Abstract
The availability of techniques to create desired genetic mutations has enabled the laboratory mouse as an extensively used model organism in biomedical research including human genetics. A new addition to this existing technical repertoire is the CRISPR/Cas system. Specifically, this system allows editing of the mouse genome much faster than the previously used techniques and more importantly multiple mutations can be created in a single experiment. Here we provide protocols for preparation of CRISPR/Cas reagents and microinjection into one cell mouse embryos to create knockout or knock-in mouse models.
Keywords: CRISPR/Cas9, sgRNA, Gene Editing, Mutant Mouse, Pronuclear and Cytoplasmic Injection
INTRODUCTION
The tools and techniques to introduce foreign genes and to mutate endogenous genes in mouse were first developed in early 1980s and those methods remained relatively standard for about three decades. In the past 4 to 5 years however, there has been a storm of newer methods and tools added to gene editing techniques and they have been elegantly used to create genetic mutations. These include designer nucleases such as Zinc Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs) and RNA guided nuclease system called Clustered Regularly Interspaced Short Palindromic Repeats/ CRISPR-associated genes (CRISPR/Cas) system. In this unit, we provide recipes and protocols to create specific mutations in mouse genome using CRISPR/Cas system that has moved to the forefront of gene editing due to its simplicity, efficiency, ease and robustness (Fujii et al., 2013, 2014; Shen et al., 2014, 2013; Wang et al., 2013; Yang et al., 2013; Zhou et al., 2014)
The CRISPR/Cas system was originally described as an adaptive immune mechanism in bacteria against invading viruses (Barrangou et al., 2007). The system constituted two RNAs and one protein component: a CRISPR RNA (crRNA), a short RNA that makes complementary binding with the foreign DNA; a tracrRNA that hybridizes with the crRNA and a Cas9 enzyme that interact with the DNA:RNA complexes and cleaves the DNA at a specific site(Horvath and Barrangou, 2010).
This system has been re-designed for gene editing purposes (Cong et al., 2013; Jinek et al., 2013; Mali et al., 2013b; Terns and Terns, 2014) by combining crRNA and tracrRNA into a chimeric unit called single guide RNA (sgRNA), and by codon optimizing the Cas9 enzyme sequence to suit mammalian expression. Hence, the CRISPR/Cas9 system used for gene editing primarily constitutes two components: a guide RNA that detects the specific sequence in the genome and the Cas9 enzyme that binds to the sgRNA and cleaves the DNA at the target site. While Cas9 mRNA is a common component, sgRNA is the unique component for each specific gene editing experiment. When DNA is cleaved, it mainly gets repaired through a mechanism called Non-Homologous End Joining (NHEJ) which is a highly error prone mechanism that causes a few base pair insertions or deletions (indels) at the cut site. Such an event, in most cases results in frame-shift mutation of the coding sequence, eventually leading to gene disruption (a knock-out). In the cases where a specific mutation is to be introduced at the cut site (called knock-in), a repair template DNA is also needed as a third component of CRISPR/Cas system, that becomes inserted at the cut site through homology directed repair (HDR). The repair template DNA can be either a single stranded oligonucleotide or a double stranded plasmid/linear DNA.
The CRISPR/Cas9 components (sgRNA, Cas9 mRNA with or without a repair DNA) can be introduced into one-cell staged mouse embryo to generate offspring that can potentially contain knockout or knock-in mutation. The process involves four major steps: 1) Designing of CRISPR targets, 2) Synthesis and purification of RNA and DNA components, 3) Isolation of one-cell staged mouse embryos, micro-injection of CRISPR/Cas components and transfer of injected embryos into pseudopregnant mice and 4) Genotyping of offspring to identify mutations. An overview of CRISPR/Cas mediated mouse genome editing steps is presented in Figure 1.
Figure 1. Overview of CRISPR/Cas mediated mouse genome editing steps.
BASIC PROTOCOL 1
Designing of CRISPR targets
The first step in gene editing using CRISPR/Cas system is to find specific CRISPR target site(s) near the genomic region of interest. The CRISPR target sequence constitutes 20 nucleotides followed by an “NGG” sequence called Protospacer Adjacent Motif (PAM).
It should be noted that due to such a short sequence requirement, there are higher risks of off-target cleavage if sequences closely similar to the target sites exist elsewhere in the genome, as recently reported by several laboratories(Fu et al., 2013; Hsu et al., 2013; Mali et al., 2013a; Pattanayak et al., 2013). In other words, Cas9 enzyme can tolerate a few base mismatches and can still cleave at such sites: particularly mismatches in the first 7 nucleotides (of the 20) are more tolerated than those in the 8th position onwards(Cong et al., 2013). Currently there are several online tools available for designing CRISPR targets (i.e.: Cong et al., 2013; Montague et al., 2014; Heigwer et al., 2014) (http://crispr.mit.edu/, https://chopchop.rc.fas.harvard.edu/index.php and http://www.e-crisp.org/E-CRISP/index.html, respectively). As an example, the tool available through http://crispr.mit.edu/, from Feng Zhang’s Laboratory at MIT/BROAD Institute, is described here. The screenshots from a CRISPR target search exercise are shown in Figure 2.
Visit http://crispr.mit.edu/
Enter the “name” for your sequence and an email address.
Select the species; e.g., “mouse”.
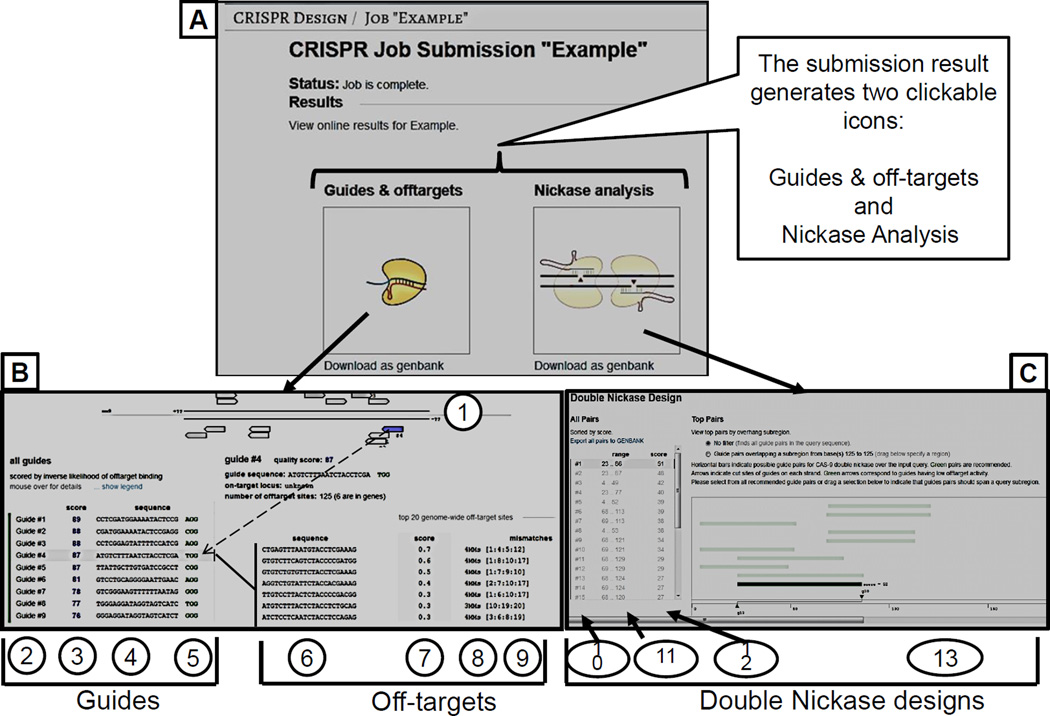
Enter the sequence in the box and click “submit”. A sequence of up to 500 bases can be entered at once. The job generates two types of clickable output files: i) guides and off-targets and ii) nickase analysis. i) Guides and off-targets: The results present a ranked list of all possible guides in the query sequence ordered by faithfulness of on-target activity computed as 100% minus a weighted sum of off-target hit-scores in the target genome. The higher the guide score, the better the guide sequence. ii) Nickase analysis lists pairs of guide sequences that can be used for double nicking approach of gene editing (discussed in Commentary section).
Guide Selection: Guides having an aggregate score of greater than 50% are colored green and should be considered candidate targeting sequences if no high-scoring off-targets fall in marked gene regions (indicated in the table to the right). Guides colored yellow should be considered backups for specific targeting in the case where no suitable green guides are clear of high-scoring, gene off-targets. Guides colored in red have many likely off-target interactions in the target genome and should be avoided.
Figure 2. CRISPR design output files.
(A) Screenshot of the CRISPER target search results window that shows two clickable icons. (B) Screenshot of the “Guides and off-targets window”. 1) Graphical display of various targets (the guide with the cursor on it gets highlighted in blue). 2) Serial number of guide sequence 3) Ranking of the guides displayed from highest to lowest. 4) Guide sequences (20 bases long) 5) PAM sequence. 6) Off-target sequences for a cursor selected guide (guide #4 on the left in this example) 7) Ranking of the off-targets displayed from highest to lowest. 8) Number of mismatches, 9) Positions of mismatches. (C) Screenshot of double nickase design. 10) Serial number of double nickase pairs, 11) the nucleotide position of pairs with respect to the input sequence 12) Ranking of double nickase pairs displayed from highest to lowest. 13) Graphical display of double nickase pairs targets (the pair with the cursor on it becomes highlighted in green)
BASIC PROTOCOL 2
Synthesis and purification of RNA and DNA components
The CRISPR/Cas system components include sgRNA and Cas9: the sgRNA guides the Cas9 enzyme to the target site on the genomic DNA and the Cas9 enzyme cleaves the target site, eventually resulting in indel mutations at the cut site. The system also includes a repair DNA as a third component if a knock-in mutation is to be inserted at the cleavage site. The microinjection components used for mouse genome editing using CRISPR/Cas system are listed in Table 1. Note that the Cas9 is introduced into the zygotes as mRNA that gets translated into the protein. This protocol describes the synthesis and purification of DNA and RNA components under the following sub-sections:
- The preparation of
- Template DNAs for RNA transcription
- RNA components from template DNAs
- Repair DNA.
Preparation of micro-injection mix
Table 1.
The microinjection components needed for mouse genome editing via CRISPR/Cas system
| Component | Molecular nature | Purpose in the CRISPR/Cas system |
Method of generation |
|---|---|---|---|
| Cas9 RNA | Capped and polyadenylated mRNA | To produce Cas9 protein | in vitro transcription followed by capping and polyadenylation |
| sgRNA | Short RNA consisting of CRISPR guide and tracer sequences | Guiding Cas9 to the target sequence | in vitro transcription |
| Repair DNA | Single stranded oligonucleotide or double stranded linear/circular plasmid DNA | Homology directed repair | Oligonucleotide: commercially synthesized Plasmid: cloning |
Materials
sgRNA expression vectors [e.g., pUC57-sgRNA expression vector (Addgene plasmid 51132 (Shen et al., 2014)), or MLM3636 (Addgene plasmid number 43860).
Suitable restriction enzyme
0.8% TAE or TBE Agarose gel
Gel-extraction kit (from Promega or Qiagen or any such vendors)
Quick Ligase (NEB)
T4 PNK (NEB)
Suitable sgRNA target sequence oligonucleotides
Suitable competent bacteria (i.e. DH10B, DH5α) and reagents for transformation
Plasmid extraction kit (from Promega or Qiagen or any such vendors)
(optional)T4 DNA Ligase (NEB)
(optional)Esp3I (Thermo Scientific)
(optional) Suitable oligonucleotides
(optional)Suitable competent bacteria (i.e. DH10B, DH5α)
(optional) Expand High Fidelity PCR system (Roche) or similar proof-reading Taq polymerase
(optional) Expand Long Template PCR System (Roche) or similar proof-reading Taq polymerase for long DNA templates
(optional) RNase-free H2O, 70% and 100% ice-cold ethanol
(optional) Oligonucleotides: T7-hCas9-Fw 5’-TAATACGACTCACTATAGGGAGAATGGACAAGAAGTACTCCATTG-3’;
hCas9-Rv 5’- CGGTAGGGATCGAACCCTTTCA-3’
T7-sgRNA-Fw 5’- TTAATACGACTCACTATAGGN20-3’ (where N20 is the selected CRISPR target, cloned in the sgRNA vector)
sgRNA-Rv 5’- AAAAGCACCGACTCGGTGCC-3’
Plasmid DNAs containing Cas9 sequence [e.g., pBGK-Cas9polyA or hCas9 (Addgene plasmid 41815 (Mali et al., 2013b)), pX260 (Addgene plasmid 42229) or pX330 (Addgene plasmid 42230)(Cong et al., 2013) or any such mammalian codon optimized Cas9 plasmid that can be used for in vitro transcription] and sgRNA expression vectors containing the desired target(s) [e.g., pUC57-sgRNA expression vector (Addgene plasmid 51132 (Shen et al., 2014)), or MLM3636 (Addgene plasmid number 43860).
Restriction enzymes that cut once downstream of the Cas9 or sgRNA sequences in the plasmids (e.g., pBGK plasmid that expresses Cas9 can be linearized with XbaI and pUC57-sgRNA vector can be linearized with DraI)
0.8% TAE or TBE Agarose gel
Gel-extraction kit (from Promega or Qiagen or any such vendors)
3 M sodium acetate, pH 5.2
mMESSAGE mMACHINE T7 ULTRA kit (Ambion, cat. no. AM1345)
MegaClear kit (Ambion, cat. no. AM1908)
RNase-free H2O, 70% and 100% ethanol
10% (w/v) sodium dodecyl sulfate (SDS)
50° and 65°C water baths or heat block
Nanodrop spectrophotometer for determination of DNA and RNA concentration
Reagents and equipment for agarose gel electrophoresis
(optional) RNAse-free microinjection buffer (TrisHCl 1mM, pH 7.5, EDTA 0.1mM, pH7.5)
(optional) NucAway Spin Columns (Life Technologies)
Preparation of template DNAs for RNA transcription – Subsection I.A
A linearized plasmid or a PCR product can be used as templates for synthesis of Cas9 mRNA and sgRNAs. Due to their convenient short size, sgRNAs can also be generated by annealing of commercially synthesized oligonucleotides.
While certain Cas9 plasmids can be used for RNA synthesis without further alterations, sgRNA target sequences need to be cloned into empty vectors before using them in in vitro transcription reactions. Two alternate sets of steps (steps 1b–6b and steps 1c – 6c) for cloning sgRNA target sequences into plasmid are given below.
Cloning sgRNA target sequences into a plasmid (template DNA) vector
Currently several CRISPR/Cas system plasmids are available through Addgene.org to clone sgRNA targets in to them to use as template DNAs. Of these, the plasmids that can be used directly, or after PCR amplifying the insert, for generating RNA molecules needed for mouse gene editing experiments are compiled in Table 2. Some of these vectors allow expression of both Cas9 mRNA and sgRNA in a single plasmid (that are typically used for gene editing in cultured cells but can also be used for embryo work) (Mashiko et al., 2013) whereas the ones that are suitable for mouse embryo injections allow synthesis of Cas9 mRNA and sgRNA separately through in vitro transcription. The sgRNA target sequences can be synthesized as DNA oligonucleotides and are cloned into a plasmid vector following standard protocols:
Digest 1ug of plasmid vector with a suitable restriction endonuclease (e.g., BbsI for pX260 or pX330 and BsaI for pUC57 sgRNA vectors) for 30 min at 37°C.
Gel purify digested vector using Promega Gel Extraction Kit and elute in EB.
Phosphorylate and anneal each pair of oligonucleotides: Mix 1 µl each of 100 µM primers, add 1 µl 10× T4 ligase buffer (NEB), 6.5 µl water + 0.5 µl T4 PNK (NEB). Anneal in a thermocycler using the following parameters: 37°C for 30 min, 95°C for 5 min and then ramp down to 25°C at 5°C/min (Note: Phosphorylation step can be optional. It can be omitted by not adding PNK and skipping the 37°C for 30 min incubation)
- Set up ligation reaction and incubate at room temperature for 10 min:
- X µl digested vector from step 2 (50ng)
- 1 µl phosphorylated and annealed oligonucleotide duplex from step 3 (1:250 dilution)
- 5 µl 2× Quick ligation Buffer (NEB)
- X µl ddH2O
- 10 µl subtotal
- µl Quick Ligase (NEB)
- 11 µl total
- (Optional but highly recommended) Treat ligation reaction with PlasmidSafe exonuclease to prevent unwanted recombination products:
11 µl ligation reaction from step 4 + 1.5 µl 10× PlasmidSafe Buffer +1.5 µl 10mM ATP + 1 µl PlasmidSafe exonuclease +15 µl total and incubate reaction at 37°C for 30 min.
Transform 1–2 µl of the final product into competent cells, pick colonies and sequence verify.
Table 2.
Examples of plasmid vectors available for synthesis of Cas9 mRNA and for generating sgRNA expression constructs
| Plasmid | Promoter | Purpose | Transcription template generation |
Reference |
|---|---|---|---|---|
| pCAG-T3-hCAS-pA | T3 | Cas9 mRNA transcription | Linearized with SphI | (Fujii et al., 2013) |
| T3 | sgRNA transcription | Linearized with DraI | (Fujii et al., 2013) | |
| pX330 | T7 or T3 in primer | Cas9 and sgRNA transcription | Used as a PCR template to amplify Cas9 and sgRNA | (Yang et al., 2013) |
| pBGK | T7 | Cas9 mRNA transcription | Linearized with XbaI | This work |
| pST1374-NLS-flag-linker-Cas9 | T7 or T3 in primer | Cas9 mRNA transcription | Used as a PCR template to amplify Cas9 and sgRNA | (Shen et al., 2013) |
| pUC57-sgRNA | T7 | sgRNA transcription | Linearized with DraI | (Shen et al., 2014) |
(Alternate Steps) Cloning sgRNA sequences into a plasmid vector by Golden Gate Cloning method
The sgRNA target sequences can also be cloned into a plasmid vector by Golden Gate Cloning method which bypasses the need to purify linearized vector (Engler and Marillonnet, 2014; Engler et al., 2008). A diagram illustrating the Golden Gate Cloning to build sgRNA expression vectors is shown in Figure 3.
-
1b
Design two oligonucleotides carrying the N20 nucleotides (identified as indicated in Basic Protocol 1) flanked by Esp3I- compatible overhangs as follows: Fw oligonucleotide ACACC-N20-G and Rv oligonucleotide AAAAC-(RC)N20-G.
-
2b
Prepare a 100µl solution of the 2 oligonucleotides at a final concentration of 10µM, in ddH20.
-
3b
Heat the oligonucleotides 95°C 5 min, let cool down 10 min at RT.
-
4bSet up golden gate cloning reaction:
- 200ng circular MLM3636 plasmid
- 2µl of the annealed oligonucleotide mix
- 2µl 10× T4 DNA ligase buffer (NEB)
- 1µl 20mM DTT
- 1µl T4 DNA ligase (NEB)
- 1µl Esp3I (Thermo Scientific)
- H20 up to 20µl
-
5bIncubate in a thermocycler with the following cycling program:
- 37°C for 5 min; 16°C for 10 min (5 times)
-
6b
Transform 1–2 µl of the final product by electroporation into TOP 10 electrocompetent cells and plate transformant on LB-Agar plates supplemented with 50µg/ml Ampicillin. Pick colonies and sequence verify.
Figure 3. Diagram illustrating the Golden Gate Cloning to build sgRNA expression vectors.
(A) Type IIs restriction enzymes are used to produce user-defined overhangs. (B) Forward and Reverse oligonucleotides corresponding to the CRISPR target site 5’N(20) are designed adding Esp3I overhangs compatible with those included in the MLM3636 vector. (C) A one-pot digestion-ligation reaction is performed with circular vector and annealed oligonucleotides to obtain the desired sgRNA vector. (D) Colony PCR of MLM3636-based sgRNA vectors. A forward primer mapping on the hU6 promoter sequence and the sgRNA Reverse oligonucleotide (previously used for the annealing reaction) are used for colony-PCR. (E) Correctly assembled plasmids yield a 93bp product: three positive colonies and a negative colony (ntc) are shown.
(Alternate Steps) Generation of PCR based templates for in vitro transcription of Cas9 and sgRNA
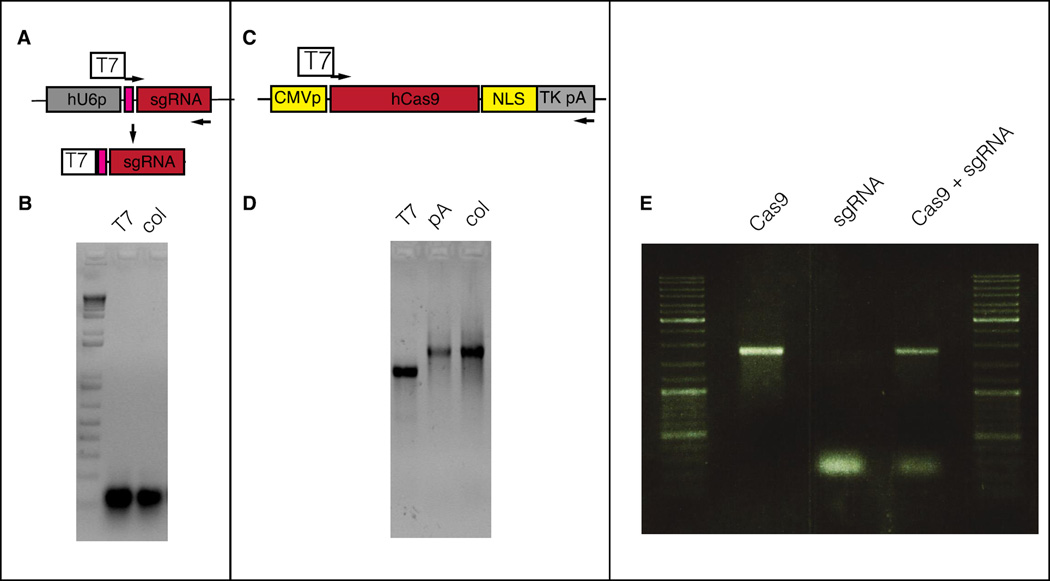
Because of its short size, sgRNA can also be synthesized directly from a double stranded DNA template obtained by annealing two oligonucleotides that contain T7 promoter sequence on 5’ end of sgRNA target sequence and therefore cloning sgRNA sequences into a plasmid vector is not absolutely necessary. Template for Cas9 mRNA transcription can also be synthesized by PCR with a T7 promoter containing primer and using any codon optimized Cas9 plasmid (Ran et al., 2013). Schematic of PCR template generation for in vitro transcription of sgRNA and Cas9 mRNA are shown in Figure 4A and 4C and the gel images of transcribed and purified RNA are shown in Figure 4B and 4C.
-
1c
Use Expand Long Template PCR System to amplify the Cas9 coding sequence with the oligonucleotides T7-hCas9-Fw and hCas9-Rv using 10ng of the hCas9 plasmid as template with the following cycling program: 94°C 5 min; 94°C 30 sec − 50°C 30 sec − 72°C 4 min (30 cycles); 72C 7 min.
Use Expand High Fidelity PCR System to amplify the sgRNA sequence with the oligonucleotides T7-sgRNA-Fw and sgRNA-Rv using 10ng of the sgRNA vector as template with the following cycling program: 94°C 5 min; 94°C 30 sec − 50°C 30 sec − 72°C 30 sec (30 cycles).
-
2c
Precipitate the PCR product with 300µl of ice-cold 100% Ethanol. Incubate 1 hour at −20°C.
-
3c
Spin 15 min at 16,000 g at 4°C. Remove the supernatant.
-
4c
Wash with 300µl of ice-cold 70% Ethanol. Spin 5 min at 16,000 g at 4°C.
-
5c
Remove the supernatant, air-dry 10 min, resuspend in 50µl RNase-free water. Estimate the DNA concentration using a Nanodrop.
-
6c
Use 500ng of purified PCR product for in vitro transcription as indicated in Step 12.
Figure 4. Representative agarose gel images of CRISPR/Cas RNA components. A and B: sgRNA in vitro transcription.
(A) The sgRNA sequence is PCR-amplified from MLM3636-based vector with primers carrying the T7 RNA polymerase promoter on the 5’ extremity. (B) The resulting PCR is used as a template for T7 RNA polymerase transcription in vitro. A typical gel electrophoresis of an sgRNA after T7 RNA polymerase transcription (T7) and after column purification with Nucaway spin columns (Col). C and D: Cas9 in vitro transcription. (C) The Cas9 ORF, including NLS, is PCR-amplified with primers carrying the T7 RNA polymerase promoter on the 5’ extremity. (D) The PCR product is used as a template for in vitro transcription, 5’capping and 3’ polyA-tailing. A representative gel electrophoresis is shown. A supershift is observed after the polyA tailing reaction (pA). Nucaway spin columns are used for RNA purification. (E) A representative gel image of Cas9 mRNA (100 ng), sgRNA (100 ng) and 1:1 injection mix of Cas9 and sgRNA (50 ng each) samples.
Preparation of RNA components from template DNAs – Subsection I.B
Regardless of the format, the RNA synthesis process starts by generating a linear DNA. If a plasmid DNA is used, the DNA is linearized using a restriction enzyme situated immediately downstream of the Cas9 or sgRNA sequence; whereas, the PCR products (generated in step 6c) can be directly used as templates for RNA synthesis (step 12).
Linearize plasmid template DNAs for in vitro transcription of Cas9 and sgRNA
-
7
Linearize 10 µg of plasmid DNA with a restriction enzyme.
Optional: Separate the plasmid digest on a 0.8% agarose gel and gel purify the band of linearized plasmid using a gel extraction kit.
-
8
Precipitate the eluted DNA by adding 0.1 volume of 3 M sodium acetate and 2.5 volumes of 100% ethanol, and incubate for 30 minutes to 1 hr at room temperature.
-
9
Pellet the DNA by centrifugation at 13,000 rpm for 10 min on a table top centrifuge at room temperature, wash the pellet with 250 µl of 70% ethanol, and air dry the pellet for 5 min.
-
10
Resuspend the DNA in 10 µl RNase-free water and incubate for 15 min at 37°C.
-
11
Estimate the DNA concentration by spectrophotometric measurement and confirm the DNA purity and integrity by agarose gel electrophoresis. (Note: approximately a total of ~1 µg DNA is required in the next step in a volume less than 6µl. Therefore, a minimum DNA concentration of 166 ng/µl is required for the in vitro transcription reaction).
In vitro transcription
-
12Set up a 20-µl in vitro transcription reaction in a 1.5-ml microcentrifuge tube using the reagents from the mMESSAGE mMACHINE T7 ULTRA kit as follows:
- 10 µl T7 2× NTP/ARCA reagent
- 2 µl 10× buffer
- 1 µg linearized template DNA (from step 5)
- Make up to 20 µl with nuclease free water
- 2 µl T7 Enzyme Mix from the kit
- Mix the reaction and incubate for 2 hr at 37°C.
Polyadenylation reaction
[Note: this step is not necessary for sgRNAs and for Cas9 transcribed from plasmids that contain multiple ‘A’ nucleotides after the coding sequence (e.g, pBGK that has 83 bases of ‘A’ nucleotides after the Cas9 coding sequence)]
-
13To each 20 µl transcription reaction add the following reagents from the mMESSAGE mMACHINE T7 ULTRA kit as follows:
- 36 µl nuclease-free H2O
- 20 µl 5× E-PAP buffer
- 10 µl 25 mM MnCl2
- 10 mM ATP
-
14
At this step, mix and take 2.5 µl as quality control sample C1.
-
15
Add 4 µl E-PAP enzyme from the Message Machine T7 Ultra kit.
-
16
Mix and incubate the reaction for 1 hr at 37°C.
RNA purification using Megaclear kit
(Note: Alternatively, follow steps 17b – 21b to use Nucaway column purification in place of the Megaclear kit.)
-
17To each 100 µl tailing reaction add reagents from the MegaClear kit as follows:
- Add 350 µl binding buffer
- Add 250 µl ethanol
- Mix and apply to spin column inserted into a collection tube (items from MegaClear kit)
-
18
Centrifuge spin column for 1 min at 16,000 × g, room temperature. Discard flow through and add 500 µl wash solution from the MegaClear kit.
-
19
Repeat step 13.
-
20
Spin the empty column for 30 sec at 16,000 × g, room temperature.
-
21
Transfer column to the top of a new collection tube, apply 50 µl elution solution (from MegaClear kit), close lid, and incubate 5 min at 65°C
-
22
Centrifuge 1 min at 16,000 × g, apply another 50 µl elution solution, and incubate 5 min at 65°C.
-
23
Collect the second eluate by centrifugation and pool both eluates.
-
24
To 100 µl of the eluate, add 10 µl 5 M ammonium acetate (from kit) and 275 µl ethanol. Incubate 30 min at −20°C.
-
25
Centrifuge 15 min at 16,000 × g, room temperature, discard supernatant, add 500 µl of 70% ethanol (prepared with embryo tested water).
-
26
Centrifuge 1 min at 16,000 × g, room temperature, repeat the washing step with 70% ethanol, discard supernatant, and air dry the pellet for 5 min.
-
27
Resuspend the RNA pellet in 50 µl T10E0.1 injection buffer, then incubate 5 min at 37°C
-
28
Estimate RNA concentration in a Nanodrop spectrophotometer. Typical yield from one in vitro transcription reaction will be ~30 µg RNA. Distribute RNA into small aliquots (~5 µl) and store at −80°C until required for the preparation of injection mix.
(optional) RNA purification using Nucaway Columns
In alternative, RNA can be purified using column-based gel chromatography. This protocol uses Nucaway columns and is provided as an alternate protocol to the Megaclear kit.
-
17b
Dissolve the powder contained in the NucAway column in 650µl of RNAse-free microinjection buffer, carefully removing all air bubbles. Cap the tube and hydrate for 5–15 min at RT.
-
18b
Remove the cap at the bottom and place the column in a collection tube. Spin for 2 min at 750 × g, room temperature.
-
19b
Place the column in a fresh 1.5ml tube, apply the RNA solution dropwise to the center of the gel bed, without touching the column wall.
-
20b
Spin the column for 2 sec at 750 × g, room temperature, placing the tube in the same orientation as in the previous centrifugation step
-
21bDiscard the column and distribute the purified RNA in small aliquots. Take a small aliquot of sample for gel electrophoresis and measure the RNA concentration using a Nanodrop device. Store RNA at −80°C until use. Typical yield of one reaction is 30–50ug of RNA.RNA used for mouse embryo injection is best assessed using gel formulations especially suited for RNA electrophoresis as described in Wefers et al., 2013. However, RNA quality can also be assessed using the TAE gels routinely used for DNA electrophoresis.Representative gel images of RNA components used in a CRISPR/Cas microinjection mix are shown in Figure 4B, 4D and 4E.
Preparation of repair DNAs – Subsection I.C
-
29
There are two types of repair DNAs; single stranded DNA with homology arms of about 60 bases long for insertion of short sequences (e.g., short immuno-affinity tags, LoxP sites) or double stranded plasmid DNA with longer homology arms of over 0.5 kb (e.g., reporter cassettes such as GFP or LacZ or minigenes). Short single stranded DNAs can be procured as oligonucleotides (primers from any commercial source such as IDT, Operon etc.). Larger double stranded targeting DNA preparation, on the other hand involve careful designing strategies and complex construction steps that are described elsewhere (Hall et al., 2009; LePage and Conlon, 2006). The primers used as repair oligonucleotides (commercially synthesized) are preferably reconstituted on the first injection session day and frozen as multiple aliquots at −80°C for later injection sessions (if necessary). The plasmid DNAs used as repair DNA are prepared using high quality plasmid DNA isolation kits (from Promega or Qiagen or any such vendors).
Preparation of micro-injection mix – Subsection II
-
30
Remove RNA aliquots from −80°C and thaw on ice. (The sgRNA and Cas9 prepared in Basic Protocol 2, sections I. A and I. B and Repair DNAs (optional).)
-
31Mix desired concentrations of Cas9 RNA, sgRNA and (optional) DNA and prepare for injection as described below.We prefer to make pre-dilutions of each of the components and mix them to obtain the final solution. For example if only one sgRNA and Cas9 are injected at 10ng/ul concentrations each, the pre-dilutions of 20ng/ul sgRNA and 20ng/ul of Cas9 mRNA are made and then equal volumes of these solutions are mixed to obtain the injection mix. If three components (e.g.; 2 sgRNAS and a Cas9 mRNA or one sgRNA, Cas9 mRNA and a repair DNA) need to be injected, each of the three components are made as 3× pre-dilutions and then a third of each of these solutions are mixed to obtain the final solution that will be 1× with respect to each component.
-
32The typical volume of injection mix prepared will be 150 to 300 µl. Centrifuge at 16,000 g for 10 minutes and then pass through Millipore column filters (UFC30VV25).Alternatively, the injection mixes can also be centrifuged at 30,000g for 1 h in which case the additional filtration may not be necessary. Spinning and/or filtering are required to eliminate any remaining solid particle to prevent clogging of the microinjection needle.
-
33
The injection mix is then loaded on to the needles as described in Basic Protocol 3. The injection needles are preferably kept in a container on ice until they are assembled on to the micro-injection set up.
BASIC PROTOCOL 3
Isolation of one-cell staged mouse embryos, micro-injection of CRISPR/Cas components and transfer of injected embryos into pseudopregnant mice
In a traditional transgenic experiment, typically about 200 to 300 fertilized oocytes are micro-injected with DNA. Whereas, due to relative high efficiency of CRISPR/Cas9 system, approximately 50–100 fertilized oocytes are sufficient to generate the desired mutations. This number of embryos can be obtained by super-ovulating 6–10 females for each microinjection session. We use FVB inbred or B6/SJL F1 hybrid strains embryos for CRISPR/Cas9 experiments. These animals can be purchased from commercial sources or can be bred in-house. All procedures involving laboratory animals should be performed according to the institutional and national guidelines and legislations (e.g., National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press, 2011. http://www8.nationalacademies.org/onpinews/newsitem.aspx?RecordID=12910 http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm)
It should be noted that the steps to generate mutant mice using CRISPR/Cas system follow the standard transgenic techniques that comprise multiple steps including embryo production, isolation, micro-injection and transfer of manipulated embryos in to recipient females that have been well established since over three decades. We suggest the reader to refer to exhaustive resources available (Behringer et al., 2014; Pease and Saunders, 2011) that describe the critical parameters and troubleshooting involved in generating transgenic mouse models which are very similar to the steps to generate mutant mice using CRISPR/Cas system.
Materials
Animals
Donor females: In our laboratory, we procure three week old B6/SJL F2 females from Jackson Laboratory (Bar Harbor, ME) or four week old FVB female from Charles River Laboratories (Wilmington, MA).
Stud males: purchased from the respective vendors at 5–6 weeks of age.
Pseudopregnant recipients: Crl: CD1(ICR) female mice purchased at 5–6 weeks of age from Charles River Laboratories, (Wilmington, MA), for pseudo-pregnant foster mother.
Vasectomized males: 5–6 week old CD-1 mice purchased from, Charles River Laboratories, (Wilmington, MA) And vasectomies performed as described (Behringer et al., 2014)
Hormones: Pregnant mares serum gonadotropin (PMSG) and Human chorionic gonadotropin (HCG)were obtained from the National Hormone and Peptide Program (Harbor–UCLA Medical Center, Torrance, CA).
Media and reagents
M2 media for embryo handling and microinjection (Millipore MR-015-D).
Hyaluronidase for dissociation of cumulus oophorus complex (Millipore MR-051-F).
KSOM + AA for embryo incubation (Millipore MR-106-D).
Light Mineral Oil (Millipore ES-005-C).
Falcon Tissue culture dish 35 × 10 mm (353001).
Falcon tissue culture dish 60 × 15mm (353002).
Falcon IVF dish (353653).
Injection Buffer (Millipore MR-095-10F).
Micro Fill 28 gauge/97mm long. (World Precision Instrument Inc. Item # MF28G).
Flexipet oocyte/embryo pipettes (Cook Medical K-FPIP-1130-10BS-5).
Tuberculin syringes
Holding pipettes. (Humagen MPH-SM-20).
Chamber slide (Lab-Tek #177372).
Equipment
Glass pipette puller:
Sutter Instrument Co. Model –P97 outfitted with 2.5mm × 2.5mm Box filament (FB255B)
Glass: World Precision instrument Item # TW100F-4 w/ Filament 1.0mm 4in. (World Precision Instrument Inc.) Sarasota FL. USA.
Nikon Eclipse TE 2000-E w/ DIC equipped with Narishige IM 300 microinjector and NT-88-V3 micromanipulators.
Condenser lens: LWD 0.52
Objectives:
PLAN 4×/0.10 WD30
PLAN APO 10×/0.45 WD4.0
PLAN FLUOR ELWD 20×/0.45 DIC L/NI
PLAN FLUOR ELWD 40×/0.60 DIC M/NI
Heating glass. Controller (CU-301) HG-T-Z002. Live Cell Instruments
Leica DM IRB equipped with Narishige IM 300 microinjector and Leica manual manipulators.
Eyepiece = #507804 HC PLAN 10×/22 w/tilt
Condenser = .30 S70
Objectives:
C PLAN 4×/.10 #506074
N PLAN L20×/0.40 CORR #506057
N PLAN L40×/0.55 CORR #506059
Heating glass. Controller (CU-301) HG-T-Z002. Live Cell Instruments
Leica MZ 9.5
Condenser lens: 10446157 PLAN 0.5×
Base: 10445367
Tilt head
Heating glass. Controller (CU-301) HG-T-Z002. Live Cell Instruments
Nikon SMZ 1000
Condenser: PLAN APO 1× WD70 lens
Base: Model C-DSDF #1002364
Mid-Piece: Model C-FMC #1009459
LV-TV Camera port
Eyepiece: Model P-BERG #1007501 w/c-w15/16 eyepiece
Heating glass. Controller (CU-301) HG-T-Z002. Live Cell Instruments
Heraeus Hera cell 150 Tri –gas incubator equipped with Coda inline filters.
Orion 350 pH meter equipped with ROSS Sure-flow Semi micro electrode (W8175BNWP).
Superovulation and collection of fertilized eggs
-
1
House mice in Individually Ventilated Cages (IVCs) on a 14–10 light cycle (on at 06:00, off at 19:00).
-
2
Inject donor female mice with 5.0 U PMSG around noon on Day 1.
-
3
Approximately 48 hours post PMSG, inject female mice with 5.0 U hCG on Day 3 and breed with stud males overnight.
-
4On day 4 morning, prepare the following dishes:
- Oviduct collection dish: 60 mm Falcon (353002) with 2ml M2 media. One per up to 10 females Hyaluronidase dish: 35 mm Falcon (353001) with 1.5ml Millipore hyaluronidase. One per up to 10 females.
- Wash dish: 35 mm Falcon (353001) with 1.5 ml M2 media. 2 per session
- KSOM rinse dish: Falcon (353001) with 1.5ml Millipore KSOM (pre-equilibrated)
- Incubation dish Falcon 353653 with 1.0ml Millipore KSOM (pre-equilibrated). 2 per injection session.
- ET dish: Falcon 3001 with 1.5ml M2 media.
- All KSOM dishes are prepared approximately 30 minutes prior to use. The dishes are not overlayed with oil to shorten equilibration time and minimize the chance of oil cross contamination. KSOM media pH changes rapidly outside the incubator.
-
5
Euthanize donor females by institution approved method. This process is performed approximately 20 hrs post HCG (about 8 am on day 4).
-
6
Dissect out the oviducts and place in Falcon 3001 culture dish containing M2. Maintain tissue samples at 37 °C on heated slide warmer. Make sure all females are processed in less than 10 minutes post euthanasia. Alternatively, females can be processed in batches to finish collection in less than 10 minutes.
-
7Once all oviducts have been collected, move to a new clean area and begin dissociating cumulus-oocyte complexes (COC).The following steps are performed under a stereomicroscope maintained at 37 °C.
-
8
Place oviducts, one at a time, in the hyaluronidase dish.
-
9
Dissect the cumulus complex out by disruption of the ampulla with a pair of fine forceps.
-
10
Continue processing the remaining oviducts working quickly. If all samples cannot be processed in <10 minutes, work with smaller sample sets.
-
11
Once the last COCs’ have been expelled from the ampulla, the first set of oocytes should have been dissociated enough to pick and collect individual oocytes from the dish.
-
12
Using a 130µm flexipet pipette, transfer the oocytes to M2 wash dish. This will inactivate the residual hyaluronidase. Transfer as little hyaluronidase/cumulus cells as possible during this process.
-
13
(optional) repeat the washing step to remove residual cumulus cells and hyaluronidase.
-
14
Pool all collected zygotes in a fresh M2 wash dish. Collect zygotes one by one with the flexipet. Count the number of zygotes and unfertilized oocytes. Record this information for fertilization efficiency.
-
15
Transfer only healthy looking zygotes to KSOM wash dish. This will dilute out any residual M2 present. Wash all the embryos in this manner and then transfer them into the incubation dish until needed (typically 30 minutes to 1 hour) and culture the dish at a proper CO2 concentration to maintain pH range of 7.23–7.42.
Microinjection needles
Injection capillaries are made fresh the morning of injection using the Sutter Model –P97 pipette puller outfitted with 2.5mm × 2.5mm Box filament (FB255B). Sterile technique is important because you will be injecting RNA molecules. The following program is used:
| Glass | Heat | Pull | Velocity | Pressure | Time |
|---|---|---|---|---|---|
| # TW100F-4 | Ramp +5 | 70 | 120 | 200 | 100delay |
Embryo microinjection
-
16
Back fill injection needles with 1 to 2 µl of injection solution using a 28 gauge micro fill connected to a 1cc tuberculin syringe. The micro fill is prewashed three times with sterile injection buffer.
-
17
Affix the injection needle in the needle holder. Remaining needles should be stored prefilled on ice as an additional precaution to prevent RNA degradation during this step). (Note: Needle storage unit is made from a Falcon 351058 culture dish outfitted with a 0.25cm diameter rod shaped model of clay. The injection needles filled with the solution are stuck to the clay and the entire storage unit is placed directly in contact with the ice bath.
-
18The following parameters are programmed into the Narishige IM 300 microinjector:
Injection pressure Balance Hold Clear Clear Hold Inj. time 20 psi 2.2 psi 14 psi 0.20 sec 0.30 sec 0.08 sec -
19
Prepare an injection slide by making two side-by-side 150µl drops of M2 media. These drops are flattened (spread-out circular) with a pipette tip to minimize their height and allow for oil overlay.
-
20
Transfer 20–50 zygotes to the depression slide (number depends on skills of injector; inject all zygotes within 10 min). The injection slide is maintained at 37 °C with the heated glass insert.
-
21
Check morphology of zygotes under the microscope (presence of zona pellucida, pronuclei, and both polar bodies). Embryos with more than two pronuclei are discarded.
-
22
Prior to injection, make sure the needle is open by placing the injection needle next to an embryo. Press the clear button on the injector. If the embryo rotates freely, the needle is free of any obstruction. If the embryo does not move, gently break the tip of the injection needle off against the holding pipette in a scrapping action. Check the needle again for flow rate. Discard if you observe too much embryo movement. With experience the proper I.D. of the needle can be ascertained by the rate of flow and movement of the embryo in this manner.
-
23
Using the holding pipette, place first zygote in position and fix by applying negative pressure.
-
24
Micro-injection:
Note: For best results, CRISPR/Cas injection mixes can be injected into cytoplasm(Horii et al., 2014), if homology directed repair DNA is not included in the mix. If the mix contains both RNA and the repair DNA, it is best to inject both into cytoplasm and pronucleus.
Cytoplasmic and pronuclear injection: Align the embryo and the holding needle so that both the opening of the needle and the pronucleus of the embryo are both in focus. If not, continue rotating/aligning the embryo. This will insure the pronucleus is not askew from the center of the holding needle. Otherwise this could cause the embryo to slightly rotate during injection resulting in missing the pronucleus. Using the injection needle, penetrate the zona pellucida and oolemma. Move forward into the closest pronucleus. Positive pressure is maintained at all times on the injection needle. Depending on ID of the needle a slight swelling of the pronucleus can be seen once the plasma membrane is penetrated. Otherwise, press the injection foot pedal to observe a slight swelling of the PN. Retract the tip of the needle to the cytoplasm and inject another volume of RNA/DNA solution into the cytoplasm. Carefully withdraw the capillary from the zygote. Often enough positive pressure is present to allow for simultaneous injection of the cytoplasm during needle removal from the pronucleus.
For the injection of Cas9 mRNA and sgRNA without targeting DNA:
Perform the same injection steps except avoid injection of the mix directly into PN. Penetrate the zona pellucida and oolemma. Inject directly into the cytoplasm (Note: with DIC and 300× one can visualize displacement of the granular structures in the cytoplasm of zygotes of certain strains (e.g., C57B6 and B6 hybrids).
-
25
Proceed with remaining zygotes.
-
26
After injection of all zygotes, use a transfer pipette to collect and transfer them to fresh M2 medium.
-
27
Sort out lysed zygotes.
-
28
Incubate surviving zygotes at 37°C in KSOM until embryo transfer. It is suggested that certain number of injected zygotes (about 30) are cultured overnight to assess the toxicity of each batch of RNA reagents prepared. A good successful injection session should result in 90 to 95% of zygotes to progress into 2 cell stage. If the batch of injection reagents is toxic, one can also notice lysis of a large number of zygotes within about 1 hour after the injection. Such testing of the RNA when thawed for use in subsequent micro-injection sessions is un-necessary.
The manipulated embryos are transferred into the oviducts of pseudopregnant foster mothers following the surgical procedures described in (Behringer et al., 2014). Pseudopregnant mice are obtained by mating 8 to 12 week old CD1 females to vasectomized CD-1 males on the day before microinjection. On the morning of the injection day, plug-positive females are used for oviduct transfers. Typically 10 to 20 CD1 females are bred in each session to obtain an average of 4 to 8 plugged females and about 15 to 25 injected embryos are transferred per female. The optimal number of embryos transferred is 18 per female, bilaterally. It should be noted that transgenic techniques that comprise steps including embryo production, isolation, micro-injection and transfer of manipulated embryos in to recipient females have been established over three decades: collective experience of several labs worldwide, has been recently surveyed with respect to standard practices followed in transgenic procedures and the expected outcomes. We recommend reading the published survey results (Fielder et al., 2010) (Pease and Saunders, 2011) that give more detailed information related to suggested practices, critical parameters and troubleshooting of transgenic procedures.
BASIC PROTOCOL 4
Genotyping of offspring
Types of mutations that can be achieved by CRISPR/Cas system are i) simple deletions and insertions, 2) replacement mutations using short repair oligonucleotides and iii) insertion of larger DNA cassettes using plasmid DNA. Genotyping assays: The offspring can be genotyped using several possible assays depending on the purpose. The Table 3 gives a synopsis of these assays. Most of these methods follow standard protocols and are well established over the years except the T7E1 assay, for which the steps are provided here. A quick overview of each is given below:
Table 3.
Genotyping methods to detect mutations in CRISPR/cas generated offspring
| Genotyping Method | Detects | Intended purpose of CRISPR/Cas system |
| Surveyor or T7E1 assay | Cas9 induced indels | Cas9 cleavage with or without repair DNA |
| Flanking PCR and RFLP | Specific small insertions or Cas9 induced deletions | Cas9 cleavage with short repair oligonucleotides |
| Int+ Ext PCR | Specific insertions | Cas9 cleavage with short repair oligonucleotides or larger plasmid DNA |
| Sequencing | All types of mutations | All of the above |
T7Endonuclease1 (T7E1) and Surveyor assay (Basic Protocol 4): T7Endonuclease 1 (T7E1) cleaves a double stranded DNA at the site if it has base pair mismatches. Another enzyme called Cel1 is also used in place of T7E1 that has similar type cleavage activity at the mismatch sites in hetero-duplex DNAs. The assay that uses Cel1 enzyme is called Surveyor assay. This property of mismatch cleaving is perfectly suited for detection of mutations in gene editing experiments where Cas9 or TALEN or ZFN mediated DNA breaks invariably lead to indels (insertions or deletions). The assay is performed by PCR amplifying the target sequence using surrounding primers and the PCR product is denatured and then annealed and incubated with Cel1 or T7E1 enzyme. The denatured and annealed dsDNAs will contain with a mixture of homo and hetero-duplexes. The enzyme digested products when resolved through agarose gel electrophoresis will show full length and expected sized cleavage products if Cas9 cleavage occurred that resulted in indels.
Flanking primer PCR and Restriction Fragment Length Polymorphism (RFLP): This method is suitable for detection of short insertions when a repair oligonucleotide is included in the micro-injection experiment to achieve targeted knock-in. If the repair oligonucleotide is designed to either contain a new restriction endonuclease (RE) site or leads to ablation of an RE site in the genomic locus, the flanking primer PCR product can be subjected to RE digestion and the products can be assessed for the desired replacement mutation. When primers surrounding the target region are used to amplify the target region, such products can show expected difference in size when subjected to agarose gel electrophoresis.
Internal + External primer PCR: In the insertion experiments, one primer that binds to the repair DNA (internal primer) and a primer that binds outside a corresponding homology arm (external primer) are used to amplify the region to detect the targeted insertion.
Sequencing: It is suggested to confirm all types of mutations by sequencing the target site. The mutations can be detected by direct sequencing of the PCR product amplified from the target region. Occasionally direct sequencing of PCR product may not detect the mutations. In such cases the PCR product can be cloned using T/A cloning method, and a few random colonies are sequenced to identify the mutation.
Materials
Taq polymerase (Roche or equivalent)
10mM solution of each nucleotide (Roche)
T7 Endonuclease I (NEB)
NEB buffer 2 (NEB)
Agarose
TAE 1X
Thermocycler and gel electrophoresis apparatus
Design two oligonucleotides to amplify a 400–700bp fragment centered on the targeted sequence.
Perform the PCR in 25µl. Make sure that the PCR amplification yields in a unique and specific product.
Run 5µl of the PCR product to verify the success of the reaction.
- Melt and anneal slowly the rest of the PCR volume, to allow the formation of heteroduplexes, as follows:
- Hold 95C 10 min
- ramp 95°C/85°C −2°C/sec, hold 85°C 1 min
- ramp 85°C/75°C −0.3°C/sec, hold 75°C 1 min
- ramp 75°C/65°C −0.3°C/sec, hold 65°C 1 min
- ramp 65°C /55°C −0.3°C/sec, hold 55°C 1 min
- ramp 55°C /45°C −0.3°C/sec, hold 45°C 1 min
- ramp 45°C /35°C −0.3°C/sec, hold 35°C 1 min
- ramp 45°C /25°C −0.3°C/sec
Digest 10µl of the PCR product with 5U of T7 Endonuclease I (NEB), in 20µl of 1× NEB buffer 2. Incubate 25 min at 37C.
- Run the digestion in a 2% agarose gel.Representative genotyping examples of T7E1 assay, flanking primer PCR, internal + external primer PCR and sequencing of target sites are shown in Figure 5.
Figure 5. Examples of genotyping.
(A) A representative T7E1 assay of a wild type and a mutant sample. The T7E1 cleavage products in mutant sample (+) are marked with asterisks. (B) A 689bp fragment centered on the CRISPR binding site is PCR amplified, cloned and sequenced. Sequence alignments and chromatogram showing a 9bp deletion in two independent clones. (C) PCR of mutation insertion site in an oligonucleotide based HDR knock-in experiment showing showing wild type band (arrow) along with higher sized bands resulting from insertion of the oligonucleotides included in the injection mix. A smaller sized band in sample 9 indicates deletion of a few nucleotides. Unequal intensities of the higher/lower sized bands with that of wild type band in some samples indicate mosaicism. (D) An example of internal + external primers PCR showing amplification of the expected band only in the positive samples (# 2 and 5).
COMMENTARY
Historic perspectives of mouse genome engineering: Genetic engineering techniques to manipulate mouse genome have been established over three decades. There are three major types of engineered mutations in mice: transgenic mouse in which an exogenous DNA is inserted in the genome, a knockout mouse in which the endogenous gene is disrupted and a knock-in mouse in which the endogenous gene segment is replaced with either a modified version of the gene or an exogenous DNA. First step in creating any of these types of mutations involves building of a DNA construct using standard molecular biology protocols. While transgenic constructs contain elements needed for expression of a coding sequence and they do not need to contain specific DNA homology arms, the targeting vectors used for knockout and knock-in, on the other hand, required to contain homology arms and they also involve careful designing strategies. Overview of transgenic and knockout vectors generation steps are described in detail elsewhere (Hall et al., 2009; Haruyama et al., 2009; LePage and Conlon, 2006; Semsarian 2002)
Creating a transgenic mouse involves direct injection of a DNA of interest into one-cell staged mouse embryos whereas knockout or knock-in mouse generation steps involve targeting of the mutation first in the mouse embryonic stem (ES) cells through homologous recombination and in the next step the manipulated ES cells are injected in to blastocyst stage embryos. In the beginning years, the knockout mutations created primarily involved complete deletion where one or more coding exons were replaced by a positive selection marker such as ‘neomycin’. In the recent two decades more sophisticated designs have been possible with the introduction of site specific recombinases such as Cre-Lox and Frt-Flp systems. Nevertheless, requirement of ES cell step to create knock-out or knock-in mutations could not be bypassed.
Recent technical advances that expedite the mouse genome engineering process
A key tool that favored the use of mouse as a model genetic organism compared to other species was the embryonic stem cells. Using embryonic stem cells and the well established procedures to introduce mutations in them through homologous recombination, several thousands of mutant alleles in mouse genome have been created. The recent technical advances that can bypass the use of ES cells, however, have made it possible to manipulate genome of any organism. These methods include ZFNs, TALENs and CRISPR/Cas systems. Among these new technologies ZFNs and TALENs have been around for about for 4 to 5 years now (Geurts et al., 2009; Panda et al., 2013; Rémy et al., 2010; Sung et al., 2013) and there are some articles that describe using these methodologies to create mutations in the mouse genome (Hermann et al., 2014; Wefers et al., 2013). Of these CRISPR/Cas is the newest technique and because of its simplicity and robustness it holds high promise for creating knockout or knock-in mutations in an unprecedented speed. CRISPR/Cas mediated knockout or knock-in mouse models can be generated in less than 3 months. More importantly, multiple mutants can be generated using CRISPR/Cas system in a single experiment which is almost impractical to perform using traditional approaches.
Critical Parameters and troubleshooting
Because the CRISPR/Cas system is a relatively new technique there are not many reports yet to make definitive comments about the critical parameters, even though the system has shown a remarkable success rate in creating genetic mutations in many organisms including the mouse. Discussed below are certain critical parameters based on the available reports and our experience in using the system.
Basic Protocol 1: Design
Input sequence: since the CRISPR target sequences are 23 nucleotide long (including PAM sequence), the system does not accept sequence less than 23 bases long. Also, the system may not find potential target sites even if the sequence entered is >23 bases.
In a standard knock-in experiment, up to about 80 extra nucleotides can be easily inserted using single stranded repair oligonucleotide that will have about 60 nucleotides homology arms on either side. Such designs force CRISPR targets to be identified very close to the desired insertion site (preferably within +/− 40 bases). This is because currently available synthesis limit of oligonucleotides is about 200 bases long (ultramers from Integrated DNA Technologies). If only point mutations are desired that does not warrant addition of extra nucleotides the target selection area can be extended up to +/− 80 bases from the desired site. As reported in cell based experiments that used double nicking approach (see below), the targeted knock-in mutation through HDR is higher when the chosen sgRNA targets are close (about +/− 30 bases) to the insertion site (Ran et al., 2013). There are currently no reports available about such limitations for direct mouse zygote experiments. If the design tool does not yield target sequences in the near vicinity (within about 40 nucleotides: and the closer, the better) of the desired site for a creating a knock-in mutation using a oligonucleotide mediated HDR, search can be extended further in which case the plasmid based HDR can be an option.
Targeting pre-existing restriction sites (or creating new ones after the genetic alteration) may be useful to facilitate the genotyping process.
The appropriate genomic DNA sequences exactly corresponding to the mouse strain that will be used in vivo should be carefully reviewed. Always take into account that the mouse genomic DNA reference is C57BL/6J, but genomic sequences of many additional inbred mouse strains are available (i.e. Ensembl-EBI, Wellcome-Trust Sanger Institute, Mouse Genomic Informatics-MGI-JAX, Phenome Database).
Basic Protocol 2: Synthesis and purification of RNA and DNA components
Quality of injection mix components (RNA, DNA and even the injection buffer) have to be of highest quality for mouse embryo injections. Utmost care should be taken in preparing the reagents in high quality nuclease free water and final injection mixes are prepared using injection buffer. Since Cas9 mRNA is a common component in all CRISPR injections, it would be ideal to prepare Cas9 mRNA in larger batches (e.g., starting reaction of 100 µl instead of 20 µl Megaclear RNA synthesis) and store the poly-adenylated and purified Cas9 mRNA in single use aliquots of about 5ug/vial in −80°C. After obtaining satisfactory results in the first injection session, single use aliquots of such batches can be used for future experiments (we have used Cas9 mRNA stored up to 5 months). Once an aliquot is thawed, re-freezing of unused mRNA is not recommended.
HDR DNA: For simple knock-in mutations such as site directed mutagenesis or insertion of short sequences, repair DNA of oligonucleotides up to 200 bases are sufficient for HDR. If larger cassettes (e.g., reporters or minigenes) need to be inserted, a targeting vector with longer homology arms, similar to those used in a ES cell based targeting, need to be designed. There are not many reports available yet regarding the critical length of homology arms needed in such vectors in the CRISPR/Cas system: we anticipate that length of homology arms largely depends on the locus in question. Such vectors need to contain longer homology arms (typically 1 kb or more on each side) but need not contain selection (positive and negative) markers. An additional important requirement in this case is that the repair plasmids should not contain the CRISPR target sequences. It is also reported that circular plasmid can be co-injected with RNA components for direct embryo targeting experiments (Mashiko et al., 2013).
Basic Protocol 3: isolation of one-cell staged mouse embryos, micro-injection of CRISPR/Cas components and transfer of injected embryos into pseudopregnant mice
The majority of mutant mice generation steps in the CRISPR/Cas system follow protocols used in traditional transgenic mouse production technology through pronuclear injection, with a few exceptions. The mouse transgenic technology has been established over several years of painstaking work from 100s of skilled technicians and researchers and are still being perfected constantly and there are multiple and exhaustive resources available that describe the critical parameters and troubleshooting (Behringer et al., 2014; International Society for Transgenic Technologies, 2011). A few parameters specifically related to CRISPR/Cas are discussed below.
Concentration of Cas9 mRNA, sgRNAs. A wide range of concentrations of Cas9 mRNA and sgRNAs has been reported to work in mouse embryos (listed in Table 4). This clearly demonstrates the flexibility and robustness of the CRISPR/Cas system. There are some indications that higher concentrations of Cas9 mRNA can yield higher number of mutants in which both alleles are targeted (Zhou et al., 2014). It is cautioned however that higher Cas9 concentration can also lead to higher off-target cleavages, at least in cell based experiments (Hsu et al., 2013). There are no such systematic reports available yet in mice.
Strain of mouse embryos: The strains in which the CRISPR/Cas system has been reported to work are C57BL6J, B6D2F1, B6SJLF1, B6/CBAF1. Many labs are currently trying CRISPR/Cas system under various strain backgrounds and as the data become available it may likely indicate that most strains are amenable to this system. While there are varying strain efficiencies as noted in standard transgenesis experiments (Auerbach et al., 2003), the efficiencies of CRISPR/Cas induced mutations may also vary among different strains. We presume that such strain variations, if occur, would result from technical challenges associated with embryo production, isolation, micro-injection and transfer steps rather than in vivo mechanistic efficiency of CRISPR/Cas system in different strains: the fact that CRISPR/Cas mediated gene editing is proven to efficiently work in other species supports this argument. It may not be an overstatement if we say “CRISPR/Cas can be successfully used to create mutants under any mouse strain background” provided the technical challenges pertaining to embryology for that strain are streamlined.
Site of micro-injection. Unlike in a typical transgenic production where the DNA is micro-injected into the pronucleus (preferably bigger of the two or both), Cas9, TALENs and ZFN systems are introduced as mRNAs which can be injected in to cytoplasm. A recent study compared the efficiency of CRISPR/Cas mediated DNA cleavage when the Cas9 mRNA and sgRNAs were injected into i) only Cytoplasm, ii) only nucleus and iii) both cytoplasm and nucleus(Horii et al., 2014). The results show that only cytoplasmic injections had highest efficiency. Nonetheless, the other two methods also yielded sufficiently high number of mutant genomes. The study, however, did not evaluate the knock-in efficiency using oligonucleotide or plasmid based repair DNA in their injection mixes. The available reports so far, that used repair DNA in their CRISPR/Cas injections, have not performed cytoplasmic-only injections and it would be hard to conclude at this point whether cytoplasmic-only injections yield desired results for the mixes that comprise repair DNA.
Table 4.
Concentrations of RNA and DNA components in published CRISPR/Cas mouse genome editing reports
| Cas9mRNA(ng/µl) | sgRNA(ng/µl) | Donor Oligonucleotide (ng/µl) |
Reference |
|---|---|---|---|
| 20 – 200 | 20 to 50 | Not Done | (Wang et al., 2013) |
| 100 | 50 | 100 ng/µl | |
| 100(Cyto) | 50(Cyto) | Plasmid 500(Cyto) | (Yang et al., 2013) |
| 100(Cyto) | 50(Cyto) | Plasmid 200(Cyto) | |
| 100(Cyto) | 50(Cyto) | Plasmid 50(Cyto) | |
| 100(Cyto) | 50(Cyto) | Plasmid 10(Cyto) | |
| 5(Nuc) | 2.5(Nuc) | Plasmid 10(Cyto) | |
| 100(Cyto) | 50(Cyto) | 50(Nuc) | |
| 100(Cyto) | 50(Cyto) | 10(Nuc) | |
| 100(Cyto) | 50(Cyto) | 2(Nuc) | |
| 20 ng/µl | 20 ng/µl | Not done | (Shen et al., 2013) |
| 20 | 2.5 to 5ng each of 5 or 10 sgRNAs | Not done | (Zhou et al., 2014) |
| 100µg/ml(Cyto) | 10 µg/ml(Cyto) | Not done | (Fujii et al., 2013) |
Cyto: site of injection is cytoplasmic
Nuc: site of injection is nuclear
Basic Protocol 4: Genotyping
Surveyor or T7E1 assay: Genotyping using Surveyor assay or T7E1 assay can be challenging in certain situations: i) mosaicism (resulting in more than two types of alleles in some samples), ii) large indels (occasional preference of PCR for shorter or larger alleles in the samples or inefficient hetero-duplex formation of strands during the assay), iii) X-linked genes (single copy genes: any mutation in such genes cannot be detected). Efficiency of this assay is also of concern in certain cases such as i) presence of non-specific bands in the PCR, ii) incomplete removal of primer dimers before the PCR product is subjected to the assay, iii) poor efficiency of Cel1 or T7E1 enzyme activity, iv) inefficient formation of heteroduplexes, v) inability to detect very short fragments if the mutation is close to one end of the PCR product. Suggested remedies in these cases would be to a) adjust PCR conditions to obtain a clean PCR product, b) purifying PCR product before the assay, c) using suggested amount (less than 200 ng) of PCR product in the assay d) redesign PCR primers to place the expected indel mutation at about the 1/3rd or 2/3rd length of the PCR product e) to consider alternate genotyping options such as RFLP and/or sequencing.
An additional point to keep in mind is the presence of Single Nucleotide Polymorphisms (SNPs), or micro-satellite variations or Simple Sequence Length Polymorphisms (SSLPs) in the genomic region used for PCR genotyping as they may lead to false positives by the T7Endouclease assay or Surveyor assay due to the associated mis-pairing of the base pairs. Therefore, we suggest including genomic DNA of the mouse strain used in these genotyping assays, to anticipate any unexpected false positives.
An example of a false negative surveyor assay result that was detected by direct sequencing of a target region PCR product is shown in Figure 6.
Figure 6. An example of Surveyor assay combined with sequencing to detect CRISPR/Cas mutations on X-chromosome.
(A) Surveyor assay of DNA samples isolated from CRISPR/Cas injected pronuclei cultured up to blastocysts stage showing negative (sample 4) and positive (remaining) samples. Red arrows indicate cleaved bands of 261 and 184 bp from the 445 bp PCR product. Notably, all samples were positive by sequencing assay including those that were surveyor negative. Note that occasionally surveyor assay result in very weak cleavage products (e.g., sample 2). (B) Direct sequencing of PCR products from wild type, a surveyor positive (sample 3) and a surveyor negative (4) sample. Sample number 4 had deletion of 8 nucleotides even though surveyor assay was negative. This could be due to the sample being male which will have only one X-chromosome. The sample 3 showed typical overlapping peaks after the cut site (arrow) indicative of two (or more; if mosaic) templates: this sample could be a female and either only one allele is mutated and/or CRISPR/Cas activity is mosaic.
Off-target effects: As discussed in the design section (Basic protocol 1), the CRISPR/Cas system is likely to have high off-target effects simply because of the very short sequence requirement in sgRNAs for target recognition. Such off-target effects can be minimized significantly by:
Careful selection of target sequences: Currently there are two online tools available for target selection for mouse genome (listed in basic protocol 1 section). It is expected that such tools will be improved further as additional experimental data are accumulated and additional tools become available. We suggest that same sequence should be queried using more than one search tool and the common best sequence(s) selected that result from multiple search tools that may decrease the likelihood of off-target effects.
Using double nicking (also called offset nicking) approach. Certain mutations in the Cas9 protein cause DNA nicking instead of double stranded breaks. This feature is elegantly used, in a similar fashion to using two molecules of ZFNs and TALENs, to cause nicking on opposites strands. A requirement for the orchestrated functioning of two independent sgRNA sequences significantly decreases the likelihood of off-target effects. An example of a Cas9 mutant that is shown to be useful in double nicking strategy is D10A (termed Cas9n: Cas9 nickase)(Fujii et al., 2014; Mali et al., 2013a; Ran et al., 2013; Shen et al., 2014).
Screening of off-target effects: Even though off-target mutations (if present) can be eliminated by breeding of the mutants in subsequent generations, it is prudent to screen the mutant(s) chosen for further experiments for presence of off-target mutations. The screening can be done, if the selected sgRNA has only a few potential off-target sites, using Surveyor or T7E1 assay of PCR products amplified from the off-target sites. Screening of the potential off-target sequences with up to 4 mismatches (particularly in the PAM distal nucleotides of the target sequence) is strongly suggested. Alternatively samples can be subjected to expensive methods such as whole genome sequencing to critically rule out all any off-target mutations.
Anticipated Results
As observed in published reports so far, CRISPR/Cas induced mutations generated occur at a higher efficiency when compared to standard transgenic mouse production. However, even though these two techniques use one-cell mouse embryo injections, they cannot be directly compared with each other (except for the number of mutants generated), because the intended end-results are quite different. Typical transgenic rates (number of transgenic mutants obtained/100 embryos injected) through pronuclear DNA injection range from 0.6 +/− 0.8 % to 2.8+/− 3.1% that depends on various parameters including strain and type of DNA(Fielder et al., 2010). All the reports that used CRISPR/Cas system so far, either to create indels or HDR knock-in mutations using short oligonucleotides, have observed a several fold higher success rate than that is achieved using traditional transgenesis. Because of such high efficiency, about a third or a quarter of starting number of embryos injected will lead to mutant pups in the CRISPR/Cas system when compared to traditional transgenesis, where typically about 200 to 300 zygotes are injected to obtain 3 to 4 independent founder mice. It is anticipated that the CRISPR/Cas system may become widely used approach and preferred method for mouse genome editing in the near future.
Time Considerations
Generation of knockout or knock-in mice using standard ES cell based approaches involve at least 2 to 3 major steps that take about a year to produce chimeras. CRISPR/Cas system on the other hand can generate mutant mice in one major step and can be completed in as less as 3 months’ time. Furthermore, multiple mutants (more than one gene or locus) can also be generated in a single experiment using CRISPR/Cas system. A typical time frame for CRISPR/Cas experimental procedures is outlined below and depicted in Figure 1:
Week 1: Searching CRISPR target sequences and designing of constructs
Weeks 1 to 2: procuring of primers for amplifying Cas9 and/or sgRNA templates and primers for genotyping assays
Weeks 2 to 3: constructing of vectors (as needed) and generating & purifying RNA and DNA components
Weeks 2 to 3: procuring of animals and testing genotyping primers on wild type genomic DNA.
Week 4: making sure that all the injection components are ready and initiation of superovulation.
Week 5: micro-injection and embryo transfer
Week 9–11: genotyping of offspring.
ACKNOWLEDGEMENT
This work was partially supported by an Institutional Development Award (IDeA) to CBG (PI: Shelley Smith) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103471, by MINECO project BIO2012-39980 to LM, by Grant-in-Aid for Scientific Research (25290035) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and 2014 Tokai University School of Medicine Research Aid to MO. DS has been supported by a PhD fellowship from La Caixa program. We greatly acknowledge the help and advice of Dr. Pawel Pelczar (Institute of Laboratory Animal Science, University of Zurich, Switzerland) in the generation of CRISPR-Cas mice. We thank Feng Zhang and David Scott (Massachusetts Institute of Technology) for commenting on the manuscript. We thank H. Miura for technical assistance in certain CRISPR/Cas experiments. MO acknowledges the people in Support Center for Medical Research and Education in Tokai University for technical assistance. RMQ, DWH and CBG acknowledge the Nebraska Research Initiative and UNMC Vice Chancellor for Research Office for supporting the mouse genome engineering core facility.
Footnotes
INTERNET RESOURCES
This site searches SpCas9 target sites within the sequence of interest and allows users to enter a 23–1000 base DNA sequence. The site is hosted and maintained by Dr. Feng Zhang’s group at Massachusetts Institute of Technology. The algorithm used by this program is based on the specificity analysis performed in Hsu et al., 2013. Details related to this website and how to use the tool are given in Basic Protocol 1 and outlined in Figure 2.
LITERATURE CITED
- Auerbach AB, Norinsky R, Ho W, Losos K, Guo Q, Chatterjee S, Joyner AL. Strain-dependent differences in the efficiency of transgenic mouse production. Transgenic research. 2003;12:59–69. doi: 10.1023/a:1022166921766. [DOI] [PubMed] [Google Scholar]
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science (New York, N.Y.) 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- Behringer R, Gertsenstein M, Nagy KV, Nagy A, editors. Manipulating the mouse embryo: a laboratory manual. 4th Edition. Cold Spring Harbor Laboratory Press; 2014. [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Kandzia R, Marillonnet S. A One Pot, One Step, Precision Cloning Method with High Throughput Capability. PLoS ONE. 2008;3:e3647. doi: 10.1371/journal.pone.0003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Marillonnet S. Golden Gate cloning. Methods in molecular biology (Clifton, N.J.) 2014;1116:119–131. doi: 10.1007/978-1-62703-764-8_9. [DOI] [PubMed] [Google Scholar]
- Fielder TJ, Barrios L, Montoliu L. A survey to establish performance standards for the production of transgenic mice. Transgenic Research. 2010;19:675–681. doi: 10.1007/s11248-009-9335-3. [DOI] [PubMed] [Google Scholar]
- Fu Y, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nature Biotechnology. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii W, Kawasaki K, Sugiura K, Naito K. Efficient generation of large-scale genome-modified mice using gRNA and CAS9 endonuclease. Nucleic Acids Research. 2013;41:e187–e187. doi: 10.1093/nar/gkt772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii W, Onuma A, Sugiura K, Naito K. Efficient generation of genome-modified mice via offset-nicking by CRISPR/Cas system. Biochemical and biophysical research communications. 2014 doi: 10.1016/j.bbrc.2014.01.141. [DOI] [PubMed] [Google Scholar]
- Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, et al. Knockout Rats via Embryo Microinjection of Zinc-Finger Nucleases. Science. 2009;325:433–433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B, Limaye A, Kulkarni AB. Overview: generation of gene knockout mice. Current protocols in cell biology / editorial board, Juan S. Bonifacino … [et al.] 2009;Chapter 19(Unit 19.12):19.12.1–19.12.17. doi: 10.1002/0471143030.cb1912s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruyama N, Cho A, Kulkarni AB. Overview: engineering transgenic constructs and mice. Current protocols in cell biology / editorial board, Juan S. Bonifacino … [et al.] 2009;Chapter 19(Unit 19.10) doi: 10.1002/0471143030.cb1910s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heigwer F, Kerr G, Boutros M. E-CRISP: fast CRISPR target site identification. Nature Methods. 2014;11:122–123. doi: 10.1038/nmeth.2812. [DOI] [PubMed] [Google Scholar]
- Hermann M, Cermak T, Voytas DF, Pelczar P. Mouse Genome Engineering Using Designer Nucleases. [Accessed April 14, 2014];Journal of Visualized Experiments. 2014 doi: 10.3791/50930. Available at: http://www.jove.com/video/50930/mouse-genome-engineering-using-designer-nucleases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii T, Arai Y, Yamazaki M, Morita S, Kimura M, Itoh M, Abe Y, Hatada I. Validation of microinjection methods for generating knockout mice by CRISPR/Cas-mediated genome engineering. [Accessed April 23, 2014];Scientific Reports. 2014 4 doi: 10.1038/srep04513. Available at: http://www.nature.com/doifinder/10.1038/srep04513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath P, Barrangou R. CRISPR/Cas, the Immune System of Bacteria and Archaea. Science. 2010;327:167–170. doi: 10.1126/science.1179555. [DOI] [PubMed] [Google Scholar]
- Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nature Biotechnology. 2013;31:827–832. doi: 10.1038/nbt.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease S, Saunders TL, editors. International Society for Transgenic Technologies. Advanced protocols for animal transgenesis: an ISTT manual. New York: Springer, Heidelberg; 2011. [Google Scholar]
- Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. eLife. 2013;2:e00471–e00471. doi: 10.7554/eLife.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePage DF, Conlon RA. Animal models for disease: knockout, knock-in, and conditional mutant mice. Methods in molecular medicine. 2006;129:41–67. doi: 10.1385/1-59745-213-0:41. [DOI] [PubMed] [Google Scholar]
- Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nature Biotechnology. 2013a;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-Guided Human Genome Engineering via Cas9. Science. 2013b;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiko D, Fujihara Y, Satouh Y, Miyata H, Isotani A, Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. [Accessed April 24, 2014];Scientific Reports. 2013 3 doi: 10.1038/srep03355. Available at: http://www.nature.com/doifinder/10.1038/srep03355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague TG, Cruz JM, Gagnon JA, Church GM, Valen E. CHOPCHOP: a CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Research. 2014 May 26; doi: 10.1093/nar/gku410. pii: gku410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda SK, Wefers B, Ortiz O, Floss T, Schmid B, Haass C, Wurst W, Kühn R. Highly efficient targeted mutagenesis in mice using TALENs. Genetics. 2013;195:703–713. doi: 10.1534/genetics.113.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nature Biotechnology. 2013;31:839–843. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease S, Saunders TL. Advanced Protocols for Animal Transgenesis. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. [Accessed June 22, 2014]. Available at: http://link.springer.com/10.1007/978-3-642-20792-1. [Google Scholar]
- Ran FA, Hsu PD, Lin C-Y, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y, et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rémy S, Tesson L, Ménoret S, Usal C, Scharenberg AM, Anegon I. Zinc-finger nucleases: a powerful tool for genetic engineering of animals. Transgenic Research. 2010;19:363–371. doi: 10.1007/s11248-009-9323-7. [DOI] [PubMed] [Google Scholar]
- Semsarian, Christopher Use of Mouse Models for the Analysis of Human Disease. Current Protocols in Human Genetics / Editorial Board, Jonathan L. Haines … [et Al.] 2002 May;Chapter 15(Unit 15.2) doi: 10.1002/0471142905.hg1502s32. [DOI] [PubMed] [Google Scholar]
- Shen B, Zhang J, Wu H, Wang J, Ma K, Li Z, Zhang X, Zhang P, Huang X. Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Research. 2013;23:720–723. doi: 10.1038/cr.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Zhang W, Zhang J, Zhou J, Wang J, Chen L, Wang L, Hodgkins A, Iyer V, Huang X, et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nature Methods. 2014;11:399–402. doi: 10.1038/nmeth.2857. [DOI] [PubMed] [Google Scholar]
- Sung YH, Baek I-J, Kim DH, Jeon J, Lee J, Lee K, Jeong D, Kim J-S, Lee H-W. Knockout mice created by TALEN-mediated gene targeting. Nature Biotechnology. 2013;31:23–24. doi: 10.1038/nbt.2477. [DOI] [PubMed] [Google Scholar]
- Terns RM, Terns MP. CRISPR-based technologies: prokaryotic defense weapons repurposed. Trends in genetics: TIG. 2014;30:111–118. doi: 10.1016/j.tig.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers B, Panda SK, Ortiz O, Brandl C, Hensler S, Hansen J, Wurst W, Kühn R. Generation of targeted mouse mutants by embryo microinjection of TALEN mRNA. Nature protocols. 2013;8:2355–2379. doi: 10.1038/nprot.2013.142. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Shen B, Zhang W, Wang J, Yang J, Chen L, Zhang N, Zhu K, Xu J, Hu B, et al. One-step generation of different immunodeficient mice with multiple gene modifications by CRISPR/Cas9 mediated genome engineering. The International Journal of Biochemistry & Cell Biology. 2014;46:49–55. doi: 10.1016/j.biocel.2013.10.010. [DOI] [PubMed] [Google Scholar]