Abstract
Background: Recent advances in research on thyroid carcinogenesis have yielded applications of diagnostic molecular biomarkers and profiling panels in the management of thyroid nodules. The specific utility of these novel, clinically available molecular tests is becoming widely appreciated, especially in perioperative decision making by the surgeon regarding the need for surgery and the extent of initial resection.
Methods: A task force was convened by the Surgical Affairs Committee of the American Thyroid Association and was charged with writing this article.
Results/Conclusions: This review covers the clinical scenarios by cytologic category for which the thyroid surgeon may find molecular profiling results useful, particularly for cases with indeterminate fine-needle aspiration cytology. Distinct strengths of each ancillary test are highlighted to convey the current status of this evolving field, which has already demonstrated the potential to streamline decision making and reduce unnecessary surgery, with the accompanying benefits. However, the performance of any diagnostic test, that is, its positive predictive value and negative predictive value, are exquisitely influenced by the prevalence of cancer in that cytologic category, which is known to vary widely at different medical centers. Thus, it is crucial for the clinician to know the prevalence of malignancy within each indeterminate cytologic category, at one's own institution. Without this information, the performance of the diagnostic tests discussed below may vary substantially.
Introduction
Standardized interpretation of fine-needle aspiration (FNA) cytology for thyroid nodules has improved since the advent of a tiered Bethesda classification scheme in 2007 (1–6). While concordance among cytopathologists is high (90%) for benign and malignant cytologic diagnoses, there is significant intra and inter-observer variability for any type of indeterminate cytologic diagnosis (75% and 64% concordance, respectively) (7). Further, lower volume cytopathologists appear more likely to categorize a nodule as indeterminate rather than benign (7,8). At present, the Bethesda FNA cytology categories have been widely adopted at high-volume thyroid surgical centers. However, ambiguity still persists regarding management of cases reported in the three indeterminate diagnostic categories of (a) atypia of uncertain significance/follicular lesion of undetermined significance (AUS/FLUS), (b) follicular neoplasm/suspicious for follicular neoplasm (FN), and (c) suspicious for malignant cells (SMC). Reported thyroid cancer risks for the indeterminate categories range greatly from 6–48% for AUS/FLUS (Category III) to 14–34% for FN (Category IV) to 53–87% for SMC (Category V) (9–12), with the greatest variability observed in the AUS/FLUS category (13–16). By contrast, the current acceptable false-negative rates for benign cytology (Category I) are <5% (9,17,18). The false-positive rate for malignant cytology (Category VI) is <1% (18,19). Use of the Bethesda criteria and categories is recommended to utilize the molecular testing described here.
Molecular Profiling Performance Varies by Differentiated Thyroid Cancer Prevalence and Institutional FNA Accuracy
The techniques and methods for molecular testing of thyroid nodules are described in detail elsewhere, and include testing for point mutations or translocations in genomic DNA from thyroid nodules (20) or gene expression profiling using RNA (21). Likewise, the relevant clinical factors (history of prior exposure to radiation, recurrent laryngeal nerve paralysis, etc.), which are important to guide decisions to perform FNA biopsy or to propose thyroid surgery otherwise, should be taken into account, but are beyond the scope of this review and are detailed elsewhere (Haugen et al. 2015 American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer; in review). Molecular assays do not currently have a demonstrated role in scenarios for which FNA biopsy is not indicated.
Utilizing molecular profiling tests requires a brief understanding of the performance statistics commonly reported in large studies. In the context of this review, sensitivity is the probability the test will classify a malignant lesion as “malignant.” Likewise, specificity is the likelihood that a molecular test will correctly classify a benign FNA specimen as “benign.” Sensitivity and specificity move in opposite directions, meaning that as the sensitivity increases, the specificity usually decreases and vice versa. Positive predictive value (PPV) is the percentage of patients with a positive test result who actually have the disease. PPV indicates how many positive test results are true positives. Conversely, negative predictive value (NPV) is the percentage of patients with a negative test who actually do not have the disease. NPV tells us how many negative tests are true negatives. The higher these numbers are, the better the performance in predicting malignancy (PPV) or excluding malignancy (NPV).
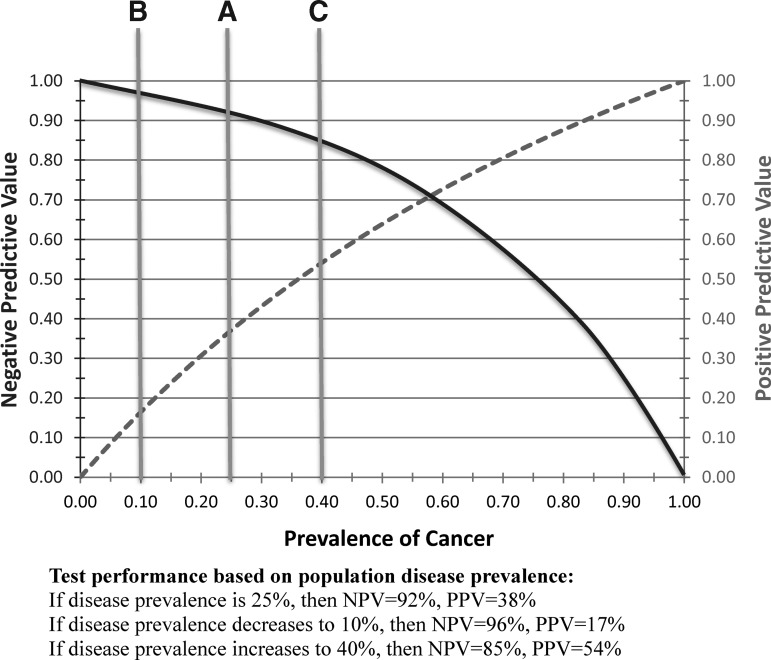
The wider the differences in prevalence of malignancy in a given Bethesda cytologic category (which varies from one institution to another based on cytologic expertise and other factors), the more variable PPV and NPV will be when using a particular test. The performance of a molecular profiling test can be quite different from that reported, and therefore one must know the local prevalence of malignancy in each cytologic category before interpreting how useful a particular molecular test will be; that is, although PPV and NPV are the most useful characteristics for clinical decision making, they are not immutable. Further, variance of disease prevalence from the values used in validation studies will necessarily and perhaps even substantially improve or degrade the predicted utility. Given the well-established and frequently dramatic variations in cancer prevalence in thyroid cytology specimens, clinicians are urged to be aware of the prevalence of disease by cytologic category in their tested patients and carefully consider how local disease prevalence may change PPV and NPV of molecular diagnostic tests when applied to their unique clinical practice (Fig. 1).
FIG. 1.
Effect of prevalence change on test predictive value. Graphical demonstration of how prevalence strongly influences positive predictive value (PPV; dashed line), that is, the ability of a test to “rule in” a disease correctly when it actually exists. A similar graph is depicted for negative predictive value (NPV; curved solid line), which is strongly influenced by prevalence in its ability to “rule out” a disease correctly. As one can observe from the figure, if the prevalence of malignancy in a thyroid nodule is 40% (vertical line “C”), the PPV of a particular test may be 54% and the NPV 85%, whereas if the prevalence of cancer is lower (10%, vertical line “A”), then the PPV drops to 17% while the NPV increases to 96%. The test (and its sensitivity, which is its ability to detect cancer when present) is conducted identically, but the prevalence of cancer makes the test perform very differently at different institutions.
Preoperative Diagnosis of Differentiated Thyroid Cancer Determines Management
Preoperative diagnosis of differentiated thyroid cancer (DTC) is important because it streamlines the extent of initial thyroidectomy by avoiding performance of two-stage surgery for indeterminate nodules (initial lobectomy followed by completion thyroidectomy) when total thyroidectomy would be recommended if the diagnosis of malignancy were known preoperatively. Conversely, a benign cytologic diagnosis can avoid diagnostic surgery for indeterminate nodules. It is generally well accepted that most thyroid cancers that are cytologically indeterminate cannot be diagnosed intraoperatively by frozen section (12,22,23). Thus, accurate preoperative diagnosis of malignancy in cytologically indeterminate nodules avoids the higher costs and patient burden associated with two surgeries, and accurate preoperative benign diagnosis avoids the costs, risks, and patient burden of surgery altogether.
Preoperative Molecular Profiling Tests for Thyroid Carcinoma
Molecular markers can enhance the diagnostic sensitivity of FNA cytology in detecting malignancy (14,24,25). Genetic alterations occur in the MAP kinase (MAPK) and PI3K/AKT pathways, including BRAFV600E and RAS point mutations, as well as translocations in the RET/PTC and PAX8/PPARγ genes (5). Together, BRAF and RAS mutations and PAX8/PPARγ and RET/PTC translocations account for the majority (about 70%) of known genetic alterations in DTC, and these mutations are often mutually exclusive, that is, a single cancer does not contain multiple redundant oncogene alterations (26). Both prognostic and genotype–phenotype correlations have also been reported for several genetic DTC markers (27–29), and specificity and PPV are even greater when BRAF or RET/PTC are positive. A seven-gene molecular panel (7-gene MT) including BRAFV600E, three isoforms of RAS point mutations, and translocations in the PAX8/PPARγ and RET/PTC genes performed on residual FNA needle hub fluid have been clinically validated to predict the presence of DTC with high specificity (86–94%) and PPV (87–100%). The 7-gene MT test was performed on >1500 indeterminate cytology specimens and correlated with histologic results to generate a real-time algorithm for management of thyroid nodules (5,6,30).
More recently, a broad gene expression classifier (GEC) was developed as a proprietary method for molecular analysis of thyroid FNA specimens diagnosed as AUS/FLUS and FN, with the intent of predicting benign pathology and thus avoiding unnecessary thyroidectomy for asymptomatic nodules. With an initially reported high negative predictive value, GEC combines an assay signature of 167 genes with commercial cytologic examination (21). The GEC and 7-gene MT are described in detail below and are the two widely available tests at the present time.
Clinical Scenarios in Which Molecular Analyses are Useful Surgically
There are several scenarios in which a molecular test may be of little benefit: (a) a nodule that does not meet the criteria for FNA, (b) cytology in a definitive Bethesda category (benign [Bethesda II] or malignant [Bethesda VI]), and (c) high prevalence of malignancy at a given institution, for instance in SMC (Bethesda V). With any diagnostic test, the clinician should only send it if it will impact her/his management.
Based on results reported for the 7-gene FNA MT panel (30), its observed value for surgical decision making in thyroid nodular disease is to allow appropriate initial oncologic total thyroidectomy rather than lobectomy with subsequent completion thyroidectomy when total thyroidectomy is clinically indicated (6); oncologic thyroidectomy may also entail a complete lobectomy for low-risk cancer (Haugen et al.; in review). In a hypothetical model, hospital-based MT also achieves significant cost efficacy by distributed risk (31) but the cost-effectiveness of using the commercial assay is unknown for the commercially available MT product (ThyGenX).
Conversely, the GEC technique is intended to avoid diagnostic thyroidectomy altogether in nodules with indeterminate cytology assessed to have a low risk of malignancy (21). The GEC is reported to attain hypothetical efficacy based on initial accuracy and cost estimates (32), but the actual cost-effectiveness is unknown.
FNA Performance Parameters for Clinically Available Molecular Profiling Tests
For preoperative use in the setting of indeterminate thyroid cytology, two different clinical tests are available and in use today (Table 1).
Table 1.
Estimated Likelihood of Malignancy in a Thyroid Nodule with Indeterminate Cytology and Recommended Management
| Bethesda cytologic category | Ancillary testing | Estimatedarisk of malignancy; range (median) | Recommendation | |
|---|---|---|---|---|
| III (AUS/FLUS) | None | 6–48% (14%) | Repeat FNA, ancillary testing, or diagnostic lobectomy | |
| GECb (reported prevalence 24%) | Suspicious | 38% | Diagnostic lobectomy | |
| Benign | 5% | Active surveillance | ||
| 7-gene MTc (reported prevalence 14%) | Positive | 88% | Oncologic thyroidectomy | |
| Negative | 6% | Active surveillance or diagnostic lobectomy | ||
| IV (FN/FL) | None | 14–34% (25%) | Ancillary testing or diagnostic lobectomy | |
| GECb (reported prevalence 25%) | Suspicious | 37% | Diagnostic lobectomy | |
| Benign | 6% | Active surveillance | ||
| 7-gene MTc (reported prevalence 27%) | Positive | 87% | Oncologic thyroidectomy | |
| Negative | 14% | Diagnostic lobectomy | ||
| ThyroSeq2.0 paneld (reported prevalence 27%) | Positive | 87% | Oncologic thyroidectomy | |
| Negative | 5% | Observation | ||
| V (SMC) | None | 53–87% (70%) | Ancillary testing or oncologic thyroidectomy | |
| GECb (reported prevalence 62%) | Suspicious | 76% | Oncologic thyroidectomy | |
| Benign | 15% | Diagnostic lobectomy | ||
| 7-gene MTc (reported prevalence 54%) | Positive | 95% | Oncologic thyroidectomy | |
| Negative | 28% | Diagnostic lobectomy | ||
Estimated risk based upon reported studies that used Bethesda classification. Estimates are highly dependent on the prevalence of disease in a given population. Thus, if the prevalence of malignancy in a category of indeterminate cytology is higher than disease prevalence reported in the referenced studies above, then the estimated likelihood of malignancy would be higher. Sonographic and clinical factors may also significantly affect these estimates in individual patients.
GEC, gene expression classifier, data as reported by Alexander et al. (21).
7-gene MT, seven-gene mutational panel, data as reported by Nikiforov et al. (30).
ThyroSeq2.0 panel, data as reported by Nikiforov et al. (36).
7-Gene MT Panel
A seven-gene panel of somatic point mutations and gene rearrangements was described in 2007 (33) and was prospectively assessed with a reference standard of histologic diagnosis; a similar panel has been independently validated (34,35). Specifically, the 7-gene MT tests for a panel of mutations (BRAF, N-/H-/K-RAS) and for translocations of the RET/PTC and PAX8/PPARγ genes. MT is a “rule in” test for DTC (5). The components of the MT are readily available CLIA-approved molecular pathology tests. The test was developed at the University of Pittsburgh, and is commercialized and offered to outside institutions through CBL Path, Inc., and in this setting has been clinically validated. The panel was also commercially marketed by Quest Diagnostics, but the results from this proprietary assay have not been validated to date. As a perioperative adjunct in surgical decision-making, the 7-gene MT panel has recently been demonstrated to add to the specificity of indeterminate FNA cytology (24) and successfully refine the initial operative management of thyroid nodules and thyroid cancer (6).
Seven-gene MT performance was examined in a large prospective single-center study (24). As detailed in Table 1, for the AUS/FLUS cytologic category, mutation identification had a PPV of 88% for histologic cancers, and the false-positive rate was 12% (24). The malignancy rate for the mutation-negative FLUS group was 6%, providing a NPV of 94% (overall accuracy for FLUS lesions was 94% with 62% sensitivity and 99% specificity). For the FN cytologic category, molecular alterations were detected by MT in 87% of resected thyroid carcinomas with a false-positive rate of 13%, while mutation-negative FN results carried a 14% cancer risk with 86% NPV (overall accuracy for 7-gene MT with FN cytology was 86%, with 57% sensitivity and 97% specificity). For the SMC category, 95% of MT-positive nodules were carcinomas with a false-positive rate of 5% (overall, 28% of mutation-negative SMC samples were malignant histologically, providing a NPV of 72% with 96% specificity, 68% sensitivity, and accuracy of 81%). In summary, for thyroid lesions with indeterminate cytology, the detection of any mutation translated into a malignancy risk for AUS/FLUS, FN, and SMC of 88%, 87%, and 95% respectively, compared to 6%, 14%, and 28% in mutation-negative lesions (24).
Based on these results, 7-gene MT positive results on FNA allow direct performance of initial therapeutic oncologic thyroidectomy for all three cytologically indeterminate Bethesda categories (6,30). Mutation negative results in AUS/FLUS lesions currently prompt diagnostic thyroidectomy when the observed prevalence of malignancy in this Bethesda category is higher than the 14% reported in the study, or when clinical and/or sonographic suspicion is intermediate or high. However, if the risk of malignancy after cytologic classification and 7-gene MT analysis yields a cancer risk of <6%, observation is also considered reasonable (30). Conversely, active surveillance or repeat FNA may be considered for patients with clinically and/or sonographically low-risk nodules especially when the local prevalence of malignancy in this Bethesda category is less than or equal to the 14% prevalence reported (24). A recent commercially sponsored blinded study of the 7-gene MT in 53 patients with indeterminate cytology verified the 7-gene MT as a “rule in” test with a specificity of 89% and PPV of 100% for a BRAF mutation or RET/PTC rearrangement, 70% for RAS mutations or PAX8-PPARγ rearrangements, and 88% overall (35). The study also observed that because of the very high cancer prevalence of 50% in the AUS/FLUS category, the NPV of the 7-gene MT was 44%, re-emphasizing that its use to exclude malignancy in cytologic categories with high cancer prevalence is not routinely recommended.
Mutation negative nodules that are cytologically indeterminate for FN currently require diagnostic lobectomy with an identical NPV of 86% in both large clinical validation studies where the prevalence of malignancy in the FN category was consistent (27% vs. 30%). Mutation-negative SMC cytologic results are much less likely to require oncologic thyroidectomy, since the risk of malignancy is reduced by approximately half to 28% when the prevalence of malignancy is 54% (30). Thus, an initial diagnostic lobectomy is generally reasonable. When the prevalence of malignancy is 67%, the risk of malignancy is approximately 56% with a mutation negative SMC nodule and patient preference regarding extent of initial surgery should be considered.
Next-Generation Sequencing Panel
Recently a comprehensive 60-gene, next-generation sequencing (NGS)-based assay was developed and implemented at the University of Pittsburgh that tests for 91% of known thyroid cancer mutations (36), increasing both sensitivity and specificity in a cohort of 143 consecutive FN/SFN cases with a cancer prevalence of 27%, and achieving a high PPV (83%), while improving the NPV significantly to 96%. Results with this panel suggest that a single test may sufficiently merge capabilities of high PPV/NPV to both “rule in” and “rule out” proficiency in a low-cost, routine, CLIA-approved test. This new test is commercially available (Thyroseq 2.0, CBL Pathology, Rye Brook, NY) and requires prospective validation in different centers, particularly those with varying prevalence of disease in the FN/SFN and AUS/LUS categories.
GEC
In 2010, Chudova et al. analyzed the amplified transcriptional profile from mRNA of FNA biopsy specimens from nodules in patients undergoing thyroidectomy, and developed a gene expression test intended to predict lesions with low risk for malignancy (37). Further mathematical analysis led to the development of a 142 gene cDNA Affymetrix cassette (Afirma) GEC (21), which appeared capable of separating “benign” from “suspicious” FNA specimens in a small independent cohort of prospectively collected samples. Initial statistical analysis with a “benign” GEC result yielded a high NPV of 96% (37). The proprietary GEC signature was prospectively tested in a commercially sponsored trial (21) with initially promising results for cytologically indeterminate nodules that were classified as AUS/FLUS (NPV 95%) and FN (NPV 94%), but results were less promising for SMC (NPV 85%) due to the higher prevalence of malignancy in this category (62%; Table 1). The thyroid GEC has been subsequently reported to exhibit a lower NPV in the setting of higher prevalence of malignancy (33%), with 2/20 GEC benign results representing undetected malignancy (NPV 90%) and an overall sensitivity of 94% and specificity of 24%, though not all patients underwent surgery (38). A small nonblinded study reported an estimated NPV of 94% for GEC, but again not all patients with benign results underwent surgical excision. Another study in 121 nodules with indeterminate cytology, “suspicious” (i.e., positive) GEC results, and surgical histology yielded a malignancy rate that varied by institution from 29% to 47%, and also reported that among 71 GEC benign nodules, there was a false-negative rate of 1.4% in very short-term follow-up (mean 8.5 months) (21).
The GEC was designed as a “rule out” test for DTC in the AUS/FLUS and FN/SFN cytologic categories. Based on the published results, GEC is not reflexively offered for the SMC cytology category, but can be specifically requested. If performed, a patient with a benign GEC result in the setting of a cytologically indeterminate nodule classified as SMC is still recommended to undergo diagnostic lobectomy; a suspicious GEC result in the SMC category adds little additional information over cytology alone with a PPV of 76%. The current GEC application is to enhance the accuracy of the cytologically indeterminate categories of AUS/FLUS and FN. A benign GEC result may be used to recommend observation and avoid a diagnostic lobectomy, especially in the absence of clinical or sonographic suspicion of malignancy. In the presence of clinical or sonographic suspicion for malignancy, and/or when the local prevalence of malignancy exceeds the 25% reported, diagnostic lobectomy is still warranted (13,28,39). However, a recent report estimated that standard application of the GEC for all indeterminate thyroid nodules would result in only a 7.2% decrease in thyroidectomy volume (40).
As mentioned above, in a prospective multicenter double-blinded study, the parameters of proprietary GEC use were analyzed (21). With a reference standard of histologic diagnosis, as detailed in Table 1, the GEC effectively ruled out DTC in half of thyroid nodules with AUS/FLUS or FN cytology. However, missed malignancies included papillary thyroid carcinoma (PTC) and oncocytic Hürthle cell cancer. In this study, there was a trend for false-negative results to correlate with inadequate follicular epithelium and smaller nodules, perhaps suggesting that sampling error was a driver of false-negative results, and subsequently, the GEC result is reported as insufficient if thyroid follicular epithelial mRNA is not detected (21). For a set of 265 AUS/FLUS, FN, and SMC cytologically indeterminate nodules with surgical histology, the GEC had an overall sensitivity of 92% and specificity of 52% with an NPV of 95%, 94%, and 85% by Bethesda category respectively and a PPV of 38%, 37%, and 76%, respectively.
Specific Scenarios for Surgical Application of Molecular Profiling Results
7 Gene MP
-
1. Atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) (Table 1):
• 7-gene MT-positive AUS/FLUS cytology should be managed by initial oncologic thyroidectomy, usually a single-stage total thyroidectomy due to the very high likelihood of malignancy (especially when positive for BRAF, RET/PTC, and PAX8-PPARγ mutations) (29). Oncologic thyroidectomy may also entail a complete lobectomy for low-risk cancer (Haugen et al.; in review). Clinical and demographic information also play a role in implementation of MT results.
• 7-gene MT-negative AUS/FLUS cytology should be managed by observation or diagnostic thyroidectomy. Diagnostic surgery is appropriate with a higher institutional cancer prevalence for AUS/FLUS lesions, or when there is clinical or sonographic suspicion for malignancy. The extent of surgery should at least be a complete lobectomy. When the local prevalence of DTC is low for AUS/FLUS lesions (e.g., prevalence of 14% yielding an MT-7 negative malignancy rate of 5.9%), or in carefully selected patients with clinically and sonographically benign or very low-risk nodules, active observation and/or repeat FNA are appropriate alternatives to diagnostic lobectomy.
-
2. Follicular or oncocytic neoplasm/suspicious for FN
• 7-gene MT-positive FN cytology should be managed by initial oncologic thyroidectomy, usually a single-stage total thyroidectomy due to the very high likelihood of malignancy. Oncologic thyroidectomy may also entail a complete lobectomy for low-risk cancer (Haugen et al.; in review).
• 7-gene MT-negative FN cytology should be managed by at least a diagnostic thyroid lobectomy. The extent of initial surgery depends upon other clinical conditions present, and may entail a total thyroidectomy.
-
3. SMC
• 7-gene MT-positive SMC cytology should be managed by initial oncologic thyroidectomy, usually an initial total thyroidectomy due to the very high likelihood of malignancy. Oncologic thyroidectomy may also entail a complete lobectomy for low-risk cancer (Haugen et al.; in review).
• 7-gene MT-negative SMC nodules should be managed by at least a diagnostic lobectomy. The initial extent of surgery depends upon other clinical conditions present, and may entail a total thyroidectomy.
-
4. Benign or nondiagnostic cytology
The 7-gene MT panel has reported utility in selected cases of cytologically benign nodules with worrisome ultrasound (US) characteristics and/or high clinical suspicion (25). Repeat US-guided FNA would be a reasonable alternative to molecular testing.
• 7-gene MT-positive cytologically benign thyroid nodules should be managed by initial oncologic thyroidectomy due to the very high likelihood of malignancy (6).
• 7-gene MT-negative cytologically benign nodules should be managed nonoperatively, unless other clinical reasons for surgery are present.
GEC
Based on the available data, GEC analysis has been shown to be potentially of value in avoiding diagnostic surgery in cytologically indeterminate thyroid nodules that are AUS/FLUS or FN and GEC benign. When cytologically AUS/FLUS or FN nodules are reported as “suspicious” using the GEC, the final decision about the extent of initial thyroidectomy should be based on a thorough discussion with the stakeholders involved (patient, endocrinologist, surgeon) and accounting for all the clinical characteristics specific to that patient. The role of the GEC in cytologically indeterminate nodules that are SFM is less clear, and the test is not reflexively performed. Two recent reports with small sample sizes have independently evaluated GEC use in single-center studies, and the results emphasize the importance of cancer prevalence in interpretation of NPV and PPV (38,41). Harrell et al. evaluated 35 patients with indeterminate cytology, histology, and GEC results, and had an observed cancer rate of 51%. Although the overall calculated sensitivity was 94% and was similar to the rate previously published, the high malignancy rate in their series resulted in a lower estimated NPV of 90% (38). In a study by McIver et al., local cytology and pathology expertise was utilized and in 36 FNAB specimens that were characterized by indeterminate cytology, GEC, and histology, an actual diagnosis of cancer was found in only 17% of these cases. Overall, the estimated sensitivity and specificity were 83% and 10%, respectively. Because of the lower pretest probability of malignancy and diminished sensitivities and specificities, decreases in estimated NPV (94%) and PPV (16%) were also observed (41).
-
1. AUS/FLUS:
• GEC-suspicious AUS/FLUS cytology should be managed by at least a diagnostic thyroid lobectomy. The initial extent of surgery depends upon other clinical conditions present, and may entail a total thyroidectomy.
• GEC-benign AUS/FLUS cytology results may be managed with active surveillance. However, diagnostic thyroid lobectomy is appropriate if otherwise clinically indicated or when institutional prevalence of malignancy in AUS/FLUS cytology is considered significant. Future clinical studies are needed to determine this threshold.
-
2. FN
• GEC-suspicious, FN cytology should be managed by at least a diagnostic lobectomy. The initial extent of surgery depends upon other clinical conditions present, and may entail a total thyroidectomy.
• GEC-benign FN cytology results may be managed with active surveillance. Diagnostic thyroid lobectomy can be offered if otherwise clinically indicated or when the institutional prevalence of malignancy in the FN cytology is significant.
-
3. SMC
• The GEC is not reflexively performed nor routinely recommended for the SMC cytologic category, but may be requested if clinically indicated.
Cautions and Modifiers to the Use of Molecular Profiling Adjuncts in Surgical Decision Making
Institutional cytologic parameters and cancer prevalence can significantly affect molecular profiling PPV and particularly NPV results. Bethesda FNA cytologic categories, which provide a variable cancer risk estimate ranging from 6% to 87% for the three different indeterminate cytologic diagnoses, are subject to variation in usage (28,30). Thus, the use of molecular profiling in cytologic indeterminate categories should be interpreted judiciously and with discretion by the clinician, who must be aware of institutional cytopathologic performance results, as well as the individual clinical and sonographic factors for each patient. Although positive MT results currently support initial oncologic thyroidectomy, which today is often total based on high cancer risk, this may not always prove to be true. As knowledge develops about genotype–phenotype correlations, gene-specific MT surgical recommendations will likely be further refined. For example, the BRAFV600E mutation is 99.5% specific for PTC (24,30), and for a lesion >1 cm in size, an initial total thyroidectomy may be appropriate. However, the BRAFK601E mutation has an approximate 80% risk of associating with low-grade encapsulated follicular variant of PTC and a 20% chance of being associated with a benign follicular adenoma. In this situation, a simple lobectomy and isthmusthectomy may be sufficient. Likewise, although the NRAS and HRAS alterations at codon 61 have an 85–87% likelihood of malignancy, many (but not all) represent low-grade follicular variant PTC histologically for which appropriately conservative surgical management may be reasonable, although RAS-positive lesions do have a high bilaterality rate (48%) and can also represent aggressive cancer types (1).
Both 7-gene MT and GEC have been proposed to be useful in “low-risk” nodules where other clinical features do not support performing surgery and in thyroid disease that would potentially be managed by lobectomy or total thyroidectomy, as detailed above. It is appealing to consider that when the 7-gene MT panel is expanded to include alterations found in the remaining 30% of thyroid cancers that it does not presently encompass, it should have even greater sensitivity and NPV, thus determining both correct initial oncologic surgical management when positive, as well as potentially avoiding surgery altogether for asymptomatic benign nodules (42,43). Similarly, improved GEC performance may one day permit determination of initial extent of surgery, facilitate the avoidance of active surveillance, or provide other clinical benefits to patients.
Currently, nonoperative management of cytologically indeterminate nodules based upon benign GEC or negative MT results requires active long-term surveillance until results become validated by a range of investigators to ensure safety of the nonoperative approach (44). As DTC is often a slow growing malignancy, the presentation of a low-grade, missed cancer may be delayed, especially for younger patients. False-negative study results may be flawed, since not all patients undergo surgery and/or the nonoperative follow-up has so far been short.
Although not yet validated or currently available commercially, further investigational approaches have been investigated for evaluating thyroid nodules (45), including whole transcriptome profiling, thyrotropin receptor (TSHR) analysis in the blood that is approaching clinical testing, and immunohistochemical (IHC) detection of galectin (27,46), and quantitative characterization of unique microRNAs associated with thyroid malignancy (47). As The Cancer Genome Atlas (TCGA) results and other novel genetic information become available, next-generation clinical tests will also improve the panel(s) available. For instance, results with the 7-gene MT panel have already led to the implementation of Thyroseq 2.0 in which additional somatic alterations in PTEN, AKT1, RET, TSHR, CTNNB1, GNAS, RET, and TP53 are examined (3,20,25,48,49), which will become even more comprehensive (hence increasing PPV and NPV) as new genetic information about thyroid carcinoma emerges (50).
Summary
Techniques for molecular profiling of thyroid cytology specimens have evolved as adjuncts to guide the appropriate management of cytologically indeterminate nodules. However, it must be stressed that the utility of any molecular test is only applicable clinically when combined with clinical and sonographic risk factors for malignancy and with understanding of the prevalence of malignancy for the Bethesda cytologic categories at the reporting institution. For example, a “rule out” test such as the GEC will perform better in a setting of lower cancer frequency, as well as in a cytologic category of low cancer frequency such as AUS/FLUS or FN, than it will in a setting of higher cancer frequency such as SMC or a site with a high prevalence of malignancy in a given cytologic category. Conversely, a “rule in” test such as the 7-gene MT will perform better in settings and categories of higher cancer frequency, for example if a clinician is specifically selecting “high risk” cases thereby enriching the prevalence of cancer in the examined population, or if the local malignancy rate is high at baseline.
Applying an anticipated PPV or NPV for either test without this information will inevitably yield variability in results from the published reports, and reduce the value and application of molecular profiling tests to surgical decision making for individual patients. Future studies on further refinements and expansion of gene sets in analytic panels, as for example informed by comprehensive analyses such as the NCI-funded TCGA (51), will likely improve the diagnostic accuracy of molecular analyses of thyroid cytology specimens and offer promise for personalizing surgical therapy, with the potential for cost and risk reduction in the diagnostic and therapeutic approaches to treating DTC.
Author Disclosure Statement
Dr. Fahey reports a patent that has been licensed to Prolias Inc. and serves on the Scientific Advisory Board of Prolias Inc. Dr. Steward declares research grant funding (clinical trial) from Veracyte. No competing financial interests exist for the remaining authors.
References
- 1.Gupta N, Dasyam AK, Carty SE, Nikiforova MN, Ohori NP, Armstrong M, Yip L, LeBeau SO, McCoy KL, Coyne C, Stang MT, Johnson J, Ferris RL, Seethala R, Nikiforov YE, Hodak SP. 2013. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab 98:E914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloos RT, Reynolds JD, Walsh PS, Wilde JI, Tom EY, Pagan M, Barbacioru C, Chudova DI, Wong M, Friedman L, LiVolsi VA, Rosai J, Lanman RB, Kennedy GC. 2013. Does addition of BRAF V600E mutation testing modify sensitivity or specificity of the Afirma Gene Expression Classifier in cytologically indeterminate thyroid nodules? J Clin Endocrinol Metab 98:E761–768 [DOI] [PubMed] [Google Scholar]
- 3.Witt RL, Ferris RL, Pribitkin EA, Sherman SI, Steward DL, Nikiforov YE. 2013. Diagnosis and management of differentiated thyroid cancer using molecular biology. Laryngoscope 123:1059–1064 [DOI] [PubMed] [Google Scholar]
- 4.Ali SZ. 2011. Thyroid cytopathology: Bethesda and beyond. Acta Cytol 55:4–12 [DOI] [PubMed] [Google Scholar]
- 5.Yip L, Ferris RL. 2014. Clinical application of molecular testing of fine-needle aspiration specimens in thyroid nodules. Otolaryngol Clin North Am 47:557–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yip L, Wharry LI, Armstrong MJ, Silbermann A, McCoy KL, Stang MT, Ohori NP, LeBeau SO, Coyne C, Nikiforova MN, Bauman JE, Johnson JT, Tublin ME, Hodak SP, Nikiforov YE, Carty SE. 2014. A clinical algorithm for fine-needle aspiration molecular testing effectively guides the appropriate extent of initial thyroidectomy. Ann Surg 260:163–168 [DOI] [PubMed] [Google Scholar]
- 7.Cibas ES, Baloch ZW, Fellegara G, LiVolsi VA, Raab SS, Rosai J, Diggans J, Friedman L, Kennedy GC, Kloos RT, Lanman RB, Mandel SJ, Sindy N, Steward DL, Zeiger MA, Haugen BR, Alexander EK. 2013. A prospective assessment defining the limitations of thyroid nodule pathologic evaluation. Ann Intern Med 159:325–332 [DOI] [PubMed] [Google Scholar]
- 8.Houlton JJ, Sun GH, Fernandez N, Zhai QJ, Lucas F, Steward DL. 2011. Thyroid fine-needle aspiration: does case volume affect diagnostic yield and interpretation? Arch Otolaryngol Head Neck Surg 137:1136–1139 [DOI] [PubMed] [Google Scholar]
- 9.Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. 2012. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol 56:333–339 [DOI] [PubMed] [Google Scholar]
- 10.Baloch ZW, Cibas ES, Clark DP, Layfield LJ, Ljung BM, Pitman MB, Abati A. 2008. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faquin WC, Baloch ZW. 2010. Fine-needle aspiration of follicular patterned lesions of the thyroid: diagnosis, management, and follow-up according to National Cancer Institute (NCI) recommendations. Diagn Cytopathol 38:731–739 [DOI] [PubMed] [Google Scholar]
- 12.Baloch ZW, LiVolsi VA. 2002. Intraoperative assessment of thyroid and parathyroid lesions. Semin Diagn Pathol 19:219–226 [PubMed] [Google Scholar]
- 13.Wang CC, Friedman L, Kennedy GC, Wang H, Kebebew E, Steward DL, Zeiger MA, Westra WH, Wang Y, Khanafshar E, Fellegara G, Rosai J, Livolsi V, Lanman RB. 2011. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid 21:243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohori NP, Nikiforova MN, Schoedel KE, LeBeau SO, Hodak SP, Seethala RR, Carty SE, Ogilvie JB, Yip L, Nikiforov YE. 2010. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance.” Cancer Cytopathol 118:17–23 [DOI] [PubMed] [Google Scholar]
- 15.Ohori NP, Schoedel KE. 2011. Variability in the atypia of undetermined significance/follicular lesion of undetermined significance diagnosis in the Bethesda System for Reporting Thyroid Cytopathology: sources and recommendations. Acta Cytol 55:492–498 [DOI] [PubMed] [Google Scholar]
- 16.Ho AS, Sarti EE, Jain KS, Wang H, Nixon IJ, Shaha AR, Shah JP, Kraus DH, Ghossein R, Fish SA, Wong RJ, Lin O, Morris LG. 2014. Malignancy rate in thyroid nodules classified as Bethesda Category III (AUS/FLUS). Thyroid 24:832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Schnadig V, Logrono R, Wasserman PG. 2007. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer 111:306–315 [DOI] [PubMed] [Google Scholar]
- 18.Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA, Moore FD, Jr, Kim BW, Nose V, Marqusee E, Larsen PR, Alexander EK. 2007. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 111:508–516 [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury J, Das S, Maji D. 2008. A study on thyroid nodules: diagnostic correlation between fine needle aspiration cytology and histopathology. J Indian Med Assoc 106:389–390 [PubMed] [Google Scholar]
- 20.Nikiforov YE. 2011. Molecular diagnostics of thyroid tumors. Arch Pathol Lab Med 135:569–577 [DOI] [PubMed] [Google Scholar]
- 21.Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, Friedman L, Kloos RT, LiVolsi VA, Mandel SJ, Raab SS, Rosai J, Steward DL, Walsh PS, Wilde JI, Zeiger MA, Lanman RB, Haugen BR. 2012. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. New Engl J Med 367:705–715 [DOI] [PubMed] [Google Scholar]
- 22.Udelsman R, Westra WH, Donovan PI, Sohn TA, Cameron JL. 2001. Randomized prospective evaluation of frozen-section analysis for follicular neoplasms of the thyroid. Ann Surg 233:716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCoy KL, Carty SE, Armstrong MJ, Seethala RR, Ohori NP, Kabaker AS, Stang MT, Hodak SP, Nikiforov YE, Yip L. 2012. Intraoperative pathologic examination in the era of molecular testing for differentiated thyroid cancer. J Am Coll Surg 215:546–554 [DOI] [PubMed] [Google Scholar]
- 24.Nikiforov YE, Nikiforova MN. 2011. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol 7:569–580 [DOI] [PubMed] [Google Scholar]
- 25.Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN. 2009. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 94:2092–2098 [DOI] [PubMed] [Google Scholar]
- 26.Mehta V, Nikiforov YE, Ferris RL. 2013. Use of molecular biomarkers in FNA specimens to personalize treatment for thyroid surgery. Head Neck 35:1499–1506 [DOI] [PubMed] [Google Scholar]
- 27.Das DK, Al-Waheeb SK, George SS, Haji BI, Mallik MK. 2013. Contribution of immunocytochemical stainings for galectin-3, CD44, and HBME1 to fine-needle aspiration cytology diagnosis of papillary thyroid carcinoma. Diagn Cytopathol 42:498–505 [DOI] [PubMed] [Google Scholar]
- 28.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V. 2013. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309:1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong MJ, Huaitao Y, Yip L, Ohori PN, McCoy KL, Stang MT, Hodak SP, Nikiforova MN, Carty SE, Nikiforov YE. 2014. PAX8/PPARγ rearrangement in thyroid nodules predicts follicular-pattern carcinomas, in particular the encapsulated follicular variant of papillary carcinoma. Thyroid 24:1369–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, Yip L, Seethala RR, Tublin ME, Stang MT, Coyne C, Johnson JT, Stewart AF, Nikiforova MN. 2011. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab 96:3390–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yip L, Farris C, Kabaker AS, Hodak SP, Nikiforova MN, McCoy KL, Stang MT, Smith KJ, Nikiforov YE, Carty SE. 2012. Cost impact of molecular testing for indeterminate thyroid nodule fine-needle aspiration biopsies. J Clin Endocrinol Metab 97:1905–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Robinson KA, Anton B, Saldanha IJ, Ladenson PW. 2011. Cost-effectiveness of a novel molecular test for cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab 96:E1719–1726 [DOI] [PubMed] [Google Scholar]
- 33.Adeniran AJ, Zhu Z, Gandhi M, Steward DL, Fidler JP, Giordano TJ, Biddinger PW, Nikiforov YE. 2006. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol 30:216–222 [DOI] [PubMed] [Google Scholar]
- 34.Moses W, Weng J, Sansano I, Peng M, Khanafshar E, Ljung BM, Duh QY, Clark OH, Kebebew E. 2010. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg 34:2589–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cantara S, Capezzone M, Marchisotta S, Capuano S, Busonero G, Toti P, Di Santo A, Caruso G, Carli AF, Brilli L, Montanaro A, Pacini F. 2010. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab 95:1365–1369 [DOI] [PubMed] [Google Scholar]
- 36.Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, Gooding WE, Hodak SP, LeBeau SO, Ohori NP, Seethala RR, Tublin ME, Yip L, Nikiforova MN. 2014. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer 120:3627–3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chudova D, Wilde JI, Wang ET, Wang H, Rabbee N, Egidio CM, Reynolds J, Tom E, Pagan M, Rigl CT, Friedman L, Wang CC, Lanman RB, Zeiger M, Kebebew E, Rosai J, Fellegara G, LiVolsi VA, Kennedy GC. 2010. Molecular classification of thyroid nodules using high-dimensionality genomic data. J Clin Endocrinol Metab 95:5296–5304 [DOI] [PubMed] [Google Scholar]
- 38.Harrell RM, Bimston DN. 2013. Surgical utility of Afirma: effects of high cancer prevalence and oncocytic cell types in patients with indeterminate thyroid cytology. Endocr Pract 20:364–369 [DOI] [PubMed] [Google Scholar]
- 39.Walsh PS, Wilde JI, Tom EY, Reynolds JD, Chen DC, Chudova DI, Pagan M, Pankratz DG, Wong M, Veitch J, Friedman L, Monroe R, Steward DL, Lupo MA, Lanman RB, Kennedy GC. 2012. Analytical performance verification of a molecular diagnostic for cytology-indeterminate thyroid nodules. J Clin Endocrinol Metab 97:E2297–2306 [DOI] [PubMed] [Google Scholar]
- 40.Dedhia PH, Rubio GA, Cohen MS, Miller BS, Gauger PG, Hughes DT. 2014. Potential effects of molecular testing of indeterminate thyroid nodule fine needle aspiration biopsy on thyroidectomy volume. World J Surg 38:634–638 [DOI] [PubMed] [Google Scholar]
- 41.McIver B, Castro MR, Morris JC, Bernet V, Smallridge R, Henry M, Kosok L, Reddi H. 2014. An Independent Study of a Gene Expression Classifier (Afirma) in the Evaluation of Cytologically Indeterminate Thyroid Nodules. J Clin Endocrinol Metab 99:4069–4077 [DOI] [PubMed] [Google Scholar]
- 42.Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. 2013. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab 98:E1852–E1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly LM, Barila G, Liu P, Evdokimova VN, Trivedi S, Panebianco F, Gandhi M, Carty SE, Hodak SP, Luo J, Dacic S, Yu YP, Nikiforova MN, Ferris RL, Altschuler DL, Nikiforov YE. 2014. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A 111:4233–4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tee YY, Lowe AJ, Brand CA, Judson RT. 2007. Fine-needle aspiration may miss a third of all malignancy in palpable thyroid nodules: a comprehensive literature review. Ann Surg 246:714–720 [DOI] [PubMed] [Google Scholar]
- 45.Wiseman SM, Haddad Z, Walker B, Vergara IA, Sierocinski T, Crisan A, Ghadessi M, Dao P, Zimmermann B, Triche TJ, Erho N, Davicioni E. 2013. Whole-transcriptome profiling of thyroid nodules identifies expression-based signatures for accurate thyroid cancer diagnosis. J Clin Endocrinol Metab 98:4072–4079 [DOI] [PubMed] [Google Scholar]
- 46.Rossi ED, Zannoni GF, Lombardi CP, Vellone VG, Moncelsi S, Papi G, Pontecorvi A, Fadda G. 2012. Morphological and immunocytochemical diagnosis of thyroiditis: comparison between conventional and liquid-based cytology. Diagn Cytopathol 40:404–409 [DOI] [PubMed] [Google Scholar]
- 47.Dettmer M, Vogetseder A, Durso MB, Moch H, Komminoth P, Perren A, Nikiforov YE, Nikiforova MN. 2013. MicroRNA expression array identifies novel diagnostic markers for conventional and oncocytic follicular thyroid carcinomas. J Clin Endocrinol Metab 98:E1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikiforov YE. 2008. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol 21:S37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivera M, Ricarte-Filho J, Tuttle RM, Ganly I, Shaha A, Knauf J, Fagin J, Ghossein R. 2010. Molecular, morphologic, and outcome analysis of thyroid carcinomas according to degree of extrathyroid extension. Thyroid 20:1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giordano T, for The Cancer Genome Atlas (TCGA) Research Network 2014. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cancer Genome Atlas Network 2015. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517:576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]