Abstract
Tendon injuries are common and present a clinical challenge to orthopedic surgery mainly because these injuries often respond poorly to treatment and require prolonged rehabilitation. Therapeutic options used to repair ruptured tendons have consisted of suture, autografts, allografts, and synthetic prostheses. To date, none of these alternatives has provided a successful long-term solution, and often the restored tendons do not recover their complete strength and functionality. Unfortunately, our understanding of tendon biology lags far behind that of other musculoskeletal tissues, thus impeding the development of new treatment options for tendon conditions. Hence, in this review, after introducing the clinical significance of tendon diseases and the present understanding of tendon biology, we describe and critically assess the current strategies for enhancing tendon repair by biological means. These consist mainly of applying growth factors, stem cells, natural biomaterials and genes, alone or in combination, to the site of tendon damage. A deeper understanding of how tendon tissue and cells operate, combined with practical applications of modern molecular and cellular tools could provide the long awaited breakthrough in designing effective tendon-specific therapeutics and overall improvement of tendon disease management.
Keywords: Tendon, Tendon repair, Growth Factors, Cell-based therapy, Mesenchymal stem cells, Embryonic stem cells, Tendon-derived cells, Natural biomaterials, Gene therapy
1. Introduction
Tendons are unique forms of connective tissue that connect and transmit forces from muscle to bone [1]. They are able to store elastic energy and withstand the high tensile forces upon which locomotion is entirely dependent [2]. This review article is designed:
(1) to provide background information on the clinical relevance of tendons and to remind the reader of the lengthy and incomplete nature of the native tendon repair process. This motivates the urgent need for improving the outcome of tendon repair; biologics offer attractive possibilities in this regard;
(2) to introduce the basic tissue and cellular organization of tendon and its major tendon-specific molecules (Sections 1.1–1.3);
(3) to summarize the results of studies based on the four main approaches - growth factors (Section 2.1), stem cells (2.2), natural biomaterials (2.3) and gene therapy (2.4);
(4) to discuss critically unresolved issues.
We have focused on in vivo studies of the repair of tendon injury, and only in some cases included in vitro examples to strengthen certain points.
1.1. Tendon clinical relevance
Primary disorders of tendons (tendinopathies), due to overuse or age-related degeneration, are widely distributed clinical problems in society, possibly resulting in acute or chronic tendon injuries. Hospital evidence and statistical data suggest that certain tendons are more prone to pathology than others; these are the rotator cuff, Achilles, tibialis posterior and patellar tendons, whose pathologies are often based on a degenerative process. In addition, the extensor and flexor tendons of the hand and fingers are frequently subjected to direct lacerations at all ages. Although there are no accurate figures specifically relating to tendon disorders, studies from primary care show that 16% of the general population suffer from rotator cuff-related shoulder pain [3] and this rises to 21% when the statistics shift to elderly hospital and community populations [3,4]. These numbers further increase in the sports community; for example, Kannus reported that 30 to 50% of all sporting injuries involve tendons [5].
Although there are a number of studies discussing this issue, there is still a need to clarify the classification and terminology of the different tendon pathologies. This situation is mainly due to the clinical problem that tendon biopsies are generally difficult to obtain and that this material is usually collected at the end-stage of the condition or after tendon rupture. In general, the major conditions affecting tendons are tendinitis and tendinosis; the first assumed to be accompanied by inflammation and pain, whereas the second can be caused by tendinous degeneration [6]. It is believed that these conditions are rarely spontaneous [7] and are not caused by single factors. Rather, they are the end result of a variety of pathological processes [8,9] which can ultimately lead to the main clinical problem: loss of tissue integrity with full or partial rupture of the tendon.
Many factors are likely to be involved in the onset and progression of tendinopathies. Intrinsic factors include age, gender, anatomical variants, body weight, and systemic disease. Extrinsic factors include sporting activities, physical loading, occupation, and environmental conditions such as walking surfaces or footwear [8,9]. In addition, it has been reported that genetic polymorphisms affecting collagen fiber formation [10] or even blood group [11] are associated with tendon injuries and tendinopathy.
Hence, tendinopathies represent major medical problems associated with physical activity and age-related degeneration. Unfortunately, due to hypocellularity and hypovascularity, the natural healing ability of tendons is extremely low and inefficient [12]. Nevertheless, healthy tendon tissue has the potential to heal by itself as long as the ruptured parts have contact and the well vascularized peritendinous tissue, the so-called paratenon, is intact [13]. There is continuing debate on whether to treat acute Achilles tendon ruptures operatively or conservatively [14]. Both options have their advantages. In the case of rotator cuff pathologies, the choice of therapy very much depends on the patient's age, degree of tendon degeneration and extent of laceration [15]. A ruptured patellar tendon needs to be treated operatively to restore the extensor apparatus of the knee [16]. The treatment of tibial tendon insufficiencies is stage-dependent [17].
The main surgical repair techniques aim to re-establish tendon alignment by suturing the ruptured ends together, which requires a non-degenerate tendon with healing potential. The reconstructions are limited by the tendon's biology. Sometimes an autograft is used to bridge certain defects, while use of allografts has increased in recent years [18,19]. When autografts are used, a certain donor side morbidity must not be neglected. And in both cases, ingrowth of the bridging graft is necessary, requiring good tissue conditions without degeneration. It is estimated that $30 billion are spent on musculoskeletal injuries in the United States each year and tendon/ligament injuries represent approximately 45% of these cases [20]. In addition, surgical repairs are often unsuccessful in which case the majority of these injuries become essentially chronic conditions that are prone to recur [21].
In summary, tendon disorders are common, debilitating conditions affecting both the working population and recreational athletes. Their etiology remains controversial, particularly in understanding which factors are primary and which are secondary to the disorder. Moreover, these conditions not only have an impact on peoples' quality of life, but also represent an enormous economic burden on the worldwide healthcare system. Therefore, it is of great importance to identify key molecular and cellular processes involved in the progression of tendinopathies and subsequent ruptures in order to develop effective therapeutic strategies for treating them.
1.2. Tendon molecular composition and cell niche
The extracellular matrix (ECM) of tendons is composed of collagen and a smaller fraction of elastin embedded in a hydrated proteoglycan matrix. The principal role of the collagen fibers is to resist to tension, whereas proteoglycans are primarily responsible for the viscoelastic properties of the tendon. The smallest structural unit is the collagen fibril. Each fibril is built from soluble tropocollagen molecules forming cross-links to create insoluble collagen molecules which then aggregate progressively into microfibrils, fibrils and finally into fibers. Bundles of fibers are bound together by thin layers of loose connective tissues known as the epi- and endotenon, which allow the fiber groups to glide on each other in an almost frictionless manner; they also carry blood vessels, nerves and lymphatics to the deeper portion of the tendon [2]. The smooth gliding of tendons as they move is aided by the lubricating molecule, lubricin [22]. Altogether this complex, three-dimensional, internal ultrastructure endows the tendon with high tensile force and resilience, while preventing damage and separation of the fibers under mechanical stress [8] (Fig. 1).
Fig. 1.
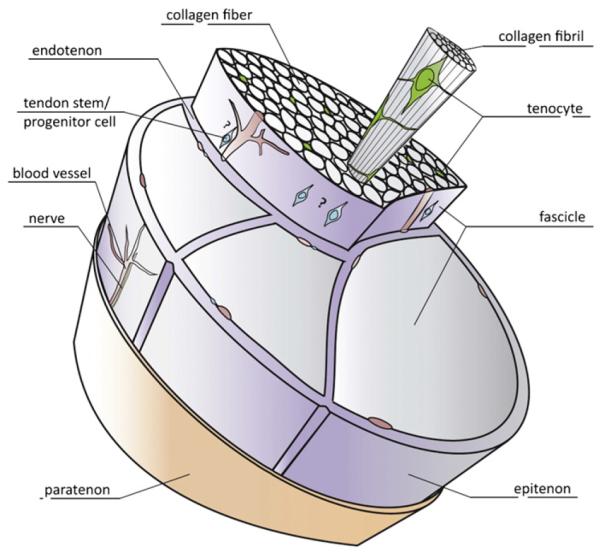
A schematic drawing of basic tendon structure. The collagen molecules are organized hierarchically in fibrils, fibers and fascicles. The cellular content is dominated by the tenocytes, which are terminally differentiated cells. Tendons contain stem and progenitor cell populations, whose exact location is still debated (therefore indicated with a?). Different sheets, endotenon and epitenon (loose connective tissues), and paratenon (fatty areolar tissue) are shown as well as blood vessels and nerves.
Based on [227].
Collagen type I is the most abundant molecule in the ECM, accounting for almost 60% of the dry mass of the tissue and approximately 95% of the total collagen [8]. Type III is the next most abundant collagen [23]. Normally, collagen type III is restricted to the tendon sheets; however, it is found abundantly in pathological tendons and it is also the first collagen to be produced in high quantity during tendon healing [8]. Other collagens in tendon include types V, VI, XII, XIV and XV.
Besides collagen fibers, the tendon ECM is composed of many other components including elastic fibers, the ground substance and inorganic components. In general, elastic fibers ensure tissue flexibility and extensibility, permitting long-range deformability and passive recoil without energy input [24]. Furthermore, they are thought to be involved in the recovery of the crimp pattern of the collagen fibers after tendon stretching [25]. The ground substance comprises hyaluronan, proteoglycans (decorin, biglycan, fibromodulin, lumican), structural glycoproteins and a wide variety of other molecules. The highly viscous and hydrophilic nature of the ground substance provides spacing and further support of the collagen fibers. Water makes up 60 to 80% of the total weight of the ground substance, whereas proteoglycans account only for 1–2% [23].
Mature tendons are normally characterized by low cellular density (Fig. 1). Approximately 90–95% of the cellular content of tendon comprises tendon-specific cell types described in the literature as tenoblasts and tenocytes, the latter being terminally differentiated [26]. Other cell types include the synovial cells of the tendon sheaths, chondrocytes at the pressure and insertion sites, and vascular cells. Tendon cells are able to synthesize all components of the tendon ECM with a peak activity during growth and a gradual decrease during aging [26]. It is thought that the low metabolic rates with anaerobic energy production typical of mature tendon cells can reduce the risk of ischemia and necrosis, especially during the extended periods of tensional stresses to which tendons are usually subjected. On the other hand, this feature is a disadvantage for tendon recovery and healing. Conversion of tenoblasts to tenocytes might occur in response to various stimuli such as exercise and trauma in which higher rates of proliferation and matrix remodeling are needed [26].
In 2007, Bi et al., identified within human hamstring tendons a novel cell population of resident tendon stem/progenitor cells (TSPC) [27]. It was shown that the TSPC exhibit classical adult mesenchymal stem cell (MSC) criteria such as presence of specific surface antigens, self-renewal, clonogenicity and three-lineage differentiation (adipogenic, osteogenic and chondrogenic), but also that they express tendon-related genes such as scleraxis and tenomodulin, and are able to form tendon and enthesis-like tissues when implanted in vivo. The existence of TSPC was further confirmed in subsequent studies with human, equine, rabbit, rat and mouse tendons [28–35]. Whether adult TSPCs represent a residual population of the embryonal tendon progenitors remains still unclear. Furthermore, there is a need for studies demonstrating the exact roles and location of TSPC during tendon maintenance and healing as well as their exact relationship to tenoblasts and tenocytes. At present, the direct comparison of TSPCs to tenoblasts and tenocytes is impeded by the lack of molecular markers allowing their precise identification and hence the isolation of pure subsets of cell populations along the tendon differentiation cascade. Despite the incomplete understanding of TSPC nature and function, they represent a potential cell source for treating injured tendons. How the tendon research field foresees using TSPC in tendon repair will be discussed later in the review.
1.3. Tendon healing
The lack of detailed molecular and histopathological studies on tendons has hampered our understanding of the mechanisms underlying tendon healing. Some evidence has been obtained from animal models of experimentally induced tendon damage [36,37]. In general, the healing process of an injured or compromised tendon passes throughout three main phases containing distinctive cellular and molecular cascades (Figs. 2 and 3). These phases can overlap and their duration is dependent on the location and severity of the disease [38–40]. The initial inflammatory stage begins with the formation of a hematoma shortly after injury. Inflammatory cells such as neutrophils, monocytes and macrophages are attracted to the injury site by pro-inflammatory cytokines [38]. Secreted angiogenic factors initiate the formation of a vascular network, which is responsible for the survival of the newly forming fibrous tissue at the injury site. Despite being profuse and haphazard, the initial vascular response is essential, since it has been shown that dimunition of blood supply impairs healing [41]. Next, components of the ECM, pre-dominantly collagen type III, are synthesized by recruited fibroblasts. After a few days, the proliferation stage takes place accompanied by the synthesis of abundant ECM components, including proteoglycans and collagens (mostly collagen type III), which are arranged in a random manner. Further features of this stage are increased cellularity and the absorption of large amounts of water. The remodeling stage includes two sub-stages; it begins 6–8 weeks after injury and takes around 1–2 years depending on the age and condition of the patient. The first sub-stage, consolidation, is characterized by a decrease in cellularity and matrix production, as the tissue becomes more fibrous through the replacement of collagen type III by collagen type I. Collagen fibers then start to organize along the longitudinal axis of the tendon, thereby restoring tendon stiffness and tensile strength. After approximately 10 weeks, the maturation stage starts, which includes an increase in collagen fibril crosslinking and the formation of more mature tendonous tissue.
Fig. 2.
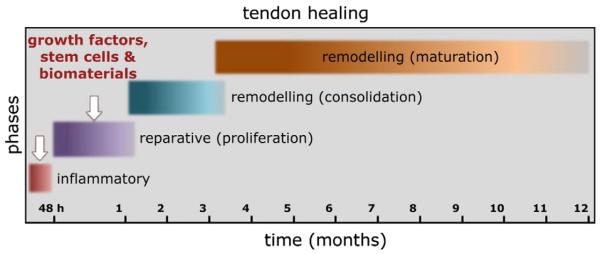
The tendon repair process in humans. The healing of ruptured tendons passes through three main phases containing distinctive cell and molecular cascades. These phases overlap and their duration depends upon the location and severity of the tendon injury. Currently, the tendon research field is actively exploring the use of growth factors, genes, stem cells and biomaterials, alone or in various combinations, for enhancing tendon healing. Mostly, the appropriate times of application are in the first two stages (indicated by white arrows), and depend on the type of growth factors, genes, stem cells or biomaterials implemented. Based on [47].
Fig. 3.
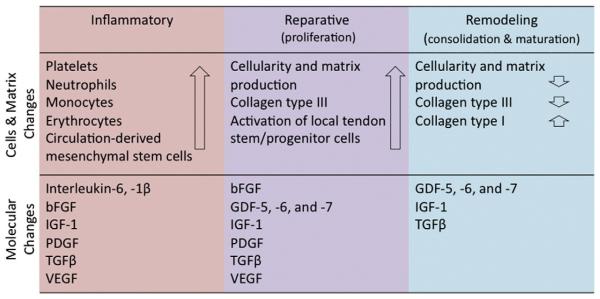
Key molecular, cellular and matrix changes occurring during the three main phases of tendon repair. Each healing stage is characterized by involvement of different growth factors, activation of certain cell types and production of essential matrix proteins, which collectively contribute to the replacement of the initial fibrous tissue with more a tendonous regenerate. Based on [45,46].
The tendon healing process is complexly orchestrated by a variety of secreted molecules [42]. Initially, certain inflammatory cytokines, such as interleukin (IL)-6 and IL-1β, are produced by the invading inflammatory cells. Later, tissue repair is facilitated by a number of growth factors, which are released by cells located at the injury site. bFGF (basic fibroblast growth factor), BMPs (bone morphogenetic proteins)-12, -13, and -14 also known as GDFs (growth and differentiation factors) -5, -6 and -7 respectively, TGFβ (transforming growth factor beta), IGF-1 (insulin-like growth factor-1), PDGF (platelet-derived growth factor) and VEGF (vascular endothelial growth factor) are involved in different phases of the healing process with diverse molecular effects (Fig. 3). During the repair process, tendon cells are activated and both synthesize and degrade ECM components, thereby participating in the slow, continuous process of tendon remodeling [39,43,44].
Two cellular mechanisms of tendon healing, known as extrinsic and intrinsic healing, have been suggested [41,45]. It is now believed that these two mechanisms normally act cooperatively. The hypothesis is that first fibroblasts and inflammatory cells from the tendon periphery, blood vessels and circulation are attracted to the injured site contributing to cell infiltration and the formation of adhesions. Thereafter, intrinsic cells from the endotenon are activated as they migrate and proliferate at the injury site, reorganizing the ECM and giving support to the internal vascular networking [38,46]. The origin of the reparative cells remains in debate. In 2007, an enlightening study from Kajikawa et al., used a model of tendon injury applied to two different chimeric rats, one expressing green fluorescent protein (GFP) in circulating mesenchymal cells, and the other in the patellar tendon. The data were consistent with the biphasic pattern of tendon healing. This comprises an initial invasion of circulating MSCs followed by the activation of local cells, which participate in the proliferative phase and carry out the long remodeling phase [45].
In most patients, especially aged individuals, the healed tendon usually does not regain the mechanical properties of the uninjured tissue. The reduced strength of the repaired tissue compared to the native tendon results from reduced integration of collagen fibers with a higher ratio of collagen type III to collagen type I. As a consequence, the tendon thickens and stiffens to overcome the lower unit mechanical strength; thus the tendon quality and its functional activity are inferior to that of healthy tendon.
2. Current biological strategies to augment tendon repair
Experimental approaches for enhancing tendon repair consist mainly of applying growth factors, singly or in combination, stem cells in native or genetically modified form, and biomaterials, alone or cell-loaded, at the site of tendon damage. In the last decade, the number of studies investigating the functionality of the above strategies has progressively increased (Fig. 4). This section of the review will focus primarily on in vivo studies. It will critically discuss progress and the remaining open questions for future research to address in order to improve the therapy of damaged tendons. Two of the most difficult tasks that researchers are facing during regeneration of tendinous tissues are: first, to achieve the regeneration of a highly specialized and three-dimensional organized matrix, whose formation implies not only biological but also mechanical constraints; and second, when using stem or progenitor cells, to prevent inappropriate plasticity of the exogenous cells, or the transdifferentiation of the local tenocytes into undesirable lineages leading to, for example, in situ adipose, cartilaginous or bone tissue formation.
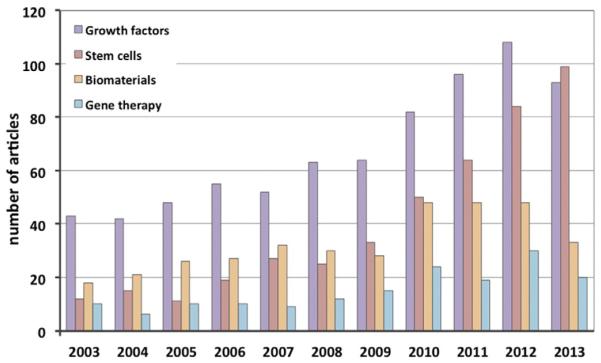
Fig. 4.
Studies on the use of biologics for tendon repair. Article counts were carried out after searching in PubMed using the following key words: tendon repair/healing in combination with growth factors, stem cells, biomaterials and gene therapy. The articles include in vivo and in vitro studies, and some articles scored in more then one category. The search results demonstrate that in the last decade the tendon research field has progressively expanded as represented by the continuous increase in the number of articles focusing on different strategies for enhancing tendon tissue healing. Such cumulative efforts may lead to the development of efficient biologics for tendon repair.
2.1. Growth factors
Tendon injury stimulates the production of a variety of growth factors at multiple stages in the healing process [40,42] leading to increased cellularity and tissue volume [47]. Increased expression of growth factors is particularly prominent in the early phases of healing [48,49]. The following growth factors are important in tendon healing: bFGF, BMP-12, -13, -14, CTGF (connective tissue growth factor), IGF-1, PDGF, TGFβ, and VEGF [49–52]. In the following section these factors are briefly introduced before describing in vitro and in vivo experiments investigating the role of the factors in tendon healing (Table 1). No human study investigating recombinant growth factors in tendon healing has been published in the literature.
Table 1.
Summary of in vitro and in vivo studies on growth factors.
| Growth factor |
Tendon | Type of study | Model | Reference |
|---|---|---|---|---|
| bFGF | Flexor tendon | In vitro | Canine | [87] |
| Flexor tendon | In vitro | Canine | [85] | |
| Flexor tendon | In vitro | Rabbit | [95] | |
| Patellar tendon | In vitro | Rat | [68] | |
| Supraspinatus tendon | In vivo | Rat | [93] | |
| BMP 12 | Achilles tendon | In vivo | Rat | [194] |
| BMP 2 | Flexor tendon | In vitro | Canine | [85] |
| Extensor tendon | In vivo | Canine | [57] | |
| Flexor tendon | In vivo | Rabbit | [58] | |
| Infraspintatus tendon | In vivo | Rabbit | [59] | |
| BMP 2, 7, 12 | Infraspinatus tendon | In vivo | Sheep | [94] |
| IGF | Flexor tendon | In vitro | Rabbit | [90] |
| Flexor tendon | In vitro | Rabbit | [91] | |
| Flexor tendon | In vitro | Rabbit | [95] | |
| Rotator cuff | In vitro | Rat | [214] | |
| PDGF | Flexor tendon | In vivo | Canine | [86] |
| Flexor tendon | In vitro | Canine | [87] | |
| Flexor tendon | In vitro | Canine | [85] | |
| Flexor and peroneal tendon | In vitro | Rabbit | [88] | |
| Flexor tendon | In vitro | Rabbit | [95] | |
| Patellar tendon | In vitro | Rat | [64] | |
| Rotator cuff | In vitro | Rat | [214] | |
| Rotator cuff | In vivo | Sheep | [78] | |
| Medial femuro-tibial ligament | Iv vivo | Rat | [92] | |
| TGFβ | Patellar tendon | In vivo | Rabbit | [80] |
| Achilles tendon | In vivo | Rat | [76] | |
| Flexor tendon | In vitro | Rabbit | [82] | |
| Flexor tendon | In vitro | Rabbit | [69] | |
| Flexor tendon | In vivo/in vitro | Rabbit | [77] | |
| Achilles tendon | In vivo | Rat | [195] | |
| Flexor tendon | In vitro | Rabbit | [65] | |
| Flexor tendon | In vivo | Rat | [71] | |
| Flexor tendon | In vivo | Murine | [72] | |
| TGFβ1 | Flexor tendon | In vitro | Murine | [73] |
| Flexor tendon | In vivo | Murine | [74] | |
| TGFβ1, 2, 3 | Infraspinatus tendon | In vivo | Sheep | [94] |
| VEGF | Flexor tendon | In vitro | Canine | [83] |
| Flexor tendon | In vitro | Canine | [84] | |
| Flexor tendon | In vitro | Canine | [85] | |
| Flexor tendon | In vitro | Rabbit | [81] | |
| ACS | Achilles tendon | In vivo | Rat | [98] |
| PRP | Achilles tendon | In vivo/In vitro | Rat | [96] |
| Flexor tendon | In vitro | Equine | [99] | |
| Flexor tendon | In vivo | Equine | [97] | |
| Review | In vivo | Human | [105] | |
| Review | In vivo | Human | [106] | |
| Review | In vivo | Human | [109] |
2.1.1. bFGF
Chang et al., found upregulated bFGF mRNA in mature tenocytes and in fibroblasts and inflammatory cells surrounding the healing site in the tendon sheath [53]. Being elevated early in the healing process [48,49], bFGF is well positioned to promote the early events in tendon healing [54].
2.1.2. BMP
BMP-12, -13, and -14, also known as GDF-7, -6, and -5 respectively, stimulate mitogenesis, and are established tenogenic factors with the potential of driving differentiation of MSC in vitro [55] and in vivo [56]. BMPs are elevated early in the tendon healing process, gradually decreasing thereafter [48,49]. BMP-2 plays a role at the enthesis, the anatomical junction of tendon and ligament to bone. New bone formation can be induced by BMP-2 within a tendon with comparable characteristics to the enthesis. However, in intratendinous healing this bone formation is clearly undesirable [57–59].
2.1.3. CTGF
In contrast to the previously described factors, CTGF exhibits a sustained increase in gene expression persisting over 21 days during healing of chicken flexor tendons [50]. In the rat supraspinatus injury model of Würgler-Hauri et al., CTGF was moderately expressed in both the insertion and midsubstance area throughout all time points [49].
2.1.4. IGF-I
IGF-1 induces tenocyte migration and increases synthesis of the ECM, including collagen [60]. Elevated IGF-1 mRNA and protein expression levels were found in healing rabbit ligaments 3 weeks after injury and in healing equine tendons after 4 to 8 weeks [61,62]. IGF-1 seems to be particularly important during the formation and remodeling stages of healing.
2.1.5. PDGF
Increased PDGF-levels have been found in healing tendons [63]. Elevated expression of the PDGF receptor β was found by Chan et al., to persist for over 6 months after tendon injury, potentially indicating the important role of PDGF during the entire tendon repair period [64].
2.1.6. TGFβ
Besides tendon cell migration and mitogenesis, TGFβ especially stimulates production of the ECM, including increases in the production of collagen types I and III by all the 3 isoforms TGFβ1, TGFβ2, and TGFβ3 [65]. High levels of expression and activity of TGFβ are found throughout the course of tendon-healing [66,67]. Resident tenocytes and infiltrating cells from the surrounding tendon sheath show increased expression of TGFβ1 mRNA [68]. Correspondingly, TGFβ1/3 receptor (CD 105; endoglin) expression was also found to be upregulated at the repair site [69]. Juneja et al., found a biphasic pattern of TGFβ expression corresponding to an early peak of TGFβ1 and a late peak of TGFβ3 expression during healing [70]. Heisterbach et al., also found early and late peaks of TGFβ1 expression [48]. However, there are also data indicating that TGFβ1 provokes increased fibrotic scar formation resulting in tendon adhesions [71,72]. In a rabbit model adhesions were reduced using an anti-TGFβ1 antibody, but were not further influenced by the addition of an antibody against the isoform TGFβ2 [66]. Possibly an imbalance between the TGFβ1-induced ECM-formation and tendon remodeling is responsible for the formation of adhesions [73,74]. Thus, defining the appropriate doses and combinations of isoforms could be essential for the successful application of TGFβ in tendon healing.
2.1.7. VEGF
Angiogenesis is important in both tendon degeneration, in cases of impaired blood supply, and in regeneration, for which the best possible capillary permeability is desirable [41]. VEGF promotes angiogenesis in tendon healing [75], and its activity rises after the inflammatory phase, especially during the proliferative and remodeling phases. In a canine model of tendon transection, VEGF mRNA peaked 10 days after surgery [76].
2.1.8. Effects of different growth factors on tendon healing
Based on the presence and influence of growth factors on tendon healing a number of studies has been published with the aim of understanding the influence of growth factors on tendon biology in vitro and on tendon healing in vivo (Table 1). For in vivo studies, the growth factors can be applied by local injection, percutaneously or operatively, or by implanting scaffolds or even suture material [77–79] containing growth factors.
Growth factors are rapidly cleared following local injection, but their persistence may be prolonged using scaffolds or coated suture material. There have been few investigations of growth factor release by coated suture material and scaffolds in tendons, but there have been several studies investigating the local application of growth factors. Local injection of TGFβ into the healing site of patellar tendons in rats significantly increased the load to failure [80]. Comparable results were found in flexor tendons of rabbit treated with VEGF, as long as the plantaris tendon was preserved. In this study expression of TGFβ was significantly elevated early in the healing course. It remains unclear whether the positive effect was caused by the VEGF therapy itself, the increased TGFβ expression provoked by VEGF, or both [81].
Interestingly native cells from different areas of the tendon tend to react differently when treated with TGFβ. Type I collagen expression is down-regulated and type III expression up-regulated in endotenon cells compared to cells from the epitenon or the tendon sheath [82]. Possibly the up-regulation of collagen type III and the down-regulation of collagen type I by cells in the endotenon marks the beginning of tendon healing induced by TGFβ [81]. As well as differential expression of collagens by epi- and endotenon cells, increased mRNA expression for VEGF was found at the healing site of flexor tendons but not at the epitenon [83,84]. Increased cell proliferation and collagen production was also provoked by PDGF and bFGF. The effect was amplified by a combination of both factors. In this study VEGF and BMP-2 did not have the same positive effect [85]. Treatment with PDGF increased total DNA, collagen crosslinking and hyaluronic acid content resulting in improved functional movement, but did not improve the tensile properties of the healed tendon [86]. Combined treatment with bFGF and PDGF increases fibronectin deposition as part of the provisional matrix and angiogenesis/revascularization in canine flexor tenocytes [87]. Moreover PDGF stimulates the synthesis of proteoglycan, collagen, non-collagenous protein and DNA [88]; bFGF was shown to accelerate intratendinous healing in patellar tendons [89]. Also IGF-1 stimulates matrix synthesis and cell proliferation of tenocytes in vitro [90,91].
The positive effects of growth factors are not limited to intratendinous lesions; PDGF increased failure load of the medial femurotibial ligament [92] and several growth factors had a positive effect on the tendon–bone healing site of the rotator cuff [93,94]. Combinations of growth factors seem more potent than individual growth factors delivered singly. For example, in an in vitro model using rabbit flexor digitorum tenocytes, the highest cell proliferation rates were achieved by combining bFGF, IGF-1, and PDGF. The combination was effective at lower doses than when the growth factors were delivered singly [95].
2.1.9. Autologous sources of growth factors
As noted above, it seems that the interaction of several different growth factors is important in tendon healing. This could explain the observed effectiveness of concentrates of autologous growth factors, such as those contained within platelet rich plasma (PRP) or autologous conditioned serum (ACS) [96–99]. In contrast to purified growth factors, PRP has already been used clinically in patients with either tendinopathy or tendon injury. Gosens et al., found significant pain reduction in 36 patients with patellar tendinopathy after PRP injection [100]. In a prospective randomized controlled study of 27 patients after ACL-reconstruction, PRP had a positive effect on donor site healing in the harvested patellar tendon leading to pain reduction and smaller defect size in MRI controls after 6 months [101]. Moreover, the patellar tendon graft itself seemed to remodel faster during ACL-reconstruction after additional application of PRP [102]. In a rat Achilles tendon model percutaneous administration of PRP 6 h after transection and resection of 3 mm tendon resulted in an increased tendon callus and strength by about 30% after 1 week, which persisted for as long as 3 weeks after injection [96]. However, after surgery to repair acute Achilles tendon rupture in patients Schepull et al., did not find improvement in healing after additional administration of PRP to the healing site [103]. In chronic Achilles tendinopathy de Vos et al. failed to find differences in pain or activity level between patients treated with PRP or saline [104].
A later systematic review de Vos et al. found no evidence of efficacy using PRP to treat chronic lateral epicondylar tendinopathy [105]. Along these lines, Hall et al. stated in their review on PRP entitled “platelet rich placebo?” that the only reasonable use for PRP was for therapy of refractory cases of lateral epicondylar tendinopathy, but not for other tendinopathies or tendon repair [106]. However, because PRP is a variable, poorly characterized cocktail of growth factors and other substances it is difficult to draw strong conclusions.
Several different devices are approved by the FDA (U.S. Food and Drug Administration) in the United States for generating PRP, resulting in different compositions of growth factors and even cells (leucocytes and erythrocytes). Moreover, PRP contains components other than growth factors, including interleukins, chemokines, proteinases, inhibitors of proteinases, adhesion molecules, sphingolipids, thromboxanes, purine nucleotides, serotonin, calcium, and many other mediators. PRP is considered to have anti-inflammatory properties, but some components, such as IL-1, -6, and -8, are pyrogens [107,108]. Thus the precise combinations and concentrations of the different factors within PRP are important determinants of the properties of this autologous blood product. This could explain the lack of activity described by Schepull et al. [103] and de Vos et al. [104]. Prospective randomized controlled trials using PRP formulations of standard, reproducible composition are needed to determine whether PRP is useful in treatment of tendon disorders [109].
2.2. Stem cells
Cell-based tissue engineering is one of the most attractive and widely explored approaches for musculoskeletal regeneration. This strategy relies on reparative cells, alone or in combination with biocompatible scaffolds, which are delivered intra-operatively to the site of tissue damage. Selecting the appropriate cell type is one of the most important factors to be considered in such applications.
With regards to tendon engineering, several cell types, including MSCs from different tissue sources (bone marrow (BM), adipose tissue (AD), embryonic stem cells (ESCs), induced pluripotent stem (iPS) cells and TSPCs) are suggested as suitable targets (reviewed in [110–115]).
2.2.1. BM-derived MSCs
MSCs for tendon tissue engineering can be easily obtained from a BM aspirate. Although they represent only 0.001–0.01% of the total cell population, they can be expanded to higher numbers in vitro [116]. When appropriately stimulated, BM-MSCs can differentiate into various mesenchymal cell types, including osteoblasts, chondrocytes and adipocytes [117]. Attempts to commit BM-MSCs to the tenogenic lineage have been based on treatment with growth factors such as GDF-5 (BMP-14) and GDF-7 (BMP-12) [118,119], or upon genetic transduction with BMP-2 and active SMAD8, BMP-12, BMP-13 or scleraxis cDNA [120–122].
Overall, these attempts have been moderately successful; although the treated BM-MSCs adopted a tendon-like cell phenotype in vitro, it is still unclear whether the phenotype remains stable when the cells are implanted into a tendon lesion. One very attractive, potential feature of BM-MSCs is the possibility that they are hypoimmunogenic, therefore allogeneic transplantation may not require immunosuppression; furthermore they can exert immunomodulatory effects on various blood cell types resulting in anti-inflammatory impact during tissue repair [123]. It has been also suggested that these cells exercise in vivo potent trophic and stimulatory functions on local progenitors, thus contributing to tissue regeneration in this alternative manner, rather then differentiating on site into tissue-specific cell types [124]. Possible difficulties when using BM-MSCs for tissue repair include painful BM harvesting procedures, lengthy periods for cell expansion, uncontrollable differentiation in vivo into undesirable cell lineages and reduced qualities with donor age [123].
In comparison to other tissue sources, BM-MSCs are the best studied and characterized, and therefore the most frequently evaluated cell type for the repair of tendon tissue [125]. The majority of the in vivo models consist of partial or complete surgical transection or collagenase-induced lesion of horse, rabbit or rat tendons. The tendon types that are typically investigated include Achilles, patellar and digital flexor tendons. A summary of relevant in vivo studies, based on BM-MSC therapy of tendon injury, and their outcomes is given in Table 2. Taken together, these studies demonstrated improved histological and biomechanical properties of the tendon, indicating an increased rate of tendon healing and maturation. However, in many of the models ectopic bone formation was described and when biomechanically tested, the regained tendon strength was approximately 20–60% that of an uninjured tendon. In addition, only few studies have examined tendon healing after 6 weeks, thus the long-term effects of therapy on tendon strength, functional quality and performance or re-occurrence of the injury are unknown.
Table 2.
Tendon repair with bone marrow-derived MSC.
| Tendon type | Model | Conclusion | Reference |
|---|---|---|---|
| Achilles tendon |
Rat; surgical cut; MSCs cultured at hypoxic and normoxic conditions, analysis at 2 and 4 weeks |
Superior biomechanical testing in tendons treated with MSC cultured in hypoxic conditions |
[228] |
| Rat; surgical cut and enthesis destroyed; suture and MSC injection; analysis at 15, 30 and 45 days |
Improved healing and biomechanical properties; enthesis comparable to control |
[229] | |
| Rat; surgical cut; total BM cell or MSC injection in DMEM; analysis at 1, 2 and 3 weeks |
Biomechanical properties of tendon treated with BM cells comparable to normal tendon; MSC second best |
[230] | |
| Rabbit; surgical transection; MSC-fibrin; follow up at 1, 3, 6 and 12 weeks |
At 3 weeks improved histological and biomechanical properties with no difference at 12 weeks |
[231] | |
| Rabbit; surgical transection; knitted poly-lactide-co-glycolide scaffold loaded with MSCs; analysis at 2, 4, 8 and 12 weeks |
Higher rate of tissue formation and remodeling was observed early on with restored function similar to native tendon |
[232] | |
| Rabbit; hallucis longus tendons transfered into calcaneal bone tunnel; MSC treatment; analysis at 2, 4 and 6 weeks |
Improved healing of the insertion of tendon to bone in the early stage | [233] | |
| Rabbit; surgical cut; MSCs in collagen gel; analysis at 12 weeks | Constructs with lower cell density displayed superior biomechanical properties |
[234] | |
| Rabbit; surgical transection; MSC-collagen implants; analysis at 4, 8 and 12 weeks |
Improved collagen organization and increased load properties | [158] | |
| Patellar tendon |
Rat; surgical transection; MSCs with fibrin injection; analysis at 10 and 20 days |
Biomechanical properties were not significantly improved but tendons displayed more mature organization without ectopic ossification |
[235] |
| Rat; surgical full thickness window defect; MSCs with fibrin injection; analysis at 10 and 20 days |
More dense collagen fibers, higher cellularity and matrix without ectopic ossification were observed |
[236] | |
| Rabbit; surgical cut; MSCs in dog decellularized tendon composites; analysis at 2 weeks |
MSC survived in multilayer composite and expressed tendon phenotype | [173] | |
| Rabbit; surgical cut; MSCs in a gel-sponge composite; analysis at 12 weeks |
Superior cellular alignment, but maximum force and stress compared to gel only |
[237] | |
| Rabbit; surgical defect; MSCs from young and aged rabbits in collagen I gels; analysis at 12 weeks |
No significant difference in biomechanical properties of tendons treated with young or aged MSC |
[238] | |
| Rabbit; surgical cut; implanted MSCs; analysis at 2, 3, 5 and 8 weeks | MSC survived and differentiated into tendon-like spindle cells | [239] | |
| Rabbit; surgical cut; MSC-collagen implants; analysis at 6, 12 and 26 weeks |
Ectopic ossification developed in approx. 25% of the tendons with MSC-collagen implant; no significant differences in mechanical properties across different seeding densities |
[159] | |
| Rabbit; surgical cut; MSCs in collagen gel; analysis at 4 weeks | Better biomechanical properties but no significant improvement of tendon microstructure |
[240] | |
| Superficial digital flexor tendon |
Horse; naturally-occurring tendon injury; MSCs with marrow supernatant; analysis at 6 months |
Treated group exhibited normalization on a biochemical, morphological and compositional level |
[241] |
| Horse; collagenase-induced tendinitis lesion; MSC injection; analysis at 8 weeks |
Increased tensile stiffness in MSC-treated group, but similar histological scores to controls without MSC |
[217] | |
| Horse; collagenase-induced tendinitis lesion; MSC injection | Repaired tendon architecture comparable to healthy tendon | [242] |
So far only few clinical trials have been conducted with BM-MSCs for therapy of tendons. Mazzocca et al. [126] isolated BM-MSCs from 11 patients during arthroscopic rotator cuff surgery. After cell expansion and treatment with insulin, the authors showed that the BM-MSCs gain features similar to those of tendon cells. In this study, however, the isolated cells were investigated in vitro and no implantation in the injured tendons was performed. Non-fractioned iliac-derived BM mononuclear cells have been injected into tendinous lesions in 14 patients with complete rotator cuff tear. After 12 months, the patients were evaluated with the UCLA (University of California, Los Angeles) score and MRI, both showing improved tendon healing and integrity. Only one patient had deterioration of tendon strength and pain after 1 year [127]. Despite the very preliminary nature of the above studies, the results suggested that BM-derived cells can be isolated, stimulated towards the phenotype of tendon cells and introduced into tendon defects. However, the tendon field is in great need of carefully designed, pre-clinical studies using large animal models aiming to: (1) monitor the fate of the implanted stem cells using different labeling techniques; (2) examine cell dose-dependent effects; (3) evaluate tendon properties after longer periods of times; and (4) standardize protocols and procedures, thus allowing direct comparison between different studies. Subsequent to this research, multicentre clinical trials can be initiated to validate the true potential and optimal mode of application of stem cells for the repair of human tendons. This approach is facilitated by the fact that BM-MSCs are already approved for human use in graft versus host disease, and are in a large number of human clinical trials for other indications. They are also used in veterinary medicine to treat several disorders, including teninopathies.
2.2.2. AD-MSCs
Subcutaneous adipose tissue is a source of stem cells that are very similar morphologically and molecularly to BM-MSCs. The AD-MSCs are also multipotent; however they are less efficient at osteogenic and chondrogenic differentiation, but excel in adipogenesis compared to BM-MSCs [128,129]. When treated with IGF-1 and TGFβ or GDF-5, AD-MSCs upregulate the expression of tendon-related gene markers, such as scleraxis and tenomodulin [55,130]. Kryger et al. [131] compared AD-MSCs to tendon-derived cells and BM-MSCs, and found comparable scaffold adherence and proliferation potential, hence suggesting AD-MSCs as alternative cell type for tendon tissue repair. When injected into horse superficial digital flexor tendons with collagenase-induced tendinitis, AD-MSCs improved collagen fiber organization and overall tendon structure [132]. Increased yield loads and energy absorption were described in a rabbit injury model of deep digital flexor tendons treated with AD-MSCs [133]. In sum, AD-MSCs are widely available, multipotent cells that are simple to obtain without high morbidity, and represent an attractive source of cells for tendon tissue engineering. Nevertheless, there has been little exploration of using AD-MSCs for tendon therapy [134]. A major difficulty is to restrict AD-MSC differentiation within the tendon defect site to the tendon cell lineage, avoiding their indigenous preference towards forming adipocytes. Nevertheless, the risk of heterotopic ossification should be less than when using BM-MSCs [134]. Like BM-MSCs, AD-MSCs are already in clinical trials for other indications.
2.2.3. ESCs and iPS cells
ESCs constitute the inner cell mass of blastocysts and are able to produce all different cell lineages from the three germ layers [135]. Unlike MSCs, they can be passaged indefinitely. Therefore in various tissue and organ regenerative models, including cardiovascular, neuronal and pancreatic repair, ESCs have considerable advantages [135]. Despite some clear benefits of ESCs over adult stem cells, their application raises social and moral issues regarding disassembly of embryonal tissues [135]. Other obstacles that have to be resolved when using ESCs include teratoma formation and spontaneous differentiation. Therefore, in the recent years iPS cells, generated by genetic reprogramming of adult lineage committed cells, have been suggested as alternatives to ESCs [136]. These cell types have overlapping characteristics and yet iPS cells can overcome many current ethical concerns in ESC-based therapy.
So far ESCs and iPS cells have not been extensively studied for tendon repair due to the lack of protocols to differentiate these cells into the tendon lineage. Non-differentiated ESCs have been injected in horse tendon lesions 1 week after collagenase-induced tendonitis, and histology and ultrasound analyses demonstrated improved lesion size [137]. Guest et al., [138] directly compared the survival of ESCs to MSCs after implantation into surgically created tendon defects in the horse. Interestingly, ESCs were detectable up to 90 days post operatively, while less then five percent of MSCs survived. However, in these studies it remains unclear if ESCs successfully differentiated into tendon progenitors. Chen et al. [139] were the first to propose stepwise differentiation of human ESCs into tenocytes via an MSC intermediate step. Cell sheets of ESC-derived MSCs were engineered into tendon-like layers under static mechanical load in vitro and used to repair a window defect in the patellar tendon. The implanted cells were detectable at least 4 weeks after surgery, and the ESC-MSC-treated tendons were larger than the controls and contained continuous collagen fibers and cells resembling tenocytes. Importantly, because of the stepwise differentiation procedure, the risk of teratoma formation is greatly reduced and, indeed, was not observed in vivo.
A study by Xu et al. [140], was the first to report a positive effect of human iPS cell-derived neural crest stem cells combined with a fibrin gel on the healing of rat patellar tendon window defects. Histological and mechanical analyses demonstrated improved matrix synthesis and superior mechanical properties of defects treated with iPS cells. Interestingly, the authors also found that the transplanted cells produced fetal tendon-related matrix proteins, stem cell recruitment factors, and tenogenic differentiation factors, and accelerated the host endogenous repair process. The above results suggest that ESCs and, presumably iPS cells, can be applied safely in tendon regeneration after controlling their differentiation pathway.
2.2.4. Tendon-derived cells
Although knowledge of the differentiated cells resident in the tendon tissue has increased, still little is known about their precursors. Furthermore, the field is still lacking clear terminology on the different subsets of tendon cells. This is mostly due to difficulties to purify, expand, maintain and compare populations of pure stem cells, progenitors, tenoblasts and tenocytes. For this reason we have given this section a unifying title.
Stem/progenitor cells of mesenchymal origin are of great interest in understanding tendon development and the healing processes. As mentioned previously, Bi et al., [27] demonstrated that human and mouse tendons harbor an unique cell population that has both stem cell but also tendon-specific characteristics. For example, these tendon-derived cells expressed high levels of scleraxis, cartilage oligomeric matrix protein (COMP), tenascin-C and tenomodulin, all tendon-related factors. Because the cells of this population showed heterogeneity in their stem cell properties, the authors named them tendon stem/progenitor cells (TSPC). When compared to BM-derived MSCs, TSPC were closely related, but not identical in terms of molecular marker profile and in vivo behavior. When the cells were applied in vivo, TSPC formed tendon- and enthesis-like structures, whereas BM-MSC formed bone- and BM-like structures. However, in this study, the cells were not used in a clinically related, tendon defect model. In addition, this study showed that TSPC reside within a unique niche, where the two extracellular proteoglycans biglycan and fibromodulin control their functions by modulating BMP signaling.
The double knockout of these two proteoglycans is characterized by higher tendon cellularity together with decreased collagen fibril thickness. TSPC isolated from these mice had augmented clonogenicity and cell proliferation, but reduced collagen type I and scleraxis expression. Lastly, Bi et al. [27] were the first to show that there is a link between distorted TSPC functions and tendon pathology, since TSPCs within the biglycan/fibromodulin-deficient tendon niche were far more responsive to BMP signaling, leading to TSPC favoring the osteogenic lineage. In turn, this resulted in so-called in-tendon ossification. Thus, the above data suggest that the molecular environment provided by the niche is essential for the correct maintenance and differentiation of the stem/progenitor cells during tendon development and repair.
Studies by Tempfer et al., [28] and Kohler et al., [29] also demonstrated the existence of a TSPC population within human supraspinatus and Achilles tendons, respectively. Several articles have suggested that tendon-derived stem cells (TDSC) can be isolated, expanded and eventually used in regenerative strategies (reviewed in [141,142]). Purification and expansion of a cell population containing only TDSCs is still difficult, because we lack molecular markers discriminating the discrete steps of tendon cell lineage differentiation from primitive stem cells via progenitors to mature tenocytes, as well as the incomplete differentiation of the primary cells. Because of this, we have used in the text the term TSPC. In order to unite and validate the existing data, the tendon field urgently requires: (1) to standardize the protocols for TSPC enrichment; (2) to develop appropriate methods to separate stem cells from progenitors; (3) to establish efficient methods for achieving terminal tenogenic differentiation in vitro which will permit validation of TSPC properties; and (4) to determine if TSPC differentiation in vitro reflects their differentiation capacity in vivo.
The discovery of TSPCs had a major impact in the field, since TSPCs might be involved in tendon tissue homeostasis and repair; alternatively, they can be used for practical purposes in tissue engineering strategies for injured tendons. Still, there remains the need to clarify whether embryonic tendon progenitors and TSPCs are identical cell populations as well as to generate solid data concerning TSPC location and function in vivo. Tempfer et al., [28] have shown that cells expressing simultaneously tendon and pericyte-associated marker genes are localized to the perivascular space of tendon tissue, hence suggesting that this niche might be the source of local stem/progenitor cells. Still, tendons are poorly vascularized, hence the contribution of perivascular cells to the regulation of tendon cell fate and functions might be less pronounced than in tissues with high blood supply. Interestingly, Mienaltowski et al., [36] reported the existence of two different stem/progenitor populations within the peritenon and tendon proper of mouse Achilles tendons. More studies are required to reconstitute carefully the regional cell composition of tendons and the interconnections between different cell types. Improving our knowledge on the above questions can provide novel, fundamental understanding not only of the development of tendon tissues, but also of their sustainability and repair.
In terms of practical application, there are several challenging issues to solve prior the use of tendon-derived cells for tendon repair. Allogeneic cells may lead to an immune reaction, whereas autologous tendon-derived cells will avoid immune complications, but may lead to a comorbid state in the patient. Furthermore, during in vitro expansion, tendon-derived cells may undergo phenotypic drift. Yao et al., [143] have shown in human tendon cells that the ratio between collagen III and I increases with progressive passaging, while decorin expression significantly decreases. Schulze-Tanzil et al., [144] suggested that the differentiated state could be preserved if the cells are grown in three-dimensional pellets. A combination of ultrastructural, biochemical and molecular analysis indeed demonstrated that the phenotypic identity of the tendon-derived cells was retained when the cells were cultured in this fashion [144]. Therefore, it will be important to establish in vitro culture systems, based on three dimensional cultivation or by using mechanical strain, which can support unaltered TSPC identity over longer periods of time.
To date, only few studies have used tendon-derived cells in tendon repair models. Cao et al., [145], used autologous tenocytes to bridge tendon partial defects in hens and observed 14 weeks postoperatively that the engineered tendons displayed histologically a structure very similar to that of normal tendons. Similar conclusions were reached by Chen et al., [146] after introducing autologous patellar tenocytes cultured on porcine bioscaffolds into massive rabbit rotator cuff defects and analyzing tissue regeneration at 4 and 8 weeks. The authors concluded that implantation of tenocyte-loaded scaffolds results in superior tendon healing when compared to the control group receiving only scaffold. However, autograft tendon controls were still better then the engineered tenocyte constructs, suggesting that further optimization of the technology is required. Stoll et al., [147] investigated the healing of a partial Achilles tendon in the rabbit after filling defects with Achilles tenocytes loaded onto polyglycolic acid (PGA)/fibrin scaffolds. The authors generated novel scoring systems for macroscopic, histological and elastic fiber assessment of the progress of tissue repair. Although no clear advantage of the tenocyte-scaffold group was detected at 6 and 12 weeks post operation, this study provides a useful multifaceted scoring system for characterization and cross-study comparison of tendon healing models.
Tenocyte-based regeneration of full size Achilles tendon defects in rats has been compared to that based on BM-MSCs [148]. For bridging the defect ends, PGA and collagen type I scaffolds seeded with one or the other cell type were used and fixed with a frame suture. After 16 weeks, DNA from the implanted cells was detected in the regenerated tissue, consistent with their long-term survival. Despite evidence of central ossification in all study groups, biomechanical tests revealed that samples loaded with tenocytes had significantly better failure strength/cross-section ratios compared to defects receiving BM-MSCs or inseeded scaffolds. Ni et al., [149], were the first to describe histologically, biomechanically and ultrasonographically that TSPCs can improve the repair of a rat patellar tendon window defect. No ectopic bone formation was detected up to 4 weeks post-injury. How implanted tendon-derived cells influence local cells at the site of tendon damage is unclear and needs further clarification. More studies are also required to understand the fate and long-term effects of tendon-derived cells during tendon repair, and to compare the benefits of using these cells rather than MSCs from different sources. The main advantage of tendon-derived cells is that, being native in origin, when re-implanted into tendon defects, they will better accommodate to familiar environment and are likely to survive longer and differentiate more easily into terminal tenocytes.
Taken together, this area holds much promise for tendon therapy after clarifying many open questions. It will be of great importance to investigate further the identity, genetic marker profile, localization and in vivo functions of TSPCs as well as to carry out well-designed pre-clinical experiments to determine the role of TSPCs during the progression of tendon diseases and subsequent repair processes.
2.2.5. Unresolved issues in cell-based therapy for tendons
The involvement of each of the above cell types has to address a number of important challenges, such as determining: the amount of cells needed; whether combination with growth factors or genetic modification is helpful and, if so, which growth factors or genes to use; which scaffolds, if any, to use; the optimum time point of delivery. To define the ideal cell number, dose-dependent in vivo studies will be important. Most likely the optimal number of cells will vary relative to the size of the tendon defect, the type of cell type used and the particular tendon in need of repair. Therefore, it is necessary in forthcoming research to define and understand the exact mechanisms underlying the in vivo performance of different cell types. Crucial questions include: do the implanted cells provide trophic support and stimulate local progenitors or do they commit on-site into the tenogenic lineage? A useful approach to address such questions will be to develop or employ strategies to label and track the implanted cells during the different healing stages. In addition to determining the optimal ratio of implanted cells to defect dimensions, the field has to resolve how to obtain and multiply in a short time frame the required amount of stem cells, without harming their innate ability to differentiate or causing uncontrolled differentiation. Co-delivery of stem cells with growth factors or using genetically altered stem cells can enhance their qualities and navigate them quicker and more effectively into the preferred differentiation cascade. However, such manipulations could have poorly controlled side effects on local stem and progenitor cell populations.
The seeding of the stem cells onto different matrices can improve their maintenance and amplification at the site of tendon injury. Furthermore, the topography and mechanical properties of the carriers can be designed in a way to direct stem cell differentiation towards tendon progenitors or even mature tenocytes. The precise timing of stem cell delivery can be determined by the cell type and carrier combination. For example, when MSCs are to be used, implantation can take place already at the inflammatory stage since this cell type might exert a beneficial effect by participating in immunomodulation, reducing inflammation and stimulating local stem cells and tenoblasts. When tendon-derived cells are to be used, it might be more appropriate to introduce them at the later, proliferative stage when they can speed the endogenous healing process.
With regards to the timing of the complete procedure the following factors should be considered: (1) hospitalization time of the patient; (2) time to obtain, enrich and multiply the cells; (3) if the cells are modified or stimulated, the time necessary to carry out such procedures; (4) if the cells are applied in combination with biomaterials, the time for scaffold loading and cell adhesion. Ideal protocols should contain only a few short steps. For example, one way to speed up the preparation will be to purify the reparative cells from the primary cell milieu with cell-specific surface antigens. Finally, stem cells of autologous, allogenic or xenogenic nature should be compared. Each of these variants holds advantages and disadvantages. In autologous applications, it is important to determine donor site morbidity and the fitness of the stem cells since tissue aging or pathology can distort stem cell functions. Recent investigations focusing on BM-MSC demonstrated apparent age-related changes such as a reduced proliferation and clonogenicity as well as altered differentiation potential [150,151]. Baxter et al., [152] observed rapid telomere shortening and earlier entry into growth arrest and senescence; Kasper et al., additionally found reduced antioxidant defense, altered cytoskeleton organization and lower migratory capacity of aged BM-MSCs [153]. We have recently reported that human TSPCs from aged and degenerated tendons have significantly reduced proliferation capacity and premature entry into senescence, decelerated motion and delayed wound closure due to dysfunctional actin dynamics [29]. Hence, similar to other tissues, aging and disease exhaust the local stem cell pool in terms of size and functional fitness. Therefore, when such autologous cells are candidates for use in tendon repair in aged individuals, various possibilities to correct their endogenous deficits or to pre-activate them ex vivo or in situ via growth factor stimulation or gene therapy have to be carefully considered [154]. The development of suitable allograft donor cells would obviate many of these problems. However, the use of allogenic cells can also be associated with difficulties such as obtaining sufficient donor material, donor background diseases, prolonged storage of the cells anda possible deterioration of cell quality during storage. One major advantage of xenograft cells is that they can be available in large numbers and ready for use at any moment. However, their use is limited while possible zoonotic diseases and xenograft reactions have to be considered and addressed.
2.3. Biomaterials
Currently, tendon repair involves the use of autologous or allogeneic tendon transfer, which can restore tendon function in the affected area. However, both options have drawbacks, the first related to donor site morbidity and the second to risk of immune rejection. In addition, rarely does the transferred tendon material match the tensile properties of the repaired tissue. Therefore, a number of biomaterials have been explored as alternatives to tendon transfer for tendon tissue engineering (reviewed in [155,156]). Some of these materials have been borrowed from the neighboring fields of cartilage and bone tissue engineering, and some have been specifically designed to resemble as close as possible the structural and biomechanical features of native tendon tissue. The ideal scaffold should cover a number of requirements such as: (1) to be biocompatible; (2) to support cell attachment and growth; (3) to have high surface area; (4) to promote tenogenic differentiation pathway; (5) not to induce host inflammatory responses; (6) when not biodegradable, to mimic native tendon architecture and mechanical properties. Furthermore, the scaffold should be easily reproducible, scalable, have good storage properties and, ideally, able to be customized.
Natural biomaterials include: collagen; silk; fibrin; hyaluronic acid; elastin; alginate; chitosan; porcine small intestine submucosa (SIS); human, porcine or bovine dermis; and decellularized tendon xenografts [155–157]. Most biomaterial studies have investigated how MSCs or tendon-derived cells respond to these materials in terms of cell adhesion, cell proliferation and survival over time, gene expression and differentiation [155–157]. Some of the studies have taken a step further into in vivo testing of the materials, alone or in combination with cells, and have examined host tissue reactions or tendon healing process (refer also to [115,157]). Some examples of studies on collagen-based scaffolds and xenografts will be discussed here.
2.3.1. Collagen-based materials
Collagen gels and composites, most frequently loaded with BM-MSCs, have been used for repair of different tendon gap models, as indicated in Table 1. In the articles of Young et al., [158] and Awad et al., [159] experimental groups treated with cell/gel implants achieved higher strength compared to suture-only controls. Interestingly, in the second study no additional benefit of increasing cell density in the collagen type I gel was found [159]. Another study showed that reducing cell to collagen ratio by 20-fold actually improved cell viability, lowered the degree of ectopic bone formation and enhanced the biomechanical properties of patellar tendon 12 weeks post-operatively [160]. It was suggested that material implants should exhibit physical properties similar to normal tendon tissue, but should be degradable. This would allow support and protection of the introduced cells in the early phases of the healing, but also replacement of the scaffold over time during de novo production of tendon matrix [160].
As mentioned earlier, critical design criteria for the ideal tendon graft requires the material to exhibit the mechanical properties of normal tendon, to facilitate functional integration and also to promote native tendon regeneration. Nanotechnology-based approaches allow development of various biomimetic scaffolds such as nanofibers and nanocomposites. Specifically, aligned nanofibers from collagen type I hold advantages because of their potential to mimic the matrix architecture of native tendon and, in turn, to regulate cellular responses. In vitro studies with cell-loaded aligned collagen I [161,162] convincingly showed that the aligned scaffold topography can induce a cell morphology similar to that of tenocytes, achieve matrix alignment and promote the upregulation of tendon-related genes such as scleraxis and collagen type XIV. Furthermore, the in vivo investigation by Kishor et al., [161] reported that braided, aligned collagen type I fibers introduced in longitudinally incised rabbit patellar tendons undergo limited degradation and associate with a low-grade granulomatous inflammation. Additionally, quantitative histology revealed that the cross-sectional areas of tendons treated with the aligned scaffolds were larger and stiffer than controls. In sum, the above studies suggested that aligned nanofibers are superior to randomly oriented biomaterials, because they are biocompatible and, moreover, can stimulate the implanted cells to differentiate towards the favorable tenogenic lineage. Thus they have the potential to be used as carriers for tendon tissue engineering applications. One critical limitation of these scaffolds is scale-up to dimensions relevant for the repair of human tendon.
2.3.2. Xenografts
A possible way to overcome the difficulty of generating stable scaffolds in large sizes is to use xenograft tissues which have matching and customized proportions similar to those of human tendon defects. FDA-approved porcine SIS devices (Restore and CuffPatch) have been used in a number of laboratory studies of rotator cuff and Achilles tendon injury models performed in dog [163–165], rabbit [166] and rat [167]. Although the properties of healthy tendon were not fully restored, the studies reported positive histological and mechanical outcomes in comparison to non-treated defects. Furthermore, upon analyzing SIS degradation patterns, it was found that SIS is subjected to rapid degradation in the first 4 weeks after surgery, which suggests that it can serve as a temporary scaffold for quick cellular infiltration [165]. Following these encouraging results, multiple clinical studies were conducted with patients undergoing rotator cuff or Achilles tendon surgery (reviewed in [155]). Earlier investigation suggested successful tendon reconstruction with SIS devices in 11 out of 12 patients up to 2 years after the surgery [168]. However, subsequent investigations found that SIS-treated groups had no augmented properties and that SIS incorporation did not improve the rate of tendon healing [169–171]. The reasons for this discrepancy are not entirely clear and the major side effect reported in the above studies was a non-infectious effusion.
Decellularized tendon, of allograft or xenograft origin, is another tissue with promise for tendon repair. This application consists of harvesting tendon pieces from cadavers or animals which, after decellularization and slicing, are re-seeded with BM-MSCs, and finally packaged together into a single scaffold. Interestingly, when cultivated on such matrices, BM-MSCs exhibit a phenotype resembling tendon cells, suggesting yet again that the appropriate nano-topography and stiffness can enforce lineage differentiation [172–174]. It is logical to conclude that the best choice for tendon repair is tendon or ligament ECM; however, there are several unresolved difficulties with the use of decellularized tendon scaffolds, such as the poor cell repopulation of the deeper tendon layers and the observation that the decellularization procedure reduces the mechanical properties of the grafts.
Scaffolds derived from human cadaver (GraftJacket), bovine (TissueMend and Bio-Blanket) and porcine dermis (Permacol) have a rich collageneous matrix, retain native dermal ECM architecture and vascular channels, and have been approved by FDA for the reinforcement of soft tissues. More than a few studies have shown their efficacy in rotator cuff and Achilles tendon repair. For example, Adams et al., [175] studied the use of human cadaver dermis in a canine infraspinatus injury model and found robust tendonous tissue formation at the site of scaffold implantation after 6 months. Bond et al., [176] reported improved shoulder mobility and decreased pain after implantation of human dermis in patients with rotator cuff tears. Positive outcomes after applying human dermis in the repair of large rotator cuff defects were also documented by Rotini et al., [177] and Snyder et al., [178]. The healing effect of human dermis materials on Achilles tendon ruptures was shown in a series of clinical studies published by Lee et al., [179] and Lee et al., [180]. Early return to physical activity, improved foot strength, reduced chronic pain and no re-ruptures were observed up to 30 months after surgery. Interestingly, Valentin et al., [181] examined histologically the host tissue response to porcine SIS and human, bovine and porcine dermis devices in a rodent model. The authors found that each biomaterial leads to a distinct form of tissue remodeling in terms of cellularity, vessels, inflammation and matrix organization. Interestingly, xenograft dermis scaffolds degraded slowly and were associated with higher inflammatory score and accumulation of denser and less organized fibrous tissue. Therefore, future controlled comparison studies are necessary to clearly define the advantages and limitation of each biomaterial.
In conclusion, natural biomaterials show significant promise for enhancing tendon repair especially in combination with reparative cells. At present, the tendon field still lacks classical biologics or tendon-specific drugs to aid repair. Therefore biomaterials, especially scaffolds mimicking native tendon architecture, represent a smart alternative option to solve one of the major problems of the field, namely to drive the reparative cells into the appropriate tendon cell lineage. However, the available data do not permit definitive conclusions, and often the use of identical materials, for example dermis scaffolds, produces variable results between different studies [175,181]. Thus, more investigations are required to improve and standardize the properties of biomaterials and to evaluate their role in the clinical practice of tendon repair.
2.4. Gene therapy
2.4.1. Concepts
It has been recognized for a long time that gene therapy has the potential to promote the repair and regeneration of damaged tissues, including tendons [182–185]. In this context, gene transfer is not used to compensate for a defined genetic defect, but instead to serve as a biological delivery system for the encoded gene products.
Gene transfer holds many advantages over traditional methods for delivering biologicals to sites of injury. In particular, it allows the local, focal production of gene products within and around the lesion in a sustained and potentially regulated fashion. Moreover, proteins synthesized locally as a result of gene transfer are likely to have undergone authentic post-translational processing leading to greater biological activity and a reduced risk of triggering neutralizing immune reactions. Gene transfer is particularly useful for delivering gene products with an intracellular site of action, including non-coding RNA molecules, signaling molecules and transcription factors; SMAD8 and scleraxis are relevant examples of the last two [120,122].
2.4.2. Vectors
Vectors are used to transfer genes (usually cDNAs) to target cells. Because viruses are naturally able to transfer with high efficiency their genes to the cells they infect, they have been widely used as vectors. For this purpose, the viral genome is manipulated to remove sequences required for replication and virulence, while retaining those needed for infectivity. (The ability to replicate is retained in certain cancer gene therapy applications.) Therapeutic genes can be spliced into the genetic space generated by these manipulations to produce a viral vector that, in principle, can infect a target cell and deliver its genetic payload to the nucleus without replicating or causing adverse events.
Recombinant viruses so far studied experimentally for gene delivery to tendons and ligaments include adenovirus, lentivirus, retrovirus, adeno-associated virus (AAV). The main properties of these four vectors are compared in Table 3, bearing in mind that the many modifications made progressively to these vectors make simple generalizations increasingly difficult. Gene transfer with a viral vector is known as transduction.
Table 3.
Salient properties of vectors used in experimental studies of tendon healing.
| Vector | Key properties | Advantages | Disadvantages | Comment |
|---|---|---|---|---|
| Adenovirus | Non-integrating Multiple serotypes Double stranded DNA genome |
Straightforward production Efficient Transduces non-dividing cells Wide host range |
Inflammatory Antigenic |
Widely used in clinical trials One well publicized death |
| Adeno-Associated Virus |
Recombinant AAV is non-integrating Wild-type AAV has single stranded DNA genome Multiple serotypes |
Transduces non-dividing cells Wild-type AAV causes no known disease Non-inflammatory |
Difficult to produce Small carrying capacity |
Possible to engineer AAV with double stranded DNA genome Increasingly popular for clinical trials because of safety |
| Retrovirus | RNA genome Integrating |
Straightforward to produce Amphotropic virus has wide host range |
Transduction requires host cell division Risk of insertional mutagenesis |
Usually used in ex vivo gene delivery Has been widely used in clinical trials Insertional mutagenesis has caused leukemia |
| Lentivirus | RNA genome Wild-type virus is integrating |
Transduces non-dividing cells Very high levels of transgene expression |
Risk of insertional mutagenesis, but non-integrating vectors developed |
Increasing use in clinical trials |
Because clinical grade viral vectors are expensive and complicated, there is continuing interest in non-viral vectors for gene delivery. These raise less safety issues, are usually simpler to manufacture, have less restrictions in carrying capacity, often lower immunogenicity and should make quicker progress through the regulatory process for human use. Non-viral vectors can be as simple as naked, plasmid DNA. Often, the efficiency of gene transfer is improved by combining the DNA with a polymeric carrier or by using a physical stimulus such as electroporation. Non-viral gene transfer is known as transfection.
The properties of viral and non-viral vectors used in regenerative orthopedics have been reviewed in several recent publications (refer to [186–188]).
2.4.3. Strategies
Regardless of the vector, there are two general gene delivery strategies, in vivo and ex vivo. For in vivo delivery, the vector is introduced directly into the body by injection or other form of direct application. Because the cellularity of tendon is low, in vivo administration in this way should not lead to high levels of transgene expression. Nevertheless, there exist several examples of its successful application in animal models of tendon healing (Table 4).
Table 4.
Use of gene transfer to promote tenogenesis in animal models of tendon injury.
| Gene | Vector | Delivery mode | Animal model | Reference |
|---|---|---|---|---|
| BMP-14/GDF-5 | Adenovirus | In vivo | Rat, Achilles | [206] |
| Adenovirus | In vivo | Rat, Achilles | [207] | |
| AAV | In vivo | Mouse, flexor tendon | [190] | |
| BMP-13/GDF-6 | Adenovirus | Ex vivo/MSC | Rat, rotator cuff | [208] |
| BMP-12/GDF-7 | Adenovirus | In vivo | Chick, flexor tendon | [205] |
| Adenovirus | Ex vivo/muscle | Rat, Achilles | [194] | |
| TGFβ, VEGF | Adenovirus | Ex vivo/MSC | Rabbit, Achilles | [213] |
| TGFβ | Adenovirus | Ex vivo/muscle | Rat, Achilles | [195] |
| Scleraxis | Adenovirus | Ex vivo/MSC | Rat, superspinatus | [211] |
| SMAD8, BMP-2 | Liposome | Ex vivo/MSC | Rat, Achilles | [120] |
| bFGF | AAV | In vivo | Chick, flexor tendon | [212] |
| PDGF-B | Liposome | In vivo | Rat, patellar tendon | [215] |
| Nanoparticle | In vivo | Rat, Achilles Tendon | [243] | |
| Retrovirus | Ex vivo/tendon fibroblasts |
Rat, rotator cuff | [214] | |
| Interleukin-10 | Lentivirus | In vivo | Mouse, patellar tendon |
[216] |
An alternative in vivo application strategy uses a scaffold impregnated with vector; this is known as a gene-activated matrix (GAM). This concept has been applied to tendons by associating adenovirus vectors with a gelatin sponge [189] and by using allograft tendon as a scaffold for AAV in a process known as “allograft revitalization” [190].
During ex vivo delivery, cells are genetically modified outside the body and then injected or otherwise implanted at the appropriate site. Ex vivo delivery combines gene therapy with cell therapy and is increasingly popular when progenitor cells, such as MSCs, are used.
Although the methods of in vivo gene delivery are simpler than ex vivo delivery, the latter is presumed to be safer because viruses are not introduced directly into the body. Because the recombinant viruses used for this type of gene therapy cannot replicate, the cells that carry them do not shed infectious particles. It can, however, be argued that the cells used in ex vivo gene delivery may have been cultured in media containing xenogeneic components, thereby introducing an element of risk, although the same would be true of ex vivo cell therapy in general.
Also, as noted, ex vivo gene delivery offers the possibility to combine the power of cell therapy with that of gene therapy. However, clinical application of such an approach is constrained by the present need to use autologous cells, which makes the process expensive and cumbersome. Development of suitable allogeneic cell lines for this purpose would greatly expedite the process.
To expedite ex vivo delivery, there is interest in developing technologies where suitable tissues that harbor accessible progenitor cells are harvested, genetically modified and reimplanted during one surgery [191,192]. Using a technique that was first developed for bone healing [193] genetically modified muscle grafts have been employed for tendon healing in animal models [194,195].
Although regulated transgene expression has not yet been explored in the context of tendon gene therapy, the availability of inducible promoters allows consideration of this approach. This reflects the likelihood that optimal healing may require the level of transgene expression to vary during the healing process. Also, such promoters allow the theoretical possibility of expressing one or more genes at different times from a polycistronic vector.
2.4.4. Progress
Early experiments confirmed the ability of various viral [196–199] and non-viral vectors [200–203] to deliver marker genes to ligaments and tendons by in vivo and ex vivo means. This work has been comprehensively reviewed by Hildebrand et al., [204]. Once marker gene delivery was achieved, it became possible to investigate the results of transferring genes with therapeutic potential.
As summarized in Table 4, most published studies using animal models of tendon repair have taken the approach of delivering a growth factor, especially one expected to promote the differentiation of progenitor cells into tenocytes. Promising results have been reported with BMP-12/GDF-7 [194,205] and BMP-14/GDF-5 [190,206,207], but not BMP-13/GDF-6 [208], even though all three of these induce tenogenesis in other systems [56,209] and BMP-13 gene transfer to MSCs induces ligamentogenesis in vitro [121]. It is possible that mechanical factors account for this discrepancy [210]. Transfer of scleraxis has been shown to promote the differentiation of MSCs into tenocytes in vitro [122] and, when used ex vivo with MSCs, to enhance healing of the rotator cuff in a rat model [211]. Similar results were reported using a combination of BMP-2 and SMAD8 cDNAs to promote tenogenesis [120].
Other investigators have transferred cDNAs encoding growth factors that are not specifically associated with differentiation of tenocytes, but which may enhance cellularity, vascularity or the deposition of extracellular matrix. Examples include TGFβ [195], bFGF [212], VEGF [213] and PDGF-B [214,215]. In general, the results have been encouraging.
Because repair and regeneration are progressive, multi-step processes, there is also interest in delivering more that one growth factor. However, in the studies of Hou et al. [213], although ex vivo transfer of TGFβ cDNA improved healing in a rabbit Achilles injury model, VEGF did not and added nothing to the effectiveness of TGFβ. It is probable that different growth factors will be needed at different times during the healing process, in which case their genetic transfer will require staged delivery of vectors with different transgenes, or single delivery of a polycistronic vector with different, inducible promoters. The latter will be very difficult to navigate through the regulatory process and into human clinical use.
Inhibiting inflammation is an alternative approach to promote repair and regeneration, and successful use of IL-10 cDNA in this regard has been reported [216]. Anti-inflammatory gene products may also be of therapeutic use in tendinitis. IGF-1 gene transfer has been explored as a way of augmenting tendon structure in tendinitis [217].
Additional uses of gene transfer in treating tendinopathies are listed in Table 5.
Table 5.
Use of gene transfer to address additional aspects of tendinopathy.
| Gene | Vector | Delivery mode | Species, indication | Reference |
|---|---|---|---|---|
| Sox-9 | Adenovirus | In vivo | Rabbit, bone–tendon junction | [222] |
| IGF-1 | Adenovirus | Ex vivo/MSC | Horse, tendinitis | [217] |
| BMP-4 | Lentivirus | In vivo | Rat, tendon insertion site | [218] |
| BMP-2 | Adenovirus | In vivo | Rabbit, tendon insertion site | [219] |
| MT1-MMP | Adenovirus | Ex vivo/MSC | Rat, tendon insertion site | [221] |
| siRNA-Runx2 | Adenovirus | In vivo | Mouse, heterotopic ossification | [223] |
| siRNA-Runx2, SMAD4 | Adenovirus | In vivo | Rat, hetertopic ossification | [224] |
| shRNA-Decorin | Lentivirus | Ex vivo/tendon cells | Rat, patellar tendon | [225] |
There is interest in using osteogenic genes to enhance the incorporation of tendon into the osseous insertion site after reconstructive surgery [218–220]. Because the tendon at this site has a fibrocartilagenous zone, there is also the possibility to improve the function of the regenerate repair by promoting the formation of cartilage in this important area using chondrogenic genes [221,222].
Another application seeks to prevent the formation of fat or bone within tendon, a risk when using multipotent progenitor cells as agents of repair. In this case, the ex vivo use of tengogenic cDNAs, such as those encoding scleraxis [122,211], SMAD8 [120] or the appropriate BMP would steer the cells towards tenogenesis and prevent them from differentiating along adipogenic or osteogenic lineages. Heterotopic ossification can also occur without the addition of such cells as a result of injury. Inhibitory species of non-coding RNA that knockdown Runx2 or SMAD4 have shown promise in blocking this process [223, 224]. Knockdown of decorin has been explored as a way of inhibiting scar formation [225].
2.4.5. Translation
Approval of clinical protocols for orthopedic applications of gene therapy is a long, expensive and tedious process [226]. Although the literature, summarized here, holds promise of success for gene-based technologies to regenerate tendons, the data are preliminary and restricted to acute, small animal models. The optimal vector, transgene, promoter and delivery mechanism still need to be determined. Efficacy then needs to be confirmed in large animal models. Safety is a major issue for all gene therapy protocols, especially those involving non-lethal pathologies; the pharmacology and toxicology testing of gene therapeutics is complicated. The cost of a therapeutic is also a large factor in today's economic environment, which is one reason to favor expedited approaches that do not require the ex vivo propagation of autologous cells [191,192].
3. Concluding remarks
Repair of tendon injuries is a lengthy process that frequently results in poor structural, mechanical and functional quality of the healed tissue. At present, the clinical options for treating tendon injuries are often unsatisfactory, especially in elderly populations. Therefore, several alternative strategies are being explored. The concept of biological therapy is attractive, because it makes use of the body's intrinsic potential to repair and heal its damaged tissues [191]. When tissue regeneration is approached in this manner, the result is expected to be natural, complete and lasting. As noted in this review, promising experimental data support the concept of using proteins, genes and cells, often in conjunction with biological scaffolds, as agents of the biological repair of tendons. However, these strategies are still in the stage of pre-clinical development and optimization. To reach their full potential and become realistic clinical options, further research focusing on solving critically important challenges is needed (Graphical abstract).
Acknowledgments
D. D. acknowledges the support for tendon research received over the years from the German Research Foundation (Grants: DFG DO1414/3-1, DO1414/1-1 and PO 1718/1-1), the Research Fund of the AO Research Foundation (Grants: S-10-47D and S-07-18D), and the Bavarian Research Foundation (Grant: DOK-100/08). C. H. E.'s work in this area has been funded by NIH grant R01 AR052809 from NIAMS. D. D. acknowledges Dr. Cvetan Popov for drawing Fig. 1 and M.Sc Sarah Dex and M.Sc Chifen Hsieh for technical assistance.
Abbreviations
- AAV
Adeno-associated virus
- ACS
Autologous conditioned serum
- ACL
Anterior cruciate ligament
- AD
Adipose tissue
- bFGF
Basic fibroblast growth factor
- BM
Bone marrow
- BMP
Bone morphogenetic protein
- COMP
Cartilage oligomeric matrix protein
- CTGF
Connective tissue growth factor
- ECM
Extracellular matrix
- ESC
Embryonic stem cell
- GAM
Gene-activated matrix
- GDF
Growth and differentiation factor
- GFP
Green fluorescent protein
- IGF-1
Insulin-like growth factor-1
- iPS
Induced pluripotent stem
- IL
Interleukin
- MSC
Mesenchymal stem cell
- MT1-MMP
Membrane-bound matrix metalloprotienase 1
- PGA
Polyglycolic acid
- PDGF
Platelet-derived growth factor
- PRP
Platelet rich plasma
- Runx2
Runt-related transcription factor 2
- SIS
Small intestine submucosa
- SMAD
Small body size mothers against decapentaplegic
- Sox-9
Sex determinating region Y - box 9
- TDSC
Tendon-derived stem cell
- TGFβ
Transforming growth factor beta
- TSPC
Tendon stem/progenitor cell
- VEGF
Vascular endothelial growth factor
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “Scaffolds, Cells, Biologics: At the Crossroads of Musculoskeletal Repair”.
References
- [1].Aslan H, Kimelman-Bleich N, Pelled G, Gazit D. Molecular targets for tendon neoformation. J. Clin. Invest. 2008;118:439–444. doi: 10.1172/JCI33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Benjamin M, Kaiser E, Milz S. Structure–function relationships in tendons: a review. J. Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Urwin M, Symmons D, Allison T, Brammah T, Busby H, Roxby M, Simmons A, Williams G. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann. Rheum. Dis. 1998;57:649–655. doi: 10.1136/ard.57.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chard MD, Hazleman R, Hazleman BL, King RH, Reiss BB. Shoulder disorders in the elderly: a community survey. Arthritis Rheum. 1991;34:766–769. doi: 10.1002/art.1780340619. [DOI] [PubMed] [Google Scholar]
- [5].Kannus P. Tendons — a source of major concern in competitive and recreational athletes. Scand. J. Med. Sci. Sports. 1997;7:53–54. [PubMed] [Google Scholar]
- [6].Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- [7].Gibson W. Are “spontaneous” Achilles tendon ruptures truly spontaneous? Br. J. Sports Med. 1998;32:266. [PubMed] [Google Scholar]
- [8].Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43:131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- [9].Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford) 2006;45:508–521. doi: 10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- [10].Mokone GG, Schwellnus MP, Noakes TD, Collins M. The COL5A1 gene and Achilles tendon pathology. Scand. J. Med. Sci. Sports. 2006;16:19–26. doi: 10.1111/j.1600-0838.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- [11].Jozsa L, Balint JB, Kannus P, Reffy A, Barzo M. Distribution of blood groups in patients with tendon rupture. An analysis of 832 cases. J. Bone Joint Surg. (Br.) 1989;71:272–274. doi: 10.1302/0301-620X.71B2.2494187. [DOI] [PubMed] [Google Scholar]
- [12].Benjamin M, Ralphs JR. Tendons and ligaments — an overview. Histol. Histopathol. 1997;12:1135–1144. [PubMed] [Google Scholar]
- [13].Maffulli NR, Leadbetter WB. Tendon Injuries. Springer-Verlag New York Inc.; 2005. P. [Google Scholar]
- [14].Wilkins R, Bisson LJ. Operative versus nonoperative management of acute Achilles tendon ruptures: a quantitative systematic review of randomized controlled trials. Am. J. Sports Med. 2012;40:2154–2160. doi: 10.1177/0363546512453293. [DOI] [PubMed] [Google Scholar]
- [15].Bishay V, Gallo RA. The evaluation and treatment of rotator cuff pathology. Prim. Care. 2013;40:889–910. doi: 10.1016/j.pop.2013.08.006. (viii) [DOI] [PubMed] [Google Scholar]
- [16].Raschke D, Schuttrumpf JP, Tezval M, Sturmer KM, Balcarek P. Extensor-mechanism-reconstruction of the knee joint after traumatic loss of the entire extensor apparatus. Knee. 2014;21:793–796. doi: 10.1016/j.knee.2014.02.003. [DOI] [PubMed] [Google Scholar]
- [17].Hintermann B, Knupp M. Injuries and dysfunction of the posterior tibial tendon. Orthopade. 2010;39:1148–1157. doi: 10.1007/s00132-010-1692-3. [DOI] [PubMed] [Google Scholar]
- [18].Drake DB, Tilt AC, DeGeorge BR. Acellular flexor tendon allografts: a new horizon for tendon reconstruction. J. Hand. Surg. [Am.] 2013;38:2491–2495. doi: 10.1016/j.jhsa.2013.03.039. [DOI] [PubMed] [Google Scholar]
- [19].Shybut TB, Pahk B, Hall G, Meislin RJ, Rokito AS, Rosen J, Jazrawi LM, Sherman OH. Functional outcomes of anterior cruciate ligament reconstruction with tibialis anterior allograft. Bull. Hosp. Jt Dis. 2013;71:138–143. [PubMed] [Google Scholar]
- [20].Liu CF, Aschbacher-Smith L, Barthelery NJ, Dyment N, Butler D, Wylie C. What we should know before using tissue engineering techniques to repair injured tendons: a developmental biology perspective. Tissue Eng. B Rev. 2011;17:165–176. doi: 10.1089/ten.teb.2010.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pennisi E. Tending tender tendons. Science. 2002;295:1011. doi: 10.1126/science.295.5557.1011. [DOI] [PubMed] [Google Scholar]
- [22].Sun Y, Berger EJ, Zhao C, An KN, Amadio PC, Jay G. Mapping lubricin in canine musculoskeletal tissues. Connect. Tissue Res. 2006;47:215–221. doi: 10.1080/03008200600846754. [DOI] [PubMed] [Google Scholar]
- [23].Kannus P. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports. 2000;10:312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- [24].Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J. Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- [25].Butler DL, Grood ES, Noyes FR, Zernicke RF. Biomechanics of ligaments and tendons. Exerc. Sport Sci. Rev. 1978;6:125–181. [PubMed] [Google Scholar]
- [26].Chuen FS, Chuk CY, Ping WY, Nar WW, Kim HL, Ming CK. Immunohistochemical characterization of cells in adult human patellar tendons. J. Histochem. Cytochem. 2004;52:1151–1157. doi: 10.1369/jhc.3A6232.2004. [DOI] [PubMed] [Google Scholar]
- [27].Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- [28].Tempfer H, Wagner A, Gehwolf R, Lehner C, Tauber M, Resch H, Bauer HC. Perivascular cells of the supraspinatus tendon express both tendon- and stem cell-related markers. Histochem. Cell Biol. 2009;131:733–741. doi: 10.1007/s00418-009-0581-5. [DOI] [PubMed] [Google Scholar]
- [29].Kohler J, Popov C, Klotz B, Alberton P, Prall WC, Haasters F, Muller-Deubert S, Ebert R, Klein-Hitpass L, Jakob F, Schieker M, Docheva D. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell. 2013;12:988–999. doi: 10.1111/acel.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng. A. 2010;16:1549–1558. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- [31].Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet. Disord. 2010;11:10. doi: 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Haasters F, Polzer H, Prall WC, Saller MM, Kohler J, Grote S, Mutschler W, Docheva D, Schieker M. Bupivacaine, ropivacaine, and morphine: comparison of toxicity on human hamstring-derived stem/progenitor cells. Knee Surg. Sports Traumatol. Arthrosc. 2011;19:2138–2144. doi: 10.1007/s00167-011-1564-3. [DOI] [PubMed] [Google Scholar]
- [33].Steinert AF, Kunz M, Prager P, Barthel T, Jakob F, Noth U, Murray MM, Evans CH, Porter RM. Mesenchymal stem cell characteristics of human anterior cruciate ligament outgrowth cells. Tissue Eng. A. 2011;17:1375–1388. doi: 10.1089/ten.tea.2010.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lovati AB, Corradetti B, Lange Consiglio A, Recordati C, Bonacina E, Bizzaro D, Cremonesi F. Characterization and differentiation of equine tendon-derived progenitor cells. J. Biol. Regul. Homeost. Agents. 2011;25:S75–S84. [PubMed] [Google Scholar]
- [35].Mienaltowski MJ, Adams SM, Birk DE. Regional differences in stem cell/progenitor cell populations from the mouse Achilles tendon. Tissue Eng. A. 2013;19:199–210. doi: 10.1089/ten.tea.2012.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Carpenter JE, Hankenson KD. Animal models of tendon and ligament injuries for tissue engineering applications. Biomaterials. 2004;25:1715–1722. doi: 10.1016/s0142-9612(03)00507-6. [DOI] [PubMed] [Google Scholar]
- [37].Warden SJ. Animal models for the study of tendinopathy. Br. J. Sports Med. 2007;41:232–240. doi: 10.1136/bjsm.2006.032342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J. Biomech. 2004;37:865–877. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- [39].Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu. Rev. Biomed. Eng. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- [40].Yang G, Rothrauff BB, Tuan RS. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res. C Embryo Today. 2013;99:203–222. doi: 10.1002/bdrc.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fenwick SA, Hazleman BL, Riley GP. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4:252–260. doi: 10.1186/ar416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].EVans CH. Cytokines and the role they play in the healing of ligaments and tendons. Sports Med. 1999;28:71–76. doi: 10.2165/00007256-199928020-00001. [DOI] [PubMed] [Google Scholar]
- [43].Sharma P, Maffulli N. Basic biology of tendon injury and healing. Surgeon. 2005;3:309–316. doi: 10.1016/s1479-666x(05)80109-x. [DOI] [PubMed] [Google Scholar]
- [44].Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J. Bone Joint Surg. Am. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- [45].Kajikawa Y, Morihara T, Watanabe N, Sakamoto H, Matsuda K, Kobayashi M, Oshima Y, Yoshida A, Kawata M, Kubo T. GFP chimeric models exhibited a biphasic pattern of mesenchymal cell invasion in tendon healing. J. Cell. Physiol. 2007;210:684–691. doi: 10.1002/jcp.20876. [DOI] [PubMed] [Google Scholar]
- [46].James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand. Surg. [Am.] 2008;33:102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- [47].Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006;6:181–190. [PubMed] [Google Scholar]
- [48].Heisterbach PE, Todorov A, Fluckiger R, Evans CH, Majewski M. Effect of BMP-12, TGF-beta1 and autologous conditioned serum on growth factor expression in Achilles tendon healing. Knee Surg. Sports Traumatol. Arthrosc. 2012;20:1907–1914. doi: 10.1007/s00167-011-1772-x. [DOI] [PubMed] [Google Scholar]
- [49].Wurgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J. Shoulder Elbow Surg. 2007;16:S198–S203. doi: 10.1016/j.jse.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen CH, Cao Y, Wu YF, Bais AJ, Gao JS, Tang JB. Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J. Hand. Surg. [Am.] 2008;33:1834–1842. doi: 10.1016/j.jhsa.2008.07.003. [DOI] [PubMed] [Google Scholar]
- [51].Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, Okada K, Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J. Shoulder Elbow Surg. 2006;15:371–377. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- [52].Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- [53].Chang J, Most D, Thunder R, Mehrara B, Longaker MT, Lineaweaver WC. Molecular studies in flexor tendon wound healing: the role of basic fibroblast growth factor gene expression. J. Hand. Surg. [Am.] 1998;23:1052–1058. doi: 10.1016/S0363-5023(98)80015-4. [DOI] [PubMed] [Google Scholar]
- [54].Muller SA, Todorov A, Heisterbach PE, Martin I, Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg. Sports Traumatol. Arthrosc. 2013 doi: 10.1007/s00167-013-2680-z. http://dx.doi.org/10.1007/s00167-013-2680-z. [DOI] [PubMed] [Google Scholar]
- [55].Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng. A. 2010;16:2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J. Clin. Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am. J. Sports Med. 1999;27:476–488. doi: 10.1177/03635465990270041201. [DOI] [PubMed] [Google Scholar]
- [58].Hashimoto Y, Yoshida G, Toyoda H, Takaoka K. Generation of tendon-to-bone interface “enthesis” with use of recombinant BMP-2 in a rabbit model. J. Orthop. Res. 2007;25:1415–1424. doi: 10.1002/jor.20447. [DOI] [PubMed] [Google Scholar]
- [59].Chen CH, Chang CH, Wang KC, Su CI, Liu HT, Yu CM, Wong CB, Wang IC, Whu SW, Liu HW. Enhancement of rotator cuff tendon-bone healing with injectable periosteum progenitor cells-BMP-2 hydrogel in vivo. Knee Surg. Sports Traumatol. Arthrosc. 2011;19:1597–1607. doi: 10.1007/s00167-010-1373-0. [DOI] [PubMed] [Google Scholar]
- [60].Trippel SB, Wroblewski J, Makower AM, Whelan MC, Schoenfeld D, Doctrow SR. Regulation of growth-plate chondrocytes by insulin-like growth-factor I and basic fibroblast growth factor. J. Bone Joint Surg. Am. 1993;75:177–189. doi: 10.2106/00004623-199302000-00004. [DOI] [PubMed] [Google Scholar]
- [61].Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J. Orthop. Res. 2005;23:84–92. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- [62].Sciore P, Boykiw R, Hart DA. Semiquantitative reverse transcription-polymerase chain reaction analysis of mRNA for growth factors and growth factor receptors from normal and healing rabbit medial collateral ligament tissue. J. Orthop. Res. 1998;16:429–437. doi: 10.1002/jor.1100160406. [DOI] [PubMed] [Google Scholar]
- [63].Duffy FJ, Jr., Seiler JG, Gelberman RH, Hergrueter CA. Growth factors and canine flexor tendon healing: initial studies in uninjured and repair models. J. Hand. Surg. [Am.] 1995;20:645–649. doi: 10.1016/S0363-5023(05)80284-9. [DOI] [PubMed] [Google Scholar]
- [64].Chan BP, Fu SC, Qin L, Rolf C, Chan KM. Supplementation-time dependence of growth factors in promoting tendon healing. Clin. Orthop. Relat. Res. 2006;448:240–247. doi: 10.1097/01.blo.0000205875.97468.e4. [DOI] [PubMed] [Google Scholar]
- [65].Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J. Hand. Surg. [Am.] 2002;27:615–620. doi: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]
- [66].Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast. Reconstr. Surg. 2000;105:148–155. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- [67].Natsu-ume T, Nakamura N, Shino K, Toritsuka Y, Horibe S, Ochi T. Temporal and spatial expression of transforming growth factor-beta in the healing patellar ligament of the rat. J. Orthop. Res. 1997;15:837–843. doi: 10.1002/jor.1100150608. [DOI] [PubMed] [Google Scholar]
- [68].Chang J, Most D, Stelnicki E, Siebert JW, Longaker MT, Hui K, Lineaweaver WC. Gene expression of transforming growth factor beta-1 in rabbit zone II flexor tendon wound healing: evidence for dual mechanisms of repair. Plast. Reconstr. Surg. 1997;100:937–944. doi: 10.1097/00006534-199709001-00016. [DOI] [PubMed] [Google Scholar]
- [69].Ngo M, Pham H, Longaker MT, Chang J. Differential expression of transforming growth factor-beta receptors in a rabbit zone II flexor tendon wound healing model. Plast. Reconstr. Surg. 2001;108:1260–1267. doi: 10.1097/00006534-200110000-00025. [DOI] [PubMed] [Google Scholar]
- [70].Juneja SC, Schwarz EM, O'Keefe RJ, Awad HA. Cellular and molecular factors in flexor tendon repair and adhesions: a histological and gene expression analysis. Connect. Tissue Res. 2013;54:218–226. doi: 10.3109/03008207.2013.787418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jorgensen HG, McLellan SD, Crossan JF, Curtis AS. Neutralisation of TGF beta or binding of VLA-4 to fibronectin prevents rat tendon adhesion following transection. Cytokine. 2005;30:195–202. doi: 10.1016/j.cyto.2004.12.017. [DOI] [PubMed] [Google Scholar]
- [72].Katzel EB, Wolenski M, Loiselle AE, Basile P, Flick LM, Langstein HN, Hilton MJ, Awad HA, Hammert WC, O'Keefe RJ. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J. Orthop. Res. 2011;29:684–693. doi: 10.1002/jor.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Farhat YM, Al-Maliki AA, Chen T, Juneja SC, Schwarz EM, O'Keefe RJ, Awad HA. Gene expression analysis of the pleiotropic effects of TGF-beta1 in an in vitro model of flexor tendon healing. PLoS One. 2012;7:e51411. doi: 10.1371/journal.pone.0051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Farhat YM, Al-Maliki AA, Easa A, O'Keefe RJ, Schwarz EM, Awad HA. TGF-beta1 suppresses plasmin and MMP activity in flexor tendon cells via PAI-1: implications for scarless flexor tendon repair. J. Cell. Physiol. 2014 doi: 10.1002/jcp.24707. http://dx.doi.org/10.1002/jcp.24707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Petersen W, Unterhauser F, Pufe T, Zantop T, Sudkamp NP, Weiler A. The angiogenic peptide vascular endothelial growth factor (VEGF) is expressed during the remodeling of free tendon grafts in sheep. Arch. Orthop. Trauma Surg. 2003;123:168–174. doi: 10.1007/s00402-002-0462-z. [DOI] [PubMed] [Google Scholar]
- [76].Zhang F, Liu H, Stile F, Lei MP, Pang Y, Oswald TM, Beck J, Dorsett-Martin W, Lineaweaver WC. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast. Reconstr. Surg. 2003;112:1613–1619. doi: 10.1097/01.PRS.0000086772.72535.A4. [DOI] [PubMed] [Google Scholar]
- [77].Hamada Y, Katoh S, Hibino N, Kosaka H, Hamada D, Yasui N. Effects of monofilament nylon coated with basic fibroblast growth factor on endogenous intrasynovial flexor tendon healing. J. Hand. Surg. [Am.] 2006;31:530–540. doi: 10.1016/j.jhsa.2005.12.003. [DOI] [PubMed] [Google Scholar]
- [78].Uggen C, Dines J, McGarry M, Grande D, Lee T, Limpisvasti O. The effect of recombinant human platelet-derived growth factor BB-coated sutures on rotator cuff healing in a sheep model. Arthroscopy. 2010;26:1456–1462. doi: 10.1016/j.arthro.2010.02.025. [DOI] [PubMed] [Google Scholar]
- [79].Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors. 2001;19:115–126. doi: 10.3109/08977190109001080. [DOI] [PubMed] [Google Scholar]
- [80].Anaguchi Y, Yasuda K, Majima T, Tohyama H, Minami A, Hayashi K. The effect of transforming growth factor-beta on mechanical properties of the fibrous tissue regenerated in the patellar tendon after resecting the central portion. Clin. Biomech. (Bristol, Avon) 2005;20:959–965. doi: 10.1016/j.clinbiomech.2005.05.012. [DOI] [PubMed] [Google Scholar]
- [81].Zhang AY, Pham H, Ho F, Teng K, Longaker MT, Chang J. Inhibition of TGF-beta-induced collagen production in rabbit flexor tendons. J. Hand. Surg. [Am.] 2004;29:230–235. doi: 10.1016/j.jhsa.2003.11.005. [DOI] [PubMed] [Google Scholar]
- [82].Klass BR, Rolfe KJ, Grobbelaar AO. In vitro flexor tendon cell response to TGF-beta1: a gene expression study. J. Hand. Surg. [Am.] 2009;34:495–503. doi: 10.1016/j.jhsa.2008.10.032. [DOI] [PubMed] [Google Scholar]
- [83].Bidder M, Towler DA, Gelberman RH, Boyer MI. Expression of mRNA for vascular endothelial growth factor at the repair site of healing canine flexor tendon. J. Orthop. Res. 2000;18:247–252. doi: 10.1002/jor.1100180212. [DOI] [PubMed] [Google Scholar]
- [84].Boyer MI, Watson JT, Lou J, Manske PR, Gelberman RH, Cai SR. Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: an investigation in a canine model. J. Orthop. Res. 2001;19:869–872. doi: 10.1016/S0736-0266(01)00017-1. [DOI] [PubMed] [Google Scholar]
- [85].Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J. Hand. Surg. [Am.] 2005;30:441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [86].Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, Kim HM, Amiel D, Gelberman RH. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J. Orthop. Res. 2009;27:1209–1215. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Harwood FL, Goomer RS, Gelberman RH, Silva MJ, Amiel D. Regulation of alpha(v)beta3 and alpha5beta1 integrin receptors by basic fibroblast growth factor and platelet-derived growth factor-BB in intrasynovial flexor tendon cells. Wound Repair Regen. 1999;7:381–388. doi: 10.1046/j.1524-475x.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- [88].Yoshikawa Y, Abrahamsson SO. Dose-related cellular effects of platelet-derived growth factor-BB differ in various types of rabbit tendons in vitro. Acta Orthop. Scand. 2001;72:287–292. doi: 10.1080/00016470152846646. [DOI] [PubMed] [Google Scholar]
- [89].Chan BP, Chan KM, Maffulli N, Webb S, Lee KK. Effect of basic fibroblast growth factor. An in vitro study of tendon healing. Clin. Orthop. Relat. Res. 1997:239–247. [PubMed] [Google Scholar]
- [90].Abrahamsson SO, Lundborg G, Lohmander LS. Recombinant human insulin-like growth factor-I stimulates in vitro matrix synthesis and cell proliferation in rabbit flexor tendon. J. Orthop. Res. 1991;9:495–502. doi: 10.1002/jor.1100090405. [DOI] [PubMed] [Google Scholar]
- [91].Abrahamsson SO. Similar effects of recombinant human insulin-like growth factor-I and II on cellular activities in flexor tendons of young rabbits: experimental studies in vitro. J. Orthop. Res. 1997;15:256–262. doi: 10.1002/jor.1100150215. [DOI] [PubMed] [Google Scholar]
- [92].Batten ML, Hansen JC, Dahners LE. Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament. J. Orthop. Res. 1996;14:736–741. doi: 10.1002/jor.1100140509. [DOI] [PubMed] [Google Scholar]
- [93].Ide J, Kikukawa K, Hirose J, Iyama K, Sakamoto H, Mizuta H. The effects of fibroblast growth factor-2 on rotator cuff reconstruction with acellular dermal matrix grafts. Arthroscopy. 2009;25:608–616. doi: 10.1016/j.arthro.2008.11.011. [DOI] [PubMed] [Google Scholar]
- [94].Rodeo SA. Biologic augmentation of rotator cuff tendon repair. J. Shoulder Elbow Surg. 2007;16:S191–S197. doi: 10.1016/j.jse.2007.03.012. [DOI] [PubMed] [Google Scholar]
- [95].Costa MA, Wu C, Pham BV, Chong AK, Pham HM, Chang J. Tissue engineering of flexor tendons: optimization of tenocyte proliferation using growth factor supplementation. Tissue Eng. 2006;12:1937–1943. doi: 10.1089/ten.2006.12.1937. [DOI] [PubMed] [Google Scholar]
- [96].Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop. Scand. 2004;75:93–99. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- [97].Bosch G, van Schie HT, de Groot MW, Cadby JA, van de Lest CH, Barneveld A, van Weeren PR. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: a placebo-controlled experimental study. J. Orthop. Res. 2010;28:211–217. doi: 10.1002/jor.20980. [DOI] [PubMed] [Google Scholar]
- [98].Majewski M, Ochsner PE, Liu F, Fluckiger R, Evans CH. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am. J. Sports Med. 2009;37:2117–2125. doi: 10.1177/0363546509348047. [DOI] [PubMed] [Google Scholar]
- [99].Schnabel LV, Mohammed HO, Miller BJ, McDermott WG, Jacobson MS, Santangelo KS, Fortier LA. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J. Orthop. Res. 2007;25:230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- [100].Gosens T, Den Oudsten BL, Fievez E, van 't Spijker P, Fievez A. Pain and activity levels before and after platelet-rich plasma injection treatment of patellar tendinopathy: a prospective cohort study and the influence of previous treatments. Int. Orthop. 2012;36:1941–1946. doi: 10.1007/s00264-012-1540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].de Almeida AM, Demange MK, Sobrado MF, Rodrigues MB, Pedrinelli A, Hernandez AJ. Patellar tendon healing with platelet-rich plasma: a prospective randomized controlled trial. Am. J. Sports Med. 2012;40:1282–1288. doi: 10.1177/0363546512441344. [DOI] [PubMed] [Google Scholar]
- [102].Seijas R, Ares O, Catala J, Alvarez-Diaz P, Cusco X, Cugat R. Magnetic resonance imaging evaluation of patellar tendon graft remodelling after anterior cruciate ligament reconstruction with or without platelet-rich plasma. J. Orthop. Surg. (Hong Kong) 2013;21:10–14. doi: 10.1177/230949901302100105. [DOI] [PubMed] [Google Scholar]
- [103].Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human Achilles tendon ruptures: a randomized single-blind study. Am. J. Sports Med. 2011;39:38–47. doi: 10.1177/0363546510383515. [DOI] [PubMed] [Google Scholar]
- [104].de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Tol JL. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303:144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- [105].de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br. J. Sports Med. 2014;48:952–956. doi: 10.1136/bjsports-2013-093281. [DOI] [PubMed] [Google Scholar]
- [106].Hall MP, Ward JP, Cardone DA. Platelet rich placebo? Evidence for platelet rich plasma in the treatment of tendinopathy and augmentation of tendon repair. Bull. Hosp. Jt Dis. 2013;71:54–59. [PubMed] [Google Scholar]
- [107].Evans CH. Platelet-rich plasma a la carte: commentary on an article by Satoshi Terada, MD, et al.: “use of an antifibrotic agent improves the effect of platelet-rich plasma on muscle healing after injury”. J. Bone Joint Surg. Am. 2013;95:e801–e802. doi: 10.2106/JBJS.M.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- [109].Yuan T, Zhang CQ, Wang JH. Augmenting tendon and ligament repair with platelet-rich plasma (PRP) Muscles Ligaments Tendons J. 2013;3:139–149. [PMC free article] [PubMed] [Google Scholar]
- [110].Obaid H, Connell D. Cell therapy in tendon disorders: what is the current evidence? Am. J. Sports Med. 2010;38:2123–2132. doi: 10.1177/0363546510373574. [DOI] [PubMed] [Google Scholar]
- [111].Longo UG, Lamberti A, Maffulli N, Denaro V. Tissue engineered biological augmentation for tendon healing: a systematic review. Br. Med. Bull. 2011;98:31–59. doi: 10.1093/bmb/ldq030. [DOI] [PubMed] [Google Scholar]
- [112].Yin Z, Chen X, Chen JL, Ouyang HW. Stem cells for tendon tissue engineering and regeneration. Expert. Opin. Biol. Ther. 2010;10:689–700. doi: 10.1517/14712591003769824. [DOI] [PubMed] [Google Scholar]
- [113].Ahmad Z, Wardale J, Brooks R, Henson F, Noorani A, Rushton N. Exploring the application of stem cells in tendon repair and regeneration. Arthroscopy. 2012;28:1018–1029. doi: 10.1016/j.arthro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- [114].Nixon AJ, Watts AE, Schnabel LV. Cell- and gene-based approaches to tendon regeneration. J. Shoulder Elbow Surg. 2012;21:278–294. doi: 10.1016/j.jse.2011.11.015. [DOI] [PubMed] [Google Scholar]
- [115].Hogan MV, Bagayoko N, James R, Starnes T, Katz A, Chhabra AB. Tissue engineering solutions for tendon repair. J. Am. Acad. Orthop. Surg. 2011;19:134–142. doi: 10.5435/00124635-201103000-00002. [DOI] [PubMed] [Google Scholar]
- [116].Bocker W, Yin Z, Drosse I, Haasters F, Rossmann O, Wierer M, Popov C, Locher M, Mutschler W, Docheva D, Schieker M. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell. Mol. Med. 2008;12:1347–1359. doi: 10.1111/j.1582-4934.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J. Cell. Mol. Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Tan SL, Ahmad RE, Ahmad TS, Merican AM, Abbas AA, Ng WM, Kamarul T. Effect of growth differentiation factor 5 on the proliferation and tenogenic differentiation potential of human mesenchymal stem cells in vitro. Cells Tissues Organs. 2012;196:325–338. doi: 10.1159/000335693. [DOI] [PubMed] [Google Scholar]
- [119].Violini S, Ramelli P, Pisani LF, Gorni C, Mariani P. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol. 2009;10:29. doi: 10.1186/1471-2121-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G, Gross G, Gazit D. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J. Clin. Invest. 2006;116:940–952. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Haddad-Weber M, Prager P, Kunz M, Seefried L, Jakob F, Murray MM, Evans CH, Noth U, Steinert AF. BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy. 2010;12:505–513. doi: 10.3109/14653241003709652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Alberton P, Popov C, Pragert M, Kohler J, Shukunami C, Schieker M, Docheva D. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012;21:846–858. doi: 10.1089/scd.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- [124].Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [125].Chaudhury S. Mesenchymal stem cell applications to tendon healing. Muscles Ligaments Tendons J. 2012;2:222–229. [PMC free article] [PubMed] [Google Scholar]
- [126].Mazzocca AD, McCarthy MB, Chowaniec D, Cote MP, Judson CH, Apostolakos J, Solovyova O, Beitzel K, Arciero RA. Bone marrow-derived mesenchymal stem cells obtained during arthroscopic rotator cuff repair surgery show potential for tendon cell differentiation after treatment with insulin. Arthroscopy. 2011;27:1459–1471. doi: 10.1016/j.arthro.2011.06.029. [DOI] [PubMed] [Google Scholar]
- [127].Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg. Sports Traumatol. Arthrosc. 2012;20:373–377. doi: 10.1007/s00167-011-1607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- [129].Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- [130].Schneider PR, Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J. Orthop. Res. 2011;29:1351–1360. doi: 10.1002/jor.21400. [DOI] [PubMed] [Google Scholar]
- [131].Kryger GS, Chong AK, Costa M, Pham H, Bates SJ, Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J. Hand. Surg. [Am.] 2007;32:597–605. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- [132].Nixon AJ, Dahlgren LA, Haupt JL, Yeager AE, Ward DL. Effect of adipose-derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. Am. J. Vet. Res. 2008;69:928–937. doi: 10.2460/ajvr.69.7.928. [DOI] [PubMed] [Google Scholar]
- [133].Behfar M, Sarrafzadeh-Rezaei F, Hobbenaghi R, Delirezh N, Dalir-Naghadeh B. Enhanced mechanical properties of rabbit flexor tendons in response to intratendinous injection of adipose derived stromal vascular fraction. Curr. Stem Cell Res. Ther. 2012;7:173–178. doi: 10.2174/157488812799859874. [DOI] [PubMed] [Google Scholar]
- [134].Uysal AC, Mizuno H. Tendon regeneration and repair with adipose derived stem cells. Curr. Stem Cell Res. Ther. 2010;5:161–167. doi: 10.2174/157488810791268609. [DOI] [PubMed] [Google Scholar]
- [135].Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol. Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- [136].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- [137].Watts AE, Yeager AE, Kopyov OV, Nixon AJ. Fetal derived embryonic-like stem cells improve healing in a large animal flexor tendonitis model. Stem Cell Res. Ther. 2011;2:4. doi: 10.1186/scrt45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Guest DJ, Smith MR, Allen WR. Equine embryonic stem-like cells and mesenchymal stromal cells have different survival rates and migration patterns following their injection into damaged superficial digital flexor tendon. Equine Vet. J. 2010;42:636–642. doi: 10.1111/j.2042-3306.2010.00112.x. [DOI] [PubMed] [Google Scholar]
- [139].Chen X, Zou XH, Yin GL, Ouyang HW. Tendon tissue engineering with mesenchymal stem cells and biografts: an option for large tendon defects? Front. Biosci. (Schol. Ed.) 2009;1:23–32. doi: 10.2741/S3. [DOI] [PubMed] [Google Scholar]
- [140].Xu W, Wang Y, Liu E, Sun Y, Luo Z, Xu Z, Liu W, Zhong L, Lv Y, Wang A, Tang Z, Li S, Yang L. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng. A. 2013;19:2439–2451. doi: 10.1089/ten.tea.2012.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lui PP, Chan KM. Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev. 2011;7:883–897. doi: 10.1007/s12015-011-9276-0. [DOI] [PubMed] [Google Scholar]
- [142].Lui PP. Identity of tendon stem cells — how much do we know? J. Cell. Mol. Med. 2013;17:55–64. doi: 10.1111/jcmm.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yao L, Bestwick CS, Bestwick LA, Maffulli N, Aspden RM. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006;12:1843–1849. doi: 10.1089/ten.2006.12.1843. [DOI] [PubMed] [Google Scholar]
- [144].Schulze-Tanzil G, Mobasheri A, Clegg PD, Sendzik J, John T, Shakibaei M. Cultivation of human tenocytes in high-density culture. Histochem. Cell Biol. 2004;122:219–228. doi: 10.1007/s00418-004-0694-9. [DOI] [PubMed] [Google Scholar]
- [145].Cao Y, Liu Y, Liu W, Shan Q, Buonocore SD, Cui L. Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast. Reconstr. Surg. 2002;110:1280–1289. doi: 10.1097/01.PRS.0000025290.49889.4D. [DOI] [PubMed] [Google Scholar]
- [146].Chen JM, Willers C, Xu J, Wang A, Zheng MH. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007;13:1479–1491. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- [147].Stoll C, John T, Conrad C, Lohan A, Hondke S, Ertel W, Kaps C, Endres M, Sittinger M, Ringe J, Schulze-Tanzil G. Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation. Biomaterials. 2011;32:4806–4815. doi: 10.1016/j.biomaterials.2011.03.026. [DOI] [PubMed] [Google Scholar]
- [148].Pietschmann MF, Frankewycz B, Schmitz P, Docheva D, Sievers B, Jansson V, Schieker M, Muller PE. Comparison of tenocytes and mesenchymal stem cells seeded on biodegradable scaffolds in a full-size tendon defect model. J. Mater. Sci. Mater. Med. 2013;24:211–220. doi: 10.1007/s10856-012-4791-3. [DOI] [PubMed] [Google Scholar]
- [149].Ni M, Lui PP, Rui YF, Lee YW, Lee YW, Tan Q, Wong YM, Kong SK, Lau PM, Li G, Chan KM. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J. Orthop. Res. 2012;30:613–619. doi: 10.1002/jor.21559. [DOI] [PubMed] [Google Scholar]
- [150].Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- [151].Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech. Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [152].Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- [153].Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kuhnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, Klose J. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27:1288–1297. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- [154].Jakob F, Ebert R, Rudert M, Noth U, Walles H, Docheva D, Schieker M, Meinel L, Groll J. In situ guided tissue regeneration in musculoskeletal diseases and aging: Implementing pathology into tailored tissue engineering strategies. Cell Tissue Res. 2012;347:725–735. doi: 10.1007/s00441-011-1237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br. Med. Bull. 2010;94:165–188. doi: 10.1093/bmb/ldp051. [DOI] [PubMed] [Google Scholar]
- [156].Zhang X, Bogdanowicz D, Erisken C, Lee NM, Lu HH. Biomimetic scaffold design for functional and integrative tendon repair. J. Shoulder Elbow Surg. 2012;21:266–277. doi: 10.1016/j.jse.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Longo UG, Lamberti A, Petrillo S, Maffulli N, Denaro V. Scaffolds in tendon tissue engineering. Stem Cells Int. 2012;2012:517165. doi: 10.1155/2012/517165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J. Orthop. Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- [159].Awad HA, Boivin GP, Dressler MR, Smith FN, Young RG, Butler DL. Repair of patellar tendon injuries using a cell–collagen composite. J. Orthop. Res. 2003;21:420–431. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- [160].Juncosa-Melvin N, Shearn JT, Boivin GP, Gooch C, Galloway MT, West JR, Nirmalanandhan VS, Bradica G, Butler DL. Effects of mechanical stimulation on the biomechanics and histology of stem cell–collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12:2291–2300. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- [161].Kishore V, Bullock W, Sun X, Van Dyke WS, Akkus O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials. 2012;33:2137–2144. doi: 10.1016/j.biomaterials.2011.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Alfredo Uquillas J, Kishore V, Akkus O. Genipin crosslinking elevates the strength of electrochemically aligned collagen to the level of tendons. J. Mech. Behav. Biomed. Mater. 2012;15:176–189. doi: 10.1016/j.jmbbm.2012.06.012. [DOI] [PubMed] [Google Scholar]
- [163].Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am. J. Sports Med. 2001;29:175–184. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- [164].Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, Kraine MR, Simmons C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J. Biomed. Mater. Res. 1995;29:977–985. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- [165].Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J. Bone Joint Surg. Am. 2007;89:621–630. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- [166].Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2005;73:61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- [167].Zalavras CG, Gardocki R, Huang E, Stevanovic M, Hedman T, Tibone J. Reconstruction of large rotator cuff tendon defects with porcine small intestinal submucosa in an animal model. J. Shoulder Elbow Surg. 2006;15:224–231. doi: 10.1016/j.jse.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [168].Metcalf MH, Savoie FH, Kellum B. Surgical technique for xenograft (SIS) augmentation of rotator-cuff repairs. Oper. Tech. Orthop. 2002;12:4. [Google Scholar]
- [169].Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J. Shoulder Elbow Surg. 2004;13:538–541. doi: 10.1016/j.jse.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [170].Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J. Bone Joint Surg. Am. 2007;89:786–791. doi: 10.2106/JBJS.F.00315. [DOI] [PubMed] [Google Scholar]
- [171].Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J. Bone Joint Surg. Am. 2006;88:1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- [172].Omae H, Zhao C, Sun YL, An KN, Amadio PC. Multilayer tendon slices seeded with bone marrow stromal cells: a novel composite for tendon engineering. J. Orthop. Res. 2009;27:937–942. doi: 10.1002/jor.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Omae H, Sun YL, An KN, Amadio PC, Zhao C. Engineered tendon with decellularized xenotendon slices and bone marrow stromal cells: an in vivo animal study. J. Tissue Eng. Regen. Med. 2012;6:238–244. doi: 10.1002/term.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Omae H, Steinmann SP, Zhao C, Zobitz ME, Wongtriratanachai P, Sperling JW, An KN. Biomechanical effect of rotator cuff augmentation with an acellular dermal matrix graft: a cadaver study. Clin. Biomech. (Bristol, Avon) 2012;27:789–792. doi: 10.1016/j.clinbiomech.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [175].Adams JE, Zobitz ME, Reach JS, Jr., An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy. 2006;22:700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- [176].Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24:403–409. doi: 10.1016/j.arthro.2007.07.033. (e401) [DOI] [PubMed] [Google Scholar]
- [177].Rotini R, Marinelli A, Guerra E, Bettelli G, Castagna A, Fini M, Bondioli E, Busacca M. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskelet. Surg. 2011;95(Suppl. 1):S13–S23. doi: 10.1007/s12306-011-0141-8. [DOI] [PubMed] [Google Scholar]
- [178].Snyder SJ, Arnoczky SP, Bond JL, Dopirak R. Histologic evaluation of a biopsy specimen obtained 3 months after rotator cuff augmentation with GraftJacket Matrix. Arthroscopy. 2009;25:329–333. doi: 10.1016/j.arthro.2008.05.023. [DOI] [PubMed] [Google Scholar]
- [179].Lee MS. GraftJacket augmentation of chronic Achilles tendon ruptures. Orthopedics. 2004;27:s151–s153. doi: 10.3928/0147-7447-20040102-15. [DOI] [PubMed] [Google Scholar]
- [180].Lee DK. A preliminary study on the effects of acellular tissue graft augmentation in acute Achilles tendon ruptures. J. Foot Ankle Surg. 2008;47:8–12. doi: 10.1053/j.jfas.2007.08.015. [DOI] [PubMed] [Google Scholar]
- [181].Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J. Bone Joint Surg. Am. 2006;88:2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- [182].Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J. Bone Joint Surg. Am. 1995;77:1103–1114. doi: 10.2106/00004623-199507000-00021. [DOI] [PubMed] [Google Scholar]
- [183].Evans CH, Robbins PD. Genetically augmented tissue engineering of the musculoskeletal system. Clin. Orthop. Relat. Res. 1999:S410–S418. doi: 10.1097/00003086-199910001-00040. [DOI] [PubMed] [Google Scholar]
- [184].Evans CH, Ghivizzani SC, Robbins PD. The 2003 Nicolas Andry Award. Orthopaedic gene therapy. Clin. Orthop. Relat. Res. 2004:316–329. doi: 10.1097/01.blo.0000148854.14399.ec. [DOI] [PubMed] [Google Scholar]
- [185].Bandara G, Robbins PD, Georgescu HI, Mueller GM, Glorioso JC, Evans CH. Gene transfer to synoviocytes: prospects for gene treatment of arthritis. DNA Cell Biol. 1992;11:227–231. doi: 10.1089/dna.1992.11.227. [DOI] [PubMed] [Google Scholar]
- [186].Evans CH. Gene delivery to bone. Adv. Drug Deliv. Rev. 2012;64:1331–1340. doi: 10.1016/j.addr.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Evans CH, Ghivizzani SC, Robbins PD. Getting arthritis gene therapy into the clinic. Nat. Rev. Rheumatol. 2011;7:244–249. doi: 10.1038/nrrheum.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Evans C. Using genes to facilitate the endogenous repair and regeneration of orthopaedic tissues. Int. Orthop. 2014;38:1761–1769. doi: 10.1007/s00264-014-2423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Dai Q, Manfield L, Wang Y, Murrell GA. Adenovirus-mediated gene transfer to healing tendon — enhanced efficiency using a gelatin sponge. J. Orthop. Res. 2003;21:604–609. doi: 10.1016/S0736-0266(02)00239-5. [DOI] [PubMed] [Google Scholar]
- [190].Basile P, Dadali T, Jacobson J, Hasslund S, Ulrich-Vinther M, Soballe K, Nishio Y, Drissi MH, Langstein HN, Mitten DJ, O'Keefe RJ, Schwarz EM, Awad HA. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Mol. Ther. 2008;16:466–473. doi: 10.1038/sj.mt.6300395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Evans CH. Advances in regenerative orthopedics. Mayo Clin. Proc. 2013;88:1323–1339. doi: 10.1016/j.mayocp.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Evans CH, Palmer GD, Pascher A, Porter R, Kwong FN, Gouze E, Gouze JN, Liu F, Steinert A, Betz O, Betz V, Vrahas M, Ghivizzani SC. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007;13:1987–1993. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- [193].Evans CH, Liu FJ, Glatt V, Hoyland JA, Kirker-Head C, Walsh A, Betz O, Wells JW, Betz V, Porter RM, Saad FA, Gerstenfeld LC, Einhorn TA, Harris MB, Vrahas MS. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur. Cells Mater. 2009;18:96–111. doi: 10.22203/ecm.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Majewski M, Betz O, Ochsner PE, Liu F, Porter RM, Evans CH. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008;15:1139–1146. doi: 10.1038/gt.2008.48. [DOI] [PubMed] [Google Scholar]
- [195].Majewski M, Porter RM, Betz OB, Betz VM, Clahsen H, Fluckiger R, Evans CH. Improvement of tendon repair using muscle grafts transduced with TGF-beta1 cDNA. Eur. Cells Mater. 2012;23:94–101. doi: 10.22203/ecm.v023a07. (discussion 101–102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Gerich TG, Kang R, Fu FH, Robbins PD, Evans CH. Gene transfer to the rabbit patellar tendon: potential for genetic enhancement of tendon and ligament healing. Gene Ther. 1996;3:1089–1093. [PubMed] [Google Scholar]
- [197].Gerich TG, Kang R, Fu FH, Robbins PD, Evans CH. Gene transfer to the patellar tendon. Knee Surg. Sports Traumatol. Arthrosc. 1997;5:118–123. doi: 10.1007/s001670050037. [DOI] [PubMed] [Google Scholar]
- [198].Hildebrand KA, Deie M, Allen CR, Smith DW, Georgescu HI, Evans CH, Robbins PD, Woo SL. Early expression of marker genes in the rabbit medial collateral and anterior cruciate ligaments: the use of different viral vectors and the effects of injury. J. Orthop. Res. 1999;17:37–42. doi: 10.1002/jor.1100170107. [DOI] [PubMed] [Google Scholar]
- [199].Pelinkovic D, Lee JY, Engelhardt M, Rodosky M, Cummins J, Fu FH, Huard J. Muscle cell-mediated gene delivery to the rotator cuff. Tissue Eng. 2003;9:143–151. doi: 10.1089/107632703762687627. [DOI] [PubMed] [Google Scholar]
- [200].Ozkan I, Shino K, Nakamura N, Natsuume T, Matsumoto N, Horibe S, Tomita T, Kaneda Y, Ochi T. Direct in vivo gene transfer to healing rat patellar ligament by intra-arterial delivery of haemagglutinating virus of Japan liposomes. Eur. J. Clin. Invest. 1999;29:63–67. doi: 10.1046/j.1365-2362.1999.00401.x. [DOI] [PubMed] [Google Scholar]
- [201].Nakamura N, Horibe S, Matsumoto N, Tomita T, Natsuume T, Kaneda Y, Shino K, Ochi T. Transient introduction of a foreign gene into healing rat patellar ligament. J. Clin. Invest. 1996;97:226–231. doi: 10.1172/JCI118395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Goomer RS, Maris TM, Gelberman R, Boyer M, Silva M, Amiel D. Nonviral in vivo gene therapy for tissue engineering of articular cartilage and tendon repair. Clin. Orthop. Relat. Res. 2000:S189–S200. doi: 10.1097/00003086-200010001-00025. [DOI] [PubMed] [Google Scholar]
- [203].Jayankura M, Boggione C, Frisen C, Boyer O, Fouret P, Saillant G, Klatzmann D. In situ gene transfer into animal tendons by injection of naked DNA and electrotransfer. J. Gene Med. 2003;5:618–624. doi: 10.1002/jgm.389. [DOI] [PubMed] [Google Scholar]
- [204].Hildebrand KA, Frank CB, Hart DA. Gene intervention in ligament and tendon: current status, challenges, future directions. Gene Ther. 2004;11:368–378. doi: 10.1038/sj.gt.3302198. [DOI] [PubMed] [Google Scholar]
- [205].Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J. Orthop. Res. 2001;19:1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- [206].Bolt P, Clerk AN, Luu HH, Kang Q, Kummer JL, Deng ZL, Olson K, Primus F, Montag AG, He TC, Haydon RC, Toolan BC. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. J. Bone Joint Surg. Am. 2007;89:1315–1320. doi: 10.2106/JBJS.F.00257. [DOI] [PubMed] [Google Scholar]
- [207].Rickert M. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. J. Bone Joint Surg. Am. 2008;90:445. (author reply 445–446) [PubMed] [Google Scholar]
- [208].Gulotta LV, Kovacevic D, Packer JD, Ehteshami JR, Rodeo SA. Adenoviral-mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am. J. Sports Med. 2011;39:180–187. doi: 10.1177/0363546510379339. [DOI] [PubMed] [Google Scholar]
- [209].Forslund C, Rueger D, Aspenberg P. A comparative dose–response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J. Orthop. Res. 2003;21:617–621. doi: 10.1016/S0736-0266(03)00010-X. [DOI] [PubMed] [Google Scholar]
- [210].Eliasson P, Fahlgren A, Aspenberg P. Mechanical load and BMP signaling during tendon repair: a role for follistatin? Clin. Orthop. Relat. Res. 2008;466:1592–1597. doi: 10.1007/s11999-008-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am. J. Sports Med. 2011;39:1282–1289. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- [212].Tang JB, Cao Y, Zhu B, Xin KQ, Wang XT, Liu PY. Adeno-associated virus-2-mediated bFGF gene transfer to digital flexor tendons significantly increases healing strength. an in vivo study. J. Bone Joint Surg. Am. 2008;90:1078–1089. doi: 10.2106/JBJS.F.01188. [DOI] [PubMed] [Google Scholar]
- [213].Hou Y, Mao Z, Wei X, Lin L, Chen L, Wang H, Fu X, Zhang J, Yu C. Effects of transforming growth factor-beta1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol. 2009;28:324–335. doi: 10.1016/j.matbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- [214].Uggen JC, Dines J, Uggen CW, Mason JS, Razzano P, Dines D, Grande DA. Tendon gene therapy modulates the local repair environment in the shoulder. J. Am. Osteopath. Assoc. 2005;105:20–21. [PubMed] [Google Scholar]
- [215].Nakamura N, Shino K, Natsuume T, Horibe S, Matsumoto N, Kaneda Y, Ochi T. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 1998;5:1165–1170. doi: 10.1038/sj.gt.3300712. [DOI] [PubMed] [Google Scholar]
- [216].Ricchetti ET, Reddy SC, Ansorge HL, Zgonis MH, Van Kleunen JP, Liechty KW, Soslowsky LJ, Beredjiklian PK. Effect of interleukin-10 overexpression on the properties of healing tendon in a murine patellar tendon model. J. Hand. Surg. [Am.] 2008;33:1843–1852. doi: 10.1016/j.jhsa.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J. Orthop. Res. 2009;27:1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- [218].Coen MJ, Chen ST, Rundle CH, Wergedal JE, Lau KH. Lentiviral-based BMP4 in vivo gene transfer strategy increases pull-out tensile strength without an improvement in the osteointegration of the tendon graft in a rat model of biceps tenodesis. J. Gene Med. 2011;13:511–521. doi: 10.1002/jgm.1604. [DOI] [PubMed] [Google Scholar]
- [219].Martinek V, Latterman C, Usas A, Abramowitch S, Woo SL, Fu FH, Huard J. Enhancement of tendon-bone integration of anterior cruciate ligament grafts with bone morphogenetic protein-2 gene transfer: a histological and biomechanical study. J. Bone Joint Surg. Am. 2002;84-A:1123–1131. doi: 10.2106/00004623-200207000-00005. [DOI] [PubMed] [Google Scholar]
- [220].Lattermann C, Zelle BA, Whalen JD, Baltzer AW, Robbins PD, Niyibizi C, Evans CH, Fu FH. Gene transfer to the tendon–bone insertion site. Knee Surg. Sports Traumatol. Arthrosc. 2004;12:510–515. doi: 10.1007/s00167-003-0482-4. [DOI] [PubMed] [Google Scholar]
- [221].Gulotta LV, Kovacevic D, Montgomery S, Ehteshami JR, Packer JD, Rodeo SA. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am. J. Sports Med. 2010;38:1429–1437. doi: 10.1177/0363546510361235. [DOI] [PubMed] [Google Scholar]
- [222].Zhu Z, Yu A, Hou M, Xie X, Li P. Effects of Sox9 gene therapy on the healing of bone–tendon junction: an experimental study. Indian J. Orthop. 2014;48:88–95. doi: 10.4103/0019-5413.125521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Lin L, Chen L, Wang H, Wei X, Fu X, Zhang J, Ma K, Zhou C, Yu C. Adenovirus-mediated transfer of siRNA against Runx2/Cbfa1 inhibits the formation of heterotopic ossification in animal model. Biochem. Biophys. Res. Commun. 2006;349:564–572. doi: 10.1016/j.bbrc.2006.08.089. [DOI] [PubMed] [Google Scholar]
- [224].Xue T, Mao Z, Lin L, Hou Y, Wei X, Fu X, Zhang J, Yu C. Non-virus-mediated transfer of siRNAs against Runx2 and Smad4 inhibit heterotopic ossification in rats. Gene Ther. 2010;17:370–379. doi: 10.1038/gt.2009.154. [DOI] [PubMed] [Google Scholar]
- [225].Lu P, Zhang GR, Cai YZ, Heng BC, Ren H, Wang LL, Ji J, Zou XH, Ouyang HW. Lentiviral-encoded shRNA silencing of proteoglycan decorin enhances tendon repair and regeneration within a rat model. Cell Transplant. 2013;22:1507–1517. doi: 10.3727/096368912X661292. [DOI] [PubMed] [Google Scholar]
- [226].Evans CH, Ghivizzani SC, Robbins PD. Orthopedic gene therapy — lost in translation? J. Cell. Physiol. 2012;227:416–420. doi: 10.1002/jcp.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Wang JH. Mechanobiology of tendon. J. Biomech. 2006;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- [228].Huang TF, Yew TL, Chiang ER, Ma HL, Hsu CY, Hsu SH, Hsu YT, Hung SC. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am. J. Sports Med. 2013;41:1117–1125. doi: 10.1177/0363546513480786. [DOI] [PubMed] [Google Scholar]
- [229].Nourissat G, Diop A, Maurel N, Salvat C, Dumont S, Pigenet A, Gosset M, Houard X, Berenbaum F. Mesenchymal stem cell therapy regenerates the native bone–tendon junction after surgical repair in a degenerative rat model. PLoS One. 2010;5:e12248. doi: 10.1371/journal.pone.0012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Okamoto N, Kushida T, Oe K, Umeda M, Ikehara S, Iida H. Treating Achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. J. Bone Joint Surg. Am. 2010;92:2776–2784. doi: 10.2106/JBJS.I.01325. [DOI] [PubMed] [Google Scholar]
- [231].Chong AK, Ang AD, Goh JC, Hui JH, Lim AY, Lee EH, Lim BH. Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit Achilles tendon model. J. Bone Joint Surg. Am. 2007;89:74–81. doi: 10.2106/JBJS.E.01396. [DOI] [PubMed] [Google Scholar]
- [232].Ouyang HW, Goh JC, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-coglycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003;9:431–439. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- [233].Ouyang HW, Goh JC, Lee EH. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am. J. Sports Med. 2004;32:321–327. doi: 10.1177/0095399703258682. [DOI] [PubMed] [Google Scholar]
- [234].Juncosa-Melvin N, Boivin GP, Galloway MT, Gooch C, West JR, Butler DL. Effects of cell-to-collagen ratio in stem cell-seeded constructs for Achilles tendon repair. Tissue Eng. 2006;12:681–689. doi: 10.1089/ten.2006.12.681. [DOI] [PubMed] [Google Scholar]
- [235].Hankemeier S, Hurschler C, Zeichen J, van Griensven M, Miller B, Meller R, Ezechieli M, Krettek C, Jagodzinski M. Bone marrow stromal cells in a liquid fibrin matrix improve the healing process of patellar tendon window defects. Tissue Eng. A. 2009;15:1019–1030. doi: 10.1089/ten.tea.2008.0046. [DOI] [PubMed] [Google Scholar]
- [236].Hankemeier S, van Griensven M, Ezechieli M, Barkhausen T, Austin M, Jagodzinski M, Meller R, Bosch U, Krettek C, Zeichen J. Tissue engineering of tendons and ligaments by human bone marrow stromal cells in a liquid fibrin matrix in immunodeficient rats: results of a histologic study. Arch. Orthop. Trauma Surg. 2007;127:815–821. doi: 10.1007/s00402-007-0366-z. [DOI] [PubMed] [Google Scholar]
- [237].Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, Butler DL. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel–collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng. 2006;12:369–379. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- [238].Dressler MR, Butler DL, Boivin GP. Effects of age on the repair ability of mesenchymal stem cells in rabbit tendon. J. Orthop. Res. 2005;23:287–293. doi: 10.1016/j.orthres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- [239].Ouyang HW, Goh JC, Lee EH. Viability of allogeneic bone marrow stromal cells following local delivery into patella tendon in rabbit model. Cell Transplant. 2004;13:649–657. [PubMed] [Google Scholar]
- [240].Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AI. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267–277. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- [241].Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One. 2013;8:e75697. doi: 10.1371/journal.pone.0075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Crovace A, Lacitignola L, De Siena R, Rossi G, Francioso E. Cell therapy for tendon repair in horses: an experimental study. Vet. Res. Commun. 2007;31(Suppl. 1):281–283. doi: 10.1007/s11259-007-0047-y. [DOI] [PubMed] [Google Scholar]
- [243].Suwalski A, Dabboue H, Delalande A, Bensamoun SF, Canon F, Midoux P, Saillant G, Klatzmann D, Salvetat JP, Pichon C. Accelerated Achilles tendon healing by PDGF gene delivery with mesoporous silica nanoparticles. Biomaterials. 2010;31:5237–5245. doi: 10.1016/j.biomaterials.2010.02.077. [DOI] [PubMed] [Google Scholar]