Abstract
Background
Malaria and intestinal helminths co-infection are major public health problems particularly among school age children in Nigeria. However the magnitude and possible interactions of these infections remain poorly understood. This study determined the prevalence, impact and possible interaction of Plasmodium falciparum and intestinal helminths co-infection among school children in rural communities of Kwara State, Nigeria.
Methods
Blood, urine and stool samples were collected from 1017 primary school pupils of ages 4–15 years. Stool samples were processed using both Kato-Katz and formol-ether concentration techniques and microscopically examined for intestinal helminths infection. Urine samples were analyzed using sedimentation method for Schistosoma haematobium. Plasmodium falciparum was confirmed by microscopy using thick and thin blood films methods and packed cell volume (PCV) was determined using hematocrit reader. Univariate analysis and chi-square statistical tests were used to analyze the data.
Results
Overall, 61.2% of all school children had at least an infection of either P. falciparum, S. haematobium, or intestinal helminth. S. haematobium accounted for the largest proportion (44.4%) of a single infection followed by P. falciparum (20.6%). The prevalence of malaria and helminth co-infection in the study was 14.4%. Four species of intestinal helminths were recovered from the stool samples and these were hookworm (22.5%), Hymenolepis species (9.8%), Schistosoma mansoni (2.9%) and Enterobius vermicularis (0.6%). The mean densities of P. falciparum in children co-infected with S. haematobium and hookworm were higher compared to those infected with P. falciparum only though not statistically significant (p = 0.062). The age distribution of both S. haematobium (p = 0.049) and hookworm (p = 0.034) infected children were statistically significant with the older age group (10–15 years) recording the highest prevalence of 47.2% and 25% respectively. Children who were infected with S. haematobium (RR = 1.3) and hookworm (RR = 1.4) have equal chances of being infected with P. falciparum as children with no worm infection. On the other hand children infected with Hymenolepis spp. (p<0.0001) are more likely to be infected with P. falciparum than Hymenolepis spp. uninfected children (RR = 2.0)
Conclusions
These findings suggest that multiple parasitic infections are common in school age children in rural communities of Kwara State Nigeria. The Hymenolepis spp. induced increase susceptibility to P. falciparum could have important consequences on how concurrent infections affect the expression or pathogenesis of these infections.
Author Summary
Malaria, schistosomiasis and intestinal helminths are parasitic diseases responsible for high morbidity and mortality in most tropical areas of the world. These parasitic diseases are common in Nigeria where people are co-infected with more than one parasite. This study investigate the prevalence and impact of these parasite co-infections in school age children in rural communities of Kwara State Nigeria. The result shows that over 61.0% of the school children had at least an infection of either P. falciparum, S. haematobium, or intestinal helminths with S. haematobium and P. falciparum accounting for the largest proportion of a single infection with 44.4% and 20.6% respectively. Children who were infected with S. haematobium (RR = 1.3) and hookworm (RR = 1.4) have equal chances of being infected with P. falciparum as children when compared with children with no infection. On the other hand children infected with Hymenolepis spp. (p<0.0001) have a higher chance of being infected with P. falciparum when compared to uninfected children (RR = 2.0). These findings suggest that Hymenolepis spp. may be associated with increase susceptibility to P. falciparum in this population of Nigeria children.
Introduction
Malaria and helminth infection are the most important public-health problems affecting children in Sub-Saharan Africa [1]. It is estimated that over a third of the world's population, mainly those individuals living in the tropics and subtropics, are infected by parasitic intestinal helminths or one or more of the species of Plasmodium [2]. Malaria caused by Plasmodium falciparum is responsible for a high burden of disease and a loss of growth in endemic countries estimated to be as high as 1.3% of gross domestic product per year [3]. Helminths infections on the other hand though not as deadly as falciparum malaria infection are associated with high morbidity and mortality occurring in sub-Saharan Africa [4]. Different environmental factors and host related activities influences the distribution of the different Intestinal helminths that includes nematodes trematodes and cestodes which make up these multiple infections [5,6]. These factors include poverty, environmental contamination with infected faeces containing helminth eggs, water bodies, inadequately cooked foods, lack of effective preventive measures [7] and immunity of the host [8].
Malaria and helminths infections are widespread and they both have similar geographical and overlapping distribution in developing countries with the major consequence of the co-infection being anaemia [9,3]. The major soil transmitted helminths (Ascaris lumbricoides, hookworm and Trichuris trichiura), coupled with schistosomiasis are responsible for more than 40% of the worldwide morbidity from all tropical infections, excluding malaria [10]. Children co-infected with these parasites have been shown to have hampered cognitive and physical development that leads to reduced learning and school achievements and are also prone to increased susceptibility to other infections [11,12]. Concomitant parasitic infections could induce modifications of the specific immune response to each pathogen and thus leading to modification of clinical expression [13]. Studies have shown that helminths can either protect [14,15] or worsen [16,17] malaria severity and young children from rural areas are the most affected.
In Nigeria, falciparum malaria and helminths infections are reportedly endemic and pose a significant health problem among children [18,19]. They are particularly more prevalent in rural communities and are closely associated with poverty [20,21]. Studies have shown that individuals co-infected with more than one parasite species are at risk of increased morbidity as well as at a risk of developing frequent and more severe disease due to interactions among the infecting parasite species [22,23]. Despite existence of contrasting evidence on the interaction of helminth and malaria infection, more results have pointed to the fact that individuals infected by helminth are more likely to develop malaria than helminth free individuals [24,17]. Considering the limited number of studies on interactions between malaria and helminth co-infections in human populations, the present study being the first study in this study area was undertaken to investigate the prevalence and impact of P. falciparum malaria parasitaemia and helminth co-infection in school children living in a setting where malaria is endemic. The results of this study could provide valuable information to local health authorities for improving existing control strategies.
Methods
Ethical considerations
The study was approved by the Kwara State ministry of Health Ethical Committee (MOH/KS/777/41). Before sample collection meetings were held with community leaders, teachers and community members. The objectives of the study including the study procedures, samples to be taken, study benefits, potential risks and discomforts were explained. Informed consent for all children who participated in the study was sought from parents and legal guardians after they have been clearly informed about the study. Parental consent given from parents/guardians were verbal. The ethical committee approved the use of oral consent as most parents were illiterates. Children were also requested to give consent and were informed of their right to refuse to participate in the study and to withdraw at any time during the study without jeopardizing their right of access to other health services. Consent was recorded as ‘yes’ or ‘no’ on an individual form designed for sample collection. The pains and the precautions of procedures such as collection of blood samples were fully explained to parents and children. Newly opened needle and syringe were used for each child. Before samples were collected, demographic data such as sex, age, weight, height and name of subject were recorded. Subjects diagnosed with malaria or helminths were treated appropriately free of charge according to national guidelines. Identification numbers were used instead of children names and information collected were kept confidential.
The study area, population and design
This cross-sectional study was conducted among primary and secondary schools in Pategi and Lafiagi communities in Nigeria. Pategi and Lafiagi are located in Pategi and Edu Local Government Areas (LGAs) in Kwara State, Nigeria. Population of Pategi is 110,852 people while that of Lafiagi is 102,780 people [25]. Both communities fall into stable malaria transmission zone where malaria is present throughout the year with a marked increase during the rainy season which normally runs from April to September. The towns stands on higher level and the soil can be described as well drained, moderately leached and with moderate humus content. Major occupations include farming (rice farming), fishing and petty trading. The study was carried out from October 2012 to May 2013, which spanned through the dry and rainy season. All school children who were willing to be part of the study were included in the study.
Sampling procedure
Detection and quantification of malaria parasites
About 2 ml of blood samples were collected by venous blood for the determination of P. falciparum parasitemia. Thick blood films were prepared, air dried, Giemsa-stained, and observed under the microscope for identification and quantification of malaria parasites. Malaria parasites were counted against 200 leukocytes, and counts were expressed as the number of parasites per microliter of blood, assuming an average leukocyte count of 8,000 cells/μl of blood [26].
Determination of packed cell volume
For Packed Cell Volume (PCV), micro hematocrit tubes filled with blood were centrifuged in a micro-hematocrit rotor for 5 minutes at 10,000g. The tubes were placed in the micro-hematocrit reader and children with PCV values ≤ 31% were considered as anaemic, which was further classified as mild (21–30%), moderate (15–20%), or severe (≤15%) [27].
Detection and quantification of helminths
Clean plastic containers were distributed for both stool and urine collection, and instructions were given for proper collection. Instructions were given to collect only terminal drops of urine The formol-ether concentration technique and Kato-Katz thick smear technique were used for quantitative determination of helminths ova [28]. Stool samples were processed within 12 hours of collection and examined microscopically within 1 hour of preparation to avoid missing hookworm ova. The intensity of infection was expressed as the number of eggs per gram of faeces (epg). Water—or urine-contaminated stools were rejected. The number of helminth eggs were counted and multiplied by 24 in order to quantify the number of eggs per gram (epg) of faeces. To ensure consistency of the result and as a form of quality control, 20% of the slides were randomly selected and read again [29].
Urine samples were analyzed using sedimentation method. Samples were left to stand on the bench for about 30 minutes. Afterwards the topmost part of the urine was discarded leaving about 10 ml in the bottle. The contents of each bottle was mixed thoroughly with the sediment and was transferred into a 20 ml centrifuge tube. The tubes were then centrifuged at 1000 r/min for 2 min. The supernatant was discarded and the residue was put on a clean glass slide and examined under 10X objective lens of the microscope. Intensity of infection was estimated as number eggs per 10ml of urine.
Statistical analysis
Data were double-entered and cross-checked in Microsoft Excel version 2013. Statistical analysis was done using SPSS version 16 for windows. For univariate analysis, frequencies were compared using the Fisher's exact tests. Prevalence of malaria, helminth and co-infection and gender were compared using χ2 tests. Relative Risk (RR) were calculated for risk estimation between P. falciparum and helminths. Logistic regression was carried out to investigate the relationship between anemia and occurrence of parasitic infection in the study population. P-value ≤ 0.05 was considered significant during the analysis
Results
A total of 1017 blood, urine and stool samples collected from primary school children aged 4 to 15 years were microscopically investigated in other to determine the prevalence and impact of S. haematobium, intestinal helminths and malaria co-infection. The mean age of the study population was 9.52 ± 1.904 years while the mean height was 124.19 ± 2.54 cm and the mean weight was 25.34 ± 4.26 kg.
The prevalence and intensities of all the parasites seen in the blood, stool and urine samples of the study subjects are shown in Table 1. Overall, 61.2% of all the school children had at least an infection of either P. falciparum, Schistosoma haematobium, hookworm, Hymenolepis specie or Enterobious vamicularis. S. haematobium accounted for the largest proportion (44.4%) of a single infection followed by P. falciparum parasitaemia (20.6%). Hookworm and Hymenolepis spp. had a prevalence of 22.5% and 9.8% respectively while E. vermicularis had the lowest prevalence of 0.6%. The intensities of infection expressed as geometric mean parasite count of positive samples is shown in Table 1.
Table 1. Overall prevalence and infection intensities (expressed as geometric mean parasite count of positive samples) of parasitic infections in school children in Kwara State Nigeria.
| Characteristics n = 1017 | P. falciparum | S. haematobium | Hookworm | Hymenolepis spp | E. vermicularis |
|---|---|---|---|---|---|
| No. Infected | 209 | 452 | 229 | 100 | 6 |
| Prevalence (%) | 20.6 | 44.4 | 22.5 | 9.8 | 0.6 |
| Geo Mean Parasite density | 316.5p/μl | 52.3/ml | 990.3/g | 476.5/g | 200/g |
| Maximum density | 14040/μl | 300/ml | 3000/g | 2200/g | 200/g |
The mean age of the study population is 9.5 ± 1.9 years. Table 2 shows the distribution of parasitic infection by age in the studied population. For P. falciparum parasitaemia, the youngest age group (4–9 years) had the highest prevalence of 22.7% but the difference was not statistically significant (p = 0.14). The distribution of S. haematobium by age was slightly significant (p = 0.049) with the older age group (10–15 years) recording the highest prevalence (47.2%). Distribution of hookworm by age also showed a significant difference (p = 0.034) with the older age group recording the highest prevalence (25%) of infection. No significant difference was observed in the age distribution of Hymenolepis spp. and E. vermicularis in the study population
Table 2. Prevalence of single parasitic infection among children by age.
| Age group | No. Exam | Prevalence of single infection (%) | ||||
|---|---|---|---|---|---|---|
| PF | SH | HW | HN | EV | ||
| 4–9 | 445 | 101 (22.7) | 182 (40.9) | 86 (19.3) | 45 (10.1) | 3 (0.7) |
| 10–15 | 572 | 108 (18.9) | 270 (47.2) | 143 (25.0) | 55 (9.6) | 3 (0.5) |
| Total | 1017 | 209 (20.6) | 452 (44.4) | 229 (22.5) | 100 (9.8) | 6 (0.6) |
| p-value | 0.14 | 0.049* | 0.034* | 0.83 | 0.73 | |
*Significant p<0.05
Key: PF = P. falciparum; SH = S. haematobium; HW = Hookworm; HN = Hymenolepis species, EV = Enterobious vermicularis
The distribution of parasitic infection according to gender among the school children is presented in Table 3. A total of 519 (51.0%) males and 498 (49.0%) females were enrolled in the study. In all, males were generally more infected with all the intestinal helminths and the difference was not significant. Similarly for P. falciparum parasitaemia, female (21.1%) were slightly more infected than males (20.0%) but the difference was not statistically significant (p = 0.69).
Table 3. Prevalence of single parasitic infection among children by sex.
| Sex | No. Exam | Prevalence of single infection (%) | ||||
|---|---|---|---|---|---|---|
| PF | SH | HW | HN | EV | ||
| Male | 519 | 104 (20.0) | 244 (47.0) | 130 (25.0) | 60 (11.6) | 4 (0.8) |
| Female | 498 | 105 (21.1) | 208 (41.8) | 99 (19.9) | 40 (8.1) | 2 (0.4) |
| Total | 1017 | 209 (20.6) | 450 (44.3) | 229 (22.5) | 102 (10.0) | 6 (0.6) |
| p-value | 0.69 | 0.10 | 0.05 | 0.07 | 0.687 | |
Key: PF = P. falciparum; SH = S. haematobium; HW = Hookworm; HN = Hymenolepis species, EV = Enterobious vermicularis
The prevalence of polyparasitism based on age and sex in the study population is shown in Table 4. Polyparasitism was generally higher among the male children but the difference was not statistically significant. No significant difference was also observed when total polyparasitism was compared between the age groups (p = 0.20)
Table 4. Prevalence of children infected with double infection by age and sex.
| Age group | Gender | No. Exam | PF+SH(%) | PF+HW(%) | PF+HN(%) | SH+HW(%) | SH+HN (%) | HW+HN (%) |
|---|---|---|---|---|---|---|---|---|
| 4–9 | Male | 221 | 28 (12.7) | 13 (5.9) | 12 (5.4) | 34 (15.4) | 14 (6.3) | 8 (3.6) |
| Female | 225 | 26 (11.6) | 12 (5.3) | 9 (4.0) | 26 (11.6) | 3 (1.3) | 3 (1.3) | |
| Total | 446 | 54 (12.1) | 25 (5.6) | 21 (4.7) | 60 (13.5) | 17 (3.8) | 11 (2.5) | |
| 10–15 | Male | 298 | 29 (9.7) | 22 (7.4) | 9 (3.0) | 58 (19.5) | 16 (5.4) | 11 (3.7) |
| Female | 274 | 23 (8.4) | 14 (5.1) | 12 (4.4) | 46 (16.8) | 16 (5.9) | 9 (.3) | |
| Total | 572 | 52 (9.1) | 36 (6.3) | 21 (3.7) | 104 (18.2) | 32 (5.6) | 20 (3.5) | |
| Overall | Male | 519 | 57 (11.0) | 35 (6.7) | 21 (4.0) | 92 (17.7) | 30 (5.8) | 19 (3.7) |
| Female | 498 | 49 (9.8) | 26 (5.2) | 21 (4.2) | 72 (14.5) | 20 (4.0) | 13 (2.6) | |
| Total | 1017 | 106 (10.4) | 61 (6.0) | 41 (4.0) | 164 (16.1) | 60 (5.9) | 32 (3.1) |
Key: PF = P. falciparum; SH = S. haematobium; HW = Hookworm; HN = Hymenolepis species, EV = Enterobious vermicularis
The association between the presence of malaria parasitaemia and intestinal helminths among the study subjects is shown in Table 5. There was a statistically significant association between P, falciparum and S. haematobium (p = 0.04), P. falciparum and hookworm (p = 0.01) with a Relative Risk of 1.3 and 1.4 respectively. Children who were infected with S. haematobium (RR = 1.3) and hookworm (RR = 1.4) have equal chances of likely being infected with P. falciparum as children with no worm infection. On the other hand, children exposed to Hymenolepis spp. (p<0.0001) are more likely to be infected with P. falciparum than Hymenolepis spp. uninfected children (RR = 2.0)
Table 5. Associations between helminths and Plasmodium falciparum infection, according to helminth species.
| Helminth Infection | P. falciparum Infection (%) | Relative Risk (CI) | p-value | |
|---|---|---|---|---|
| Present n = 209 | Absent n = 808 | |||
| S. haematobium | 106 (50.7) | 346 (42.8) | 1.3 (1.0–1.6) | 0.04 |
| Hookworm | 61 (29.2) | 168 (20.8) | 1.4 (1.0–1.8) | 0.01 |
| Hymenolepis spp | 42 (20.1) | 58 (7.2) | 2.0 (1.6–2.7) | <0.0001 |
A logistic regression analysis was carried out to determine the occurrence of anemia among the school children using the single and double parasitic infection as predictors (Table 6). Plasmodium falciparum (P = <0.001), S. haematobium (p < 0.001) and hookworm (p < 0.001) infections showed strong significant association with anemia. Also the occurrence of P. falciparum + S. haematobium (P = <0.001), P. falciparum + hookworm (p = 0.004), S. haematobium + hookworm (P = <0.001) and P. falciparum + Hymenolepis spp. (P = <0.001) co- infections were strong predictors of the occurrence of anemia in the study population
Table 6. Logistic regression predicting the occurrence of anemia in relation to parasitic infection among the school children.
| Predictor | No. positive (%) | Odds | Wald χ2 | p-value | Odds Ratio |
|---|---|---|---|---|---|
| PF | 209 (20.6) | 1.417 | 61.697 | <0.001* | 4.126 |
| SH | 450 (44.3) | 0.907 | 32.363 | <0.001* | 2.476 |
| HW | 229(22.5) | 1.601 | 82.657 | <0.001* | 4.957 |
| HN | 102 (10.0) | 0.492 | 4.025 | 0.045* | 1.636 |
| PF+SH | 106 (10.4) | 1.009 | 15.109 | <0.001* | 2.744 |
| PF+HW | 61 (6.0) | 1.203 | 8.466 | 0.004* | 3.328 |
| SH+HW | 164 (16.1) | 1.724 | 73.282 | <0.001* | 5.606 |
| PF+HN | 41(4.0) | 1.562 | 15.422 | <0.001* | 4.768 |
| SH+HN | 60 (5.9) | -0.421 | 0.804 | 0.370 | 0.656 |
| HW+HN | 32 (3.1) | 0.680 | 1.390 | 0.238 | 1.973 |
*Significant at p<0.05
Key: PF = P. falciparum; SH = S. haematobium; HW = Hookworm; HN = Hymenolepis species, EV = Enterobious vermicularis
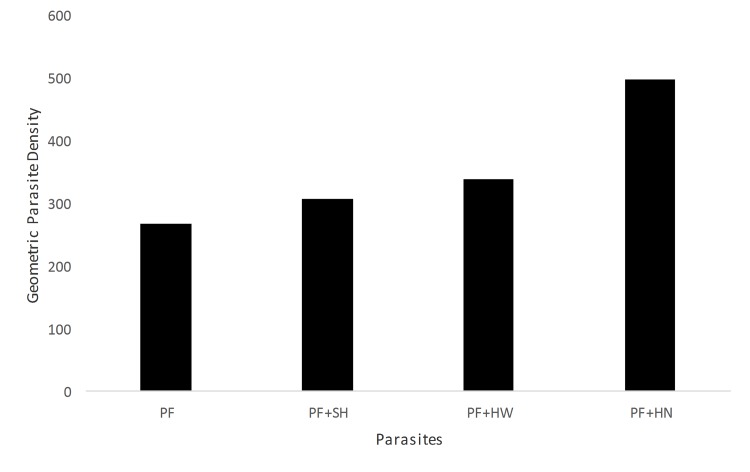
Children infected exclusively with P. falciparum alone had the lowest geometric mean parasite density (GMPD) per microliter of blood in the study population. Significantly high GMPD was observed among children co-infected with P. falciparum + Hymenolepis spp. as compared to those with P. falciparum alone. Also P. falciparum + hookworms and P. falciparum + S. haematobium co-infections also recorded a significantly higher GMPD in comparison to P. falciparum alone (p < 0.001) (Fig 1).
Fig 1. Geometric parasite density per microlitre of blood by type of infection.
PF vs PF+HN = p< 0.001; PF + HW Vs PF + SH = p< 0.001;Key: PF = P. falciparum; SH = S. haematobium; HW = Hookworm; HN = Hymenolepis species.
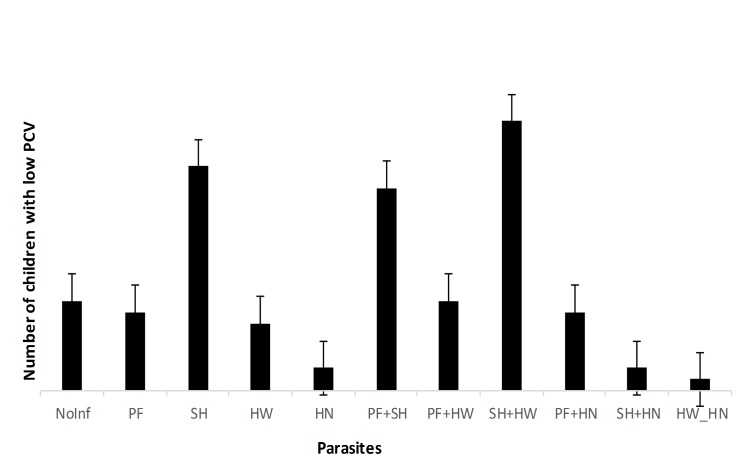
Fig 2 showed the proportion of children with low PCV with respect to the different parasites. Children infected with S. haematobium alone, co-infection of P. falciparum and S. haematobium, co-infection of S. haematobium and hookworm had significantly higher proportion of children with low PCV when compared to children without infection. None of the children in the study population presented with severe anemia while the prevalence of moderate and mild anemia was 1.2% and 30.0% respectively.
Fig 2. The proportion of children with anemia by type of infection.
Key: PF = P. falciparum; SH = S. haematobium; HW = Hookworm; HN = Hymenolepis species.
Discussion
This study suggests that underlying infection with Hymenolepis spp. may increase the chance of being infected with P. falciparum and also increase the geometric mean parasite count of P. falciparum. The implication of this is that Hymenolepis spp. modestly increases the degree of susceptibility against P. falciparum malaria among school children in rural communities of Ilorin, Nigeria. To the best of our knowledge, this work provides the first report of the prevalence of Hymenolepis spp. and its association to P. falciparum infection. Hymenolepis spp. is not a frequently encountered helminth infection in Nigeria. Previous authors have reported a higher prevalence of 18.3% in the 80s in the Niger Delta area of the country [30] while lower prevalence has been reported in other parts of the country [31]. Hymenolepis spp. often remain asymptomatic and severe infections can occur in individuals with more than 20,000 eggs per gram of feces. Symptoms of severe diseases include enteritis with or without diarrhea, abdominal pain, and loss of appetite. Children may experience more severe intestinal symptoms, which can include epilepsy, probably due to the toxic action that the products of the inflammation cause [32]. Further work on the immunological interaction will be needed to further elucidate the impact of this parasite in this population.
A near to equal association was observed between P. falciparum infection and S. haematobium infection and P. falciparum infection and hookworm infection. In many previous studies, observation for the interaction between P. falciparum infection and S. haematobium infection had yielded contrasting reports. While some studies reported increased susceptibility to P. falciparum infection in the presence of S. haematobium [16] others have shown protective effect of S. haematobium on P. falciparum [15]. These contrasting results have left important questions unanswered about the biological associations between P. falciparum and helminths. Explanations for these contrasting results have been given in terms of immunonological interactions and microgeographical variation [33]. The rates of co-infection may be influenced not only on chance, but also on the spatial distribution of environmental conditions that favor the transmission of multiple species [7,34]. Also immunological interactions and common factors that affect genetic susceptibility or host behavior can also influence co-infection rate [34]. In all, this study demonstrated that malaria, schistosomiasis and soil-transmitted helminth infections are of public health importance among primary schools pupils in Pategi and Lafiagi, Kwara State, Nigeria and co-infections of these parasites are common and interactions could have a negative effect on the infected school children. The findings of P. falciparum, schistosomiasis and helminth co-infection observed in this study are supported by other previous studies in Sub-Saharan Africa [27,22].
The most prevalent parasites in this study were S. haematobioum. P. falciparum, hookworm and Hymenolepis spp. The prevalence of urinary schistosomiasis (44.4%) reported in this study suggests that Pategi and Lafiagi LGAs of Kwara State are endemic for urinary schistosomiasis. Earlier study in Bida Niger State, Nigeria a community that shares both socio-cultural and geographical attributes with Pategi and Lafiagi showed high prevalence of urinary schistosomiasis [35]. The prevalence of co-infection of malaria and helminths (14.4%) observed is an indication of high polyparasitism among school children in the study area. Co-infection of P. falciparum with S. haematobium was the highest, followed by P. falciparum and hookworm. Both falciparum malaria and helminths have similar geographical distribution and it is estimated that over a third of the world's population, mainly those living in the tropics and subtropics are infected [3]. Factors that may support co-infections which were present in the area of study include the abundant presence of mosquito and snail vectors, temperature, humidity, stagnant bodies of water, bushy environment, unhygienic environment and poor drainage. Others include poor sanitary disposal, open air defecation, usage of night soil as fertilizer in farming, barefoot walking which can predispose subjects to penetration of infective larvae of hookworm.
The observed prevalence of both S. haematobium and hookworm are significant with respect to age, with the older age group (10–15 years) being more infected. This reflects the fact that older age groups are involved in more water activity contact and also have higher duration of exposure to infection of other soil transmitted helminths. For P. falciparum parasitaemia, the prevalence was higher among the younger age group. It was previously observed that the majority of malaria infections in individuals living in endemic regions are asymptomatic with the young children bearing the highest burden of disease and carrying asymptomatic infections for most of the time [36,18]. The decline of P. falciparum parasite prevalence with age, observed in this study is in agreement with our previous study [18] and it is the characteristic of both symptomatic and asymptomatic falciparum infection in endemic regions [37,38]. Ascaris lumbricoides which was one of the commonest intestinal parasite in Nigeria was not detected in the current study. This observation has been previously made by some authors who have reported this specie to be rare in some areas in Sub-Saharan Africa [22,4].
About 39% of the children in the study were classified as anemic with majority being moderately anemic. Many studies have reported anemia as one of the major health problems resulting from malaria [39,40], hookworm and S. haematobium [41,4] infection in Nigeria. The highest proportion of school children with low PCV was observed among those co-infected with hookworm and schistosomiasis followed by schistosomiasis alone and P. falciparum schistosomiasis co-infected school children. Also the logistic regression analysis predicts that the occurrence of anemia among the school children is strongly influenced by the presence of single or co-infection of P. falciparum, hookworm and S. haematobium. These observations further confirms previous reports of a positive relationship that exist between parasitic infections and anemia and also the possible synergistic interaction of these parasites as a strong etiology of anemia [40,41,42].
Higher mean geometric parasite density of P. falciparum was observed in co-infection with helminths when compared to P. falciparum alone. This observation has been previously reported by other authors [43,44]. Higher mean geometric parasite density was observed in individuals simultaneously co-infected with both P. falciparum and S. haematobium, P. falciparum and hookworms or P. falciparum and Hymenolepis spp. It thus appears that S. haematobium, hookworm or Hymenolepis spp. are associated with higher prevalence of P. falciparum in this population of Nigeria children.
There is an urgent call by WHO and its collaborating partners to scale up interventions to control and eliminate Neglected Tropical Diseases (NTDs) in the WHO African region. Schistosomiasis and soil transmitted helminths are among the NTDs targeted for elimination. In other for this to materialize, the affected areas must be identified and its burden must be assessed. This remains a crucial and urgent roadmap in other to achieve complete control and elimination of the NTDs in Africa. This study is therefore important for policy makers and stakeholders in the control of NTDs in Africa.
Conclusively, high prevalence of co-infection of P. falciparum, S. haematobium and intestinal helminths was observed among school children in Pategi and Lafiagi communities of Kwara State Nigeria. This is the first study to document the prevalence of these co-infections and also highlighting the impact of Hymenolepis spp. and P. falciparum co-infection among children in the middle belt of Nigeria. The effort of Kwara State Government in reducing the morbidity caused by S. haematobium and intestinal helminths through yearly mass deworming with Albendazole and praziquantel treatment should be complimented with adequate health education. Toilets should be provided in the schools with water for hand washing and personal hygiene enforced in other to create attitudes that will break the cycle of this infections.
Supporting Information
(DOC)
Acknowledgments
The authors are grateful to the Kwara State Ministries of Health and the Edu and Lafiagi Local government areas of Kwara State for their permission and cooperation. We also appreciate the community leaders, the head teachers and the school teachers for their cooperation. Finally we thank community volunteers that worked with us.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Adegnika AA, Kremsner PG. Epidemiology of malaria and helminth interaction: a review from 2001 to 2011. Curr Opin HIV AIDS. 2012;7: 221–224. 10.1097/COH.0b013e3283524d90 [DOI] [PubMed] [Google Scholar]
- 2. Salazar-Castañon VH, Legorreta-Herrera M, Rodriguez-Sosa M. Helminth Parasites Alter Protection against Plasmodium Infection. BioMed Res Int. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434: 214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hotez PJ, Kamath A. Neglected Tropical Diseases in Sub-Saharan Africa: Review of Their Prevalence, Distribution, and Disease Burden. PLoS Negl Trop Dis. 2009;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brooker S, Clements ACA. Spatial heterogeneity of parasite co-infection: Determinants and geostatistical prediction at regional scales. Int J Parasitol. 2009;39: 591–597. 10.1016/j.ijpara.2008.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salim N, Knopp S, Lweno O, Abdul U, Mohamed A, Schindler T, et al. Distribution and Risk Factors for Plasmodium and Helminth Co-infections: A Cross-Sectional Survey among Children in Bagamoyo District, Coastal Region of Tanzania. PLoS Negl Trop Dis. 2015;9: e0003660 10.1371/journal.pntd.0003660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Booth M. The role of residential location in apparent helminth and malaria associations. Trends Parasitol. 2006;22: 359–362. [DOI] [PubMed] [Google Scholar]
- 8. Ouf EA, Ojurongbe O, Akindele AA, Sina-Agbaje OR, Van Tong H, Adeyeba AO, et al. Ficolin-2 levels and FCN2 genetic polymorphisms as a susceptibility factor in schistosomiasis. J Infect Dis. 2012;206: 562–570. 10.1093/infdis/jis396 [DOI] [PubMed] [Google Scholar]
- 9. Naing C, Whittaker MA, Nyunt-Wai V, Reid SA, Wong SF, Mak JW, et al. Malaria and soil-transmitted intestinal helminth co-infection and its effect on anemia: a meta-analysis. Trans R Soc Trop Med Hyg. 2013;107: 672–683. 10.1093/trstmh/trt086 [DOI] [PubMed] [Google Scholar]
- 10. Finkelstein JL, Schleinitz MD, Carabin H, McGarvey ST. Decision-Model Estimation of the Age-Specific Disability Weight for Schistosomiasis Japonica: A Systematic Review of the Literature. PLoS Negl Trop Dis. 2008;2: e158 10.1371/journal.pntd.0000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131: 649S–666S; discussion 666S–668S. [DOI] [PubMed] [Google Scholar]
- 12. Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, et al. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg. 2005;72: 540–548. [PMC free article] [PubMed] [Google Scholar]
- 13. Sokhna C, Le Hesran J-Y, Mbaye PA, Akiana J, Camara P, Diop M, et al. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar J. 2004;3: 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Briand V, Watier L, LE Hesran J-Y, Garcia A, Cot M. Coinfection with Plasmodium falciparum and schistosoma haematobium: protective effect of schistosomiasis on malaria in senegalese children? Am J Trop Med Hyg. 2005;72: 702–707. [PubMed] [Google Scholar]
- 15. Nacher M, Gay F, Singhasivanon P, Krudsood S, Treeprasertsuk S, Mazier D, et al. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol. 2000;22: 107–113. [DOI] [PubMed] [Google Scholar]
- 16. Le Hesran J-Y, Akiana J, Ndiaye EHM, Dia M, Senghor P, Konate L. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Trans R Soc Trop Med Hyg. 2004;98: 397–399. [DOI] [PubMed] [Google Scholar]
- 17. Spiegel A, Tall A, Raphenon G, Trape JF, Druilhe P. Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2003;97: 198–199. [DOI] [PubMed] [Google Scholar]
- 18. Ojurongbe O, Adegbayi AM, Bolaji OS, Akindele AA, Adefioye OA, Adeyeba OA. Asymptomatic falciparum malaria and intestinal helminths co-infection among school children in Osogbo, Nigeria. J Res Med Sci Off J Isfahan Univ Med Sci. 2011;16: 680–686. [PMC free article] [PubMed] [Google Scholar]
- 19. Ogungbamigbe TO, Ojurongbe OO, Ogunro PS, Olowe OA, Elemile PO. Prevalence and transmission pattern of Plasmodium falciparum infection in Osogbo metropolis, southwest, Nigeria. Afr J Med Med Sci. 2007;36: 305–310. [PubMed] [Google Scholar]
- 20. Ajayi I. O, Afonne C, Dada-Adegbola H, Falade C. O. Prevalence of Asymptomatic Malaria and Intestinal Helminthiasis Co-infection among Children Living in Selected Rural Communities in Ibadan Nigeria. Am J Epidemiol Infect Dis. 2015;3: 15–20. [Google Scholar]
- 21.Dada-Adegbola H, Oluwatoba O, Falade C. Asymptomatic malaria and intestinal helminth co-infection among children in a rural community in Southwest Nigeria. 2013;4: 6 pages. [DOI] [PMC free article] [PubMed]
- 22. Kinung’hi SM, Magnussen P, Kaatano GM, Kishamawe C, Vennervald BJ. Malaria and Helminth Co-Infections in School and Preschool Children: A Cross-Sectional Study in Magu District, North-Western Tanzania. PLoS ONE. 2014;9: e86510 10.1371/journal.pone.0086510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brooker S, Clements AC, Hotez PJ, Hay SI, Tatem AJ, Bundy DA, et al. The co-distribution of Plasmodium falciparum and hookworm among African schoolchildren. Malar J. 2006;5: 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nacher M, Singhasivanon P, Treeprasertsuk S, Vannaphan S, Traore B, Looareesuwan S, et al. Intestinal helminths and malnutrition are independently associated with protection from cerebral malaria in Thailand. Ann Trop Med Parasitol. 2002;96: 5–13. [DOI] [PubMed] [Google Scholar]
- 25.Kwara State—Cities By Population. In: 2014 Population List [Internet]. 2014. Available: http://www.populationlist.com/Kwara_State/30/2041103/state-population
- 26. Ojurongbe O, Ogungbamigbe TO, Fagbenro-Beyioku AF, Fendel R, Kremsner PG, Kun JF. Rapid detection of Pfcrt and Pfmdr1 mutations in Plasmodium falciparum isolates by FRET and in vivo response to chloroquine among children from Osogbo, Nigeria. Malar J. 2007;6: 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nkuo-Akenji TK, Chi PC, Cho JF, Ndamukong KKJ, Sumbele I. Malaria and helminth co-infection in children living in a malaria endemic setting of mount Cameroon and predictors of anemia. J Parasitol. 2006;92: 1191–1195. [DOI] [PubMed] [Google Scholar]
- 28. Idris MA, Al-Jabri AM. Usefulness of Kato-Katz and trichrome staining as diagnostic methods for parasitic infections in clinical laboratories. J Sci Res Med Sci Sultan Qaboos Univ. 2001;3: 65–68. [PMC free article] [PubMed] [Google Scholar]
- 29. Adefioye O, Efunshile A, Ojurongbe O, Akindele A, Adewuyi I, Bolaji O, et al. Intestinal Helminthiasis among School Children in Ilie, Osun State, Southwest, Nigeria. Sierra Leone J Biomed Res. 2011;3: 36–42. [Google Scholar]
- 30. Arene FO, Akabogu OA. Intestinal parasitic infections in pre-school children in the Niger Delta. J Hyg Epidemiol Microbiol Immunol. 1986;30: 99–102. [PubMed] [Google Scholar]
- 31. Agi PI. Pattern of infection of intestinal parasites in Sagbama community of the Niger Delta, Nigeria. West Afr J Med. 1995;14: 39–42. [PubMed] [Google Scholar]
- 32. Fortunato S, Castagna B, Monteleone MR, Pierro R, Cringoli G, Bruschi F. Parasite prevalence in a village in Burkina Faso: the contribution of new techniques. J Infect Dev Ctries. 2014;8: 670–675. 10.3855/jidc.3660 [DOI] [PubMed] [Google Scholar]
- 33. Mwangi TW, Bethony JM, Brooker S. Malaria and helminth interactions in humans: an epidemiological viewpoint. Ann Trop Med Parasitol. 2006;100: 551–570. 10.1179/136485906X118468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hillier SD, Booth M, Muhangi L, Nkurunziza P, Khihembo M, Kakande M, et al. Plasmodium falciparum and Helminth Coinfection in a Semiurban Population of Pregnant Women in Uganda. J Infect Dis. 2008;198: 920–927. 10.1086/591183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnabas B, Aliyu M, Gbate M, Obi P, Ezeako I. Prevalence of Schistosomiasis and Other Intestinal Helminth Parasites among School Children in Bida, Niger State, Nigeria. Eur J Sci Res. 2011;48: 621–626. [Google Scholar]
- 36. Greenwood BM. Asymptomatic malaria infections—do they matter? Parasitol Today Pers Ed. 1987;3: 206–214. [DOI] [PubMed] [Google Scholar]
- 37. Bruce MC, Donnelly CA, Packer M, Lagog M, Gibson N, Narara A, et al. Age- and species-specific duration of infection in asymptomatic malaria infections in Papua New Guinea. Parasitology. 2000;121 (Pt 3): 247–256. [DOI] [PubMed] [Google Scholar]
- 38. Njama-Meya D, Kamya MR, Dorsey G. Asymptomatic parasitaemia as a risk factor for symptomatic malaria in a cohort of Ugandan children. Trop Med Int Health. 2004;9: 862–868. [DOI] [PubMed] [Google Scholar]
- 39. Noland GS, Graves PM, Sallau A, Eigege A, Emukah E, Patterson AE, et al. Malaria prevalence, anemia and baseline intervention coverage prior to mass net distributions in Abia and Plateau States, Nigeria. BMC Infect Dis. 2014;14: 168 10.1186/1471-2334-14-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gayawan E, Arogundade ED, Adebayo SB. Possible determinants and spatial patterns of anaemia among young children in Nigeria: a Bayesian semi-parametric modelling. Int Health. 2014;6: 35–45. 10.1093/inthealth/iht034 [DOI] [PubMed] [Google Scholar]
- 41. Osazuwa F, Ayo OM, Imade P. A significant association between intestinal helminth infection and anaemia burden in children in rural communities of Edo state, Nigeria. North Am J Med Sci. 2011;3: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Getachew M, Yewhalaw D, Tafess K, Getachew Y, Zeynudin A. Anaemia and associated risk factors among pregnant women in Gilgel Gibe dam area, Southwest Ethiopia. Parasit Vectors. 2012;5: 296 10.1186/1756-3305-5-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mboera LEG, Senkoro KP, Rumisha SF, Mayala BK, Shayo EH, Mlozi MRS. Plasmodium falciparum and helminth coinfections among schoolchildren in relation to agro-ecosystems in Mvomero District, Tanzania. Acta Trop. 2011;120: 95–102. 10.1016/j.actatropica.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 44. Sangweme DT, Midzi N, Zinyowera-Mutapuri S, Mduluza T, Diener-West M, Kumar N. Impact of schistosome infection on Plasmodium falciparum Malariometric indices and immune correlates in school age children in Burma Valley, Zimbabwe. PLoS Negl Trop Dis. 2010;4: e882 10.1371/journal.pntd.0000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.