Abstract
BACKGROUND
Recovering upper-limb motor function has important implications for improving independence of patients with tetraplegia after traumatic spinal cord injury (SCI).
OBJECTIVE
To evaluate the feasibility, safety and effectiveness of robotic-assisted training of upper limb in a chronic SCI population.
METHODS
A total of 10 chronic tetraplegic SCI patients (C4 to C6 level of injury, American Spinal Injury Association Impairment Scale, A to D) participated in a 6-week wrist-robot training protocol (1 hour/day 3 times/week). The following outcome measures were recorded at baseline and after the robotic training: a) motor performance, assessed by robot-measured kinematics, b) corticospinal excitability measured by transcranial magnetic stimulation (TMS), and c) changes in clinical scales: motor strength (Upper extremity motor score), pain level (Visual Analog Scale) and spasticity (Modified Ashworth scale).
RESULTS
No adverse effects were observed during or after the robotic training. Statistically significant improvements were found in motor performance kinematics: aim (pre 1.17 ± 0.11 radians, post 1.03 ± 0.08 radians, p = 0.03) and smoothness of movement (pre 0.26 ± 0.03, post 0.31 ± 0.02, p = 0.03). These changes were not accompanied by changes in upper-extremity muscle strength or corticospinal excitability. No changes in pain or spasticity were found.
CONCLUSIONS
Robotic-assisted training of the upper limb over six weeks is a feasible and safe intervention that can enhance movement kinematics without negatively affecting pain or spasticity in chronic SCI. In addition, robot-assisted devices are an excellent tool to quantify motor performance (kinematics) and can be used to sensitively measure changes after a given rehabilitative intervention.
Keywords: Spinal cord injury, transcranial magnetic stimulation, robotic training, kinematics
1. Introduction
Following an upper motor neuron lesion such as stroke and spinal cord injury (SCI), survivors are often left with reduced voluntary muscle activation. Restoration or improvement of residual muscle control is of major importance in rehabilitation since this can improve functional independence and quality of life (Simpson, Eng, Hsieh, & Wolfe, 2012). While most literature focuses on post-stroke rehabilitation efficacy, available evidence suggests that motor training can improve motor function in SCI, greater than spontaneous recovery alone (Barbeau, Nadeau, & Garneau, 2006; Behrman & Harkema, 2007; Spooren, Janssen-Potten, Kerckhofs, & Seelen, 2009; Wirth, Van Hedel, & Curt, 2008). Recovery depends on a number of factors, including the extent of injury, post-injury medical care, surgical intervention, and the type of rehabilitative therapy (Lynskey, Belanger, & Jung, 2008).
Behavioral therapies have been shown to improve motor function, and usually those therapies have key elements of repetition and feedback. Rehabilitative therapies involving intense repetitive training, or massed practice have been shown to improve motor strength and function (Beekhuizen & Field-Fote, 2005). Although the mechanisms responsible for these modifications are not fully understood, activity-dependent brain and spinal plasticity is likely to play a major role (Lynskey et al., 2008).
Robot-assisted rehabilitation has been described as an effective method of promoting motor recovery compared to traditional physiotherapy alone due to its ability to deliver highly reproducible control movement sequences (Kadivar et al., 2011; Volpe et al., 2009). Robotic devices represent a sophisticated and interactive rehabilitation system, appealing for their ability to objectively quantify various aspects of movement (Edgerton & Roy, 2009; H. I. Krebs, Volpe, & Hogan, 2009; Volpe, Krebs, & Hogan, 2003). The most compelling clinical changes in stroke patients were observed after twelve-weeks of training (Lo et al., 2010), although kinematic changes can be observed with as little as six-weeks of training.
Despite the abundant literature supporting the efficacy of upper-limb robotic training in stroke population, there are a limited number of studies showing feasibility, safety and efficacy in chronic SCI population (Kadivar et al., 2011, 2012; H. Krebs et al., 2008; Sledziewski, Schaaf, & Mount, 2012; Yozbatiran et al., 2012). We proposed that chronic SCI patients with some residual upper-limb function should also be able to improve voluntary motor control with the same robotic upper extremity training. The objective of this study was to determine the feasibility, including safety and compliance, of 6-weeks upper-extremity robot-assisted training in chronic SCI individuals with tetraplegia and residual arm motor function. We hypothesized that robot-assisted upper-limb training would be well-tolerated and lead to improve quantitative measures of motor performance in subjects considered to have reached a motor recovery plateau, that would allow an improvement in their quality of life.
2. Methods
2.1. Participants and study design
A total of 10 chronic SCI subjects volunteered for the study. The inclusion criteria were: history of traumatic spinal cord injury at the cervical level; presence of impaired motor function in the right extensor carpi radialis (ECR) muscle with a motor power score of 1 to 4 over 5; time since injury greater than one year; tolerance to sitting upright for at least one hour; and cognitively and behaviorally capable of complying with the robotic protocol. Patients were excluded from the study if they presented with any of the following exclusion criteria: progressive neurodegenerative disorder; concomitant traumatic brain injury or stroke; uncontrolled pain in the affected limb or exercise intolerance; severely limited range of joint motion; irreversible muscle contractures; clinically significant cognitive impairment; ongoing use of central nervous system (CNS)-active medications; medically unstable; contraindication for transcranial magnetic stimulation (TMS; past medical history of seizures or epilepsy, presence of metallic implants in the brain, presence of pacemaker, pregnancy).
Consenting participants underwent a 6-week wrist-robot training protocol, three times per week. Robotic kinematics, clinical evaluations and corticospinal excitability were the outcome measures performed at baseline and at the conclusion of the robotic training period. The study was approved by the Burke Medical Rehabilitation Institutional Review Board and all participants provided written informed consent.
2.2. Robotic training intervention
2.2.1. Upper-limb robotics: Set up
Participants remained seated in their own wheelchairs and were maneuvered into a wheelchair accessible version of the InMotion 3.0 Wrist robot (Interactive Motion Technologies, Massachusetts, USA). The participant’s right wrist was lightly fastened to the robotic arm, allowing both multi-directional reaching movements of the wrist (flexion/extension, adduction/abduction), and bi-directional uni-planar wrist movements (pronation/supination). Participants were required to perform these movements under unassisted (wrist point-to-point), forceful (wrist round-dynamic), or resisted (wrist playback static) conditions within a two-dimensional workspace corresponding to targets on a screen. For a detailed description of the design and characterization of the wrist robot see Krebs (2007).
2.2.2. Robotic training protocol
The study consisted of a 6-week wrist-robot training intervention, three days per week, totaling 18 training sessions per participant. The InMotion 3.0 Wrist robot was used to provide a customized, goal-directed, robot assisted wrist therapy session, specifically targeting wrist flexion/extension and supination/pronation. Each therapeutic session was started with a wrist stretch regimen for flexors and extensors (120 repetitions) around a “clock-face” format. Following the stretch, the participants completed three one-way recordings consisting of 16 unassisted point-to-point movements (48 in total) for quantification. The first two one-way recordings were succeeded by a therapeutic wrist adaptive regimen consisting of 320 point-to-point movements. This was followed by a therapeutic wrist adaptive regimen for supination/pronation along the horizontal plane of a rectangular box (320 point-to-point movements in total) and a one-way recording (4 unassisted movements in total). During each therapeutic regimen, the robot provided assistance if the participant was unable to initiate or complete the movement independently. Participants were allowed one minute of rest after each adaptive regimen and were given up to 1 hour (maximum) to complete a total of 1000 movements per therapeutic session. If the time allotted expired without the participant completing the required movements, the number of active movements completed were recorded and the session for the day was terminated.
2.3. Transcranial magnetic stimulation and electromyography
Bipolar surface electromyography (EMG) electrodes (1 cm diameter, 2 cm inter-pole distance; Biometrics Ltd, UK) were placed over the right ECR wrist muscle. A reference (grounding) strap was positioned around the wrist. All EMG activity was recorded by Biometrics electromyography (Biometrics Ltd, UK) and signals were amplified and filtered (×1000 gain, band-pass filter 20–400 Hz) into a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK) using Spike 2.6 for further off-line analysis. Measurements were performed at rest and the responses were measured as the peak-to-peak amplitude of the non-rectified signal. During the experiments, EMG silence was continuously monitored with visual feedback to ensure complete muscle relaxation.
Participants remained seated in their own wheelchairs with their right forearm placed in a pronated position supported by a cushion. A comfortably fitted cloth cap was placed on the participant’s head and the vertex of the participants’ skull was identified, recorded and marked to ensure consistent positioning of the cap throughout the study. Stimulation sites were pre-marked on the cap in 1-cm steps lateral to the vertex over the contralateral primary motor cortex in both coronal and sagittal planes. A figure-of-eight coil (Model DB-80, Tonika Elektronik A/S, Farum, Denmark) was connected to a MagPro X100 series (MagVenture A/S, Farum, Denmark) magnetic stimulator and placed flat on the head, congruent with the curved under-surface of the coil, and positioned over the left primary motor cortex. The coil handle was oriented posteriorly, and rotated 45° lateral to the mid-line of the head, and a biphasic pulse shape was used. The optimum site to elicit motor evoked potentials (MEPs) from the right ECR muscle was determined and marked on the cap. The optimal site was identified using a systematic search pattern in 1 cm steps, starting 5 cm lateral to the vertex, and yielding the largest MEP amplitude for a given suprathreshold stimulus intensity.
2.4. Outcome measures
Three independent raters conducted the robotic, clinical and TMS evaluations, respectively. All outcomes were recorded at baseline and at the end of the 6-week training period.
2.4.1. Kinematics: Quantifying motor performance
Recordings were obtained by the robot, from 80 unconstrained multi-directional pointing movements of the wrist (flexion/extension, adduction/abduction), and 20 unconstrained forearm movements (supination/pronation). As detailed later only pointing movements were included in the analysis and the specific kinematic measures derived from the robot data included: a) aim; b) deviation; c) mean speed, d) peak speed, e) movement smoothness, and f) duration of the movement.
2.4.2. Clinical outcomes
The clinical assessment consisted of: a) an upper-limb motor strength evaluation using the upper extremity motor score (UEMS); b) the Modified Ashworth scale (MAS) used to characterize muscle tone; and c) the Visual Analog Scale (VAS) to evaluate changes in pain.
The UEMS measured motor function of five upper extremity key muscles on each side of the body. A score ranging from 0 to 5 was awarded for each muscle, with a score of 0 representing no detectable contraction and a score of 5 for normal muscle strength. An overall motor score out of a possible 25, indicating normal motor function, was derived for each arm.
The MAS was used to measure hypertonia, and rated the resistance to passive stretch in four different upper extremity muscle groups (biceps, triceps, wrist extensors, wrist flexors) bilaterally. The MAS was measured using an ordinal scale (0, 1, 2, 3, 4, 5), and evaluations were conducted by moving the limb about a joint at different speeds and noting the muscular response, for example, speed and position dependence throughout the range of motion.
Pain was assessed using a self-evaluation VAS. Each SCI participant was asked to rate their pain during the previous 24 hours from 0 to 10, where 0 indicates no pain and 10 represents the worst pain possible.
2.4.3. Neurophysiological outcomes
The neurophysiology evaluation consisted of three measures: a) resting motor threshold (RMT), b) Motor evoked potential (MEP) amplitude and latency at rest and c) MEP facilitation. The RMT was defined as the minimum TMS intensity required to elicit a reliable MEP amplitude of >50 μV in at least 50% of consecutive trials and determined by stimulating the optimal site for the targeted muscle using 2% increments in stimulator output. The TMS stimulus intensity was expressed as percentage of maximal stimulator output (MSO). For the MEP facilitation measure, corticospinal excitability was measured during an attempted maximal voluntary contraction (MVC) of the ECR muscle, where a single TMS pulse set at 120% of RMT was applied to the optimal scalp site. Five responses were recorded with an intertrial interval of approximately 5 seconds. MEP facilitation was calculated by expressing the MEP amplitude during voluntary activity relative to the MEP amplitude at rest.
2.5. Data analysis
Kinematic analysis included pointing movements from the central target to and from the outer targets. A movement was considered to begin when the speed first became greater than 2% of the peak speed and was considered to end after the speed dropped and remained below the 2% threshold. Lateral deviation D of subjects’ movements from a straight line connecting the targets was calculated as:
where s(i) is the wrist position at sample i, p(i) is the coordinate of the intersection between the straight line connecting the targets and its perpendicular passing through s(i), and N is the total number of samples. This metric has been widely used in motor learning experiments on reaching and pointing movements. Note that the hand paths produced during wrist rotations lie on a roughly spherical surface surrounding the wrist joint. However, for wrist rotations of the magnitudes used in this study, the spherical surface is shallow and the hand paths can be approximated as lying in a plane tangential to this spherical surface. In other words, for the moderate-sized wrist rotations we used, the difference between a path on this sphere and its projection onto the tangential plane is small: the maximum error associated with this approximation is 1.6° in FE (5% of FE at that point) and 1.0° in RUD (10% of RUD at that point).
In addition, the following metrics were extracted from speed profiles of the pointing movements, which were calculated as summed squares of the first order difference of the X and Y trajectory components smoothed with a 0–4 Hz bandwidth FIR filter: Movement Aim A was calculated as:
where s(i) is the wrist velocity at sample i, ref(j) is the angle of the straight line connecting the center target to the outer target j, and N is the total number of samples.
Movement Mean speed M was calculated as:
Movement Peak speed P was calculated as:
One smoothness metric was also computed as mean speed divided by peak speed. This smoothness metric is dimensionless and increases monotonically with movement smoothness.
Group data for the all the kinematic parameters were tested for significant differences pre- to post-training using a one-tailed paired t-test and an alpha level of 0.05.
Peak-to-peak MEP amplitude was calculated on individual waveforms using Spike 2 software (Cambridge Electronic Design, Cambridge, UK). Two-tailed paired t-tests, or signed-rank for non-parametric variables, were used to analyze clinical and neurophysiological data. We examined the correlation between changes in motor performance kinematics with baseline clinical (UEMS and spasticity) and TMS outcome measures. Results are presented as mean ± standard error of mean (SEM) unless otherwise noted.
3. Results
3.1. Participant characteristics
Table 1 summarizes demographic and clinical characteristics. A total of 10 subjects with SCI (8 males; aged 44.8 ± 16.3 years, range 17–64 years) were included in the study. All but one was right-handed, and the average time since injury was 4.7 ± 2.5 years (mean ± standard deviation, range 2–8 years). For each patient, the neurological level of the lesion was determined as the most caudal spinal segment with normal sensory and motor function and each patient was classified according to the American Spinal Injury Association (ASIA) Impairment Scale; from grade A through to grade D (for a more detailed description see Burns et al., 2011). All 10 subjects presented cervical lesions between levels C4 and C6; three patients were graded ASIA-A complete, four were ASIA-B incomplete, one was ASIA-C incomplete and two were ASIA-D incomplete. All patients tolerated the robotic training. No adverse effects were reported (pain, increased spasticity or other discomfort) during or after the training period.
Table 1.
Clinical and demographic characteristics
| Subject number | Age (yrs) | Gender | Handedness | Time since injury (yrs) | AIS grade | Level of injury |
|---|---|---|---|---|---|---|
| 1 | 29 | F | R | 3 | B | C4 |
| 2 | 32 | M | R | 8 | B | C5 |
| 3 | 43 | M | R | 2 | C | C4 |
| 4 | 55 | F | R | 3 | A | C6 |
| 5 | 54 | M | R | 2 | A | C6 |
| 6 | 17 | M | R | 4 | B | C4 |
| 7 | 70 | M | R | 3 | D | C4 |
| 8 | 64 | M | L | 8 | D | C5 |
| 9 | 42 | M | R | 7 | B | C5 |
| 10 | 42 | M | R | 7 | A | C4 |
AIS: American Spinal Injury Association (ASIA) Impairment Scale.
3.2. Robotic kinematics outcomes: motor performance
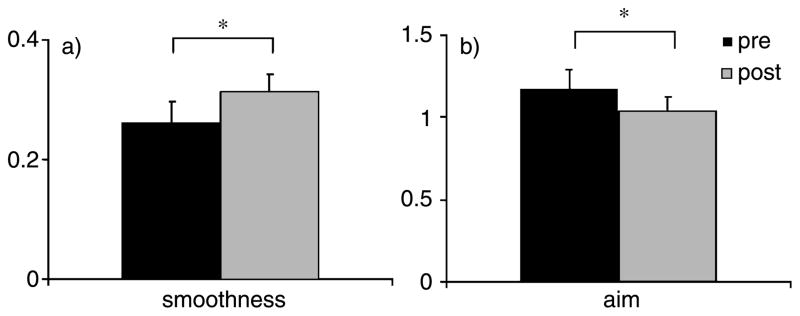
Significant improvements were found in kinematic variables aim and smoothness following the 6-week robotic training (aim: pre 1.17 ± 0.11 radians, post 1.03 ± 0.08 radians; p = 0.03; smoothness: pre 0.26 ± 0.03, post 0.31 ± 0.02, p = 0.03). No changes in deviation, mean speed, peak speed and duration of the movement were found (Figs. 1 and 2).
Fig. 1.
Robotic kinematic outcomes. a) Movement smoothness, b) and aim.
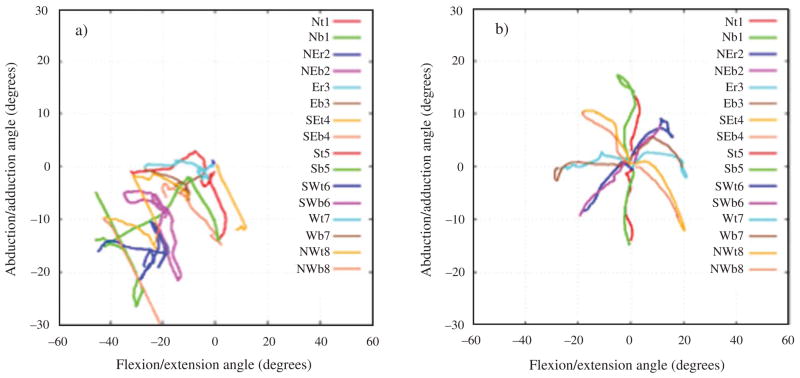
Fig. 2.
Motor performance of one subject after 6-weeks of upper-limb robotic training showing the robotics kinematics a) pre and b) post-training.
3.3. Clinical outcomes after robotic training
There were no changes in the motor strength of the trained right arm (UEMS: pre 8.3 ± 1.1, post 8.4 ± 1.1; p = 0.4) or in the untrained left arm (pre 11.6 ± 1.5, post 12.1 ± 1.4; p = 0.41) after the 6-weeks training. Upper limb spasticity in the four assessed upper limb muscles of either arm was unaltered following training (MAS: right trained arm pre 5.2 ± 1.4, post 4.9 ± 1.1; p = 0.43; left untrained arm pre 4.0 ± 0.7; post 4.5 ± 0.9; p = 0.34). No changes were observed in the pain levels after the training (VAS: pre 0.9 ± 0.5, post 0.9 ± 0.4; p = 0.99; see Table 2).
Table 2.
Clinical outcome results
| Subject number | UEMS (0–25) |
Modified Ashworth Scale (0–20)
|
Visual Analog Scale (0–10)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Right |
Left |
Right |
Left |
PRE | POST | |||||
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | |||
| 1 | 10 | 10 | 12 | 13 | 0 | 4 | 1 | 5 | 0 | 0 |
| 2 | 5 | 5 | 9 | 11 | 1 | 5 | 2 | 6 | 0 | 0 |
| 3 | 6 | 7 | 12 | 12 | 10 | 9 | 8 | 7 | 0 | 0 |
| 4 | 14 | 13 | 14 | 14 | 2 | 2 | 1 | 2 | 5 | 4 |
| 5 | 9 | 10 | 9 | 10 | 4 | 4 | 4 | 4 | 0 | 0 |
| 6 | 6 | 8 | 5 | 6 | 5 | 5 | 5 | 5 | 0 | 0 |
| 7 | 4 | 3 | 21 | 21 | 6 | 9 | 3 | 8 | 2 | 3 |
| 8 | 10 | 11 | 17 | 17 | 16 | 11 | 8 | 8 | 2 | 2 |
| 9 | 14 | 13 | 11 | 11 | 4 | 0 | 4 | 0 | 0 | 0 |
| 10 | 5 | 4 | 6 | 6 | 4 | 0 | 4 | 0 | 0 | 0 |
| Mean | 8.3 | 8.4 | 11.6 | 12.1 | 5.2 | 4.9 | 4 | 4.5 | 0.9 | 0.9 |
| SEM | 1.1 | 1.1 | 15 | 1.4 | 1.4 | 1.1 | 0.7 | 0.9 | 0.5 | 0.4 |
UEMS: Upper eternity motor score; SEM: Standard error of mean.
3.4. TMS outcomes after robotic training
There were no changes in any neurophysiological parameters after the 6-weeks of training. The average RMT of the ECR muscle did not significantly vary (pre 42% MSO, post 42.5% MSO, p = 0.4). The MEP amplitude and latency remained unchanged after the training (amplitude: pre 0.32 ± 0.5 mV, post 0.27 ± 0.06 mV, p = 0.35; latency pre 17.4 ± 0.7 ms, post 16.9 ± 0.74 ms, p = 0.28). There was a slight but not significant increase in MEP facilitation after training (pre 173.2 ± 43.2% of change, post 194.7 ± 36.3 % of change; p = 0.2).
3.5. Correlation analysis
Despite the small sample size we assessed the correlation between the two kinematic parameters that improved after the robotic training (smoothness and aim) and clinical neurophysiologic characteristics at baseline (UEMS, MAS and MEP amplitude). There was a strong positive correlation between change in smoothness according to the initial spasticity level (R2 = 0.403), and change in aim was positively correlated with the initial spasticity in the trained arm (R2 = 0.123). The initial UEMS and MEP amplitude had no correlation with the change in smoothness and aim.
4. Discussion
4.1. Upper-limb robotic training and motor performance
In the present study we evaluated the feasibility and effectiveness of a 6-week robot assisted upper-limb training protocol on the motor performance of chronic cervical SCI patients who had reached a plateau in their motor recovery. The proposed robotic training intervention used a high number of repetitions (>1000 per session) with an established session frequency (3 times per week) for 6-weeks. Our findings confirm that the repetitive visual-motor training protocol led to significant improvements in motor performance of the participants in terms of kinematic components of the arm movement: smoothness and aim. As expected, the changes in motor performance were not accompanied by changes in muscle strength probably due to an insufficient number of sessions; since the stroke literature suggests 36 sessions three times per week over a period of 12 weeks is the optimal robotic training dose in stroke patients to achieve meaningful functional gains in the upper-extremity (Lo et al., 2010).
4.2. Robotic training and clinical outcomes
Other mechanisms involved in improved motor performance after the robotic training could include improved peripheral muscle strength and muscle endurance (Yozbatiran et al., 2012). The SCI participants included in the study showed a positive tendency towards a greater UEMS after the robotic training that did not reach statistical significance, possibly due to a deficient amount of robotic rehabilitation delivered (dose and duration, only 6-weeks of training), which may have been insufficient to produce a measurable change. The initial motor strength in the trained arm seemed to have no correlation with the degree of improvement in motor performance, as has been suggested by Zariffa and colleagues, when comparing robotic training with conventional therapy in subacute patients (Zariffa et al., 2012). Nevertheless, the presence of overall less spasticity in the group data at the beginning of the training showed the strongest correlation with the greater improvement in smoothness and aim. This suggests spasticity is an important factor to consider during the rehabilitation process, since it can limit the upper-limb functional improvement.
4.3. Robotic training and neurophysiology changes
The specific factors that contributed to the measured gains remain unclear; however, potential mechanisms may include activity-dependent neuroplastic changes, such as reorganization of available intact circuits, or the formation of new circuits either at a cortical or subcortical level or in the spinal cord below the lesion (Kadivar et al., 2011). Nonetheless, in the current study, the basic TMS measures of corticomotor excitability and conduction did not change significantly. It is likely that additional measures of excitation, inhibition or cortical motor mapping may be necessary to detect the neurophysiological correlates of these kinematics.
4.4. Feasibility
The robotic training study was well tolerated by the SCI participants. They did not present any undesirable secondary effect, such as an increase in the baseline pain level or spasticity in either of the arms. None of the participants dropped out of the study. Some case reports have described the safety and feasibility of the upper limb robotic training in this population with similar results, high tolerance and no increase in pain or spasticity (Yozbatiran et al., 2012; Sledziewski et al., 2012; Kadivar et al., 2012), using different robotic devices (Armeo, Geo Ro).
The participants complied nicely with the training schedule. All subjects completed the same number of training sessions (eighteen sessions) and the length of the training period ranged from 42 to 51 days. Therefore all subjects finished within 7 and one half weeks.
4.5. Limitations of the study
A shortcoming of the study was the lack of a control group. Although it was not the intention of this study to clarify the superior effectiveness of the robotic intervention over other interventions, future studies intending to do this should incorporate a blinded randomized controlled design. A previous study from our group compared robotic training in neurological patients to a passive movement protocol also on the same robots, showing that indeed volitional engagement in the robotic training (as in the present study) was necessary for motor performance improvements, since the control group did not improve (Volpe 2005). Another important issue is the sample size tested. The number of subjects in this preliminary study was insufficient to demonstrate statistical significance for the clinical improvement observed. Future studies should test an increased sample size, and aim to test a more homogeneous patient population.
Based on upper-limb robotic training studies with stroke patients where the length of the training was 12 to 18-weeks, we conclude that the number of sessions performed were not enough to show clinically significant improvements and cortical excitability reorganization. It has recently been suggested that when patients present with severely limited capabilities, a longer (>36 weeks) or alternative form of therapy, for example, one combined with stimulation, may be warranted (Kadivar et al., 2012). Lastly, the study lacks adequate follow-up measurements to ensure that the functional gain is retained over time.
Other aspects of the robotic training-induced upper-limb recovery as timing (when), intensity (how much) and dose (how long) of the training, as well as the characteristics of the patients that would benefit more should also be further investigated (Benito et al., 2012).
5. Conclusion
Improving motor control with robotic assisted devices in patients with chronic SCI is feasible and safe when performed in a supervised and controlled environment. Robotic training is a novel and reliable method and appears to be a particularly powerful way to promote functional recovery, but the optimal dose of training remains unknown. Since the initial functional capabilities of patients can influence the benefits measured after robotic rehabilitation training, future studies incorporating a larger homogeneous sample of participants is warranted.
Acknowledgments
This work was supported by the NIH Grant R01HD069776 for DJE, MC, AR; NIH grant K24 RR018875 for APL; the New York State Center of Research Excellence in Spinal Cord Injury Grant CO19772; and the Burke Foundation.
Footnotes
Declaration of interest
Dr Hermano Igo Krebs is a co-inventor of the Massachusetts Institute of Technology (MIT) held patent for the robotic devices used for the training protocol in this study. He holds an equity position in Interactive Motion Technologies, Inc, a company that manufactures this type of technology under license to the MIT. The remaining authors have no conflict of interest in the submission of this manuscript.
References
- Barbeau H, Nadeau S, Garneau C. Physical determinants, emerging concepts, and training approaches in gait of individuals with spinal cord injury. [Review] Journal of Neurotrauma. 2006;23(3/4):571–585. doi: 10.1089/neu.2006.23.571. [DOI] [PubMed] [Google Scholar]
- Beekhuizen KS, Field-Fote EC. Massed practive versus massed practice with stimulation: Effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabilitation and Neural Repair. 2005;19:33–45. doi: 10.1177/1545968305274517. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Harkema SJ. Physical rehabilitation as an agent for recovery after spinal cord injury. Physical Medicine and Rehabilitation Clinics of North America. 2007;18(2):183–202. doi: 10.1016/j.pmr.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Burns S, Biering-Sørensen F, Donovan W, Graves DE, Jha A, Johansen M, Waring W. International standards for neurological classification of spinal cord injury, Revised 2011. Topics in Spinal Cord Injury Rehabilitation. 2011;18(1):85–99. doi: 10.1310/sci1801-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR. Robotic training and spinal cord plasticity. [Review] Brain Research Bulletin. 2009;78(1):4–12. doi: 10.1016/j.brainresbull.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadivar Z, Sullivan JL, Eng DP, Pehlivan AU, O’Malley MK, Yozbatiran N, Francisco GE. Robotic training and kinematic analysis of arm and hand after incomplete spinal cord injury: A case study. Paper presented at the International Conference on Rehabilitation Robotics; Zurich, Switzerland. 2011. [DOI] [PubMed] [Google Scholar]
- Kadivar Z, Sullivan JL, Eng DP, Pehlivan AU, O’Malley MK, Yozbatiran N, Francisco GE. RiceWrist robotic device for upper limb training: Feasibility study and case report of two tetraplegic persons with spinal cord injury. [Case Report] International Journal of Biological Engineering. 2012;2(4):27–38. doi: 10.5923/j.ijbe.20120204.01. [DOI] [Google Scholar]
- Krebs H, Dipietro L, Levy-Tzedek S, Fasoli S, Rykman-Berland A, Zipse J, Hogan N. A paradigm shift for rehabilitation robotics: Therapeutic robots enhance clinician productivity in facilitating patient recovery. IEEE Engineering in Medicine and Biology Magazine. 2008;27(4):61–70. doi: 10.1109/MEMB.2008.919498. [DOI] [Google Scholar]
- Krebs HI, Volpe B, Hogan N. A working model of stroke recovery from rehabilitation robotics practitioners. Journal of NeuroEngineering and Rehabilitation. 2009;6:6. doi: 10.1186/1743-0003-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HI, Volpe BT, Williams D, Celestino J, Charles SK, Lynch D, Hogan N. Robot-aided neurorehabilitation: A robot for wrist rehabilitation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2007;15(3):327–335. doi: 10.1109/TNSRE.2007.903899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Peduzzi P. Robot-assisted therapy for long-term upper-limb impairment after stroke. The New England Journal of Medicine. 2010;362(19):1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey JV, Belanger A, Jung R. Activity-dependent plasticity in spinal cord injury. Journal of Rehabilitation Research and Development. 2008;45(2):229–240. doi: 10.1682/JRRD.2007.03.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson LA, Eng JJ, Hsieh JT, Wolfe DL. The health and life priorities of individuals with spinal cord injury: A systematic review. [Research Support, Non-U.S. Gov’t Review] Journal of Neurotrauma. 2012;29(8):1548–1555. doi: 10.1089/neu.2011.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledziewski L, Schaaf RC, Mount J. Use of robotics in spinal cord injury: A case report. [Case Report] American Journal of Occupational Therapy. 2012;66(1):51–58. doi: 10.5014/ajot.2012.000943. [DOI] [PubMed] [Google Scholar]
- Spooren AI, Janssen-Potten YJ, Kerckhofs E, Seelen HA. Outcome of motor training programmes on arm and hand functioning in patients with cervical spinal cord injury according to different levels of the ICF: A systematic review. [Review Article] Journal of Rehabilitation Medicine. 2009;41(7):497–505. doi: 10.2340/16501977-0387. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Huerta PT, Zipse JL, Rykman A, Edwards D, Dipietro L, Krebs HI. Robotic devices as therapeutic and diagnostic tools for stroke recovery. [Neurological Review] Archives of Neurology. 2009;66(9):1086–1090. doi: 10.1001/archneurol.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N. Robot-aided sensorimotor training in stroke rehabilitation. Advances in Neurology. 2003;92:429–433. [PubMed] [Google Scholar]
- Wirth B, Van Hedel HJ, Curt A. Changes in corticospinal function and ankle motor control during recovery from incomplete spinal cord injury. Journal of Neurotrauma. 2008;25(5):467–478. doi: 10.1089/neu.2007.0472. [DOI] [PubMed] [Google Scholar]
- Yozbatiran N, Berliner J, O’Malley MK, Pehlivan AU, Kadivar Z, Boake C, Francisco GE. Robotic training and clinical assessment of upper extremity movements after spinal cord injury: A single case report. [Case Report] Journal of Rehabilitation Medicine. 2012;44:186–188. doi: 10.2340/16501977-0924. [DOI] [PubMed] [Google Scholar]
- Zariffa J, Kapadia N, Kramer JL, Taylor P, Alizadeh-Meghrazi M, Zivanovic V, Willms R, Townson A, Curt A, Popovic MR, Steeves JD. Feasibility and efficacy of upper limb robotic rehabilitation in a subacute cervical spinal cord injury population. Spinal Cord. 2012;50(3):220–226. doi: 10.1038/sc.2011.104. [DOI] [PubMed] [Google Scholar]