Abstract
Objectives
A functional vacuolar adenosine triphosphatase (v-ATPase) complex regulates canonical Wnt/β-catenin signaling. The goal of this study was to identify the distribution of the v-ATPase in human and murine models of pancreatic intraepithelial neoplasms (PanINs) and assess its role in Wnt/β-catenin signaling.
Methods
We evaluated the immunolabeling pattern of the v-ATPase in human PanIN specimens and murine PanIN-1 and PanIN-2 lesions obtained from Ptf1aCre/+; LSL-KrasG12D mice. Wnt/β-catenin signaling was interrogated in primary PanIN cells by examining the phosphorylated levels of its surface coreceptor, low-density lipoprotein receptor-related protein-6 (LRP6), and its intracellular effector, nonphosphorylated β-catenin. The response of primary PanIN cells to epidermal growth factor (EGF) was assessed in the absence and presence of the v-ATPase inhibitor, concanamycin.
Results
In advanced (PanIN-2), but not early (PanIN-1), lesions, the v-ATPase assumed a polarized phenotype. Blocking the v-ATPase disrupted Wnt/β-catenin signaling in primary PanIN cells despite significantly higher levels of the total and activated Wnt cell surface coreceptor, LRP6. Vacuolar adenosine triphosphatase blockade significantly decreased the total and activated levels of EGF receptor, a determinant of PanIN progression. The activation of EGF receptor and its intracellular mediator, p44/42 mitogen-activated protein kinase, was also reduced by v-ATPase blockade. This led to diminished proliferation in response to EGF ligand.
Conclusions
The v-ATPase regulates Wnt/β-catenin and EGF receptor signaling in PanINs.
Keywords: v-ATPase, PanIN, Wnt/β-catenin
Cancer cells exist in hypoxic and metabolically challenged conditions.1 This microenvironment evokes compensatory changes in the cancer cell that confer survival properties. One such mechanism is the activity of the vacuolar adenosine triphosphatase (v-ATPase).2–4 This proton transporter mediates multiple pH-dependent processes, including vesicular trafficking, coupled ion gradient-molecular transport, and protease activation.5–8 One explanation of how the v-ATPase contributes to cancer cell growth and invasion may be its ability to provide a localized proton efflux, thereby rating an acidic extracellular microenvironment that favors protease activation. We and others previously noted that v-ATPase expression is increased in human pancreatic cancer specimens and localizes to plasma membranes in certain pancreatic cancer cell lines.9,10 However, the functional outcome of v-ATPase blockade was mixed, with specific protease activities diminished while the activities of others increased.10 This suggests that proton efflux may not be the sole mechanism by which the v-ATPase promotes cancer behavior.
Recently, other mechanisms by which v-ATPase function may drive cancer survival and growth have been proposed.11–13 Cellular migration and developmental signaling require coordinated trafficking of surface receptors and proper control of signal transduction networks. For instance, inhibiting the v-ATPase altered the localization of epidermal growth factor receptor (EGFR), reduced cellular motility, and impeded metastatic spread of cancer cells in vivo.11 Because the presence of EGFR is essential for acinar-to-ductal metaplasia stimulated by mutant Kras or chemically induced pancreatitis,14,15 this suggests that the v-ATPase may adopt a polarized cellular pattern in a manner analogous to EGFR to facilitate receptor recycling and signal transduction. Moreover, the proper function of developmental pathways such as Wnt and Notch requires an intact v-ATPase for proper receptor trafficking and signal transduction.12,13
To address these issues further, v-ATPase localization during different stages of pancreatic intraepithelial neoplasms (PanINs) was assessed. We further evaluated whether the function of the Wnt/β-catenin pathway was v-ATPase dependent in PanIN cells, as has recently been proposed.12 Our work demonstrates that advanced, but not early, precancerous PanIN lesions show polarized labeling for the v-ATPase. We further demonstrate that the v-ATPase regulates canonical Wnt/β-catenin signaling in a primary PanIN cell line. In the presence of v-ATPase blockade, increased levels of the Wnt surface receptor, low-density lipoprotein receptor-related protein-6 (LRP6), occurred in conjunction with decreased intracellular levels of its nuclear effector, β-catenin. Thus, our work reveals that the v-ATPase regulates the normal signal transduction of a developmental pathway at the advanced PanIN stage.
MATERIALS AND METHODS
Human PanIN Lesions
Paraffin-embedded specimens from patients who underwent surgery for pancreatic ductal adenocarcinoma were immunolabeled to evaluate v-ATPase intensity and labeling (n = 16). The institutional review board of the VA CT Healthcare System approved the study.
Mouse Models of PanIN Lesions
The LSL-KrasG12D and Ptf1aCre/+ mice were previously described.16 The strains were interbred to obtain Ptf1aCre/+; LSL-KrasG12D mice, which served as the models of advanced (PanIN-2) lesions. Ptf1aCre/+ littermates served as controls. The mice used in the study were 24 weeks of age.
Antibodies and Reagents
Antibodies to p44/42 mitogen-activated protein kinase (MAPK), No. 9102; phospho-p44/42 MAPK, No. 4370; EGFR, No. 2232; phospho-EGFR, No. 3777 (Cell Signaling, Danvers, Mass); V1E (Genway, Sigma, St Louis, Mo); as well as V0a1, V0a2, and V0a3 (gift of Dr Dennis Stone, University of Texas Southwestern Medical Center, Dallas, Tex) were used to assess v-ATPase isoform specificity and responses to v-ATPase-dependent cellular proliferation signals. Antibodies to phospho-low-density lipoprotein receptor-related protein-6 (LRP6) No. 1490; total LRP6, No. 2560; nonphosphorylated (active) β-catenin, No. 4270; total β-catenin, No. 8480; phospho-GSK3β, No. 9327; and total glycogen synthase kinase 3β (GSK3β) No. 9315 (Cell Signaling) were used to interrogate canonical Wnt/β-catenin signaling. Secondary fluorescent antibodies were purchased from Invitrogen. Chemical reagents were purchased from Sigma, unless specified. The inhibitor of v-ATPase function, concanamycin (10–50 nM), was used in cell culture experiments.
Cell Culture
A primary PanIN line, PI34, was generated from Pdx-Cre; LSL-KrasG12D; p16fl/fl; yellow fluorescent protein mice at 6 weeks of age when only PanIN-1, PanIN-2, as well as PanIN-3 lesions and no invasive carcinoma were detected on histology. Yellow fluorescent protein + pancreas epithelial lineage-labeled cells were isolated by flow cytometry as previously described.17 The cells were maintained in Dulbecco’s modified eagle medium + fetal calf serum 10%. To obtain conditioned medium, the cells were grown to 80% to 90% confluence, washed twice with serum-free media, and then incubated with serum-free media overnight. Conditioned medium was obtained after 18 to 20 hours under control and v-ATPase blockade conditions.
Immunohistochemistry and Immunofluorescence
Immunohistochemistry was performed as described.18 Sections were deparaffinized, treated to inhibit endogenous peroxidase, and subjected to antigen retrieval. Slides were washed in tris-buffered saline and incubated with primary antibodies. Sections were washed as well as incubated with biotinylated anti-serum and then with streptavidin complexed with horseradish peroxidase followed by diaminobenzidine. The sections were then counterstained with hematoxylin and eosin.
Immunofluorescence labeling was performed on tissue sections and primary PanIN cells. In brief, the tissue sections and the PanIN cells on coverslips were rinsed with phosphate-buffered saline, permeabilized with 0.05% Triton-X for 5 to 10 minutes, and blocked in 2% bovine serum albumin. Samples were incubated with primary antibody and then with the corresponding secondary antibodies. Slides were mounted with ProLong Gold with DAPI (Invitrogen). Control slides were incubated in secondary antibody only. Pancreatic intraepithelial neoplasm lesions with v-ATPase labeling were stained with Alcian blue to assess acidic mucins and counterstained with nuclear fast red solution.19 Immunofluorescence and differential interference contrast microscopy images were obtained on a Zeiss Axiovert microscope and adjusted on Adobe Photoshop, version 9.0.
Immunoblotting
Immunoblotting was performed as described.18 Protein content was determined by Pierce assay. Proteins were run on a gradient gel and transferred to polyvinylidene difluoride membranes. After blocking in a 5% milk solution, membranes were incubated overnight with primary antibodies. After washing, the primary antibodies were labeled using a peroxidase-conjugated goat IgG against the host species of the primary antibody. Peroxidase was detected by a chemiluminescence assay (Pierce). Equivalence of loading was confirmed using β-actin (Sigma) or Coomassie stain of conditioned medium. Blots were developed by film or visualized on a Bio-Rad imager and integrated densitometry assessed by Image J. Values were expressed as a percentage of the control or vehicle for ease of comparison.
Cellular Proliferation
WST-1 assay was performed according to manufacturer’s instructions. Briefly, 5 × 103 primary PanIN (PI34) cells were plated in a 96-well plate, placed in 0.1% fetal calf serum media after reaching 70% confluence, and then challenged with EGF 10 pg/mL to 100 ng/mL for 24 hours to assess optimal proliferative responses to EGF. The PanIN cells were then challenged with EGF 1 ng/mL in the absence and presence of concanamycin 10 nM.
Statistical Analysis
P values were calculated using 2-tailed Student t tests on Prism software version 4.0, with P < 0.05 deemed statistically significant. Values were stated as mean ± SEM.
RESULTS
V-ATPase Undergoes Basolateral Localization in Advanced PanIN Lesions in Humans
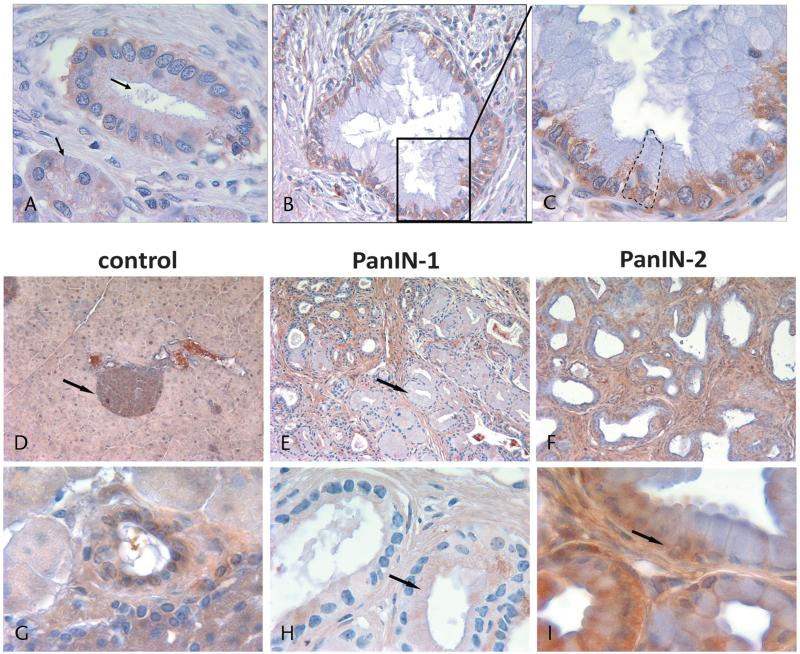
Regions of cancer cells reflecting the leading edge or invasive front are responsible for focal protease release required for matrix degradation and cell invasion.10,20 Alternatively, the localization of receptors initiates growth and developmental pathways known to promote cell survival and proliferation. We investigated whether these changes occur in human PanIN lesions. Low-grade PanIN lesions (Fig. 1A, arrow) with typical columnar morphology displayed diffuse labeling of the V1E subunit. This diffuse labeling pattern was also seen in a section of normal acinar cells (Fig. 1A, arrowhead). With more neoplastic features such as a cribriform pattern of cells and increased nuclear-cytoplasmic ratio seen in advanced PanIN lesions, V1E labeling was situated along the basolateral membranes (Figs. 1B, C; magnified view of inset in 1B with the outline of polarized labeling of columnar cell). We next addressed whether this change in v-ATPase localization occurs in murine models of PanINs.
FIGURE 1.
Human pancreatic tissue demonstrates v-ATPase labeling in advanced, but not early, PanIN lesions. A, Low-grade PanIN lesions displayed diffuse labeling of the V1E subunit (arrow). A diffuse labeling pattern was also seen in a normal section of acinar cells (arrowhead). B and C, In advanced PanIN lesions, V1E labeling was situated along the basolateral membranes (C, magnified view of inset in B with the outline of columnar-appearing cell). D, In the control mice, v-ATPase staining was notable in islets (arrow). E and H, The PanIN-1 lesions demonstrated heterogeneous V1E labeling with predominantly diffuse staining pattern (arrow). F and I, The PanIN-2 lesions display more robust staining of the v-ATPase and prominent basolateral distribution of V1E labeling in some cells (arrow). Low-magnification images, ×10; high-magnification images, ×40.
V-ATPase Localizes to the Basolateral Domain in Murine Models of Advanced PanINs
In the control Ptf1aCre/+ mice, labeling of the v-ATPase was prominent in islets (Fig. 1D, arrow), a finding that is consistent with its known localization.21 Under higher magnification, acinar cells and ducts displayed homogeneous labeling of the V1E subunit (Fig. 1G). Pancreatic intraepithelial neoplasm 1 lesions from the Ptf1aCre/+; LSL-KrasG12D mice also demonstrated heterogeneous V1E labeling, with some ducts labeling more robustly than others (Fig. 1E, arrow). In early PanIN lesions, V1E staining of cells was diffuse rather than polarized (Fig. 1H, arrow). In more advanced PanIN-2 lesions, characterized by heterogeneity of cells and a cribriform pattern, V1E staining became more prominent (Fig. 1I), with a prominent basolateral distribution of cellular labeling in some cells (Fig. 1I, arrow). Thus, labeling of the v-ATPase subunit V1E in murine models of PanIN formation recapitulates those found in human PanIN specimens.
Distinct cellular localization of the v-ATPase in the early versus advanced PanIN lesions in human pancreatic tissue was further delineated. Alcian blue staining confirmed the presence of acidic mucins in the early and advanced PanIN lesions (Figs. 2A, D; ×20). Differential interference contrast microscopy and Alcian blue staining of the early (Figs. 2B, C) and advanced PanIN (Figs. 2F, G) lesions confirmed columnar morphology but with pseudopalisading cells, irregularly oriented nuclei, and increased nuclear atypia in the advanced PanIN lesions (Figs. 2F–H). The early PanIN lesions demonstrated v-ATPase localization in both apical and basolateral cellular compartments (Fig. 2D). The advanced PanIN specimens demonstrated v-ATPase localization confined mainly to the basolateral region (Fig. 2H; arrow). These findings indicate that, with advancing PanIN stage, the v-ATPase adopts a basolateral distribution within the cell that may play a role in PanIN progression.
FIGURE 2.
Advanced PanIN lesions in humans reveal a basolateral distribution of the v-ATPase. A to D, Early PanIN lesions demonstrate a diffuse pattern of v-ATPase labeling: A, Alcian blue labeling confirms the presence of acidic mucins, ×20. B, Differential interference contrast microscopy. C, Alcian blue labeling showing uniform nuclei of the early PanIN lesion, ×40. D, The V-ATPase labeling (red) of the early PanIN showing both apical (arrow) and basolateral labeling. E to H, More advanced PanIN lesions show a polarized basolateral distribution of the v-ATPase: E, Alcian blue labeling, ×20. F, Differential interference contrast microscopy. G, Alcian blue stain with pronounced nuclear atypia and pseudopalisading morphology. H, Pronounced basolateral v-ATPase distribution (arrow). The images acquired at ×20 and ×40 magnification. Scale bar represents 40 μM. L indicates lumen.
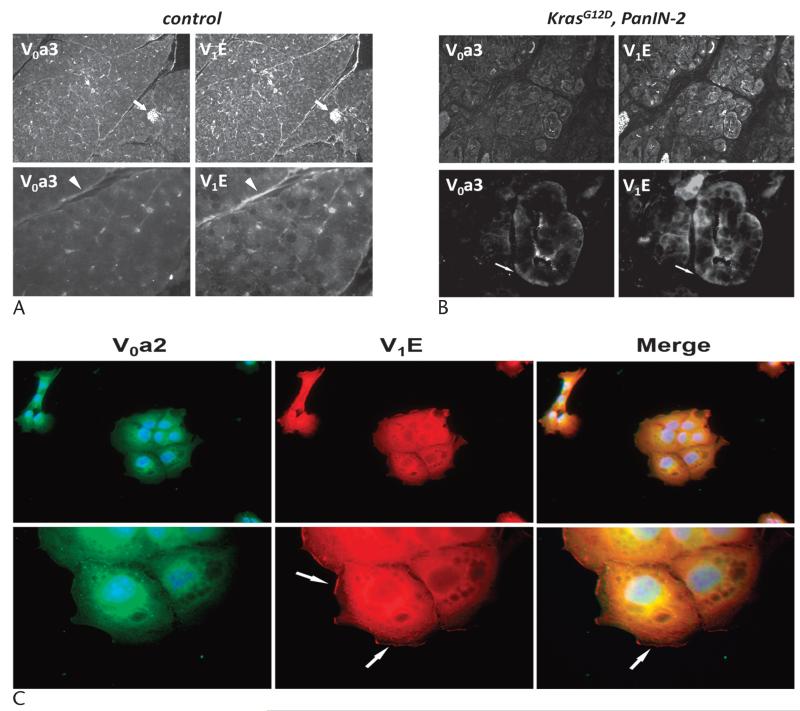
Specific subunits of the v-ATPase are known to localize to plasma membranes. Thus, we next investigated whether the V0a3 and V1E subunits shared localization in the control and PanIN-2 murine pancreas. In the control specimens, both subunits prominently labeled islets (Fig. 3A, arrows), as previously described, as well as ductal structures (Fig. 3A, arrowheads). In the PanIN-2 lesions, V0a3 and V1E subunits localized to the basolateral domain in specific regions (Fig. 3B, arrows). In addition, primary PanIN cells, PI34, were stained for V1E and also noted to have plasma membrane localization (Fig. 3C, arrow). These results indicate that, in advanced PanIN lesions and cells, v-ATPase localization is polarized.
FIGURE 3.
Murine models of PanIN-2 lead to polarized v-ATPase expression. A, Pancreatic tissue from the control mice shows prominent V0a3 and V1E labeling of islets (arrows) and ducts (arrowheads). B, Pancreatic tissue from PanIN-2 models shows polarized labeling in some ducts (arrows). C, Primary PanIN cells show peripheral labeling of the V1E subunit (arrows) without labeling for the V0a2 subunit. Low-magnification images, ×40; high-magnification images, ×100.
V-ATPase Regulates Canonical Wnt/β-catenin Signaling in Primary PanIN Cells
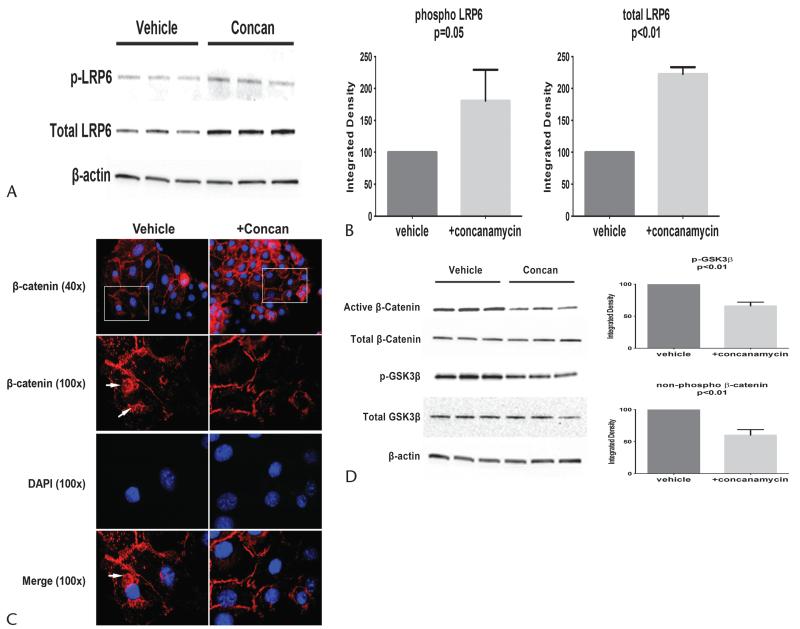
Because canonical Wnt/β-catenin signaling is implicated in pancreatic carcinogenesis and is regulated by a functional v-ATPase network,12,22 we examined the effects of v-ATPase blockade in primary PanIN cells. Vacuolar adenosine triphosphatase blockade led to a more than 2-fold increase in the levels of the cell surface receptor, total LRP6, and a nearly 80% increase in its active form, phospho-LRP6 (Figs. 4A, B).
FIGURE 4.
The v-ATPase is necessary for normal Wnt/β-catenin signaling in primary PanIN cells. A, Phospho-LRP6 and total LRP6 levels increased with concanamycin exposure. B, Quantification of blots showing approximately 80% increase in phospho-LRP6 with concanamycin. C, Blockade of the v-ATPase led to diminished intracellular levels of β-catenin in primary PanIN cells compared with vehicle-treated cells. D, Immunoblots of active (nonphosphorylated) β-catenin show reduced levels with concanamycin 10 nM exposure despite increased representative blot from a total of 3 separate experiments.
Increased phospho-LRP6 should result in enhanced levels of its downstream effector, nonphosphorylated (active) β-catenin. Immunofluorescent images of PI34 cells revealed cell surface labeling of β-catenin under control and concanamycin conditions (Fig. 4C). Cytoplasmic and perinuclear staining of β-catenin (Fig. 4C, arrows) was notable under control conditions but was minimal in the presence of concanamycin. This was confirmed by immunoblotting where v-ATPase blockade led to reduced nonphosphorylated β-catenin by nearly 50% despite similar levels of total β-catenin (Fig. 4D).
Central to the control of canonical Wnt signaling is the constitutive negative regulation of intracellular β-catenin. Phosphorylation of GSK3β leads to the inhibition of GSK3β activity on β-catenin and the stabilization of β-catenin.23 Under concanamycin conditions, phosphorylated (inactive) GSK3β was reduced by approximately 60%, amounting to an appropriate decrease in active β-catenin levels (Fig. 4D). Although LRP6 activation should result in increased GSK3β inhibition, the opposite effect occurred. These effects indicate that blocking the v-ATPase uncouples the activation of Wnt cell surface receptor from its downstream effectors, GSK3β and β-catenin.
V-ATPase Blockade Decreases EGFR Levels and PanIN Proliferation in Response to EGF
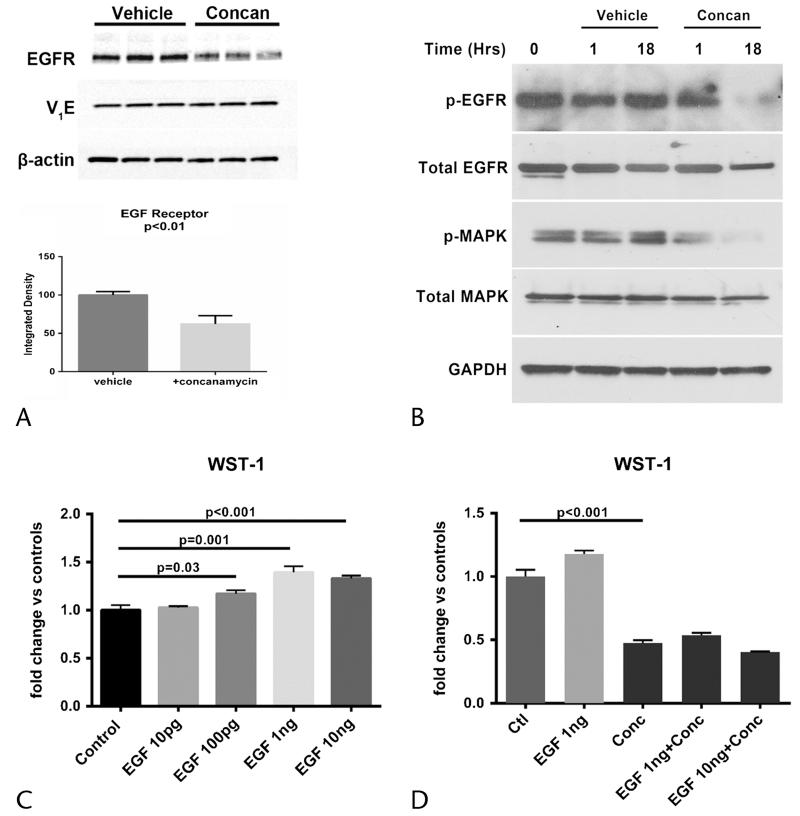
A recently detailed driver mechanism toward carcinogenesis in mutant Kras cells is the presence of EGFR signaling.15 Previous studies on breast cancer cells have described changes in EGFR distribution with v-ATPase blockade,11 but whether EGFR levels are altered by v-ATPase inhibition in PanIN cells is unknown. The EGFR levels decreased by 40% in concanamycin-treated PI34 cells without affecting the levels of the V1E subunit (Fig. 5A). Blocking v-ATPase activity for 1 hour did not alter the levels of phospho-EGFR, total EGFR, or intracellular effectors such as p44/42 MAPK (Fig. 5B). At 18 hours, however, the activation of phospho-EGFR, total EGFR, and MAPK was diminished in concanamycin-treated cells (Fig 5B). Functionally, diminished levels of EGFR and its activated form led to reduced proliferation in response to EGF ligand. PI34 cells demonstrated a significant proliferative response to EGF from 100 pg/mL to 10 ng/mL (Fig. 5C), which was significantly blocked by concanamycin (Fig. 5D). These findings indicate that PanIN proliferative responses to EGF are v-ATPase dependent.
FIGURE 5.
Effects of v-ATPase blockade on EGFR and EGF-mediated proliferation. A, Immunoblot and corresponding quantification show that concanamycin 10 nM decreases EGFR levels by 40% without affecting the levels of v-ATPase subunit V1E. B, Immunoblot of P134 cells shows phospho-EGFR and phospho-MAPK levels 1 and 18 hours after v-ATPase inhibition (10-nM concanamycin). C, P134 cells show a concentration-dependent proliferative response to EGF. D, Proliferative responses to EGF in PanIN cells are blocked in the presence of concanamycin 10 nM. Representative results from a total of 3 to 5 separate experiments.
DISCUSSION
Previous studies on human pancreatic cancer specimens had found higher gene expression and immunolabeling of various v-ATPase subunits in pancreatic ductal adenocarcinoma compared with the normal pancreas and with the benign cystic pancreatic tumors.9,10 We previously showed that plasma membrane localization of the v-ATPase in pancreatic cancer cells lines occurred with differential effects on protease activation.10 The current study examined experimental PanIN lesions to determine whether basolateral and plasma membrane localization of the v-ATPase occurred in premalignant lesions, whether it corresponded to a specific stage of PanIN development, and whether this might affect the regulators of neoplasia. In this study, we found that the v-ATPase localized in a basolateral labeling pattern in advanced (PanIN-2), but not early (PanIN-1), experimental PanIN lesions. We also found that a functional v-ATPase is essential for canonical Wnt/β-catenin signaling in primary PanIN cells.
The polarized staining pattern of the v-ATPase at the advanced PanIN stage and its role in Wnt/β-catenin signal transduction correlate with findings from clinical studies. Tissue microarray analysis of human PanIN lesions has shown cytoplasmic and nuclear β-catenin in advanced, but not early, PanIN lesions.24 Combined with the current study, which shows a polarized v-ATPase at a defined stage of PanIN development, this suggests a role for the v-ATPase in regulating developmental signaling pathways that advance the neoplastic phenotype. In fact, a functional v-ATPase has been implicated in canonical Wnt/β-catenin signaling and Notch activation.12 In the Wnt signaling study, v-ATPase blockade abrogated LRP6 phosphorylation, activation, and downstream signaling. Interestingly, our data in PanIN cells and pancreatic cancer cells (data not shown) demonstrate that Wnt/β-catenin signaling is disrupted but not through impairment of LRP6 phosphorylation, which actually increased with v-ATPase blockade. Rather, increased LRP6 receptor activation with v-ATPase blockade resulted in decreased levels of its nuclear effector, the activated β-catenin. This indicates that a functional v-ATPase is necessary for proper signal transduction from surface receptor activation to active intracellular β-catenin. In the context of v-ATPase localization on basolateral membranes in advanced, but not early, PanINs, this lends additional evidence for a temporally defined role for the v-ATPase in mediating developmental signaling pathways such as canonical Wnt signaling.
Although the current study shows that Wnt/β-catenin signaling depends on a functional v-ATPase in PanIN cells, the role of this developmental pathway in PanIN lesion development and progression remains unclear. Experimental models using pancreas lineage-specific expression of constitutively active β-catenin lead to indolent solid-pseudopapillary neoplasms rather than PanIN formation.25 Even more surprising, constitutively active β-catenin negates the ability of mutant KrasG12D to induce PanIN formation in mice.25 This indicates that Wnt/β-catenin signaling regulates acinar cell differentiation in models using mutant KrasG12D, raising the possibility that Wnt signaling must be temporally regulated for PanINs to develop.26 In the current context, where a functional v-ATPase was necessary for normal Wnt signal transduction, the specific polarization of the v-ATPase at the advanced PanIN stage suggests the involvement of the Wnt pathway at a late stage of PanIN differentiation.
The current study also highlights the role of the v-ATPase in regulating EGFR levels and suggests additional regulatory mechanisms that control its levels. Studies using pancreas-specific mutant Kras mice demonstrated that EGFR was absolutely required for PanIN and pancreatic cancer development in this model.15 Although our study did not define a direct link between the Wnt/β-catenin signaling and the suppression of EGFR, it has been previously shown that overexpressing β-catenin increases EGF and EGFR expression in other organs.27 Thus, our results point to the possibility that v-ATPase blockade through the negative regulation of Wnt/β-catenin signaling may modulate EGFR levels.
In this study, we addressed the localization of the v-ATPase at the advanced PanIN stage. In a previous study, we identified a role for v-ATPase activity in mediating protease activation in pancreatic cancer cells, thereby contributing to matrix turnover.10 Because matrix degradation by proteases can also liberate ligands such as EGF,28 the results in this study suggest that v-ATPase activity may promote cellular tumor growth by providing sources of growth factors and maintaining signaling networks in response to ligands such as EGF.
In summary, v-ATPase staining of experimental PanIN formation in a well-characterized mutant Kras model demonstrates a loss of polarity that occurs with advanced, but not early, PanIN grades. These distinct changes are seen in grade 2 PanIN lesions, suggesting a temporal role for v-ATPase polarization at this stage of malignant transformation. Because the orchestrated activation of Wnt/β-catenin is v-ATPase dependent and necessary for pancreatic tumorigenesis, these findings suggest a role for the v-ATPase in regulating Wnt/β-catenin at the PanIN stage.
ACKNOWLEDGMENT
The authors thank Dr Arijeet Gattu for technical assistance.
This study was supported by NIH Liver Center Core DK34989, VA Merit Award (C.C.); and NIH DK088945 (A.D.R.).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinton A, Bond S, Forgac M. V-ATPase functions in normal and disease processes. Pflugers Arch. 2009;457:589–598. doi: 10.1007/s00424-007-0382-4. [DOI] [PubMed] [Google Scholar]
- 3.Sennoune SR, Martinez-Zaguilan R. Plasmalemmal vacuolar H + −ATPases in angiogenesis, diabetes and cancer. J Bioenerg Biomembr. 2007;39:427–433. doi: 10.1007/s10863-007-9108-8. [DOI] [PubMed] [Google Scholar]
- 4.Marshansky V, Futai M. The V-type H + −ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol. 2008;20:415–426. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson N. Structure, molecular genetics, and evolution of vacuolar H + −ATPases. J Bioenerg Biomembr. 1989;21:553–571. doi: 10.1007/BF00808113. [DOI] [PubMed] [Google Scholar]
- 6.Arvan P, Castle JD. Isolated secretion granules from parotid glands of chronically stimulated rats possess an alkaline internal pH and inward-directed H + pump activity. J Cell Biol. 1986;103:1257–1267. doi: 10.1083/jcb.103.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orci L, Ravazzola M, Amherdt M, et al. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol. 1986;103:2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyenbach KW, Wieczorek H. The V-type H + ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol. 2006;209:577–589. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- 9.Ohta T, Numata M, Yagishita H, et al. Expression of 16 kDa proteolipid of vacuolar-type H(+)-ATPase in human pancreatic cancer. Br J Cancer. 1996;73:1511–1517. doi: 10.1038/bjc.1996.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung C, Mader CC, Schmitz JC, et al. The vacuolar-ATPase modulates matrix metalloproteinase isoforms in human pancreatic cancer. Lab Invest. 2011;91:732–743. doi: 10.1038/labinvest.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiedmann RM, von Schwarzenberg K, Palamidessi A, et al. The V-ATPase-inhibitor archazolid abrogates tumor metastasis via inhibition of endocytic activation of the Rho-GTPase Rac1. Cancer Res. 2012;72:5976–5987. doi: 10.1158/0008-5472.CAN-12-1772. [DOI] [PubMed] [Google Scholar]
- 12.Cruciat CM, Ohkawara B, Acebron SP, et al. Requirement of prorenin receptor and vacuolar H + −ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 13.Vaccari T, Duchi S, Cortese K, et al. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development. 2010;137:1825–1832. doi: 10.1242/dev.045484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardito CM, Gruner BM, Takeuchi KK, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304–317. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navas C, Hernandez-Porras I, Schuhmacher AJ, et al. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318–330. doi: 10.1016/j.ccr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eser S, Messer M, Eser P, et al. In vivo diagnosis of murine pancreatic intraepithelial neoplasia and early-stage pancreatic cancer by molecular imaging. Proc Natl Acad Sci U S A. 2011;108:9945–9950. doi: 10.1073/pnas.1100890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung C, Shugrue C, Nagar A, et al. Ethanol exposure depletes hepatic pigment epithelium-derived factor, a novel lipid regulator. Gastroenterology. 2009;136:331–340. doi: 10.1053/j.gastro.2008.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasca di Magliano M, Sekine S, Ermilov A, et al. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 21.Sun-Wada GH, Toyomura T, Murata Y, et al. The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J Cell Sci. 2006;119:4531–4540. doi: 10.1242/jcs.03234. [DOI] [PubMed] [Google Scholar]
- 22.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Al-Aynati MM, Radulovich N, Riddell RH, et al. Epithelial-cadherin and beta-catenin expression changes in pancreatic intraepithelial neoplasia. Clin Cancer Res. 2004;10:1235–1240. doi: 10.1158/1078-0432.ccr-03-0087. [DOI] [PubMed] [Google Scholar]
- 25.Heiser PW, Cano DA, Landsman L, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288–1300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puri S, Hebrok M. Cellular plasticity within the pancreas—lessons learned from development. Dev Cell. 2010;18:342–356. doi: 10.1016/j.devcel.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan X, Apte U, Micsenyi A, et al. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129:285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grahovac J, Wells A. Matrikine and matricellular regulators of EGF receptor signaling on cancer cell migration and invasion. Lab Invest. 2014;94:31–40. doi: 10.1038/labinvest.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]