Synopsis
Spinal cord injuries (SCI) can disrupt communications between the brain and the body, leading to a loss of control over otherwise intact neuromuscular systems. The use of electrical stimulation (ES) of the central and peripheral nervous system can take advantage of these intact neuromuscular systems to provide therapeutic exercise options, to allow functional restoration, and even to manage or prevent many medical complications following SCI. The use of ES for the restoration of upper extremity, lower extremity and truncal functions can make many activities of daily living a potential reality for individuals with SCI. Restoring bladder and respiratory functions and preventing pressure ulcers may significantly decrease the morbidity and mortality following SCI. Many of the ES devices are already commercially available and should be considered by all SCI clinicians routinely as part of the lifelong rehabilitation care plan for all eligible individuals with SCI.
Keywords: Electrical stimulation; Electrodes; Spinal cord injuries; Rehabilitation; Paralysis, spastic; Respiratory paralysis; Pressure ulcer; Urinary bladder, neurogenic
An injury to the spinal cord can disrupt communications between the brain and body, leading to a loss of control over otherwise intact neuromuscular systems. By taking advantage of these intact neuromuscular systems, a number of neuroprostheses have been developed to restore functions through electrical stimulation (ES) or functional electrical stimulation (FES) of the central and peripheral nervous system. Neuroprostheses employing ES to control the paralyzed muscles can postpone or prevent many secondary medical complications and improve functional independence by providing a means to exercise and negotiate physical barriers. Improvements in multiple body systems and functions have been reported through the use of FES, and they will be discussed in this chapter. These devices range in complexity, and include components such as power supplies (which may be completely external to the body or implanted and recharged with radio frequency (RF) waves), a control circuit (i.e., the “brains” of the device), lead wires, connectors, external braces, and sensors. In this chapter, we will describe the basic properties of the electrodes, the current ES and FES systems being developed in research and in clinical practice, and the future of these devices.
The Basic Properties of Electrodes for Nerve Stimulation
In neuroprostheses, electrodes are the interface between the external circuitry and the tissue, delivering a charge that stimulates the nerves connected to the muscles of interest. This charge perturbs the resting potential of the neuron (typically around −65 mV); if this value is raised beyond a threshold, membrane depolarization occurs. This results in an influx of Na+ ions, initiating an action potential that can travel spatially down the length of an axon. A coordinated group of action potentials can lead to a muscle contraction1. By targeting nerves rather than the muscle fibers themselves (which can also be stimulated electrically), substantially smaller charge densities may be used, consuming less power and avoiding tissue damage2.
Provided that the neuromuscular system is intact, stimulation may be achieved at a variety of locations (from the origin of the neuron in the spinal cord, to the peripheral nerve, to the skin above the muscle), using various types of electrodes. The simplest configuration uses large (cm2) electrodes placed on the surface of the skin. The electrodes are easily replaced, however, achieving accurate and precise positioning can be challenging, and charge is distributed over a large area. A more invasive approach is to implant needle-like electrodes percutaneously into the muscle of interest. This method is considered a precursor to fully implanted systems, although subcutaneous electrodes themselves can remain functional for years3. When electrodes are fully implanted in close proximity to the nerve, even more precise targeting can be achieved using even smaller current densities, which are less likely to damage the tissue.
Electrodes have been designed to wrap around individual nerves, with a range of geometries, including spiral4, helical5 and rectangular6. To selectively address smaller groups of axons within a nerve and to reach areas which are not readily accessible from the surface, intrafascicular electrodes may be inserted into the nerve itself7. Pools of neurons may also be stimulated directly in the spinal cord in intraspinal microstimulation8. While implanted devices offer superior targeting, the obvious drawback is the invasiveness of the insertion process and the potential risk of infection, though this has not been reported as a significant issue9.
In FES, the electrode typically acts as a conductor, delivering electrical charge from a power supply to the tissue. Charge transfer occurs when voltage applied between the active electrode and a second electrode (called the reference electrode) generates an electric field, which in turn, forces electrical charge to flow. In systems in which multiple stimulation channels are utilized, a single reference electrode may be used. When a voltage is applied, the energy can drive a number of unwanted chemical reactions. To avoid generating H2 gas from water, the voltage generated between the electrodes must not exceed the amount required to electrolyze water (~ −0.6 V to −0.8 V depending on electrode type10). The amount of charge that can be delivered within these limits depends on the impedance of the material, which should be low to maximize the current delivered. To balance the charge injected to stimulate the neurons and prevent the electrochemical decomposition of tissue, a secondary pulse of opposite polarity should be included in the stimulation profile (i.e., a biphasic pulse should be applied). The electrodes themselves must be selected to be resistant to corrosion under physiological conditions, even under an applied voltage. Common electrode materials for implanted devices include corrosion-resistant stainless steel, and noble metals such as PtIr, or Pt (which have highly stable atomic configurations and therefore are resistant to chemical processes such as corrosion or oxidation). Other metals (including silver, iron, and copper) are known to elicit dramatic inflammatory response in vivo and should be avoided11.
The time-dependent failure of neural interfaces in vivo is an impediment to long-term use, particularly for recording electrodes, and stimulating electrodes, which inject small currents into small target areas. The principal cause of failure of these devices is the encapsulation, which occurs as a part of the foreign body response, insulating the electrodes from their surroundings12. To avoid scar formation initiated by mechanical mismatch between stiff electrodes and soft tissues, there is an increasing interest in fabricating electrodes and arrays from soft (low modulus) materials such as silicone elastomer13. Beyond this, a number of strategies have been undertaken to modify the surface properties of electrodes to improve the interactions which take place with surrounding tissue and reduce glial scar formation14. When developing new electrodes, arrays, and coatings, in vitro testing may be utilized initially to screen the cellular response, but they must be tested in vivo following the standard ISO 10993.
Upper Extremity Functional Restoration with FES
For individuals with cervical level spinal cord injury (SCI), restoration of hand function is their top priority15. Neuroprostheses using FES provide the most promising method for significant gain in hand and arm function for this population. Muscle contractions can be orchestrated to produce coordinated grasp opening and closing; thumb opening, closing and positioning; wrist extension/flexion; forearm pronation; and elbow extension for individuals with C5/C6 level SCI. Neuroprostheses can be coupled with tendon transfers in order to maximize function16. The objectives of these neuroprostheses are to reduce the need to rely on assistance from others, the need for adaptive equipment, braces or other orthotic devices, and the time it takes to perform tasks. Neuroprostheses make use of the patient’s own paralyzed musculature to provide the power for grasp and the patient’s voluntary musculature to control the grasp. Typically, individuals with SCI use the neuroprosthesis for eating, personal hygiene, writing and office tasks.
Neuroprostheses have been clinically implemented and investigated using systems based on surface electrodes, percutaneous electrodes and implanted devices. Surface and percutaneous systems have potential application in muscle conditioning and in short-term research or clinical applications17. Implanted systems are generally utilized for long-term functional enhancement.
All existing upper extremity neuroprosthetic systems consist of 1) a stimulator that activates the muscles of the forearm and hand, and 2) an input transducer and control unit. The control signal for grasp is derived from an action that the user has retained voluntary control over, which can include joint movement, muscle activity, respiration, or voice control18. A coordinated stimulation pattern is developed so that the muscles are activated in a sequence that produces a functional grasp pattern as the user typically has control over grasp opening and closing, but does not have direct control over the activation of each muscle.
Surface stimulation of the forearm and hand can be used to exercise and to produce functional movements. Nathan19 developed a splint that incorporates surface electrodes for grasp. This system is commercially available [NESS H200, Bioness, Valencia, CA] and is primarily intended for therapeutic applications following stroke or SCI such as building muscle strength, preventing joint contractures, and improving tissue viability. Popovic et al20 have developed a surface stimulation system called the ETHZ-ParaCare neuroprosthesis. This system is capable of four channels of stimulation and can be interfaced with a variety of control inputs. Early functional results indicate that subjects can use the system to perform a variety of activities of daily living (ADL) in the home21.
Implanted FES systems have been utilized for long-term functional enhancement for individuals with cervical SCI. The largest clinical trial of an upper extremity neuroprosthesis was the Freehand trial, initiated by the Cleveland Functional Electrical Stimulation (FES) Center in 199222. The Freehand® neuroprosthesis used an implanted eight channel receiver-stimulator and control of grasp opening and closing was achieved through graded elevation of the user’s contralateral shoulder. Using the neuroprosthesis, 100% of the participants (n= 28) improved in independence in at least one task, and 78% were less dependent in at least three tasks. More than 90% were satisfied with the neuroprosthesis23. The Freehand system was transferred to industry (NeuroControl Corp. (NCC)), and was implemented successfully in over 200 SCI users24. Despite the clinical success, the company exited the SCI market in 2001 and no longer markets the Freehand System.
A second generation implanted neuroprosthesis has been developed, improving on the features of the Freehand System25. This system, called the Implanted Stimulator Telemeter Twelve-channel System (IST-12), has twelve stimulation channels and two channels of myoelectric signal recording acquisition26. To date, twelve SCI subjects have been implanted with the IST-12 system, including three subjects with systems for restoring movement in both hands. Subjects successfully use the processed myoelectric signal from a wrist extensor for proportional control of grasp opening and closing. Every subject has demonstrated improvement in at least two activities, and as many as eleven activities. Most commonly, improvement was demonstrated in eating with a fork and writing with a pen. Other tasks in which subjects showed improvement included: office tasks, using a cell phone, getting money out of a wallet, and embroidery25, as illustrated in Figure 1.
Figure 1.

Functional activities performed using the IST-12 myoelectrically-controlled neuroprosthesis. From left to right: eating with a fork, holding a pen to write, holding a cup, needle embroidery, holding a tennis racquet.
Availability
At present, commercially available FES systems for grasp function in cervical SCI are limited to surface stimulation systems. Specifically, the NESS H200 is available by prescription at multiple sites throughout the world (www.bioness.com). Other systems, such as the Compex system, are primarily targeted for exercise training rather than function benefit. Efforts are currently underway to increase the availability of implanted neuroprostheses to individuals with SCI [http://casemed.case.edu/ifr/].
Future directions
Future directions for FES hand systems include the development of fully-implanted systems that eliminate the need to don and doff components27 and the expanded use of myoelectric control algorithms to control multiple functions at the same time28. The use of signals derived directly from the brain (brain-computer interface), either externally or through implanted electrodes, is expected to result in more natural hand system control29. In addition, systems are being developed to provide whole arm function for those with C4 or higher SCI30.
Lower Extremity Functional Restoration with FES
The inability to stand or step significantly limits the performance of SCI individuals’ many ADL such as washing dishes at a counter or reaching items on high shelves. For individuals with thoracic-level complete SCI, stimulated contractions of the lower extremity muscles can enable standing and stepping, increase personal mobility, and improve general health and quality of life31. In persons with incomplete injuries walking performance can be improved32.
Eight channels of continuous stimulation to the knee, hip and trunk extensors can power the sit-to-stand transition and support the body vertically against collapse (Figure 2)33. Stimulation to the hip ab/adductors and ankle plantar/dorsiflexors has been included in experimental systems for sensor-based control of standing balance in the coronal and sagittal planes34. Existing neuroprostheses for lower extremity function currently utilize maximal levels of constant stimulation at the hips and knees35. Recipients of a neuroprosthesis with epimysial and intramuscular electrodes that continuously activated the vasti, gluteals, hamstrings and lumbar erector spinae exhibited mean and median standing times of 10 minutes and 3 minutes, respectively33. This is sufficient for facilitating transfers to high surfaces, performing swing-to gait for short distances in wheelchair inaccessible environments and participating in other social, work and personal activities. Some implant recipients in a Phase II clinical trial of the system were able to stand for more than 20 minutes, and all were able to release one hand from a walker or assistive device to reach objects overhead (Figure 3). On average, 90% of body weight was placed on the legs, reducing requirements on the arms to only light touch to maintain balance. System performance and patterns of usage were maintained following discharge for at least one year follow-up. While there were no discernible interactions between injury level, degree of preserved sensation or time post-injury and system performance, outcomes appear to be inversely proportional to height and weight, implying that body mass index may be an important clinical factor for determining expectations35. Long term use of neuroprostheses for standing was safe and effective, and had no adverse physiological effects.
Figure 2.
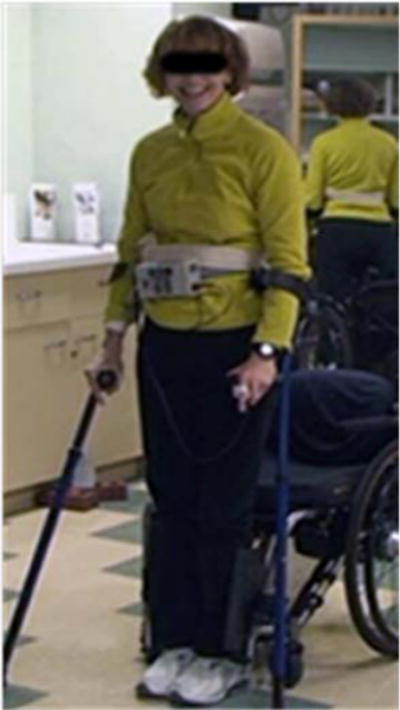
Implant recipient (C7 AIS C) standing with FES to the knee, hip and trunk extensors, and hip/trunk ab/adductors. Multi-contact cuff electrodes on the femoral nerves selectively activate the uniarticular heads of the quadriceps (vastus lateralis, intermedius and medialis).
Figure 3.

Eight channel implant recipient (T9 AIS A) releases one had for overhead reaching activities while standing with the neuroprosthesis.
Stepping of up to 100m has also been achieved after paralysis with simple pre-programmed patterns of open-loop stimulation delivered from the surface or via 8- and 16-channel implanted pulse generators36. Once initiated by the user, stepping motions can cycle continuously while the appropriate adjustments are made with the upper body until the pattern is stopped. Alternatively, the stimulation for sequential steps can be triggered from successive depressions of ring- or walker-mounted switches or automatically from body-mounted sensors such as inclinometers, accelerometers, gyroscopes or foot/heel switches37. The largest potential impact of stimulation may be for people with motor incomplete injuries (Figure 4) who require activation of a small number of muscles during the gait cycle in order to become household or community ambulators38. In such cases, gait training with stimulation can have a therapeutic effect in terms of improved voluntary strength, walking speed, stride length and cadence even after completion of aggressive conventional therapies39. Interactive use of stimulation to assist gait resulted consistently in an additional 20% improvement in walking speed and six minute distance, as well as a more than three-fold increase in maximum walking distance, illustrating a significant neuroprosthetic effect. Walking with stimulation was also more dynamic as evidenced by decreased time spent in the double support phases of gait. The electromyographic activity of muscles under volitional control has also been exploited as a command source to control stimulation in individuals with incomplete injuries. This has the potential to coordinate stimulated contractions with voluntary motor function, and in so doing reinforce voluntary movement patterns and provide a mechanism to continuously modulate walking speed and cadence40.
Figure 4.
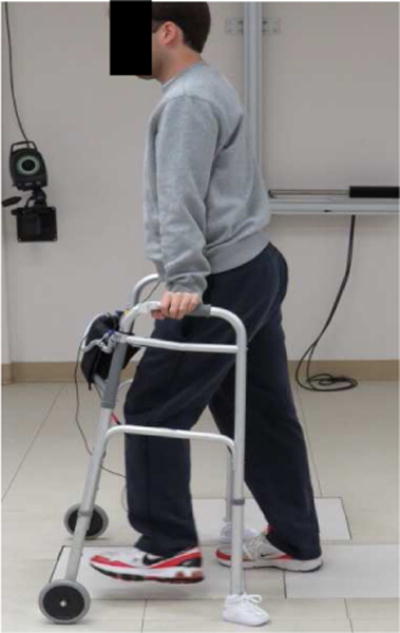
Subject with incomplete SCI (C5 AIS D) walking with an eight channel implanted receiver stimulator for activation of hip flexors and ankle dorsiflexors.
Surface FES to the lower extremity muscles with intact innervation has allowed cycling movement which simulates exercise training, leading to increase in oxygen consumption during exercise41, muscle mass and strength, and quality of life in individuals with chronic SCI42.
Availability
While implanted standing and walking systems clearly provide significant functional and clinical benefits, such systems are currently only available on a research basis. Limited lower extremity function is possible with commercially available surface stimulators with reduced channel counts43.
FES cycling devices are available through Restorative Therapies, Inc. (www.restorative-therapies.com) and Therapeutic Alliances, Inc. (www.musclepower.com) in the US.
Future Directions
Standing performance with implanted neuroprostheses can be improved significantly by utilizing nerve-based electrodes which more fully recruit the target muscles. Continuous stimulation of the femoral nerve with a multi-contact cuff electrode below the branches to the rectus femoris and sartorius was shown to extend standing time and accelerate progress through reconditioning rehabilitation and balance training with the system44. The potential to delay the effects of fatigue by alternating activation of independent motor unit pools within a muscle via multi-contact nerve cuffs or multiple independent nerve- or muscle-based electrodes is also being investigated45. Current neuroprostheses are generally unresponsive to environmental disturbances, necessitating use of the arms for balance on an assistive device. Additional research is also focusing on automatically modulating stimulation in response to perturbations in order to reduce reliance on the upper extremities, allow users to alter their postures in advance of anticipated disturbances, and minimize the risk of falls while standing, or using advanced biomechanical modeling techniques to optimize stimulus patterns during walking or while assuming various task-dependent standing postures46. Another promising development involves the combination of FES with exoskeletal bracing that can lock, unlock or couple the joints as necessary to avoid fatigue and smoothly shape limb trajectories, or that can inject small amounts of assistive power when the stimulated responses are too weak or fatigued to complete a motion47. With such an approach, users would be able to walk under their own power, therefore accrue the physiological benefits of exercising the paralyzed muscles in addition to those of standing, weight bearing and mobilization.
Trunk Control and Posture with FES
Following SCI, trunk muscles can oftentimes not provide the necessary forces to adequately control trunk posture due to a lack of innervation48 and/or muscle atrophy49, significantly limiting their performance during ADL50 and even leading to secondary health complications such as reduced respiratory capacity51. To compensate for insufficient muscle control during sitting, individuals with SCI usually tilt their pelvis further backward to increase stability in the anterior direction52. When reaching, they oftentimes use one arm thrown over the back of their chair to provide the external forces necessary to keep the trunk from bending forward uncontrollably. Compensational sitting arrangements can, however, lead to kyphosis53 and pressure ulcers (PU) that arise from asymmetric trunk orientation and infrequent weight redistribution. It is therefore not surprising that individuals with SCI have prioritized the recovery of trunk control over the recovery of walking function and other essential functional abilities15.
Bracing devices such as corsets are perhaps the most common items for stabilizing the trunk after SCI. In order to improve reaching and wheelchair propulsion, some individuals with SCI use chest straps54. In the general case of reaching from a wheelchair during ADL, chest straps or other restraints are highly undesirable as they hinder free and spontaneous movement, decrease available trunk range of motion, and draw undue attention to themselves. Also, other studies have shown that the large forces exerted upon the abdomen by a fabric corset might cause abnormal increases in the intra-abdominal pressure, potentially leading to disturbance of the viscera55.
Stiffening the paralyzed trunk and hip extensors with continuous electrical stimulation has a multitude of benefits: it can correct kyphotic seated postures, normalize lateral vertebral alignment, improve ventilation and respiratory volumes, and alter interface pressures56. It can also expand bimanual workspace57, statically stabilize the torso (Figure 5), increase the forces that can be exerted on objects with the upper extremities, return users to erect sitting from a fully forward-flexed posture, and improve manual wheelchair propulsion efficiency at comfortable speeds58. Independent bed turning and wheelchair transfers can also be facilitated by more rigidly coupling the pelvis to the shoulders when the paralyzed core trunk muscles are continuously activated with stimulation to stiffen the torso59. In addition, activating the quadratus lumborum with surface or implanted electrodes has been shown to enhance medio-lateral stability and assist with attaining side leaning postures, whereas coactivation with the abdominal muscles can further stiffen the trunk while seated or assist in attaining forward leaning postures. Some of the required muscles to achieve these clinical outcomes can be accessed via surface stimulation; however, strong and isolated contractions are robustly and repeatably achieved by exciting the T12–L2 spinal nerves associated with the lumbar erector spinae and other muscles (Figure 6) using intramuscular electrodes and surgically implanted pulse generators60. It should be emphasized that the strategy of continuously activating the core trunk and hip muscles only substitutes one statically stable posture for another. Upper extremity effort is still required to stabilize the body during transitions between non-stimulated and stimulated postures, and to maintain balance or restore erect sitting when exposed to internal or external perturbations.
Figure 5.
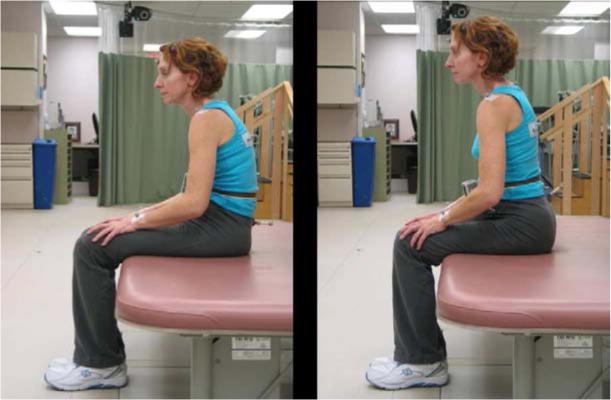
Effect of FES on seated posture. By stimulating the trunk and hip muscles, consistent significant changes in posterior pelvic tilt and shoulder height were recorded.
Figure 6.
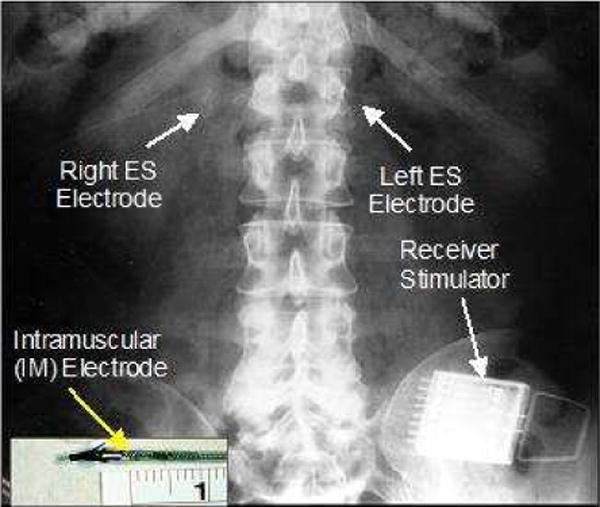
X-ray of an implanted trunk system showing intramuscular electrodes (inset) inserted into T12-L1 to activate the lumbar erector spinae muscles.
Extensive studies have been carried out to assess the strategy used by the intact central nervous system to mediate trunk balance in neurologically intact individuals. Such studies mainly involve biomechanical simulations and experimental observations of the static and dynamic behavior of trunk posture in a seated pose61. These studies confirmed the initial feasibility of utilizing continuous stimulation to increase trunk stiffness, vary trunk posture, and resist static perturbations. Moreover, they resulted in tools for evaluating more sophisticated control systems that might allow users to set their own task-dependent postures, and maintain balance during internal or external perturbations. Recent studies have established the feasibility of a self-righting control system that works on the dynamic movement of the trunk to automatically return to an erect posture from forward-flexed positions by monitoring trunk tilt and modulating stimulation to the trunk and hip extensors appropriately (Figure 7)62. In this study, five individuals with SCI volunteered to test a simple threshold-based set-point controller. The controller worked consistently across all subjects despite considerable inter-subject variability in terms of SCI level, and motor and sensory impairment.
Figure 7.
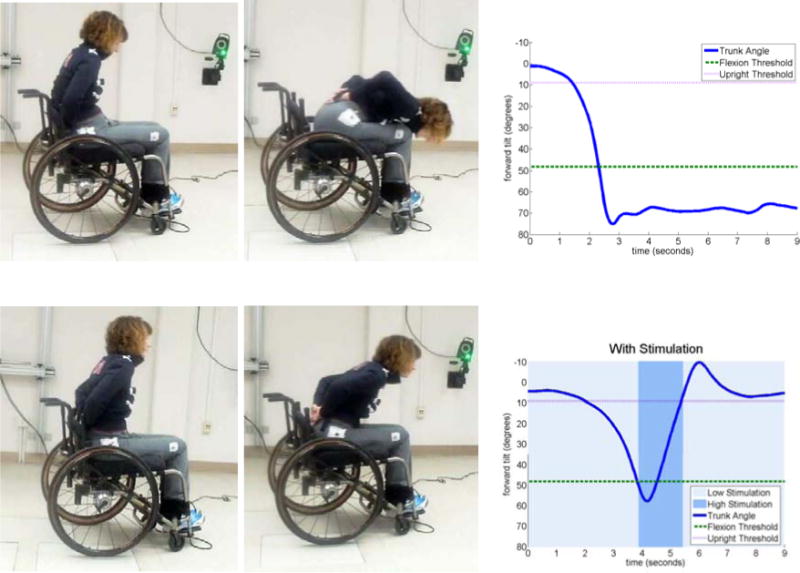
Simple threshold-based control of seated balance based on trunk tilt in a subject with C8 tetraplegia. Without stimulation (top) of the hip and trunk extensors, the subject cannot return to erect sitting from a fully forward-flexed position without use of the arms. With the controller active (bottom), forward trunk tilt is arrested prior to a forward fall, and upright posture is automatically restored.
Availability
Currently, neuroprostheses for controlling the paralyzed torso and enhancing seated function can only be obtained through research and development studies, while attempts to commercialize such systems are ongoing.
Future Directions
Advanced systems to control seated posture and trunk balance have the potential to prevent falls from the wheelchair while performing ADL, during sudden collisions and unexpected stops, and while negotiating bumpy or uneven terrain, thus, eliminating the need for chest straps or other constraints that would hinder function. New systems that can sense trunk and wheelchair position, velocity, or acceleration as well as communicate the user’s intent to closed-loop controllers need to be developed. Important requirements of such systems are that they are portable, appear natural, and can be easily integrated with any residual motor and/or sensory function. Such systems also need to be translated into routine clinical use and disseminated widely in home and community environments. Future directions also include the timing of the stimulation to coincide with different phases of the manual wheelchair propulsion cycle to improve efficiency during ramp ascent or varying speeds, utilization during rowing exercise, and early introduction of trunk control systems soon after injury to prevent the development of spinal deformities and help vary posture to augment pressure relief maneuvers.
FES Techniques to Restore Respiratory Muscle Function
The use of functional electrical stimulation to improve respiratory muscle function is discussed in depth in the section entitled “Diaphragm Pacing in Spinal Cord Injury”, authored by Kevin L. Dalal, MD and Anthony F.DiMarco, MD.
Prevention of pressure ulcers through electrical stimulation
Pressure ulcers (PU) are a common complication following SCI. They cause psychological distress, have a detrimental impact on quality of life and place a significant burden on health care systems with costs recently estimated at $6 to $15 billion per year in the US63. Preventing PU from developing in the first place will reduce patient suffering, improve patient outcomes and quality of life and reduce the large health care costs associated with treating them. Indeed, it has been estimated that prevention of pressure ulcers is approximately 2.5 times more economical than treating them64.
Pressure ulcers can develop in one of two ways. They can originate at the surface of the skin and progress inwards if unattended. Skin inspections are often effective in detecting these ulcers at an early stage of development. If unattended, these ulcers can progressively affect deeper tissue layers ending at the bone. PU can also originate at deep muscle-bone interfaces and progress outwards. These ulcers have only recently been acknowledged clinically and are now referred to as deep tissue injury (DTI). Sustained pressure leads to unrelieved mechanical deformation, tissue ischemia and ischemia-reperfusion injury. Muscle is more susceptible to breakdown due to mechanical deformation and ischemia-reperfusion injury than skin; thus damage originates within muscle tissue around bony prominences much sooner than in the skin. Skin inspections are ineffective in detecting DTI at their earliest stages of development and there are currently no clinically viable methods for the early detection of DTI. Therefore, these ulcers often develop unbeknownst to the affected individual or their caregiver. Once DTI exhibit obvious skin signs; e.g., purple discoloration, extensive damage in the underlying soft tissue had already occurred. Current prevention strategies such as pressure re-distributing surfaces (mattresses and seating cushions) and periodical weight shifts have not decreased the incidence of PU, in fact, the prevalence of PU, particularly DTI, is on the rise65. Therefore, other approaches are necessary. ES through surface stimulation and implanted electrodes are two novel ways to prevent PU, each having their own specific advantages and disadvantages. Both systems require intact innervation to the gluteal muscles.
Intermittent electrical stimulation for the prevention of DTI
Intermittent electrical stimulation66 (IES) was developed for the prevention of DTI. This method applies brief ES through surface electrodes to muscles around bony prominences that are loaded during sitting or lying down (e.g., the gluteus maximus muscles) every few minutes causing them to contract. These periodical contractions mimic the subconscious postural adjustments conducted by able-bodied individuals in response to discomfort while sitting or lying down. Ten seconds of IES causing fused muscle contractions in the gluteus muscles every 10 minutes while sitting redistributes surface pressure away from the ischial tuberosities have shown to significantly increase tissue oxygenation in study participants independent of gluteal muscle mass67 68. IES-induced contractions significantly redistribute internal pressure away from the bony prominences69 and reduce tissue deformation in the muscles between the ischial tuberosity and skin even when loading levels as high at 75% of body weight in adult pigs with SCI were applied70. Most importantly, IES is effective in significantly reducing or completely eliminating the formation of DTI in adult rats and pigs71; thus establishing a strong scientific support for the utility of IES as a means for preventing DTI in clinical settings.
Implanted Neuromuscular Stimulation for Tissue Health and Pressure Ulcer Prevention
Another approach of ES for PU prevention is through stimulation of the inferior gluteal nerve, which innervates the gluteus maximus muscle, and lies relatively deep to the buttock surface and close to the sciatic nerve. Surface electrode placement for preferential recruitment of the inferior gluteal nerve can be difficult for users to achieve. Moreover, repeatable electrode placement in the upper buttock region may be hard to accomplish for either independent users or their carers. Implanted neuromuscular electrical stimulation (NMES) systems for long-term therapeutic use have dual advantages. The stimulating tip of the electrode can be located close to the motor point of the nerve of interest. This reduces the charge required to elicit a contractile response and ensures that the response is repeatable and predicable. The user does not have to replace the stimulating electrode every day so the system is both reliable and simple to use.
The gluteal stimulation v1 (GSTIM I) system utilizing implanted electrodes with percutaneous leads provides both concurrent bilateral and alternating gluteal stimulation to deliver muscle conditioning and regular weight-shifting to the user. GSTIM I has been shown to have a positive impact on multiple aspects of tissue health. Subjects who received GSTIM I have shown statistically significant changes between baseline and post-intervention ischial region interface pressure (Figure 8). Maximum gluteal muscle thickness significantly increased and was maintained with regular use of gluteal NMES72. Tissue oxygen levels also improved with regular use of dynamic stimulation but decreased on withdrawal.
Figure 8.
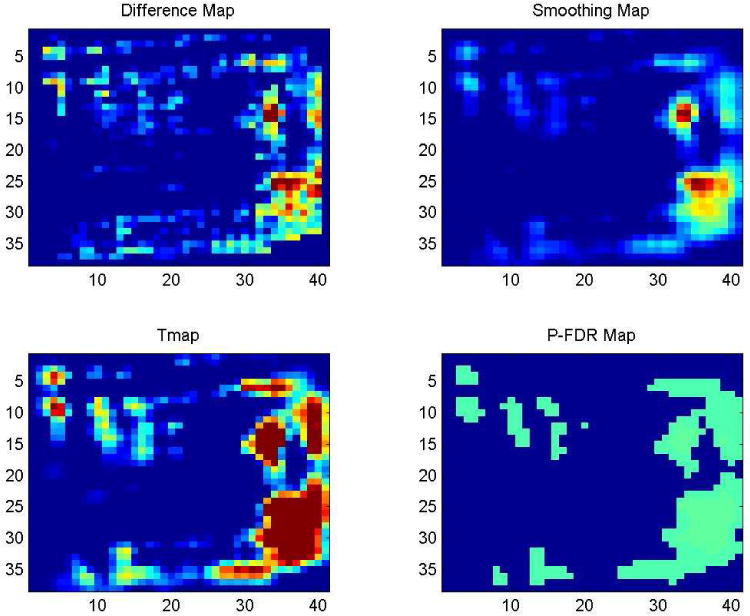
Multistage longitudinal analysis and self-registration (LASR) analysis maps showing areas of significant change in seated interface pressures over time (output adjusted for simultaneous testing at multiple locations)
In addition to the long-term changes in muscle characteristics, weight-shifting induced by gluteal NMES dynamically alters conditions at the seating support interface facilitated by stimulated muscular contractions. This dynamic effect increases over time as the paralyzed muscles become stronger with regular use of implanted gluteal NMES. Chronic application of gluteal stimulation is thus uniquely able to affect the intrinsic properties of paralyzed muscle through contractile responses to repeated stimulation, increasing muscle thickness and blood flow together with reducing regional interface pressures73,74. Use of GSTIM I also increased sitting tolerance and minimized the impact of minor incidents such as skin tears due to poor transfers which were reported to be resolved in days rather than weeks.
Therapeutic implanted NMES provides a unique intrinsic approach to reducing the risk of PU development for persons with SCI. Daily use of NMES is indicated in order to maintain hypertrophy of paralyzed muscles. Long-term use of gluteal NMES using implanted systems may provide an adjunctive method to ensure a regular pressure relief regimen in high-risk individuals. This can reduce the risk of PU development and allow users to participate more fully in ADL.
Availability
Both the IES system and the fully implanted NMES for the prevention of pressure ulcers are currently under research protocol use only.
Future Directions
Further research is currently underway to examine the efficacy and effectiveness of the approach for PU prevention with both the surface stimulation and implanted systems. A system for clinical use to deliver IES to the gluteal region, known as Smart-e-Pants75 (Smart-electronic-Pants) (Figure 9) was developed. It is composed of a garment, surface electrodes and a small battery-operated stimulator. The electrodes are placed on mesh panels in the garment. Safety, feasibility and acceptability of Smart-e-Pants have been tested in a wide range of healthcare settings, including 50 volunteers in an acute rehabilitation unit, tertiary rehabilitation hospital, a long-term care facility, and homecare. Study participants used the system for at least 4 weeks, 12 hours per day. The system proved to be safe and feasible in all four clinical settings. No PU was observed in any of the participants. Donning and doffing of the Smart-e-Pants system took between 7 and 18 minutes. Importantly, patients and caregivers did not find the application of Smart-e-Pants nor IES to be disruptive and indicated that the stimulation was acceptable as part of their daily routine in over 97% of the time. These preliminary clinical studies on IES as a preventative treatment strategy are very promising. Further refinement of the stimulator and garment is also necessary to promote usability.
Figure 9.

“Smart-e-pants” system showing garment, mesh panel for surface electrodes and stimulator.
Future development of the fully implanted NMES system will utilise a small, rechargeable stimulator customized to provide two synchronized channels of stimulation to automatically produce the regular weight-shift maneuvers recommended for periodic pressure relief when seated in the wheelchair.
FES for Restoring Bladder Control
The lower urinary tract (LUT) functions in the storage and emptying of urine. Following SCI with an upper motor neuron injury to the sacral nerve roots, volitional control of these functions is frequently lost and the LUT becomes hyper-reflexive. Incontinence can occur when the detrusor produces large, uninhibited reflex contractions at low volumes of stored urine. Simultaneously with detrusor contractions, the external urethral sphincter (EUS) may reflexively contract as pressure builds in the urethra during voiding, producing detrusor-sphincter dyssynergia (DSD). This uncoordinated reflex and the subsequent high bladder pressures can result in inefficient voiding, incontinence, and ureteric reflux causing renal injury. In addition, DSD can also cause autonomic dysreflexia (AD), which can be life-threatening if not resolved. Finally, loss of bladder control has a severe impact on quality of life and self-image. Individuals with SCI list bladder function restoration among the highest priority for restoration, above standing and ambulation15.
Individuals with SCI frequently report ineffectiveness with existing bladder management, medication side effects, challenges associated with bladder catheterization strategies, and complications associated with surgical solutions. Similar to many other complications of SCI discussed above, there remains a critically unmet need to restore bladder function lost to SCI and the use of FES may offer an effective solution.
FES offers a means to restore LUT function by activating the bladder and inhibiting the urethral sphincter to produce voiding, or by inhibiting the bladder to provide urinary continence and reduce triggers for AD and restore LUT function76. The Brindley approach was the first widely clinically available FES system for bladder function77. This approach produces bladder contractions by stimulating bladder motor efferents in the sacral roots. To avoid co-contraction of the EUS and detrusor preventing fluid flow, stimulation is delivered in repeated bursts. After each burst, the striated EUS muscle relaxes, but the smooth-muscle bladder relaxes more slowly, maintaining bladder pressure and creating a pressure gradient that causes post-stimulus urine flow. This system has been implanted in thousands of individuals with SCI and is both medically and cost-effective78. However, this approach requires transection of the dorsal spinal roots (dorsal rhizotomy) to eliminate unwanted bladder and urethral reflexes due to sensory feedback. This rhizotomy also eliminates desirable reflexes that affect sexual and bowel functions, and removes the opportunity for future clinical therapies, markedly reducing acceptance of this approach by individuals with SCI79.
Stimulation of peripheral sensory pathways can access and influence the spinal neural circuits that control pelvic reflexes and function. Afferent-mediated neural prostheses take advantage of natural nervous system processes and are potentially less invasive than spinal-root based approaches such as the Brindley system. This approach has the potential to provide more natural function than motor driven approaches, though it is more dependent on stimulation patterns and other inputs to the spinal circuits. One such approach uses genital nerve stimulation to achieve direct spinal level bladder inhibition. This approach has primarily been used acutely, but it has also shown to improve urinary continence and bladder capacity in persons with SCI during short duration use80,81. If longer term use is effective, then this approach may provide both a non-invasive and implanted option. Bladder inhibition via implanted electrodes on the pudendal nerve82 and sacral roots83 can also provide bladder inhibition in individuals with SCI.
Availability
There are several neural prostheses in development to restore pelvic functions for individuals with SCI to activate or inhibit the bladder and urethral sphincter and provide a “rhizotomy-free Brindley system”. They are not commercially available yet.
Future Directions
Some approaches have been shown to be effective in animal models and may be promising for human studies. Bladder activation and voiding via pudendal urethral afferent stimulation has been demonstrated in animal models and human studies suggest that bladder excitation can be achieved84. This approach may provide a peripheral based alternative to sacral root based bladder activation.
Urethral sphincter inhibition and bladder voiding can be obtained with patterned afferent stimulation of sacral dermatomes85. This approach has achieved clinical daily voiding of awake animals with chronic SCI. It may potentially provide a less invasive alternative in humans. Finally, high (kHz) frequency stimulation can provide temporary, reversible complete motor block of the pudendal nerve and allow bladder voiding equivalent to nerve transection86. Bilateral pudendal nerve block can provide clinical daily voiding of awake animals with chronic SCI. If this motor based approach is effective in humans, it could be combined with pudendal bladder inhibition to restore bladder function with a single implant.
Intraspinal Microstimulation for Gait Restoration
Apart from the above systems which are either commercially available or closer to clinical availability, one novel experimental approach is worth noting. A significant limitation of the surface stimulation system to restore walking is that many of the key muscles required for walking lie deep in the leg and are not accessible with surface electrodes. Even with the percutaneous implantation system, many channels will be required to stimulate these different muscles. Mushahwar’s group has pioneered the use of implanted electrodes in the spinal cord to overcome these problems87. Intraspinal microstimulation (ISMS) involves implantation of ultrafine microwires precisely into the anterior horn of the lumbar enlargement. A single electrode can stimulate a synergistic group of muscles; thus, routing electrodes widely through the body to each member of a muscle group is not necessary. The levels of stimulation are orders of magnitude less than those required for stimulation through the skin. Moreover, the levels required for generating functional limb movements generated no signs of discomfort or pain in conscious experimental animals implanted with ISMS microwires. By stimulating the motor pools innervating hindlimb of an anesthetized cat, walking of more than 1 kilometer along a 4 meter walkway has been produced without appreciable muscle fatigue. The trunk and forequarters are partly supported, as would be true in a person using a walker or other assistive devices. The system currently uses external sensors to control stimulation, but single unit recordings from dorsal root ganglion will ultimately be used for control.
There are several surgical considerations in planning for a proof of principle study of ISMS in humans, the most critical of which is patient selection. Instrumentation, fusion and/or SCI at T12-L1, the lumbar enlargement, will preclude ISMS. Yet one of the commonest sites of traumatic SCI is the thoracolumbar junction with mid-thoracic paraplegia being less common. While younger individuals with SCI are generally better candidates for any experimental therapy, a temporary implant or even surgical mapping procedure to determine the ability of ISMS to activate motor pools may preclude these individuals from undergoing permanent implantation in the future. Multiple penetrations of the spinal cord may result in gliosis, and opening the dura alone is enough to scar it to the pia-arachnoid layers, making surgical re-exploration higher risk. Therefore, younger individuals with SCI may not be the best for a short-term feasibility trial. Other surgical issues include the ease or difficulty of implanting very fine <100 μm wires into the spinal cord not only so that they do not bend, but also so they are placed exactly perpendicular to the dorsal surface of the cord and reach the anterior horn motor cell pools. Specific instrumentation has been designed to inject stem cells successfully into anterior horn of lumbar spinal segments88 and could be adapted to insert electrodes as well. As with stem cell injection, anticipated complications include cerebrospinal fluid leak, wound dehiscence and possibly long-term kyphosis. While minimally invasive insertion methods would be optimal for such surgery, it is best to start with an open approach to target the correct motor pool levels fully. Fusion may be undertaken as part of the procedure to secure wires emanating from the dura and prevent significant motion at the level of implant. However, fusing a mobile segment is not without long-term risk of failure or more degenerative change at adjacent segments. Utilizing microelectrodes with multiple contact sites along their shaft will reduce the number of penetrations required to reach an excitable motor pool for ISMS, and these are currently under development. A wireless system with only a thumb-tack type of receiver on the surface of the cord, linked wirelessly to a transmitter implanted subcutaneously would be ideal.
Key Points.
Electrical stimulation of the peripheral and central nervous system may be used for rehabilitation and management of complications following spinal cord injury.
Electrical stimulation may improve the functional status and quality of life of many persons with spinal cord injuries.
Many of the electrical stimulation strategies are already commercially available, while others are being tested in human and laboratory studies.
Electrical stimulation should be routinely considered as part of the rehabilitation and medical management of eligible persons with spinal cord injuries.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chester H. Ho, Email: chester.ho@albertahealthservices.ca.
Ronald J. Triolo, Email: ronald.triolo@case.edu.
Anastasia L. Elias, Email: aelias@ualberta.ca.
Kevin L. Kilgore, Email: klk4@case.edu.
Anthony F. DiMarco, Email: afd3@case.edu.
Kath Bogie, Email: kath.bogie@case.edu.
Albert H. Vette, Email: albert.vette@ualberta.ca.
Musa Audu, Email: mxa93@case.edu.
Rudi Kobetic, Email: rkobetic@fescenter.org.
Sarah R. Chang, Email: sarah.r.chang@case.edu.
K. Ming Chan, Email: kming@ualberta.ca.
Sean Dukelow, Email: sean.dukelow@albertahealthservices.ca.
Dennis J. Bourbeau, Email: dbourbeau@fescenter.org.
Steven W. Brose, Email: steven.brose@va.gov.
Kenneth J. Gustafson, Email: kjg@case.edu.
Zelma Kiss, Email: zkiss@ucalgary.ca.
Vivian K. Mushahwar, Email: Vivian.mushahwar@ualberta.ca.
References
- 1.Popovic MR, Keller T, Papas IPI, Dietz V, Morari M. Surface-stimulation technology for grasping and walking neuroprostheses. IEEE Eng Med Biol Mag. 2001;20(1):82–93. doi: 10.1109/51.897831. [DOI] [PubMed] [Google Scholar]
- 2.Ragnarsson KT. Functional electrical stimulation after spinal cord injury: current use, therapeutic effects and future directions. Spinal Cord. 2007 Sep;46(4):255–274. doi: 10.1038/sj.sc.3102091. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal S, Kobetic R, Nandurkar S, Marsolais EB. Functional electrical stimulation for walking in paraplegia: 17-year follow-up of 2 cases. J Spinal Cord Med. 2003;26(1):86–91. doi: 10.1080/10790268.2003.11753666. [DOI] [PubMed] [Google Scholar]
- 4.Naples GG, Mortimer JT, Scheiner A, Sweeney JD. A spiral nerve cuff electrode for peripheral nerve stimulation. 1988:905–916. doi: 10.1109/10.8670. [DOI] [PubMed] [Google Scholar]
- 5.Agnew WF, McCreery DB, Yuen TG, Bullara LA. Histologic and physiologic evaluation of electrically stimulated peripheral nerve: considerations for the selection of parameters. Ann Biomed Eng. 1989;17(1):39–60. doi: 10.1007/BF02364272. [DOI] [PubMed] [Google Scholar]
- 6.Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. 2002:294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- 7.Tyler DJ, Durand DM. A slowly penetrating interfascicular nerve electrode for selective activation of peripheral nerves. IEEE Trans Rehabil Eng. 1997;5(1):51–61. doi: 10.1109/86.559349. [DOI] [PubMed] [Google Scholar]
- 8.Bamford JA, Mushahwar VK. Intraspinal microstimulation for the recovery of function following spinal cord injury. Prog Brain Res. 2011:227–239. doi: 10.1016/B978-0-444-53815-4.00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal S, Triolo RJ, Kobetic R, Miller M, Bieri C, Kukke S, Rohde L, Davis JA., Jr Long-term user perceptions of an implanted neuroprosthesis for exercise, standing, and transfers after spinal cord injury. J Rehabil Res Dev. 2003 May-Jun;40(3):241–52. [PubMed] [Google Scholar]
- 10.Cogan SF. Neural Stimulation and Recording Electrodes. Annu Rev Biomed Eng. 2008;10(1):275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 11.Geddes LA, Roeder R. Criteria for the selection of materials for implanted electrodes. Ann Biomed Eng. 2003 Aug;31(7):879–890. doi: 10.1114/1.1581292. [DOI] [PubMed] [Google Scholar]
- 12.Prasad A, Sanchez JC. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J Neural Eng. 2012 Apr;9(2):026028. doi: 10.1088/1741-2560/9/2/026028. [DOI] [PubMed] [Google Scholar]
- 13.Khaled I, Cheng C, Elmallah S, Moussa W, Mushahwar V, Elias A. A Flexible Base Electrode Array for Intraspinal Microstimulation. IEEE Trans Biomed Eng. 2013 Jun; doi: 10.1109/TBME.2013.2265877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grill WM, Norman SE, Bellamkonda RV. Annual Review of Biomedical Engineering. Vol. 11. Palo Alto: Annual Reviews; 2009. Implanted Neural Interfaces: Biochallenges and Engineered Solutions; pp. 1–24. [DOI] [PubMed] [Google Scholar]
- 15.Anderson K. Targeting recovery: Priorities of the spinal cord injured population. Journal of Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 16.Keith MW, Kilgore KL, Peckham PH, Wuolle KS, Creasey G, Lemay M. Tendon Transfers And Functional Electrical Stimulation for Reconstruction of Hand Function in Spinal Cord Injury. J Hand Surg. 1996;21a:89–99. doi: 10.1016/s0363-5023(96)80160-2. [DOI] [PubMed] [Google Scholar]
- 17.Chae J, Kilgore KL, Triolo RJ, Creasey G. Functional Neuromuscular Stimulation in Spinal cord Injury. Physical Medicine and Rehabilitation Clinics of North America. 2000;11(1):209–226. [PubMed] [Google Scholar]
- 18.Scott TR, Haugland M. Command and control interfaces for advanced neuroprosthetic applications. Neuromodulation. 2001 Oct;4(4):165–75. doi: 10.1046/j.1525-1403.2001.00165.x. [DOI] [PubMed] [Google Scholar]
- 19.Nathan RH. Functional electrical stimulation of the upper limb: charting the forearm surface. Med Biol Eng Comput. 1979 Nov;17(6):729–36. doi: 10.1007/BF02441554. [DOI] [PubMed] [Google Scholar]
- 20.Popovic M, Popovic D, Keller T. Neuroprostheses for Grasping. Neurological Research. 2002 Jul;24:443–452. doi: 10.1179/016164102101200311. [DOI] [PubMed] [Google Scholar]
- 21.Popovic R, Thrasher T, Adams M, Takes V, Zivanovic V, Tonack M. Functional electrical therapy: Retraining grasping in spinal cord injury. Spinal Cord. 2006;44(3):143–151. doi: 10.1038/sj.sc.3101822. [DOI] [PubMed] [Google Scholar]
- 22.Peckham PH, Keith MW, Kilgore KL, Grill JH, Wuolle KS, Thrope GB, Gorman P, Hobby J, Mulcahey MJ, Carroll S, Hentz V, Wiegner A. Efficacy of an Implanted Neuroprosthesis for Restoring Hand Grasp in Tetraplegia: A Multicenter Study. Arch Physical Medicine and Rehabilitation. 2001;82:1380–8. doi: 10.1053/apmr.2001.25910. [DOI] [PubMed] [Google Scholar]
- 23.Wuolle KS, Van Doren CL, Bryden AM, et al. Satisfaction and usage of a hand neuroprosthesis. Arch Phys Med Rehabil. 1999;80:206–213. doi: 10.1016/s0003-9993(99)90123-5. [DOI] [PubMed] [Google Scholar]
- 24.Taylor P, Esnouf J, Hobby J. The functional impact of the Freehand system on tetraplegic hand function, clinical results. Spinal Cord. 2002;40:560–566. doi: 10.1038/sj.sc.3101373. [DOI] [PubMed] [Google Scholar]
- 25.Kilgore KL, Hoyen HA, Bryden AM, Hart RL, Keith MW, Peckham PH, Montague FW, Sams CJ, Bhadra N. An Implanted Upper Extremity Neuroprosthesis Utilizing Myoelectric Control. Journal of Hand Surgery. 2008;33A:539–550. doi: 10.1016/j.jhsa.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart RL, Bhadra N, Montague FW, Kilgore KL, Peckham PH. Design and testing of an advanced implantable neuroprosthesis with myoelectric control. IEEE Trans Neural Syst Rehabil Eng. 2011 Feb;19(1):45–53. doi: 10.1109/TNSRE.2010.2079952. Epub 2010 Sep 27. [DOI] [PubMed] [Google Scholar]
- 27.Peckham PH, Kilgore KL. Challenges and opportunities in restoring function after paralysis. IEEE Trans BME. 2013;60(3):602–609. doi: 10.1109/TBME.2013.2245128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss CW, Kilgore KL, Peckham PH. A novel command signal for motor neuroprosthetic control. Neural Rehabilitation and Neural Repair. 2011 Nov-Dec;25(9):847–54. doi: 10.1177/1545968311410067. [DOI] [PubMed] [Google Scholar]
- 29.Donoghue JP, Nurmikko A, Black M, Hochberg LR. Assistive technology and robotic control using motor cortex ensemble-based neural interface systems in humans with tetraplegia. J Physiol. 2007 Mar 15;579(Pt 3):603–11. doi: 10.1113/jphysiol.2006.127209. Epub 2007 Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams MR, Kirsch RF. Evaluation of head orientation and neck muscle EMG signals as command inputs to a human-computer interface for individuals with high tetraplegia. IEEE Trans Neural Systems & Rehabil Eng. 2008;16(5):485–496. doi: 10.1109/TNSRE.2008.2006216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohde L, Bonder B, Triolo R. An exploratory study of perceived quality of life with implanted standing neuroprostheses. Journal of Rehabilitation Research & Development. 2012;49(2):265–278. doi: 10.1682/jrrd.2010.08.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creasey GH, Ho CH, Triolo RJ, Gater DR, DiMarco AF, Bogie KM, Keith MW. Clinical applications of electrical stimulation after spinal cord injury. J Spinal Cord Med. 2004;27:365–75. doi: 10.1080/10790268.2004.11753774. [DOI] [PubMed] [Google Scholar]
- 33.Triolo RJ, Bailey SN, Miller ME, Rohde L, Anderson J, Davis JA, Abbas JJ, Diponio LA, Forrest GP, Gater DR, Yang LJ. Longitudinal performance of a surgically implanted neuroprosthesis for lower extremity exercise, standing, and transfers after spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2012;93(5):896–904. doi: 10.1016/J.APMR.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nataraj R, Audu M, Triolo R. Comparing joint kinematics and center of mass acceleration for feedback control of standing by functional neuromuscular stimulation. Journal of NeuroEngineering and Rehabilitation. 2012;9:25. doi: 10.1186/1743-0003-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mushahwar VK, Jacobs PL, Normann RA, Triolo RJ, Kleitman N. New functional electrical stimulation approaches to standing and walking. J Neural Eng. 2007;4:S181–97. doi: 10.1088/1741-2560/4/3/S05. [DOI] [PubMed] [Google Scholar]
- 36.Kobetic R, Triolo RJ, Uhlir J, Bieri C, Wibowo M, Polando G, Marsolais EB, Davis JA, Ferguson K, Sharma M. Implanted functional electrical stimulation system for mobility in paraplegia: a follow-up case report. IEEE Transactions on Rehabilitation Engineering. 1999;7(4):390–398. doi: 10.1109/86.808942. [DOI] [PubMed] [Google Scholar]
- 37.Cikajlo I, Matjacic Z, Bajd T, Futami R. Sensory supported FES control in gait training of incomplete spinal cord injury persons. Artificial Organs. 2005;29(6):459–461. doi: 10.1111/j.1525-1594.2005.29077.x. [DOI] [PubMed] [Google Scholar]
- 38.Hardin E, Kobetic R, Murray L, Corado-Ahmed M, Pinault G, Sakai J, Nogan S, Ho C, Triolo R. Walking after incomplete spinal cord injury with an implanted FES system. Journal of Rehabilitation Research and Development. 2007;44(3):333–346. doi: 10.1682/jrrd.2007.03.0333. [DOI] [PubMed] [Google Scholar]
- 39.Bailey SN, Hardin E, Kobetic R, Boggs L, Pinault G, Triolo R. Neuroprosthetic and neurotherapeutic effects of implanted electrical stimulation for ambulation after incomplete spinal cord injury. Journal of Rehabilitation Research & Development. 2010;47(1):7–16. doi: 10.1682/jrrd.2009.03.0034. [DOI] [PubMed] [Google Scholar]
- 40.Dutta A, Kobetic R, Triolo R. Gait initiation with electromyographically triggered electrical stimulation in people with partial paralysis. ASME Journal of Biomechanical Engineering. 2009;131081002(8):1–9. doi: 10.1115/1.3086356. [DOI] [PubMed] [Google Scholar]
- 41.Hettinga DM, Andrews BJ. Oxygen consumption during functional electrical stimulation-assisted exercise in persons with spinal cord injury: implications for fitness and health. Sports Med. 2008;38(10):825–38. doi: 10.2165/00007256-200838100-00003. [DOI] [PubMed] [Google Scholar]
- 42.Sadowsky CL, Hammond ER, Strohl AB, Commean PK, Eby SA, Damiano DL, Wingert JR, Bae KT, McDonald JW., 3rd Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J Spinal Cord Med. 2013 Nov;36(6):623–31. doi: 10.1179/2045772313Y.0000000101. Epub 2013 Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutta A, Kobetic R, Triolo R. Ambulation after incomplete spinal cord injury with EMG-triggered functional electrical stimulation. IEEE Transactions on Biomedical Engineering. 2008;55(2):791–794. doi: 10.1109/TBME.2007.902225. [DOI] [PubMed] [Google Scholar]
- 44.Fisher L, Miller M, Nogan S, Davis J, Anderson J, Murray L, Tyler D, Triolo R. Standing after spinal cord injury with four contact nerve-cuff electrodes for quadriceps stimulation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2008;16(5):473–478. doi: 10.1109/TNSRE.2008.2003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher L, Tyler D, Triolo R. Optimization of selective stimulation parameters for multi-contact electrodes. Journal of NeuroEngineering and Rehabilitation. 2013;10:25. doi: 10.1186/1743-0003-10-25. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Audu M, Nataraj R, Gartman S, Triolo R. Posture shifting after spinal cord injury using functional neuromuscular stimulation – a computer simulation study. Journal of Biomechanics. 2011;44:1639–1645. doi: 10.1016/j.jbiomech.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobetic R, To C, Schnellenberger J, Audu M, Bulea T, Gaudio R, Tashman S, Triolo RJ. Development of a hybrid orthosis for standing, walking and stair climbing after spinal cord injury. Journal of Rehabilitation Research & Development. 2009;46(3):447–462. [PubMed] [Google Scholar]
- 48.Kukke SN, Triolo RJ. The effects of trunk stimulation on bimanual seated workspace. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2004;12:177–185. doi: 10.1109/TNSRE.2004.827222. [DOI] [PubMed] [Google Scholar]
- 49.Spungen AM, Adkins RH, Steward CA, Wang J, Pierson RN, Waters RL, Bauman WA. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. Journal of Applied Physiology. 2003;95:2398–2407. doi: 10.1152/japplphysiol.00729.2002. [DOI] [PubMed] [Google Scholar]
- 50.Potten YJM, Seelen HAM, Drukker J, Reulens JPH, Drost MR. Postural muscle responses in the spinal cord injured persons during forward reaching. Ergonomics. 1999;42:1200–1215. doi: 10.1080/001401399185081. [DOI] [PubMed] [Google Scholar]
- 51.Hart N, Laffont I, de La Sota A, Lejaille M, Macadou G, Polkey M, Denys P, Lofaso F. Respiratory effects of combined truncal and abdominal support in patients with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2005;86:1447–1451. doi: 10.1016/j.apmr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 52.Hobson DA, Tooms RE. Seated lumbar/pelvic alignment. A comparison between spinal cord-injured and noninjured groups. Spine. 1992;17:293–298. [PubMed] [Google Scholar]
- 53.Hobson DA, Tooms RE. Seated lumbar/pelvic alignment. A comparison between spinal cord-injured and noninjured groups. Spine. 1992;17:293–298. [PubMed] [Google Scholar]
- 54.Curtis KA, Kindlin CM, Reich KM, White DE. Functional reach in wheelchair users: the effects of trunk and lower extremity stabilization. Archives of Physical Medicine and Rehabilitation. 1995;76:360–367. doi: 10.1016/s0003-9993(95)80662-8. [DOI] [PubMed] [Google Scholar]
- 55.Ueyoshi A, Shima Y. Studies on spinal braces. With special reference to the effects of increased abdominal pressure. International Orthopaedics. 1985;9:255–258. doi: 10.1007/BF00266512. [DOI] [PubMed] [Google Scholar]
- 56.Wu GA, Lombardo LM, Triolo RJ, Bogie KM. The effects of combined trunk and gluteal neuromuscular electrical stimulation on posture and tissue health in spinal cord injury. Physical Medicine and Rehabilitation. 2013;5:688–696. doi: 10.1016/j.pmrj.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 57.Kukke SN, Triolo RJ. The effects of trunk stimulation on bimanual seated workspace. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2004;12:177–185. doi: 10.1109/TNSRE.2004.827222. [DOI] [PubMed] [Google Scholar]
- 58.Triolo RJ, Bailey SN, Miller ME, Lombardo LM, Audu ML. Effects of Stimulating Hip and Trunk Muscles on Seated Stability, Posture, and Reach After Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation. 2013;94:1766–1775. doi: 10.1016/j.apmr.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Triolo RJ, Bailey SN, Lombardo LM, Miller ME, Foglyano K, Audu ML. Effects of Intramuscular Trunk Stimulation on Manual Wheelchair Propulsion Mechanics in 6 Subjects with Spinal Cord Injury. Archives of Physical Medicine and Rehabilitation. 2013;94:1997–2005. doi: 10.1016/j.apmr.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis JA, Triolo RJ, Uhlir JP, et al. Surgical technique for installing an 8-channel neuroprosthesis for standing. Clinical Orthopaedics and Related Research. 2001;4:237–252. doi: 10.1097/00003086-200104000-00035. [DOI] [PubMed] [Google Scholar]
- 61.Vette AH, Yoshida T, Thrasher TA, Masani K, Popovic MR. A comprehensive three-dimensional dynamic model of the human head and trunk for estimating lumbar and cervical joint torques and forces from upper body kinematics. Medical Engineering & Physics. 2012;34(5):640–649. doi: 10.1016/j.medengphy.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 62.Murphy JO, Audu ML, Lombardo LM, Foglyano KM, Triolo RJ. Feasibility of a Closed-Loop Controller for Righting Seated Posture after Spinal Cord Injury. Accepted by Journal of Rehabilitation Research and Development. 2013 doi: 10.1682/JRRD.2013.09.0200. [DOI] [PubMed] [Google Scholar]
- 63.Markova A, Mostow EN. US skin disease assessment: ulcer and wound care. Dermatol Clin. 2012;30(1):107–11. ix. doi: 10.1016/j.det.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Lyder CH, Wang Y, Metersky M, Curry M, Kliman R, Verzier NR, Hunt DR. Hospital-acquired pressure ulcers: results from the national Medicare Patient Safety Monitoring System study. J Am Geriatr Soc. 2012;60:1603–1608. doi: 10.1111/j.1532-5415.2012.04106.x. [DOI] [PubMed] [Google Scholar]
- 65.VanGilder C, MacFarlane GD, Harrison P, Lachenbruch C, Meyer S. The demographics of suspected deep tissue injury in the United States: an analysis of the International Pressure Ulcer Prevalence Survey 2006–2009. Adv Skin Wound Care. 2010;23:254–261. doi: 10.1097/01.ASW.0000363550.82058.7f. [DOI] [PubMed] [Google Scholar]
- 66.Mushahwar VK, Solis L. Mitigation of pressure ulcers using electrical stimulation. [patent] 2009 [Google Scholar]
- 67.Solis LR, Gyawali S, Seres P, Curtis CA, Chong SL, Thompson RB, Mushahwar VK. Effects of intermittent electrical stimulation on superficial pressure, tissue oxygenation, and discomfort levels for the prevention of deep tissue injury. Ann Biomed Eng. 2011;39:649–663. doi: 10.1007/s10439-010-0193-1. [DOI] [PubMed] [Google Scholar]
- 68.Gyawali S, Solis L, Chong SL, Curtis C, Seres P, Kornelsen I, Thompson R, Mushahwar VK. Intermittent electrical stimulation redistributes pressure and promotes tissue oxygenation in loaded muscles of individuals with spinal cord injury. J Appl Physiol. 2011;110:246–255. doi: 10.1152/japplphysiol.00661.2010. [DOI] [PubMed] [Google Scholar]
- 69.Solis LR, Liggins A, Uwiera RR, Poppe N, Pehowich E, Seres P, Thompson RB, Mushahwar VK. Distribution of internal pressure around bony prominences: implications to deep tissue injury and effectiveness of intermittent electrical stimulation. Ann Biomed Eng. 2012;40:1740–1759. doi: 10.1007/s10439-012-0529-0. [DOI] [PubMed] [Google Scholar]
- 70.Solis LR, Liggins A, Uwiera RR, Poppe N, Pehowich E, Seres P, Thompson RB, Mushahwar VK. Distribution of internal pressure around bony prominences: implications to deep tissue injury and effectiveness of intermittent electrical stimulation. Ann Biomed Eng. 2012 Aug;40(8):1740–59. doi: 10.1007/s10439-012-0529-0. Epub 2012 Feb 22. [DOI] [PubMed] [Google Scholar]
- 71.Solis LR, Twist E, Seres P, Thompson RB, Mushahwar VK. Prevention of deep tissue injury through muscle contractions induced by intermittent electrical stimulation after spinal cord injury in pigs. J Appl Physiol (1985) 2013 Jan 15;114(2):286–96. doi: 10.1152/japplphysiol.00257.2012. Epub 2012 Nov 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bogie KM, Wang X, Triolo RJ. Long term prevention of pressure ulcers in high risk individuals: a single case study of the use of gluteal neuromuscular electrical stimulation. Arch Phys Med Rehabil. 2006;87(4):585–91. doi: 10.1016/j.apmr.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 73.Bogie KM, Triolo RJ. The effects of regular use of neuromuscular electrical stimulation on tissue health. Journal of Rehabilitation Research and Development. 2003;40(6):469–475. doi: 10.1682/jrrd.2003.11.0469. [DOI] [PubMed] [Google Scholar]
- 74.Bogie K, Ho CH, Chae J, Triolo RJ. Dynamic therapeutic neuromuscular electrical stimulation for pressure relief. Am J Phys Med Rehabil. 83240(3):2004. [Google Scholar]
- 75.Mushahwar VK, Isaacson G, Ahmetovic A, Sommer R. Apparatus and method for electrically stimulating pressure-loaded muscles [patent] 2013 [Google Scholar]
- 76.Gaunt RA, Prochazka A. Control of urinary bladder function with devices: successes and failures. Prog Brain Res. 2006;152:163–94. doi: 10.1016/S0079-6123(05)52011-9. Review. [DOI] [PubMed] [Google Scholar]
- 77.Brindley GS. An implant to empty the bladder or close the urethra. J Neurol Neurosurg Psychiatry. 1977;40:358–69. doi: 10.1136/jnnp.40.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Creasey GH, Dahlberg JE. Economic consequences of an implanted neuroprosthesis for bladder and bowel management. Arch Phys Med Rehabil. 2001 Nov;82(11):1520–5. doi: 10.1053/apmr.2001.25912. [DOI] [PubMed] [Google Scholar]
- 79.Sanders PM, Ijzerman MJ, Roach MJ, Gustafson KJ. Patient preferences for next generation neural prostheses to restore bladder function. Spinal Cord. 2011 Jan;49(1):113–9. doi: 10.1038/sc.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farag FF, Martens FM, Rijkhoff NJ, Heesakkers JP. Dorsal genital nerve stimulation in patients with detrusor overactivity: a systematic review. Curr Urol Rep. 2012 Oct;13(5):385–8. doi: 10.1007/s11934-012-0273-x. [DOI] [PubMed] [Google Scholar]
- 81.Lee YH, Kim SH, Kim JM, Im HT, Choi IS, Lee KW. The effect of semiconditional dorsal penile nerve electrical stimulation on capacity and compliance of the bladder with deformity in spinal cord injury patients: a pilot study. Spinal Cord. 2012 Apr;50(4):289–93. doi: 10.1038/sc.2011.141. [DOI] [PubMed] [Google Scholar]
- 82.Possover M, Schurch B, Henle KP. New strategies of pelvic nerves stimulation for recovery of pelvic visceral functions and locomotion in paraplegics. Neurourol Urodyn. 2010 Nov;29(8):1433–8. doi: 10.1002/nau.20897. [DOI] [PubMed] [Google Scholar]
- 83.Kirkham AP, Knight SL, Craggs MD, Casey AT, Shah PJ. Neuromodulation through sacral nerve roots 2 to 4 with a Finetech-Brindley sacral posterior and anterior root stimulator. Spinal Cord. 2002 Jun;40(6):272–81. doi: 10.1038/sj.sc.3101278. [DOI] [PubMed] [Google Scholar]
- 84.Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Multiple pudendal sensory pathways reflexly modulate bladder and urethral activity in patients with spinal cord injury. J Urol. 2011 Feb;185(2):737–43. doi: 10.1016/j.juro.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCoin JL, Bhadra N, Gustafson KJ. Electrical stimulation of sacral dermatomes can suppress aberrant urethral reflexes in felines with chronic spinal cord injury. Neurourol Urodyn. 2013 Jan;32(1):92–7. doi: 10.1002/nau.22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boger AS, Bhadra N, Gustafson KJ. High frequency sacral root nerve block allows bladder voiding. Neurourol Urodyn. 2012 Jun;31(5):677–82. doi: 10.1002/nau.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holinski BJ, Mazurek KA, Everaert DG, Stein RB, Mushahwar VK. Restoring stepping after spinal cord injury using intraspinal microstimulation and novel control strategies. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:5798–801. doi: 10.1109/IEMBS.2011.6091435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Riley J, Federici T, Polak M, Kelly C, Glass J, Raore B, Taub J, Kesner V, Feldman EL, Boulis NM. Neurosurgery. 2012 Aug;71(2):405–16. doi: 10.1227/NEU.0b013e31825ca05f. discussion 416. [DOI] [PubMed] [Google Scholar]


