Abstract
Aims
To examine if menstrual phase affects relapse in women attempting to quit smoking.
Design
An intent-to-treat randomized smoking cessation trial where women were assigned to quit smoking in either the follicular (F) or luteal (L) menstrual phase and were followed for up to 26 weeks. They were assessed for relapse by days to relapse and relapse phase to determine if those who begin a quit attempt during the F phase were more successful than those who begin during the L phase.
Setting
Tobacco Use Research Center, University of Minnesota, Minneapolis, Minnesota.
Participants
A total of 202 women.
Measurements
Latency to relapse from continuous and prolonged abstinence, point prevalence, phase of relapse, first slip within the first 3 and 5 days post-quit date, subject completion rates and symptomatology (i.e. withdrawal and craving).
Findings
The mean days to relapse from continuous abstinence and relapse from prolonged abstinence for the F group were 13.9 and 20.6 days, respectively, and 21.5 and 39.2 days, respectively, for the L group. Using point prevalence analysis at 14 days, 84% of the F group had relapsed compared with 65% of the L group [χ2 = 10.024, P = 0.002; odds ratio (OR) = 2.871, 95% confidence interval (CI), 1.474– 5.590]. At 30 days, 86% of the F group relapsed, compared with 66% of the L group (χ2 = 11.076, P = 0.001; OR = 3.178, 95% CI, 1.594–6.334).
Conclusion
Women attempting to quit smoking in the F phase had less favorable outcomes than those attempting to quit in the L phase. This could relate to ovarian hormones, which may play a role in smoking cessation for women.
Keywords: Gender differences, menstrual cycle, ovarian hormones, relapse, smoking cessation
INTRODUCTION
In 2001, more than three-quarters of female smokers in the United States reported wanting to quit smoking, and nearly half (46.6%) tried to do so during the previous year [1]. There is evidence that, compared to men, women experience greater difficulty quitting, have less success in sustaining initial quit attempts [2–6] and are more likely to relapse faster [7,8]. The reasons for this are not well understood. Several barriers to smoking cessation appear to be more pronounced in women than in men. These include low confidence in quitting [3,9,10], smoking to relieve negative affect [5,8,11–13], fear of gaining weight after quitting [14–21] and higher rates of current and past depression [22–25]. Sex-based differences in the efficacy of pharmacological aids are conflicting. Some studies report that nicotine replacement therapy is less effective at suppressing some nicotine withdrawal responses in women than in men [26,27] and that women are less successful than men in quitting smoking when nicotine patches are used [28], while other studies [29,30] do not report a difference. Furthermore, it has been suggested that subjective and reinforcing effects of some non-nicotine smoking cues may be greater in women than in men [20,29]. The understanding of the behavioral and physiological basis for this apparent gender difference is still evolving.
A growing body of literature, although inconsistent and conflicting is emerging on whether sex steroid hormones estradiol and progesterone, which fluctuate throughout the menstrual cycle, may have a role. Some studies on smoking behavior report smoking rates to be higher during menses [31,32] and luteal phases [33,34] of the cycle, whereas other studies report no phase effect [18,35]. Under ad libitum smoking conditions, withdrawal symptomatology and craving were reported to be higher during the late luteal phase versus the follicular phase of the menstrual cycle [36].
There is evidence in some, but not all, studies to suggest that quitting smoking is more difficult in one phase versus the other. A few observational studies [34,37] report that nicotine withdrawal symptoms were more pronounced in the luteal phase. Experimental studies also report increased intensity of tobacco withdrawal symptoms during the late luteal phase [38,39]. On the other hand, experimental studies of short-term abstinence with a strictly defined cycle phase did not find that withdrawal symptomatology was affected by menstrual cycle phase [40,41], but that craving was elevated during post-menses [41]. Still another study [42] reported attenuation of withdrawal when exogenous progesterone was given early in the follicular phase. Desire to smoke and relieve negative affect has also been reported to be greater in the late luteal phase [39,40,43]. Furthermore, cue-induced craving in acute smoking abstinence was reported to be more prominent in the late luteal phase than the follicular phase [44]. In addition, response to nicotine patch in acute smoking cessation has been shown to diminish craving and certain premenstrual symptoms in the late luteal phase compared to the follicular phase [45]. The literature is clearly mixed, but does suggest that sex hormones may play a role. Contributing to the confusion are the varying definitions of phase used by investigators and the unclear role of premenstrual symptomatology, which is correlated moderately with withdrawal symptoms [40].
Even fewer studies investigated menstrual cycle phase-related differences in the relapse rates of women smokers, but again results are conflicting. Women who quit smoking during the premenstrual phase were significantly less successful than women who quit at mid-cycle, and less successful compared to men who quit smoking [43]. Other studies have offered evidence suggesting that quitting smoking is more difficult during the luteal phase [34,37,44]. One investigation found that women were most likely to relapse during menses regardless of phase of quit date [46].
These discordant findings result probably from small sample sizes, the difficulty and variability in accurately determining the menstrual cycle phase of study participants and the influence of study setting (i.e. ‘laboratory’ versus ‘naturalistic’) on smoking behavior. Additional controlled clinical investigations with rigorous definition of cycle phase are needed to discern how sex steroids influence smoking cessation symptomatology and the propensity to relapse. Such studies will help to determine whether women have specific biological needs that should be addressed in smoking cessation treatment programs.
In response to the need for strong, consistent data on the effect of menstrual cycle phase on women’s smoking relapse rates, we conducted a randomized, out-patient, intent-to-treat trial of 202 female smokers. Our primary aim was to determine how smoking relapse rates are influenced by the menstrual phase in which a quit attempt is initiated. Women were assigned randomly to quit smoking in the follicular (F) or luteal (L) phases of the menstrual cycle and followed for 26 weeks. These two phases differ with respect to sex hormone levels (higher estrogen compared to progesterone in the F phase and higher progesterone compared to estrogen in the L phase [47]) and factors potentially affecting relapse (i.e. measurable withdrawal and premenstrual symptoms) [34,37,45]. We hypothesized that women randomized to begin a quit smoking attempt during the F phase would be more successful than women randomized to quit during the L phase. We expected that women with F phase quit dates would remain abstinent for a greater number of days until their first relapse to smoking, and would be less likely to relapse at all, than women with L phase quit dates. Withdrawal symptomatology was also measured to investigate the effect of menstrual phase on factors that might contribute to relapse.
METHODS
This study was approved by the University of Minnesota Institutional Review Board. Informed consent was obtained before enrollment and data collection.
Recruitment and screening
Participants were recruited through flyers and local advertising. Eligible for inclusion were 18–40-year-old women who smoked greater than 10 cigarettes per day for at least 1 year and had regular menstrual cycles every 24–36 days for the previous 6 months. Women were excluded if they were obtaining nicotine from sources other than cigarettes (e.g. nicotine replacement, cigars, pipes, smokeless tobacco, etc.); were pregnant, breastfeeding or intending to become pregnant within 6 months; were using hormones (prescribed and herbal); were receiving treatment for psychiatric or emotional problems or for severe premenstrual symptoms; or were not motivated to quit smoking, as indicated by a score of less than 7 on a 10-point motivation scale. This scale has been used previously by the authors to determine motivation level for quitting smoking [48]. Women with a shortened premenstrual assessment form (PAF) score of < 10, indicating no premenstrual symptoms [49], were also excluded (< 1%) because premenstrual symptomatology was a dependent measure.
Women were screened first by telephone, then at a clinic visit. After informed consent was obtained, study eligibility was confirmed by assessing women’s medical and menstrual history, smoking histories, blood levels of estrogen and progesterone, breath carbon monoxide (CO) and saliva cotinine levels (to verify smoking status) and written measures of premenstrual symptoms. As part of the screening visit, women also completed the Fagerstrom Test for Nicotine Dependence [50] and provided demographic information.
Randomization
After the initial clinic visit, equal numbers of women were randomized via a computer model to each quit group. Enrolled women tracked their menstrual cycle prospectively and were asked to quit smoking in either the F phase (days 4–6) or L phase (6–8 days after luteinizing hormone (LH) surge) of their menstrual cycle, with day 1 defined as the first day of menses. The LH surge was identified using home urine testing kits (described below). A 3-day span for the assigned quit day was used to ensure that the first 2–5 days after quitting—when peak symptoms of withdrawal usually occur [51]—fell within a participant’s assigned quit phase. This also kept follow-up clinic visits on a fixed weekday schedule.
Protocol
Within 2–6 weeks after enrollment and randomization, participants attended a baseline clinic visit. The visit occurred approximately 7 days in advance of their randomized phase quit date. Women were instructed to quit smoking at midnight on their quit day. During the baseline visit, women completed written measures of withdrawal, premenstrual symptoms and smoking urges. Trained counselors provided participants with 30 minutes of behavioral counseling (15 minutes at each subsequent clinic visit) that focused on personally relevant coping strategies. Participants also received self-help manuals [52].
From their quit date, participants were followed for 26 weeks, with eight clinic visits and two telephone contacts. Participants used home urine LH testing kits during the first 2 months of the study. Participants kept daily records: menstrual cycle calendars (to track menses and LH testing results), smoking diaries (to track the number and time of cigarettes smoked each day) and written measures of withdrawal, premenstrual symptoms and smoking urges. In an effort to reduce inaccurate reporting, clinic staff instructed the participants not to go back and complete the forms if they missed a day and that there would be no penalty if forms were not completed. Clinical staff collected and reviewed forms at each clinic visit.
Clinic visits occurred in weeks 1 (days 2 and 5), 2 (days 9 and 12), 4, 8, 12 and 26. At each visit, smoking counseling (approximately 15 minutes) was provided, calendars and diaries were reviewed, daily forms were collected, blood samples were taken for hormone testing and abstinence was confirmed via breath CO and saliva cotinine testing. Participants who relapsed were encouraged to set another quit date. Relapsers continued in the protocol the same as abstainers. Participants received up to $310 in compensation for study participation.
Menstrual cycle monitoring
Three methods were used to assign participants to an initial quit date by phase (F or L) and to monitor phase throughout the treatment period: daily menstrual calendars [47], urine LH testing (First Response Ovulation Test kit, performed during the first 2 months of the study) and serum hormone level monitoring. The completion rate for daily menstrual calendars was 98.8%. Ovulation can be predicted accurately by urine LH testing [53–56], and we have used this method successfully in previous trials to define the L phase [35,40,45,57]. Participants were instructed by study staff to start testing for an LH surge on approximately days 7–10 of their menstrual cycle; they continued testing for 10 days or until the LH surge was detected, whichever came first. Serum levels of estradiol (E2) and progesterone were assessed from blood samples collected at clinic visits. Ratios of these hormones were used to confirm phase of assigned quit date; a higher estradiol/progesterone ratio indicated F phase; and a lower estradiol/progesterone ratio indicated L phase. Analyses were performed at the Fairview University Diagnostic Laboratory, 2001.
Outcome measures
Relapse
Relapse to smoking was our primary outcome. Three definitions of relapse were applied: relapse from continuous abstinence (CA), relapse from prolonged abstinence (PA) and point prevalence (PP) [58]. Relapse from CA is defined as a single puff of a cigarette. Some assert that this is the most representative definition of abstinence, given that most people who slip initially return to regular smoking [59]. PA refers to sustained abstinence after a grace period. We defined relapse from PA as seven consecutive slips without a 24-hour period of abstinence between any slip [60], where a ‘slip’ was any isolated episode consisting of at least one puff of a cigarette when the cigarette was lit, then extinguished. The day of relapse was defined retrospectively as the first day of a slip. This measure has been used successfully to capture long-term abstainers who initially slip [61,62]. With PP, relapse was defined as any smoking during a 7-day window prior to the time of follow-up. PP is less stringent than PA or CA measures and is intended to be a cross-sectional snapshot measure. For this analysis, we assessed PP at 14 days and 30 days after quit date.
Participants’ smoking status (relapsed or abstinent) was determined retrospectively using three measures, as follows. (1) Daily smoking diaries: participants recorded abstinence or a smoking slip (i.e. any isolated episode consisting of one or more puffs of a cigarette when the cigarette was lit, then extinguished) [51]. The completion rate for daily smoking diaries was 99.9%. (2) Time-line follow-back (TLFB): this is a daily estimation method for retrospective collection of cigarettes smoked per day between clinic visits. This item is validated to collect data for up to 12 months prior to the interview [63,64]. TLFB results were reviewed by staff at each clinic visit. (3) Biochemical verification of abstinence: breath samples were collected at all visits using CO monitoring devices (Bedfont Scientific Limited Company, Kent, UK), calibrated weekly for accuracy. CO levels > 5 parts per million (p.p.m.) indicated acute smoking. Saliva samples for cotinine measurement were collected at all visits to verify self-reports of abstinence. Participants placed a dental roll in their month for approximately 5 minutes for sample collection. Cotinine levels less than 15 ng/ml indicated abstinence. Samples were analyzed in batches at the Minneapolis Medical Research Foundation, Division of Toxicology using gas chromatography. If there was discordance between CO biochemical verification and self-report, participants were questioned at the clinic. Participants who reported their smoking status inaccurately or used alternative forms of nicotine such as smokeless tobacco or nicotine replacement therapy use were dropped and replaced (< 1%).
Symptomatology
Withdrawal and craving were measured with the 11-item Minnesota Nicotine Withdrawal Scale (MNWS) [56,65]. Premenstrual symptoms were measured with the 10-item Shortened Premenstrual Assessment Form (PAF) [49]. Participants completed the MNWS and PAF at screening, at baseline and then daily (approximately 5 minutes per day) for 8 weeks beginning on quit day. Smoking urges were assessed by the 32-item Questionnaire on Smoking Urges (QSU) [66]. Participants completed the QSU at screening, at baseline and then daily for 4 weeks, beginning on quit day. Daily forms were collected at each clinic visit. Completion rates were 77.7% for MNWS and PAF questionnaires and 76.7% for QSU questionnaire.
Analyses
SPSS version 14.0 was used for statistical analyses. An alpha level of P < 0.05 indicated statistical significance.
Latency to relapse (from continuous and prolonged abstinence)
Kaplan–Meier survival analyses with right censoring were used to compare survival curves for the F and the L quit group.
The effect of quit date (F or L) on the log10 days of initial abstinence was tested using a t-test. This log transformation allowed us to normalize the Poisson distribution that we observed when looking at consecutive days of abstinence. Separate tests were conducted for relapse from continuous abstinence (CA) and for relapse from prolonged abstinence (PA).
Because Fagerstrom scores and number of cigarettes smoked were significantly different for the two quit groups at baseline, we repeated the previous analyses using a one-way analysis of covariance (ANCOVA), controlling for the baseline Fagerstrom score and number of cigarettes smoked. We also ran Cox regressions, controlling for the baseline Fagerstrom score and number of cigarettes, to compare the survival curves for the F and L quit groups. Adding these two covariates left our results essentially unchanged in all cases.
Point prevalence
For 14 and 30 days after the initial quit date, we compared the proportion of relapsers (any smoking in the previous 7 days) in the F and L quit groups using χ2 tests of independence.
Time to relapse
For each quit phase group, we calculated the percentage of participants who relapsed < 24 hours after their quit date, relapsed ≥ 24 hours after their quit date or never relapsed, and used a χ2 test to compare them.
Phase of relapse
Among all participants who relapsed, we calculated the percentage that relapsed within their assigned quit phase and the percentage that relapsed during the alternative menstrual cycle phase.
Post hoc analysis
Using χ2, we compared the proportion of first slip (CA) between the quit-phase groups (F and L), within the first 3 days and 5 days after quit date. For participants whose first slip occurred within 3 days of their quit date (that is, definitely within their initial quit phase), we also tested the mean number of days between this first slip and relapse (defined as PA) using a t-test. Participants who did not relapse within 3 days were not included. This latter test examines a possible difference in the response to the first cigarette (i.e. first slip) between the two menstrual phases.
Symptomatology
We used t-tests for all symptomatology analyses. First, we compared the mean symptom scores (for MNWS minus craving, craving, QSU factors 1 and 2) of the two quit phase groups at two time-points: on quit day and on day of relapse, using both CA and PA definitions of relapse. Secondly, among women who relapsed, we compared the mean symptom scores on day of relapse using both CA and PA definitions of relapse for women who relapsed less than 24 h after quitting and women who were abstinent for at least 1 day. Thirdly, we compared the average symptom scores for the first 7 days post-quit of women who relapsed using the PA definition of relapse and women who did not.
RESULTS
Participant characteristics
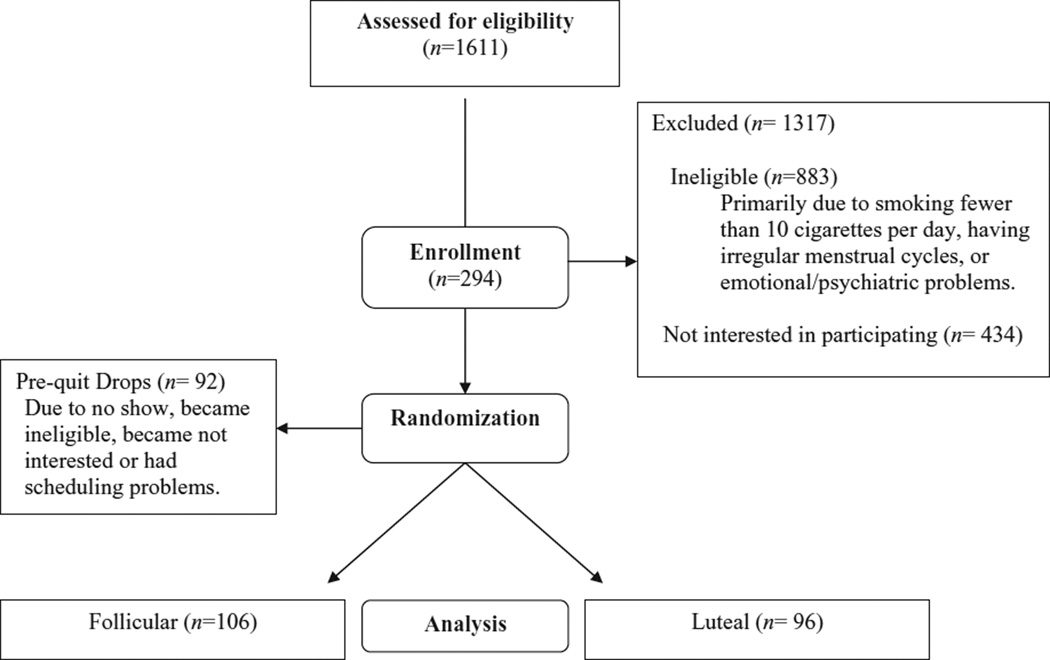
The participant flow diagram is presented in Fig. 1. From 2002 until 2005, 1611 women were screened for study eligibility. Of these, 434 chose not to participate and 883 did not meet inclusion criteria. The main reasons for exclusion were smoking fewer than 10 cigarettes per day, having irregular menstrual cycles and experiencing current emotional or psychological issues. Subsequently, 294 participants met inclusion criteria, agreed to enroll and were randomized to a smoking quit date in either the F or L phase. Women randomized to the L group smoked more cigarettes per day than women randomized to the F group (17.5 ± 6.5 and 15.8 ± 4.7, respectively, P = 0.033).
Figure 1.
Participant paradigm
Depending on when participants were screened, they had to wait from 2 to 6 weeks to reach their assigned quit date. During this period, some (n = 92) of the randomized participants (F group = 41; L group =51) lost interest in the study, reported scheduling problems or subsequently became ineligible. This left 202 participants who reached their quit date and were thus eligible for inclusion in the analyses. Compared to the 202 (F group =106; L group = 96) women who met their quit date, the 92 prequit dropouts had lower affect subscale scores on the PAF (as measured at the screening clinic visit), a shorter mean duration of previous quit attempts, fewer years of education and lower yearly family incomes (data not shown). The differential drop-out rate was not different between the two pre-quit dropout groups (χ2 = 1.58, P = 0.210), but they differed by Fagerstrom score (pre-quit L group = 4.8 ± 2.2 versus pre-quit F group = 3.9 ± 2.1, P = 0.03).
The demographic and smoking history characteristics for the 202 women included in our outcome analyses are reported in Table 1. Women randomized to a F quit date (n = 106) smoked fewer cigarettes per day and had higher levels of nicotine dependence (Fagerstrom scores) than women randomized to a L quit date (n = 96).
Table 1.
Demographic characteristics and smoking behavior history for participants (n = 202) at screening.
| Characteristic | Total enrolled (n = 202) | Follicular quit group (n = 106) | Luteal quit group (n = 96) | P-value |
|---|---|---|---|---|
| Demographics† | ||||
| Age (years) | 29.8 ± 6.6 | 29.0 ± 6.4 | 30.6 ± 6.7 | 0.101 |
| Education (years) | 13.7 ± 2.0 | 13.7 ± 2.2 | 13.7 ± 1.7 | 0.974 |
| Ethnic originठ| ||||
| African American | 4% | 6% | 2% | 0.460 |
| American Indian | 9% | 11% | 7% | |
| Asian | 3% | 2% | 4% | |
| White | 82% | 79% | 84% | |
| Other | 3% | 3% | 2% | |
| Hispanic¶ | 2% | 2% | 1% | 0.614 |
| Marital status§ | ||||
| Single | 54% | 58% | 50% | 0.333 |
| Divorced | 14% | 12% | 16% | |
| Separated | 2% | 1% | 3% | |
| Married | 30% | 29% | 31% | |
| Yearly family income§ | ||||
| ≤ $30 000 | 49% | 52% | 45% | 0.779 |
| $30 001–60 000 | 34% | 29% | 38% | |
| > $60 000 | 18% | 19% | 17% | |
| Smoking history† | ||||
| Years of smoking | 13.1 ± 7.0 | 12.6 ± 7.6 | 13.6 ± 7.0 | 0.316 |
| Number cigarettes/day | 16.6 ± 5.6 | 15.8 ± 4.7 | 17.5 ± 6.5 | 0.033 |
| Fagerstrom score (1–11)†† | 4.1 ± 2.0 | 4.4 ± 1.9 | 3.8 ± 2.1 | 0.031 |
| Number of quit attempts | 2.7 ± 2.1 | 2.7 ± 1.9 | 2.8 ± 2.3 | 0.786* |
| Longest days abstinent | 208.0 ± 489.7 | 141.5 ± 242.9 | 279.6 ± 653.5 | 0.578* |
| Screening measures† | ||||
| PAF total | 25.1 ± 9.5 | 25.1 ± 9.5 | 25.1 ± 9.6 | 0.993 |
| PAF affect | 10.0 ± 4.2 | 10.1 ± 4.1 | 9.9 ± 4.3 | 0.777 |
| PAF pain | 7.8 ± 3.6 | 8.0 ± 3.8 | 7.6 ± 3.5 | 0.418 |
| PAF water | 7.3 ± 3.4 | 7.0 ± 3.4 | 7.6 ± 3.5 | 0.240 |
| MNWS total | 5.3 ± 4.8 | 5.3 ± 4.7 | 5.2 ± 4.9 | 0.857 |
| MNWS craving | 2.7 ± 0.9 | 2.8 ± 0.7 | 2.6 ± 1.0 | 0.068 |
| QSU factor 1 | 4.9 ± 1.2 | 4.9 ± 1.2 | 4.9 ± 1.2 | 0.965 |
| QSU factor 2 | 2.8 ± 1.1 | 2.8 ± 1.1 | 2.8 ± 1.1 | 0.864 |
| CO | 17.7 ± 8.7 | 17.2 ± 8.0 | 18.3 ± 9.4 | 0.350 |
| Cotinine | 224.6 ± 112.4 | 210.1 ± 95.2 | 241.1 ± 128.8 | 0.235 |
t-test performed on the log of these numbers.
Statistics reported as means ± standard deviation unless otherwise noted.
Reported as frequency (%).
P-value for categorical items is the Spearman’s correlation.
Reported separately from race.
Fagerstrom (1978). PAF: Premenstrual Assessment Form; MNWS: Minnesota Nicotine Withdrawal Scale; QSU: Questionnaire on Smoking Urges; CO: carbon dioxide.
Latency to relapse
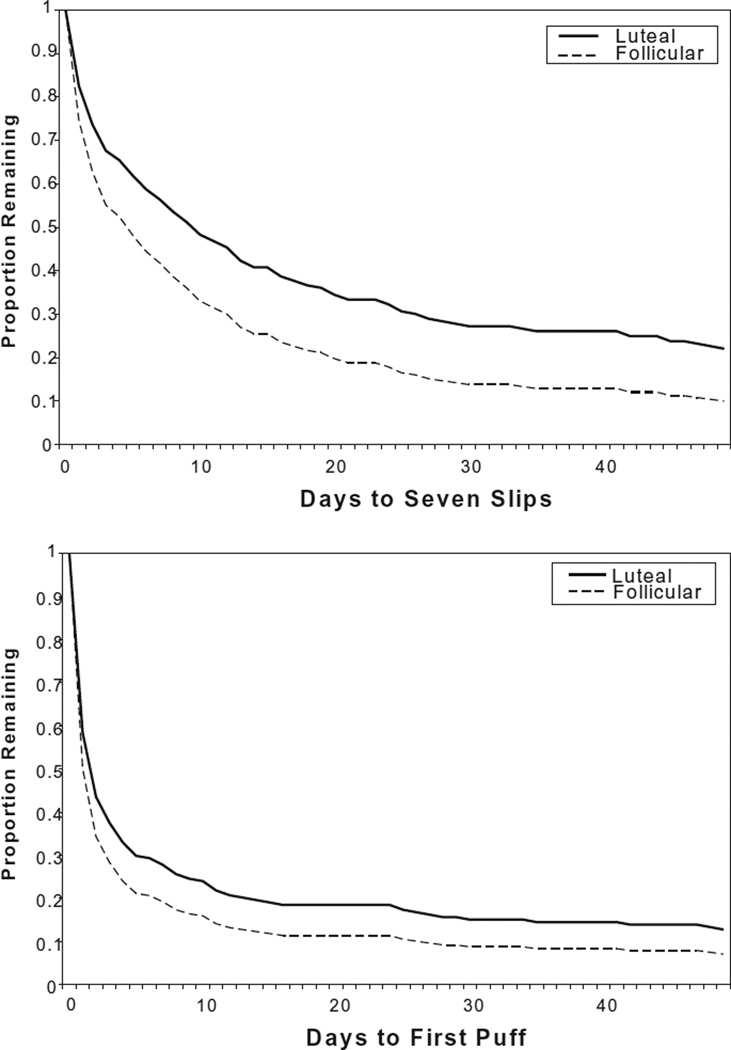
For all 202 participants who reached their assigned quit day, we compared days to relapse for the F quit and L quit groups using the PA (seven slips in 7 days) and CA (first slip) definitions of relapse. Kaplan–Meier survival analyses with right censoring were used to compare survival curves for the two groups (Fig. 2). For PA, the L quit group experienced more days to relapse than the F quit group (eb = 1.234, P = 0.007). For CA, the difference between the groups was not as large, and the results were not statistically significant (eb= 1.134, P = 0.093). Because there was a significant difference at baseline between the two quit groups on the Fagerstrom scores and number of cigarettes smoked, the above analyses were re-run as Cox regressions, with two covariates. The results were essentially unchanged (eb = 1.230, P = 0.009 and eb = 1.141, P = 0.081, respectively).
Figure 2.
Survival curves using ‘seven slips’ (prolonged abstinence: PA) and ‘one puff’ (continuous abstinence: CA) as relapse definitions
We compared the log of days to relapse for the F quit and L quit groups using a t-test (Table 2). For PA, the L quit group experienced slower relapse (L = 39.2 days, F = 20.6 days, t200 = 2.447, P = 0.015). The difference for CA was not significant (L = 21.5 days, F = 13.9 days, t200 = 1.908, P = 0.058). We compared log of days to relapse rather than mean days because latency to relapse is distributed Poisson and is extremely skewed. These tests were re-run as analyses of covariance, controlling for Fagerstrom scores and number of cigarettes smoked, but the results were little changed (PA: F1,198 = 3.882, P = 0.050; CA: F1,198 = 2.821, P = 0.095).
Table 2.
Mean (± standard deviation) days to relapse and point prevalence for each quit phase group.
| Relapse definition | Follicular quit group (n = 106) |
Luteal quit group (n = 96) | P-value | Odds ratio (confidence interval) |
|---|---|---|---|---|
| One slip (CA)* | 13.9 (43.4) | 21.5 (46.3) | 0.058* | NA |
| Seven slips (PA)* | 20.6 (45.8) | 39.2 (59.0) | 0.015* | NA |
| 14 days PP | 84% relapsed | 65% relapsed | 0.002 | 2.871 (1.474–5.590) |
| 30 days PP | 86% relapsed | 66% relapsed | 0.001 | 3.178 (1.594–6.334) |
t-test on log of days to relapse. CA: continuous abstinence; PA: prolonged abstinence; PP: point prevalence; NA: not applicable.
Point prevalence
PP at 14 and 30 days is reported in Table 2. At both time-points, a higher percentage of women in the F group had relapsed than in the L group.
Time to relapse
For the F quit and L quit groups, we calculated the percentage of participants who relapsed within 24 hours, relapsed ≥ 24 hours and never relapsed (Table 3). For PA, the L quit group stayed abstinent longer (χ2(2) = 7.03, P = 0.030), with 23% of the L quit group never relapsing as opposed to 9% of the F quit group. The difference was not significant for CA (χ2(2) = 2.74, P = 0.255).
Table 3.
Percentage of participants in each quit phase group who relapsed < 24 hours after quitting, relapsed ≥ 24 hours after quitting, and never relapsed.
| Relapse definition | Follicular quit group (n = 106) % (n) |
Luteal quit group (n = 96) % (n) |
P-value |
|---|---|---|---|
| One slip (CA) | 0.255* | ||
| Relapsed < 24 h | 48% (51) | 44% (42) | |
| Relapsed ≥ 24 h | 45% (48) | 43% (41) | |
| Never relapsed | 7% (7) | 14% (13) | |
| Seven slips (PA) | 0.030† | ||
| Relapsed < 24 h | 25% (26) | 19% (18) | |
| Relapsed ≥ 24 h | 66% (70) | 58% (56) | |
| Never relapsed | 9% (10) | 23% (22) |
χ2 = 2.74, d.f. = 2.
χ2 = 7.03, d.f. = 2.
CA: continuous abstinence; PA: prolonged abstinence.
Phase of relapse
Of the 181 participants who relapsed, 162 women (89%) relapsed while in their assigned quit phase using the CA definition of relapse. Ninety-one per cent (90 of 99) of the F quit group relapsed in the F phase and 9% (nine of 99) relapsed in the L phase. For the L quit group, 88% (72 of 82) relapsed in the L phase and 12% (10 of 82) relapsed in the F phase. For PA, 73% of the participants who relapsed did so within their assigned quit phase. For the L group 69% (51 of 74) relapsed in the L phase, and for the F group 76% (73 of 96) relapsed in the F phase (please see Table 4).
Table 4.
Percentage of participants who relapsed in each quit phase group in a particular phase (follicular or luteal).
| Phase of relapse | Follicular quit group (n = 99 relapsed) % (n) |
Luteal quit group (n = 82 relapsed) % (n) |
|---|---|---|
| Continuous abstinence | ||
| Follicular | 91% (90) | 12% (10) |
| Luteal | 9% (9) | 88% (72) |
| Phase of relapse |
Follicular quit group (n = 96) % (n) |
Luteal quit group (n = 74) % (n) |
| Prolonged abstinence | ||
| Follicular | 76% (73) | 31% (23) |
| Luteal | 24% (23) | 69% (51) |
The latency to relapse analyses were re-run, for both CA and PA, substituting relapse phase for quit phase as the independent variable. The results were essentially unchanged. Considering that the make-up of these groups (quit phase and relapse phase) is virtually identical, this outcome is not surprising.
Post hoc analysis
In post hoc analysis of first slip occurrence (CA), 77% (82 of 106) of women in the F quit group and 66% (63 of 96) of women in the L quit group had a first slip within 3 days of their randomized assigned quit date. This group difference is statistically significant χ2(1) = 4.04, P = 0.04). This held true for first slips occurring within 5 days of the randomized quit phase date (χ2(1) = 4.87, P = 0.03). These time-periods were chosen so we could be sure that all participants were examined in their original, randomized menstrual phase.
The mean time-period between the first slip (within 3 days of quit day to assure same phase) and relapse (PA) was 4.6 ± 7.6 days for the F quit group and 5.0 ± 9.5 days for the L quit group (P > 0.50). The two quit groups did not differ on the time between first slip and eventual relapse (PA).
Symptomatology
We observed no phase-related differences in symptom severity. At screening, there were no significant differences in the withdrawal or premenstrual symptomatology scores for women in the F quit group and women in the L quit group (Table 1). Similarly, on quit day and on day of relapse for both CA and PA, there were no significant differences in MNWS, craving and QSU factor scores for women in the F quit group and women in the L quit group (data not shown). Among women who relapsed, mean symptom scores on day of relapse did not differ for women who relapsed less than 24 hours after quitting and women who were abstinent for at least 1 day. This was true for both CA and PA (data not shown). The only significant differences in symptomatology that we observed were between relapsers and non-relapsers (Table 5). Women who relapsed defined by PA (n = 170) had higher symptoms of withdrawal, craving and urge to smoke than women who never relapsed (n = 32).
Table 5.
Mean symptom scores during the first 7 days post-quit for relapsers and non-relapsers.*
| Symptom measure | Relapsers (n= 170) Mean±SD |
Never relapsed (n = 32) Mean±SD |
t-value | P-value |
|---|---|---|---|---|
| Total MNWS minus craving† | 11.19 ± 5.34 | 9.12 ± 5.17 | −2.01 | 0.046 |
| Craving‡ | 2.83 ± 0.79 | 2.46 ± 0.69 | −2.45 | 0.015 |
| QSU factor 1§ (desire to smoke) | 4.24 ± 1.10 | 3.45 ± 1.00 | −3.76 | 0.000 |
| QSU factor 2§ (smoking to relieve negative affect) | 3.45 ± 1.27 | 2.71 ± 1.16 | −3.05 | 0.003 |
Relapse is defined as seven consecutive slips without 24 h between.
Total Minnesota Nicotine Withdrawal Scale (MNWS) minus craving (range 0–28).
Craving (range 0–4).
Questionnaire on Smoking Urges (QSU) factors 1 and 2 (range 1–7). SD: standard deviation.
DISCUSSION
Our original hypothesis is not supported by the results of this prospective treatment trial. Rather, we observed that women who were randomized to quit smoking in the F phase of their menstrual cycle had worse smoking cessation outcomes than women who were randomized to quit in the L phase. Specifically, the F quit group experienced fewer days to relapse (from CA and PA, and from PP abstinence at 14 and 30 days) than the L quit group. Regardless of their quit phase, many participants did not remain abstinent beyond 24 hours of their quit attempt, and the percentage of women who never relapsed was low. Most women relapsed in their assigned quit phase group. Overall, the F quit group had a less favorable outcome than the L quit group.
The reason for these observed phase-related differences in quit smoking success is not clear. One possibility is that phase-related differences in withdrawal symptom severity are influencing women’s quit success. Some observational clinical studies [34,37] have reported that women who quit in the luteal phase experience more withdrawal. Further, there is some empirical evidence to suggest that escalation of withdrawal symptoms contributes to relapse during a smoking cessation attempt. For example, in several studies withdrawal symptoms were reported to begin within 4–24 hours after cessation and were suggested to have contributed to risk for early relapse [65,67–70]. In this trial, however, we measured women’s withdrawal symptoms daily and found no phase effect on their magnitude. Women’s mean withdrawal symptoms on both quit day and relapse day did not differ for the two quit groups. In addition, we have conducted previously an analysis of data from a subset of women from this study—the first 137 women who completed 30 days of their protocol [48]. From this analysis, we found that their withdrawal symptoms, nicotine craving and smoking urges increased 1–2 days prior to relapse, peaked at relapse and then diminished. However, this marked symptom effect was not related to menstrual phase; the same patterns were observed consistently for women in both the F and L phases.
Although contrary to our original hypothesis and the findings from some studies [34,37,43,44,46], our phase-relapse results are congruent with other clinical studies of addictive drugs [42,71–75]. For example, there is evidence that ovarian hormones influence subjective and mood altering responses to nicotine [76], amphetamines [77] and cocaine [71,74]. Consistently, the effects of these drugs are enhanced in the follicular phase (when estrogen levels are higher than progesterone levels). In other research, the interaction between progesterone and cocaine in male and female cocaine users was examined using subjective, physiological and behavioral outcomes [78]. Progesterone attenuated some of the physiological and subjective effects in both genders. In other studies, participants’ responses to d-amphetamines varied with the menstrual cycle [77,79]. Their subjective responses were enhanced in the late follicular phase (when estrogen is moderate), but not in the luteal phase (when progesterone is prominent). We examined the effects of administering a single dose of micronized progesterone to a small sample of women smokers (n = 12) in the early follicular phase (lower estrogen and progesterone) [42]. We found an association between progesterone treatment and attenuated subjective smoking effects, as well as a trend toward decreased smoking behavior. Our data are more in line with these studies of addictive drugs, i.e. enhanced effect of estrogen (follicular phase) compared to more attenuated effect of progesterone (luteal phase), resulting in a poorer outcome in the follicular quit group.
It has also been suggested that ovarian hormones alter nicotine metabolism in women, and that this may affect smoking cessation. In pregnant women (for whom estrogen and progesterone are elevated), the clearance of nicotine was significantly higher than in non-pregnant women [80]. Additionally, women taking oral contraceptives had significantly higher rates of nicotine metabolism than women who were not taking the contraceptives. Interestingly, among oral contraceptive users, nicotine metabolism was accelerated only in those taking estrogen-only and combined (estrogen and progesterone) contraceptives. These data suggest that estrogen may be responsible for enhanced nicotine metabolism in women [81].If estrogen does alter nicotine metabolism, this could affect nicotine dependence and ability to quit.
Another possible explanation for our results is that ovarian hormones are altering the reinforcing effects of nicotine. Studies in animal models of drug self-administration provide strong evidence of this, and it may occur via alterations in dopamine functioning in the reward areas of the brain where estrogen has been suggested to enhance dopamine functioning [82,83]. For example, a relationship was observed between elevated levels of estrogen and increased self-administration of heroin, cocaine and nicotine [84–87]. Estrogen also enhanced the reinstatement of cocaine self-administration [88], whereas progesterone attenuated cocaine’s effects [89] and suppressed relapse to cocaine in rats [90]. These findings suggest that estrogen and progesterone may moderate drug-seeking behavior.
Based on this literature, one could posit that a first slip during the follicular phase will contribute to quicker relapse (i.e. estrogen-enhancing reinforcement). Because first slip predicts relapse [91], the occurrence of first slip and the time from slip to relapse by menstrual cycle phase may help us to understand estrogen’s role in augmenting the reinforcing effects of nicotine. In this study, although the percentages of first slip were higher in the first 3 and 5 days post-quit for women in the F group versus the L group, there was no difference in the mean days from first slip to relapse between groups. However, our study was not designed to measure this; therefore, our results do not refute estrogen’s effect in enhancing nicotine as a reinforcer.
Additional research is needed to define the mechanism(s) by which sex steroid hormones might modulate nicotine response and abstinence. In the follicular phase, does estrogen increase dopamine levels and trigger greater difficulty quitting? Does it increase the metabolism of nicotine associated with greater craving for nicotine? The natural fluctuations in hormonal ratios across the menstrual cycle may also play a role in relapse. A more rigorous study design is needed to control for the fluctuating hormone levels, where exogenous hormones are administered during the early follicular phase when endogenous hormones are low. Then nicotine response systems and pharmacokinetics could be studied more precisely to help elucidate the role of sex hormones in smoking cessation in women.
This is the first randomized study of female smokers designed to test if menstrual phase affects smoking cessation. No other studies have compared relapse rates directly by assigned menstrual quit phase. This study used rigorous criteria to identify phase and define relapse. Consequently, this study contributes new data in this evolving field to support a role of sex hormones in smoking cessation for women. This potential role needs clearer definition with a more controlled study design to investigate the mechanism of sex hormones in nicotine dependence.
There are some limitations to the present study. Self-report measures are limiting and daily measurements are subject to being invalid. However, our data show that women’s premenstrual symptoms increased prior to onset of menses. This suggests that forms were not completed randomly. In addition, daily measures of craving, withdrawal symptoms and smoking urges all peaked at the time of relapse, as one might expect [48]. The timing of the cycle and quit date meant that participants had to wait 2–6 weeks to reach their quit date. While unavoidable, this delay probably increased our number of prequit dropouts. However, the rate of dropout for the two randomized quit groups was not different, so the final randomized groups were not biased. Relapse rates in our study were high, although comparable to those reported in other behavioral intervention studies where no pharmacological smoking cessation treatments were used [92–94]. The low success rate we observed was not a result of women merely not attempting to quit. Most participants (86%, 173 of 202) made at least one serious quit attempt (≥ 24 hours) in the first 30 days following their assigned quit day. In addition, women had to rate their motivation at least 7 on a 10-point scale to be included in this ‘intent-to-quit study’. We purposefully did not include pharmacological treatment in our design, to avoid confounders of the dependent variables. Nicotine replacement has been shown to diminish craving and some premenstrual symptoms, especially in the late luteal phase [45]. Chantix was not available when this study was being conducted. It would be important to repeat this study with pharmacotherapy to improve abstinence. Our results are not generalizable to women without premenstrual symptoms as they were excluded from the study, although most women (85%) [95] report experiencing some premenstrual symptoms.
In conclusion, among women enrolled in a randomized 26-week intent-to-treat smoking cessation study, those who attempted to quit in the F phase had less favorable outcomes (i.e. shorter days to relapse and fewer numbers who did not relapse) than women who attempted to quit in the L phase. More research is needed to understand the biological mechanisms underlying these observed phase-related differences. Our findings add to existing literature that supports an important role for ovarian hormones in nicotine addiction and smoking cessation. Future clinical trials of smoking cessation in women may want to control for menstrual cycle phase. Our results suggest that the success of a quit attempt could be affected by quit date timing (by menstrual cycle phase) and is an intervention that could be delivered in a clinical setting or used for smoking cessation programs.
Acknowledgements
This work was funded by NIDA grant 2-R01-DA08075. We thank our research staff—Alicia Allen, Nicole Cordes and Roshan Paudel—for help with subject recruitment, data measurement and data entry; and Marc Mooney, Anne Marie Weber-Main and Megan Roth for critical review and editing of manuscript drafts.
References
- 1.United States Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2001. [Google Scholar]
- 2.Bjornson W, Rand C, Connett JE, Lindgren P, Nides M, Pope F, et al. Gender differences in smoking cessation after 3 years in the Lung Health Study. Am J Public Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blake SM, Klepp KI, Pechacek TF, Folsom AR, Luepker RV, Jacobs DR, et al. Differences in smoking cessation strategies between men and women. Addict Behav. 1989;14:409–418. doi: 10.1016/0306-4603(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 4.Grunberg NE, Winders SE, Wewers ME. Gender differences in tobacco use. Health Psychol. 1991;10:143–153. [PubMed] [Google Scholar]
- 5.Ockene JK. Smoking among women across the life span: prevalence, interventions, and implications for cessation research. Ann Behav Med. 1993;15:135–148. [Google Scholar]
- 6.United States Department of Health and Human Services. The Health Consequences of Smoking for Women: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Office of the Assistant Secretary for Health, Office on Smoking and Health; 1980. [Google Scholar]
- 7.Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of 28-day relapse in smokers. Addict Behav. 1996;21:481–490. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 8.Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addict Behav. 1997;22:521–533. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 9.Audrain J, Gomez-Caminero A, Robertson AR, Boyd R, Orleans CT, Lerman C. Gender and ethnic differences in readiness to change smoking behavior. Womens Health. 1997;3:139–150. [PubMed] [Google Scholar]
- 10.Sorensen G, Goldberg R, Ockene J, Klar J, Tannenbaum T, Lemeshow S. Heavy smokers among a sample of employed women. Am J Prev Med. 1992;8:207–214. [PubMed] [Google Scholar]
- 11.Borland R. Slip-ups and relapse in attempts to quit smoking. Addict Behav. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- 12.Borrelli B, Niaura R, Keuthen NJ, Goldstein MG, DePue JD, Murphy C, et al. Development of major depressive disorder during smoking-cessation treatment. J Clin Psychiatry. 1996;57:534–538. doi: 10.4088/jcp.v57n1106. [DOI] [PubMed] [Google Scholar]
- 13.Glassman AH, Covey LS, Dalack GW, Stetner F, Rivelli SK, Fleiss J, et al. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther. 1993;54:670–679. doi: 10.1038/clpt.1993.205. [DOI] [PubMed] [Google Scholar]
- 14.Jarry JL, Coambs RB, Polivy J, Herman CP. Weight gain after smoking cessation in women: the impact of dieting status. Int J Eat Disord. 1998;24:53–64. doi: 10.1002/(sici)1098-108x(199807)24:1<53::aid-eat5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. J Consult Clin Psychol. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- 16.Perkins KA, Levine MD, Marcus MD, Shiffman S. Addressing women’s concerns about weight gain due to smoking cessation. J Subst Abuse Treat. 1997;14:173–182. doi: 10.1016/s0740-5472(96)00158-4. [DOI] [PubMed] [Google Scholar]
- 17.Pomerleau CS, Kurth CL. Willingness of female smokers to tolerate postcessation weight gain. J Subst Abuse. 1996;8:371–378. doi: 10.1016/s0899-3289(96)90215-1. [DOI] [PubMed] [Google Scholar]
- 18.Pomerleau CS, Teuscher F, Goeters S, Pomerleau OF. Effects of nicotine abstinence and menstrual phase on task performance. Addict Behav. 1994;19:357–362. doi: 10.1016/0306-4603(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 19.Talcott GW, Fiedler ER, Pascale RW, Klesges RC, Peterson AL, Johnson RS. Is weight gain after smoking cessation inevitable? J Consult Clin Psychol. 1995;63:313–316. doi: 10.1037//0022-006x.63.2.313. [DOI] [PubMed] [Google Scholar]
- 20.Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchinson S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res. 2001;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- 21.Whitlock EP, Vogt TM, Hollis JF, Lichtenstein E. Does gender affect response to a brief clinic-based smoking intervention? Am J Prev Med. 1997;13:159–166. [PubMed] [Google Scholar]
- 22.Borrelli B, Bock B, King T, Pinto B, Marcus BH. The impact of depression on smoking cessation in women. Am J Prev Med. 1996;12:378–387. [PubMed] [Google Scholar]
- 23.Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- 24.Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet. 2001;357:1929–1932. doi: 10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- 25.Niaura R, Abrams DB. Stopping smoking: a hazard for people with a history of major depression? Lancet. 2001;357:1900–1901. doi: 10.1016/S0140-6736(00)05089-3. [DOI] [PubMed] [Google Scholar]
- 26.Hatsukami DK, Skoog K, Allen SS, Bliss R. Gender and the effects of different doses of nicotine gum on tobacco withdrawal symptoms. Exp Clin Psychopharmacol. 1995;3:163–173. [Google Scholar]
- 27.Wetter DW, Fiore MC, Young TB, McClure JB, de Moor CA, Baker TB. Gender differences in response to nicotine replacement therapy: objective and subjective indexes of tobacco withdrawal. Exp Clin Psychopharmacol. 1999;7:135–144. doi: 10.1037//1064-1297.7.2.135. [DOI] [PubMed] [Google Scholar]
- 28.Swan GE, Jack LM, Ward MM. Subgroups of smokers with different success rates after use of transdermal nicotine. Addiction. 1997;92:207–217. [PubMed] [Google Scholar]
- 29.Benowitz NL, Hatsukami D. Gender differences in the pharmacology of nicotine addiction. Addiction. 1998;3:383–404. doi: 10.1080/13556219871930. [DOI] [PubMed] [Google Scholar]
- 30.Shiffman S, Sweeney CT, Dresler CM. Nicotine patch and lozenge are effective for women. Nicotine Tob Res. 2005;7:119–127. doi: 10.1080/14622200412331328439. [DOI] [PubMed] [Google Scholar]
- 31.Marks JL, Hair CS, Klock SC, Ginsburg BE, Pomerleau CS. Effects of menstrual phase on intake of nicotine, caffeine, and alcohol and nonprescribed drugs in women with late luteal phase dysphoric disorder. J Subst Abuse. 1994;6:235–243. doi: 10.1016/s0899-3289(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 32.Steinberg JL, Cherek DR. Menstrual cycle and cigarette smoking behavior. Addict Behav. 1989;14:173–179. doi: 10.1016/0306-4603(89)90045-2. [DOI] [PubMed] [Google Scholar]
- 33.Mello NK, Mendelson JH, Palmieri SL. Cigarette smoking by women: interactions with alcohol use. Psychopharmacology (Berlin) 1987;93:8–15. doi: 10.1007/BF02439579. [DOI] [PubMed] [Google Scholar]
- 34.O’Hara P, Portser SA, Anderson BP. The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav. 1989;14:595–600. doi: 10.1016/0306-4603(89)90001-4. [DOI] [PubMed] [Google Scholar]
- 35.Allen SS, Hatsukami D, Christianson D, Nelson D. Symptomatology and energy intake during the menstrual cycle in smoking women. J Subst Abuse. 1996;8:303–319. doi: 10.1016/s0899-3289(96)90170-4. [DOI] [PubMed] [Google Scholar]
- 36.Allen SS, Hatsukami D, Christianson D, Nelson D. Symptomatology and energy intake during the menstrual cycle in smoking women. J Subst Abuse. 1996;8:303–319. doi: 10.1016/s0899-3289(96)90170-4. [DOI] [PubMed] [Google Scholar]
- 37.Perkins KA, Levine M, Marcus M, Shiffman S, D’Amico D, Miller A, et al. Tobacco withdrawal in women and menstrual cycle phase. J Consult Clin Psychol. 2000;68:176–180. doi: 10.1037/0022-006X.68.1.176. [DOI] [PubMed] [Google Scholar]
- 38.DeBon M, Klesges RC, Klesges LM. Symptomatology across the menstrual cycle in smoking and nonsmoking women. Addict Behav. 1995;20:335–343. doi: 10.1016/0306-4603(94)00070-f. [DOI] [PubMed] [Google Scholar]
- 39.Pomerleau CS, Garcia AW, Pomerleau OF, Cameron OG. The effects of menstrual phase and nicotine abstinence on nicotine intake and on biochemical and subjective measures in women smokers: a preliminary report. Psycho-neuroendocrinology. 1992;17:627–638. doi: 10.1016/0306-4530(92)90021-x. [DOI] [PubMed] [Google Scholar]
- 40.Allen SS, Hatsukami DK, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res. 1999;1:129–142. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- 41.Pomerleau CS, Mehringer AM, Marks JL, Downey KK, Pomerleau OF. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addict Behav. 2000;25:483–497. doi: 10.1016/s0306-4603(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 42.Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 43.Craig D, Parrott A, Coomber JA. Smoking cessation in women: effects of the menstrual cycle. Int J Addict. 1992;27:697–706. doi: 10.3109/10826089209068761. [DOI] [PubMed] [Google Scholar]
- 44.Franklin TR, Napier K, Ehrman R, Gariti P, O’Brien CP, Childress AR. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res. 2004;6:171–175. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- 45.Allen SS, Hatsukami D, Christianson D, Brown S. Effects of transdermal nicotine on craving, withdrawal and premenstrual symptomatology in short-term smoking abstinence during different phases of the menstrual cycle. Nicotine Tob Res. 2000;2:231–241. doi: 10.1080/14622200050147493. [DOI] [PubMed] [Google Scholar]
- 46.Frye CA, Ward KD, Bliss RE, Garvey AJ. Influence of the menstrual cycle on smoking relape and withdrawal symptoms. In: Keefe FJ, editor. Society of Behavioral Medicine. Thirteenth Annual Scientific Sessions. New York/Rockville, MD: Society of Behavioral Medicine; 1992. p. 107. [Google Scholar]
- 47.Speroff L, Glass RH, Kase NG. Clinical Gynecologic Endocrinology and Infertility. Baltimore: Lippincott Williams & Wilkins; 1994. pp. 97–114. [Google Scholar]
- 48.Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tob Res. 2008;10:35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- 49.Allen SS, McBride CM, Pirie PL. The shortened premenstrual assessment form. J Reprod Med. 1991;36:769–772. [PubMed] [Google Scholar]
- 50.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 51.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 52.United States Department of Health and Human Services. Clearing the Air. Rockville, MD: US Department of Health and Human Services. National Institute of Health; 2003. [Google Scholar]
- 53.Grinsted J, Jacobsen JD, Grinsted L, Schantz A, Stenfoss HH, Nielsen SP. Prediction of ovulation. Fertil Steril. 1989;52:388–393. doi: 10.1016/s0015-0282(16)60904-4. [DOI] [PubMed] [Google Scholar]
- 54.Luciano AA, Peluso J, Koch EI, Maier D, Kuslis S, Davison E. Temporal relationship and reliability of the clinical, hormonal, and ultrasonographic indices of ovulation in infertile women. Obstet Gynecol. 1990;75:412–416. [PubMed] [Google Scholar]
- 55.Schiphorst LE, Collins WP, Royston JP. An estrogen test to determine the times of potential fertility in women. Fertil Steril. 1985;44:328–334. doi: 10.1016/s0015-0282(16)48856-4. [DOI] [PubMed] [Google Scholar]
- 56.Yen SS, Jaffe RB, Barbieri RL. Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Philadelphia, PA: W.B. Saunders Company; 1999. [Google Scholar]
- 57.Allen SS, Hatsukami D, Christianson D, Brown S. Energy intake and energy expenditure during the menstrual cycle in short-term smoking cessation. Addict Behav. 2000;25:559–572. doi: 10.1016/s0306-4603(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 58.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 59.Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- 60.Ossip-Klein DJ, Bigelow G, Parker SR, Curry S, Hall S, Kirkland S. Classification and assessment of smoking behavior. Health Psychol. 1986;5:3–11. [PubMed] [Google Scholar]
- 61.Killen JC, Maccoby N, Taylor CB. Nicotine gum and self-regulation training in smoking relapse prevention. Behav Ther. 1984;15:234–248. [Google Scholar]
- 62.Nides MA, Rakos RF, Gonzales D, Murray RP, Tashkin DP, Bjornson-Benson WM, et al. Predictors of initial smoking cessation and relapse through the first 2 years of the Lung Health Study. J Consult Clin Psychol. 1995;63:60–69. doi: 10.1037//0022-006x.63.1.60. [DOI] [PubMed] [Google Scholar]
- 63.Sobell L, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. New Jersey: Humana Press; 1991. [Google Scholar]
- 64.Sobell LC, Sobell MB. Timeline Follow-Back User’s Guide. Toronto, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- 65.Stitzer ML, Gross J. Smoking relapse: the role of pharmacological and behavioral factors. Prog Clin Biol Res. 1988;261:163–184. [PubMed] [Google Scholar]
- 66.Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- 67.Carey MP, Kalra DL, Carey KB, Halperin S, Richards CS. Stress and unaided smoking cessation: a prospective investigation. J Consult Clin Psychol. 1993;61:831–838. doi: 10.1037//0022-006x.61.5.831. [DOI] [PubMed] [Google Scholar]
- 68.Gritz ER, Carr CR, Marcus AC. The tobacco withdrawal syndrome in unaided quitters. Br J Addict. 1991;86:57–69. doi: 10.1111/j.1360-0443.1991.tb02629.x. [DOI] [PubMed] [Google Scholar]
- 69.Shiffman S. Relapse following smoking cessation: a situational analysis. J Consult Clin Psychol. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- 70.Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassal JD. Temptations to smoke after quitting: a comparison of lapsers and maintainers. Health Psychol. 1996;15:455–461. doi: 10.1037//0278-6133.15.6.455. [DOI] [PubMed] [Google Scholar]
- 71.Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berlin) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- 72.Marks JL, Pomerleau CS, Pomerleau OF. Effects of menstrual phase on reactivity to nicotine. Addict Behav. 1999;24:127–134. doi: 10.1016/s0306-4603(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 73.Masson CL, Gilbert DG. Cardiovascular and mood responses to quantified doses of cigarette smoke in oral contraceptive users and nonusers. J Behav Med. 1999;22:589–604. doi: 10.1023/a:1018793729594. [DOI] [PubMed] [Google Scholar]
- 74.Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- 75.Sofuoglu M, Poling J, Mouratidis M, Kosten T. Effects of topiramate in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berlin) 2006;184:645–651. doi: 10.1007/s00213-005-0296-9. [DOI] [PubMed] [Google Scholar]
- 76.Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berlin) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- 78.Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Justice AJ, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 80.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 81.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 82.Disshon KA, Dluzen DE. Use of in vitro superfusion to assess the dynamics of striatal dopamine clearance: influence of estrogen. Brain Res. 1999;842:399–407. doi: 10.1016/s0006-8993(99)01863-6. [DOI] [PubMed] [Google Scholar]
- 83.Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- 84.Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- 85.Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 86.Roth ME, Casimir AG, Carroll ME. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav. 2002;72:313–318. doi: 10.1016/s0091-3057(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 87.Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 88.Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 89.Morissette M, Di Paolo T. Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J Neurochem. 1993;60:1876–1883. doi: 10.1111/j.1471-4159.1993.tb13415.x. [DOI] [PubMed] [Google Scholar]
- 90.Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking in female rats. Exp Clin Psychopharmacol. 2007;6:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- 91.Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: a report from the Normative Aging Study. Addict Behav. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- 92.Group TNS. Transdermal nicotine for smoking cessation. J Am Med Assoc. 1991;266:3133–3138. [PubMed] [Google Scholar]
- 93.Osler M, Prescott E, Godtfredsen N, Hein HO, Schnohr P. Gender and determinants of smoking cessation: a longitudinal study. Prev Med. 1999;29:57–62. doi: 10.1006/pmed.1999.0510. [DOI] [PubMed] [Google Scholar]
- 94.Swan GE, Ward MM, Carmelli D, Jack LM. Differential rates of relapse in subgroups of male and female smokers. J Clin Epidemiol. 1993;46:1041–1053. doi: 10.1016/0895-4356(93)90172-w. [DOI] [PubMed] [Google Scholar]
- 95.United States Department of Health and Human Services. [accessed 1 June 2007];Premenstrual Syndrome. 2007 Available at: http://www.4women.gov/FAQ/pms.htm.