SUMMARY
New and more promising therapies for chronic hepatitis C (CHC) genotype 1 (G1) naive patients have recently been approved in the United States and Europe, and several more regimens are expected to become available within the next several years. While this scenario unfolds, it is necessary to develop a rational method to allocate current treatment in CHC G1 patients. We performed a cost-effectiveness analysis of boceprevir (BOC)- and telaprevir (TVR)-based triple therapy according to different patients’ selection strategies. A semi-Markov model of CHC natural history and progression towards end-stage liver disease was built. We considered 3 selection strategies based on METAVIR fibrosis stage: (i) treat all patients with F1–F4 fibrosis, (ii) only F2–F4 and (iii) only F3–F4. For each strategy, TVR interleukin-28B-guided (IL28B-guided) and BOC rapid virologic response-guided (RVR-guided) therapies were applied. The model assessed the costs and outcomes, using a lifetime and 5-year time horizon, and adopting the Italian National Health System perspective. The incremental cost-effectiveness ratio (ICER) for F1–F4 strategy relative to F3–F4 was €5132 per quality-adjusted life years gained, across TVR IL-28B-guided therapy, and €7042 in the BOC RVR-guided therapy. Conversely, in the 5-year scenario, the ICER for F1–F4 strategy relative to F3–F4 was €1 818 679 (TVR IL28B-guided) and €1 866 437 (BOC RVR-guided) per end-stage liver disease or death (ESLD-D) avoided. In view of anticipated improvement in the efficacy of future regimens, selective treatment of only patients with advanced fibrosis and cirrhosis with TVR or BOC could represent the most cost-effective strategy to optimize resource utilization.
Keywords: cost-effectiveness, fibrosis stage, HCV genotype 1, naïve patients, sustained virologic response, triple therapy
INTRODUCTION
Hepatitis C virus (HCV) infection is a major global health problem with an estimated worldwide prevalence of 170 million persons. Untreated chronic HCV infection evolves through progressive fibrosis stages to cirrhosis and eventually liver decompensation and hepatocellular carcinoma (HCC). These conditions are characterized by high morbidity and mortality, and significant economic impact on health systems, as reflected by frequent high-intensity hospitalizations, interventional procedures and liver transplantation [1,2]. The natural history of chronic hepatitis C (CHC) can be radically changed by successful antiviral therapy (i.e. achievement of sustained virologic response, SVR).
Achievement of SVR reduces disease progression, occurrence of hard clinical outcomes (liver failure, HCC and death) and associated health costs [3]. Nevertheless, efforts to reduce morbidity and mortality of CHC have been historically limited by low SVR rates in genotype 1 (G1) patients treated with pegylated interferon alfa and ribavirin (dual therapy – DT) [4]. Triple therapy (TT) with DT plus one of two protease inhibitors (PI), boceprevir (BOC) or telaprevir (TVR), significantly improves SVR rates to 63–75% vs 38–44% with DT alone in treatment-naïve individuals [5,6]. Despite improved SVR, the increased complexity, pill burden and adverse effect profile of TT have limited treatment eligibility and utilization. Two cost-effectiveness analyses of TT in G1 CHC naïve patients have demonstrated that these new treatment options are cost-effective [7,8], particularly when treatment strategy is determined by pretreatment (interleukin-28B gene polymorphism, IL28B) or on-treatment viral kinetics (rapid virologic response, RVR). Through these strategies, PIs can be avoided in a subgroup of patients who respond well to DT alone (IL28B C/C). Although two new DAA-based triple therapy regimens for genotype 1 infection (sofosbuvir or simeprevir to be used with DT) have been approved by the U.S. Federal Drug Administration as of December 2013 [9,10], new therapies are not expected to be approved globally until the end of 2014, even in midst of very promising data suggesting that interferon-free regimens may emerge as a new standard of care for genotype 1 HCV within the next 12 months. Considering the many challenges associated with TT, including pill burden (12–18 pills/day), q8 h dosing, multisystem toxicity, drug interactions, resistance and significant cost, and in face of limited economic resources and promising drugs in development, it is necessary to develop a rational method to selectively allocate current treatment in CHC G1 patients. Therefore, we performed a cost-effectiveness analysis of BOC- and TVR-based TT according to different patients’ selection strategies driven by fibrosis stage.
METHODS
Overview
A decision-analytic semi-Markov model of HCV natural history and progression towards end-stage liver diseases was built to assess the cost-effectiveness of patient selection strategies for treatment-naïve patients with G1 CHC (Fig. 1). The cohort was defined by initial fibrosis stage (Metavir score of F0, F1, F2, F3 or F4/compensated cirrhosis), age (stratified by fibrosis stage), IL-28B genotype (CC and non-CC types) and weight distribution. We considered three different strategies to allocate treatment: (i) treat all patients with F1–F4 fibrosis, (ii) treat only patients with F2–F4 and (iii) treat only patients with F3–F4. Each strategy was applied to two alternative BOC- or TVR-based treatment protocols which have been identified in previous studies as the most cost-effective: IL28B-guided, TVR-based TT and RVR-guided, BOC-based TT [7,8]. The model estimated the costs related to the treatment with DT and TT, the costs associated with each health state, the life years (LYSs), the quality-adjusted life years (QALYs), the incremental cost per LYS gained and incremental cost per QALY gained. The model assessed the costs and outcomes using a lifetime time horizon in alignment with the Italian National Health System (NHS) perspective. Future costs and clinical benefits (QALYs) were discounted at 3% per year.
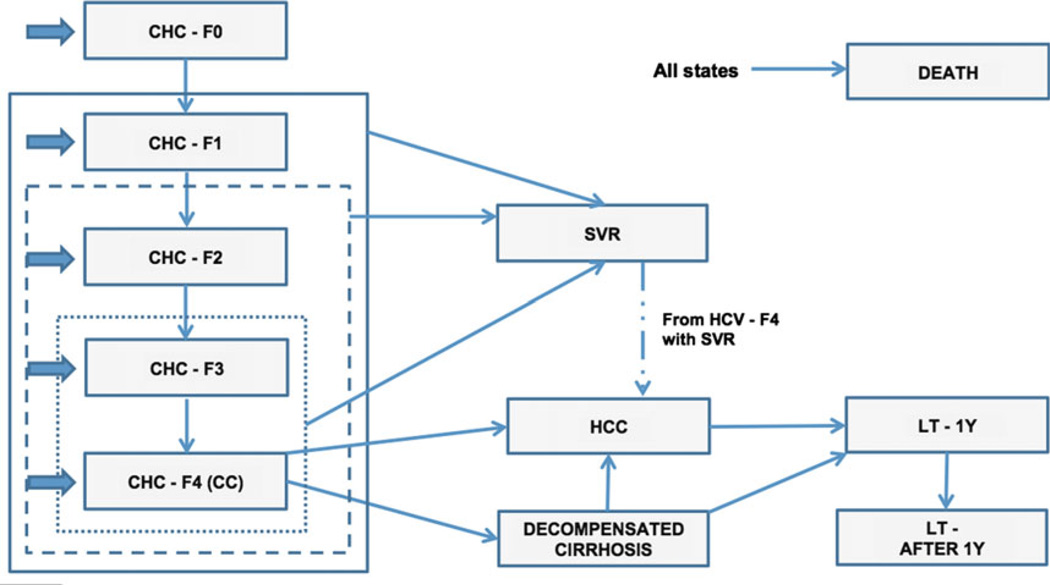
Fig. 1.
Diagrammatic representation of the Markov model. The states of the model are represented by the rectangles, transitions between states are denoted by the thin arrows, and entry points were denoted by large arrows. The continuous line represents the F1–F4 selection strategy, the dash line the F2–F4 and the dotted line the F3–F4.
Because the landscape of HCV treatment is changing and very promising interferon-free treatment will be available soon, the cost-effectiveness analysis was performed with an alternative scenario using 5 years as the time horizon. This time horizon was chosen to simulate a reasonable maximum time before interferon-free treatments become available globally, with the hypothesis that it is only cost-effective to treat patients with the highest likelihood of liver failure in the next 5 years without treatment (F3–F4), and furthermore stratify patients who are unlikely to progress within 5 years for whom treatment deferral to future interferon-free regimens may be acceptable. In the 5-year simulation, the model assessed costs, number of end-stage liver diseases (decompensated cirrhosis, HCC or liver transplant) or death (ESLD-D) and incremental cost per ESLD-D avoided as outcome measures.
Model structure
The model simulated the disease progression of a treatment-naïve CHC G1 subject (Fig. 1). The model included the following health states: patients achieving SVR (SVR), no fibrosis (CHC-F0), portal fibrosis with no septa (CHCF1), portal fibrosis with few septa (CHC-F2), numerous septa without cirrhosis (CHC-F3), compensated cirrhosis (CHC-F4 or CC), decompensated cirrhosis (DC), HCC, liver transplantation (LT-1Y) and liver transplant after the first year (LT-AFTER 1Y).
Treatment strategies
Three selection strategies were simulated in the model. Patients undergo treatment immediately if their fibrosis stage is included in the selection strategy considered, or later when they progress from a less severe fibrosis state not included in the selection strategy to a more severe and included state (e.g. in the F2–F4 strategy, a F1 patient was treated only when he progressed to a F2 state). The selection strategies were as follows:
F1–F4 strategy: treating all patients having fibrosis stage between F1 and F4 (including extremes) and all patients who progressed from F0 to F1;
F2–F4 strategy: treating all patients having fibrosis stage between F2 and F4 and all patients who progressed from F0 and F1 to F2;
F3–F4 strategy: treating all patients having fibrosis stage between F3 and F4 and all patients who progressed from F0, F1 and F2 to F3.
With this structure, the model compared the impact of more restricted strategies (e.g. F3–F4) and the effects of watchful waiting to treat the less severe fibrosis stage, with the impact of more comprehensive strategies that assumed treatment of all patients with any fibrosis (F1–F4). The goal of treatment was to achieve SVR. The treatment protocols are described in more details in Supplement 1.
Input parameters
Cohort characteristics
Initial model parameters for cohort age, fibrosis stage and weight distributions were developed using characteristics of an existing cohort of predominantly Caucasian G1 treatment-naïve CHC patients from northern Italy who were enrolled in the VBMH study (Value Based Medicine in Hepatology), an ongoing Italian prospective observational study [11]. The VBMH study includes 3213 patients consecutively recruited in three major referral centres during an 18-month period, including 111 G1 treatment-naïve CHC patients. The cohort characteristics are shown in Table 1.
Table 1.
Model parameter values and ranges
| Model parameters | Base-case value (Range) | Distribution | Reference |
|---|---|---|---|
| Model assumption | |||
| Discount rate cost/outcomes | 0.03 (0–0.06) | – | [24] |
| Time horizon | Lifetime | – | – |
| Perspective | NHS | – | – |
| Cohort characteristics | |||
| Age F0, year | 51 (41–61) | Normal | [11] |
| Age F1, year | 52 (42–62) | Normal | [11] |
| Age F2, year | 55 (44–66) | Normal | [11] |
| Age F3, year | 59 (47–71) | Normal | [11] |
| Age F4, year | 57 (46–72) | Normal | [11] |
| Body weight, kg | 71 | Normal | [11] |
| Stage of fibrosis distribution | |||
| F0 | 0.06 | Dirichlet | [11] |
| F1 | 0.17 | Dirichlet | [11] |
| F2 | 0.51 | Dirichlet | [11] |
| F3 | 0.04 | Dirichlet | [11] |
| F4 | 0.22 | Dirichlet | [11] |
| Proportion with IL-28B genotype, CC type | 0.33 (0.29–0.38) | Beta | [25] |
| HCV natural history | |||
| Fibrosis progression (annual probability) | |||
| F0–F1 | 0.12 (0.107–0.127) | Beta | [26] |
| F1–F2 | 0.09 (0.078–0.093) | Beta | [26] |
| F2–F3 | 0.12 (0.112–0.130) | Beta | [26] |
| F3–F4 | 0.12 (0.107–0.123) | Beta | [26] |
| F4 to Decompensated cirrhosis | 0.04 (0.030–0.055) | Beta | [27] |
| F4–HCC | 0.03 (0.024–0.042) | Beta | [27] |
| Decompensated cirrhosis to HCC | 0.06 (0.026–0.091) | Beta | [28] |
| Decompensated cirrhosis to liver transplant | 0.01 (0–0.022) | Beta | [28] |
| HCC to liver transplant | 0.11 (0.076–0.141) | Beta | [11] |
| Mortality relative risk from nonliver causes in patients F0–F3 | 1.79 (0.8–4.0) | LogNormal | [13] |
| Hazard ratio for HCC development in patients F4 with SVR | 0.24 (0.18–0.31) | LogNormal | [15] |
| Liver-related mortality (annual probability) | |||
| F4 | 0.03 (0.023–0.041) | Beta | [27] |
| Decompensated cirrhosis | 0.13 (0.085–0.179) | Beta | [29] |
| HCC* | 0.58 (0.530–0.630) | Beta | [14] |
| Liver transplant | 0.13 (0.123–0.136) | Beta | [29] |
| Liver transplant, after 1st year | 0.033 (0.032–0.034) | Beta | [29] |
F0, Metavir fibrosis stage 0; F1, Metavir fibrosis stage 1; F2, Metavir fibrosis stage 2; F3, Metavir fibrosis stage 3; F4, Metavir fibrosis stage 4; SVR, Sustained virologic respond; HCC, hepatocellular carcinoma.
The mortality rate included also the nonliver-related mortality.
Mortality
In the states CHC-F0, F1, F2 and F3, we applied a nonliver-related mortality rate, inferred from the general Italian population [12]. Specifically, based on data from El-Kamary et al. [13], the overall mortality applied to each fibrosis stages from F0 to F3 was adjusted by a mortality relative risk of 1.79. The model also estimated specific liver-related mortality when they progressed to advanced liver disease including CC, DC, etc. (Table 1). In the HCC state, all-cause mortality rate was applied due to an increase in both liver- and nonliver-related mortality compared to the general population [14] (Table 1). The mortality rate of the general Italian population was applied to patients who achieved SVR.
Transition probabilities between states
A probability associated to each state transition was estimated (Table 1). From the health state of SVR, the only transition allowed was to death for non-CHC-related causes, except for CHC-F4 patients who remain at risk for complications of cirrhosis and HCC [15]. The transition probability from HCC to liver transplant was inferred from the VBMH study [11]. Furthermore, the transition from decompensated cirrhosis and HCC to liver transplant was assumed in the model only for subjects aged less than or equal to 65 years as recommended by Italian guidelines for liver transplantation.
When transition probabilities were estimated from meta-analyses that included studies on Caucasian, Black and Asian patients, we reassessed the probability including only European studies to derive probability estimates from populations similar to our Italian cohort (Supplement 2).
Effectiveness of treatment
The effectiveness of the two alternative BOC- or TVR-based treatment protocols (stratified by fibrosis) is reported in Supplement 3. When possible, we used the efficacy estimates related to Caucasian patients to be more specific for a European context based on data reported from phase 3 clinical trials [5,6,16,17] and from a meta-analysis [18]. In the BOC protocol, we estimated the RVR probability using a weighted mean between pegylated interferon alfa-2a and alfa-2b in the G1 and G4 patients reported in the meta-analysis by Romero-Gómez et al. [19]. Similarly, to estimate the SVR probability in patients with RVR, we used the SVR weighted mean between standard dose pegylated interferon alfa-2b and alfa-2a from the IDEAL study [4].
When data stratified by fibrosis stage were not available, we made the following assumptions: the prevalence of IL-28 CC and the probability of eRVR were assumed equal in F1, F2 and F3, the probability of SVR was assumed equal in F1 and F2 IL-28 CC patients, the probability of RVR and eRVR was assumed equal in F1, F2 and F3 patients, while the probability of SVR was assumed equal in F1 and F2 treated or retreated with TT. On the basis of the efficacy ratio observed in the TT with BOC between F1–F2 (70%) and F3 (54%) patients, we estimated that the final SVR rates would be 22.9% lower in F3 patients with RVR compared to F1–F2 patients.
Health outcomes
Utility weights associated to each state were derived from the EuroQol Five-Dimensional 3 Levels (EQ-5D-3L) data collected in the VBMH study (Table 2) [20]. To assess the utility weights, we applied the recently estimated EQ-5D-3L Italian algorithm [21]. The patients included in this analysis were those with CHC, cirrhosis-CHC and end-stage liver diseases associated to HCV. Disutility associated to the side effects of DT and TT was estimated and applied in the model (Table 1). We used the treatment disutility from Liu et al. [7] to assess the potential disutility applicable in our model.
Table 2.
Utilities and costs
| Model parameters | Base-case value (Range) | Distribution | Reference |
|---|---|---|---|
| Utility weights | |||
| F0–F3 | 0.904 (0.723–1) | Beta | [20] |
| SVR after F0–F3 fibrosis | 0.912 (0.730–1) | Beta | [21] |
| F4 | 0.877 (0.702–1) | Beta | [20] |
| SVR after F4 | 0.912 (0.730–1) | Beta | [20] |
| Decompensated cirrhosis | 0.848 (0.678–1) | Beta | [20] |
| HCC | 0.867 (0.694–1) | Beta | [20] |
| Liver transplant | 0.852 (0.682–1) | Beta | [20] |
| After liver transplant | 0.910 (0.728–1) | Beta | [20] |
| DT annualized decrement | 0.029 (0.023–0.035) | Beta | [7] |
| TT annualized decrement | 0.044 (0.035–0.052) | Beta | [7] |
| Cost (2013 €) | |||
| F0–F3 | 522 (±20%) | Gamma | [30] |
| SVR after F0–F3 fibrosis | 0 | – | Assumption |
| F4 | 1512 (±20%) | Gamma | Assumption |
| SVR after F4 | 1512 (±20%) | Gamma | [31] |
| Decompensated cirrhosis | 6350 (±20%) | Gamma | [31] |
| HCC | 12 744 (±20%) | Gamma | [31] |
| Liver transplant | 90 986 (±20%) | Gamma | [8] |
| After liver transplant | 17 612 (±20%) | Gamma | [30] |
| IL-28B testing | 67 | – | [23] |
F0, Metavir fibrosis stage 0; F1, Metavir fibrosis stage 1; F2, Metavir fibrosis stage 2; F3, Metavir fibrosis stage 3; F4, Metavir fibrosis stage 4; SVR, sustained virologic respond; HCC, hepatocellular carcinoma; DT, dual therapy; TT, triple therapy.
Costs
Using the Italian NHS point of view, only direct costs were considered. The average annual cost per patient was assessed for each state (Table 1). The costs related to a time period before 2013 were adjusted for a discount factor associated with inflation in Italian healthcare costs [22]. The cost associated to the SVR state (those cured by treatment) was assumed to be 0 € for patients between F1 and F3, while the cost associated to F4 patients with SVR was equal to CHC F4 patients without SVR. Methods used to estimate the drug costs and the weekly treatment cost are reported in Supplement 4. The cost related to genetic testing for the IL-28B polymorphism was included in the TVR protocol [23].
Analysis
The model estimated costs, LYs and QALYs under each treatment protocol and selection strategy. Results are presented as incremental cost-effectiveness ratios (ICERs), incremental cost per QALY and LY gain. To be consider cost-effective, a strategy should be under the willingness to pay threshold of 37 000 € per QALY gained, considered acceptable by leading regulatory and decision-making agencies such as NICE-UK, AHRQ–USA and CADTH-Canada. To test the robustness of the model’s assumption and specific parameters, we performed a one-way sensitivity analysis by changing parameters using plausible range or the 95% confidence interval (Table 1). To test the impact of fibrosis stage distributions, we reperformed the analysis using the data by Poynard et al. [1]. A probabilistic sensitivity analysis was also performed to address the uncertainty of the model parameters. Statistical distributions were assigned to the model parameters to evaluate the uncertainty around the point estimates. Tables 1 and 2 and Supplement 3 report the type of distributions assigned to each parameter. Uncertainty in all model parameters was assessed using a Monte Carlo simulation, drawing parameter values at random 1000 times from the appropriate corresponding distributions.
As mentioned before, we also used an alternative scenario with a time horizon of 5 years and ESLD-D as outcomes to assess the potential cost-effectiveness of different patients’ selection strategies in a short-term scenario. Results were presented as incremental cost per ESLD-D avoided, to show the impact in terms of patients save from the development of an end-stage liver diseases or death per € spent using different patients selection strategies. The model was validated to compare the different patients’ selection strategies (Supplement 5).
RESULTS
Base-case analysis
In accordance with literature, the likelihood of SVR varied by fibrosis stage, with a higher probability of SVR in the mild stages compared to advanced stages (Supplement 6).
The results showed that F1–F4 and F2–F4 selection strategies were superior to F3–F4, in terms of QALYs and LYs gained but more expensive (Table 3). The ICERs for F1–F4 and F2–F4 strategies relative to F3–F4 were €5132 and €3798 per QALY gained, respectively, across TVR IL-28B-guided therapy and €7042 and €5944 in the BOC RVR-guided therapy (Table 3).
Table 3.
Base-case results
| Patient’s selection strategy |
LYs | Discounted QALYs |
Discounted Cost (€) |
LYs gained |
QALYs gained |
Incremental cost (€) |
ICER (€/LYG) |
ICER (€/QALY) |
|---|---|---|---|---|---|---|---|---|
| Telaprevir IL-28B-guided triple therapy | ||||||||
| F3–F4 | 23.27 | 14.15 | €39 922.17 | – | – | – | – | – |
| F2–F4 | 24.48 | 14.73 | €42 115.49 | 1.21 | 0.58 | €2193.33 | €1800.11 | €3798.43 |
| F1–F4 | 24.76 | 14.86 | €43.549.14 | 1.49 | 0.71 | €3626.97 | €2426.62 | €5132.13 |
| Boceprevir RVR-guided therapy | ||||||||
| F3–F4 | 22.73 | 13.89 | €36 681.69 | – | – | – | – | – |
| F2–F4 | 23.95 | 14.46 | €40 091.19 | 1.22 | 0.57 | €3409.49 | €2798.91 | €5944.90 |
| F1–F4 | 24.14 | 14.56 | €41 366.78 | 1.41 | 0.67 | €4685.08 | €3326.88 | €7042.49 |
F3–F4, Metavir fibrosis stage 3 and 4; F2–F4, Metavir fibrosis stage 2, 3 and 4; F1–F4, Metavir fibrosis stage 1, 2, 3 and 4; LYs, life years; QALYS, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; RVR, rapid virologic response.
The F1–F4 strategy was also cost-effective when compare to F2–F4; the ICERs were €11 089 and €13 904 per QALY gained, in the TVR IL-28B- and in the BOC RVR-guided therapies, respectively.
Sensitivity analyses
The results of one-way sensitivity analysis are showed in the Supplement 7. Results were sensitive to change in the annual discount rate costs, in the F1–F3 SVR utility value, in the mortality RR from nonliver causes in patients F0–F3, age of F2 patients and in the SVR probability for patient with no RVR and fibrosis stage F2.
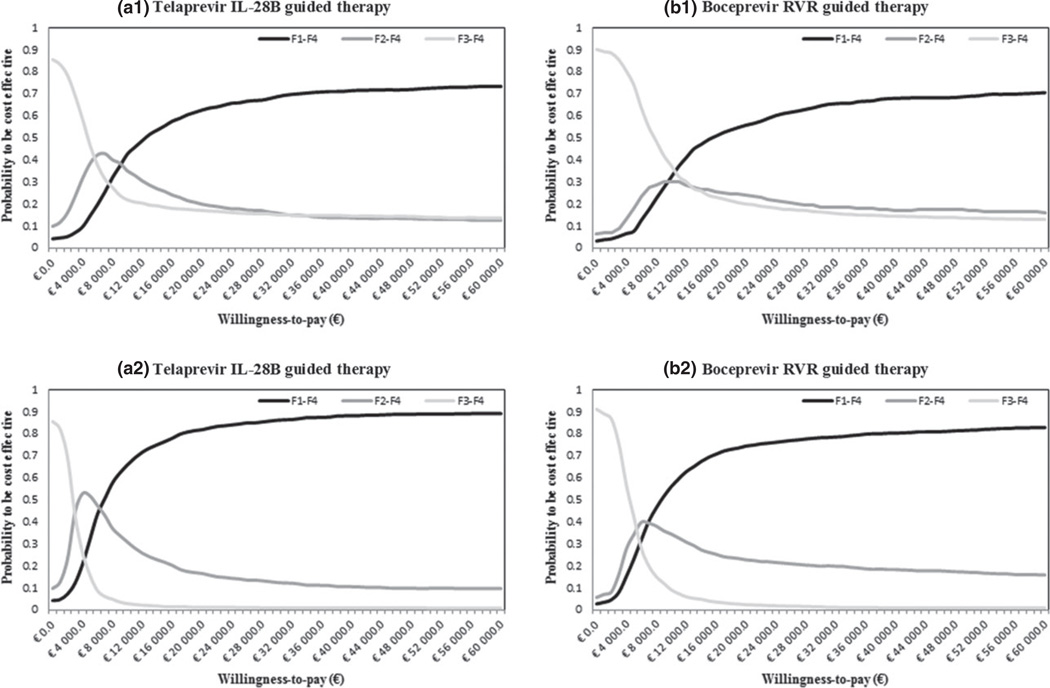
The results of the probabilistic sensitivity analyses are showed in the Fig. 2. These results suggested F1–F4 strategy to be cost-effective with a willingness to pay of €9000 per QALY in the TVR IL-28B-guided therapy (a1) and €10 000 per QALY in the BOC RVR-guided therapy (b1).
Fig. 2.
Cost-effectiveness acceptability curve (a1) Telaprevir IL-28B-guided therapy (quality-adjusted life years – QALYs as outcome), (a2) Telaprevir IL-28B-guided therapy (LYs as outcome), (b1) Boceprevir rapid virologic response (RVR)-guided therapy (QALYs as outcome) and (b2) Boceprevir RVR-guided therapy (LYs as outcome).
Short-time horizon analysis
Using a time horizon of 5 years, F1–F4 and F2–F4 selection strategies were slightly superior to F3–F4 in terms of ESLD-D avoided with a difference between 0.5 and 0.9 in a 100 patient simulation cohort (Table 3). However, the F3–F4 selection strategy was more cost-effective than F1–F4 and F2–F4 strategies, with a difference in cost savings between €1 062 969 and €1 559 465 (Table 3). The ICERs for F1–F4 and F2–F4 strategies relative to F3–F4 were €1 818 679 and €1 614 462 per ESLD-D avoided, respectively, across TVR IL-28B-guided therapy and €1 866 437 and €1 648 676 in the BOC RVR-guided therapy (Table 4).
Table 4.
Alternative scenario results (time horizon 5 years). Costs and effectiveness are given per 100 patients
| Patient’s selection strategy |
ESLD-D | Discounted Cost (€) |
ESLD-D avoided |
Incremental cost (€) |
ICER (€/ESLD-D avoided) |
|---|---|---|---|---|---|
| Telaprevir IL-28B-guided triple therapy | |||||
| F3–F4 | 8.43 | €2 092 216 | – | – | |
| F2–F4 | 7.75 | €3 196 311 | 0.53 | €1 104 095 | €1 614 462 |
| F1–F4 | 7.57 | €3 651 681 | 0.86 | €1 559 465 | €1 818 679 |
| Boceprevir RVR-guided therapy | |||||
| F3–F4 | 9.08 | €1 876 160 | – | – | |
| F2–F4 | 8.43 | €2 939 129 | 0.64 | €1 062 969 | €1 648 676 |
| F1–F4 | 8.28 | €3 362 551 | 0.80 | €1 486 391 | €1 866 437 |
F3–F4, Metavir fibrosis stage 3 and 4; F2–F4, Metavir fibrosis stage 2, 3 and 4; F1–F4, Metavir fibrosis stage 1, 2, 3 and 4; ESLD-D, end-stage liver diseases or death; ICER, incremental cost-effectiveness ratio.
DISCUSSION
To our knowledge, this is the first model aimed at assessing the cost-effectiveness of patient selection strategies stratified by fibrosis stage, and using a 5-year time horizon (rather than lifetime), with the intention of comparing the relative benefit of broad (F1–F4) vs selective (F3–F4) treatment of CHC G1 infection. This model provides physicians and policymakers a rational method to allocate first-generation protease inhibitor-containing treatments in CHC G1 patients in a context of limited economic resources and expectation of promising new drugs in the future.
The results of our model confirmed that the cost-effectiveness of a F1–F4 treatment strategy was significantly decreased when the time horizon of the analysis was switched from lifetime to 5 years, a time frame in which we postulated newer and more effective HCV treatments would become broadly available.
In the lifetime scenario, the F1–F4 strategy provided an ICER of €5132 and €7043 per QALY in the TVR and BOC treatment protocols, respectively, compared to the F3–F4 strategy. Conversely, using the 5-year time horizon, the F1–F4 strategy provided an incremental cost for end-stage liver disease or death avoided of €1 818 679 pin the TVR IL-28B-guided therapy and €1 866 437 in the BOC RVR-guided therapy, compared with the F3–F4 strategy. When a 5-year time horizon was considered, the model showed that treating all F1–F4 patients had a cost of €33 625 or €36 518 per patient, with BOC and TVR, respectively, compared to €18 761 or €20 922 when only F3–F4 patients were treated. These increments in cost are related to a decreased number of end-stage liver disease or death of 0.0086 and 0.0080 per patient and an ICER of about €1 850 000 per ESLD-D avoided.
Because of the slow progression of CHC to cirrhosis/HCC and liver failure [1], the impact of HCV eradication may not be observed for several years. Accordingly, our model demonstrates that although treating F1–F4 CHC patients is cost-effective across a lifetime time horizon, only the treatment of advanced fibrosis/cirrhosis is economically acceptable across a 5-year time horizon. This is of high clinical relevance in context of emerging therapies for G1 HCV which are associated with shorter treatment duration, decreased interferon requirement, and significantly improved safety and efficacy profiles. Although sofosbuvir (nucleotide polymerase inhibitor) and simeprevir (protease inhibitor) were approved by the U.S. FDA on 6 December 2013 [9] and 22 November 2013 [10], respectively, and on 17 January 2014 in the European Union, broader global access to these new DAAs is not expected for several years. Due to the very high cost of these treatments, physicians, health payers and health policymakers are confronting difficult questions regarding how to prioritize patients for whom treatment should be given. This model provides stronger evidence to support a selective treatment strategy focused on the use of antiviral therapy for patients with advanced liver disease (F3–F4).
Several limitations of our study require clarification. First, all estimates for SVR with TT treatment were derived from phase 3 clinical trials rather than real-world practice due to higher quality of evidence; although we cannot exclude the possibility of lower SVR rates in nontrial settings, relative differences in SVR by fibrosis stage would be expected to be similar [4–6,18]. Second, we focused on a cohort of Caucasian patients using efficacy and transition probabilities related to this group of subjects which is representative of a European cohort and may not be applicable to other populations. Furthermore, when specific data on different stages of fibrosis were not available, we used literature-based assumptions on IL-28B CC prevalence, eRVR, RVR and SVR probability; sensitivity analyses showed that these parameters did not change results. Instead, our results were more sensitive to a variation in the discount cost rate, utility value associated to a SVR state and to nonliver-related mortality relative risk in CHC patients. The model did not include the possibility of reinfection after SVR which represents a rare but plausible scenario in high-risk populations such as injection drug users. Finally, a direct comparison of BOC and TVR was not performed in our model due to a primary aim of comparing fibrosis-based selection strategies rather than antiviral regimens and due to the absence of head-to-head comparative data.
In conclusion, our study provides important new evidence confirming that selective treatment of CHC G1 naïve patients with advanced fibrosis or compensated cirrhosis (F3–F4) is more cost-effective than broader treatment of all patients with CHC G1 (F1–F4) within a 5-year time horizon, which takes into account the rapidly changing dynamics of drug development for CHC infection. These results must be interpreted with caution, and individual patient treatment decisions must address the balance of risks between the safety/efficacy profiles of current treatment regimens vs the risks of deferral until future therapies are available. The authors anticipate that cost-effectiveness across the spectrum of liver fibrosis will improve as multiple safe, highly effective, interferon-free regimens become available within the next 5 years, at which time drug and overall treatment costs may decrease and justify broader treatment of CHC infection.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Elisabeth Fenwick (Professor of Health Economics, Health Economics and Health Technology Assessment, University of Glasgow, Glasgow, UK) for her support and advice during the model development.
This work was an independent research and was not funded. MS and JL were funded by the NIH Grant DK34989: Silvio O. Conte Digestive Diseases Research Core Centers. JL has received consulting/honoraria from Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead and Merck and has received research contracts from Abbott, Achillion, Boehringer-Ingelheim, Bristol-Myers Squibb, Genentech, Gilead, Janssen and Vertex.
Abbreviations
- BOC
boceprevir
- CHC
chronic hepatitis C
- DC
decompensated cirrhosis
- DT
dual therapy
- ESLD-D
end-stage liver disease or death
- HCC
hepatocellular carcinoma
- HCV
Hepatitis C virus
- ICERs
incremental cost-effectiveness ratios
- NHS
National Health System
- QALYs
quality-adjusted life years
- RVR
rapid virologic response
- SVR
sustained virologic response
- TT
Triple therapy
- TVR
telaprevir
Footnotes
CONFLICT OF INTEREST
Nothing to declare for the other authors in connection with the treatment modalities included in the current article.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Data S1: Treatment protocols.
Data S2: Reassessed transition probabilities.
Data S3: Effectiveness of treatments.
Data S4: Drugs and treatments costs.
Data S5: Model validation.
Data S6: SVR probabilities.
Data S7: Sensitivity analysis.
REFERENCES
- 1.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 3.Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 4.McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C. Ann Intern Med. 2012;156:279–290. doi: 10.1059/0003-4819-156-4-201202210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cammà C, Petta S, Enea M, et al. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2012;56:850–860. doi: 10.1002/hep.25734. [DOI] [PubMed] [Google Scholar]
- 9.FDA. Food and Drug Administration. [accessed 11 December 2013]; [Internet]. Available at: http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377920.htm.
- 10.FDA. Food and Drug Administration. [accessed 11 December 2013]; [Internet]. Available at: http://www.fda.gov/forconsumers/byaudience/forpatientadvocates/ucm377234.htm.
- 11.Okolicsanyi S, Ciaccio A, Rota M, et al. Generation and Performance of Outcome Indicators in Liver Disease: the Value Based Medicine in Hepatology Study (V.B.M.H.) Hepatology. 2013;58(S1):1195A. [Google Scholar]
- 12.Italian National Institute of Statistics (ISTAT) [accessed 15 May 2013]; [Internet]. Available at: http://demo.istat.it/ [Google Scholar]
- 13.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53(2):150–157. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.op den Winkel M, Nagel D, Sappl J, et al. Prognosis of patients with hepatocellular carcinoma. Validation and ranking of established staging-systems in a large western HCC-cohort. PLoS ONE. 2012;7(10):e45066. doi: 10.1371/journal.pone.0045066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 16.Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 18.Vierling JM, Zeuzem S, Poordad F, et al. Safety and efficacy of Boceprevir/Peginterferon/Ribavirin (BOC/P/R) combination therapy for chronic HCV G1 patients with compensated cirrhosis: a meta-analysis of five phase 3 clinical trials. J Hepatol. 2013;58(S1):s567–s568. [Google Scholar]
- 19.Romero-Gómez M, Planas R, Ampuero J, et al. Meta-analysis: pegylated interferon α-2a achieves higher early virological responses than α-2b in chronic hepatitis C. Aliment Pharmacol Ther. 2013;37(11):1065–1073. doi: 10.1111/apt.12314. [DOI] [PubMed] [Google Scholar]
- 20.Cortesi PA, Scalone L, Ciampichini R, et al. The impact of type of liver conditions on the patients’ health related quality of life. Value Health. 2013;16(7):A500. [Google Scholar]
- 21.Scalone L, Cortesi PA, Ciampichini R, et al. Italian population-based values of EQ- 5D health states. Value Health. 2013;16(5):814–822. doi: 10.1016/j.jval.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Italian National Institute of Statistics (ISTAT) [accessed 15 May 2013]; [Internet]. Available at: http://dati.istat.it/Index.aspx. [Google Scholar]
- 23.National agency for regional healthcare services (AGENAS) [accessed 15 May 2013]; [internet]. Available at: http://www.agenas.it/ [Google Scholar]
- 24.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 25.Jacobson LM, Catlett I, Marcellin P, et al. Telaprevir substantially Improved SVR rates across all IL28B Genotypes in the advance trial. J Hepatol. 2011;54(S1):s542–s543. [Google Scholar]
- 26.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 27.Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32(3):344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 28.Planas R, Ballesté B, Alvarez MA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40(5):823–830. doi: 10.1016/j.jhep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122(4):889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 30.Fagiuoli S, Scalone L, Ciampichini R, et al. Societal burden in Hepatits C patients: the COME study results. J Hepatol. 2012;56(2):S11–S12. [Google Scholar]
- 31.Cucchetti A, Trevisani F, Cescon M, et al. Cost-effectiveness of semiannual surveillance for hepatocellular carcinoma in cirrhotic patients of the Italian Liver Cancer population. J Hepatol. 2012;56(5):1089–1096. doi: 10.1016/j.jhep.2011.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.