Abstract
Background
Tissue kallikrein is a serine proteinase that generates the vasoactive kinin peptide, which produces vasodilatory, angiogenic and anti-apoptotic effects. In this study, we investigated the effect of a stable supply of kallikrein/kinin on ventricular remodeling and blood vessel growth in rats after myocardial infarction (MI).
Methods and Results
At 1 week after coronary artery ligation, tissue kallikrein or kinin was infused via minipump for 4 weeks. At 5 weeks after MI, kallikrein and kinin infusion significantly improved cardiac contractility and reduced diastolic dysfunction without affecting systolic blood pressure. Kallikrein and kinin significantly increased capillary density in the non-infarcted region. Kallikrein and kinin also reduced heart weight/body weight ratio, cardiomyocyte size, and ANP and BNP expression in the non-infarcted area. Moreover, kallikrein and kinin infusion inhibited interstitial collagen deposition, collagen fraction volume, and collagen I and collagen III mRNA levels, TGF-β1 and PAI-1 expression and Smad2 phosphorylation. The effects of kallikrein and kinin on cardiac remodeling were associated with increased NO levels and reduced NADPH oxidase expression and activity, superoxide formation and malondialdehyde levels. Furthermore, in cultured cardiac fibroblasts, kinin inhibited Ang II-stimulated TGF-β1 production, and the effect was blocked by icatibant.
Conclusion
These results indicate that a sub-depressor dose of kallikrein or kinin can restore impaired cardiac function in rats post-infarction heart failure by inhibiting hypertrophy and fibrosis and promoting angiogenesis through increased NO formation and suppression of oxidative stress and TGF- β1 expression.
Keywords: kallikrein, kinin, cardiac remodeling, fibrosis, hypertrophy, angiogenesis, TGF-β1, PAI-1, oxidative stress
Introduction
Post myocardial infarction (MI) remodeling including left ventricular (LV) hypertrophy, fibrosis and endothelial dysfunction, may contribute to cardiac dysfunction and lead to heart failure. Mediators such as oxidative stress, angiotensin II (Ang II), transforming growth factor (TGF-β1) and plasminogen activator inhibitor-1 (PAI-1) are the main stimulators of cardiac hypertrophy, extracellular matrix deposition and loss of blood vessels after MI.1,2 Evidence from both animal and human studies showed that increased oxidative stress plays an important role in the pathogenesis of heart failure after MI.3 Previous studies showed that Ang II-induced cardiac remodeling was mediated by reactive oxygen species (ROS) formation and suppression of antioxidant defenses, suggesting that Ang II-ROS signaling may act as a potent stimulator of cardiomyocyte hypertrophy.4 Furthermore, increased oxidative stress along with activation of TGF- β1 may lead to endothelial dysfunction due to decreased bioavailability of endothelium-derived nitric oxide (NO).5 In addition, overexpression of TGF-β1 in transgenic mice has been shown to result in interstitial fibrosis and hypertrophy of cardiomyocytes.6 These findings suggest that oxidative stress and TGF-β1 may underlie the development and progression of heart failure.
Tissue kallikrein is a serine proteinase that specifically processes low molecular weight kininogen to produce the potent vasoactive kinin peptides bradykinin (BK) and Lys-BK (kallidin).7 Intact kinins bind to the kinin B2 receptor, and kinin metabolites, such as des-Arg9-BK and des-Arg10-kallidin, bind to the B1 receptor. Activation of kinin receptors, with subsequent stimulation of nitric oxide (NO)-cGMP and prostacyclin-cAMP pathways, modulates a broad spectrum of biological functions. Kinin B2 receptor knockout mice exhibit a gradual development of dilated cardiomyopathy, in association with peri-vascular and reparative fibrosis.8 In contrast, transgenic rats overexpressing human tissue kallikrein are more resistant to isoproterenol-induced cardiac hypertrophy and fibrosis compared to normal rats, but these effects are abolished by icatibant, a kinin B2 receptor antagonist.9 Using somatic gene transfer approaches, we have shown that kallikrein gene delivery reduces cardiac hypertrophy and fibrosis in pressure-induced hypertensive rats and in rats after myocardial infarction.10, 11 We recently reported that tissue kallikrein infusion through kinin B2 receptor activation protected against inflammatory cell accumulation in the heart associated with reduced NF-κB activation and chemokine and adhesion molecule expression at 1 week after myocardial infarction.12 In addition, we have shown that kinin infusion reduced salt-induced inflammatory cell recruitment in the kidney without affecting blood pressure in hypertensive Dahl-salt-sensitive rats.13 In the present study, we examined the potential therapeutic effects of a stable supply of kallikrein and kinin protein infusion at a sub-depressor dose on ventricular remodeling and neovascularization in rats after MI. We found that kallikrein and kinin can prevent cardiac dysfunction in the failing myocardium by promoting blood vessel growth and inhibiting hypertrophy and fibrosis through increased NO formation and suppression of oxidative stress and TGF-β1 expression.
Methods
Animals and Treatments
Wistar rats (male, 220 to 250 g body weight, Harlan) were employed in this study. The study complied with the Guides for the Care and Use of Laboratory Animals (Institute of Laboratory Resources, National Academy of Sciences, Bethesda, MD). Myocardial infarction was established by ligation of the left coronary artery as previously described.14 Briefly, a thoracotomy was performed via the fourth intercostal space, the heart was exposed and ECG was then monitored. A 6-0 polypropylene suture (Ethicon) was passed loosely around the left anterior descending (LAD) coronary artery near its origin. Once hemodynamics was stabilized, LAD coronary artery occlusion was performed by irreversible tightening of the suture loop. Acute myocardial ischemia was deemed successful on the basis of regional cyanosis of the myocardial surface distal to the suture, accompanied by elevation of the ST segment on ECG. After one week, rats with MI were randomly divided into three groups, and received infusion of saline (n=7), purified tissue kallikrein (1μg/h) (n=8) or bradykinin (0.5 μg/h, Sigma) (n=6) by osmotic minipumps (ALZET) implanted subcutaneously in the back.
Hemodynamic Parameters
At 5 weeks after infarction, cardiac function and physiological parameters were measured by carotid cannulation11 using a 2.5 French micro-manometer (Millar Instrument, Houston, TX) by advancement into the left ventricle. Heart rate (HR), mean arterial pressure (MAP), left ventricular end diastolic pressure (LVEDP), cardiac contractility (dP/dt maximum and dP/dt minimum) were recorded and analyzed by a model 7E polygraph (BIOPAC).
Histological Analysis
At the end of the procedure, cardiac tissues were fixed in 4% paraformaldehyde and embedded. The left ventricle was cut into 3 transversal slices on basal, middle and apex levels. Four-micron (4 μm) sections were obtained for morphological analyses. Masson’s Trichrome staining was performed to determine the percentage of scar length to total LV circumference. Cardiomyocyte size was determined by Gordon and Sweet silver staining. One hundred cardiomyocytes were chosen randomly per slide and traced with the use of NIH Image software (version 1.61). Myocardial fibrosis was observed by Sirius red staining and analyzed by Adobe PhotoShop software. Collagen fraction volume was calculated as the percentage of staining to total LV tissue.
Immunohistochemical analyses were performed to visualize capillaries and collagen deposition in non-infarcted myocardium using an immunostaining kit according to the manufacturer’s instructions (Universal Elite ABC, Vector). Primary antibodies against CD-31, factor VIII (endothelial cell antigen, Santa Cruz, 1:200 dilution), collagen I, collagen III (Sigma,1:200) were used. To determine capillary density, the number of positive staining was counted in a double blind fashion from 20 different fields of each section (n=6 or 7). Actual neovascularization was derived from an increased capillary-to-myocyte ratio, which was calculated as capillary density divided by myocyte density.
Western Blot Analysis
Left ventricular tissue in the non-infarct region (0.2 grams) was homogenized (Polytron, Brinkmann Instruments) in l ml protein lysis buffer and centrifuged at 12 000 × g for 30 min at 4°C. The supernatant (the cytosolic fraction) was removed and protein concentrations were measured by a protein assay kit (Bio-Rad). Western blot analysis was performed using cytosolic fraction to detect the total and/or phosphorylated forms of Smad2 (Cell Signaling), TGF-β1 and PAI-1 (Santa Cruz). GAPDH (Advanced Immunochemical) was employed as internal control. Membranes were incubated with secondary antibody conjugated to LumiGLO chemiluminescent reagent. Chemiluminescence was detected using an ECL-Plus kit (Perkin Elmer Life Science) and visualized by Kodak X-ray film. The bands were quantified by densitometry.
Nitrite/Nitrate (NOx), NADPH Oxidase Activity and Superoxide Assays
Nitrite/nitrate levels in the non-infarct region of cardiac extracts were measured by a fluorometric assay as previously described.15 NADPH oxidase activities were measured by chemiluminescent detection of superoxide using a luminometer (Turner Designs). Superoxide was measured by a spectrophotometric assay based on rapid reduction of ferricytochrome c to ferrocytochrome c. Non-superoxide-dependent reduction of cytochrome c was corrected for by deducting the activity not inhibited by superoxide dismutase.
Measurement of Malondialdehyde Levels
Lipid peroxidation was determined as an indicator of oxidative stress by measurement of malondialdehyde (MDA) levels. Cytosolic protein (500 μg) was mixed with 2% butylated hydroxytoluene and quintanilla reagent and boiled for 15 min. The reaction mixture was centrifuged at 3000 × g for 10 min. The soluble phase was measured with a spectrophotometer at 535 nm using MDA standards (0 to 30 μmol/mL) (Sigma).
Quantitative Real-time PCR (qRT-PCR)
Total RNA was extracted from myocardium using Trizol reagent (Invitrogen). cDNA was transcribed using a cDNA Archive Kit (Applied Biosystems). The qRT-PCR reaction was carried out using the Gene Expression Assay Rn005616661_ml for ANP, Rn00580641_ml for BNP, Rn00561717_ml for PAI-1, Rn01475962_ml for TGF-β1, Rn00801649_gl for collagen I, and Rn01437650_gl for collagen III (Applied Biosystems) running on a 7300 real time PCR system (Applied Biosystems). Transcription of the housekeeping gene GAPDH was determined by specific primer/probe mix (Applied Biosystems). The final quantification was determined by Relative Quantification software (Applied Biosystems).
Cell Culture
Cardiac ventricular fibroblasts were obtained from hearts of adult male Sprague-Dawley rats by using a modification of published methods.16 The purity of cardiac fibroblasts was estimated to be >95% as identified by positive immunostaining with vimentin and negative staining with desmin (myocytes), α-smooth muscle actin (vascular smooth muscle cells), and von Willebrand factor (endothelial cells). The cells were then seeded in six-well plates (1 × 105 cells/well) and incubated overnight in growth medium. After 48 h serum free medium starvation, kinin and/or Ang II (Sigma) was added to the medium at a final concentration of 1 μM, 0.1μM. For blocking kinin B2 receptor, icatibant (1.0 μM) was added to the medium 30 min before the treatment. After 24 h of incubation, TGF-β levels the cultured medium were measured by a quantitative sandwich enzyme immunoassay technique, using commercial kits (R&D Systems).
Statistical Analysis
Data were expressed as mean ± SEM and were compared between experimental groups with the use of ANOVA followed by Fisher’s PLSD. Probability values of P<0.05 were considered statistically significant.
Results
Kallikrein and Kinin Infusion Improves Cardiac Function at 5 Weeks after MI
Effects of kallikrein and kinin on hemodynamic parameters were measured at 5 weeks after MI (Table 1). Initiation of kallikrein and kinin infusion at 1 week after permanent coronary artery ligation had no apparent effect on myocardial infarct size at 5 week after MI. Mean arterial pressure (MAP) and heart rate were markedly reduced after MI, but was partially restored by kallikrein and kinin treatment. Moreover, kallikrein and kinin infusion had no significant effect on systolic blood pressure measured by the indirect tail cuff method at 2 to 5 weeks after MI (data not shown). Characteristic impairments in contractility (dP/dt max) and diastolic function (LVEDP, dP/dt min) after MI were significantly improved after kallikrein or kinin infusion. Heart weight/body weight and lung weight/body weight ratios were increased 5 weeks after MI, but were reduced by kallikrein and kinin infusion. Similarly, the LV long axis, a measure of LV enlargement, was also significantly reduced in rats receiving kallikrein or kinin infusion. Successful kallikrein infusion was determined by observing elevated circulating tissue kallikrein levels at 1 and 5 weeks after MI (data not shown). In addition, we observed a marked increase of kinin B1 and B2 receptor mRNA levels (5 and 7 fold, respectively) in the non-infarct region of the myocardium at 5 weeks after MI (data not shown).
Table 1.
Effect of Kallikrein and Kinin Infusion on Heart Function and Physiological Parameters
| Sham (n=6) |
MI (n=7) |
MI/TK (n=8) |
MI/Kinin (n=6) |
|
|---|---|---|---|---|
| Infarct area (% of LV) | - | 28.2±2.8 | 27 ±3.4 | 27.2±2.4 |
| HR, bpm | 348.1 ± 7.3 | 308 ± 11.7* | 355.4 ± 11.7 | 340.8 ± 11.3 |
| MAP (mmHg) | 112.1 ± 4.9 | 66.9 ± 4.1* | 81.2 ± 4.7 | 79.4 ± 3.9 |
| LVEDP (mmHg) | 1.9 ± 0.6 | 12.4 ± 2.1* | 7.5 ± 1.4 | 8.1± 2.0 |
| dP/dt max (mmHg/s) | 3437.6 ± 119.4 | 1998.5 ± 154.5* | 2492.2 ± 164 | 2241.3 ± 110.7 |
| dP/dt min (mmHg/s) | 2850.8 ± 94.2 | 1405±126* | 1857.8 ± 135.3 | 1680±109 |
| LW/BW (mg/g) | 4.0 ±1.8 | 8.16 ±3.7* | 4.9 ±1.2 | 5.0 ±1.5 |
| LV Axis Length (mm) | 11.8 ±1.5 | 15.2 ±1.4* | 13.2 ± 0.8 | 13.2 ±1.4 |
Value are expressed to mean ±SEM
P<0.05 vs. sham &MI/TK. MI/Kinin;
P<0.05 vs. sham & MI/TK
HW:heart weight; BW:body weight ; LW:lung weight; LV Axis Length:length from apex to aortic valve;
HR : heart rate; bpm : beats per minute; MAP: mean arterial pressure; LVEDP: left ventricula end-diastolic pressure; dP/dt max : maximum first derivative of pressure; dP/dt min: minimun first derivative of pressure.
Kallikrein and Kinin Increases Capillary Density
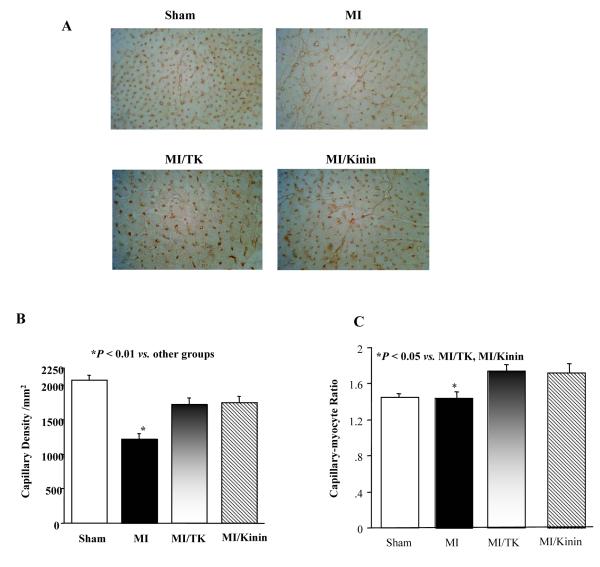
Representative photomicrograph images show that kallikrein and kinin infusion increased capillary number in the non-infarct area and arteriole number in peri-infarct area at 5 weeks after MI as identified by immunostaining with antibodies to CD-31 (Fig. 1A). Quantitative analysis indicated that the capillary density was significantly reduced in the MI group compared to the sham group, and kallikrein and kinin prevented this decrease (Fig. 1B). Furthermore, a 21% and 19% increase in capillary-to-myocyte ratio was observed in the kallikrein and kinin groups, respectively (Fig. 1C). These results indicate that kallikrein and kinin are capable of promoting capillary growth after MI.
Fig. 1.
Kallikrein and kinin promote neovascularization 5 weeks after MI. (A) Representative micrograph images of heart sections stained with antibodies against CD31 (400× magnification). (B) Capillary density in number of capillaries per mm2. (C) Quantification of capillary-to-myocyte ratio. Data are expressed as mean ± SEM (n=6-8).
Kallikrein and Kinin Inhibits Cardiac Hypertrophy and Collagen Accumulation
Representative micrograph images of cardiomyocyte surface stained with Gordon & Sweet silver and quantitative analysis showed that kallikrein and kinin infusion reduced cardiomyocyte size as compared to the MI control in the non-infarct area (Fig. 2A & B). In addition, kallikrein and kinin significantly reduced MI-induced ANP and BNP expression (Fig. 2C & D). These results indicate that both kallikrein and kinin significantly inhibited MI-induced cardiac hypertrophy.
Fig. 2.
Effect of kallikrein and kinin on cardiac hypertrophy in the non-infarcted region. (A) Representative histological left ventricular sections stained with Gordon and Sweet’s silver (400× magnification). (B) Quantification of cardiomyocyte area. Real-time PCR analysis of (C) ANP and (D) BNP mRNA levels. Data are expressed as mean ± SEM (n=6-8).
Sirius red staining showed that cardiac collagen accumulation increased markedly after MI and the effect was markedly reduced by kallikrein and kinin treatment (Fig. 3A). Collagen I and collagen III immunohistochemical staining further verified the collagen deposition. Kallikrein and kinin treatment reduced MI-induced collagen I and collagen III accretion in the non-infarcted area (Fig. 3A). Quantitative analysis showed that kallikrein and kinin significantly reduced collagen fraction volume after MI (Fig. 3B). Increased collagen type I and type III mRNA levels after MI, as measured by real-time PCR, were also significantly reduced by kallikrein and kinin (Fig. 3 C & D).
Fig. 3.
Effect of kallikrein and kinin on cardiac fibrosis in the non-infarcted region. (A) Sirius Red staining and immunohistochemical staining of collagens I and III (200× magnification). (B) Collagen fraction volume was quantified from Sirius Red staining using Adobe Photoshop. Real-time PCR analysis of (C) collagen I and (D) collagen III mRNA levels. Data are expressed as mean ± SEM (n=6-8).
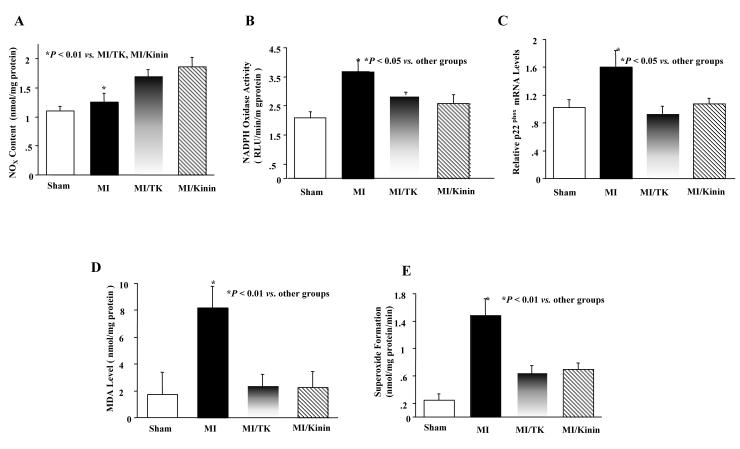
Kallikrein and Kinin Increases Cardiac NOx Levels and Reduces Oxidative Stress
Kallikrein and kinin infusion significantly increased cardiac NOx levels compared to the MI group (Fig.4A). NADPH oxidase activities and malondialdehyde-bis (MDA) levels were measured in the no-infarct myocardium to evaluate oxidative stress after MI. As shown in Fig. 4B, NADPH oxidase activities were significantly increased after MI compared to sham, and kallikrein and kinin blocked the increase. Similarly, MI induced p22phox (a subunit of NADPH oxidase) mRNA levels compared to the sham, whereas kallikrein and kinin reduced p22phox expression (Fig. 4C). Moreover, kallikrein and kinin completely prevented the increase in cardiac MDA levels induced by MI damage (Fig. 4D). In addition, MI significantly induced superoxide formation compared to the sham group, but was suppressed significantly in the animals receiving kallikrein or kinin infusion (Fig. 4E).
Fig. 4.
Effect of kallikrein and kinin on (A) cardiac NO levels, (B) NADPH oxidase activity, (C) p22phox mRNA levels, (D) MDA in the non-infarct myocardium and (E) superoxide formation. Data are expressed as mean ± SEM (n=6-7).
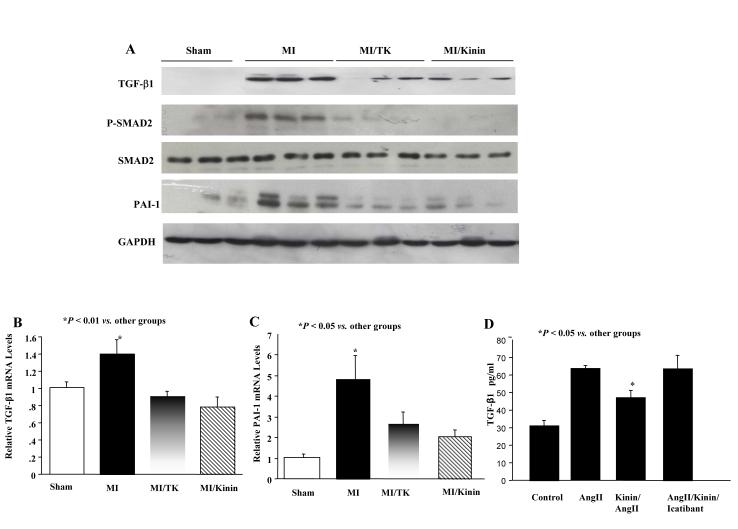
Kallikrein/kinin reduces TGF-β1, PAI-1 and Smad-2 levels. Western blot analysis showed that MI induced elevated TGF-β1 and plasminogen activator inhibitor (PAI-1) levels of these pro-fibrotic mediators (Fig. 5A). Kallikrein and kinin also decreased TGF-β1 and PAI-1 mRNA levels, as determined by real-time PCR (Fig. 5B & C).
Fig. 5.
Effect of kallikrein and kinin on the expression of pro-fibrotic molecules. (A) Representative Western blots of myocardial TGF-β1, PAI-1 and Smad2 levels. Real-time PCR analysis of (B) TGF-β1 and (C) PAI-1 mRNA levels. Data are expressed as mean ± SEM (n=6). (D) Effect of AngII and kinin on TGF-β1 levels in adult rat cardiac fibroblasts. Data are expressed as mean ± SEM (n=3). The data are representative of 3 independent experiments.
Kinin Decreases TGF-β Levels in Stimulated Adult Cardiac Fibroblasts
In cultured rat cardiac fibroblasts, TGF-β levels were elevated by Ang II treatment, and the increase was significantly reduced by kinin administration (Fig. 5D). Co-treatment with the kinin B2 receptor antagonist icatibant significantly blocked kinin’s effect.
Discussion
This study demonstrates that kallikrein and kinin protein infusion can rescue cardiac dysfunction after chronic myocardial infarction by inhibiting hypertrophy and fibrosis and promoting angiogenesis. At 5 weeks after MI, kallikrein or kinin infusion had no effect on myocardial infarct size. A constant supply of kallikrein and kinin improved cardiac remodeling in the non-infarct area after MI through inhibition of extracellular matrix accumulation and cardiomyocyte hypertrophy independent of its blood pressure-lowering effect. Kallikrein and kinin also improved neovascularization and limited ventricular remodeling through suppression of oxidative stress, TGF-β1 and PAI-1 expression as well as increased NO formation and VEGF expression.
It is well recognized that kinins exert their effects via kinin B2 and B1 receptors. Consistent with other studies, we showed that both B2 and B1 receptors are up-regulated in the non-infarcted hearts after MI. In our previous reports, we have demonstrated that cardiac protective effects of tissue kallikrein can be blocked by the kinin B2R antagonist, indicating an effect mediated by the kinin B2 receptor.14 On the other hand, the role of kinin B1 receptor in cardiac function is controversial. A detrimental effect of the B1 receptor in myocardial ischemia was observed using both pharmacological blockade and gene knockout mice.17 However, a recent study indicated that kinin B1 receptor knockout mice have a larger left ventricular diastolic chamber dimension after myocardial infarction, and blockade of kinin B2 receptors worsened this condition, suggesting that the kinin B1 receptor may serve a protective role in cardiac dysfunction.18 Our recent studies showed that myocardial hypertrophy induced by aortic occlusion is worsened in B2R knockout mice, but not in B1R knockout mice.19 These combined results indicate a protective role of the kinin B2 receptor against cardiac injury.
It has been established that increased NADH/NADPH oxidase activity and superoxide formation play a critical role in functional and structural damage of cardiac myocytes after myocardial ischemia. In contrast, NO has been shown to act as a potent antioxidant by abolishing mitochondrial oxidant damage in adult rat cardiomyocytes.20 Moreover, NO is capable of inhibiting neutrophil superoxide anion production via a direct action on the membrane components of the NADPH oxidase and the assembly of NADPH oxidase subunits.21 NO has also been shown to attenuate cardiac remodeling and oxidative stress after myocardial infarction.22 Our present study showed that kallikrein and kinin treatment protects against cardiac dysfunction by increased NO levels in conjunction with reduced NADPH oxidase activity and expression, superoxide anion formation and MDA levels. In this study, co-infusion of kallikrein with icatibant was not performed, as our recent study showd that the cardiac protective effect of tissue kallikrein by inhibiting apoptosis and inflammation after kallikrein infusion was blocked by icatibant.12 Taken together, these results suggest that kallikrein and kinin, through NO formation, limits cardiac remodeling via suppression of oxidative stress-mediated signaling pathways.
Increased capillary density is especially important for cardiac repair after MI due to the increase demand of oxygen and nutrition. A previous study showed that reduced capillary density in the non-infarcted area occurs after MI and a kinin B2 receptor antagonist abolished the protective effect of ACE inhibition, indicating a role of kinin in promoting capillary growth.23 ACE inhibition was first shown to improve survival after MI in a rat model 24 and was further shown to have beneficial effects in reduction of morbidity and mortality rates and improvement in the quality of life in patients with MI and chronic heart failure.25 The beneficial effect of ACE inhibition can be partially blocked by icaticant, a kinin B2 receptor antagonist, suggesting a role of kinin in cardioprotection exerted by ACE inhibition.26 In this study, we further demonstrate that a stable supply of tissue kallikrein/kinin has direct cardioprotective effects in hemodynamics and cardiac remodeling post-ischemic heart failure. Kinin has also been shown to stimulate capillary tube formation by transactivation of the VEGF receptor through NO formation in cultured endothelial cells.27 This is consistent with the finding that kinin can promote NO release and proliferation in cultured endothelial cells.28 Furthermore, a recent study reported that downregulation of cardiac PAI-1 activity augments neovascularization and improves functional recovery.29 In the current study, kallikrein and kinin infusion increased capillary density in the ischemic myocardium in association with elevated cardiac NO content and reduced PAI-1 levels. These combined results indicate that the angiogenic effect of kallikrein/kinin may be due to increased NO levels and decreased PAI-1 expression.
Cardiac hypertrophy after MI is accompanied by the expression of a “fetal” gene program, including the up-regulation of genes such as atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP).30 We showed that infusion of tissue kallikrein/kinin was able to block hypertrophy in rats with MI, as evidenced by reduction of the ratio of left ventricle weights and heart weights, cardiomyocyte size and the expression of ANP and BNP. In adult rat cardiomyocytes co-cultured with bovine aortic endothelial cells, kinin was shown to prevent Ang II-induced hypertrophy by increasing NO levels.31 Therefore, the beneficial effect of kallikrein/kinin on MI-induced cardiac fibrosis and hypertrophy may be contributed to increased nitric oxide bioavailability.
In our study, we showed that kallikrein/kinin infusion inhibited MI-induced oxidative stress, the expression of TGF-β1 and PAI-1, and deposition of collagen types I and III in the myocardial interstitium. Oxidative stress initiates a series of signaling cascades leading to cardiac fibroblast proliferation and progressive accumulation of ECM proteins via activation of transcription and production of TGF-β1.32 TGF-β1 plays a crucial role in promoting hypertrophy and fibrosis in several cardiac diseases. The elevation of TGF-β1 could directly stimulate fibroblast proliferation and ECM protein accumulation, resulting in interstitial fibrosis. TGF-β1 acts as an important profibrotic cytokine by signaling through phosphorylation (activation) of Smad2 and Smad3, which translocate to the nucleus to modulate cell synthesis of most matrix proteins.33 In cultured cardiac fibroblasts, kinin has been observed to down-regulate ECM protein production via NO-related signaling molecule cGMP.34 ECM degradation may also be due to kinin’s ability to stimulate plasminogen activator release from endothelial cells.35 Moreover, kinin has been shown to decrease TGF-β1-induced PAI-1 expression in cultured proximal tubular cells, thereby facilitating matrix degradation and inducing interstitial fibrosis.36 Our present study also shows that kinin inhibits Ang II-induced TGF-β1 production in the cardiac fibroblasts and the effect is blocked by icatibant. Therefore, these combined studies indicate that kallikrein/kinin through kinin B2 receptor activation inhibits MI-induced cardiac fibrosis by suppression of oxidative stress and TGF-β1-mediated signaling mechanisms.
Our study shows that a stable supply of purified tissue kallikrein or kinin infusion can improve cardiac remodeling after chronic myocardial infarction. The infusion rates were based on the results of previous published and unpublished studies. For example, infusion of kinin at a rate of 500 ng/h caused a more pronounced protective effect against salt-induced renal injury in rats than kinin at 100 ng/h.13 In addition, tissue kallikrein was delivered at 1 μg/h based on the dose used in an ischemic stroke model. Protein infusion has several advantages over adenovirus-mediated delivery. For example, protein infusion provides a stable supply of the therapeutic product and can be terminated at any time during the experiment, whereas the expression of recombinant gene product after adenovirus-mediated gene delivery is transient with highest levels around 5 days. In addition, local injection of adenovirus can possibly produce an inflammatory response and is therefore no longer effective after a second injection. In conclusion, the present study provides important new insights that a sub-depressor dose of kallikrein/kinin can rescue failing myocardium by promoting angiogenesis and inhibiting hypertrophy and fibrosis through increased NO formation, and suppression of oxidative stress and TGF-β1 expression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paul M, Stock P, Langheinrich M, Liefeldt L, Schonfelder G, Bohm M. Role of the cardiac renin-angiotensin system in human heart failure. Adv Exp Med Biol. 1995;377:279–83. doi: 10.1007/978-1-4899-0952-7_17. [DOI] [PubMed] [Google Scholar]
- 2.Takeshita K, Hayashi M, Iino S, Kondo T, Inden Y, Iwase M, et al. Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol. 2004;164:449–56. doi: 10.1016/S0002-9440(10)63135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. 1997;96:2414–20. doi: 10.1161/01.cir.96.7.2414. [DOI] [PubMed] [Google Scholar]
- 4.Lu L, Quinn MT, Sun Y. Oxidative stress in the infarcted heart: role of de novo angiotensin II production. Biochem Biophys Res Commun. 2004;17:943–51. doi: 10.1016/j.bbrc.2004.10.106. [DOI] [PubMed] [Google Scholar]
- 5.Hogg N, Browning J, Howard T, Winterford C, Fitzpatrick D, Gobe G. Apoptosis in vascular endothelial cells caused by serum deprivation, oxidative stress and transforming growth factor-beta. Endothelium. 1999;7:35–49. doi: 10.3109/10623329909165310. [DOI] [PubMed] [Google Scholar]
- 6.Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1) Am J Physiol Heart Circ Physiol. 2002;283:1253–62. doi: 10.1152/ajpheart.00578.2001. [DOI] [PubMed] [Google Scholar]
- 7.Regoli D, Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- 8.Emanueli C, Maestri R, Corradi D, Marchione R, Minasi A, Tozzi MG, et al. Dilated and failing cardiomyopathy in bradykinin B(2) receptor knockout mice. Circulation. 1999;7:2359–65. doi: 10.1161/01.cir.100.23.2359. [DOI] [PubMed] [Google Scholar]
- 9.Silva JA, Araujo RC, Baltatu O, Oliveira SM, Tschöpe C, Fink E, et al. Reduced cardiac hypertrophy and altered blood pressure control in transgenic rats with the human tissue kallikrein gene. FASEB J. 2000;14:1858–60. doi: 10.1096/fj.99-1010fje. [DOI] [PubMed] [Google Scholar]
- 10.Bledsoe G, Chao L, Chao J. Kallikrein gene delivery attenuates cardiac remodeling and promotes neovascularization in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2003;285:H1479–88. doi: 10.1152/ajpheart.01129.2002. [DOI] [PubMed] [Google Scholar]
- 11.Agata J, Chao L, Chao J. Kallikrein gene delivery improves cardiac reserve and attenuates remodeling after myocardial infarction. Hypertension. 2002;40:653–9. doi: 10.1161/01.hyp.0000036035.41122.99. [DOI] [PubMed] [Google Scholar]
- 12.Yao YY, Yin H, Shen B, Chao L, Chao J. Kallikrein infusion minimizes myocardial infarction and inflammation through suppression of oxidative stress and mitogen-activated protein kinase signaling pathway. Regulatory Peptides. 2007;140:12–20. doi: 10.1016/j.regpep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao J, Li HJ, Yao YY, Shen B, Gao L, Bledsoe G, et al. Kinin infusion attenuates salt-induced inflammation, apoptosis and renal fibrosis through inhibition of oxidative stress and mitogen-activated protein kinase activity. Hypertension. 2007;49:490–7. doi: 10.1161/01.HYP.0000255925.01707.eb. [DOI] [PubMed] [Google Scholar]
- 14.Yin H, Chao L, Chao J. Kallikrein/kinin protects against myocardial apoptosis after ischemia/reperfusion via Akt-glycogen synthase kinase-3 and Akt-Bad.14-3-3 signaling pathways. J Biol Chem. 2005;280:8022–30. doi: 10.1074/jbc.M407179200. [DOI] [PubMed] [Google Scholar]
- 15.Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem. 1993;214:11–6. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 16.Brilla CG, Scheer C, Rupp H. Angiotensin II and intracellular calcium of adult cardiac fibroblasts. J Mol Cell Cardiol. 1998;30:1237–46. doi: 10.1006/jmcc.1998.0689. [DOI] [PubMed] [Google Scholar]
- 17.Lagneux C, Bader M, Pesquero JB, Demenge P, Ribuot C. Detrimental implication of B1 receptors in myocardial ischemia: evidence from pharmacological blockade and gene knockout mice. Int Immunopharmacol. 2002;2:815–22. doi: 10.1016/s1567-5769(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Carretero OA, Sun Y, Shesely EG, Rhaleb NE, Liu YH, et al. Role of the B1 kinin receptor in the regulation of cardiac function and remodeling after myocardial infarction. Hypertension. 2005;45:747–53. doi: 10.1161/01.HYP.0000153322.04859.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li HJ, Yin H, Yao YY, Shen B, Chao L, Chao J. Tissue kallikrein protects against pressure overload-induced cardiac hypertrophy through kinin B2 receptor and glycogen synthase kinase-3beta activation. Cardiovasc Res. 2007;3:130–42. doi: 10.1016/j.cardiores.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z, Park SS, Mueller RA, Bagnell RC, Patterson C, Boysen PG. Adenosine produces nitric oxide and prevents mitochondrial oxidant damage in rat cardiomyocytes. Cardiovasc Res. 2005;65:803–12. doi: 10.1016/j.cardiores.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Clancy RM, Leszczynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–21. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith RS, Agata J, Xia CF, Chao L, Chao J. Human endothelial nitric oxide synthase gene delivery protects against cardiac remodeling and reduces oxidative stress after myocardial infarction. Life Sci. 2005;76:2457–71. doi: 10.1016/j.lfs.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, et al. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99:1926–35. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeffer MA, Pfeffer JM, Steinberg C, Finn P. Survival after an experimental myocardial infarction: beneficial effects of long-term therapy with captopril. Circulation. 1985;72:406–12. doi: 10.1161/01.cir.72.2.406. [DOI] [PubMed] [Google Scholar]
- 25.Mulder P, Devaux B, Richard V, Henry JP, Wimart MC, Thibout E, et al. Early versus delayed angiotensin-converting enzyme inhibition in experimental chronic heart failure: effects on survival, hemodynamics, and cardiovascular remodeling. Circulation. 1997;95:1314–9. doi: 10.1161/01.cir.95.5.1314. [DOI] [PubMed] [Google Scholar]
- 26.Wollert KC, Studer R, Doerfer K, Schieffer E, Holubarsch C, Just H, et al. Differential effects of kinins on cardiomyocyte hypertrophy and interstitial collagen matrix in the surviving myocardium after myocardial infarction in the rat. Circulation. 1997;95:1910–7. doi: 10.1161/01.cir.95.7.1910. [DOI] [PubMed] [Google Scholar]
- 27.Miura S, Matsuo Y, Saku K. Transactivation of KDR/Flk-1 by the B2 receptor induces tube formation in human coronary endothelial cells. Hypertension. 2003;41:1118–23. doi: 10.1161/01.HYP.0000064345.33807.57. [DOI] [PubMed] [Google Scholar]
- 28.Yang SW, Lee WK, Lee EJ, Kim KY, Lim Y, Lee KH, et al. Effect of bradykinin on cultured bovine corneal endothelial cells. Ophthalmologica. 2001;215:303–8. doi: 10.1159/000050879. [DOI] [PubMed] [Google Scholar]
- 29.Xiang G, Schuster MD, Seki T, Witkowski P, Eshghi S, Itescu S. Downregulated expression of plasminogen activator inhibitor-1 augments myocardial neovascularization and reduces cardiomyocyte apoptosis after acute myocardial infarction. J Am Coll Cardiol. 2005;46:536–41. doi: 10.1016/j.jacc.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Yoshiyama M, Omura T, Yoshida K, Izumi Y, Takeuchi K, et al. Beneficial effects of combination of ACE inhibitor and angiotensin II type 1 receptor blocker on cardiac remodeling in rat myocardial infarction. Cardiovasc Res. 2003;7:48–54. doi: 10.1016/s0008-6363(02)00644-2. [DOI] [PubMed] [Google Scholar]
- 31.Rosenkranz AC, Dusting GJ, Ritchie RH. Endothelial dysfunction limits the antihypertrophic action of bradykinin in rat cardiomyocytes. J Mol Cell Cardiol. 2000;32:1119–26. doi: 10.1006/jmcc.2000.1149. [DOI] [PubMed] [Google Scholar]
- 32.Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-beta 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276:2531–7. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- 33.Savage C, Das P, Finelli AL, Townsend SR, Sun CY, Baird SE, et al. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc Natl Acad Sci U S A. 1996;93:790–4. doi: 10.1073/pnas.93.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim NN, Villegas S, Summerour SR, Villarreal FJ. Regulation of cardiac fibroblast extracellular matrix production by bradykinin and nitric oxide. J Mol Cell Cardiol. 1999;31:457–66. doi: 10.1006/jmcc.1998.0887. [DOI] [PubMed] [Google Scholar]
- 35.Minai K, Matsumoto T, Horie H, Ohira N, Takashima H, Yokohama H, et al. Bradykinin stimulates the release of tissue plasminogen activator in human coronary circulation: effects of angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2001;37:1565–70. doi: 10.1016/s0735-1097(01)01202-5. [DOI] [PubMed] [Google Scholar]
- 36.Okada H, Watanabe Y, Kikuta T, Kobayashi T, Kanno Y, Sugaya T, et al. Bradykinin decreases plasminogen activator inhibitor-1 expression and facilitates matrix degradation in the renal tubulointerstitium under angiotensin-converting enzyme blockade. J Am Soc Nephro. 2004;15:2404–13. doi: 10.1097/01.ASN.0000136132.20189.95. [DOI] [PubMed] [Google Scholar]