Abstract
Feline oral squamous cell carcinoma (OSCC) is the most common oral tumor in cats. There is no effective treatment, and the average duration of survival after diagnosis is only 2 months. Feline OSCC is frequently associated with osteolysis; however, the mechanisms responsible are unknown. The objective of this study was to characterize the epidemiology and pathology of bone-invasive OSCC in cats and to determine the expression of select bone resorption agonists. In sum, 451 cases of feline OSCC were evaluated. There was no sex or breed predisposition, although there were more intact cats in the OSCC group compared to the control group. Gingiva was the most common site, followed by the sublingual region and tongue. Cats with lingual OSCC were younger (mean, 11.9 years) compared to cats with gingival OSCC (mean, 13.6 years). In addition to osteolysis, there was periosteal new bone formation, osseous metaplasia of tumor stroma, and direct apposition of OSCC to fragments of bone, suggestive of bone-binding behavior. Eighty-two cases were selected for immunohistochemical detection of parathyroid hormone-related protein (PTHrP). Specimens with osteolysis had increased PTHrP expression and nuclear localization, compared to OSCC without osteolysis. Thirty-eight biopsies of OSCC with osteolysis were evaluated for tumor necrosis factor α expression, and only 4 biopsies had such expression in a small proportion of tumor cells. Increased tumor expression of PTHrP and increased localization of PTHrP to the nucleus were associated with osteolysis and may play an important role in bone resorption and tumor invasion in cats with OSCC.
Keywords: bone resorption, feline, immunohistochemistry, oral squamous cell carcinoma, parathyroid hormone-related protein, osteolysis, tumor necrosis factor alpha, cat, domestic mammals, species, digestive tract, tissue, oncology, disease process, technology
Oral squamous cell carcinoma (OSCC) is the most commonly diagnosed tumor of the oral cavity in cats.8,30 It is extremely aggressive, as characterized by destruction of oral tissues and bone invasion, and it is often associated with tooth loss, ulceration, and secondary bacterial infections.20 Although early surgical removal may be curative, most cats have invasive disease at the time of diagnosis and require extensive resections that are associated with morbidity,13,22 or the tumors are so extensive that they are no longer surgical candidates.20 There is still no effective treatment for feline OSCC, and average duration of survival after diagnosis rarely exceeds 2 months.11
The purpose of this study was to describe the epidemiology and pathology of cats with OSCC, with emphasis on characterizing OSCC-associated bone resorption, and to determine the expression of various candidate regulators of osteoclastic activity. We evaluated 451 surgical biopsies, in which we characterized the patient signalment and history, clinical signs, size of lesion, histopathology, and type of bone interaction.
Factors that can play an important role in bone resorption include parathyroid hormone–related protein (PTHrP), interleukin 1α (IL-1α), tumor necrosis factor α (TNFα), receptor activator of nuclear factor κB ligand (RANKL), osteoprotegerin (OPG), and transforming growth factor β1 (TGFβ1). RANKL is a direct stimulator of osteoclast activation normally expressed by osteoblasts, but it is also expressed by a number of bone-invasive tumors, including feline1 and human OSCC.6 PTHrP, IL-1α, and TNFα stimulate osteoblast expression of RANKL and are expressed in human OSCC.29 PTHrP expression has also been demonstrated in a feline OSCC cell line.33 OPG is a soluble receptor for RANKL and inhibits activation of osteoclasts. OPG is expressed at low levels in human OSCC.6 TGFβ1 is a growth factor that has stimulatory and inhibitory effects on osteoclastic bone resorption and has been shown to be expressed by human OSCC epithelial cells25 and stroma.27
Materials and Methods
Biopsy Material and Patient Signalment
In sum, 451 hematoxylin and eosin–stained (HE-stained) tissue sections from surgical biopsies of feline OSCC were acquired from Veterinary Diagnostics Ltd (Columbus, OH), IDEXX Laboratories, Inc (Worthington, OH), and Clinilab, Inc (Valpariaso, IN). Biopsies were submitted between 1998 and 2006 from 282 veterinary practices and practitioners from 11 states. In total, 309 cases (68%) were from Ohio. Tumors were assigned to the following anatomic locations based on information provided: tongue, sublingual (includes ventral tongue, frenulum, mouth floor), gingiva (includes tumors associated with teeth, mandible, maxilla, and jaw), hard palate, soft palate, tonsils, pharynx, larynx, buccal mucosa, lip, and not reported. Clinical history was summarized, including symptoms, size of tumor, and evidence of bone resorption. The breed, hair length (long, short, or undetermined based on reported breed), sex, and reproductive status (intact or neutered) of 389 cats (≥ 8 years of age) with OSCC were compared to 391 cats (≥ 8 years of age) that had tissue submitted to IDEXX Laboratories in 2005, regardless of diagnosis. Limiting the cases to this age group was performed to minimize the effect of age on neuter status, particularly in the control group, which included a greater proportion of immature cats compared to the OSCC group. The HE-stained tissue sections were evaluated microscopically to confirm the presence of OSCC and to determine the presence of bone in the biopsy material. Specimens were evaluated for evidence of new bone formation (high osteocyte density and woven collagen pattern), osteoclastic bone resorption (eroded bone surfaces with or without osteoclasts), and evidence of OSCC adhesion to bone (OSCC in direct contact with bone).
Immunohistochemistry for the Detection of Bone Resorption Mediators in Feline Tissues
Immunohistochemical protocols were designed for the detection of several candidate factors known to regulate osteoclastic bone resorption, including PTHrP, TNFα, IL-1α, RANKL, OPG, and TGFβ1. The primary antibodies were polyclonal rabbit anti-human PTHrP (34–53, AB-2, Calbiochem, La Jolla, CA), polyclonal goat anti-human TNFα (R&D Systems, Minneapolis, MN), polyclonal goat anti-human IL-1α (R&D Systems), monoclonal mouse anti-human RANKL (clone 70525, R&D Systems), polyclonal goat anti-human OPG (R&D Systems), and polyclonal rabbit anti-human TGFβ1 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA). The secondary antibodies were biotin-labeled goat anti-rabbit immunoglobulin G (IgG; Zymed, San Francisco, CA), biotin-labeled horse anti-goat IgG (Vector Laboratories, Burlingame, CA), and biotin-labeled horse anti-mouse IgG (Vector Laboratories). Feline skin served as positive control tissue for PTHrP and TGFβ1; mouse skin and feline ovary were also used for TGFβ1; feline mammary carcinoma, for TNFα; feline bone and lymph node, for RANKL and OPG; and feline lymph node, for IL-1α. A range of primary antibody dilutions were evaluated, ranging from 0.2 to 5.0 μg/ml for all antibodies except PTHrP (0.2 to 2.0 μg/ml), TGFβ1 (0.05 to 4.0 μg/ml) and RANKL (1 to 25 μg/ml). Blocking peptides for PTHrP (Calbiochem), TNFα (R&D Systems), and TGFβ1 (Santa Cruz Biotechnology) were preincubated with the primary antibodies (10:1, peptide:anti-body) to determine the primary antibody specificity. Blocking peptides for RANKL, OPG, and IL-1α were not used, because these antibodies did not work on feline control tissues and further optimization was not pursued. Two forms of antigen retrieval were evaluated: target retrieval solution (Dako, Carpinteria, CA) at 95°C for 20 minutes, followed by room temperature cooling for 20 minutes, and digestion with 0.02 units/ml recombinant α2,3-neuraminidase (Calbiochem) for 60 minutes at room temperature. Specificity of secondary antibodies was evaluated by omitting the primary antibody from the protocol. Protocols that yielded signals on positive control tissue and those that demonstrated specificity of the primary and secondary antibodies were selected for evaluating feline OSCC specimens (PTHrP and TNFα). Positive and negative controls were run with all OSCC slides. Pancytokeratin (AE1/AE3) immunohistochemistry was performed on select tissues with evidence of OSCC adhesion to bone to confirm the direct apposition of tumor cells with the bone matrix; it was performed in the Histology and Immunohistochemistry Laboratory in the Department of Veterinary Biosciences.
Immunohistochemistry for PTHrP and TNFα in Feline OSCC Biopsies
Forty-four feline OSCC tumors with microscopic evidence of osteolysis were selected, as were 38 feline OSCC tumors without microscopic evidence or reported clinical history of osteolysis. Tissues were routinely processed, sectioned, and mounted on glass slides. Sections were dewaxed and rehydrated through stepwise washes of xylene and descending concentrations of ethanol followed by water. For PTHrP immunohistochemistry, antigen retrieval was achieved by neuraminidase digestion as described above. Endogenous peroxidases were quenched with a ready-to-use peroxidase blocking reagent (Dako) for 30 minutes at room temperature. Nonspecific binding was blocked with serum-free, ready-to-use protein block (Dako) for 20 minutes at room temperature. Protein block was replaced by PTHrP primary antibody diluted to 1 μg/ml in serum-free protein block. Tissues were incubated with primary antibody in a humidified chamber at 4°C for 16 hours. After being washed in phosphate buffered saline, tissue sections were incubated with biotin-labeled goat anti-rabbit IgG. The avidin–biotin–peroxidase method (Vector Laboratories) was then applied according to the manufacturer’s instructions. Color development was achieved with Dako diaminobenzidine chromogen system. Feline skin served as a positive control. For negative control, the primary antibody was preadsorbed (4°C for 16 hours) with PTHrP blocking peptide before being applied to the tissues. TNFα immunohistochemistry was performed as for PTHrP except that antigen retrieval was achieved with heated target retrieval solution as described above; the primary antibody (or blocked antibody, for negative controls) was applied for 40 hours in a 4°C humidified chamber; the secondary antibody was biotin-labeled horse anti-goat IgG; and the positive control tissue was feline mammary carcinoma. Tissue sections were not counterstained. Slides were imaged using Nomarski bright field microscopy and black-and-white photography.
To determine if PTHrP was differentially expressed in the oral cavity by anatomic site, immunohistochemistry was performed on normal feline gingiva and lingual tissue collected from 12 cats during postmortem examination. All tissues were fixed in 10% buffered formalin, paraffin embedded, and sectioned at 5 μm before immunohistochemical evaluation.
Grading PTHrP Immunohistochemistry
Specimens that were evaluated using immunohistochemistry for the detection of PTHrP were scored on the basis of a subjective assessment of overall signal intensity in OSCC cells (absent, light, moderate, or heavy), proportion of PTHrP-positive OSCC cells (regardless of cellular localization; 0 to 24%, 25 to 49%, 50 to 74%, and 75 to 100%), and the proportion of OSCC cells with PTHrP-positive nuclei (0 to 24%, 25 to 49%, 50 to 74%, and 75 to 100%). The distribution of PTHrP intensity, cellular staining, and nuclear staining scores were compared between specimens with and without bone resorption. Only a small number of tumors with osteolysis demonstrated TNFα positivity (4 of 38) and in only a small proportion of cells (less than 5%). Comparison to tumors without osteolysis was not pursued, because evaluation of this bone resorption agonist in tumors with osteolysis was predominantly negative.
Statistical Analysis
Age and tumor site were displayed as histograms. Average age by tumor site was displayed as mean ± standard error. Age of cats with lingual OSCC was compared to age of cats with gingival, sublingual, or other sites of OSCC using the t test when data were normally distributed (determined with the Shapiro–Wilk test) or the Wilcoxon rank–sum (Mann–Whitney) test when not normally distributed. The P value of .05 divided by the number of comparisons (3) was accepted as significant (P = .017). Categorical data (breed, hair length, sex, neuter status, and PTHrP scores) were analyzed with the Fisher exact test. All analyses were performed with STATA 10 Intercooled software (Cary, NC).
Results
Epidemiology of Feline OSCC
Age was reported for 416 cats. The average age of cats with OSCC was 13 years (range, 1.5 to 22.0) but was not normally distributed (Shapiro–Wilk, P = .00017) (Fig. 1). Gingiva was the most common location (51%) (Fig. 2). Forty percent of gingival tumors were specified as mandibular and 37% as maxillary. The locations of the remaining 23% of gingival tumors were not provided. Twenty-three percent of feline OSCC biopsies were sublingual and 11% were lingual. Tumors from buccal mucosa, hard palate, soft palate, larynx, pharynx, tonsil, and lip each represented ≤ 2% of the total. Anatomic location was not provided for the remaining 5%.
Figure 1.
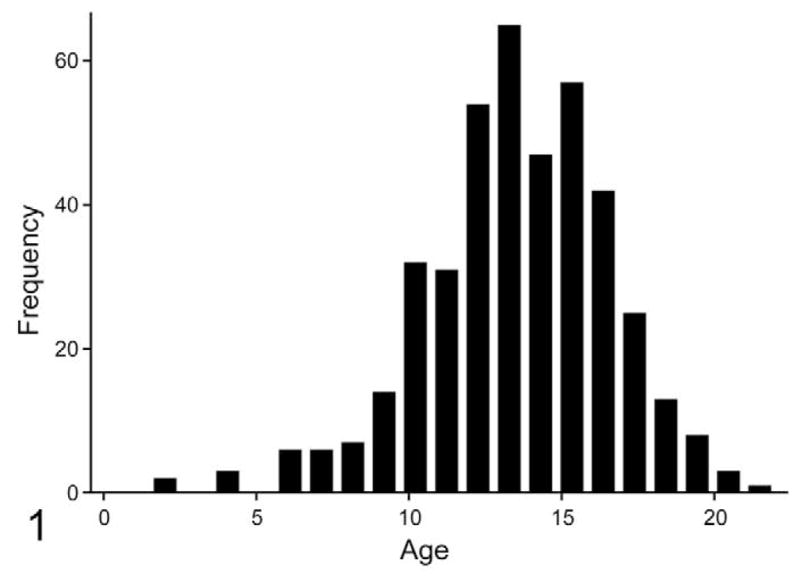
Age distribution of cats with oral squamous cell carcinoma (OSCC). The average age of cats diagnosed with OSCC was 13 years (n = 541), with a range of 1.5 to 22.0 years. Age was not normally distributed (skewed toward older cats).
Figure 2.
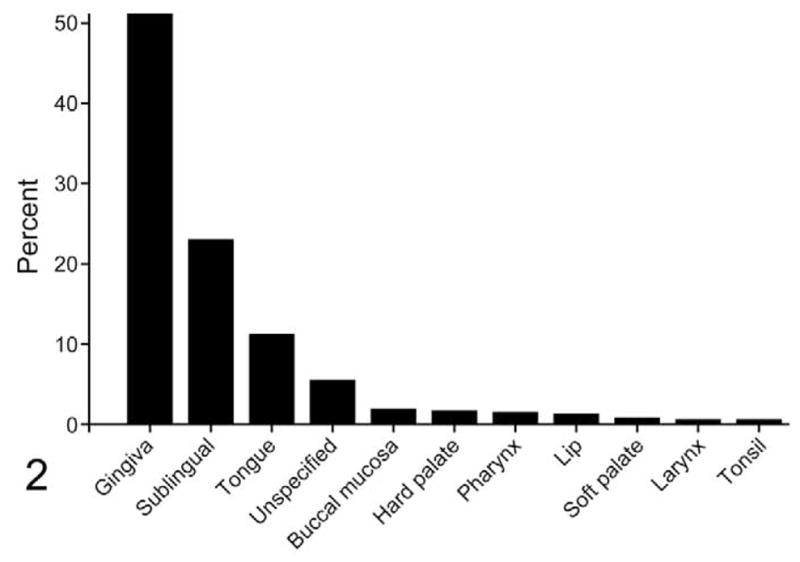
Anatomic location of feline OSCC. The most common location of OSCC was gingiva (including tumors associated with teeth, mandible, maxilla, and jaw), followed by sublingual (includes ventral tongue, frenulum, mouth floor) and tongue.
The average age of cats with lingual OSCC was 11.9 years old, younger than cats with gingival OSCC (13.6 years; P = .0004, 2-tailed t test) and cats with OSCC from other sites (13.5 years; combined hard and soft palate, buccal, tonsil, larynx, pharynx, lip, and unspecified location; P = .0094, 2-tailed t test). Although cats with lingual OSCC were, on average, younger than cats with sublingual OSCC (13.0 years), the difference was not statistically significant when adjusted for multiple comparisons (Wilcoxon rank–sum, P = .028) (Fig. 3).
Figure 3.
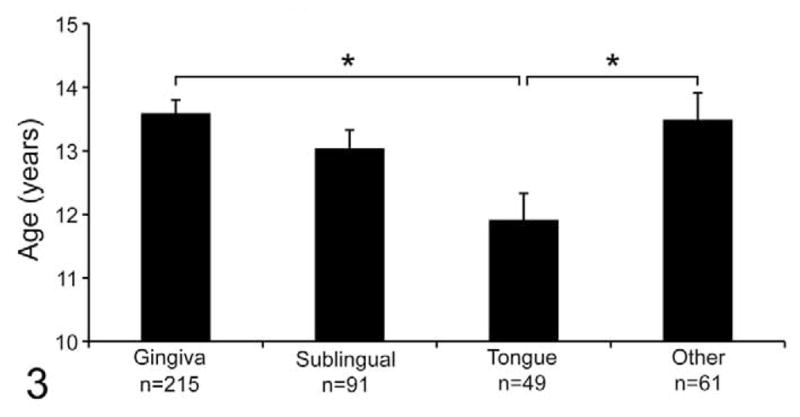
Cats with lingual OSCC were younger than cats with OSCC of the gingiva and other sites. The average age of cats with lingual OSCC was 11.9 years old, younger than cats with gingival OSCC (13.6 years; *P = .0004) and cats with OSCC from other sites (13.5 years; combined hard and soft palate, buccal, tonsil, larynx, pharynx, lip, and unspecified; *P = .0094). Although cats with lingual OSCC were, on average, younger than cats with sublingual OSCC (13.0 years), the difference was not statistically significant when adjusted for multiple comparisons (Wilcoxon rank–sum, *P = .028).
There was no significant difference in breed, hair length, or sex distribution between cats in the control group and the OSCC group (Table 1); however, there were more intact cats in the OSCC group (21%) compared to the control group (15%; P = .015, Fisher exact test). Ulceration was the most common symptom reported (36%), followed by loose teeth (17%) and facial swelling (13%) (n = 230). Average duration of symptoms was 5.2 weeks (range, 0.1 to 36.0 weeks, n = 72). Average tumor size was 2.4 cm in diameter (range, 1 to 8 cm, n = 32). Clinical evidence of bone involvement (radiography, tooth loss, clinical observation) was reported in 95 cases.
Table 1.
Comparison of Breed, Hair Length, Sex, and Neuter Status Between Control Cats and Cats With Oral Squamous Cell Carcinoma (OSCC)a
| Control, % | OSCC, % | |
|---|---|---|
| Breedb | ||
| DSH | 68 | 69 |
| DLH | 17 | 19 |
| DMH | 3 | 4 |
| Siamese | 3 | 1 |
| Himalayan | 2 | 3 |
| Persian | 2 | 3 |
| Mixed | 2 | 1 |
| Otherc | 3 | 0 |
| Hair lengthd | ||
| Short | 73 | 70 |
| Long | 25 | 29 |
| Unspecified | 2 | 1 |
| Sex | ||
| Female | 7 | 12 |
| Spayed female | 47 | 42 |
| Male | 8 | 10 |
| Castrated male | 38 | 36 |
| Neuter statuse | ||
| Intact | 15 | 21 |
| Neutered | 85 | 78 |
Control group consisted of 391 cats; OSCC group consisted of 389 cats. Only cats at least 8 years old were included.
DSH, domestic short hair; DMH, domestic medium hair; DLH, domestic long hair.
Other breeds that represented less than 1% of both groups included Main Coon, Abyssinian, Rag Doll, Balinese, Egyptian Mau, Munchkin, Bengal, Cornish Rex, Manx, Angora, and Burmese.
Hair length was designated on the basis of breed. Short = DSH, Siamese, Abyssinian, Balinese, Egyptian Mau, Munchkin, Bengal, Cornish Rex, Manx, and Burmese. Long = DMH, DLH, Himalayan, Persian, Main Coon, Rag Doll, and Angora. Unspecified = mixed breed.
There were more intact cats in the OSCC group than the control group (P = .01).
Pathology and Characterization of Bone Involvement
Thirty-seven percent of biopsies contained bone. Of bone-containing specimens, 87% had evidence of bone resorption, 46% had evidence of new bone formation, and 49% demonstrated direct contact of OSCC cells with eroded bone surfaces, suggestive of OSCC adhesion to bone. Identification of new bone was based on high osteocyte density and woven collagen pattern, in contrast to the low osteocyte density and lamellar collagen pattern characteristic of mature cortical bone. In some cases, new bone was organized in a radiating pattern of trabeculae extending from the surface of mature bone consistent with reactive periosteal new bone formation (Figs. 4, 5). In other specimens, immature bone existed as irregular spicules within dense bands of fibroplasia between nests and islands of OSCC, interpreted as tumor-induced metaplastic bone formation (Figs. 6, 7). Bone resorption was characterized by eroded bone with irregular, scalloped surfaces (Fig. 8) and occasionally with large multinucleated osteoclasts in resorption pits on the bone surface (Fig. 9). In almost all specimens with new bone formation, osteoclastic resorption of the immature bone was observed. OSCC cells occasionally surrounded fragments of eroded bone (Fig. 10) and were in direct contact with the bone surface with no intervening stromal cells (Fig. 11).
Figure 4.
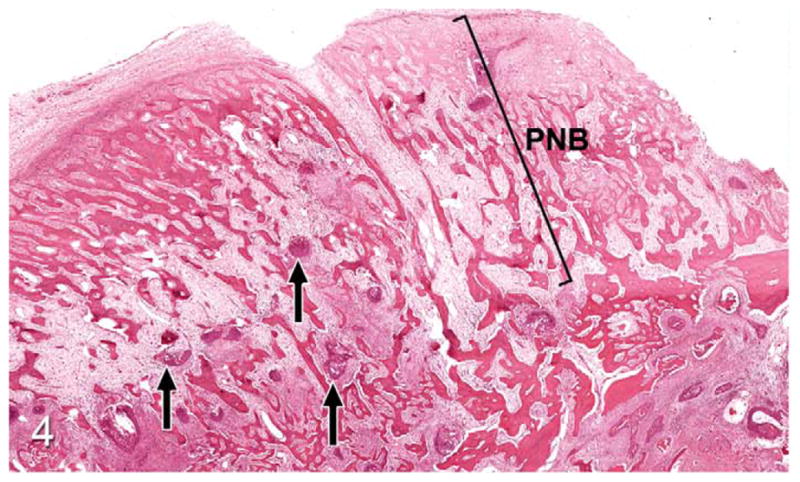
Gingival oral squamous cell carcinoma (OSCC); cat, case No. 1. Low-power magnification revealing small islands of OSCC (arrows) with radiating trabeculae of periosteal new bone (PNB). HE.
Figure 5.
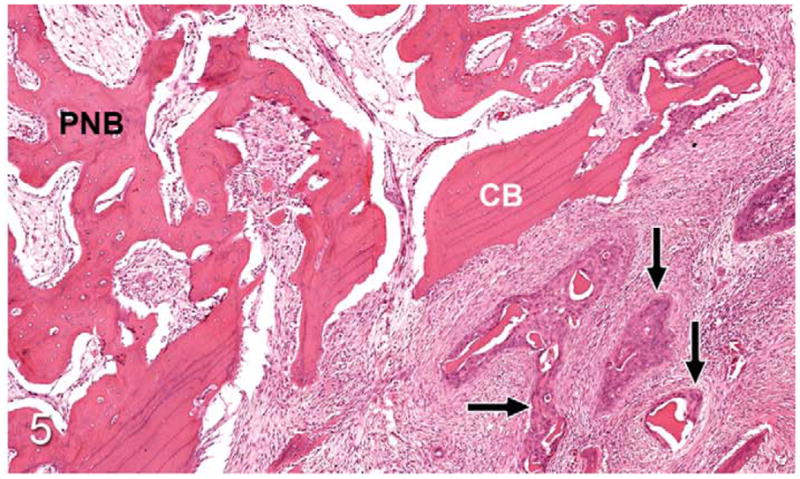
Gingival OSCC; cat, case No. 1. Higher magnification of Figure 4 revealing OSCC (arrows) surrounding small fragments of bone adjacent to lamellar cortical bone (CB) and periosteal new bone (PNB). HE.
Figure 6.
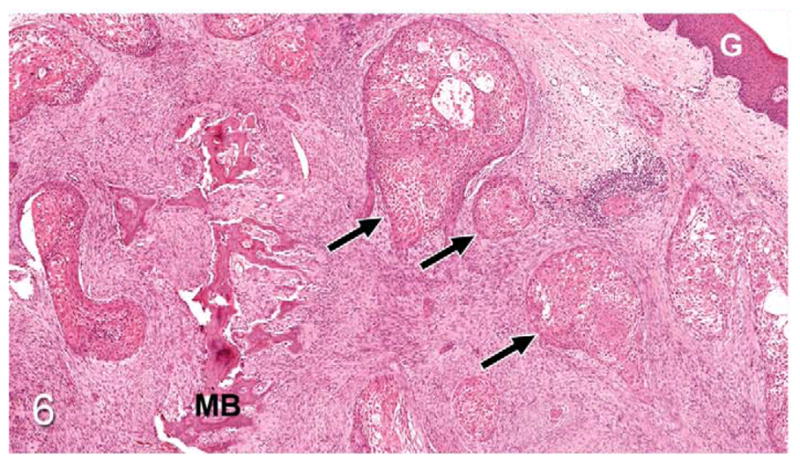
Gingival OSCC; cat, case No. 2. Low magnification revealing islands of OSCC (arrows) deep to the gingival surface (G) with desmoplasia and irregular trabeculae of immature metaplastic bone (MB). HE.
Figure 7.
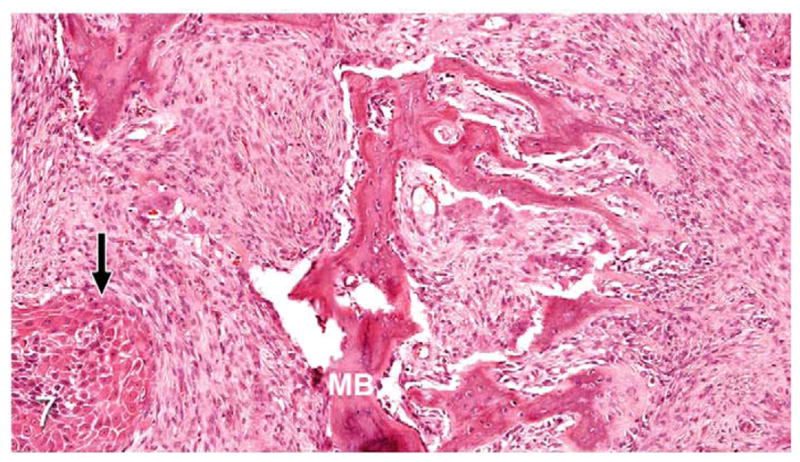
Gingival OSCC; cat, case No. 2. Higher magnification of Figure 6 demonstrating metaplastic bone (MB) formation within the tumor stroma adjacent to OSCC (black arrow). HE.
Figure 8.
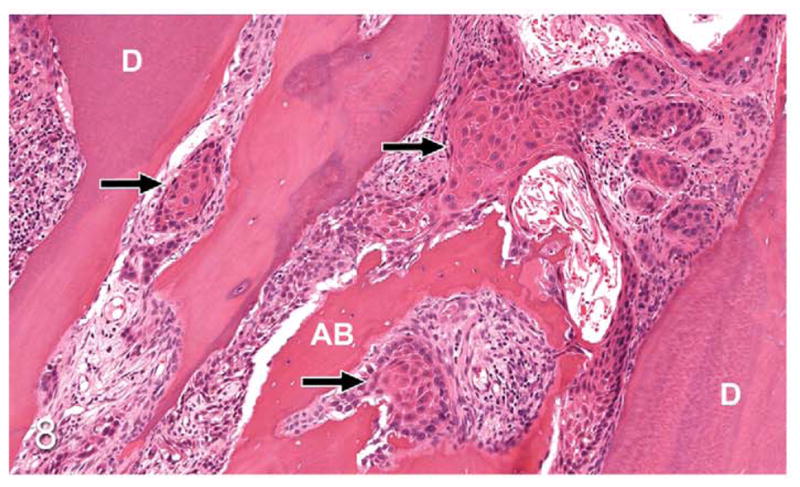
Gingival OSCC; cat, case No. 3. Islands of OSCC (arrows) infiltrating between tooth root dentin (D) and irregularly surfaced (eroded) alveolar bone (AB). HE.
Figure 9.
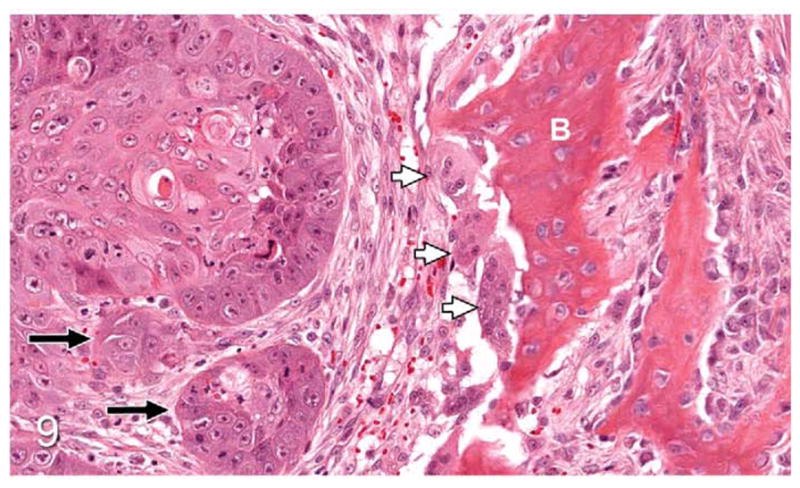
OSCC (site unspecified); cat, case No. 4. OSCC (black arrows) adjacent to immature bone (B) with numerous multinucleated osteoclasts in resorption pits (white arrows). HE.
Figure 10.
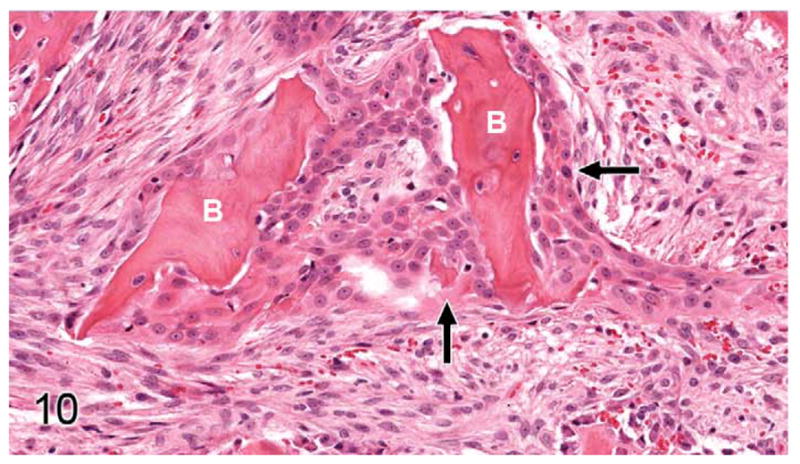
Gingival OSCC; cat, case No. 5. OSCC cells (arrows) surround small fragments of previously eroded bone (B). HE.
Figure 11.
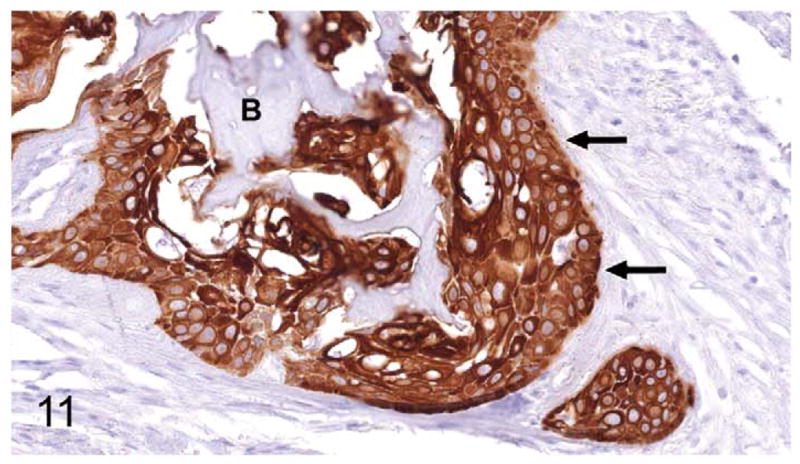
Gingival OSCC; cat, case No. 5. Cytokeratin-positive (AE1/AE3) OSCC cells (arrows) surrounding previously eroded bone (B). Diaminobenzidine chromogen and hematoxylin counterstain.
Immunohistochemistry Optimization
RANKL, OPG, and IL-1α were not detectable in positive control tissues, and evaluation of feline OSCC specimens with these antibodies was not performed. The TGFβ1 antibody produced strong signal in feline skin, feline ovary, and mouse ovary, but specificity could not be demonstrated, because pre-adsorption of the antibody with TGFβ1 blocking peptide failed to block the signal; therefore, TGFβ1 was not evaluated in the feline OSCC specimens. The TNFα antibody produced strong cytoplasmic signal in feline mammary carcinoma (data not shown), and the PTHrP antibody produced moderately intense cytoplasmic signal in feline epidermal cells (Fig. 12). Both TNFα and PTHrP positive reactions were successfully blocked with their respective blocking peptides.
Figure 12.
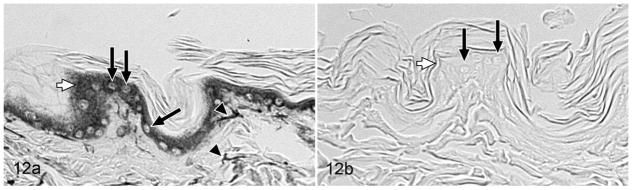
Skin; cat, case No. 6, control tissue. (a) Parathyroid hormone-related protein–positive (PTHrP-positive) control. PTHrP was detected in the cytoplasm of epidermal keratinocytes (white arrow) and in dermal fibroblasts (arrowheads) but not in epithelial cell nuclei (black arrows). Diaminobenzidine, no counterstain. (b) PTHrP-negative control. Preincubation of the PTHrP primary antibody with PTHrP blocking peptide eliminated staining in the positive control tissue (white arrow = epidermis, black arrows = nuclei). Diaminobenzidine, no counterstain.
Expression of TNFα in Feline OSCC Specimens With Bone Resorption
The optimized TNFα protocol was used to evaluate 32 cases of feline OSCC with evidence of osteolysis. Twenty-eight cases (88%) of feline OSCC with osteolysis were negative for TNFα expression; the remaining 4 cases demonstrated positivity for TNFα in only 1 to 5% of tumor cells (data not shown). Evaluation of additional biopsies with and without bone resorption were not performed.
Expression of PTHrP in Feline OSCC Specimens With and Without Bone Resorption
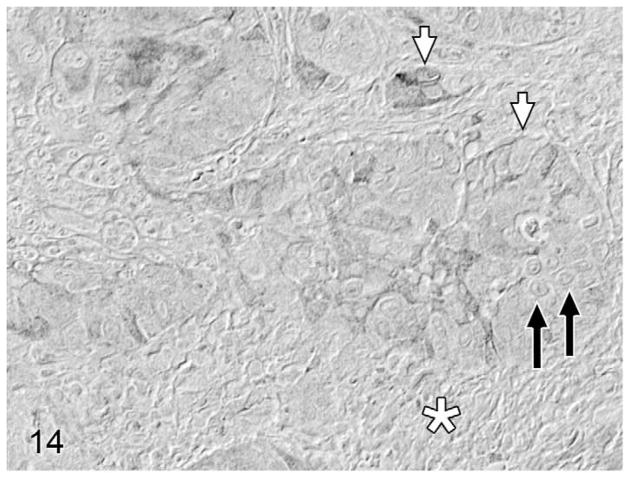
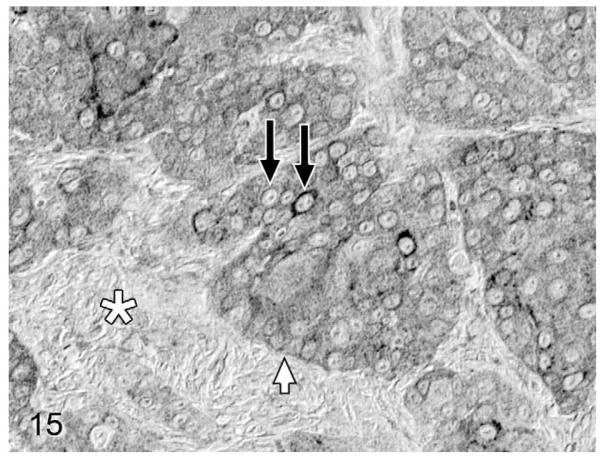
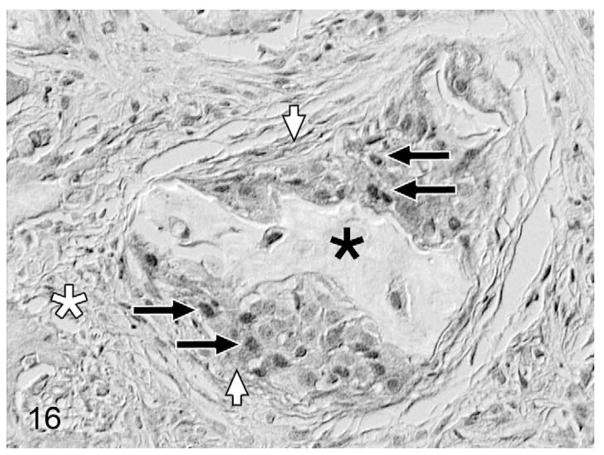
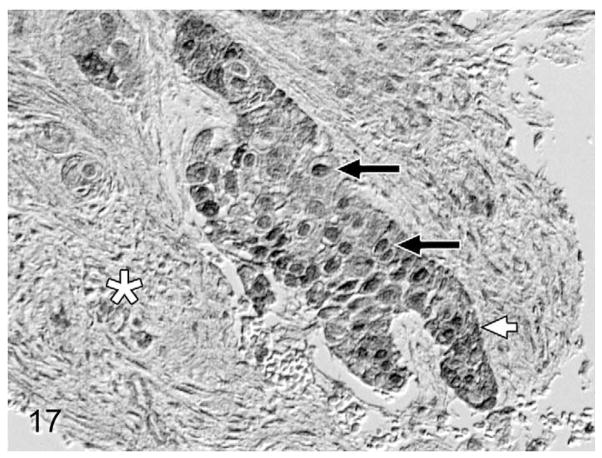
Overall, PTHrP was expressed in most OSCC cells in a diffuse, finely granular cytoplasmic pattern; however, a subset of PTHrP-expressing cells also demonstrated nuclear localization of PTHrP (Fig. 13). Most biopsies without osteolysis (Figs. 14, 15) had light to moderate cytoplasmic staining for PTHrP. Most biopsies with osteolysis demonstrated moderate to heavy PTHrP staining that frequently localized to the nucleus in addition to the cytoplasm (Figs. 16, 17). Fibroblasts in tumor stroma often demonstrated light to moderate staining; bone-lining cells, osteoblasts, and osteocytes in bone-containing biopsies were also PTHrP positive. PTHrP staining in postmortem samples of feline gingiva and tongue (Figs. 18, 19) was light to moderate intensity in epithelial cells.
Figure 13.
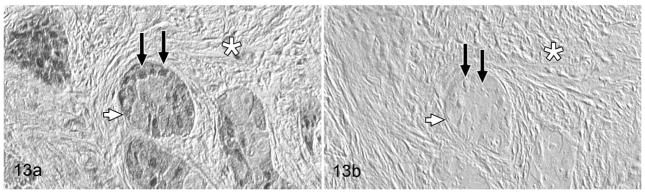
Gingival oral squamous cell carcinoma; cat, case No. 7. (a) PTHrP-positive reaction. Moderate PTHrP staining was detected in the cytoplasm (white arrow) and nuclei (black arrows) of OSCC cells. Fibrous stroma (desmoplasia) indicated by white asterisk. (b) PTHrP-negative control. Preincubation of the PTHrP primary antibody with PTHrP blocking peptide eliminated staining (white arrow = OSCC cell, black arrows = nuclei, white asterisk = desmoplasia). Diaminobenzidine, no counterstain.
Figure 14.
Lingual oral squamous cell carcinoma (OSCC) without osteolysis; cat, case No. 8. Light parathyroid hormone-related protein (PTHrP) staining (cytoplasmic) in a low proportion of OSCC cells (white arrows) without nuclear localization (black arrows). Desmoplasia indicated by white asterisk. Diaminobenzidine, no counterstain.
Figure 15.
Lingual OSCC without osteolysis; cat, case No. 9. Moderate cytoplasmic PTHrP staining (white arrow) in a high proportion of OSCC cells without nuclear localization (black arrows). Desmoplasia indicated by white asterisk. Diaminobenzidine, no counterstain.
Figure 16.
Gingival OSCC with osteolysis; cat, case No. 10. Moderate PTHrP staining of a high proportion of OSCC cells (white arrows) with nuclear localization (black arrows) in OSCC cells surrounding a fragment of previously eroded bone (black asterisk). Desmoplasia indicated by white asterisk. Diaminobenzidine, no counterstain.
Figure 17.
Gingival OSCC with osteolysis; cat, case No. 11. Heavy PTHrP staining of a high proportion of OSCC cells (white arrow) demonstrating nuclear localization (black arrows). Desmoplasia indicated by white asterisk. Bone is out of field of view. Diaminobenzidine, no counterstain.
Figure 18.
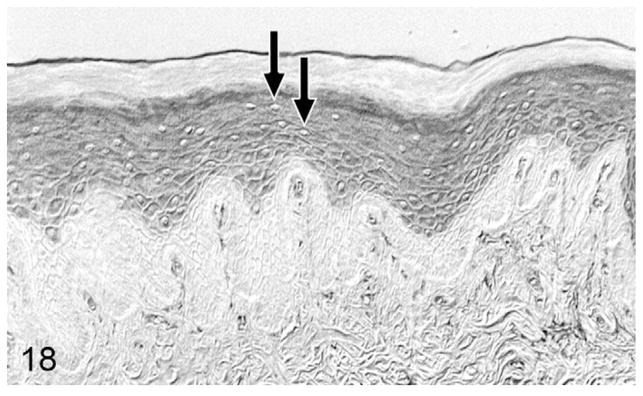
Normal gingiva; cat, case No. 12. Moderate parathyroid hormone-related protein (PTHrP) staining of gingival suprabasalar epithelial cells without nuclear localization. Unstained nuclei indicated by black arrows. Diaminobenzidine, no counterstain.
Figure 19.
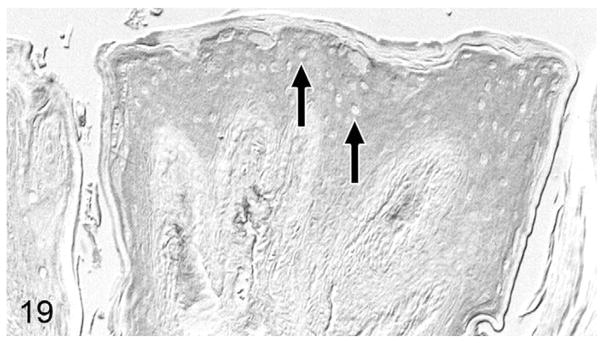
Normal tongue; cat, case No. 12. Moderate parathyroid hormone-related protein (PTHrP) staining of lingual suprabasalar epithelial cells without nuclear localization. Unstained nuclei indicated by black arrows. Diaminobenzidine, no counterstain.
The distribution and intensity of PTHrP localization was scored and compared between tumors with and without evidence of osteolysis. Heavy PTHrP staining intensity was more common in feline OSCC with osteolysis, compared to OSCC without osteolysis (P = .004, Fisher exact test, data not shown). PTHrP-positive tumor cells were more common in biopsies with osteolysis compared to OSCC without osteolysis (Fisher exact test, P = .001) (Fig. 20). Eighty percent of OSCC biopsies with osteolysis had 75 to 100% PTHrP-positive cells, compared to only 39% of the OSCC specimens without evidence of osteolysis. Positive nuclei were more common in biopsies of feline OSCC with osteolysis, compared to OSCC biopsies without osteolysis (Fisher exact test, P < .0001) (Fig. 20). Forty-one percent of OSCC specimens with bone resorption had nuclear localization of PTHrP in 75 to 100% of OSCC cells, compared to only 8% of specimens without bone resorption. There was no statistical difference in epithelial cell expression of PTHrP between normal feline gingiva and tongue (postmortem samples) in terms of intensity, percentage positive epithelial cells, and percentage positive nuclei (Fisher exact test, data not shown).
Figure 20.
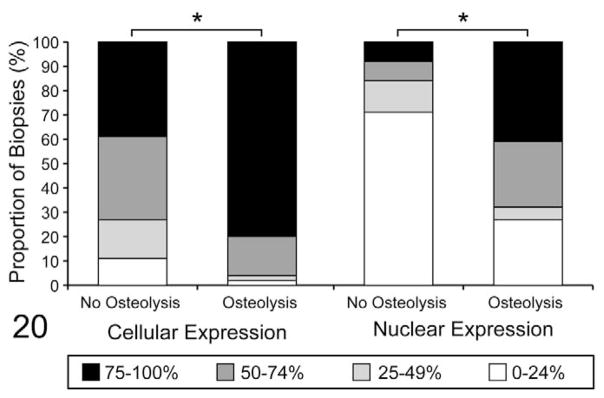
Parathyroid hormone-related protein–positive (PTHrP-positive) oral squamous cell carcinoma (OSCC) cells and PTHrP-positive nuclei are more frequent in biopsies with evidence of osteolysis. Each bar represents 100% of biopsies in each group (with and without evidence of osteolysis). Each bar is subdivided to demonstrate the proportion of biopsies with 0 to 24%, 25 to 49%, 50 to 74%, and 75 to 100% PTHrP-positive cells or PTHrP-positive nuclei, as indicated. PTHrP-positive tumor cells were more common in biopsies with osteolysis, compared to OSCC without osteolysis (*P = .001). PTHrP-positive tumor nuclei were more common in biopsies with osteolysis, compared to OSCC without osteolysis (*P < .0001).
Discussion
Epidemiology
The population of cats with OSCC in this study was similar in age (mean, 13 years; range, 1.5 to 22.0 years) and sex distribution (53.7% female and 46.3% male) to a cohort of 227 cats with OSCC reported in 1989 by Stebbins et al (mean, 12.5 years; range, 3 to 21 years; 55.5% female and 44.5% male).30 Although female cats appear to be slightly overrepresented in these studies, there was no statistical difference between cats with OSCC and the control cats in this study. OSCC most often affects older humans; however, there is a clear sex association, with a male:female ratio of 2.0:0.1.36 Most human cases occur in men older than 45 years who use tobacco and/or consume alcohol.28 Whereas cats do not share the same risk factors as most human patients with OSCC (although some cats are exposed to environmental tobacco smoke in their homes),2 they may have similarities to a small subgroup of human patients with OSCC who are unique in that they do not smoke or consume alcohol, they tend to be female, they have tumors of the oral cavity (as opposed to the oropharynx, a common site among people who smoke and consume alcohol), and they are older than patients who smoke and consume alcohol. Risk factors for the development of OSCC in this subgroup of human patients are unknown.9
There was no significant difference in breed, sex, or hair length between control cats and cats with OSCC. These findings are similar to what Bertone et al reported in 2003, when they compared patient signalment and history between 36 cats with OSCC and 112 cats with renal disease.2 Similarly, no breed predisposition was detected in the study performed by Stebbins et al.30 Interestingly, our study demonstrated that there were more intact cats (regardless of sex) in the OSCC group than in the control group (21% compared to 15%). The reason why intact cats are overrepresented in the OSCC group is unknown, but it may involve either the influence of hormones such as estrogen and testosterone on the development and progression of OSCC or the role of other factors associated with neuter status, such as living indoors or outdoors, type of diet, and exposure to infectious agents and environmental carcinogens.
Cats with lingual OSCC were significantly younger than cats with OSCC of the gingiva and other sites, excluding the sublingual area; however, the reason for this is unknown. Lingual OSCC may progress more rapidly than OSCC of other sites in the oral cavity, or the clinical signs are more rapidly detectable in lingual OSCC, although this would mean that cats with gingival OSCC were living an average of 1.5 years longer than cats with lingual OSCC, which is highly unlikely given the aggressive and rapidly progressive nature of this tumor. It is more likely that lingual OSCC actually developed at a younger age, which suggests that the pathogenesis of lingual OSCC differs from that of other locations.
We hypothesize that lingual epithelium differs in susceptibility to the development of OSCC, compared to other sites in the oral cavity. In humans with OSCC, variation in the expression of polymorphic xenobiotic metabolizing enzymes in oral mucosa, such as those of the cytochrome P450 family, has been implicated as a reason for increased individual susceptibility to oral cancer as a result of exposure to carcinogens in tobacco smoke.36 Site-specific expression of xenobiotic metabolizing enzymes within the oral cavity has not been reported in cats or humans; however, a study by Robinson et al demonstrated that carboxylesterase activity in the oral cavity of mice varied, depending on location, with an apparent correlation between lesion distribution following exposure to a high concentrations of vinyl acetate (a carcinogen) and high carboxylesterase activity in different areas of the oral cavity.26 Bertone et al demonstrated a 2-fold increased risk for the development of OSCC in cats exposed to environmental tobacco smoke.2 Perhaps the expression of polymorphic and site-specific xenobiotic metabolizing enzymes in the lingual mucosa makes some cats more susceptible to carcinogenesis, thereby developing OSCC of the tongue at an earlier age. Cats may be exposed to carcinogens in environmental tobacco smoke or other sources as they are deposited on the hair coat and introduced to the tongue through grooming.33
Pathology
The most common location of OSCC was gingiva (including tumors associated with teeth, mandible, maxilla, and jaw), followed by the sublingual region (including ventral tongue, frenulum, mouth floor) and tongue. These findings differ from reports stating that the sublingual area11 and tongue8 are the most common sites. This discrepancy may be due to differences in how tumor sites were classified; for example, Dorn et al did not have a site designation for sublingual tumors but did separate gingival tumors from those of the dental alveolus.8 We did not designate dental alveolus as a site separate from gingiva, and we did not separate maxillary tumors from mandibular tumors.
Bone invasion was most commonly found in gingival OSCC, as expected given the close proximity of the gingiva to underlying bone. Bone invasion was characterized by osteoclastic bone resorption, new bone formation, and desmoplasia. A proportion of biopsies that contained bone demonstrated islands of OSCC surrounding, and in direct apposition with, the surface of eroded bone, suggesting that OSCC cells may be capable of adhering to bone. To our knowledge, OSCC adherence to bone has not been reported; however, human breast and prostate cancer cells have been shown to adhere to bone matrix in vitro.15,31 Carcinoma cells adhering to bone matrix was mediated by the interaction of integrin receptors on the tumor cells with collagen 115 and bone sialoprotein.31 TGFβ1, a growth factor stored in bone matrix and released in an active form during bone resorption, has been shown to stimulate prostate carcinoma cell adhesion.16 Although certain carcinoma cells appear to adhere to bone, it is unlikely that carcinomas directly resorb bone.10,34 The significance of OSCC–bone binding is not known; however, exposed bone surfaces may serve as a scaffold upon which OSCC can migrate, much like perineural invasion. OSCC adhesion to bone may be particularly significant if it promotes tumor cell migration and proliferation, as has been demonstrated with human breast31 and prostate cancer cells,15 and so warrants further investigation to confirm the adhesion of OSCC cells to bone matrix.
Immature bone in feline OSCC occurred as interwoven, radiating trabeculae of periosteal new bone or as irregular spicules of metaplastic bone arising within tracts of tumor-associated desmoplasia adjacent to islands and cords of tumor cells. The induction of periosteal new bone formation is an expected event, resulting from activation of the periosteum during expansile and invasive growth of the tumor. In contrast, the formation of immature bone within areas of tumor-induced desmoplasia is likely the result of OSCC cells inducing the expression of bone formation agonists (eg, bone morphogenetic proteins, isoforms of TGFβ12) in the adjacent stroma, in conjunction with metaplastic transition of stromal cells from a fibroblastic to osteoblastic phenotype.
Expression of TNFα and PTHrP
A role for tumor-expressed TNFα in the pathogenesis of OSCC-induced bone resorption was not supported in this study, because only a few biopsies with evidence of bone resorption had detectable TNFα (4 of 32 biopsies) and in only a small number of cells. These findings are in contrast to the work of Shibahara et al, who demonstrated that TNFα expression was increased in cases of bone-invasive human OSCC;29 however, a more recent study by Van Cann et al revealed that TNFα was not differentially expressed in human OSCC with and without invasion into the mandibular medullary canal.35
PTHrP was detected in feline OSCC, with and without evidence of bone resorption, using an antibody directed toward midregion (34–53) PTHrP. Anti-PTHrP antibodies directed toward this epitope have been used to detect PTHrP in a feline OSCC cell line32 and in various canine tissues (epidermis, myoepithelial cells of dermal apocrine glands, mammary gland, anal sac epithelium).23 Staining intensity of PTHrP expression in OSCC cells varied from absent to heavy and was detected in stromal cells surrounding islands of OSCC, in osteoblasts lining trabeculae of new bone, in osteocytes, and in overlying oral epithelium. This staining pattern is not unexpected, because PTHrP is known to be ubiquitous in expression,7 and PTHrP has been expressed by canine dermal fibroblasts,4 canine prostate stromal cells,4 mouse,19 and canine osteoblasts23 and in rat preosteoblasts and osteocytes.24
Immunohistochemistry has been used to detect PTHrP in feline neoplasms, including lung carcinomas, a thyroid carcinoma, and lymphoma.5 Additionally, an immunoradiomimetic assay for human PTHrP was used to detect elevated plasma levels of PTHrP in cats with humoral hypercalcemia of malignancy.5 It is unknown if the increased PTHrP expression observed in feline OSCC with osteolysis is the cause or result of bone resorption. It is likely that both relationships are occurring. PTHrP is known to stimulate osteoclastic bone resorption by increasing the expression of RANKL in osteoblasts, which in turn leads to the maturation and activation of osteoclasts, resulting in bone resorption. Bone resorption leads to the liberation of activated growth factors from the bone matrix, such as TGFβ1, in addition to the release of calcium. Both calcium and TGFβ1 can stimulate increased production of PTHrP17. In fact, TGFβ1 has been shown to increase PTHrP expression in a feline OSCC cell line, SCCF1.32 The prognostic significance of increased PTHrP is controversial. On one hand, PTHrP expression in human colorectal cancer and gastric cancer correlated with depth of invasion and mestastasis.21 On the other, PTHrP expression in human non–small cell lung carcinomas was associated with a survival advantage among female patients, and increased PTHrP expression has been associated with longer survival in patients with ductal mammary carcinoma.21 The effect of PTHrP expression on prognosis in veterinary patients has not been studied.
PTHrP is capable of stimulating osteoclastic bone resorption by increasing osteoblast expression of RANKL and subsequent recruitment and activation of osteoclasts; it also stimulates bone formation by promoting the maturation and survival of matrix-producing osteoblasts.17,18 PTHrP expression in murine osteoblasts has been shown to be necessary for bone formation,17,19 and expression of PTHrP in human OSCC cells has been shown to induce the in vitro formation of osteoclasts by stimulating RANKL expression in murine osteoblasts.14 It is likely that tumor-derived PTHrP had a stimulatory role in formation of new bone and osteoclastic bone resorption observed in the feline OSCC biopsies.
OSCC biopsies with evidence of bone resorption demonstrated an increased percentage of cells with nuclear localization of PTHrP. The significance of nuclear localization of PTHrP in feline OSCC with osteolysis is unknown, but it may support tumor cell proliferation, survival, adhesion, and invasion, as has been shown for human breast cancer cell lines.3 PTHrP is known to localize to the nucleus and nucleolus during the G1 phase of the cell cycle, and it has been shown to inhibit apoptosis of chondrocytes and tumor cells.17
Feline OSCC was most commonly located in the gingiva and frequently demonstrated bone involvement, as characterized by osteoclastic bone resorption, formation of new bone (periosteal new bone and metaplastic bone), and direct apposition of OSCC cells with bone, suggesting bone adhesion. OSCC expression of TNFα was not important to the pathogenesis of bone resorption, because only a small proportion of biopsies demonstrated TNFα expression in OSCC cells. In contrast, PTHrP expression was commonly expressed in OSCC biopsies with and without evidence of osteolysis. The proportion of PTHrP-positive cells and nuclei, in addition to overall staining intensity, was increased in tumors with evidence of osteolysis. These results support a relationship between PTHrP expression in feline OSCC cells and osteoclastic bone resorption; however, further investigation is necessary to determine the significance of PTHrP expression and nuclear localization in feline OSCC.
Acknowledgments
We thank IDEXX Laboratories, Veterinary Diagnostics Ltd, Clinilab Inc, HistoTechniques Ltd, and Dr Steven Weisbrode and Dr Paul Stromberg in the Department of Veterinary Biosciences at The Ohio State University for providing case material; the Histology/Immunohistochemistry Core in the Department of Veterinary Biosciences for preparation of slides; and Tim Vojt at The Ohio State University for assistance with figures.
Funding
This work was supported by the Morris Animal Foundation (grant No. D03FE-029). One of the authors (C.K.M.) was supported by an F32 from the National Cancer Institute (CA130458).
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- 1.Barger AM, Fan TM, de Lorimier LP, Sprandel IT, O’Dell-Anderson K. Expression of receptor activator of nuclear factor kappa-B ligand (RANKL) in neoplasms of dogs and cats. J Vet Intern Med. 2007;21:133–140. doi: 10.1892/0891-6640(2007)21[133:eoraon]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Bertone ER, Snyder LA, Moore AS. Environmental and lifestyle risk factors for oral squamous cell carcinoma in domestic cats. J Vet Intern Med. 2003;17:557–562. [PubMed] [Google Scholar]
- 3.Bhatia V, Saini MK, Falzon M. Nuclear PTHrP targeting regulates PTHrP secretion and enhances LoVo cell growth and survival. Regul Pept. 2009;158:149–155. doi: 10.1016/j.regpep.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomme EA, Werkmeister JR, Zhou H, Kartsogiannis V, Capen CC, Rosol TJ. Parathyroid hormone-related protein expression and secretion in a skin organotypic culture system. Endocrine. 1998;8:143–151. doi: 10.1385/ENDO:8:2:143. [DOI] [PubMed] [Google Scholar]
- 5.Bolliger AP, Graham PA, Richard V, Rosol TJ, Nachreiner RF, Refsal KR. Detection of parathyroid hormone-related protein in cats with humoral hypercalcemia of malignancy. Vet Clin Pathol. 2002;31:3–8. doi: 10.1111/j.1939-165x.2002.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 6.Chuang FH, Hsue SS, Wu CW, Chen YK. Immunohistochemical expression of RANKL, RANK, and OPG in human oral squamous cell carcinoma. J Oral Pathol Med. 2009;38:753–758. doi: 10.1111/j.1600-0714.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 7.Clemens TL, Cormier S, Eichinger A, Endlich K, Fiaschi-Taesch N, Fischer E, Friedman PA, Karaplis AC, Massfelder T, Rossert J, Schlüter KD, Silve C, Stewart AF, Takane K, Helwig JJ. Parathyroid hormone-related protein and its receptors: nuclear functions and roles in the renal and cardiovascular systems, the placental trophoblasts and the pancreatic islets. Br J Pharmacol. 2001;134:1113–1136. doi: 10.1038/sj.bjp.0704378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorn CR, Priester WA. Epidemiologic analysis of oral and pharyngeal cancer in dogs, cats, horses, and cattle. J Am Vet Med Assoc. 1976;169:1202–1206. [PubMed] [Google Scholar]
- 9.Farshadpour F, Hordijk GJ, Koole R, Slootweg PJ. Non-smoking and non-drinking patients with head and neck squamous cell carcinoma: a distinct population. Oral Dis. 2007;13:239–243. doi: 10.1111/j.1601-0825.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- 10.Goltzman D. Osteolysis and cancer. J Clin Invest. 2001;107:1219–1220. doi: 10.1172/JCI13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes AM, Adams VJ, Scase TJ, Murphy S. Survival of 54 cats with oral squamous cell carcinoma in United Kingdom general practice. J Small Anim Pract. 2007;48:394–399. doi: 10.1111/j.1748-5827.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 12.Heliotis M, Ripamonti U, Ferretti C, Kerawala C, Mantalaris A, Tsiridis E. The basic science of bone induction. Br J Oral Maxillofac Surg. 2009;47:511–514. doi: 10.1016/j.bjoms.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Hutson CA, Willauer CC, Walder EJ, Stone JL, Klein MK. Treatment of mandibular squamous cell carcinoma in cats by use of mandibulectomy and radiotherapy: seven cases (1987–1989) J Am Vet Med Assoc. 1992;201:777–781. [PubMed] [Google Scholar]
- 14.Kayamori K, Sakamoto K, Nakashima T, Takayanagi H, Morita K, Omura K, Nguyen ST, Miki Y, Iimura T, Himeno A, Akashi T, Yamada-Okabe H, Ogata E, Yamaguchi A. Roles of interleukin-6 and parathyroid hormone-related peptide in osteoclast formation associated with oral cancers: significance of interleukin-6 synthesized by stromal cells in response to cancer cells. Am J Pathol. 2010;176:968–980. doi: 10.2353/ajpath.2010.090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiefer JA, Farach-Carson MC. Type I collagen-mediated proliferation of PC3 prostate carcinoma cell line: implications for enhanced growth in the bone microenvironment. Matrix Biol. 2001;20:429–437. doi: 10.1016/s0945-053x(01)00159-7. [DOI] [PubMed] [Google Scholar]
- 16.Kostenuik PJ, Sanchez-Sweatman O, Orr FW, Singh G. Bone cell matrix promotes the adhesion of human prostatic carcinoma cells via the alpha 2 beta 1 integrin. Clin Exp Metastasis. 1996;14:19–26. doi: 10.1007/BF00157682. [DOI] [PubMed] [Google Scholar]
- 17.Liao J, McCauley LK. Skeletal metastasis: established and emerging roles of parathyroid hormone-related protein (PTHrP) Cancer Metastasis Rev. 2006;25:559–571. doi: 10.1007/s10555-006-9033-z. [DOI] [PubMed] [Google Scholar]
- 18.Martin TJ. Osteoblast-derived PTHrP is a physiological regulator of bone formation. J Clin Invest. 2005;115:2322–2324. doi: 10.1172/JCI26239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao D, He B, Jiang Y, Kobayashi T, Soroceanu MA, Zhao J, Su H, Tong X, Amizuka N, Gupta A, Genant HK, Kronenberg HM, Goltzman D, Karaplis AC. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1–34. J Clin Invest. 2005;115:2402–2411. doi: 10.1172/JCI24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris J, Dobson J. Head and neck. In: Morris J, Dobson J, editors. Small Animal Oncology. New York, NY: 2001. pp. 94–124. [Google Scholar]
- 21.Nishihara M, Kanematsu T, Taguchi T, Razzaque MS. PTHrP and tumorigenesis: is there a role in prognosis? Ann N Y Acad Sci. 2007;1117:385–392. doi: 10.1196/annals.1402.046. [DOI] [PubMed] [Google Scholar]
- 22.Northrup NC, Selting KA, Rassnick KM, Kristal O, O’Brien MG, Dank G, Dhaliwal RS, Jagannatha S, Cornell KK, Gieger TL. Outcomes of cats with oral tumors treated with mandibulectomy: 42 cases. J Am Anim Hosp Assoc. 2006;42:350–360. doi: 10.5326/0420350. [DOI] [PubMed] [Google Scholar]
- 23.Okada H, Nishijima Y, Yoshino T, Grone A, Capen CC, Rosol TJ. Immunohistochemical localization of parathyroid hormone-related protein in canine mammary tumors. Vet Pathol. 1997;34:356–359. doi: 10.1177/030098589703400414. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki K, Jingushi S, Ikenoue T, Urabe K, Sakai H, Iwamoto Y. Expression of parathyroid hormone-related peptide and insulin-like growth factor I during rat fracture healing. J Orthop Res. 2003;21:511–520. doi: 10.1016/S0736-0266(02)00161-4. [DOI] [PubMed] [Google Scholar]
- 25.Pries R, Wollenberg B. Cytokines in head and neck cancer. Cytokine Growth Factor Rev. 2006;17:141–146. doi: 10.1016/j.cytogfr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Robinson DA, Bogdanffy MS, Reed CJ. Histochemical localisation of carboxylesterase activity in rat and mouse oral cavity mucosa. Toxicology. 2002;180:209–220. doi: 10.1016/s0300-483x(02)00375-x. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal E, McCrory A, Talbert M, Young G, Murphy-Ullrich J, Gladson C. Elevated expression of TGF-beta1 in head and neck cancer-associated fibroblasts. Mol Carcinog. 2004;40:116–121. doi: 10.1002/mc.20024. [DOI] [PubMed] [Google Scholar]
- 28.Scully C, Bagan J. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009;15:388–399. doi: 10.1111/j.1601-0825.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 29.Shibahara T, Nomura T, Cui NH, Noma H. A study of osteoclast-related cytokines in mandibular invasion by squamous cell carcinoma. Int J Oral Maxillofac Surg. 2005;34:789–793. doi: 10.1016/j.ijom.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Stebbins KE, Morse CC, Goldschmidt MH. Feline oral neoplasia: a ten-year survey. Vet Pathol. 1989;26:121–128. doi: 10.1177/030098588902600204. [DOI] [PubMed] [Google Scholar]
- 31.Sung V, Stubbs JT, 3rd, Fisher L, Aaron AD, Thompson EW. Bone sialoprotein supports breast cancer cell adhesion proliferation and migration through differential usage of the alpha(v)beta3 and alpha(v)beta5 integrins. J Cell Physiol. 1998;176:482–494. doi: 10.1002/(SICI)1097-4652(199809)176:3<482::AID-JCP5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Tannehill-Gregg S, Kergosien E, Rosol TJ. Feline head and neck squamous cell carcinoma cell line: characterization, production of parathyroid hormone-related protein, and regulation by transforming growth factor-beta. In Vitro Cell Dev Biol Anim. 2001;37:676–683. doi: 10.1290/1071-2690(2001)037<0676:FHANSC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Tannehill-Gregg SH, Levine AL, Rosol TJ. Feline head and neck squamous cell carcinoma: A natural model for the human disease and development of a mouse model. Veterinary and Comparative Oncology. 2006;4:84–97. doi: 10.1111/j.1476-5810.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 34.Tomita A, Kasaoka T, Inui T, Toyoshima M, Nishiyama H, Saiki H, Iguchi H, Nakajima M. Human breast adenocarcinoma (MDA-231) and human lung squamous cell carcinoma (Hara) do not have the ability to cause bone resorption by themselves during the establishment of bone metastasis. Clin Exp Metastasis. 2008;25:437–444. doi: 10.1007/s10585-008-9148-4. [DOI] [PubMed] [Google Scholar]
- 35.Van Cann EM, Slootweg PJ, de Wilde PC, Otte-Holler I, Koole R, Stoelinga PJ, Merkx MA. The prediction of mandibular invasion by squamous cell carcinomas with the expression of osteoclast-related cytokines in biopsy specimens. Int J Oral Maxillofac Surg. 2009;38:279–284. doi: 10.1016/j.ijom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Walker DM, Boey G, McDonald LA. The pathology of oral cancer. Pathology. 2003;35:376–383. doi: 10.1080/00310290310001602558. [DOI] [PubMed] [Google Scholar]