Abstract
OBJECTIVES
To determine how well the interview-based, clinic-friendly International Academy of Nutrition and Aging (FRAIL) frailty scale predicts future disability and mortality in the African American Health (AAH) cohort compared with the clinic-friendly Study of Osteoporotic Fractures (SOF) frailty scale, the phenotype-based Cardiovascular Health Study (CHS) frailty scale, and the comprehensive Frailty Index (FI).
DESIGN
Longitudinal cohort study.
SETTING
Metropolitan St. Louis, Missouri.
PARTICIPANTS
African American Health is a population-based panel study of African Americans (baseline age 49–65) from St. Louis, Missouri. Participants completed in-home assessments at baseline (N = 998) and 3- (n = 853) and 9- (n = 582) year follow-up.
MEASUREMENTS
Outcomes included activity of daily living (ADL) and instrumental ADL difficulties at 3 and 9 years and 9-year mortality. Frailty measures included the FRAIL, SOF, and CHS scales and the FI.
RESULTS
The FRAIL, SOF, CHS, and FI measures predicted new 3- and 9-year disability, and the FRAIL and FI scales predicted 9-year mortality. Receiver operating characteristic (ROC) contrasts showed that the FRAIL scale performed as well as (9-year disability and mortality) or better than (3-year disability) the CHS and SOF scales and the FI better than the FRAIL, CHS, and SOF scales for all outcomes except the FRAIL and CHS scales for 9-year ADL difficulties. The CHS and SOF scales were equivalent for all outcomes in ROC contrasts.
CONCLUSION
Overall the FI and the FRAIL scale exhibited the strongest predictive validity for disability and mortality in AAH. The best prediction tool to identify frail individuals at risk of disability and mortality may be one that includes a comorbidity measure. The FRAIL scale includes a comorbidity item and is a brief interview-based measure that is easy to administer, score, and interpret. The FRAIL scale has demonstrated validity and may prove to be a valuable scale for use by clinicians.
Keywords: frailty, ADLs, IADLs, mortality, African American
Frailty is a condition that places older persons at risk of poor outcomes when exposed to stressful events.1 Frailty is present in approximately 5% of the population aged 60 and older.2 Numerous studies have suggested that frailty is a predictor of functional deterioration and mortality.3–5 Models of frailty have been developed using three different domains: functional, deficit accumulation, and biological.6–11 The Frailty Index (FI) was developed based on the concept that deficit accumulation—a combination of symptoms, diseases, conditions, and disability—can predict frailty.9 A biological model of frailty based on five components—weight loss, exhaustion, low energy expenditure, slowness, and weakness—is known as the Cardiovascular Health Study (CHS) scale.12 The Study of Osteoporotic Fractures (SOF) scale included one functional and two biological factors in its frailty index—inability to rise from a chair five times without using the arms, weight loss, and reduced energy level.13 The CHS and FI measures are not practical for use in a busy clinic. The CHS scale requires measured performance (walking speed, grip strength) and is scored based on relative values in a population. The FI includes numerous items, typically 40 or more, and may include measured performance (e.g., cognition, physical performance). The SOF scale is brief and easy to administer in a clinic but requires measured performance (chair stands).
The FRAIL scale was recently developed as a simple measure that combines components of functional, deficit accumulation, and biological frailty models.14,15 The FRAIL scale was constructed to include only interview questions and require minimal administration time so that physicians and other health professionals can easily use it in clinical practice. Initial studies have shown that the FRAIL scale predicts adverse health outcomes,16–18 but research directly comparing different models of frailty is limited,19,20 and these studies have not included the FRAIL scale.
African Americans have a higher level of functional impairment and disability than do Caucasians21 and have been shown to have a higher prevalence of frailty than Caucasians using the CHS model.22 Thus there is a need for a brief frailty tool that can be used to identify African Americans at risk of disability and mortality so that early interventions can be developed. African American Health (AAH) is a population-based study of late middle-aged African Americans from two socioeconomically diverse areas of St. Louis. The AAH population has been demonstrated to have excess disability21 and represents a population that could benefit greatly from early frailty identification by clinicians.
The objective of this study was to investigate how well the interview-based, clinic-friendly FRAIL scale predicts future disability and mortality compared with the clinic-friendly SOF scale, the phenotype-based CHS scale, and the comprehensive FI in the AAH cohort. It was hypothesized that the FRAIL, CHS, and SOF measures and the FI would have predictive validity for disability and mortality and that the predictive validity of the FRAIL scale for disability and mortality would be similar to that of the SOF and CHS scales and the FI using receiver operating characteristic (ROC) contrasts.
METHODS
Study Sample
African American Health has been described in detail previously.21 In brief, it is a population-based panel study of African Americans from two socioeconomically diverse areas of St. Louis. Participants were born between 1936 and 1950 and were aged 49 to 65 at the Wave 1 baseline assessment. Inclusion criteria involved community-dwelling, self-reported black or African-American race, and Mini-Mental State Examination (MMSE) scores of 16 or greater. Recruitment proportion (participants/enumerated eligible persons) was 76%. Wave 1 was conducted at participants’ homes in 2000/01. Interviewers completed 26 hours of training on study-specific interviewing and physical performance measurements. In-home assessments were repeated 36 months after baseline during Wave 4 in 853 of the 947 survivors (90.1%). In-home assessments also were repeated 9 years after baseline during Wave 10 in 582 of the 835 surviving participants (70%). The analytical sample for this report includes 779 baseline respondents with valid scores on all frailty scales in analyses that include only respondents with no dependency in any baseline activity of daily living (ADL). The institutional review board at Saint Louis University approved this study.
Frailty Scales
The FRAIL scale includes five components: fatigue, resistance, ambulation, illness, and loss of weight.14,15 FRAIL scale scores range from 0 to 5 (one point for each component; 0 = best to 5 = worst) and represent frail (3–5), prefrail (1–2), and robust (0) health status. AAH Wave 1 data were used to construct the FRAIL scale for this study. Fatigue was measured by asking respondents how much time during the previous 4 weeks they felt tired, with responses of all of the time or most of the time scored one point. Resistance was assessed by asking participants whether they had any difficulty walking up 10 steps alone without resting and without aids and ambulation by asking whether they had any difficulty walking several hundred yards alone and without aids; yes responses were each scored as one point. Illness was scored 1 for respondents who reported five or more illnesses out of 11 total. Loss of weight was scored 1 for respondents with a weight decline of 5% or more within the previous 12 months. Fatigue and weight loss represent biological factors, resistance and ambulation represent function, and illness represents deficit accumulation.
The CHS scale consists of five components—unintentional weight loss, exhaustion, low activity, weakness, and slowness—and scores range from 0 to 5.12 A modified 0 to 5 CHS scale was constructed using AAH Wave 1 available variables. Weight loss of 5% or more in the previous year was scored as one point. Feeling tired all or most of the time in the previous 4 weeks was scored as one point. Yale Physical Activity Scale23 scores in the lowest quintile stratified according to sex (<16 for women, <17.2 for men) were coded as one point. The lowest quintile of grip strength stratified according to sex and body mass index (BMI) quartiles (female: BMI < 26.61 kg/m2 and grip strength < 22 kg, BMI 26.61–30.89 kg/m and grip strength < 22 kg, BMI 30.89–36.10 kg/m2 and grip strength < 24 kg, BMI > 36.10 kg/m2 and grip strength < 20 kg; male: BMI < 24.54 kg/m2 and grip strength < 32.2 kg, BMI ≥ 24.54–27.60 kg/m2 and grip strength < 35.4 kg, BMI > 27.6–30.68 kg/m2 and grip strength < 39.1 kg, BMI > 30.68 kg/m2 and grip strength < 35.5 kg) was scored as one point. Reporting a lot of difficulty or being unable to walk for one-quarter of a mile was scored as one point. CHS scale scores are classified as follows: frail (3–5), prefrail (1–2), and robust (0) health status.
SOF includes three items (weight loss, chair stands, energy level), with a score range of 0 to 3.20 SOF items were scored as weight loss of 5% or more in the previous year (one point), inability to complete five consecutive chair rises (one point), and reported having a lot of energy in the previous 4 week none or a little of the time (one point). SOF scale scores are categorized as frail (2–3), prefrail (1), and robust (0).
The FI was constructed using a standardized procedure24 and included 25 items from the AAH study. A summary score (0–25; one point per item) was used to construct the FI as total score/25 (0–1). FI items included self-rated health; difficulty walking several hundred yards, walking 1 mile, climbing one flight of stairs, climbing several flights of stairs, doing moderate activities, and doing vigorous activities; accomplished less than would like because of physical health, cut down on time spent at work or other activities because of emotional problems, did not feel full of life, felt downhearted and depressed, and felt tired in the past 4 weeks; restless sleep in the past week; diabetes mellitus; stroke; heart attack; cancer; chronic obstructive pulmonary disease; heart failure; angina pectoris; asthma; kidney disease; MMSE worst quintile; and Falls Efficacy Scale worst quintile. FI scores are classified as frail (>0.25), prefrail (0.25–0.20), and robust (<0.20).
Outcomes
Disability was assessed using ADL scales. ADL difficulties included seven items (0–7; bathing, dressing, eating, transferring bed or chair, walking across a room, getting outside, and using toilet).25 One or more new ADL difficulties at 3 and 9 years was coded as 1. IADL difficulties included eight items (0–8; preparing meals, shopping for groceries, managing money, making telephone calls, doing light housework, doing heavy housework, getting to places out-side walking distance, and managing medications).25,26 One or more new IADL difficulties at 3 and 9 years was coded as 1. ADL and IADL items were obtained at baseline and 3 and 9 years. Mortality was determined by tracking efforts throughout the study, including contacting proxies, search of multiple databases, and visits to the last known neighborhood. Deaths were coded as 1.
Statistical Analysis
Data were analyzed using SPSS Statistics version 20.0 (IBM Corp., Somers, NY) and SAS version 9.2 (SAS Institute, Inc., Cary, NC). Descriptive statistics are reported as means ± standard deviations (SD) or percentages. SPSS logistic regression was used to investigate the associations between each frailty scale category (frail, prefrail, robust (reference)) with study outcomes. Odds ratios were adjusted for age and sex (AORs), and 95% confidence intervals (CIs) are reported for logistic regression. SAS was used to generate receiver operator characteristic (ROC) curves and ROC contrasts to compare the area under the ROC curve (AUC) for study outcomes of the frailty scales. Frailty scale total scores for FRAIL (0–5), CHS (0–5), SOF (0–3), and FI (0–1) were used for ROC curve analyses. Sensitivity analyses were conducted including participants with baseline ADL dependencies (n = 834) and by examining outcomes according to tertiles for age. Results did not differ substantively for sensitivity analyses, so only the main analyses (n = 779) are presented.
RESULTS
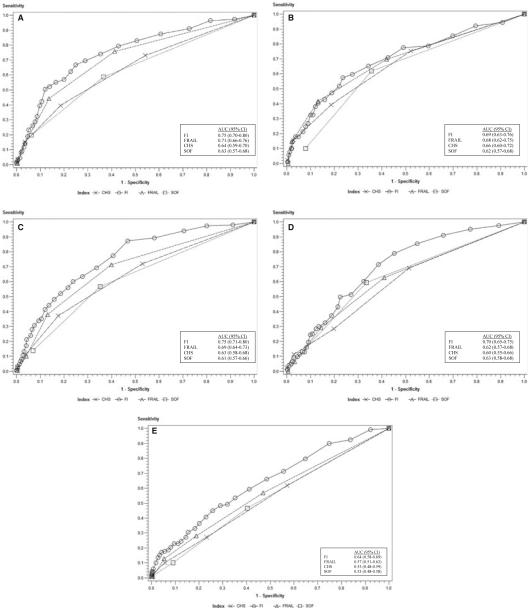
Participant baseline age according to tertile was 51.4 ± 1.1 (n = 263), 56.0 ± 1.5 (n = 254), and 61.5 ± 2.0 (n = 262), and the percentages of participants aged 60 and older were 26%, 46%, and 95% at baseline and 3- and 9-year follow-up, respectively. Classification at baseline on the FRAIL, SOF, and CHS scales and the FI was 42.0%, 32.0%, 51.6%, and 9.4%, respectively, for prefrail and 6.4%, 9.2%, 6.3%, and 22.6%, respectively, for frail. Table 1 demonstrates that frail or prefrail status on all scales predicted one or more new ADL difficulties at 3 and 9 years (except for FI prefrail at 9 years). AUC comparisons for frailty scales show that the FI (AUC = 0.75) was more predictive of one or more new ADL difficulties at 3-year follow-up than all other scales and that the FRAIL scale (AUC = 0.71) performed better than the CHS (AUC = 0.64) and SOF (AUC = 0.63) scales (Figure 1A). The FI (AUC = 0.69) and the FRAIL scale (AUC = 0.68) were better predictors than the SOF scale (AUC = 0.62) and equivalent to the CHS scale (AUC = 0.66) for one or more new ADL difficulties at 9-year follow-up (Figure 1B).
Table 1.
Frailty Measures and Disability
| ≥1 New Activity of Daily Living Difficulties |
≥1 New Instrumental Activity of Daily Living Difficulties |
|||
|---|---|---|---|---|
| 3-Year, n = 668 | 9-Year, n = 470 | 3-Year, n = 667 | 9-Year, n = 457 | |
|
|
||||
| Measure | Adjusted Odds Ratio (95% Confidence Interval) P-Value | |||
| International Academy of Nutrition and Aging (FRAIL) frailty scale | ||||
|
| ||||
| Prefrail | 4.14 (2.5–6.8) <.001 | 2.82 (1.7–4.8) <.001 | 3.56 (2.4–5.4) <.001 | 2.29 (1.5–3.6) <.001 |
|
| ||||
| Frail | 10.25 (4.7–22.3) <.001 | 14.93 (5.6–40.0) <.001 | 5.07 (2.4–10.6) <.001 | 3.08 (1.2–8.1) .02 |
|
| ||||
| Study of Osteoporotic Fractures (SOF) frailty scale | ||||
|
| ||||
| Prefrail | 1.98 (1.3–3.1) .004 | 3.18 (1.9–5.3) <.001 | 2.23 (1.5–3.3) <.001 | 2.79 (1.8–4.4) <.001 |
|
| ||||
| Frail | 5.19 (2.8–9.8) <.001 | 2.36 (1.0–5.50) .047 | 2.94 (1.6–5.4) .001 | 3.22 (1.6–6.7) .002 |
|
| ||||
| Cardiovascular Health Study (CHS) frailty scale | ||||
|
| ||||
| Prefrail | 1.94 (1.2–3.1) .005 | 2.38 (1.4–4.1) .002 | 2.05 (1.4–3.1) .001 | 1.84 (1.2–2.9) .009 |
|
| ||||
| Frail | 7.11 (3.3–15.2) <.001 | 10.39 (4.2–25.9) <.001 | 4.49 (2.2–9.3) <.001 | 5.60 (2.3–13.7) <.001 |
|
| ||||
| Frailty Index (FI) | ||||
|
| ||||
| Prefrail | 2.28 (1.1–4.7) .03 | 2.04 (0.9–4.4) .07 | 2.55 (1.4–4.7) .003 | 2.19 (1.1–4.3) .02 |
|
| ||||
| Frail | 7.90 (4.9–12.7) <.001 | 5.00 (2.9–8.6) <.001 | 5.58 (3.6–8.6) <.001 | 2.94 (1.8–4.9) <.001 |
Logistic regression adjusted for age and sex. Reference category is robust health status for each measure.
Figure 1.
(A) One or more new activity of daily living (ADL) difficulties at 3 years. Receiver operating characteristic (ROC) contrasts: Frailty Index (FI) versus International Academy of Nutrition and Aging (FRAIL) frailty scale, P = .047; FI versus Cardiovascular Health Study (CHS) frailty scale, P < .001; FI versus Study of Osteoporotic Fractures (SOF) frailty scale, P < .001; FRAIL scale versus CHS scale, P = .002; FRAIL scale versus SOF scale, P < .001; CHS scale versus SOF scale, P = .62. (B) One or more new ADL difficulties at 9 years. ROC contrasts: FI versus SOF scale, P = .03; FRAIL scale versus SOF scale, P = .03; FI versus FRAIL scale, P = .71; FI versus CHS scale, P = .22; FRAIL versus CHS scale, P = .31; CHS scale versus SOF scale, P = .30. (C) One or more new instrumental activity of daily living (IADL) difficulties at 3 years. ROC contrasts: FI versus FRAIL scale, P < .001; FI versus CHS scale, P < .001; FI versus SOF scale, P < .001; FRAIL scale versus CHS scale, P = .02; FRAIL scale versus SOF scale, P < .001; CHS scale versus SOF scale, P = .40. (D) One or more new IADL difficulties at 9 years. ROC contrasts: FI versus FRAIL scale, P = .001; FI versus CHS scale, P < .001; FI versus SOF scale, P = .01; FRAIL scale versus CHS scale, P = .38, FRAIL scale versus SOF scale, P = .72; CHS scale versus SOF scale, P = .28. (E) 9-year mortality. ROC contrasts: FI versus FRAIL scale, P = .001; FI versus CHS scale, P = <.001; FI versus SOF scale, P = <.001; FRAIL scale versus CHS scale, P = .22; FRAIL scale versus SOF scale, P = .10; CHS scale versus SOF scale, P = .92.
Table 1 shows that frail or prefrail status on all scales predicted one or more new IADL difficulties at 3 and 9 years. The FI (AUC = 0.75) was a better predictor of one or more new IADLs at 3 years than the FRAIL (AUC = 0.69), CHS (AUC = 0.63), and SOF (AUC = 0.61) scales, and the FRAIL scale outperformed the CHS and SOF scales (Figure 1C). For one or more new IADL difficulties at 9 years, the FI (AUC = 0.70) was superior to the FRAIL (AUC = 0.62), CHS (AUC = 0.60), and SOF (AUC = 0.63) scales (Figure 1D). There were no differences between the FRAIL, CHS, and SOF scales.
Table 2 shows the predictive values of scales for 9-year mortality. Frail status on the FI and the FRAIL scale predicted mortality, but prefrail status did not. The mortality ROC curves are shown in Figure 1D. The FI (AUC = 0.64) was a better predictor than the FRAIL (AUC = 0.57), CHS (AUC = 0.53), and SOF (AUC = 0.53) scales.
Table 2.
Frailty Measures and Mortality (N = 779)
| Measure | Adjusted Odds Ratio (95% Confidence Interval) |
P-Value | Mortality, % |
|---|---|---|---|
| International Academy of Nutrition and Aging (FRAIL) frailty scale | |||
|
| |||
| Robust | 1 (reference) | 12.7 | |
|
| |||
| Prefrail | 1.45 (0.94–2.23) | .09 | 15.9 |
|
| |||
| Frail | 3.24 (1.62–6.47) | <.001 | 30.0 |
|
| |||
| Study of Osteoporotic Fractures (SOF) frailty scale | |||
|
| |||
| Robust | 1 (reference) | 13.8 | |
|
| |||
| Prefrail | 1.36 (0.89–2.10) | .26 | 17.3 |
|
| |||
| Frail | 1.42 (0.71–2.84) | .32 | 16.7 |
|
| |||
| Cardiovascular Health Study (CHS) frailty scale | |||
|
| |||
| Robust | 1 (reference) | 13.7 | |
|
| |||
| Prefrail | 1.21 (0.79–1.84) | .38 | 15.2 |
|
| |||
| Frail | 1.95 (0.93–4.09) | .08 | 24.5 |
|
| |||
| Frailty Index (FI) | |||
|
| |||
| Robust | 1 (reference) | 11.5 | |
|
| |||
| Prefrail | 1.77 (0.92–3.41) | .08 | 19.2 |
|
| |||
| Frail | 2.28 (1.46–3.55) | <.001 | 24.4 |
Logistic regression adjusted for age and sex.
DISCUSSION
In AAH, prefrail or frail status according to the FRAIL, SOF, and CHS scales and the FI predict new disability; and frail status on the FI and the FRAIL scale predicts mortality. Contrary to the first hypothesis, frail status on the CHS and SOF scales and prefrail status on all measures do not predict mortality, although the AORs are in the expected direction. The European Male Aging Study of men aged 40 to 79 has also shown that prefrail and frail status on the FRAIL, CHS, and FI measures predict mortality.18 Another recent longitudinal study in a Chinese cohort of adults aged 65 and older showed that total FRAIL, CHS, and FI scale scores are associated with physical limitations and mortality.16 Overall initial studies with the FRAIL indicate that this interview-only tool has good predictive validity for disability and mortality across diverse population groups.
ROC contrasts for the AUC show that the simple FRAIL is a better predictor of new disability at 3 years than the CHS and SOF scales and at least an equivalent predictor of 9-year disability and mortality. The FRAIL scale includes a comorbidity item (which the CHS and SOF scales do not), which may explain these findings. The FI demonstrated better predictive validity than any of the other scales for new disability and mortality except new ADL difficulties at 9 years in ROC contrasts. The comprehensive FI9,24 captures the critical components of the FRAIL, CHS, and SOF scales in addition to other important health outcomes (e.g., falls, mood, cognition), which may be why it has superior predictive validity for disability and mortality. The CHS and SOF scales did not differ in any ROC contrast. Equivalent AUCs for the CHS and SOF measures, including mortality and disability, have also been reported in the prediction of adverse health outcomes.19,20 One explanation for this is that the SOF physical performance item (chair stands) could provide predictive capacity akin to the CHS low activity, weakness, and slowness components.
This study has limitations. The late middle-age AAH cohort results may not generalize to an older population of African Americans, although sensitivity analyses did not reveal substantive differences across age tertiles. Study outcomes were limited to disability and mortality. The AUCs indicate only a moderate ability of frailty measures to discriminate outcomes. Future research should include older African Americans and other outcomes such as fractures, falls, hospitalization, and institutionalization. Cut points of greater than 0.21 (frail),18 0.13 to 0.21 (prefrail), and 0 to 1.12 (robust)18 or 0.25 or greater (frail) and less than 0.25 (robust)27 have previously been used to categorize the FI. Slightly different cut points were used in the present study, which may have influenced the results of hypothesis 1.
The FRAIL meets criteria for a scale that would make a successful diagnostic test of frailty.28 Specifically, it shows strong content (face validity) by including factors that investigators have considered to represent the basic principles necessary to define frailty. Construct validity has been demonstrated because the FRAIL correlates with measures of disability even when they are excluded as part of the definition.17 This is important because frailty is not the same as disability.29 The FRAIL scale has also been shown to correlate with self-rated health and falls efficacy.17 Predictive validity exists in that the FRAIL scale predicts new disabilities and mortality.16–18 The FRAIL scale is clinically sensible30 in that it is easily used and useful in that it earmarks areas of potential treatment, and it can be broadly acceptable to a general practitioner audience.
In conclusion, the FI and the FRAIL scale exhibited the strongest predictive validity for new disability and mortality in AAH. The best prediction tool to identify frail individuals at risk of disability and mortality may be one that includes a comorbidity measure. The FRAIL scale is brief; is interview based; is easy to administer, score, and interpret; includes a comorbidity measure; and has demonstrated validity, so it may prove to be valuable for use by clinicians in the office.
ACKNOWLEDGMENTS
This research was supported by a grant from the National Institute on Aging to Dr. D.K. Miller (R01 AG010436).
Sponsor’s Role: The funding source had no role in the study design, data collection, data analysis, interpretation of results, or write-up.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: All authors contributed to study concept and design, analysis and interpretation of data, and preparation of manuscript.
REFERENCES
- 1.Morley JE, Vellas B, Abellan van Kan G, et al. Frailty consensus: A call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilhelm-Leen ER, Hall YN, Tamura MK, et al. Frailty and chronic kidney disease: The Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–671. doi: 10.1016/j.amjmed.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeulan J, Neyens JC, vanRossum E, et al. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: A systematic review. BMC Geriatr. 2011;11:33. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham JE, Snih SA, Berges IM, et al. Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology. 2009;55:644–651. doi: 10.1159/000235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong CH, Weiss D, Sourial N, et al. Frailty and its association with disability and comorbidity in a community-dwelling sample of seniors in Montreal: A cross-sectional study. Aging Clin Exp Res. 2010;22:54–62. doi: 10.1007/BF03324816. [DOI] [PubMed] [Google Scholar]
- 6.Strawbridge WJ, Shema SJ, Balfour JL, et al. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998;53B:S9–S16. doi: 10.1093/geronb/53b.1.s9. [DOI] [PubMed] [Google Scholar]
- 7.Morley JE, Perry HM, III, Miller DK. Editorial: Something about frailty. J Gerontol A Biol Sci Med Sci. 2002;57A:M698–M704. doi: 10.1093/gerona/57.11.m698. [DOI] [PubMed] [Google Scholar]
- 8.Gobbens RJ, van Assen MA, Luijkx KG, et al. The Tilburg Frailty Indicator: Psychometric properties. J Am Med Dir Assoc. 2010;11:344–355. doi: 10.1016/j.jamda.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62A:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 10.Syddall H, Roberts HC, Evandrou M, et al. Prevalence and correlates of frailty among community-dwelling older men and women: Findings from the Hertfordshire Cohort Study. Age Ageing. 2010;39:197–203. doi: 10.1093/ageing/afp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avila-Funes JA, Helmer C, Amieva H, et al. Frailty among community-dwelling elderly people in France: The Three-City Study. J Gerontol A Biol Sci Med Sci. 2008;63A:1089–1096. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56A:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Cawthon PM, Marshall LM, Michael Y, et al. Osteoporotic Fractures in Men Research Group. Frailty in older men: Prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- 14.Abellan van Kan G, Rolland YM, Morley JE, et al. Frailty: Toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Abellan van Kan G, Rolland Y, Bergman H, et al. The I.A.N.A. Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12:29–37. doi: 10.1007/BF02982161. [DOI] [PubMed] [Google Scholar]
- 16.Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J Am Geriatr Soc. 2012;60:1478–1486. doi: 10.1111/j.1532-5415.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 17.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravindrarajah R, Lee DM, Pye SR, et al. The ability of three different models of frailty to predict all-cause mortality: Results from the European Male Aging Study (EMAS) Arch Gerontol Geriatr. 2013;57:360–368. doi: 10.1016/j.archger.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Ensrud KE, Ewing SK, Cawthon PM, et al. Osteoporotic Fractures in Men Research Group. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57:492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 21.Miller DK, Wolinsky FD, Malmstrom TK, et al. Inner city, middle-aged African Americans have excess frank and subclinical disability. J Gerontol A Biol Sci Med Sci. 2005;60A:207–212. doi: 10.1093/gerona/60.2.207. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch C, Anderson ML, Newman A, et al. Cardiovascular Health Study Research Group The association of race with frailty: The Cardiovascular Health Study. Ann Epidemiol. 2006;16:545–553. doi: 10.1016/j.annepidem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Dipietro L, Caspersen CJ, Ostfeld AM, et al. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;25:628–642. [PubMed] [Google Scholar]
- 24.Tajar A, O’Connell MD, Mitnitski AB, et al. Frailty in relation to variations in hormone levels of the hypothalamic-pituitary-testicular axis in older men: Results from the European Male Aging Study. J Am Geriatr Soc. 2011;59:814–821. doi: 10.1111/j.1532-5415.2011.03398.x. [DOI] [PubMed] [Google Scholar]
- 25.National Center for Health Statistics . Data File Documentation, National Health Interview, Second Supplement on Aging, 1994 (machine readable data file and documentation) National Center for Health Statistics; Hyattsville, MD: 1998. [Google Scholar]
- 26.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 27.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62A:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 28.Rockwood K. What would make a definition of frailty successful? Age Ageing. 2005;34:432–434. doi: 10.1093/ageing/afi146. [DOI] [PubMed] [Google Scholar]
- 29.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59A:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 30.Feinstein AR. Clinimetrics. Yale University Press; New Haven, CT: 1987. [Google Scholar]