in budding yeast. Cell Motil Cytoskeleton. 1993;25(2):129–142. doi: 10.1002/cm.970250203. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990 May 18;61(4):723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht B., Schmidt M. F. Membrane fusion induced by influenza virus hemagglutinin requires protein bound fatty acids. FEBS Lett. 1986 Jun 23;202(1):127–132. doi: 10.1016/0014-5793(86)80662-7. [DOI] [PubMed] [Google Scholar]
- Latterich M., Schekman R. The karyogamy gene KAR2 and novel proteins are required for ER-membrane fusion. Cell. 1994 Jul 15;78(1):87–98. doi: 10.1016/0092-8674(94)90575-4. [DOI] [PubMed] [Google Scholar]
- Latterich M., Schekman R. The karyogamy gene KAR2 and novel proteins are required for ER-membrane fusion. Cell. 1994 Jul 15;78(1):87–98. doi: 10.1016/0092-8674(94)90575-4. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Orci L., Glick B. S., Block M. R., Rothman J. E. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988 Jul 15;54(2):221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner W., Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996 Apr 5;85(1):83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Mellman I. Enigma variations: protein mediators of membrane fusion. Cell. 1995 Sep 22;82(6):869–872. doi: 10.1016/0092-8674(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Moehle C. M., Aynardi M. W., Kolodny M. R., Park F. J., Jones E. W. Protease B of Saccharomyces cerevisiae: isolation and regulation of the PRB1 structural gene. Genetics. 1987 Feb;115(2):255–263. doi: 10.1093/genetics/115.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Is NSF a fusion protein? Trends Cell Biol. 1995 Sep;5(9):335–339. doi: 10.1016/s0962-8924(00)89059-5. [DOI] [PubMed] [Google Scholar]
- Morgan A., Dimaline R., Burgoyne R. D. The ATPase activity of N-ethylmaleimide-sensitive fusion protein (NSF) is regulated by soluble NSF attachment proteins. J Biol Chem. 1994 Nov 25;269(47):29347–29350. [PubMed] [Google Scholar]
- Mundy D. I., Warren G. Mitosis and inhibition of intracellular transport stimulate palmitoylation of a 62-kD protein. J Cell Biol. 1992 Jan;116(1):135–146. doi: 10.1083/jcb.116.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve C. W., Williams D. Fatty acids on the A/Japan/305/57 influenza virus hemagglutinin have a role in membrane fusion. EMBO J. 1990 Dec;9(12):3857–3866. doi: 10.1002/j.1460-2075.1990.tb07604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Nuoffer C., Balch W. E. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Ostermann J., Orci L., Tani K., Amherdt M., Ravazzola M., Elazar Z., Rothman J. E. Stepwise assembly of functionally active transport vesicles. Cell. 1993 Dec 3;75(5):1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Glick B. S., Arden S. R., Rothman J. E. Fatty acylation promotes fusion of transport vesicles with Golgi cisternae. J Cell Biol. 1990 Apr;110(4):955–961. doi: 10.1083/jcb.110.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Orci L., Glick B. S., Amherdt M., Arden S. R., Malhotra V., Rothman J. E. Fatty acyl-coenzyme A is required for budding of transport vesicles from Golgi cisternae. Cell. 1989 Oct 6;59(1):95–102. doi: 10.1016/0092-8674(89)90872-6. [DOI] [PubMed] [Google Scholar]
- Quesnel S., Silvius J. R. Cysteine-containing peptide sequences exhibit facile uncatalyzed transacylation and acyl-CoA-dependent acylation at the lipid bilayer interface. Biochemistry. 1994 Nov 15;33(45):13340–13348. doi: 10.1021/bi00249a021. [DOI] [PubMed] [Google Scholar]
- Rabouille C., Levine T. P., Peters J. M., Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 1995 Sep 22;82(6):905–914. doi: 10.1016/0092-8674(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Raymond C. K., Roberts C. J., Moore K. E., Howald I., Stevens T. H. Biogenesis of the vacuole in Saccharomyces cerevisiae. Int Rev Cytol. 1992;139:59–120. doi: 10.1016/s0074-7696(08)61410-2. [DOI] [PubMed] [Google Scholar]
- Rexach M. F., Schekman R. W. Distinct biochemical requirements for the budding, targeting, and fusion of ER-derived transport vesicles. J Cell Biol. 1991 Jul;114(2):219–229. doi: 10.1083/jcb.114.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L., Stirling C. J., Woodman P. G. Multiple N-ethylmaleimide-sensitive components are required for endosomal vesicle fusion. Mol Biol Cell. 1994 Jul;5(7):773–783. doi: 10.1091/mbc.5.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Schimmöller F., Riezman H. Involvement of Ypt7p, a small GTPase, in traffic from late endosome to the vacuole in yeast. J Cell Sci. 1993 Nov;106(Pt 3):823–830. doi: 10.1242/jcs.106.3.823. [DOI] [PubMed] [Google Scholar]
- Shaw J. M., Wickner W. T. vac2: a yeast mutant which distinguishes vacuole segregation from Golgi-to-vacuole protein targeting. EMBO J. 1991 Jul;10(7):1741–1748. doi: 10.1002/j.1460-2075.1991.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida M., Hong R. M., Tagaya M. Role of two nucleotide-binding regions in an N-ethylmaleimide-sensitive factor involved in vesicle-mediated protein transport. J Biol Chem. 1994 Aug 12;269(32):20636–20641. [PubMed] [Google Scholar]
- Sztul E., Colombo M., Stahl P., Samanta R. Control of protein traffic between distinct plasma membrane domains. Requirement for a novel 108,000 protein in the fusion of transcytotic vesicles with the apical plasma membrane. J Biol Chem. 1993 Jan 25;268(3):1876–1885. [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Wada Y., Ohsumi Y., Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. I. Isolation and characterization of two classes of vam mutants. J Biol Chem. 1992 Sep 15;267(26):18665–18670. [PubMed] [Google Scholar]
- Wattenberg B. W., Raub T. J., Hiebsch R. R., Weidman P. J. The activity of Golgi transport vesicles depends on the presence of the N-ethylmaleimide-sensitive factor (NSF) and a soluble NSF attachment protein (alpha SNAP) during vesicle formation. J Cell Biol. 1992 Sep;118(6):1321–1332. doi: 10.1083/jcb.118.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidman P. J., Melançon P., Block M. R., Rothman J. E. Binding of an N-ethylmaleimide-sensitive fusion protein to Golgi membranes requires both a soluble protein(s) and an integral membrane receptor. J Cell Biol. 1989 May;108(5):1589–1596. doi: 10.1083/jcb.108.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S., Bacallao R., Wickner W. Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J Cell Biol. 1987 Oct;105(4):1539–1547. doi: 10.1083/jcb.105.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S., Emr S. D., Wickner W. T. Mutants of Saccharomyces cerevisiae that block intervacuole vesicular traffic and vacuole division and segregation. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1076–1080. doi: 10.1073/pnas.87.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S., Wickner W. Intervacuole exchange in the yeast zygote: a new pathway in organelle communication. Science. 1988 Jul 29;241(4865):589–591. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Brunner M., Wilson D. W., Wiedmann M., Rothman J. E. Soluble N-ethylmaleimide-sensitive fusion attachment proteins (SNAPs) bind to a multi-SNAP receptor complex in Golgi membranes. J Biol Chem. 1992 Jun 15;267(17):12239–12243. [PubMed] [Google Scholar]
- Whiteheart S. W., Kubalek E. W. SNAPs and NSF: general members of the fusion apparatus. Trends Cell Biol. 1995 Feb;5(2):64–68. doi: 10.1016/s0962-8924(00)88948-5. [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Rossnagel K., Buhrow S. A., Brunner M., Jaenicke R., Rothman J. E. N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J Cell Biol. 1994 Aug;126(4):945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann H., Hengst L., Gallwitz D. Endocytosis in yeast: evidence for the involvement of a small GTP-binding protein (Ypt7p). Cell. 1992 Dec 24;71(7):1131–1142. doi: 10.1016/s0092-8674(05)80062-5. [DOI] [PubMed] [Google Scholar]
- Wilson D. W., Whiteheart S. W., Wiedmann M., Brunner M., Rothman J. E. A multisubunit particle implicated in membrane fusion. J Cell Biol. 1992 May;117(3):531–538. doi: 10.1083/jcb.117.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
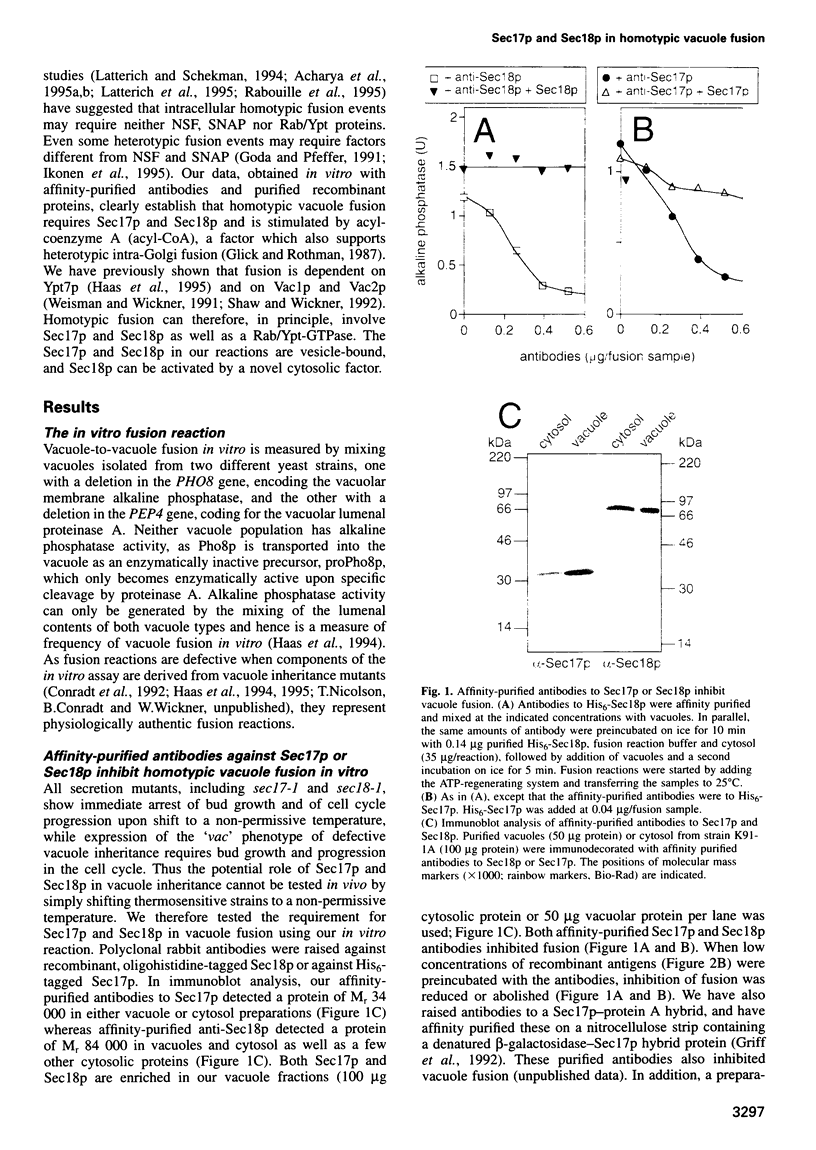
- Xu Z., Wickner W. Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1996 Mar;132(5):787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]