Abstract
The inflammasomes are innate immune system receptors/sensors that regulate the activation of caspase-1 and induce inflammation in response to infectious microbes and molecules derived from host proteins. It has been implicated in a host of inflammatory disorders. Recent developments have greatly enhanced our understanding of the molecular mechanisms by which different inflammasomes are activated. Additionally, increasing evidence in mouse models, supported by human data, strongly implicates an involvement of the inflammasome in the initiation or progression of diseases with a high impact on public health such as metabolic disorders and neurodegenerative diseases. Finally, recent developments pointing toward promising therapeutics that target inflammasome activity in inflammatory diseases have been reported. This review will focus on these three areas of inflammasome research.
INTRODUCTION
Inflammation is a protective immune response mounted by the evolutionarily-conserved innate immune system to harmful stimuli, such as pathogens, dead cells, or irritants, and is tightly regulated by the host. Insufficient inflammation can lead to persistent infection of pathogens while excessive inflammation can cause chronic or systemic inflammatory diseases. Innate immune function depends upon recognition of pathogen-associated molecular patterns (PAMPs), derived from invading pathogens, and danger-associated molecular patterns (DAMPs), induced as a result of endogenous stress, by germline-encoded pattern-recognition receptors (PRRs). Activation of PRRs by PAMPs or DAMPs triggers downstream signaling cascades and leads to production of type I interferon (interferon-α and interferon-β) and proinflammatory cytokines. Of note, DAMP-triggered inflammation, which is particularly important in inflammatory diseases, is termed sterile inflammation when it occurs in the absence of any foreign pathogens1.
Activation of the inflammasome is a key function mediated by the innate immune system, and recent advances have greatly increased our understanding of the macromolecular activation of inflammasomes. Several families of PRRs are important components in the inflammasome complex including the nucleotide-binding domain, leucine-rich repeat containing proteins (NLRs, also known as NOD-like receptors) and absent in melanoma 2-like receptors (ALRs, AIM2-like receptors) in both mice and humans2. Upon sensing certain stimuli, the relevant NLR or AIM2 can oligomerize to be a caspase-1-activating scaffold. Active caspase-1 subsequently functions to cleave the proinflammatory IL-1 family of cytokines into their bioactive forms, IL-1β and IL-18, and cause pyroptosis, a type of inflammatory cell death3,4.
Inflammasomes have been linked to a variety of autoinflammatory and autoimmune diseases, including neurodegenerative diseases (multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease) and metabolic disorders (atherosclerosis, type-2 diabetes, and obesity)4. In inflammatory disease initiation, inflammasomes play either causative or contributing roles, and also exaggerate the pathology in response to host-derived factors. This review will focus on the current understanding of inflammasome activation, the roles of inflammasomes in several prevalent diseases that are increasingly recognized as having an inflammatory contribution, such as neurodegenerative diseases and metabolic disorders, and advances in potential therapies targeting inflammasomes.
MECHANISMS OF INFLAMMASOME ACTIVATION
General principles of inflammasome activation
Recent developments in our understanding of the mechanisms of inflammasome activation have been expertly reviewed in depth4–8. However, here, we give a brief overview of recent advances in the mechanisms of inflammasome activation in order to best explain their link with disease.
Inflammasomes are multimeric protein complexes that assemble in the cytosol after sensing PAMPs or DAMPs7,9. While there are fundamental differences between inflammasomes dependent upon stimuli, in general, canonical inflammasomes serve as a scaffold to recruit the inactive zymogen pro-caspase-1 (Figures 1 and 2). Oligomerization of pro-caspase-1 proteins induces their auto-proteolytic cleavage into active caspase-110. Active caspase-1 is a cysteine-dependent protease that cleaves precursor cytokines pro-IL-1β and pro-IL-18 generating biologically active cytokines IL-1β and IL-18, respectively11–13. Active caspase-1 is also able to induce an inflammatory form of cell death known as pyroptosis5–7.
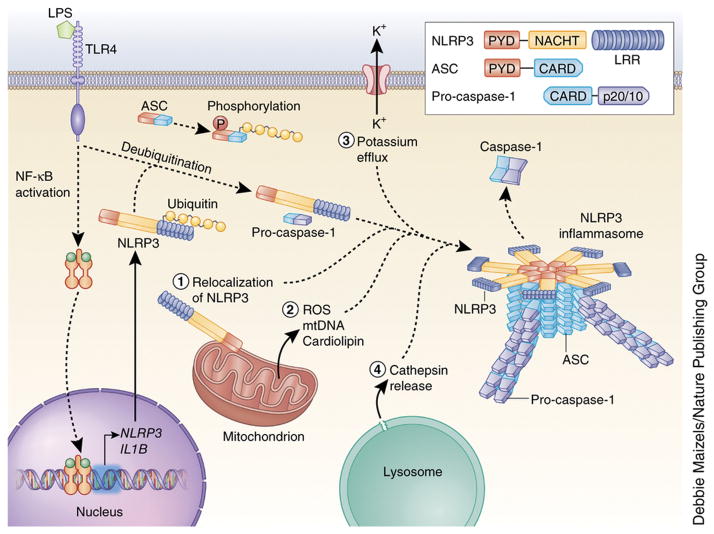
Figure 1. Mechanisms of NLRP3 inflammasome activation.
NLRP3 must be primed before activation. Priming involves two distinct steps. First, an NF-κB–activating stimulus, such as LPS binding to TLR4, induces elevated expression of NLRP3 (as well as IL1B), which leads to increased expression of NLRP3 protein. Additionally, priming immediately licenses NLRP3 by inducing its deubiquitination. The adaptor protein ASC must become linearly ubiquitinated and phosphorylated for inflammasome assembly to occur. After priming, canonical NLRP3 inflammasome activation requires a second, distinct signal to activate NLRP3 and lead to the formation of the NLRP3 inflammasome complex. The most commonly accepted activating stimuli for NLRP3 include relocalization of NLRP3 to the mitochondria, the sensation of mitochondrial factors released into the cytosol (mitochondrial ROS, mitochondrial DNA, or cardiolipin), potassium efflux through ion channels, and cathepsin release following destabilization of lysosomal membranes. Recent studies have determined that activated NLRP3 nucleates ASC into prion-like filaments through PYD-PYD interactions. Pro-caspase-1 filaments subsequently form off of the ASC filaments through CARD-CARD interactions, allowing autoproteolytic activation of pro-caspase-1. Inset shows domain arrangement of the NLRP3 inflammasome components. Pro-caspase-1 and caspase-1 domains are simplified for clarity, the CARD domain is actually removed by cleavage, and two heterodimers form with the p20 and p10 effector domains (p20/10).
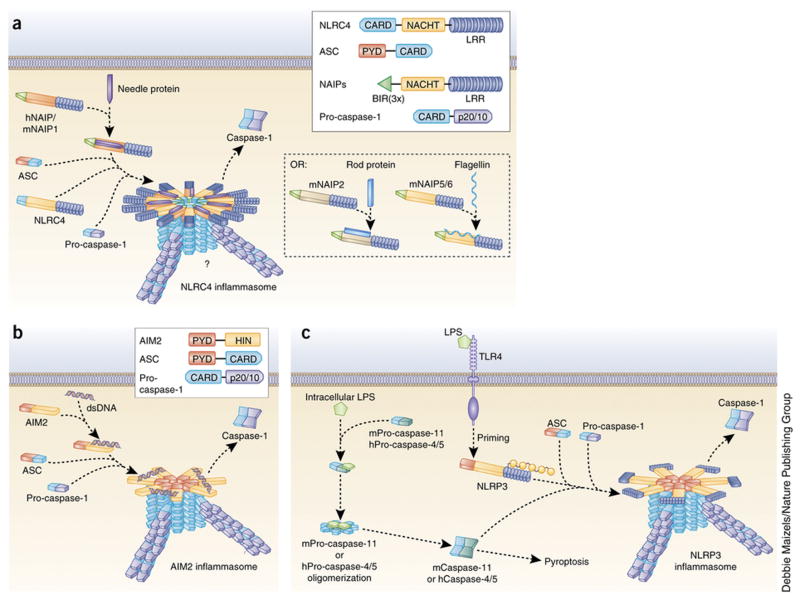
Figure 2. Mechanisms of NLRC4, AIM2 and noncanonical NLRP3 inflammasome activation.
(a) NLRC4 inflammasome agonists such as the bacterial needle protein bind directly to regions within the NACHT domains of the NAIP subfamily of proteins. hNAIP and mNAIP1 bind needle protein, mNAIP2 binds rod protein, and both mNAIP5 and mNAIP6 bind flagellin. Ligand-bound NAIP proteins then oligomerize with NLRC4 to form a caspase-1–activating inflammasome. Though NLRC4 can directly oligomerize with caspase-1 through CARD-CARD interactions, ASC is required for caspase-1 activation by the NLRC4 inflammasome, possibly through the formation of prion-like filaments (blue) by ASC. However, ASC is dispensable for the induction of pyroptosis. Inset shows domain arrangement of NLRC4 inflammasome components. NAIP proteins have three N-terminal BIR domains. hNAIP, human NAIP; mNAIP, mouse NAIP. (b) The mechanism of AIM2 inflammasome activation is well defined. The HIN domain of AIM2 directly binds cytosolic dsDNA, displacing the PYD and relieving autoinhibition. This allows oligomerization of AIM2 PYD with ASC PYD, converting ASC into its prion form. Prion-like filaments of pro-caspase-1 (violet) are then able to form off of the ASC filaments, inducing caspase-1 activation. Inset shows domain arrangement of AIM2 inflammasome components. (c) Studies have determined that mouse pro-caspase-11 (mPro-caspase-11) and human pro-caspases-4 and -5 (hPro-caspase-4/5) can directly bind intracellular LPS and activate a noncanonical NLRP3 inflammasome. This induces oligomerization of these pro-caspases, leading to their proximity-induced activation. This is sufficient for the induction of pyroptosis but not for the processing of pro-IL-1β. However, active mCaspase-11 and hCaspase-4 can promote full assembly and activation of the NLRP3 inflammasome following a priming signal.
Inflammasome names denote the protein forming the scaffold. Most inflammasomes are formed with one or two NLR family members, and NLRC4 requires interaction with an NLR member of the NAIP subfamily of proteins6,14 (Figures 1 and 2A). However, non-NLR proteins such as AIM2 (Figure 2B) and pyrin can also form inflammasomes. NLRC4 can directly associate with caspase-1 through CARD-CARD interactions15. NLRs containing an amino-terminal pyrin domain (PYD) are shown to associate with apoptosis-associated speck-like protein containing a CARD (ASC) in order to recruit pro-caspase-1 to the inflammasome9,16 (Figures 1).
Inflammasome activation occurs when the scaffold protein senses or binds its activating stimuli. How this occurs is starting to be clarified for certain inflammasome proteins6, prominent among these are the roles of ASC, AIM2, and NAIP/NLRC4. For example, AIM2 can directly bind to its stimulus, double-stranded DNA (dsDNA)17. However, many questions remain regarding inflammasome activation. We will now briefly discuss the mechanism of activation of the most well-characterized inflammasomes where major advances have been made. The readers can refer to recent reviews where all of the NLR inflammasomes have been reviewed5–7, including evidence supporting the existence of less-characterized inflammasomes, such as NLRP6, NLRP7, NLRP12, and IFI16 inflammasomes. Additionally, though NLRP1, which has many genetic variants in mice and rats, forms well-defined inflammasomes in these rodent models, activation of the single human NLRP1 paralog into an inflammasome is less well understood18.
NLRP3 inflammasome
The NLRP3 inflammasome (Figure 1) is activated in response to the widest array of stimuli, leading to the theory that the dissimilar agonists induce similar downstream events which are sensed by NLRP38,19,20. The mechanisms of NLRP3 activation supported by the most studies include potassium efflux out of the cell, the generation of mitochondrial reactive oxygen species (ROS), translocation of NLRP3 to the mitochondria, the release of mitochondrial DNA or cardiolipin, or the release of cathepsins into the cytosol after lysosomal destabilization6–8 (Figure 1). However, not all of these events are induced by all NLRP3 agonists, so the precise mechanism of NLRP3 activation is still debated. Additionally, increases in intracellular calcium can activate the NLRP3 inflammasome21,22, but this is also not a requirement of all NLRP3 agonists23. Though many published studies support the involvement of lysosomal cathepsins, proteases that degrade internalized proteins, in NLRP3 inflammasome activation, it is important to note that this is not without some controversy24.
In most cell types, NLRP3 must be primed, and a prototypical example of such a priming event is the binding of LPS to TLR4. Priming has long been known to increase cellular expression of NLRP3 through NF-κB signaling25. However, recent findings have shown that priming rapidly licenses mouse NLRP3 inflammasome activation by inducing the deubiquitination of NLRP3 independent of new protein synthesis, while inhibition of deubiquitination inhibits human NLRP3 activation26,27. Once primed, NLRP3 can respond to its stimuli and assemble the NLRP3 inflammasome. Additionally, ASC must be linearly ubiquitinated for NLRP3 inflammasome assembly28. Current stimuli recognized as NLRP3 agonists that induce NLRP3 inflammasome formation include ATP, pore-forming toxins, crystalline substances, nucleic acids, hyaluronan, and fungal, bacterial, or viral pathogens6,7. These stimuli can be encountered during infection, either produced by pathogens or released by damaged host cells. Additionally, pathologic conditions in the body may promote formation of these stimuli in the absence of infection, such as the formation of inflammatory cholesterol crystals, as discussed in more detail later.
Recent studies identified that the NLRP3 NBD oligomerizes the NLRP3 PYD, which serves as a scaffold to nucleate ASC proteins through PYD-PYD interactions29,30. This causes ASC to convert to a prion-like form and generate long ASC filaments that are crucial to inflammasome activation. Pro-caspase-1 then interacts with ASC through CARD-CARD interactions and forms its own prion-like filaments that branch off of the ASC filaments. The close proximity of pro-caspase-1 proteins then induces auto-proteolytic maturation of pro-caspase-1 into active caspase-1.
Additionally, increasing evidence has identified a crucial role for caspase-8 in inflammasome activation and pro-IL-1β processing. Caspase-8 is a pro-apoptotic protease that initiates the external apoptosis pathway in response to external stimuli, such as FasL and TNF, and protects against an inflammatory form of cell death termed necroptosis31. It is now also recognized that caspase-8 is required for both the transcriptional priming and activation of the canonical and noncanonical NLRP3 inflammasomes in mice in response to pathogenic stimuli and ligands stimulating various different TLRs32–34. Thus, inflammatory diseases in which TLR ligands are generated could lead to caspase-8-mediated NLRP3 priming or activation.
Additionally, caspase-8 was shown to bind and localize to ASC specks, further suggesting that caspase-8 is an important component of inflammasome complexes35. However, the exact molecular mechanism of how caspase-8 promotes caspase-1 activation has yet to be elucidated. Importantly, caspase-8 also has an identified role in NLRC4 and AIM2 inflammasome activation35,36 and has even been shown to directly promote pro-IL-1β processing in a noncanonical caspase-8 inflammasome induced by the binding of certain extracellular pathogens to dectin-137. Notably, the exact role of caspase-8-mediated inflammasome activation is somewhat controversial38.
NLRC4 inflammasome
In contrast to the diverse stimuli that activate NLRP3, the NLRC4 inflammasome responds to a more limited set of stimuli. A major advance in our understanding of the NLRC4 inflammasome is that NLRC4 forms a complex with various NAIP proteins, and NLRC4-activating ligands are bound by these NAIP components rather than by NLRC4 (Figure 2A). This raises the question of whether NLRC4 is a scaffolding protein and not a receptor14,39. In mice, NAIP1 binds the bacterial type III secretory system (T3SS) needle protein40,41, NAIP2 binds the bacterial T3SS rod protein42, and both NAIP5 and NAIP6 bind bacterial flagellin42,43. T3SS is found in several gram negative bacteria and allows the bacteria to inject effector molecules into infected host cells. By contrast to mice, only one human NAIP protein has been characterized, and it was found to bind only the T3SS needle protein40, suggesting a far more restrictive repertoire of ligands for the NLRC4 inflammasome in human cells than NLRP3, which responds to a plethora of stimuli.
Once NAIP proteins bind their ligands, they can oligomerize with NLRC4 and form a NAIP/NLRC4 inflammasome14. In order for NLRC4 to be activated, its autoinhibition must be relieved to allow oligomerization with NAIP proteins, but how this occurs is unclear14. However, two new gain-of-function mutations have recently been identified in humans that cause severe spontaneous autoimmune syndrome, suggesting that the helical domain is responsible for this autoinhibition44,45. Though some reports indicate that mouse NLRC4 must be phosphorylated prior to inflammasome activation46,47, there are also conflicting reports indicating that phosphorylation is dispensable14.
Though NLRC4 contains a CARD domain, ASC is required for maximal inflammasome activation7 (Figure 2A). A possible explanation might be the formation of NLRC4 filaments, as there is evidence that the CARD domain can convert ASC to its prion-like form31.
AIM2 inflammasome
The non-NLR AIM2 can also form a caspase-1-containing inflammasome, but, unlike the NLRs, the HIN-200 domain of AIM2 can directly bind its stimulus, cytosolic dsDNA, which may be encountered in the cytosol during pathogenic infection (Figure 2B)17. The autoinhibitory conformation of AIM2 is created by interactions of its two domains and relieved by the sugar phosphate backbone of dsDNA48. DNA binding displaces the PYD domain48, freeing the PYD domain to recruit ASC to the complex17,49. AIM2 cannot interact with ASC unless autoinhibition is relieved50 and, thus, AIM2 maintains itself in an inactive state until its ligand binds.
Interestingly, AIM2 does not appear to recognize a specific sequence or structure of dsDNA but instead requires a dsDNA strand of at least 80 base pairs for optimal inflammasome activation48. Similar to NLRP3, oligomerized AIM2 nucleates ASC through PYD-PYD interactions and converts ASC to its prion form, leading to the development of long PYD-PYD ASC filaments29,30.
Recently, a noncanonical AIM2 inflammasome was shown to mediate protection against Francisella novicida51. F. novicida infection is detected by cGAS and STING, inducing the expression of the transcription factor IRF1. IRF1 increases the expression of guanylate binding proteins, which increase the intracellular killing of the bacterium. This releases dsDNA into the cytosol and induces AIM2 inflammasome activation.
Noncanonical inflammasomes
A developing area of interest in the inflammasome field is the noncanonical inflammasome formed by caspase-11 in mice (Figure 2C). Caspase-11 was initially found to be important for the activation of caspase-1 and caspase-352. Recently, it was shown that caspase-11 promotes NLRP3 inflammasome activation to indirectly enhance processing of pro-IL-1β or pro-IL-1853. More remarkably, caspase-11 detects intracellular LPS and some intracellular bacteria, directly mediating cell death and IL-1α secretion, but not IL-1β secretion, in a mechanism independent of the traditional LPS receptor TLR47,54,55. Though humans do not express caspase-11, recent studies indicate that caspase-4 and caspase-5 in human cells serve a similar function56,57 (Figure 2C). Notably, active caspase-4 can promote the activation of the primed NLRP3 inflammasome without a need for a canonical NLRP3 activating stimulus57. As caspase-11-deficient mice are protected from endotoxic shock53, further study of the noncanonical inflammasome in human cells is of great interest.
Mechanisms of inflammasome spreading
ASC has long been recognized to redistribute upon inflammasome activation from the nucleus to the cytosol and form a large perinuclear aggregate in cells58,59. In a recent breakthrough, ASC specks were reported to be released by dying cells, leading to cleavage of extracellular pro-IL-1β and activating caspase-1 in macrophages internalizing the specks60. Importantly, as activation of all major inflammasomes is associated with speck formation59, this suggests that inflammasome activation propagates inflammation from cell to cell. The buildup of specks at sites of inflammation has serious implications for inflammatory diseases, as injection of purified ASC specks into mice in vivo was shown to propagate inflammation60.
Additionally, phosphorylation of ASC was recently identified to be a key checkpoint in ASC speck formation. The kinases Syk and JNK, which activate in response to a vast array of stimuli and lead to the phosphorylation of many downstream targets, mediate phosphorylation of ASC upon NLRP3 inflammasome activation, and inhibition of these kinases prevented ASC speck formation and blocked caspase-1 activation61. Importantly, phosphorylation was dispensable for NLRP3 and ASC oligomerization. This suggests that phosphorylation of ASC may be necessary for ASC to switch to its prion form and form self-propagating filaments. This also suggests that kinase inhibition may have potential therapeutic use against inflammatory diseases in the absence of more targeted inhibitors.
INFLAMMASOMES IN DISEASE
Here we focus on neurologic disorders and metabolic diseases, both of which are not traditionally considered to be inflammatory diseases, but are increasingly recognized as having an inflammatory component that contributes significantly to the disease process. Misfolded protein aggregates and aberrant accumulation of certain metabolites accompanied with those diseases are endogenous DAMPs that have been proved to be direct activators of the NLRP3 inflammasome, which plays a critical role in the initiation and progress of those diseases.
The inflammasome and multiple sclerosis
Multiple sclerosis (MS), one of the most common autoimmune/inflammatory diseases, is characterized by myelin-reactive CD4+ T cells that infiltrate the central nervous system (CNS), attack oligodendrocytes and induce demyelination62. Demyelination partially disrupts the communication of the nervous system, resulting in physical, mental, and psychiatric challenges, among other issues. Presently, MS has no cure and shortens the lifespan of patients approximately 5 to 10 years63.
Experimental autoimmune encephalomyelitis (EAE) is a commonly-used animal model to mimic MS. To induce EAE, mice are immunized with the peptide myelin oligodendrocyte glycoprotein (MOG) emulsified in adjuvant, inducing infiltration of MOG-specific T cells and other inflammatory cells into the CNS64.. Prior to the discovery of NLRs, the inflammasome products caspase-1, IL-1β, and IL-18 had been shown to contribute to EAE progression. Casp1−/−, Il1a−/−, Il1b−/− and Il18−/− mice are resistant to EAE, accompanied by reductions in IFN-γ and/or IL-17 levels65–67. Recently, Nlrp3 expression has been shown to increase in the spinal cord during EAE progression and Nlrp3-deficient mice showed a dramatically delayed course and reduced severity of disease, accompanied by fewer infiltrating inflammatory cells and reduced astrogliosis64,68. In addition, a study using a cuprizone model of MS also showed that Nlrp3-deficient mice had delayed demyelination and oligodendrocyte loss69. Additionally, in EAE mice there was increased IL-18 levels, compared with controls and Il18-deficient mice phenocopied the reduced disease seen in Nlrp3-deficient mice, suggesting NLRP3 functions through IL-18 to promote EAE64,68.
Despite these findings, the role of NLRP3 in EAE progression is complicated. Expression of Nlrp3 in antigen-presenting cells (APCs) was required to stimulate T helper type 1 (Th1) and Th17 cells to respond to brain autoantigen in one study64. Additionally, Nlrp3 and Asc (also known as Pycard) deficiency caused reduced expression of many chemokines and chemokine receptors, such as Ccr2 and Ccr6, in both APCs and Th cells, reducing migration of Th1 and Th17 cells into the CNS of Nlrp3- and Asc-deficient mice following EAE induction by MOG peptide immunization. However, direct delivery of CD4+ T cells from EAE-induced WT, Nlrp3−/− or Asc−/− mice into the brain and spinal cord of recipient Rag2−/− mice, which lack mature T cells, induced the same extent of disease68. In summay, while these results suggest that the NLRP3 inflammasome contributes to both Th1 and Th17 cell responses and migration during EAE, the function of the NLRP3 inflammasome is not an inherent function of T cells. In the clinic, peripheral blood mononuclear cells (PBMCs) from relapsing-remitting MS patient had higher levels of NLRP3, IL-1β, and caspase-1 than were found in PBMCs from healthy controls. Intriguingly, soluble factors secreted by human PBMCs upon NLRP3 activation skew the cytokine profile of CD4+ T cells toward a pro-inflammatory Th17 phenotype, supporting a link between MS and the NLRP3 inflammasome70.
However, a role for NLRP3 and ASC in EAE is not found by all studies and varies with variations in the disease model. Aggressive immunization of mice with heat-killed mycobacteria (Mtb) was able to induce EAE even in the absence of NLRP3 or ASC, whereas lower-dose Mtb immunization required NLRP3 and ASC for EAE induction71. Another study found no difference in MOG-induced EAE disease between WT and Nlrp3-deficient mice. In the same study, ASC promoted EAE progression in an inflammasome-independent manner through a mechanism of maintaining CD4+ T cell survival. In agreement with this, Asc-deficient mice were even more resistant to EAE than Casp1-deficient mice72. Part of the differences in inflammasome dependency may be explained by recent findings showing that IFN-β inhibited IL-1β production by macrophages, and only NLRP3-dependent EAE was ameliorated by IFN-β treatment. This suggests that IFN-β may therapeutically inhibit the NLRP3 inflammasome-IL-1β/IL-18 axis in MS71. Though IFN-β has been used therapeutically for more than 15 years, one third of MS patients fail to respond to IFN-β, echoing heterogeneity in the disease.
In addition to the NLRP3 inflammasome, a recent study using the pertussis toxin (PTX)-induced EAE model showed that TLR4 was required for pro-IL-1β induction, and the pyrin-dependent inflammasome contributed to bioactive IL-1β formation. IL-1β stimulated nearby stromal cells to secret IL-6, which can promote leukocyte adhesion and migration. Pyrin (also known as Mefv)-deficient 2D2 mice (MOG-specific T cell receptor transgenic mice) had lower EAE incidence and delayed and less severe disease following PTX injection. However, the pyrin inflammasome only functions at the initial stage of EAE induced by PTX, as comparable infiltration of CD3+ cells was observed in the spinal cord of mice with similar clinical scores regardless of their genotype. In line with this, adoptive transfer of MOG-specific T cells into WT and pyrin-deficient mice induced similar EAE73.
The inflammasome and Alzheimer’s disease
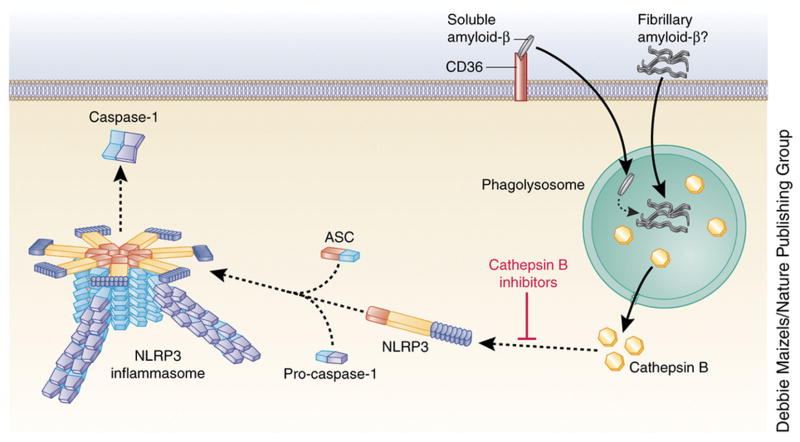
Accumulation of amyloid-β plaques in the cerebrum is a characteristic of Alzheimer’s disease (AD) Amyloid-β peptide is regularly formed in cerebral tissue by cleavage of the amyloid precursor protein, but it can form prion-like misfolded oligomers in the case of AD74. Amyloid-β was the first molecule associated with neurodegenerative disease models that was found to activate the murine NLRP3 inflammasome, resulting in IL-1β production75. Fibrillary amyloid-β induces NLRP3-inflammasome-dependent caspase-1 activation through a mechanism dependent on endosomal rupture and cathepsin B release in LPS-primed murine macrophages75 (Figure 3). Interestingly, administration of cathepsin B inhibitors significantly improved memory deficit and reduced amyloid plaque load in the brain in the AD mouse model, suggesting a potential therapeutic approach for Alzheimer’s treatment in which the inflammasome is targeted76. Importantly, a recent pivotal study in mice identified that the cell-surface receptor CD36 mediates the internalization of soluble amyloid-β, which then undergoes intracellular conversion to fibrillary amyloid-β to activate the NLRP3 inflammasome77. A direct link between the NLRP3 inflammasome and the development of AD has been shown in APP/PS1 mice (transgenic mice developing chronic deposition of amyloid-β) with NLRP3 and caspase-1 deficiency. These mice have reduced AD-related pathogenesis, reflected by reduced chronic amyloid-β secretion, neuronal inflammation, and cognitive impairment. In these mice, NLRP3-inflammasome deficiency skewed microglial cells to an M2 phenotype (characterized by elevated expression of arginase-1 and IL-4 ), resulting in the reduced deposition of amyloid-β and enhanced tissue remodeling in the AD mouse model78. In addition to the mouse study, a recent study found enhanced active caspase-1 expression in human brains with AD, suggesting that there is a link between inflammasome activation and Alzheimer’s in humans78. Therefore, in vitro and in vivo studies suggest a potentially important role for the NLRP3 inflammasome in the pathogenesis of AD and identify the NLRP3-caspase-1 axis as a potential target for AD therapy.
Figure 3. Mechanisms of NLRP3 inflammasome action in Alzheimer’s disease.
In Alzheimer’s disease, CD36 mediates the internalization of soluble amyloid-β and its intracellular conversion to fibrillary amyloid-β. This leads to disruption of the phagolysosome and activation of the NLRP3 inflammasome due to cathepsin B release. However, this does not exclude the possibility that phagocytosis of extracellular fibrillary amyloid-β also activates the NLRP3 inflammasome. Cathepsin B inhibition prevents amyloid-β–induced NLRP3 activation.
Inflammasome and Parkinson’s disease model
Parkinson’s disease (PD) results in the death of dopamine-generating neurons in the substantia nigra and the presence of aggregated inclusions mainly composed of α-synuclein (αSyn) in neurons79. αSyn can form fibrils with a cross β-sheet structure, morphologically similar to the amyloid fibrils from AD80. Through multiple mechanisms, intracellular αSyn can be released into extracellular spaces81. Extracellular αSyn activates primary microglia, astrocytes, as well as transformed microglia and astrocyte cell lines and induces the production of the cytokine IL-1β81,82. In a rat model of PD, chronic expression of exogenous IL-1β introduced in an adenoviral vector in the region of the substantia nigra was shown to induce cell death in dopamine neurons and to promote PD progression83. Recently it was found that both fibrillary and monomeric αSyn induce pro-IL-1β expression via TLR2 signaling in human primary monocytes, but only fibrillary αSyn fully activated the inflammasome by inducing caspase-1 activation and mature IL-1β production84. This activation of caspase-1 required phagocytosis, cathespin B, and ROS. Cathepsin B and ROS are thought to lie upstream of NLRP3 activation, suggesting that αSyn activated the NLRP3 inflammasome84. However, this study did not use the more relevant microglial cells and astrocytes, and the involvement of NLRP3 was not directly proven by an in vivo animal model.
In a PD model mouse in which PD is induced by loss of nigral dopaminergic neurons caused by treatment with neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), mice lacking Nlrp3 are resistant to developing PD. This provides in vivo evidence for a link between the NLRP3 inflammasome and PD85. Interestingly, dopamine was found to negatively regulate NLRP3 activation in both primary microglia and astrocytes via a dopamine D1 receptor (DRD1)-cyclic adenosine monophosphate (cAMP) signaling pathway85. Moreover, cAMP was found to directly bind to NLRP3 and promote its ubiquitination-dependent degradation via the E3 ubiquitin ligase MARCH785. Furthermore mice lacking DRD1 are more susceptible to MPTP-induced neuroinflammation, reflected by enhanced NLRP3 activation-dependent IL-1β and IL-18 production and increased loss of dopaminergic neurons85. These studies suggest that dopamine-producing neurons and the NLRP3 inflammasome regulate each other in a bidirectional fashion, where the inflammasome can damage these neurons, while dopamine from these neurons can inhibit NLRP3 function.
NLRP3 inflammasome and atherosclerosis
Chronic inflammation plays an essential role in the initiation and progression of metabolic disorders such as type 2 diabetes (T2D), obesity, gouty arthritis, and atherosclerosis86. Atherosclerosis accounts for 70% of morbidity in T2D patients and is a chronic disease that results in progressive narrowing of arterial vessels due to imbalanced lipid metabolism. Cholesterol crystals and white blood cells accumulate on the arterial wall, limiting the flow of oxygen-rich blood to the organs87. It is commonly referred to as a hardening or furring of the arteries, which can lead to life-threatening complications such as heart attack and stroke.
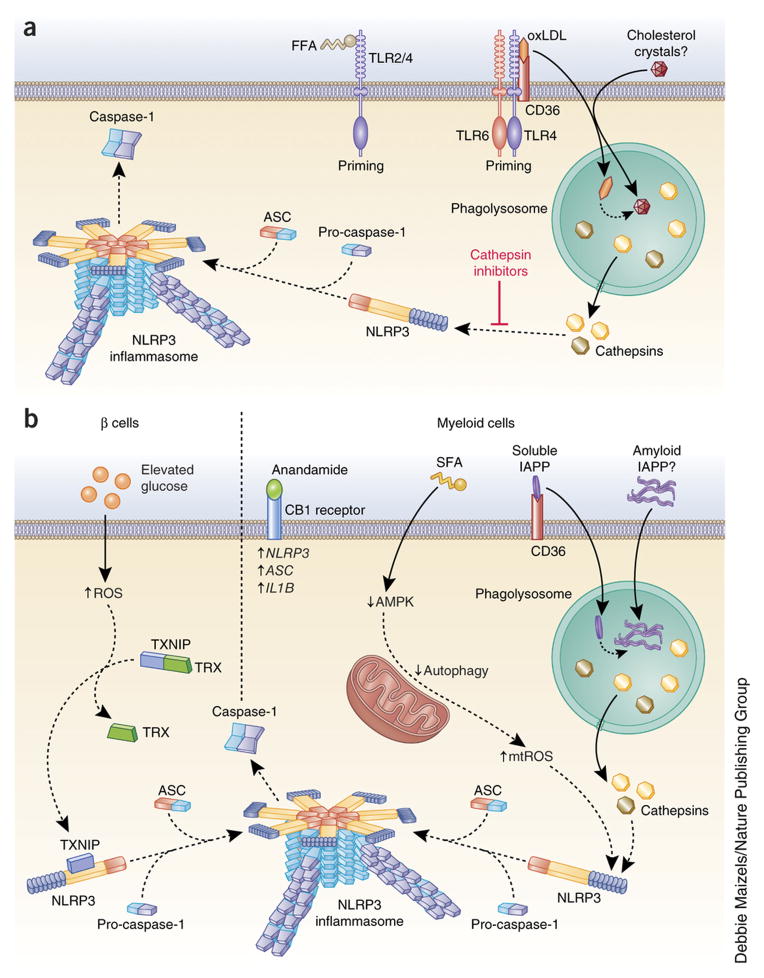
It has long been suggested, on the basis of evidence from mouse models88–90, that IL-18, a product of inflammasome activation, may have crucial roles in the initiation and progression of atherosclerosis. Furthermore, human atherosclerotic plaques have elevated concentrations of IL-18 and IL-18 receptors compared to disease-free arterial tissues. Apolipoprotein E (ApoE) is important for proper cholesterol metabolism. In ApoE-deficient mice, which spontaneously develop atherosclerotic lesions, elevated IL-18 levels have been shown to cause vascular inflammation and enhance the instability of atherosclerotic plaques, while IL-18-deficiency resulted in reduced atherosclerotic lesion size89,91,92. Elevation of low density lipoprotein (LDL) and free fatty acids (FFAs) in human blood due to imbalanced lipid metabolism is able to induce pro-IL-1β production through TLRs, providing the first signal for inflammasome activation93 (Figure 4A). Recent studies indicate that the cell surface receptor CD36 facilitates internalization of oxidized LDL (ox-LDL) and intracellular conversion of ox-LDL to cholesterol crystals77. These cholesterol crystals formed intracellularly activate the NLRP3 inflammasome in vitro in both mouse and human cells through phagolysosomal damage, a mechanism dependent on both cathepsin B and cathepsin L88 (Figure 4A). In vivo, intraperitoneal injection of cholesterol crystals in mice induced acute inflammation that was attenuated by the deficiency of NLRP3 inflammasome components, cathepsin B, and cathepsin L. In this model, IL-1β was released through NLRP3 inflammasome activation and in turn promoted rupture of atherosclerotic plaques.
Figure 4. Mechanism of inflammasome activation in inflammatory disease.
(a) In atherosclerosis, free fatty acids (FFA) can prime the NLRP3 inflammasome through TLR2-TLR4 signaling. Additionally, oxidized low-density lipoprotein (oxLDL) primes the NLRP3 inflammasome through a CD36-TLR4-TLR6 signaling complex. CD36 also facilitates the internalization of oxLDL and its intracellular conversion to cholesterol crystals, which disrupt the phagolysosome and activate the NLRP3 inflammasome through cathepsin release. Phagocytosis of extracellular cholesterol crystals may also contribute to inflammasome activation. Cathepsin inhibition prevents the NLRP3 inflammasome activation induced by cholesterol crystals. (b) In type 2 diabetes (T2D), the NLRP3 inflammasome is activated in both islet β-cells and myeloid cells. In β-cells, elevated glucose increases thioredoxin (TRX)-interacting protein (TXNIP). Intracellular ROS also causes a conformational change in TXNIP, leading to its dissociation from TRX. TXNIP then binds NLRP3 and promotes NLRP3 inflammasome activation. In myeloid cells, the endocannabinoid anandamide binds the CB1 receptor to increase the expression of NLRP3, ASC and IL1B. Saturated fatty acid (SFA) inhibits intracellular AMP-activated protein kinase (AMPK). This decreases autophagy, which leads to an increase in mitochondrial ROS (mtROS), a known NLRP3 inflammasome stimulus. CD36 facilitates the internalization of soluble islet amyloid polypeptide (IAPP), which is converted intracellularly to its amyloid form. This disrupts the phagolysosome and activates the NLRP3 inflammasome due to cathepsin release. As the amyloid form of IAPP builds up in the pancreatic islets of individuals with T2D, phagocytosis of extracellular amyloid IAPP may also contribute to NLRP3 inflammasome activation.
Mice lacking the LDL receptor are prone to developing atherosclerotic plaques. When these mice are fed a high-cholesterol diet, they have markedly reduced lesion size if the bone marrow cells lack Nlrp3, Asc, or Il1a and Il1b88. Similarly, in the ApoE-deficient mouse model of atherosclerosis, lack of IL-1β significantly decreases the size of atherosclerotic lesions94. In line with this, another study showed that blockade of IL-1β inhibited atherosclerotic plaque formation in the ApoE-deficient mouse model95. However, other studies have failed to link NLRP3 and IL-1β to atherosclerosis but instead found that IL-1α played an essential role in mice96,97. Further studies are required to clarify the contributions of IL-1α and IL-1β to atherogenesis.
NLRP3 inflammasome and type 2 diabetes
Type 2 diabetes (T2D) is a major global health threat resulting in insulin resistance and is a chronic inflammatory disease characterized by elevated circulating levels of TNF, interleukins, and cytokine-like proteins known as adipokines released from adipose tissue98. IL-1β in particular has been strongly linked to the pathogenesis of T2D by promoting insulin resistance and causing β-cell functional impairment and apoptosis. In cell culture, IL-1β dampens insulin sensitivity by inducing JNK-dependent serine phosphorylation of insulin receptor substrate-1 (IRS-1), resulting in the disruption of insulin-induced PI3K-Akt signaling in insulin-targeted cells. At the same time, IL-1β induces the expression of TNF-α99, which could independently impair insulin signaling100. Together with elevated FFAs in circulation due to imbalanced lipid metabolism, IL-1β induces metabolic stressors, such as ER stress and oxidative stress, both of which are involved in induction of inflammation and β-cell loss, thereby leading to the pathogenesis of T2D86,101. Furthermore, clinical trials reported that either IL-1 receptor antagonist (IL-1RA) or anti-IL-1β neutralizing antibody improved control of glucose levels and β-cell function102,103. Data also show that fatigue in T2D patients was reduced by IL-1β blockade. Trials with larger patient numbers should strengthen the argument for IL-1β-targeted therapy in T2D104.
Elevation of NLRP3 inflammasome activity in myeloid cells from T2D patients when compared with those from unaffected individuals has been described105. Multiple studies have found that NLRP3-, ASC-, and/or caspase-1-deficient mice show improved glucose tolerance and insulin sensitivity when exposed to a high fat diet (HFD)99,106–109. This is accompanied by reduced inflammatory cytokine levels in the serum and metabolic tissues such as liver and adipose tissue in conjunction with increased insulin-PI3K-Akt signaling99,106–108. These studies provide a direct link between the NLRP3 inflammasome, chronic inflammation, and insulin resistance.
As regards the role of the NLRP3 inflammasome and IL-1β in T2D pathogenesis, extensive studies have identified endogenous and exogenous stimulators of the NLRP3 inflammasome during T2D. Islet amyloid polypeptide (IAPP), a 37-amino-acid peptide hormone secreted from β-cells along with insulin, can form an amyloid structure that builds up in the pancreatic islets of patients with T2D110. As in the conversion of oxLDL to cholesterol crystals, the surface receptor CD36 also facilitates the conversion of soluble IAPP to its amyloid form (Figure 4B). In vitro, IAPP induces NLRP3 activation through a mechanism involving phagolysosome perturbation as well as cathepsin-B and cathepsin-L that leads to IL-1β production in macrophages and dendritic cells in culture111 (Figure 4B). In a transgenic mouse model in which human IAPP is overexpressed in mouse β-cells, pancreatic macrophages showed strong induction of IL-1β111,112. Elevated blood glucose was reported to induce IL-1β expression in β-cells, possibly through inflammasome activation mediated by thioredoxin (TRX)-interacting protein (TXNIP)108,113. Glucose can upregulate TXNIP expression in islets, and increased ROS due to oxidative stress in T2D has been proposed to cause conformational changes in TXNIP, leading to dissociation from thioredoxin and, in turn, association with NLRP3 for inflammasome activation108 (Figure 4B). Even though those studies could link oxidative stress with NLRP3 activation and IL-1β production in islets, the data were not reproducible in Txnip-deficient macrophages by another research group111.
The neuromodulatory lipids known as endocannabinoids were recently found to induce NLRP3 inflammasome-dependent IL-1β production by pancreatic infiltrating macrophages through the peripheral CB1 receptor (CB1R), resulting in pancreatic β-cell death in a paracrine manner114 (Figure 4B). Endocannabinoid anandamide increased ASC protein levels and caspase-1 activation in rat islets and markedly increased IL-1β secretion from a mouse macrophage cell line, RAW264.7. Anandamide-induced IL-1β production is dependent on Nlrp3 and Cb1r (also known as Cnr1). Intriguingly, blockade of CB1R by an inhibitor delayed the progress of T2D in the Zucker diabetic fatty rat which carries a spontaneous mutation of the leptin receptor gene and develops hyperglycemia progressively with aging accompanied by reduced β-cell apoptosis and hyperglycemia. This finding implicates CB1R to be a therapeutic target in T2D114.
Finally saturated fatty acids such as palmitate and ceramide that arise from a high fat diet and induce type 2 diabetes can induce NLRP3 inflammasome activation99,107 (Figure 4B). In mouse macrophages, palmitate inhibits AMP-activated protein kinase (AMPK) activity, leading to defective autophagy and the generation of mitochondrial ROS, which is a proposed mechanism of NLRP3 inflammasome activation99. Ceramide is also sensed by NLRP3 resulting in NLRP3-dependent caspase-1 activation in both mouse bone marrow-derived macrophages (BMDM) and mouse epididymal adipose tissue explants107. Interestingly, replacement of saturated fatty acid (SFA) with monounsaturated fatty acid (MUFA) in HFDs improves insulin sensitivity by reducing IL-1β production via preserved AMPK activity in the mouse model115. Recently, omega 3 fatty acids (ω-3 FAs) which are polyunsaturated fatty acids, have been shown to inhibit NLRP3 inflammasome activity through a G protein-coupled receptor (GPR120)/GPR40-β-arrestin-2 signaling pathway116. More importantly, ω-3 FAs prevented insulin resistance in a HFD-induced T2D model, suggesting the potential dietary use of ω-3 FAs in the amelioration of T2D and other inflammatory diseases116. Using the human THP-1 cell line, others have shown that unsaturated fatty acid can prevent NLRP3 activation, presenting another way to reduce inflammation117.
NLRP3 inflammasome and obesity
Obesity is characterized by excessive expansion of adipose tissue due to adipocyte hypertrophy and immune cell infiltration98. Obesity-associated inflammation leads to functional abnormality of adipocytes, resulting in elevated circulating levels of FFAs and ectopic lipid accumulation118. This can subsequently give rise to multiple metabolic disorders such as atherosclerosis and T2D, as discussed previously. In this section, we will focus on discussing the involvement of inflammasome components in the development of obesity and adipose inflammation.
The expression of human NLRP3 and ASC/PYCARD is upregulated in adipocytes from obese patients119. Caspase-1 expression was found in both human and mouse adipose tissues and increases with adipocyte differentiation and obesity development120. Blockade of caspase-1 and IL-1β, but not IL-18, improves adipogenic gene expression in vitro, indicating that caspase-1 regulates adipogenesis potentially via IL-1β. Differentiated adipocytes with caspase-1 deficiency also have improved adipogenesis and insulin sensitivity compared to wild-type control cells. 120.
To establish the direct link between inflammasome activity and obesity development, HFD- or genetically-induced obese animals lacking inflammasome components have been studied106,120. It was initially reported that caspase-1 contributes to adipose tissue formation, as mice lacking Casp1 have reduced adipocyte size, reduced fat mass, increased adipogenic gene expression and improved insulin sensitivity. Furthermore in the HFD-induced obesity model, mice lacking Casp1 gained less weight than wild-type controls did. In the spontaneously obese mouse model (ob/ob mice), caspase-1 inhibition reduced the body weight of ob/ob mice. Interestingly, caspase-1 blockade resulted in decreased lipogenesis and higher fat oxidation than in control mice but did not affect food intake, suggesting the potential mechanism by which caspase-1 promotes obesity120. Similarly, it was also observed that NLRP3, ASC, and caspase-1 deficiency protected from HFD-induced obesity106. However, a recent study reported contradictory results that mice lacking Casp1 were more obese than control mice including having increased fat mass compared with controls121. The difference may be due to the variation in intestinal microbiota in mice raised in different animal facilities, as intestinal microbiota has been demonstrated to play a significant role in metabolic diseases122. Additionally, IL-18, one of the products of inflammasome activation, has been shown to protect mice from obesity as mice lacking Il18 developed obesity due to increased food intake123. This provides another possibility for the discrepancy in obesity phenotypes observed in Casp1-deficient mice.
Recently, it was shown that the lack of inhibitor of κB kinase epsilon (IKBKE), a downstream mediator of TLR and cytokine signals, in ApoE-deficient mice fed a Western-type diet (high in saturated fat) caused enhanced expression of inflammasome-related genes and low-grade chronic inflammation124. Hence, IKBKE functions as an endogenous negative regulator of the NLRP3 inflammasome under an obesity-inducing condition.
As regards the role of caspase-1 in obesity, studies have shown that it is likely that caspase-1 contributes to obesity through various mechanisms. It was previously thought that macrophages accumulate within inflamed adipose tissue to produce caspase-1125. However, recent studies in mice have shown that a major source of caspase-1 in adipose tissue is independent of infiltrating macrophages120. Recently, caspase-1 was shown to prevent lipid clearing in non-hematopoietic cells by an NLRP3-dependent but IL-1α/β- and IL-18-independent manner126. Furthermore, sirtuin 1 (SIRT1), a deacetylase which can regulate metabolism and protect from obesity, was recently shown to be a caspase-1 substrate. Adipocyte-specific Sirt1 knockout resulted in spontaneous obesity, and SIRT1 protein was cleaved and inactivated in adipose tissues by active caspase-1 under the HFD stress127. However, the mechanism of inflammasome and caspase-1 activation in adipocytes needs clarification.
A strong association between obesity and leukocytosis exists, and inflamed adipose tissue from obese mice was recently found to induce monocytosis in recipient wild type mice128. NLRP3 played an essential role in obesity-induced leukocytosis, as Nlrp3−/− bone marrow reconstituted in ob/ob recipient mice resulted in significantly-reduced numbers of circulating leukocytes128.
THERAPIES THAT TARGET INFLAMMASOMES
Inappropriate inflammasome activity has been incriminated in the pathogenesis of neurodegenerative disease and metabolic disorders. Many reagents that target the inflammasome products IL-1β and IL-18, including recombinant IL-1RA anakinra, the neutralizing IL-1β antibody canakinumab, the soluble decoy IL-1 receptor rilonacept, IL-18–binding protein, soluble IL-18 receptors and anti-IL-18 receptor monoclonal antibodies, have been developed to treat autoinflammatory diseases such as cryopyrin-associated autoinflammatory syndrome (CAPS)129,130. However, independently of IL-1β and IL-18, inflammasome-dependent pyroptosis is a type of inflammatory cell death that will release DAMPs to induce more inflammation and also is important in the pathology of CAPS131. Therefore, inhibitors of the inflammasomes could offer greater therapeutic promise for this condition.
A small-molecule inhibitor, named glyburide, that is commonly used for treatment of T2D was the first compound identified to inhibit NLRP3- but not NLRC4- and NLRP1-dependent IL-1β production132. Glyburide is able to inhibit ATP-, nigericin-, and IAPP-induced NLRP3 inflammasome activation111. However, glyburide’s mechanism of action remains elusive, though it is known to function downstream of the P2X7 receptor and upstream of NLRP3. Importantly, glyburide has been shown to efficiently prevent endotoxic-shock-induced lethality in the animal model of this disease132. A recently identified group of NLRP3 inhibitors targeting P2X7 signaling is the nucleoside reverse transcriptase inhibitors (NRTIs), which are mainly used to block retrovirus replication. NRTIs have efficacy on several inflammatory and autoimmune diseases in mouse models133. Several other small-molecule inhibitors targeting NLRP3, NLRP1, NLRC4 or AIM2, including parthenolide134, Bay 11–708134, CRID3135, auranofin136, isoliquiritigenin137, 3,4-methylenedioxy-β-nitrostyrene138, cyclopentenone prostaglandin 15d-PGJ2139 and 25-Hydroxycholesterol (25-HC)140, have been characterized, even though their potency for in vivo usage needs more evaluation. The large majority of these are pharmacologic inhibitors that have been repurposed to target the inflammasome.
Recently, two additional small-molecule inhibitors that reduced NLRP3 activation have been reported. It was found that the ketone body β-hydroxybutyrate (BHB), which serves as an alternative source of ATP during energy-deficit status, specifically inhibits a variety of stimuli triggering NLRP3 inflammasome activation but not NLRC4 or AIM2 inflammasome activation141. Importantly, in animal models of NLRP3-mediated diseases such as Muckle-Wells syndrome, familial cold autoinflammatory syndrome, and urate crystal-induced peritonitis, BHB-complexed nanolipogels and a ketogenic diet strikingly attenuated caspase-1 activation and IL-1β secretion. It was shown that BHB inhibits the NLRP3 inflammasome by preventing potassium efflux and reducing ASC oligomerization and speck formation, although the direct target of BHB is still under exploration141.
Another study found that the compound MCC950 is a highly selective inhibitor of the NLRP3 inflammasome142. MCC950 blocked both canonical (ATP, nigericin, and monosodium urate) and noncanonical (cytosolic LPS) NLRP3-dependent inflammasome activation at nanomolar concentrations, with no effect on NLRC4, NLRP1, or AIM2 inflammasomes. In vivo, MCC950 has been shown to reduce IL-1β production and attenuate the severity of EAE, a disease model of multiple sclerosis described earlier which is known to be exacerbated by the NLRP3 inflammasome64,68,71. MCC950 rescued the neonatal lethality in a mouse model of CAPS while blockade of IL-1β alone did not prevent lethality, providing evidence for a benefit of inflammasome inhibitors beyond the sole inhibition of IL-1β. Even though the mechanism of NLRP3 inhibition by MCC950 is not fully understood, an extensive assessment of in vitro and in vivo pharmacokinetics of MCC950 has been performed, making significant strides toward therapeutic application142.
Type I interferon has been shown to suppress inflammasome activation with a poorly understood mechanism143. Recent studies showed that an IFN-stimulated gene product cholesterol 25-hydroxylase (Ch25h) antagonizes both Il1b transcription and NLRP3, NLRC4, and AIM2 inflammasome activation, suggesting Ch25h has a broad inhibitory activity of different inflammasomes. More importantly, the Ch25h substrate 25-hydroxycholesterol is able to inhibit NLRP3 inflammasome activation and IL-1β production140.
CONCLUSIONS AND PERSPECTIVES
The new understanding of how inflammasomes are activated in health and disease raises new questions. Can post-translational modifications of inflammasome components be targeted to modulate inflammasome activation? For example, therapies that specifically promote NLRP3 ubiquitination could quell pathologic inflammation driven by NLRP3 inflammasome activation by promoting NLRP3 degradation. What are the contributory roles of inflammasomes in the myeloid lineage compared to other cell types such as endothelial cells, epithelial cells or even adipocytes in inflammatory diseases? Can drugs that directly target inflammasome components, rather than those that target the end products of inflammasomes such as IL-1β, be identified? Two new gain-of-function mutations of NLRC4, Val341Ala and Thr337Ser, causing severe spontaneous autoimmune syndromes have recently been identified in humans44,45. Establishment of the mouse model with similar mutations in NLRC4 will be a powerful tool to study the mechanism of NLRC4 auto-activation-induced autoimmune diseases and evaluate NLRC4 inhibitors in vivo.
Importantly, a greater understanding of the balance between beneficial versus detrimental inflammasome activation is also needed. Indeed, inflammasome activity is critical for host response to microbial pathogens and possibly for optimal response to vaccine adjuvants, as cytokine production by the innate immune system shapes the adaptive immune response. Thus, all inflammasome activation cannot be considered harmful, and the therapeutic inhibition of this pathway has to be balanced against its beneficial contribution. As the mechanistic insight of the inflammasomes increases, opportunities to create new therapies for patients with inflammatory diseases are expected to enhance proportionately.
References
- 1.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature reviews. Immunology. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annual review of cell and developmental biology. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 4.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 5.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015 doi: 10.1016/j.tcb.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Chang HY, Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell. 1998;1:319–325. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 11.Howard AD, et al. IL-1-converting enzyme requires aspartic acid residues for processing of the IL-1 beta precursor at two distinct sites and does not cleave 31-kDa IL-1 alpha. J Immunol. 1991;147:2964–2969. [PubMed] [Google Scholar]
- 12.Gu Y, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 13.Ghayur T, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 14.Vance RE. The NAIP/NLRC4 inflammasomes. Current opinion in immunology. 2015;32C:84–89. doi: 10.1016/j.coi.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poyet JL, et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276:28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasula SM, et al. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 17.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavarría-Smith J, Vance RE. The NLRP1 inflammasomes. Immunol Rev. 2015;265:22–34. doi: 10.1111/imr.12283. [DOI] [PubMed] [Google Scholar]
- 19.Ratsimandresy RA, Dorfleutner A, Stehlik C. An Update on PYRIN Domain-Containing Pattern Recognition Receptors: From Immunity to Pathology. Front Immunol. 2013;4:440. doi: 10.3389/fimmu.2013.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami T, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109:11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee GS, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsnelson MA, Rucker LG, Russo HM, Dubyak GR. K+ Efflux Agonists Induce NLRP3 Inflammasome Activation Independently of Ca2+ Signaling. J Immunol. 2015;194:3937–3952. doi: 10.4049/jimmunol.1402658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dostert C, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juliana C, et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J Biol Chem. 2012;287:36617–36622. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Molecular cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers MA, et al. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J Exp Med. 2014;211:1333–1347. doi: 10.1084/jem.20132486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu A, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai X, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvesen GS, Walsh CM. Functions of caspase 8: the identified and the mysterious. Semin Immunol. 2014;26:246–252. doi: 10.1016/j.smim.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganesan S, et al. Caspase-8 modulates dectin-1 and complement receptor 3-driven IL-1β production in response to β-glucans and the fungal pathogen, Candida albicans. J Immunol. 2014;193:2519–2530. doi: 10.4049/jimmunol.1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurung P, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allam R, et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982–990. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sagulenko V, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Man SM, et al. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1β production. J Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gringhuis SI, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 38.Monie TP, Bryant CE. Caspase-8 functions as a key mediator of inflammation and pro-IL-1β processing via both canonical and non-canonical pathways. Immunol Rev. 2015;265:181–193. doi: 10.1111/imr.12284. [DOI] [PubMed] [Google Scholar]
- 39.Tenthorey JL, Kofoed EM, Daugherty MD, Malik HS, Vance RE. Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Mol Cell. 2014;54:17–29. doi: 10.1016/j.molcel.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Zhao Y, Shi J, Shao F. Human NAIP and mouse NAIP1 recognize bacterial type III secretion needle protein for inflammasome activation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:14408–14413. doi: 10.1073/pnas.1306376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rayamajhi M, Zak DE, Chavarria-Smith J, Vance RE, Miao EA. Cutting edge: Mouse NAIP1 detects the type III secretion system needle protein. J Immunol. 2013;191:3986–3989. doi: 10.4049/jimmunol.1301549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 44.Canna SW, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nature genetics. 2014;46:1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romberg N, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nature genetics. 2014;46:1135–1139. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qu Y, et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature. 2012;490:539–542. doi: 10.1038/nature11429. [DOI] [PubMed] [Google Scholar]
- 47.Matusiak M, et al. Flagellin-induced NLRC4 phosphorylation primes the inflammasome for activation by NAIP5. Proc Natl Acad Sci U S A. 2015;112:1541–1546. doi: 10.1073/pnas.1417945112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin T, et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity. 2012;36:561–571. doi: 10.1016/j.immuni.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin T, Perry A, Smith P, Jiang J, Xiao TS. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J Biol Chem. 2013;288:13225–13235. doi: 10.1074/jbc.M113.468033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Man SM, et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 2015;16:467–475. doi: 10.1038/ni.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang SJ, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149:613–622. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 54.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 55.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi J, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 57.Kajiwara Y, et al. A critical role for human caspase-4 in endotoxin sensitivity. J Immunol. 2014;193:335–343. doi: 10.4049/jimmunol.1303424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang MT, et al. Critical role of apoptotic speck protein containing a caspase recruitment domain (ASC) and NLRP3 in causing necrosis and ASC speck formation induced by Porphyromonas gingivalis in human cells. J Immunol. 2009;182:2395–2404. doi: 10.4049/jimmunol.0800909. [DOI] [PubMed] [Google Scholar]
- 59.Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol. 2009;182:3173–3182. doi: 10.4049/jimmunol.0802367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franklin BS, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hara H, et al. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol. 2013;14:1247–1255. doi: 10.1038/ni.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goverman J. Autoimmune T cell responses in the central nervous system. Nature reviews. Immunology. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 64.Gris D, et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 2010;185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. International immunology. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 66.Furlan R, et al. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination. J Immunol. 1999;163:2403–2409. [PubMed] [Google Scholar]
- 67.Shi FD, Takeda K, Akira S, Sarvetnick N, Ljunggren HG. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J Immunol. 2000;165:3099–3104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- 68.Inoue M, Williams KL, Gunn MD, Shinohara ML. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10480–10485. doi: 10.1073/pnas.1201836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jha S, et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15811–15820. doi: 10.1523/JNEUROSCI.4088-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peelen E, et al. Increased inflammasome related gene expression profile in PBMC may facilitate T helper 17 cell induction in multiple sclerosis. Molecular immunology. 2015;63:521–529. doi: 10.1016/j.molimm.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Inoue M, et al. Interferon-beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Science signaling. 2012;5:ra38. doi: 10.1126/scisignal.2002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw PJ, et al. Cutting edge: critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J Immunol. 2010;184:4610–4614. doi: 10.4049/jimmunol.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dumas A, et al. The inflammasome pyrin contributes to pertussis toxin-induced IL-1beta synthesis, neutrophil intravascular crawling and autoimmune encephalomyelitis. PLoS pathogens. 2014;10:e1004150. doi: 10.1371/journal.ppat.1004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat Immunol. 2015;16:229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 75.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nature immunology. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hook VY, Kindy M, Hook G. Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer disease mice expressing the wild-type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. The Journal of biological chemistry. 2008;283:7745–7753. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- 77.Sheedy FJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature immunology. 2013;14:812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heneka MT, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: genetics and pathogenesis. Annual review of pathology. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 80.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annual review of biochemistry. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 81.Lee SJ. Origins and effects of extracellular alpha-synuclein: implications in Parkinson’s disease. Journal of molecular neuroscience : MN. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- 82.Beraud D, Maguire-Zeiss KA. Misfolded alpha-synuclein and Toll-like receptors: therapeutic targets for Parkinson’s disease. Parkinsonism & related disorders. 2012;18 (Suppl 1):S17–20. doi: 10.1016/S1353-8020(11)70008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrari CC, et al. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiology of disease. 2006;24:183–193. doi: 10.1016/j.nbd.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 84.Codolo G, et al. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PloS one. 2013;8:e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan Y, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 86.Robbins GR, Wen H, Ting JP. Inflammasomes and metabolic disorders: old genes in modern diseases. Molecular cell. 2014;54:297–308. doi: 10.1016/j.molcel.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nature medicine. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 88.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elhage R, et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovascular research. 2003;59:234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 90.Mallat Z, et al. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circulation research. 2001;89:E41–45. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- 91.Tan HW, et al. IL-18 overexpression promotes vascular inflammation and remodeling in a rat model of metabolic syndrome. Atherosclerosis. 2010;208:350–357. doi: 10.1016/j.atherosclerosis.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 92.de Nooijer R, et al. Overexpression of IL-18 decreases intimal collagen content and promotes a vulnerable plaque phenotype in apolipoprotein-E-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:2313–2319. doi: 10.1161/01.ATV.0000147126.99529.0a. [DOI] [PubMed] [Google Scholar]
- 93.Masters SL, Latz E, O’Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Science translational medicine. 2011;3:81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- 94.Kirii H, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 95.Bhaskar V, et al. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011;216:313–320. doi: 10.1016/j.atherosclerosis.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 96.Freigang S, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nature immunology. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 97.Menu P, et al. Atherosclerosis in ApoE-deficient mice progresses independently of the NLRP3 inflammasome. Cell death & disease. 2011;2:e137. doi: 10.1038/cddis.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature reviews. Immunology. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 99.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature immunology. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 101.Legrand-Poels S, et al. Free fatty acids as modulators of the NLRP3 inflammasome in obesity/type 2 diabetes. Biochemical pharmacology. 2014;92:131–141. doi: 10.1016/j.bcp.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 102.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England journal of medicine. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 103.Mandrup-Poulsen T, Pickersgill L, Donath MY. Blockade of interleukin 1 in type 1 diabetes mellitus. Nature reviews. Endocrinology. 2010;6:158–166. doi: 10.1038/nrendo.2009.271. [DOI] [PubMed] [Google Scholar]
- 104.Cavelti-Weder C, et al. Inhibition of IL-1beta improves fatigue in type 2 diabetes. Diabetes care. 2011;34:e158. doi: 10.2337/dc11-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee HM, et al. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stienstra R, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature medicine. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature immunology. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 109.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell metabolism. 2012;15:10–18. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 110.Cooper GJ, et al. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nature immunology. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Janson J, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7283–7288. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maedler K, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. The Journal of clinical investigation. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jourdan T, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nature medicine. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Finucane OM, et al. Monounsaturated Fatty Acid-Enriched High-Fat Diets Impede Adipose NLRP3 Inflammasome-Mediated IL-1beta Secretion and Insulin Resistance Despite Obesity. Diabetes. 2015 doi: 10.2337/db14-1098. [DOI] [PubMed] [Google Scholar]
- 116.Yan Y, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 117.L’Homme L, et al. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. Journal of lipid research. 2013;54:2998–3008. doi: 10.1194/jlr.M037861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature reviews. Molecular cell biology. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yin Z, et al. Transcriptome analysis of human adipocytes implicates the NOD-like receptor pathway in obesity-induced adipose inflammation. Molecular and cellular endocrinology. 2014;394:80–87. doi: 10.1016/j.mce.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell metabolism. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang H, Capell W, Yoon JH, Faubel S, Eckel RH. Obesity development in caspase-1-deficient mice. Int J Obes (Lond) 2014;38:152–155. doi: 10.1038/ijo.2013.59. [DOI] [PubMed] [Google Scholar]
- 122.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 123.Netea MG, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nature medicine. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 124.Patel MN, et al. Hematopoietic IKBKE limits the chronicity of inflammasome priming and metaflammation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:506–511. doi: 10.1073/pnas.1414536112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kotas ME, et al. Role of caspase-1 in regulation of triglyceride metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4810–4815. doi: 10.1073/pnas.1301996110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell metabolism. 2012;16:180–188. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nagareddy PR, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell metabolism. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jesus AA, Goldbach-Mansky R. IL-1 blockade in autoinflammatory syndromes. Annual review of medicine. 2014;65:223–244. doi: 10.1146/annurev-med-061512-150641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Frontiers in immunology. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brydges SD, et al. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. The Journal of clinical investigation. 2013;123:4695–4705. doi: 10.1172/JCI71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lamkanfi M, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. The Journal of cell biology. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fowler BJ, et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 2014;346:1000–1003. doi: 10.1126/science.1261754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Juliana C, et al. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. The Journal of biological chemistry. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Coll RC, Robertson A, Butler M, Cooper M, O’Neill LA. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PloS one. 2011;6:e29539. doi: 10.1371/journal.pone.0029539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Isakov E, Weisman-Shomer P, Benhar M. Suppression of the pro-inflammatory NLRP3/interleukin-1beta pathway in macrophages by the thioredoxin reductase inhibitor auranofin. Biochimica et biophysica acta. 2014;1840:3153–3161. doi: 10.1016/j.bbagen.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 137.Honda H, et al. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. Journal of leukocyte biology. 2014;96:1087–1100. doi: 10.1189/jlb.3A0114-005RR. [DOI] [PubMed] [Google Scholar]
- 138.He Y, et al. 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. The Journal of biological chemistry. 2014;289:1142–1150. doi: 10.1074/jbc.M113.515080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maier NK, Leppla SH, Moayeri M. The Cyclopentenone Prostaglandin 15d-PGJ2 Inhibits the NLRP1 and NLRP3 Inflammasomes. J Immunol. 2015 doi: 10.4049/jimmunol.1401611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reboldi A, et al. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345:679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Youm YH, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nature medicine. 2015 doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Coll RC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nature medicine. 2015 doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guarda G, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]