Abstract
Melanoma often spreads to cutaneous or subcutaneous sites that are amenable to direct, intralesional injection. As such, developing effective injectable agents has been of considerable interest. Talimogene laherperepvec (T-VEC) is an injectable modified oncolytic herpes virus being developed for the treatment of advanced melanoma. Pre-clinical studies have shown that T-VEC preferentially infects melanoma cells and exerts antitumor activity through directly mediating cell death and by augmenting local and even distant immune responses. T-VEC has now been assessed in Phase II and III clinical trials and has demonstrated a tolerable side-effect profile and promising efficacy, showing an improved durable response rate and a trend toward superior overall survival compared to granulocyte-macrophage colony-stimulating factor. Despite these promising results, responses have been uncommon in patients with visceral metastases. T-VEC is currently being evaluated in combination with other immune therapies (ipilimumab and pembrolizumab) with early signs of activity. In this review, we discuss the preclinical rationale, the clinical experience, and future directions for T-VEC in advanced melanoma.
Keywords: GM-CSF, HSV, immune therapy, injectable, melanoma, oncolytic, talimogenelaherperepvec, T-VEC
Melanoma is an aggressive cutaneous malignancy that is responsible for >9000 deaths in the USA annually [1]. The prognosis for advanced melanoma has traditionally been quite poor with a median overall survival of 6–9 months [2]. Local or regional disease may be cured with surgical treatment in many patients, but relapse is common in patients with high risk disease. Five-year survival rates vary widely by American Joint Committee on Cancer (AJCC) stage, ranging from >95% for stage IA (<1-mm thickness) to <30% for stage IIIc (spread to 4+ lymph nodes or both nodal and in-transit metastasis) [3]. Talimogene Laherperepvec (T-VEC), an engineered oncolytic herpes simplex type 1 virus, is directly injected into melanoma tumors with regional or cutaneous metastatic spread, and is reviewed in detail in this manuscript. First, however, we will discuss factors that influence T-VEC use in the context of other melanoma therapeutics.
Two clinical presentations relatively unique to melanoma directly influence the use of injectable immune therapies such as T-VEC. First, in-transit metastases occur when melanoma cells spread to the dermal lymphatics and present as cutaneous or subcutaneous lesions, generally between a primary tumor and its regional lymphatic basin [4]. While this represents only regional disease, lesions can be quite numerous, making surgical resection difficult. Second, melanoma metastases have tropism for the skin, and may metastasize only to subcutaneous or cutaneous sites. In these cases, metastatic spread may range from a single site of disease to multifocal, disseminated skin and soft tissue involvement. In both of these clinical scenarios, all melanoma lesions are directly visible or accessible, but surgical therapy may not be optimal due to the extent of disease and high risk of relapse. Understanding how to treat these patients has been a major research focus and is particularly relevant for T-VEC therapy.
Overview of regional & systemic melanoma therapies
Many patients with in-transit disease have disease con-fined to a single limb, which is amenable to regional therapy. This may consist of either isolated limb perfusion (ILP) or isolated limb infusion (ILI). Both techniques involve the administration of high-dose chemotherapy (typically, single agent melphalan) to the isolated vascular system of the limb with hyperthermia. The vascular system is isolated by surgical cannulation (ILP), or percutaneous catheterization and tourniquet (ILI). Overall response rates of up to 79% for ILP and 84% for ILI have been reported [5,6]. Complete response rates are reported to be as high as 54% for ILP and 38% for ILI, many of which are durable. Unfortunately, many patients fail to respond or have disease outside the perfusion field that is not amenable to therapy. The morbidity of these interventions is also significant, with major complication rates of up to 20% and amputation rates of up to 3% reported in some historical series [5,6].
Other therapies have been proposed for in-transit disease and distant skin and soft tissue metastases, including electrochemotherapy and topical immunotherapy. Electrochemotherapy involves systemic administration of low-dose chemotherapy and concurrent intralesional electroporation that renders tumor cells permeable to chemotherapy and induces direct cell death. Responses of up to 80% have been reported in Phase II trials from Europe, but the long-term efficacy of this therapy remains uncertain [7]. This approach also requires a complex and expensive device, and few clinicians are trained to perform this therapy effectively. Topical therapy with agents such as imiquimod (Aldara®) has also been reported [8]. This strategy, in our experience, is only useful in a subset of patients with low-volume, superficial dermal in-transit metastases. These factors limit the use of this therapy and confirm the need for additional therapeutic options in this setting.
Several novel systemic therapies have been developed over the last several years and have transformed therapy for advanced and metastatic melanoma. Molecular-targeted therapeutics are effective for approximately 50% of patients that harbor activating BRAF mutations (with BRAF and/or MEK inhibitors) or more rarely, KIT mutations (KIT inhibitors) [9–11]. Immune therapies, particularly immune checkpoint inhibitors targeting PD-1 (nivolumab, pembrolizumab) and CTLA4 (ipilimumab), also improve progression-free and overall survival in metastatic melanoma [12–14]. These agents induce durable, antitumor immune responses in an increasing number of patients. Unfortunately, primary or acquired resistance to both targeted and immune therapy occurs in the majority of patients, highlighting the need for more effective therapies.
Rationale for injectable immune therapy
Melanoma’s propensity for cutaneous/subcutaneous spread provides a unique opportunity for clinically feasible direct injection of tumors. This strategy is potentially attractive, given the decreased side effects of intradermal compared with systemic therapy, but leaves the question of whether this approach will address the systemic nature of the disease. Fortunately, systemic immune responses may be induced by intralesional immune therapy in some cases, causing regression of noninjected lesions. Historically, this has been observed in clinical trials of intralesional Bacilus Calmette Guerin (BCG) and various cytokine-based intralesional therapies. A longitudinal experience from the 1970s reported regression of 90% of lesions injected with BCG and 17% of noninjected lesions [15]. A study of adjuvant BCG for high-risk, resected disease did not show improved clinical outcomes, so this therapy is not used clinically as an adjuvant therapy [16]. Injectable granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, and interferon alpha have also demonstrated responses in injected and noninjected melanoma lesions in less than half of treated patients [17,18]. These agents may be useful for local control in selected cases, but their cutaneous toxicity and lack of a systemic therapeutic benefit have limited their clinical utility.
Oncolytic virotherapy
Oncolytic viruses selectively recognize, infect and destroy malignant cells with minimal effects on normal human cells [19,20]. All viruses have unique tissue-specific tropism (e.g., influenza for respiratory epithelium). Some naturally occurring viruses are cancer-specific; others, including HSV-1, may be engineered to preferentially infect cancer cells. Once infected, the virus replicates and causes cancer cell death via several mechanisms. These include cellular lysis from viral replication, hijacking of cellular death pathways, and promotion of cellular immunity [21]. A number of oncolytic viruses, including HSV-1, adenovirus, coxsackie virus and vaccinia virus have been used in various preclinical studies and clinical trials. In many studies across several cancer types, these agents were administered systemically, often in combination with other antineoplastic therapies [22]. Although some activity has been observed in these early phase trials, the presence of concurrent therapies and low objective response rates highlights the need for randomized clinical trials [23–27]. Systemic delivery of oncolytic viruses is limited by antibody and complement coating causing sequestration and clearance in the liver and spleen [21]. Given the disseminated nature of metastatic cancer, systemic administration may ultimately be preferable if these barriers can be overcome given the disseminated nature of metastatic cancer. Intradermal injection of the oncolytic virus bypasses these neutralizing effects and, at least in melanoma, appears to be more effective at this time.
T-VEC
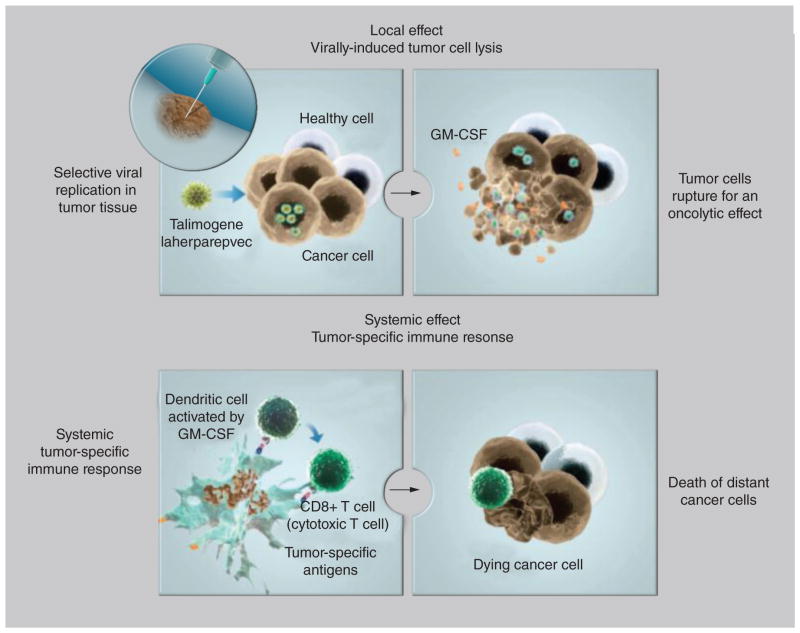
T-VEC (previously known as OncoVEXGM-CSF) is an engineered, oncolytic herpes simplex virus type 1 (HSV-1). T-VEC is injected locally into the tumor and then selectively recognizes, infects, and destroys malignant cells with minimal effects on normal human cells [28]. T-VEC is an HSV-1 with several modifications which confer its oncolytic properties. The neurovirulence factor ICP34.5 is inactivated to prevent neuronal involvement [28]. This neurovirulence factor is replaced by the coding sequence for GM-CSF, enhancing local production of GM-CSF. GM-CSF functions to recruit antigen presenting cells to the tumor microenvironment, enhance dendritic cell function and promote cytotoxic T-cell responses to tumor-associated antigens [29–31]. Direct intratumor injection of single-agent GM-CSF has demonstrated preclinical and limited clinical activity [17,32–34]. Furthermore, in mouse models, intratumoral inoculation of GM-CSF with various inactivated HSV vectors (including the eventual T-VEC system) resulted in greater tumor inhibition and improved mouse survival compared with HSV alone [28,35]. Intratumoral injection or production of GM-CSF likely augments local expression and enhances its immune effects. Finally, the ICP47 region is also deleted which promotes viral replication, enhances antigen presentation and improves oncolysis [28,36]. As with other injectable agents, local oncolysis is thought to activate T cells that may induce a distant immune response (Figure 1) [37]. Several preclinical studies support this by demonstrating tumor infiltration by CD8+ T cells in noninjected metastases [38].
Figure 1. Proposed mechanism of action for talimogene laherparepvec.
GM-CSF: Granulocyte-macrophage colony-stimulating factor.
T-VEC is administered intralesionally at a dose of up to 4 ml of 106–108 pfu/ml in phosphate-buffered saline. The administered volume varies with lesion size: up to 0.1 ml for tumors up to 0.5 cm in longest dimension, up to 0.5 ml for 0.5–1.5 cm tumors, up to 1 ml for 1.5–2.5 cm tumors, up to 2 ml for 2.5–5 cm tumors, and up to 4 ml for tumors >5 cm. For superficial dermal lesions that are easily palpable, intralesional administration is relatively straightforward. The needle is placed in the central portion of the lesion and the correct volume of drug is administered as a single injection. Many patients with locally and regionally advanced melanoma and distant skin-soft tissue metastases have some lesions that are located in the deep dermis, subcutaneous tissue, intramuscular sites, or regional nodes. These lesions cannot be accurately injected visually or by palpation. We have injected these lesions effectively under ultrasound guidance in the clinic. Familiarity with ultrasound and ultrasound-guided localization techniques are needed, but many surgeons or interventional radiologists are able to perform such injections without difficulty. The use of ultrasound-guided injection expands the population of patients who may be considered for intralesional therapy, and ensures accurate delivery of drug to the tumor.
Clinical efficacy of T-VEC in advanced melanoma
T-VEC is the only oncolytic viral therapy that has been tested in a randomized clinical trial. An initial Phase II study enrolled 50 patients treated with T-VEC every 3 weeks (Table 1) [39]. Importantly, this study included patients with unresectable regional disease (stage IIIc; n = 10), skin or lymph node only metastases (stage IV M1a; n = 16), lung-only metastases (stage IV M1b; n = 4), and visceral involvement (stage IV M1c; n = 20). Most patients (74%) had received prior systemic therapies. Patients could have up to 10 lesions injected. In this study, the objective response rate by RECIST 1.0 criteria was 26%; 8 of 13 responding patients experienced complete responses, and 12 responses lasted for >6 months. Both injected and uninjected lesions responded in this study. Only 3 of 20 patients (15%) with stage IV M1c disease experienced an objective response. This included two patients with durable responses rendered disease-free by subsequent surgery and a patient with liver metastases who experienced a complete response. Several patients experienced disease progression prior to response (‘pseudo-progression’), a phenomenon that has been observed with other immune therapies [12,40]. Similar to other immunotherapy studies, survival rates appeared to ‘plateau’ after 1 year, with 58 and 52% of patients surviving to 1 and 2 years, respectively. Ninety-three percent of responding patients were alive 1 year after starting therapy.
Table 1.
Clinical activity of talimogene laherparepvec in advanced melanoma.
| Phase II T-VEC study | Phase III T-VEC study | |
|---|---|---|
| Patient numbers | 50 | 295 (T-VEC arm) |
| Stage | IIIc, 20% IVa, 32% IVb, 8% IVc, 40% |
IIIb/c, 30% IVa, 27% IVb, 21% IVc, 22% |
| Objective response rate | 26% | 26.4% (16.3% durable response rate) |
| Overall survival | 58% 1 year 52% 2 year |
23.3 months (median) |
| Toxicities | Grade 1–2: fever 52%, chills 48%, fatigue 32%, nausea 30% No Grade 3 AEs noted |
All grades: fatigue 50%, chills 49%, fever 43%, nausea 36% Grade 3/4: cellulitis 2%, fatigue 2%, vomiting 2%, dehydration 2% |
| NCT trial ID, Reference | NCT00289016 [27] | NCT00769704 [29] |
T-VEC: Talimogene laherparepvec.
A Phase III study was then conducted (OPTiM) which randomized subjects 2:1 to T-VEC or GM-CSF (administered subcutaneously, 125 μg/m2 for 14 of every 28 days). Durable response rate by a blinded adjudication committee was the primary endpoint, and was defined as the proportion of patients experiencing a response lasting 6 months or more at any time in their clinical course. Key secondary endpoints included objective response rate by RECIST 1.1 criteria, and overall survival (OS). Of 430 patients enrolled, stage distribution was fairly similar to the Phase II study including 30% with stage III disease, 27% with IV M1a, 21% with IV M1b and 22% with IV M1c (Table 1) [37]. Durable response rate was higher in the T-VEC arm (16.3 vs 2.1%; p < 0.001), as was objective response rate (26.4 vs 5.7%). Although durable responses occasionally occurred in stage IV M1c patients (8% durable response rate), most were seen in stage III (33%) and stage IV M1a (16%) disease. A strong trend toward improved overall survival was noted on the interim analysis (median 23.3 vs 19 months; p = 0.07) and the primary analysis (median 23.3 vs 18.9 months; p = 0.051). In exploratory analyses, overall survival was superior in the T-VEC arm for patients with stage IIIB/C or IV M1a (HR: 0.57, p < 0.001) but not in those with IV M1b/c disease (HR: 1.07, p = 0.71). In addition, survival was superior for patients receiving T-VEC as first-line therapy (HR: 0.5, p < 0.001) but not in those receiving second line therapy or later (HR: 1.13, p = 0.46).
The T-VEC monotherapy studies clearly show that this agent may stimulate distant responses in noninjected lesions, presumably through immune activation against tumor antigens or neoantigens. Responses in lung and especially other visceral metastases, however, occur somewhat infrequently. Given the activity of other systemic therapies across metastatic stages, the role of single agent T-VEC may be largely restricted to stage IIIc/IV M1a disease.
Combination strategies
In view of the promising activity and favorable toxicity profile (discussed below) of T-VEC alone, it was hypothesized that this agent should be combined with other melanoma therapeutics. One intriguing strategy is to combine with another immune activating agent. Although some studies suggest that GM-CSF may stimulate local immune suppressive cells (myeloid-derived suppressor cells) [41,42], the combination of oncolytic viral therapy and immune checkpoint blockade has significant preclinical support [38,43]. For example, in a B16 melanoma mouse model, an oncolytic Newcastle disease virus induced local and distant tumor infiltration with CD8+ and CD4+ T cells [38]. Addition of CTLA-4 blockade induced effective anti-tumor responses and established protection from tumor rechallenge, even in poorly immunogenic models.
Accordingly, a Phase I study of ipilimumab + T-VEC (NCT01740297) has been conducted and results were presented at the American Society of Clinical Oncology Meeting in May, 2014 [44]. Nineteen patients were enrolled; therapy consisted of intralesional T-VEC at up to 4 ml of 106 PFU/ml at week 1, then up to 4 ml of 108 PFU/ml at week 4, and then every 2 weeks thereafter. Ipilimumab was added at week 6 and given at the FDA approved dose of 3 mg/kg IV every 3 weeks for four doses. Patients had fairly even distribution across stage III (n = 4), stage IV M1a (n = 4), stage IV M1b (n = 5) and stage IV M1c (n = 6) disease; 11 (58%) harbored a BRAF mutation. Among the 18 evaluable patients, the objective response rate was 56% by immune-related response criteria, including a 33% complete response rate. No patients with stage IV M1c disease responded, although two had stable disease with reduction in tumor diameter nearly reaching a partial response. Of note, activated CD8+ T cells in the peripheral blood (defined as HLA-DR+CD3+CD4-T cells) increased by ≥40% after two doses of T-VEC in 10 of 12 patients with responses or stable disease compared with only 1 of 5 patients with primary disease progression. Although the results are preliminary, the degree of clinical activity observed in this study is impressive and is greater than reported in other large studies of either agent alone. Based on these results, a randomized Phase II study of ipilimumab + T-VEC versus ipilimumab alone is ongoing, with target accrual of 200 patients (NCT01740297). A randomized Phase I/II study of pembrolizumab with or without T-VEC is also underway (NCT02263508).
Safety of T-VEC
Adverse events largely arise from the inflammatory response induced by T-VEC. A theoretical concern with oncolytic viral therapy is that the virus could mutate and regain pathogenicity [21]. This has not been observed clinically with T-VEC or with other oncolytic viruses over several decades of clinical trials with various agents [45]. In the Phase II clinical trial, drug-related adverse events occurred in 85% of patients, primarily a grade I–II flu-like syndrome (Table 1). Patients experienced pyrexia (52%), chills (48%), fatigue/malaise (32%), nausea (30%), localized pain (24%) and headache (20%). Vitiligo occurred in three patients who experienced a response, a well-described phenomenon that also occurs with IL-2 and other immune therapies. The side effect profile was similar in the Phase III randomized study with frequent low-grade flu-like symptoms. No grade III toxicities occurred in >3% of the study population.
In combination with ipilimumab, no dose limiting toxicities were observed during the DLT evaluation period. Among 19 patients, 26% (n = 5) experienced grade 3 toxicities attributed to either T-VEC or ipilimumab, including fever, hypophysitis, influenza-like illness and adrenal insufficiency. One patient also experienced grade 4 elevations of amylase and lipase, attributed to ipilimumab.
Other intralesional therapy
Several other intralesional agents have been investigated in early clinical studies. Velimogene aliplasmid is a lipid-based formulation containing a plasmid encoding major histocompatibility complex (MHC) class I and HLA-B7 and B2 microglobulin light chains. While this agent induced objective responses in 11.8% of patients treated in a Phase II study (including noninjected lesions), survival and response rates were inferior to cytotoxic chemotherapy [36,46]. Another oncolytic virus, Coxsackie A21 (CAVATAK) is a genetically unmodified virus that preferentially infects I-CAM-1 expressing cells. Melanoma cells preferentially upregulate I-CAM-1 and once infected, cellular death ensues by direct cytolysis [47]. A Phase II study is ongoing; preliminary results have shown activity in injected and non-injected lesions [48]. Rose Bengal disodium (PV-10) is a xanthine dye that activates T cells through an unclear mechanism [49]. A Phase II study of 80 patients demonstrated an objective response rate of 51% with 26% experiencing a complete response. In a subset of 28 patients who had all lesions injected, 71% had objective responses [50].
Conclusion
Over the past few years, several new therapies have been developed and approved for advanced melanoma. The exact role for T-VEC in this environment is still being elucidated. T-VEC is active as a single agent and is particularly effective in advanced stage III or stage IV M1a melanoma. This indicates that it may ultimately play an important role in the multimodality management of these patients, particularly for control of skin, soft tissue, or lymph node metastases that are not amenable or responsive to surgical resection or regional chemotherapy. It may also have a role in pre-surgical or neo-adjuvant therapy of patients with locally advanced disease, although this has not been studied thus far.
A more intriguing, and broadly applicable, hypothesis is that T-VEC may augment the antitumor response to other systemic therapeutic agents. The most likely role will be as a combination partner with ipilimumab or an anti-PD-1 agent. Two randomized clinical trials are ongoing to elucidate the safety and efficacy of this strategy. A Phase II study of ipilimumab with or without T-VEC has accrued rapidly, and results are expected soon (NCT01740297). This combination is promising, with an impressive 56% response rate in Phase I results. These results come with the caveat that only 18 patients were assessed and only five had stage IV M1c disease. A Phase I study of pembrolizumab and T-Vec has been initiated, and a randomized study comparing pembrolizumab + T-VEC versus pembrolizumab alone is planned (NCT02263508). In addition, a clinical trial investigating the neoadjuvant use of T-VEC in resectable melanoma will provide important insight into immunologic microenvironment changes caused by T-VEC (NCT02211131).
Future perspective
T-VEC is a genetically modified GM-CSF-secreting oncolytic HSV-1 virus that has been developed as an intralesional immunotherapy for melanoma. The drug has substantial activity as a single agent in patients with skin and soft tissue metastases, with durable complete response rates of 16% reported in all patients, and up to 33% in patients with stage III disease in a randomized clinical trial. The clinical utility of single agent T-VEC in multimodality management of advanced melanoma is unclear, but it may ultimately play an important role in local–regional control of skin and soft tissue metastases. The potential benefit of combinations of T-VEC and other immunotherapies or targeted therapies is significant, and is being actively investigated. If these trials show significant improvement in outcome relative to single agent therapy, T-VEC may become an important component of the management of many patients with metastatic melanoma.
Executive summary.
Mechanisms of action
Talimogene laherparepvec (T-VEC) is an oncolytic, engineered herpes virus with specific modifications that confer its anticancer properties.
Removal of the neurovirulence factor (ICP34.5) prevents neurotoxicity, replacement with granulocyte-macrophage colony-stimulating factor (GM-CSF) promotes dendritic cell function, and deletion of ICP47 region promotes oncolysis and viral replication.
Pharmacokinetic properties
T-VEC is administered intradermally, limiting traditional pharmacokinetic measurements.
T-VEC viral DNA was detected in 28.3 and 20.4% of blood and urine samples tested, mostly obtained within 24 h of injection.
GM-CSF expression was detected in 11 of 13 fine-needle aspirates of tumor samples but was not detected in serum samples.
Clinical efficacy
T-VEC was compared with subcutaneous GM-CSF in a randomized Phase III study (OpTIM).
Durable response rate lasting ≥6 months (16.2 vs 2.1%, p < 0.001) was superior in the T-VEC arm. A trend toward improved median overall survival was also noted (23.3 months vs 18.9 months, p = 0.051).
T-VEC combined with ipilimumab (anti-CTLA4) is a promising combination, with a 56% response rate.
Safety & tolerability
Adverse events are largely due to an inflammatory response in the acute setting, and include pyrexia (52%), chills (48%), fatigue (32%) and nausea (30%) and localized pain (24%).
Toxicities are largely grades 1–2 with no individual grade 3 toxicities occurring in >3% of patients.
Dosage & administration
T-VEC is injected directly into cutaneous and subcutaneous lesions.
Phase II/III study doses were up to 4 ml at 106 pfu/ml for the first dose, then up to 4 ml at 108 pfu/ml every 2 weeks.
0.5 ml was injected into tumors 0.5–1.5 cm; 1 ml into tumors 1.5–2.5 cm; 2 ml into tumors >2.5 cm.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by NIH K12 CA 0906525 (DB Johnson). Amgen – Consultant – Review Board – MC Kelley. In addition to the peer-review process, with the authors consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made at the discretion of the authors and based on scientific or editorial merit only. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of Phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future Phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Read RL, Haydu L, Saw RP, et al. In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol. 2015;22:475–481. doi: 10.1245/s10434-014-4100-0. [DOI] [PubMed] [Google Scholar]
- 5.Vrouenraets BC, Hart GA, Eggermont AM, et al. Relation between limb toxicity and treatment outcomes after isolated limb perfusion for recurrent melanoma. J Am Coll Surg. 1999;188:522–530. doi: 10.1016/s1072-7515(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JF, Kam PC, Waugh RC, et al. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14:238–247. doi: 10.1002/(sici)1098-2388(199804/05)14:3<238::aid-ssu8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Campana LG, Testori A, Mozzillo N, et al. Treatment of metastatic melanoma with electrochemotherapy. J Surg Oncol. 2014;109:301–307. doi: 10.1002/jso.23512. [DOI] [PubMed] [Google Scholar]
- 8.Micali G, Lacarrubba F, Nasca MR, et al. Topical pharmacotherapy for skin cancer: part II. Clinical applications. J Am Acad Dermatol. 2014;70:979, e1–12. doi: 10.1016/j.jaad.2013.12.037. quiz 9912. [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2014;372(1):30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182–3190. doi: 10.1200/JCO.2012.47.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (Anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morton DL, Eilber FR, Holmes EC, et al. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg. 1974;180:635–643. doi: 10.1097/00000658-197410000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agarwala SS, Neuberg D, Park Y, et al. Mature results of a Phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I–III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer. 2004;100:1692–1698. doi: 10.1002/cncr.20166. [DOI] [PubMed] [Google Scholar]
- 17.Si Z, Hersey P, Coates AS. Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Res. 1996;6:247–255. doi: 10.1097/00008390-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 18.von Wussow P, Block B, Hartmann F, et al. Intralesional interferon-alpha therapy in advanced malignant melanoma. Cancer. 1988;61:1071–1074. doi: 10.1002/1097-0142(19880315)61:6<1071::aid-cncr2820610603>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 19.Guo ZS, Thorne SH, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. An excellent review of oncolytic viral therapy for cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Pol J, Bloy N, Obrist F, et al. Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology. 2014;3:e28694. doi: 10.4161/onci.28694. This is a summary of current and previous trials of oncolytic viruses for cancer therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KH, Dmitriev IP, Saddekni S, et al. A Phase I clinical trial of Ad5/3-Delta24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol Oncol. 2013;130:518–524. doi: 10.1016/j.ygyno.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanerva A, Nokisalmi P, Diaconu I, et al. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res. 2013;19:2734–2744. doi: 10.1158/1078-0432.CCR-12-2546. [DOI] [PubMed] [Google Scholar]
- 25.Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a Phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 26.Liikanen I, Ahtiainen L, Hirvinen ML, et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212–1223. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bramante S, Koski A, Kipar A, et al. Serotype chimeric oncolytic adenovirus coding for GM-CSF for treatment of sarcoma in rodents and humans. Int J Cancer. 2014;135:720–730. doi: 10.1002/ijc.28696. [DOI] [PubMed] [Google Scholar]
- 28•.Liu BL, Robinson M, Han ZQ, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. Preclinical validation and discovery of talimogene laherparepvec system. [DOI] [PubMed] [Google Scholar]
- 29.Mach N, Gillessen S, Wilson SB, et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 30.Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hercus TR, Thomas D, Guthridge MA, et al. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114:1289–1298. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeller C, Jansen B, Heere-Ress E, et al. Perilesional injection of r-GM-CSF in patients with cutaneous melanoma metastases. J Invest Dermatol. 2001;117:371–374. doi: 10.1046/j.0022-202x.2001.01427.x. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman HL, Ruby CE, Hughes T, et al. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer. 2014;2:11. doi: 10.1186/2051-1426-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toda M, Martuza RL, Rabkin SD. Tumor growth inhibition by intratumoral inoculation of defective herpes simplex virus vectors expressing granulocyte-macrophage colony-stimulating factor. Mol Ther. 2000;2:324–329. doi: 10.1006/mthe.2000.0130. [DOI] [PubMed] [Google Scholar]
- 36.Hersey P, Gallagher S. Intralesional immunotherapy for melanoma. J Surg Oncol. 2014;109:320–326. doi: 10.1002/jso.23494. [DOI] [PubMed] [Google Scholar]
- 37.Kaufman HL, Andtbacka RHI, Collichio FA, Amatruda T, Senzer NN, et al. Primary overall survival (OS) from OPTiM, a randomized Phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol. 2014;32:9008_abstr. [Google Scholar]
- 38•.Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. Preclinical support for combining oncolytic virotherapy with immune checkpoint blockade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Senzer NN, Kaufman HL, Amatruda T, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. Phase II clinical trial evaluatingtalimogene laherperepvec. [DOI] [PubMed] [Google Scholar]
- 40.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 41.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 42.Tarhini AA, Butterfield LH, Shuai Y, et al. Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA4 antibody and interferon-alpha or TLR-9 agonist and GM-CSF with peptide vaccination. J Immunother. 2012;35:702–710. doi: 10.1097/CJI.0b013e318272569b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engeland CE, Grossardt C, Veinalde R, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther. 2014;22:1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puzanov I, Milhem MM, Andtbacka RH, Minor DR, Hamid O, et al. Primary analysis of a Phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin Oncol. 2014;32:9029_abstr. [Google Scholar]
- 45.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 46.Bedikian AY, Richards J, Kharkevitch D, et al. A Phase 2 study of high-dose Allovectin-7 in patients with advanced metastatic melanoma. Melanoma Res. 2010;20:218–226. doi: 10.1097/CMR.0b013e3283390711. [DOI] [PubMed] [Google Scholar]
- 47.Shafren DR, Au GG, Nguyen T, et al. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus a21. Clin Cancer Res. 2004;10:53–60. doi: 10.1158/1078-0432.ccr-0690-3. [DOI] [PubMed] [Google Scholar]
- 48.Andtbacka RH, Shafren DR, Grose M, Post L, Weisberg J. Abstract 2939: CAVATAK-mediated oncolytic immunotherapy in advanced melanoma patients. Cancer Res. 2014;74:2939. [Google Scholar]
- 49.Toomey P, Kodumudi K, Weber A, et al. Intralesional injection of rose bengal induces a systemic tumor-specific immune response in murine models of melanoma and breast cancer. PLoS ONE. 2013;8:e68561. doi: 10.1371/journal.pone.0068561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwala SS, Thompson JF, Smithers BM, Ross MI, Scoggins CR, et al. Efficacy of intralesional Rose Bengal in patients receiving injection of all existing melanoma in Phase II study PV-10-MM-02. J Clin Oncol. 2014;32:9027. [Google Scholar]