Abstract
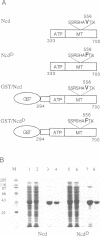
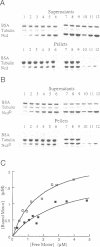
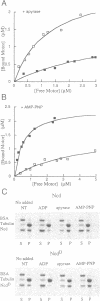
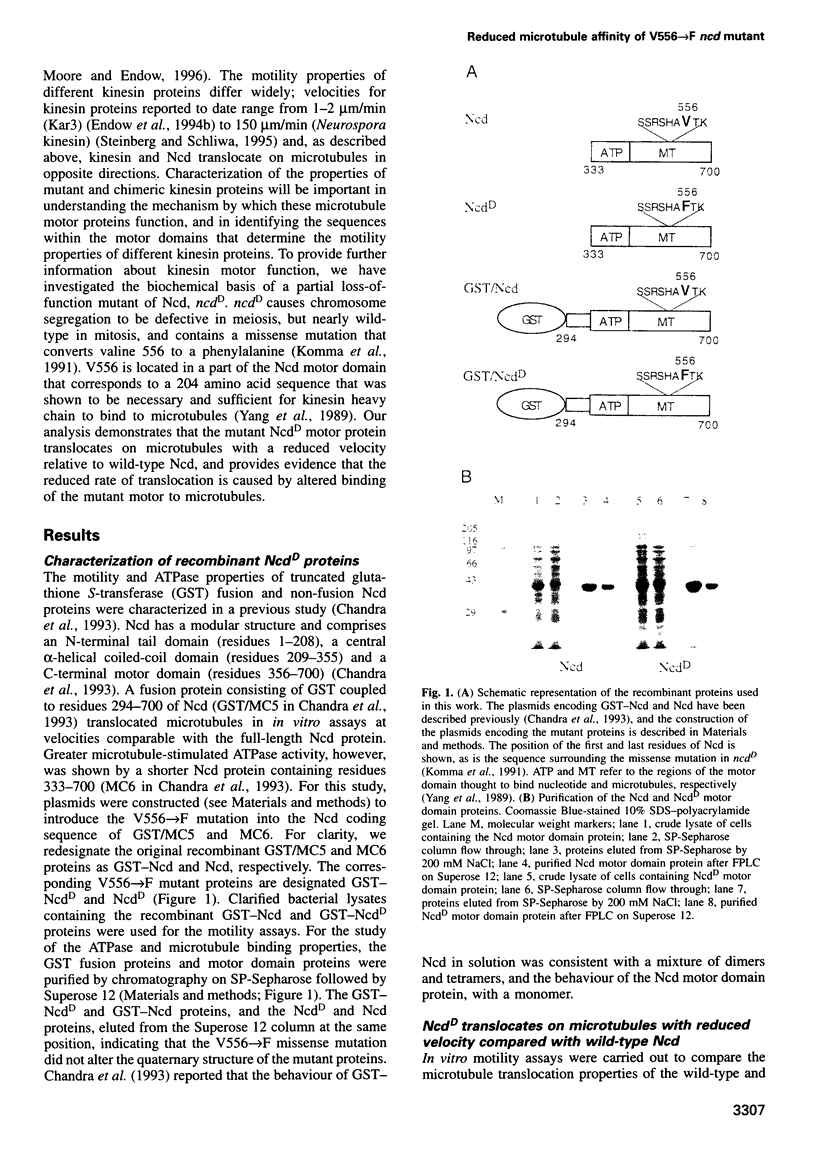
Non-claret disjunctional (Ncd) is a kinesin-related microtubule motor protein in Drosophila that functions in meiotic spindle assembly in oocytes and spindle pole maintenance in early embryos. The partial loss-of-function mutant ncdD retains mitotic, but not meiotic, function. The predicted NcdD mutant protein contains a V556-->F mutation in the putative microtubule binding region of the Ncd motor domain. Here we report an analysis of the properties of recombinant Ncd and NcdD proteins. A GST-NcdD fusion protein translocated microtubules approximately 10-fold more slowly than the corresponding wild-type protein in gliding assays. The maximum microtubule-stimulated ATPase activity of an NcdD motor domain protein was reduced approximately 3-fold and an approximately 3-fold greater concentration of microtubules was required for half-maximal stimulation of ATPase activity, compared with the corresponding wild-type protein. The Km for ATP and basal rate of ATP turnover were, in contrast, similar for the NcdD mutant and wild-type Ncd motor domain proteins. Pelleting assays demonstrated that the binding of the mutant NcdD motor protein to microtubules was reduced in the absence of nucleotide, relative to wild-type. The reduced velocity of NcdD translocation on microtubules is therefore correlated with reductions in microtubule-stimulated ATPase activity and affinity of the mutant motor for microtubules. The characteristics of the NcdD motor explain its meiotic loss of function, and are consistent with partial motor activity of Ncd being sufficient for its mitotic, but not its meiotic, role.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brady S. T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985 Sep 5;317(6032):73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Chandra R., Endow S. A. Expression of microtubule motor proteins in bacteria for characterization in in vitro motility assays. Methods Cell Biol. 1993;39:115–127. doi: 10.1016/s0091-679x(08)60165-x. [DOI] [PubMed] [Google Scholar]
- Chandra R., Salmon E. D., Erickson H. P., Lockhart A., Endow S. A. Structural and functional domains of the Drosophila ncd microtubule motor protein. J Biol Chem. 1993 Apr 25;268(12):9005–9013. [PubMed] [Google Scholar]
- Crevel I. M., Lockhart A., Cross R. A. Weak and strong states of kinesin and ncd. J Mol Biol. 1996 Mar 22;257(1):66–76. doi: 10.1006/jmbi.1996.0147. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Chandra R., Komma D. J., Yamamoto A. H., Salmon E. D. Mutants of the Drosophila ncd microtubule motor protein cause centrosomal and spindle pole defects in mitosis. J Cell Sci. 1994 Apr;107(Pt 4):859–867. doi: 10.1242/jcs.107.4.859. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Henikoff S., Soler-Niedziela L. Mediation of meiotic and early mitotic chromosome segregation in Drosophila by a protein related to kinesin. Nature. 1990 May 3;345(6270):81–83. doi: 10.1038/345081a0. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Kang S. J., Satterwhite L. L., Rose M. D., Skeen V. P., Salmon E. D. Yeast Kar3 is a minus-end microtubule motor protein that destabilizes microtubules preferentially at the minus ends. EMBO J. 1994 Jun 1;13(11):2708–2713. doi: 10.1002/j.1460-2075.1994.tb06561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. P., Webb M. R., Brune M., Johnson K. A. Pathway of processive ATP hydrolysis by kinesin. Nature. 1995 Feb 23;373(6516):671–676. doi: 10.1038/373671a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. C., von Hippel P. H. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989 Nov 1;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Gliksman N. R., Salmon E. D. Microtubule-associated motility in cytoplasmic extracts of sea urchin eggs. Cell Motil Cytoskeleton. 1993;24(3):167–178. doi: 10.1002/cm.970240304. [DOI] [PubMed] [Google Scholar]
- Hackney D. D. Kinesin ATPase: rate-limiting ADP release. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6314–6318. doi: 10.1073/pnas.85.17.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsumi M., Endow S. A. Mutants of the microtubule motor protein, nonclaret disjunctional, affect spindle structure and chromosome movement in meiosis and mitosis. J Cell Sci. 1992 Mar;101(Pt 3):547–559. doi: 10.1242/jcs.101.3.547. [DOI] [PubMed] [Google Scholar]
- Hatsumi M., Endow S. A. The Drosophila ncd microtubule motor protein is spindle-associated in meiotic and mitotic cells. J Cell Sci. 1992 Dec;103(Pt 4):1013–1020. doi: 10.1242/jcs.103.4.1013. [DOI] [PubMed] [Google Scholar]
- Huang T. G., Hackney D. D. Drosophila kinesin minimal motor domain expressed in Escherichia coli. Purification and kinetic characterization. J Biol Chem. 1994 Jun 10;269(23):16493–16501. [PubMed] [Google Scholar]
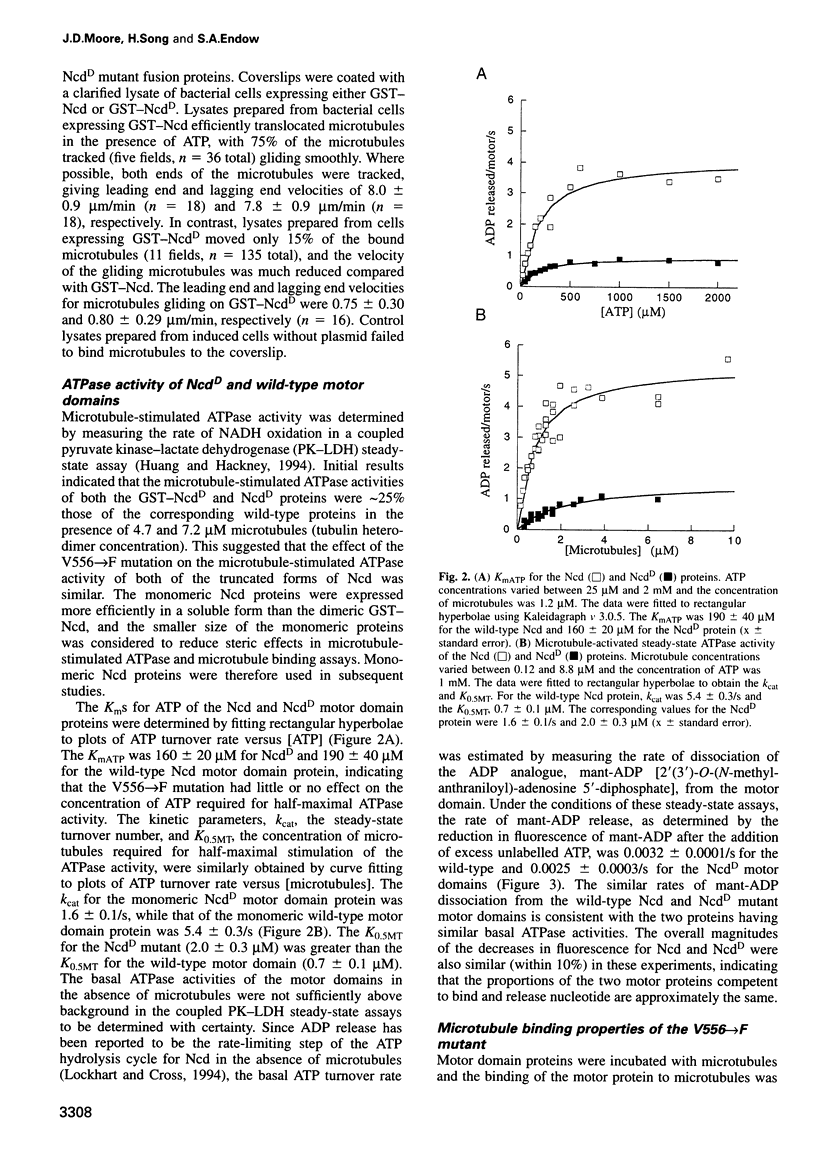
- Kalfayan L., Wensink P. C. Developmental regulation of Drosophila alpha-tubulin genes. Cell. 1982 May;29(1):91–98. doi: 10.1016/0092-8674(82)90093-9. [DOI] [PubMed] [Google Scholar]
- Komma D. J., Horne A. S., Endow S. A. Separation of meiotic and mitotic effects of claret non-disjunctional on chromosome segregation in Drosophila. EMBO J. 1991 Feb;10(2):419–424. doi: 10.1002/j.1460-2075.1991.tb07963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
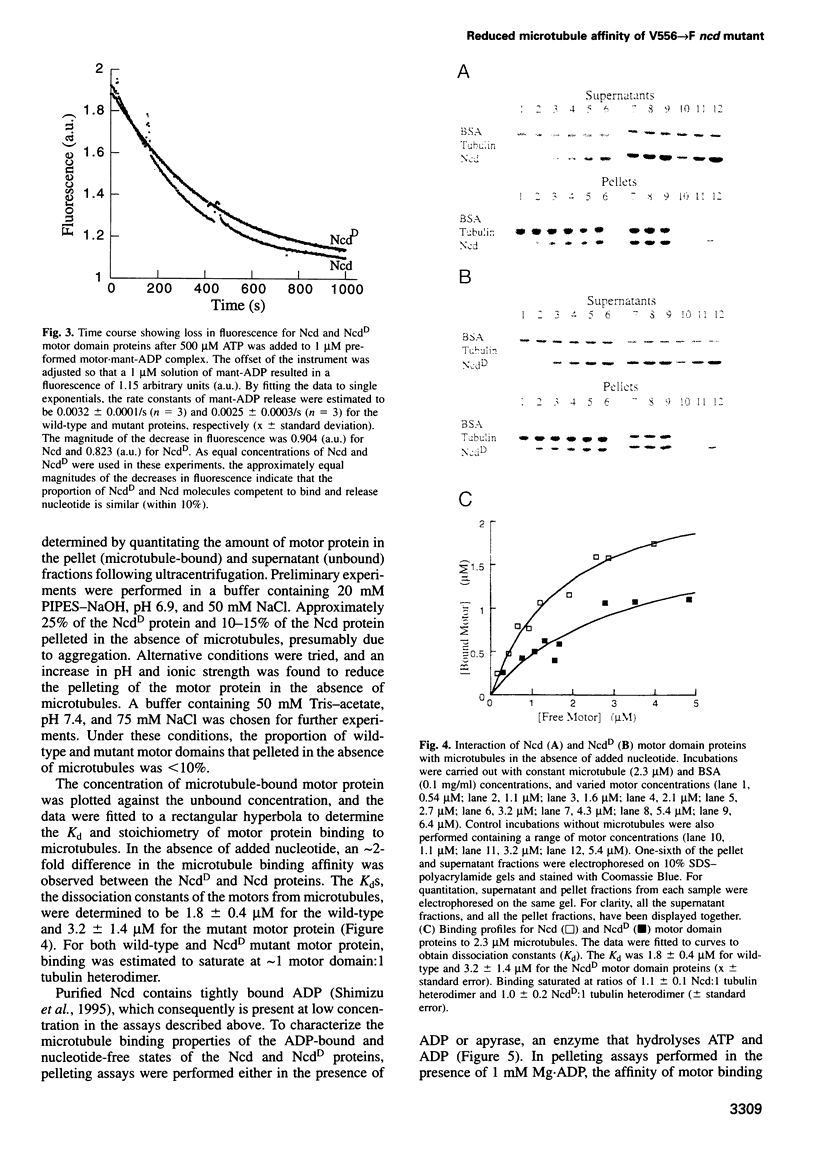
- Lockhart A., Crevel I. M., Cross R. A. Kinesin and ncd bind through a single head to microtubules and compete for a shared MT binding site. J Mol Biol. 1995 Jun 16;249(4):763–771. doi: 10.1006/jmbi.1995.0335. [DOI] [PubMed] [Google Scholar]
- Lockhart A., Cross R. A., McKillop D. F. ADP release is the rate-limiting step of the MT activated ATPase of non-claret disjunctional and kinesin. FEBS Lett. 1995 Jul 24;368(3):531–535. doi: 10.1016/0014-5793(95)00723-m. [DOI] [PubMed] [Google Scholar]
- Lockhart A., Cross R. A. Origins of reversed directionality in the ncd molecular motor. EMBO J. 1994 Feb 15;13(4):751–757. doi: 10.1002/j.1460-2075.1994.tb06317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. Z., Taylor E. W. Mechanism of microtubule kinesin ATPase. Biochemistry. 1995 Oct 10;34(40):13242–13251. doi: 10.1021/bi00040a040. [DOI] [PubMed] [Google Scholar]
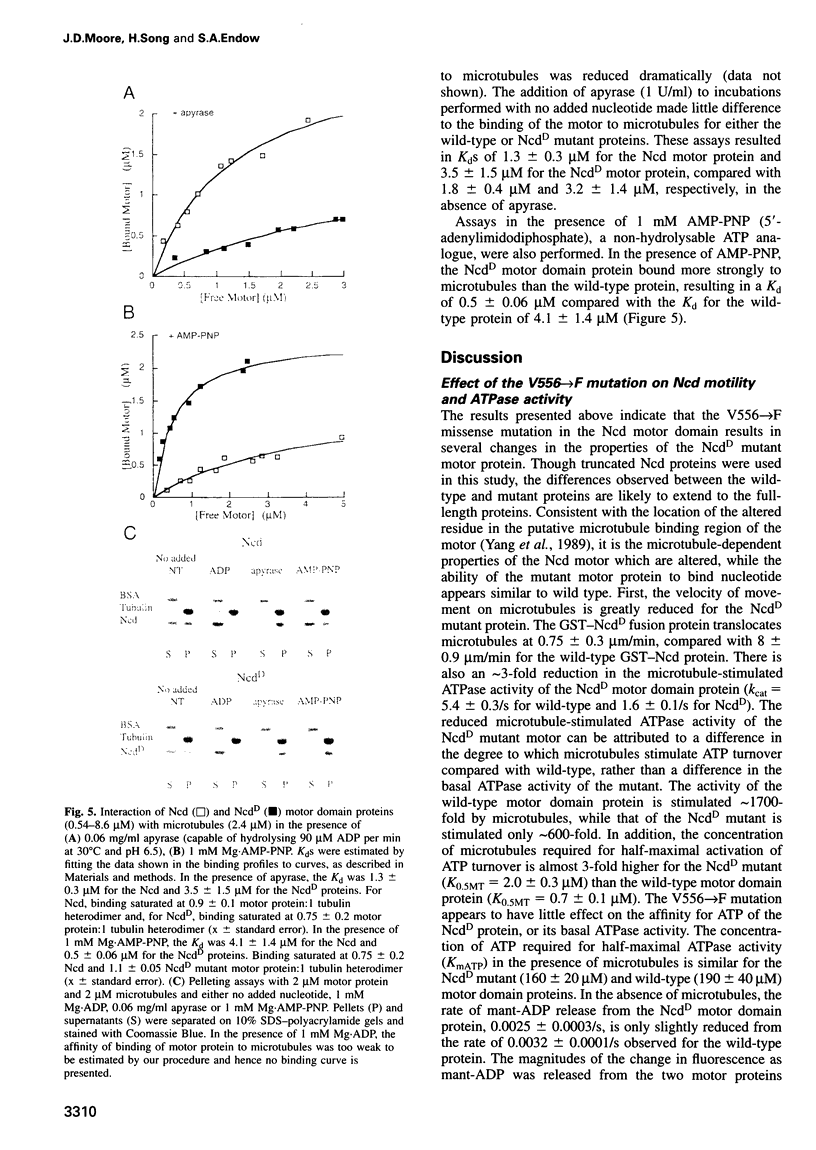
- Matthews K. A., Rees D., Kaufman T. C. A functionally specialized alpha-tubulin is required for oocyte meiosis and cleavage mitoses in Drosophila. Development. 1993 Mar;117(3):977–991. doi: 10.1242/dev.117.3.977. [DOI] [PubMed] [Google Scholar]
- McDonald H. B., Stewart R. J., Goldstein L. S. The kinesin-like ncd protein of Drosophila is a minus end-directed microtubule motor. Cell. 1990 Dec 21;63(6):1159–1165. doi: 10.1016/0092-8674(90)90412-8. [DOI] [PubMed] [Google Scholar]
- Moore J. D., Endow S. A. Kinesin proteins: a phylum of motors for microtubule-based motility. Bioessays. 1996 Mar;18(3):207–219. doi: 10.1002/bies.950180308. [DOI] [PubMed] [Google Scholar]
- Romberg L., Vale R. D. Chemomechanical cycle of kinesin differs from that of myosin. Nature. 1993 Jan 14;361(6408):168–170. doi: 10.1038/361168a0. [DOI] [PubMed] [Google Scholar]
- Saxton W. M., Porter M. E., Cohn S. A., Scholey J. M., Raff E. C., McIntosh J. R. Drosophila kinesin: characterization of microtubule motility and ATPase. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1109–1113. doi: 10.1073/pnas.85.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Sablin E., Vale R. D., Fletterick R., Pechatnikova E., Taylor E. W. Expression, purification, ATPase properties, and microtubule-binding properties of the ncd motor domain. Biochemistry. 1995 Oct 10;34(40):13259–13266. doi: 10.1021/bi00040a042. [DOI] [PubMed] [Google Scholar]
- Steinberg G., Schliwa M. The Neurospora organelle motor: a distant relative of conventional kinesin with unconventional properties. Mol Biol Cell. 1995 Nov;6(11):1605–1618. doi: 10.1091/mbc.6.11.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Mitchison T., Steuer E., Reese T. S., Sheetz M. P. Different axoplasmic proteins generate movement in opposite directions along microtubules in vitro. Cell. 1985 Dec;43(3 Pt 2):623–632. doi: 10.1016/0092-8674(85)90234-x. [DOI] [PubMed] [Google Scholar]
- Vernos I., Karsenti E. Chromosomes take the lead in spindle assembly. Trends Cell Biol. 1995 Aug;5(8):297–301. doi: 10.1016/s0962-8924(00)89045-5. [DOI] [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988 Oct;107(4):1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., Salmon E. D., Endow S. A. The Drosophila claret segregation protein is a minus-end directed motor molecule. Nature. 1990 Oct 25;347(6295):780–782. doi: 10.1038/347780a0. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lee J. C. Preparation of tubulin from brain. Methods Enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Laymon R. A., Goldstein L. S. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989 Mar 10;56(5):879–889. doi: 10.1016/0092-8674(89)90692-2. [DOI] [PubMed] [Google Scholar]