Summary
Obesity is closely associated with cardiovascular diseases and type 2 diabetes, but some obese individuals, despite having excessive body fat, exhibit metabolic health that is comparable with that of lean individuals. The ‘healthy obese’ phenotype was described in the 1980s, but major advancements in its characterization were only made in the past five years. During this time, several new mechanisms that may be involved in health preservation in obesity were proposed through the use of transgenic animal models, use of sophisticated imaging techniques and in vivo measurements of insulin sensitivity. However, the main obstacle in advancing our understanding of the metabolically healthy obese phenotype and its related long-term health risks is the lack of a standardized definition. Here, we summarize the proceedings of the 13th Stock Conference of the International Association of the Study of Obesity. We describe the current research and highlight the unanswered questions and gaps in the field. Better understanding of metabolic health in obesity will assist in therapeutic decision-making and help identify therapeutic targets to improve metabolic health in obesity.
Keywords: insulin sensitive, metabolic syndrome, metabolically healthy, obesity
Introduction
In October 2013, the International Association of the Study of Obesity (now known as the World Obesity Federation) convened in Punta Cana in the Dominican Republic to discuss the metabolically healthy and unhealthy obese phenotypes. The 2013 Stock Conference was the 13th annual conference to honour the contribution of Dr Mike Stock to the field of obesity research. The meeting was co-chaired by Eric Ravussin and Samuel Klein and attended by 11 outstanding speakers and invited delegates with diverse clinical and basic expertise and a common interest in the aetiology of obesity-associated metabolic disease.
Worldwide obesity has nearly doubled since 1980, and World Health Organization data from 2008 show that over 200 million men and nearly 300 million women are obese. Overweight and obesity are estimated to contribute to 44% of the diabetes burden and 23% of the ischaemic heart disease burden (1). But not all obese individuals develop metabolic disease. In fact, the presence of obesity-related metabolic disturbances varies widely among obese individuals. While most obese individuals present a range of cardio-metabolic complications, including diabetes, hypertension, dyslipidaemia and insulin resistance, some remain free of these abnormalities despite similar fat mass and age. The ‘healthy obese’ phenotype was described as a subtype of obesity in 1982 by Sims (2), after Keyes (3) and Andres (4) in analyses of epidemiological data concluded that overweight and obesity are not always associated with cardiovascular disease and mortality (5). Since then, much research has been devoted to characterizing the metabolically healthy obese (MHO) phenotype, its associated metabolic disease and mortality risks, and to understanding the mechanisms that provide protection from metabolic disease in obesity (as reviewed (6–8) ). This paper is a summary of the body of work presented by the conference speakers and highlights the major obstacles and unanswered questions in the field.
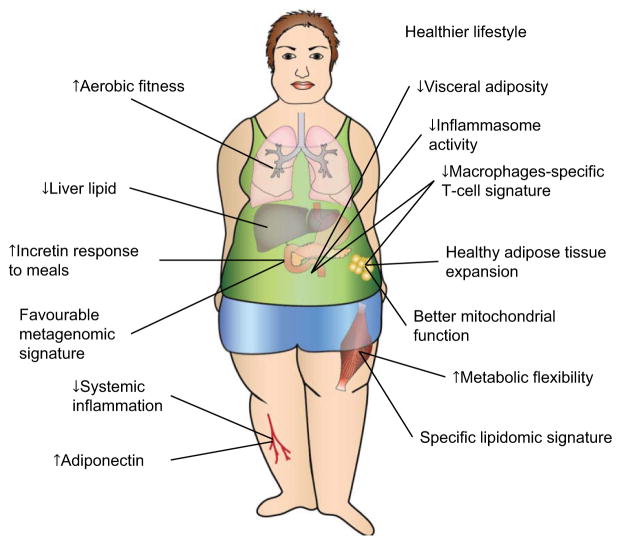
Dr Rabasa-Lhoret opened the meeting with an overview of the key metabolic characteristics of the healthy obese individual, who despite having excessive body fat, displays high levels of insulin sensitivity, no hypertension as well as a favourable lipid, inflammation, hormonal, liver enzyme and immune profile (6). Two key characteristics have been reported by most groups (Fig. 1): for a similar total fat mass, MHO individuals present less visceral and ectopic fat, in particular liver steatosis than patients with metabolic abnormalities (6–8). The MHO phenotype is also associated with a lower degree of systemic inflammation (e.g. as measured by lower plasma high-sensitivity C-reactive protein [CRP] (9–11) ) (Fig. 1). Dr Rabasa-Lhoret indicated that longitudinal data clearly show that not all MHO individuals remain metabolically healthy over time with up to 30% converting to metabolically unhealthy obese (MUHO) with cardio-metabolic complications over a 5–10-year time frame (12–14). Two important risk factors have been suggested to be associated with such conversion: ageing and additional weight gain; conversely, higher physical activity and weight loss are associated with a higher likelihood to preserve the MHO status (13).
Figure 1.
Proposed features of preserved metabolic health in obese humans. One of the most consistent characteristics of metabolic health in obesity across studies in humans is reduced liver lipid. This is likely the consequence of increased capacity for storing fat coupled with improved mitochondrial function in adipose tissue and decreased de novo lipogenesis in liver. This can also result in decreased deposition of lipids, including bioactive species, in skeletal muscle. Decreased adipose tissue inflammation with decreased macrophages and a unique T-cell signature with an anti-inflammatory circulating milieu were also suggested to characterize metabolic health in obesity. Anecdotal data support a possible role for healthier lifestyle, including increased level of physical activity and healthier diet. It remains to be established whether a favourable metagenomic signature is a characteristic of metabolic health in obesity. Reprinted and adapted from Samocha-Bonet et al., ‘Insulin-sensitive obesity in humans – a “favourable fat” phenotype?’ Trends Endocrinol Metab (2012), with permission from Wiley.
Dr Rabasa-Lhoret emphasized that mechanisms that could explain the favourable metabolic profile of individuals who are MHO are poorly understood, but implied that favourable abdominal fat mass distribution, as well as preservation of ‘healthy’ subcutaneous adipose tissue function and structure may explain the improved ability to store the excessive fat mass without (or with less) associated metabolic complications. The role of healthy adipose tissue expansion during energy excess in metabolic health in obesity was discussed later in the meeting by Dr Scherer.
MHO – inconsistent definition stirs confusion
Dr Rabasa-Lhoret stressed that the main obstacle in advancing our understanding of the MHO phenotype and its long-term metabolic fate is the inconsistent definition of metabolic health across studies. The problem is twofold: first, the terminology used to define this clinical entity is based on either insulin sensitivity (predominantly by the surrogate homeostasis model assessment of insulin resistance, with subjective cut-offs) or the metabolic syndrome (MS). Adding to the confusion is the use of different sets of criteria to define the MS (15), and the inconsistent requirements in different studies to be either free of or have fewer of the MS features. Defining metabolic health on the basis of different criteria resulted in a wide range of prevalence reported for the phenotype from less than 10% of the obese population to almost 50% (6,7). Dr Rabasa-Lhoret and others showed considerable differences in metabolic characteristics (16) and all-cause mortality (17) for MHO defined on the basis of different sets of criteria. This, of course, leads to disagreement regarding the benefit of therapeutic interventions. A recent meta-analysis indicated that a healthier metabolic obese profile may not translate into a lower risk of mortality (18). Despite the fact that significant methodological concerns have been raised regarding the analysis, Dr Rabasa-Lhoret suggested that it probably indicates that patients who are MHO are not entirely protected from metabolic disease. Nonetheless, the MHO group was at significantly lower health risk when compared with MUHO (18). Dr Rabasa-Lhoret concluded that resolution of these uncertainties will only be possible when a consensus about a definition for a preserved cardio-metabolic health in obesity is reached.
Gene–environment interactions in insulin resistance and metabolic disease
The next presentations by Dr Kahn and Dr Pietiläinen focused on gene–environment interactions in metabolic disease in animal models and humans. Dr Kahn presented compelling data from his longstanding research in animal models emphasizing the importance of both genetic disposition and environmental factors in determining insulin resistance. Both type 2 diabetes and obesity are the result of a complex interaction between genes and the environment. While the relative importance of these components is very difficult to sort out in humans, mouse models provide a powerful tool to investigate the contributions of genetic and environmental factors involved in disease susceptibility. Kahn and colleagues have previously shown that C57BL/6J (B6J) and 129X1SvJ or 129S1/SvImg (129J) mice have remarkably different susceptibility to obesity and MS. Thus, on a regular chow diet, B6J mice gain more weight, have higher levels of insulin and leptin, and show greater glucose intolerance than 129J mice, and these phenotypic differences are further exacerbated with high-fat feeding (19). Likewise, genetically induced insulin resistance by heterozygous deletion of the insulin receptor and insulin receptor substrate 1 lead to more than 90% of B6J mice developing diabetes, but only less than 5% of 129J mice (20). Genome-wide scanning of an intercross between these strains indicated that at least four loci on three chromosomes are involved in this process, and one of these corresponds to be the PKCδ gene (19,21). By comparing 129J mice with another closely related substrain of 129 mice from Taconic Farms, Dr Kahn suggested critical roles for diet and gut microbiome composition in metabolic health and disease, as well. The topic of the microbiome was also covered in detail by Dr Nieuwdorp.
Dr Pietiläinen agreed that metabolically benign obesity in humans results from an interaction between beneficial genes and healthy lifestyle. Age, sex and cumulative amount of years exposed to excess weight may also play a role in an individual’s metabolic response to obesity. Dr Pietiläinen suggested that the study of weight-discordant monozygotic (MZ) twin pairs is a powerful tool to appreciate gene–environment interaction in humans. In this design, the obesity-discordant twins are fully matched for genes, age and sex, and partially matched for environmental factors, including intrauterine and childhood family environment. Thereby, comparison of weight- and obesity-discordant MZ twins allows the study of long-term metabolic consequences of acquired weight gain within each genotype, but without the confounding effects of ageing in longitudinal studies. On the other hand, comparison of pairs with different metabolic responses to weight gain highlight biological pathways associated with the development of healthy or unhealthy obesity.
Using this approach, Dr Pietiläinen and her colleagues made significant contributions to understanding of metabolic health in obesity. The first set of experiments showed that overall, a number of pre-diabetic and pre-atherosclerotic changes were observed in the more obese compared with the less obese co-twins: reduced insulin sensitivity evaluated by the gold-standard hyperinsulinaemic-euglycaemic clamp, increased fasting insulin, higher liver lipid, dyslipidaemia, pro-inflammatory serum and differences in adipose tissue lipidomics profile and arterial and endothelial dysfunction (22–25). In a recent twin collection, Dr Pietiläinen reported that metabolic phenotypes in acquired obesity were polarized to two distinct groups (26). The average weight difference between the co-twins was 17 kg. Age (23–36 years), sex distribution and age of onset of obesity (19–20 years) was similar in the two groups. While in one group, the obese co-twins exhibited a typical response to obesity with markedly elevated liver fat, insulin resistance, hypertension, dyslipidaemia, and blunted incretin response, in the other group, the obese co-twins were metabolically healthy as their lean co-twins (26–28). Remarkably, in the MHO group, the percentage of liver fat was very low (~1%) and was similar to that of the lean co-twins, highlighting the liver contribution to the unhealthy obese phenotype.
Dr Pietiläinen continued by describing a global transcriptomics analysis of subcutaneous adipose tissue, where it became evident that the fundamental feature of MHO seemed to be intact mitochondrial function (26). The role of mitochondria in metabolic health and disease in obesity was described in detail by Dr Roden later in the meeting. Dr Pietiläinen explained that three energy dissipation pathways, oxidative phosphorylation, fat oxidation and amino acid catabolism showed preserved pathway activities in subjects who are MHO at a level similar to their lean counterparts (26). In contrast, these pathways were significantly down-regulated in adipose samples from obese twins with metabolic disturbances. Another potential hallmark of metabolic health, a favourable inflammatory profile of the adipose tissue was also observed in the MHO twins (26). Also, the fat cells of the MHO twins were smaller with evidence of more active differentiation processes within the fat tissue. As multiple mitochondrial pathways are vital in adipocyte differentiation (29), it is possible that mitochondrial malfunction impairs the development of new fat cells, which in turn results in an inability of the adipose tissue to expand under conditions of energy excess. This failure of fat cell proliferation has long been suspected to constitute the framework for ectopic fat storage, insulin resistance and type 2 diabetes. Based on her twin studies, Dr Pietiläinen suggested that one possible mechanism behind impaired adipocyte differentiation could be decreased catabolism of branched-chain amino acids (BCAAs) (22,26). Mitochondrial metabolism of BCAAs, especially that of leucine, stimulates adipocyte growth and differentiation by activating the mammalian target of rapamycin signalling (30). Therefore, decreases in BCAA catabolism reduce the building blocks necessary for this signalling route. The decrease in adipose tissue BCAA catabolism can also lead to increases in plasma BCAA (31), a phenomenon closely linked to systemic insulin resistance (32) and development of type 2 diabetes (33). Further, the down-regulation of mitochondrial biogenesis seemed to coincide with up-regulation of inflammatory pathways in the fat of MUHO twins, with significantly elevated serum CRP concentrations (26). As discussed later, the circulating inflammatory environment in itself, could lead to metabolic disturbances in obesity.
Dr Pietiläinen concluded that the contributions of genes vs. environmental factors to the large metabolic variation in the response to obesity are not known. In the obesity-discordant MZ twins, the theoretical role of genetic point mutations (heteroplasmy) of mitochondrial genome to explain the twins’ weight differences have so far been disputed (22). However, it is not ruled out that genetic factors play a role in the large group differences in the development of MHO or MUHO. Nonetheless, there was some evidence for the role of lifestyle in MHO: the MUHO twins were less physically active and tended to increased alcohol consumption compared with their lean co-twins, patterns not found in the MHO twins (26). Based on the twin study, no predictive signs of the fate of obesity were found in lean co-twins. All lean twin pair members were healthy, and the metabolic abnormalities, if any, only appeared after development of obesity (26).
The next talk, presented by Dr Lin, focused on metabolic disease in China. Dr Lin presented intriguing data from a population-based cohort of men and women from urban and rural areas in China. China is facing an epidemic of metabolic disease because of rapid nutrition and lifestyle transition in the last few decades. The prevalence of overweight and obesity increased from 23% to 31% and from 7% to 12%, respectively from 2002 to 2010 (34,35). Similarly, in 1980 less than 1% of adult Chinese had diabetes (36) and estimated prevalence reached 11.6%, or 113.9 million, in 2012 (37). Dr Lin indicated that compared with Caucasians, Asians are known to have increased genetic predisposition for type 2 diabetes and tend to present the metabolically unhealthy phenotype, in particular increased visceral adiposity at lower body mass index (BMI) (38). Few studies have prospectively investigated the relationships between environmental factors and genetic variants with obesity and related metabolic disease in Chinese. Lin and colleagues in a cohort of men and women aged 50–70 years from urban and rural areas of Beijing and Shanghai, found significant north–south and urban–rural differences in the prevalence of the MS and type 2 diabetes. Specifically, at a given level of overweight/obesity, northern participants had higher risk of MS and/or type 2 diabetes (39). Ethnical differences in the linkage disequilibrium patterns and risk allele frequencies of GRK5, RASGRP1 and FTO loci were noticed between Chinese Hans and White Europeans (40). As an example, the association between FTO and type 2 diabetes was independent of BMI in Chinese Hans, but not in Caucasians (41). Interestingly, plasma CRP concentrations in the Chinese population were lower, with approximately 50% of participants with MS with CRP below ‘low risk’ level according to the American Heart Association criteria (predominantly generated from data in Caucasian populations) (42). Similar to studies in Caucasian populations; however (38), larger leg fat was associated with a better adipokine and inflammatory cytokine profile and with a reduced risk of MS, in contrast to adverse effects of trunk fat (43,44). Interesting differences were revealed by gas chromatography analysis of erythrocyte fatty acids between different geographical areas. Generally, Shanghai or urban participants had significantly higher concentrations of total and most of n−3 fatty acids compared with their Beijing or rural counterparts. Also, erythrocyte docosahexaenoic acid, but not eicosapentaenoic was inversely associated with MS risk (45). A gene variant on FADS1, an essential desaturase in polyunsaturated fatty acid metabolism, was associated with lower high-density lipoprotein cholesterol only when erythrocyte 18:2n−6 or 18:3n−3 were lower (46). Overall, erythrocyte trans-fatty acids concentrations were lower in Chinese population than those reported in Western studies. The major trans-fatty acid, trans-18:1 was significantly associated with dairy products intake (47). Interestingly, increased levels of trans-18:1 isomers and diary intake were associated with 30–35% reduced 6-year type 2 diabetes incidence, but the significant association between trans-18:1 isomers and type 2 diabetes risk was abolished by adjusting for dairy intake, suggesting that trans-18:1 may be a potential biomarker for dairy intake in this population (48). On the other hand, erythrocyte trans-18:2 levels were associated with dyslipidaemia (47). In addition, increased erythrocyte 16:1n−7 (palmitoleic acid) and other de novo lipogenesis-associated fatty acids associated with a high carbohydrate low fat diet and with an increased 6-year incidence of metabolic disease (49). Overall, Dr Lin’s work revealed unique relationships between genetic disposition, environmental factors and metabolic disease in Chinese individuals, and will assist in guiding future recommendations to improve metabolic health in this population.
Role of the adipose tissue studies in MHO and MUHO animal models
Dr Scherer focused on the role of the adipose tissue in determining the metabolic fate of obesity and presented fascinating data generated in obese animal models with a beneficial metabolic profile that mimic the clinical MHO situation. He opened with an overview of the processes that take place in adipose tissue in states of energy excess. During the progression from the lean to the obese state, adipose tissue undergoes hyperplasia as well as hypertrophy in an attempt to cope with the increased demand for triglyceride storage. This requires a high degree of plasticity at both the cellular and tissue levels. Even though adipose tissue as a whole seems to be a relatively static tissue containing many adipocytes that turn over slowly, these cells are embedded in an environment that can rapidly adapt to the needs of expanding and newly differentiating adipocytes. The extracellular matrix of adipose tissue faces unique challenges with respect to adjusting to the need for remodelling and expansion (50). In parallel, the vasculature has to adapt to altered requirements for nutrient and oxygen exchange (51). A decreased plasticity of these processes leads to metabolic dysfunction. Furthermore, to maintain a healthy, non-inflamed phenotype, complex regulatory mechanisms are in place to ensure adipocytes and stromal vascular cells efficiently crosstalk to allow adipose tissue to expand upon increased demand for storage of triglycerides. Dr Scherer proposed a model of stepwise adipose tissue dysfunction that is initiated by rapid expansion of existing adipocytes to accommodate triglycerides during excess caloric intake. This leads very quickly to an acute, and eventually chronic, state of hypoxia in adipose tissue (52). The Scherer lab hypothesized that vascular endothelial growth factor-A (VEGF-A)-induced stimulation of angiogenesis enables sustained and sufficient oxygen and nutrient exchange during fat mass expansion, thereby improving adipose tissue function. Utilizing a doxycycline-inducible adipocyte-specific VEGF-A overexpression model, they demonstrate improved vascularization in parallel with ‘browning’ of white adipose tissue, and massive up-regulation of uncoupling protein 1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha, associated with increased energy expenditure and resistance to high-fat diet-mediated metabolic insults (53). Conversely, inhibition of VEGF–A-induced activation of vascular endothelial growth factor receptor 2 (VEGFR2) during the early phase of high-fat diet-induced weight gain, caused systemic insulin resistance. However, the same VEGF-A–VEGFR2 blockade in ob/ob mice lead to reduced body weight gain, improvement in insulin sensitivity, decreased inflammation and increased incidence of adipocyte death. The consequences of modulation of angiogenic activity are therefore context-dependent. Pro-angiogenic activity during adipose tissue expansion is beneficial, associated with potent protective effects on metabolism, while anti-angiogenic action in the context of pre-existing adipose tissue dysfunction leads to improvements in metabolism, an effect likely mediated by the ablation of dysfunctional pro-inflammatory adipocytes (53).
Dr Scherer proposed that changes during the adipose expansion process also affect adipocyte-derived secretory factors (adipokines), such as adiponectin (54). Adiponectin promotes insulin sensitivity, decreases inflammation and promotes cell survival (55), and its levels are frequently down-regulated in the obese state. Avoiding the obesity-associated down-regulation of adiponectin by genetic manipulation allows the adipose tissue to further expand and leads to a ‘healthy’ expansion, with enlarged subcutaneous adipose tissue, improved vascularization and enhanced adipogenesis. As a result, the Scherer lab showed that systemic insulin sensitivity improved, that is an overall phenotype reminiscent of chronic exposure to pharmacological doses of peroxisome proliferator-activated receptor gamma agonists (56). In a similar fashion, overexpression of the mitochondrial protein mitoNEET in the adipocyte results in alteration in mitochondrial iron content and a reduced rate of β oxidation. This results in adiponectin overexpression as well and in an overall phenotype very similar to what is observed in the adiponectin-overexpressing mouse (57).
Dr Scherer reasoned that these phenotypes of healthy adipose tissue expansion have a positive effect on the systemic lipotoxic environment that prevails in the obese state (58). This may be particularly relevant for sphingolipids that tend to accumulate and prompt a high level of cytotoxicity under high-fat diet conditions. Adiponectin potently stimulates a ceramidase activity associated with its two receptors, adipoR1 and adipoR2, and enhances ceramide catabolism and formation of its anti-apoptotic metabolite – sphingosine-1-phosphate, independently of adenosine monophosphate-activated protein kinase (59). These observations suggest a novel role of adipocyte-derived factors that have beneficial systemic effects, with sphingolipid metabolism as its core upstream component (60). As the overall levels of adiponectin are reduced in the obese state, the prevailing increase in ceramides can lead to a significant increase in insulin resistance, hence contributing to the lipotoxic environment in a number of different tissues.
Dr Scherer concluded that preserving healthy adipose tissue with the ability to further expand with proper vascularization and a rate of adipogenesis that keeps pace with the need for additional fat cells is key to preserving elevated adiponectin levels, reduce cytotoxicity, giving rise to the MHO phenotype. It was agreed that the data from the preclinical models presented translate very well into the clinical setting.
Mitochondrial function and metabolic health
The term mitochondrial function comprises different features of the mitochondria such as oxidative phosphorylation capacity, submaximal adenosine diphosphate (ADP)-stimulated oxidative phosphorylation and basal as well as insulin-stimulated adenosine triphosphate ((ATP) synthesis). These features of the mitochondria can be quantified in humans using noninvasive and invasive methods. Dr Roden presented compelling evidence generated by sophisticated in vivo measurements for the need of a healthy mitochondrial function to preserve metabolic health. Substrate oxidation in all tissues requires adequate mitochondrial function. The Roden lab uses in vivo (31) P magnetic resonance spectroscopy to asses unidirectional flux through ATP synthase from saturation transfer as well as submaximal ADP-stimulated oxidative phosphorylation from phosphocreatinine recovery upon exercise (61).
During the last decade, alterations in oxidative metabolism have been described and linked to impaired insulin-stimulated glucose metabolism in human skeletal muscle and liver. These alterations frequently occur in parallel with augmented lipid storage suggesting impaired lipid oxidation capacity. Insulin-resistant states, such as type 2 or long-standing type 1 diabetes, but also obesity, family history of type 2 diabetes or gestational diabetes, may exhibit acquired or inherited reductions of oxidative phosphorylation. Severely insulin-resistant first-degree relatives of patients with type 2 diabetes, for instance, have lower mitochondrial density/number and function compared with matched persons without family history of type 2 diabetes (62). Patients with overt type 2 diabetes, however, do not generally have markedly lower mitochondrial density or function than controls, indicating that acquired factors also contribute to alteration in energy metabolism (63). Indeed, higher concentrations of circulating free fatty acids and glucose determine both mitochondrial function and insulin sensitivity.
Although mitochondrial function can be altered in insulin-resistant states, this does not necessarily hold true for all tissues or explain all forms of insulin resistance. Roden and colleagues showed that muscle flux through ATP synthase negatively relates not only to insulin sensitivity and glycaemia, but particularly to liver fat content (64). Of note, impaired mitochondrial function can be present in liver of patients with overt type 2 diabetes. On the other hand, there is some evidence that the oxidative capacity and production of reactive oxygen species (ROS) can be even increased in hepatic mitochondria of obese insulin-resistant humans (65). Thus, hepatic mitochondria are likely able to oxidize excess intracellular lipids in early stages of obesity, but concomitant production of ROS may accelerate insulin resistance, fat deposition and impair organ function in the long term (66).
Some lifestyle modifications and pharmacological interventions can enhance mitochondrial content and function, which is not necessarily related to changes in insulin sensitivity. Dr Roden gave the example of a gene variant, (G/G single nucleotide polymorphism of the nicotinamide adenine dinucleotide dehydrogenase [ubiquinone] 1 β sub-complex 6 [NDUFB6] rs540467 gene) that increased the response of muscle ATP synthesis, but not of insulin sensitivity, to physical exercise training. These findings hint at interaction of inherited factors with responsiveness to the metabolic effects of lifestyle modifications in humans (67,68). On the other hand, Dr Roden suggested that even short-term inhibition of lipolysis with reduction of circulating free fatty acids by acipimox improves insulin sensitivity and ROS production, but does not affect oxidative capacity in skeletal muscle (69).
Dr Roden concluded that mitochondrial function contributes to metabolic health by adapting to substrate availability under various conditions. States of reduced insulin sensitivity and inadequate insulin secretion lead to greater lipolysis and increased fluxes of triglycerides and free fatty acids to the muscles and the liver. Particularly, the liver can adapt its energy metabolism by increasing mitochondrial oxidation, thereby preventing the accumulation of hepatic lipids. Inadequate adaptation because of inherited factors and/or long-term exposure to high lipid and glucose levels will raise cellular ROS production, which activates inflammatory pathways such as c-Jun N-terminal kinase and nuclear factor kappa-light-chain-enhancer of activated B cells, and toxic lipid metabolites such as diacylglycerols or ceramides, which cause insulin resistance. These mechanisms contribute to the development and progression of non-alcoholic fatty liver disease and to systemic insulin resistance by modifying the release of tissue-specific cytokines such as hepato-, myo- and adipokines, i.e. a metabolically unhealthy phenotype.
Dys-regulation of immune system by chronic obesity
During obesity, adipose tissue acquires immunological properties that are exemplified by increased infiltration of haematopoietic lineage cells such as activated macrophages, neutrophils, eosinophils, mast cells, innate lymphoid cells, and T and B cells (70,71). Dr Dixit presented topical data from his lab and others on the involvement of the immune system in metabolic disease in obesity. New data demonstrate that the reciprocal interactions between immune and metabolic systems may play physiological roles in adaptation to chronic energy excess through adipose tissue remodelling, restoration of tissue homeostasis and potential effects on fuel utilization (72,73). However, an ever-growing body of work demonstrates that immune–metabolic interactions can also lead to low-grade inflammation that impairs tissue function and links obesity to chronic disease (70,71). Among several innate immune sensors that can detect obesity-associated ‘danger signals’, the NLRP3 inflammasome, a multimolecular complex of proteins that controls caspase-1 activation and downstream pro-inflammatory cytokines interleukin (IL)-1β and IL-18 appears to play a major role in the development of metabolic inflammation (74–76). Dr Dixit and others showed that reduction of NLRP3 inflammasome activity in patients with obesity and type 2 diabetes by metformin (77) as well as weight loss (74) is linked to improved glucose homeostasis. In addition, genetic inactivation of the NLRP3 in mice protects against atherosclerosis, type 2 diabetes, gout as well as other sterile inflammatory diseases such as Alzheimer’s disease (70,75). Notably, multiple new inflammasome complexes including the NLRP6-, AIM2-, and NLRP12-inflammasomes and non-canonical caspase-11 inflammasomes have recently been described. But their role in the pathogenesis of obesity remains to be established. Dr Dixit suggested that future investigations that address stimulatory and inhibitory inflammasome pathways may enhance our understanding of the aetiology of obesity-associated conditions and aid in the design of novel therapeutics.
In addition, diet-induced obesity in mice impairs CD4 and CD8 T–cell-mediated adaptive immunity that is manifested by increased severity and death from influenza infections (78–80). Consistent with this, data from 19 countries during the H1N1 pandemic indicated that obese individuals had significantly higher morbidity and mortality from H1N1 influenza (81). In addition to influenza, obesity can also compromise vaccination efficacy against hepatitis B and tetanus (70). Dr Dixit therefore hypothesized that in a future event of infectious disease outbreaks, obese individuals may be highly susceptible, and existing vaccination strategies may not be efficacious in a large segment of the at-risk population; this should raise public health concern. Studies are therefore needed to identify the mechanism of obesity-associated reduction in immune surveillance and immune reconstitution to improve the prevention and treatment strategies against re-emergence of infectious diseases.
Dr Dixit added that it is now evident that obesity not only impacts innate immune cells such as macrophages, but also compromises central immunity by impairing generation of new T-cells from thymus. The genetic obesity driven by lack of leptin or melanocortin 4 receptor leads to increased damage to thymic stromal cells that leads to reduced production of naïve T-cells from thymus (81). Furthermore, diet-induced chronic obesity accelerates the thymic atrophy process by inducing lipotoxicity within the thymic microenvironment that leads to reduced naïve T-cell production and restriction of T-cell receptor (TCR) repertoire diversity (81). The major mechanism of obesity-induced lipotoxicity is by alterations in thymic epithelial cells, as well as reductions in T-cell progenitors in the thymus and bone marrow (70,81). The thymus is responsible for the development and selection of T-cells that are tolerant to self-antigens and possess broad TCR repertoire to generate an immunocompetent response against foreign antigens. Obesity in humans is also associated with reduced generation of naïve T-cells. Importantly, compared with lean healthy subjects, individuals who are obese and non-diabetic and diabetic displayed reduced thymic T-cell production, but no difference was found in naïve T-cells between the metabolically healthy and unhealthy obese individuals in this study (81). Dr Dixit suggested that additional studies using multi-colour flow cytometry in a large group of people are necessary to fully delineate effect of metabolically healthy vs. unhealthy obesity on indicators of adaptive immune responses. In conclusion, Dr Dixit argued that as the obesity epidemic evolves and threatens to shorten the health span and lifespan, the identification of the immunological processes that are sensitive to caloric restriction or caloric excess offers exciting new avenues to manage chronic diseases.
Specific T-cell signature in adipose tissue in individuals who are MHO
Building on Dr Dixit’s presentation, Dr Klein, in the first part of his talk, presented clinical data supporting the involvement of a specific immune cell signature in metabolic disease in obesity. Reduced adipose tissue inflammation has long been suggested to partly explain insulin sensitivity in obesity in humans (82–86). In the last decade, a growing body of work demonstrated that obesity is associated with macrophage infiltration into adipose tissue (87,88) in conjunction with a switch from anti- to pro- inflammatory macrophage phenotype (89). Recently, studies in Dr Klein’s laboratory and others (90,91) have extended the evidence for an immune system involvement in obesity-related metabolic disease in humans to specific T-cell signature in adipose tissue. Individuals with obesity who are insulin-sensitive had decreased proportions of IL-22 and IL-17 producing cells in CD4+ T-cells expanded from subcutaneous adipose tissue, similar to lean controls (90). This was related to decreases in plasma IL-6, the IL that stimulates lymphocyte polarization towards the Th17/Th22 phenotype. Further, Klein and colleagues have demonstrated that insulin action was inhibited in IL–17- and IL–22-treated rat soleus muscle strips and primary human hepatocytes in vitro, suggesting a link between circulating IL-17 and IL-22 and peripheral insulin resistance (90). Furthermore, others have reported that the percentage of anti-inflammatory regulatory T-cells in visceral adipose tissue was increased in MHO, similar to levels observed in lean healthy individuals (91). Altogether, these findings suggest a unique T-cell signature in adipose tissue that may be involved in preserving metabolic health in obesity in humans. The second part of Dr Klein’s talk focused on lessons from body weight alterations in humans (see ‘Metabolically healthy obesity – Lessons from weight loss and gain studies’).
Role of microbiota in metabolic health
Dr Nieuwdorp focused on the involvement of gut micro-biota in determining metabolic health, presenting fascinating data from his group and others. A multitude of microbial cells reside in the human intestinal tract and their association with human health and disease is now well recognized (92). The beneficial impact of the gut’s microbes on human metabolism has been discovered only recently and alterations in gut microbiota have been described in human obesity, type 2 diabetes and insulin resistance (as reviewed (93)). Causal relationships between certain intestinal micro-organisms and obesity were established when colonization of germ-free mice with ‘obese microbiota’ resulted in significant increases in body fat compared with colonization with ‘lean microbiota’ (94). The human intestinal microbiota is extremely complex and includes thousands of species with close to 5 million genes, termed the metagenome (92). High-throughput sequencing of the metagenome from stool samples is now regularly used to analyse the intestinal microbiota. Metagenomic data combined with clinical information give rise to metagenome-wide association study (MGWAS). Karlsson and colleagues (95) and Qin and colleagues (96) in MGWAS studies in European and Chinese populations, respectively reported highly significant correlations of specific intestinal microbes and their genes in type 2 diabetes. Strikingly, similar type 2 diabetes metagenomic signatures were reported in the European and Chinese individuals. Specifically, fewer Clostridiales bacteria that produce the short-chain fatty acid butyrate was highly discriminant of type 2 diabetes (92), underscoring the well-established role of butyrate producing bacteria as regulators of glucose and lipid metabolism (93). The Nieuwdorp lab, in a breakthrough study has recently showed that faecal transplantation from lean insulin-sensitive donors in male insulin-resistant recipients improved peripheral and hepatic insulin-sensitivity 6 weeks post transplantation (97). This was associated with increased gut microbiota diversity and in particular, increases in specific butyrate producing bacterial species (97). Dr Nieuwdorp suggested that these findings may translate to exciting therapeutic avenues for metabolic disease, but whether preserved metabolic health in obesity is associated with a favourable metagenomic signature remains to be established.
MHO – lessons from weight loss and gain studies
Dr Leibel, Dr Ravussin and Dr Klein talks focused on data gathered in experimental caloric restriction or excess interventions in humans. It is well known that weight loss achieved through caloric restriction improves cardiovascular disease risk and insulin sensitivity in overweight and obese subjects (98–100). This is clearly the case for individuals who are MUHO. However, MHO individuals present better cardio-metabolic profile at baseline and whether further improvement in metabolic health is achieved with weight loss is unclear. For a comparable weight loss through caloric restriction and exercise, MUHO consistently correct cardio-metabolic risk profile, while findings in MHO remain controversial, with either increase (101), no change (102,103) or decrease (104) in insulin sensitivity. An overall reduced metabolic benefit of caloric restriction in the more healthy obese population may reflect their initial improved health, but further study in large cohorts is required to guide clinical recommendations. Bariatric surgery with dramatic weight loss in severely obese individuals corrects oral glucose tolerance, insulin sensitivity and β-cell function (105), and with comparable weight loss both MHO and MUHO exhibit improvements in blood pressure, lipid profile and fasting glucose 6–12 months post-surgery (106,107). While these findings suggest that bariatric surgery is effective in correcting cardio-metabolic risk profile irrespective of metabolic health in the short-term, a recent long-term follow-up of approximately 14 years reported that obese individuals with higher baseline fasting insulin showed a greater relative treatment benefit in cardiovascular events (108). No such differences in relative treatment benefits were noted when the cohort was stratified based on BMI, waist or waist–hip ratio. Overall, these findings support the addition of markers of insulin sensitivity to better guide selection for treatment in severely obese individuals, but further study with other disease and mortality end points is required.
Overfeeding and weight gain, on the other hand increases insulin resistance in healthy individuals (109,110). Dr Ravussin suggested that similar to studies in animal models, experimental short-term overfeeding challenge is employed as means to trace the temporal order of events leading to metabolic disturbances in humans. Using this approach, work in the Ravussin lab and others have demonstrated that short-term experimental overfeeding for 4–8 weeks with 4–10% weight gain increases abdominal subcutaneous and visceral fat depots, without increases in subcutaneous adipose tissue inflammation (110–112). Adipose tissue extracellular matrix remodelling genes, however, are dramatically induced by short-term overfeeding in healthy individuals (110,112). To the surprise of the Ravussin lab, 8 weeks overfeeding with 10% weight gain resulted in a dramatic up-regulation of extracellular matrix remodelling and inflammation-associated genes in skeletal muscle in healthy young men. These findings add dysfunctional extracellular matrix remodelling in skeletal muscle to the pathogenic milieu associated with energy excess and obesity. Overall, data in humans and time course analysis in high–fat-fed animal models (113,114) are consistent with a secondary role of adipose tissue inflammation in metabolic disturbances, while abnormal adipose extracellular matrix development and fibrosis are early consequences of weight gain (114). These data may support the hypothesis that prolonged repeated bouts of energy excess are necessary to reach a critical threshold from which low-grade inflammation in adipose tissue is maintained (89). Indeed, weight cycling in mice enhances adipose tissue and systemic inflammation, which may contribute to metabolic dysfunction (115,116). Dr Ravussin hypothesized that less weight cycling and maintenance of stable body weight may explain health preservation in obesity, but this requires further study. Also, it remains to be established whether individuals who are MHO are protected from the adverse effects of overfeeding.
Concluding remarks
Despite the uncertainty about the exact degree of protection related to the metabolically healthy or insulin-sensitive obese status, identifying underlying factors and mechanisms associated with this phenotype will eventually be invaluable in helping the scientific and medical community understand factors that predispose, delay or protect obese individuals from metabolic disturbances. It is essential to underscore that the MHO concept presently only address the cardio-metabolic risks associated with obesity; it is therefore important that patients who are MHO are still very likely to present many other obesity-related complications such as altered physical and/or physiological functional status, sleep problems, articulation and postural problems, stigma, etc. Importantly, the MHO concept supports the fact that classification based on excess adiposity per se (e.g. BMI or body composition if available) should be supplemented with obesity-related comorbidities, e.g. with fasting insulin (108) as proposed by the Edmonton obesity classification system (117). A greater understanding of the MHO phenotype has important implications for therapeutic decision-making, characterization of subjects in research protocols and medical education. Patients who are MHO need medical attention, but the most appropriate care for these patients remains to be established. Above all, because of all the other associated disorders associated with obesity, it is imperative to also enrol patients who are MHO in weight management therapy.
Acknowledgments
The authors would like to thank Dr David York who has coordinated the Stock Conferences for many years. In addition, we would like to thank the sponsors xxx and yyy for making this meeting possible. We also thank our speakers and all of the participants attending the 2013 Stock Conference for their intellectual contributions and thoughtful discussions during the meeting.
References
- 1.World Health Organization Media center. Fact Sheet: Obesity and Overweight. Health Organization World: WHO; 2013. [Google Scholar]
- 2.Sims EAH. Characterization of the syndromes of obesity. In: Brodoff BN, Bleicher SJ, editors. Diabetes Mellitus and Obesity. Williams & Wilkins; Baltimore, MD: 1982. pp. 219–226. [Google Scholar]
- 3.Keyes A. Overweight and the risk of sudden heart attack and sudden death, in obesity in perspective. DHEW Publication No (NIH) 1973:75–708. [Google Scholar]
- 4.Andres R. Effect of obesity on total mortality. Int J Obes. 1980;4:381–386. [PubMed] [Google Scholar]
- 5.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 6.Primeau V, Coderre L, Karelis AD, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 7.Samocha-Bonet D, Chisholm DJ, Tonks K, Campbell LV, Greenfield JR. Insulin-sensitive obesity in humans – a ‘favorable fat’ phenotype? Trends Endocrinol Metab. 2012;23:116–124. doi: 10.1016/j.tem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Stefan N, Häring H-U, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 9.Karelis AD, Faraj M, Bastard JP, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 10.Karelis AD, Rabasa-Lhoret R. Obesity: can inflammatory status define metabolic health? Nat Rev Endocrinol. 2013;9:694–695. doi: 10.1038/nrendo.2013.198. [DOI] [PubMed] [Google Scholar]
- 11.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 12.Appleton SL, Seaborn CJ, Visvanathan R, et al. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36:2388–2394. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y, Ryu S, Suh BS, Yun KE, Kim CW, Cho SI. Impact of BMI on the incidence of metabolic abnormalities in metabolically healthy men. Int J Obes. 2012;36:1187–1194. doi: 10.1038/ijo.2011.247. [DOI] [PubMed] [Google Scholar]
- 14.Soriguer F, Gutierrez-Repiso C, Rubio-Martin E, et al. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J Clin Endocrinol Metab. 2013;98:2318–2325. doi: 10.1210/jc.2012-4253. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 16.Messier V, Karelis AD, Prud’homme D, Primeau V, Brochu M, Rabasa-Lhoret R. Identifying metabolically healthy but obese individuals in sedentary postmenopausal women. Obesity (Silver Spring, Md. 2010;18:911–917. doi: 10.1038/oby.2009.364. [DOI] [PubMed] [Google Scholar]
- 17.Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care. 2013;36:2294–2300. doi: 10.2337/dc12-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions? A systematic review and meta-analysis. Ann Intern Med. 2013;159:758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
- 19.Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni RN, Almind K, Goren HJ, et al. Impact of genetic background on development of hyperinsulinemia and diabetes in insulin receptor/insulin receptor substrate-1 double heterozygous mice. Diabetes. 2003;52:1528–1534. doi: 10.2337/diabetes.52.6.1528. [DOI] [PubMed] [Google Scholar]
- 21.Almind K, Kulkarni RN, Lannon SM, Kahn CR. Identification of interactive loci linked to insulin and leptin in mice with genetic insulin resistance. Diabetes. 2003;52:1535–1543. doi: 10.2337/diabetes.52.6.1535. [DOI] [PubMed] [Google Scholar]
- 22.Pietilainen KH, Rissanen A, Kaprio J, et al. Acquired obesity is associated with increased liver fat, intra-abdominal fat, and insulin resistance in young adult monozygotic twins. Am J Physiol Endocrinol Metab. 2005;288:E768–E774. doi: 10.1152/ajpendo.00381.2004. [DOI] [PubMed] [Google Scholar]
- 23.Pietilainen KH, Sysi-Aho M, Rissanen A, et al. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects – a monozygotic twin study. PLoS ONE. 2007;2:e218. doi: 10.1371/journal.pone.0000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietilainen KH, Rog T, Seppanen-Laakso T, et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biol. 2011;9:e1000623. doi: 10.1371/journal.pbio.1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietilainen KH, Bergholm R, Rissanen A, et al. Effects of acquired obesity on endothelial function in monozygotic twins. Obesity. 2006;14:826–837. doi: 10.1038/oby.2006.96. [DOI] [PubMed] [Google Scholar]
- 26.Naukkarinen J, Heinonen S, Hakkarainen A, et al. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia. 2014;57:167–176. doi: 10.1007/s00125-013-3066-y. [DOI] [PubMed] [Google Scholar]
- 27.Kaye SM, Maranghi M, Bogl LH, et al. Acquired liver fat is a key determinant of serum lipid alterations in healthy monozygotic twins. Obesity. 2013;21:1815–1822. doi: 10.1002/oby.20228. [DOI] [PubMed] [Google Scholar]
- 28.Matikainen N, Bogl LH, Hakkarainen A, et al. GLP-1 responses are heritable and blunted in acquired obesity with high liver fat and insulin resistance. Diabetes Care. 2014;37:242–251. doi: 10.2337/dc13-1283. [DOI] [PubMed] [Google Scholar]
- 29.Hackl H, Burkard TR, Sturn A, et al. Molecular processes during fat cell development revealed by gene expression profiling and functional annotation. Genome Biol. 2005;6:R108. doi: 10.1186/gb-2005-6-13-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lackey DE, Lynch CJ, Olson KC, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–E1187. doi: 10.1152/ajpendo.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem. 2010;285:11348–11356. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, Xie G, Jia W. Insulin resistance and the metabolism of branched-chain amino acids. Front Med. 2013;7:53–59. doi: 10.1007/s11684-013-0255-5. [DOI] [PubMed] [Google Scholar]
- 33.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinese Center for Disease Control and Prevention. [accessed March 2014];Major findings in chronic diseases and risk factors of national DSPs in 2010. 2011 http://www.chinacdc.cn/gwswxx/mbsqc/201109/t20110906_52141.htm.
- 35.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31:177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 36.Pan XR, Yang WY, Li GW, Liu J. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative GROUP. Diabetes Care. 1997;20:1664–1669. doi: 10.2337/diacare.20.11.1664. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 38.Hocking S, Samocha-Bonet D, Milner K-L, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34:463–500. doi: 10.1210/er.2012-1041. [DOI] [PubMed] [Google Scholar]
- 39.Yu Z, Lin X, Haas JD, et al. Obesity related metabolic abnormalities: distribution and geographic differences among middle-aged and older Chinese populations. Prev Med. 2009;48:272–278. doi: 10.1016/j.ypmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Li H, Gan W, Lu L, et al. A genome-wide association study identifies GRK5 and RASGRP1 as type 2 diabetes loci in Chinese Hans. Diabetes. 2013;62:291–298. doi: 10.2337/db12-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Kilpelainen TO, Liu C, et al. Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia. 2012;55:981–995. doi: 10.1007/s00125-011-2370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye X, Yu Z, Li H, Franco OH, Liu Y, Lin X. Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Am Coll Cardiol. 2007;49:1798–1805. doi: 10.1016/j.jacc.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 43.Wu H, Qi Q, Yu Z, et al. Independent and opposite associations of trunk and leg fat depots with adipokines, inflammatory markers, and metabolic syndrome in middle-aged and older Chinese men and women. J Clin Endocrinol Metab. 2010;95:4389–4398. doi: 10.1210/jc.2010-0181. [DOI] [PubMed] [Google Scholar]
- 44.Wu H, Qi Q, Yu Z, Sun L, Li H, Lin X. Opposite associations of trunk and leg fat depots with plasma ferritin levels in middle-aged and older Chinese men and women. PLoS ONE. 2010;5:e13316. doi: 10.1371/journal.pone.0013316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang G, Sun Q, Hu FB, et al. Erythrocyte n-3 fatty acids and metabolic syndrome in middle-aged and older Chinese. J Clin Endocrinol Metab. 2012;97:E973–E977. doi: 10.1210/jc.2011-2997. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Sun Q, Zong G, et al. Interaction between a common variant in FADS1 and erythrocyte polyunsaturated fatty acids on lipid profile in Chinese Hans. J Lipid Res. 2013;54:1477–1483. doi: 10.1194/jlr.P027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu DX, Sun Q, Ye XW, et al. Erythrocyte trans-fatty acids, type 2 diabetes and cardiovascular risk factors in middle-aged and older Chinese individuals. Diabetologia. 2012;55:2954–2962. doi: 10.1007/s00125-012-2674-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zong G, Sun Q, Yu D, et al. Dairy consumption, type 2 diabetes, and changes in cardiometabolic traits: a prospective cohort study of middle-aged and older Chinese in Beijing and Shanghai. Diabetes Care. 2014;37:56–63. doi: 10.2337/dc13-0975. [DOI] [PubMed] [Google Scholar]
- 49.Zong G, Zhu J, Sun L, et al. Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am J Clin Nutr. 2013;98:319–326. doi: 10.3945/ajcn.113.061218. [DOI] [PubMed] [Google Scholar]
- 50.Khan T, Muise ES, Iyengar P, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun K, Wernstedt Asterholm I, Kusminski CM, et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 55.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metabol. 2013;2:133–141. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JY, van de Wall E, Laplante M, et al. Obesity-improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kusminski CM, Holland WL, Sun K, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012;18:1539–1549. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14:1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 59.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Unger RH, Scherer PE, Holland WL. Dichotomous roles of leptin and adiponectin as enforcers against lipotoxicity during feast and famine. Mol Biol Cell. 2013;24:3011–3015. doi: 10.1091/mbc.E12-10-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 62.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szendroedi J, Schmid AI, Chmelik M, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med. 2007;4:e154. doi: 10.1371/journal.pmed.0040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szendroedi J, Kaul K, Kloock L, et al. Lower fasting muscle mitochondrial activity relates to hepatic steatosis in humans. Diabetes Care. 2014;37:468–474. doi: 10.2337/dc13-1359. [DOI] [PubMed] [Google Scholar]
- 65.Sunny Nishanth E, Parks Elizabeth J, Browning Jeffrey D, Burgess Shawn C. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–810. doi: 10.1016/j.cmet.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koliaki C, Roden M. Hepatic energy metabolism in human diabetes mellitus, obesity and non-alcoholic fatty liver disease. Mol Cell Endocrinol. 2013;379:35–42. doi: 10.1016/j.mce.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Kacerovsky-Bielesz G, Kacerovsky M, Chmelik M, et al. A single nucleotide polymorphism associates with the response of muscle ATP synthesis to long-term exercise training in relatives of type 2 diabetic humans. Diabetes Care. 2012;35:350–357. doi: 10.2337/dc11-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kacerovsky-Bielesz G, Chmelik M, Ling C, et al. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes. 2009;58:1333–1341. doi: 10.2337/db08-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phielix E, Jelenik T, Nowotny P, Szendroedi J, Roden M. Reduction of non-esterified fatty acids improves insulin sensitivity and lowers oxidative stress, but fails to restore oxidative capacity in type 2 diabetes: a randomised clinical trial. Diabetologia. 2014;57:572–581. doi: 10.1007/s00125-013-3127-2. [DOI] [PubMed] [Google Scholar]
- 70.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 71.Shu CJ, Benoist C, Mathis D. The immune system’s involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012;24:436–442. doi: 10.1016/j.smim.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases – did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Youm YH, Adijiang A, Vandanmagsar B, Burk D, Ravussin A, Dixit VD. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152:4039–4045. doi: 10.1210/en.2011-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298–306. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–1243. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 80.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010;184:3127–3133. doi: 10.4049/jimmunol.0903220. [DOI] [PubMed] [Google Scholar]
- 81.Yang H, Youm YH, Vandanmagsar B, et al. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farb MG, Bigornia S, Mott M, et al. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011;58:232–237. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kolak M, Westerbacka J, Velagapudi VR, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 84.Koska J, Stefan N, Dubois S, et al. mRNA concentrations of MIF in subcutaneous abdominal adipose cells are associated with adipocyte size and insulin action. Int J Obes. 2009;33:842–850. doi: 10.1038/ijo.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qatanani M, Tan Y, Dobrin R, et al. Inverse regulation of inflammation and mitochondrial function in adipose tissue defines extreme insulin sensitivity in morbidly obese patients. Diabetes. 2013;62:855–863. doi: 10.2337/db12-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sears DD, Hsiao G, Hsiao A, et al. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc Natl Acad Sci USA. 2009;106:18745–18750. doi: 10.1073/pnas.0903032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ortega Martinez de Victoria E, Xu X, Koska J, et al. Macrophage content in subcutaneous adipose tissue: associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes. 2009;58:385–393. doi: 10.2337/db08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 90.Fabbrini E, Cella M, McCartney SA, et al. Association between specific adipose tissue CD4+T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–74. e1–3. doi: 10.1053/j.gastro.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Esser N, L’homme L, Roover A, et al. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56:2487–2497. doi: 10.1007/s00125-013-3023-9. [DOI] [PubMed] [Google Scholar]
- 92.de Vos WM, Nieuwdorp M. Genomics: a gut prediction. Nature. 2013;498:48–49. doi: 10.1038/nature12251. [DOI] [PubMed] [Google Scholar]
- 93.Vrieze A, Holleman F, Zoetendal EG, Vos WM, Hoekstra JBL, Nieuwdorp M. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53:606–613. doi: 10.1007/s00125-010-1662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 95.Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 96.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 97.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 98.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. NEJM. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Unick JL, Beavers D, Jakicic JM, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the look ahead trial. Diabetes Care. 2011;34:2152–2157. doi: 10.2337/dc11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care. 2010;33:1957–1959. doi: 10.2337/dc10-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kantartzis K, Machann J, Schick F, et al. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia. 2011;54:864–868. doi: 10.1007/s00125-010-2006-3. [DOI] [PubMed] [Google Scholar]
- 103.Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes. 2006;30:1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 104.Karelis AD. Metabolically healthy but obese individuals. Lancet. 2008;372:1281–1283. doi: 10.1016/S0140-6736(08)61531-7. [DOI] [PubMed] [Google Scholar]
- 105.Bradley D, Conte C, Mittendorfer B, et al. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest. 2012;122:4667–4674. doi: 10.1172/JCI64895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jimenez A, Perea V, Corcelles R, Moize V, Lacy A, Vidal J. Metabolic effects of bariatric surgery in insulin-sensitive morbidly obese subjects. Obes Surg. 2013;23:494–500. doi: 10.1007/s11695-012-0817-7. [DOI] [PubMed] [Google Scholar]
- 107.Sesti G, Folli F, Perego L, Hribal ML, Pontiroli AE. Effects of weight loss in metabolically healthy obese subjects after laparoscopic adjustable gastric banding and hypocaloric diet. PLoS ONE. 2011;6:e17737. doi: 10.1371/journal.pone.0017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sjostrom L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 109.Samocha-Bonet D, Campbell LV, Mori TA, et al. Overfeeding reduces insulin sensitivity and increases oxidative stress, without altering markers of mitochondrial content and function in humans. PLoS ONE. 2012;7:e36320. doi: 10.1371/journal.pone.0036320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tam CS, Covington JD, Bajpeyi S, et al. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J Clin Endocrinol Metabol. 2014;99:1749–1757. doi: 10.1210/jc.2013-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tam CS, Viardot A, Clement K, et al. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59:2164–2170. doi: 10.2337/db10-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alligier M, Meugnier E, Debard C, et al. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J Clin Endocrinol Metab. 2012;97:E183–E192. doi: 10.1210/jc.2011-2314. [DOI] [PubMed] [Google Scholar]
- 113.Turner N, Kowalski GM, Leslie SJ, et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56:1638–1648. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 114.Sun K, Tordjman J, Clément K, Scherer Philipp E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–3188. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS ONE. 2012;7:e39837. doi: 10.1371/journal.pone.0039837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. Can Med Assoc J. 2011;183:E1059–E1066. doi: 10.1503/cmaj.110387. [DOI] [PMC free article] [PubMed] [Google Scholar]