Abstract
SPAK (Ste20-related proline alanine rich kinase) and OSR1 (oxidative stress responsive kinase) are members of the germinal center kinase VI sub-family of the mammalian Ste20 (Sterile20)-related protein kinase family. Although there are 30 enzymes in this protein kinase family, their conservation across the fungi, plant and animal kingdom confirms their evolutionary importance. Already, a large volume of work has accumulated on the tissue distribution, binding partners, signaling cascades, and physiological roles of mammalian SPAK and OSR1 in multiple organ systems. After reviewing this basic information, we will examine newer studies that demonstrate the pathophysiological consequences to SPAK and/or OSR1 disruption, discuss the development and analysis of genetically-engineered mouse models, and address the possible role these serine/threonine kinases might have in cancer proliferation and migration.
I. INTRODUCTION
Serine/threonine kinases are proteins which hydrolyze a nucleoside triphosphate (e.g. ATP) and transfer the inorganic phosphate to the side chain (R-groups) of serine and threonine residues. Two parameters which define a kinase are whether the catalytic domain is located in the amino or carboxyl terminal portion and the mechanism of regulation. Seven major categories of protein kinase regulation include: subcellular localization, binding of non-protein ligands, phosphorylation inside and outside of the activation loop, protein-protein binding, protein accumulation, and dephosphorylation (246). Approximately 2% of all eukaryotic genes encode protein kinases, making them one of the largest protein families (173). The Sterile 20 (Ste20)-related kinases are a relatively large family of proteins conserved across fungi, plant, and animal kingdoms (25). This review will focus on the molecular characteristics and physiological roles of two mammalian members of the germinal center kinase (GCK) VI subfamily of Ste20-related kinases, SPAK (Ste20/SPS1-related proline-alanine rich kinase) and OSR1 (oxidative stress-responsive kinase).
II. OVERVIEW OF MAMMALIAN STE20-RELATED KINASES
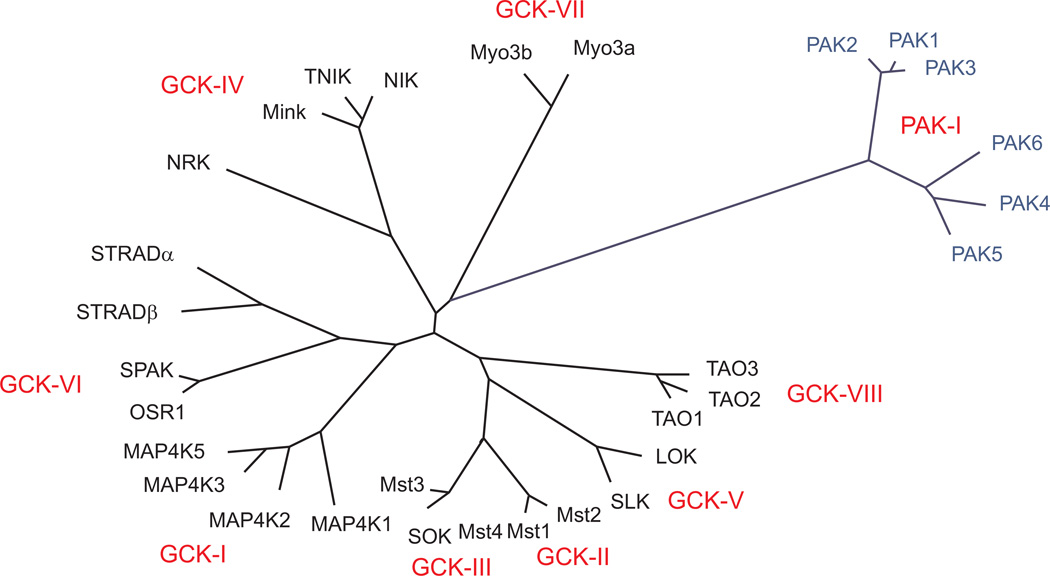
There are currently 30 enzymes in the mammalian Ste20-related protein kinase family, sub-divided into the p21 activated kinases (PAKs) which have a 3' or carboxyl-terminal catalytic domain, and the germinal center kinases (GCKs) which have a 5' or amino-terminal catalytic domain (Figure 1, Table 1, and (25, 49)). In addition to having a 3' catalytic domain, PAKs have a conserved amino-terminal Cdc42/Rac interactive binding (CRIB) domain, and an autoinhibitory mechanism involving the catalytic and CRIB domains (119, 152, 288). Although present in multiple organ systems, PAKs are highly expressed in brain, muscle, and spleen, and regulate multiple cellular processes (e.g. cytoskeletal rearrangement, energy homeostasis, cell transformation, and cancer invasiveness).
Figure 1. Cluster dendrogram of mammalian Ste20 kinases.
The amino acid sequences of 30 mammalian Ste20 kinases were aligned using VectorNti Suite 6.0 (Invitrogen/Life Technologies, Grand Island, NY), saved as a text file, and then reformatted for use with Promlk, a software component of the Phylogeny Inference Package (PHYLIP) from http://evolution.gs.washington.edu/phylip.html. Promlk estimates phylogenies from protein amino acid sequences by maximum likelihood and assumes a molecular clock. Length of tree branches can be compared to the reference bar which represents 0.1 amino acid substitutions per site. Mouse kinase (sequence accession numbers) used: PAK1 (NP_035165), PAK2 (NP_796300), PAK3 (NP_032804), PAK4 (NP_081746), PAK5 (AAR37415), PAK6 (BAE34725), TAO1 (NP_659074), TAO2 (NP_001157246), TAO3 (NP_001074777), LOK (BAA24073), SLK (NP_001158111), MST1 (NP_067395), MST2 (NP_062609), MST3 (NP_663440), MST4 (NP_598490), SOK (AAH52913), MAPK1 (NP_032305), MAPK2 (NP_033032), MAPK3 (NP_001074826), MAPK4 (NP_001239131), MAPK5 (NP_958927), SPAK (NP_058562), OSR1 (NP_598746), STRADα (NP_001239377), STRADβ (NP_766244), NRK (BAA84943), MINK (AAH52474), TNIK (AAI58060), NIK (NP_058592), Myo3ab (EDL08135), Myo3b (AAX59999).
Table 1.
Diversity of mammalian Ste20 kinases.
| Name | Other names | Family | Function |
|---|---|---|---|
| MAP4K1 | HPK1 | GCK-I | negative regulator of T cell function |
| MAP4K2 | GCK-I | unknown | |
| MAP4K3 | GLK | GCK-I | autoimmunity, NFKB signaling |
| MAP4K5 | GCKR, KHS | GCK-I | Wnt mediated JNK activation |
| Mst1 | STK4, KRS2 | GCK-II | oxidative stress-induced apoptosis |
| Mst2 | STK3 | GCK-II | oxidative stress-induced apoptosis |
| Mst3 | STK24 | GCK-III | cell migration, cell cycle progression |
| Mst4 | GCK-III | cell growth transformation | |
| SOK1 | STK25 | GCK-III | induces programmed cell death, involved in cell migration |
| NIK | MAP4K4 | GCK-IV | activates SAPK pathway |
| NRK | GCK-IV | activates JNK pathway | |
| TNIK | GCK-IV | Rap2 (small Rho GTPase)-mediated disassembly of F-actin | |
| Mink | GCK-IV | Rap2 (small Rho GTPase)-mediated disassembly of F-actin | |
| LOK | STK10 | GCK-V | cell cycle control |
| SLK | STK2, LOSK | GCK-V | induces programmed cell death |
| SPAK | STK39, PASK | GCK-VI | Modulates epithelial transport, cell volume homeostasis |
| OSR1 | OXSR1 | GCK-VI | Modulates epithelial transport, cell volume homeostasis |
| STRADα | GCK-VI | pseudokinase, adaptor to LKB1 kinase a tumor suppressor | |
| STRADβ | GCK-VI | pseudokinase, adaptor to LKB1 kinase a tumor suppressor | |
| Myo3a | DFNB30 | GCK-VII | actin interaction, involved in deafness |
| Myo3b | GCK-VII | actin interaction, involved in deafness | |
| TAO1 | PSK2, KFC-B | GCK-VIII | mitosis, cell cycle progression |
| TAO2 | PSK1, KFC-C | GCK-VIII | activates MAPK, stabilize microtubules |
| TAO3 | JIK, DPK, KFC-A | GCK-VIII | inhibits JNK/SAPK |
| PAK1 | PAK-I | interact with small Rho-GTPases, actin depolymerization | |
| PAK2 | PAK65 | PAK-I | cell survival, matrix invasion |
| PAK3 | OPHN3 | PAK-I | actin myosin organization, synaptic transmission |
| PAK4 | PAK-II | matrix invasion, neurite outgrowth, actin polymerization | |
| PAK5 | PAK7 | PAK-II | neurite outgrowth |
| PAK6 | PAK-II | unknown |
The eight sub-groups of the GCKs are based on their localization, structure, and function (see (49, 56) for reviews). The mitogen-activated protein kinases (MAPKs) within the GCK-I sub-group participate in signaling cascades to regulate a diverse array of physiological processes (for extensive reviews, see (31, 308)). The mammalian Ste20-related kinases (Mst1/Mst2) in GCK-II promote the programmed cell death (apoptotic) pathway when activated by oxidative stress (157, 165). Mst1 also negatively regulates cardiac myocyte hypertrophy through phosphorylation of another serine/threonine kinase Lats2 (177). There are two additional Mst kinases (Mst3/Mst4), however, because these do not possess the autoinhibitory and dimerization domains found in Mst1/Mst2, they form a third sub-group of Ste20-related kinases, GCK-III. Mst3 inhibits cell migration and regulates cell cycle progression (170, 265). Mst4 also influences cell growth and transformation (163) and affects cytoskeletal rearrangement, morphogenesis, and apoptosis through MAPK signaling cascades (171). A third member of GCK-III is the Ste20 oxidant stress response kinase (SOK1), which localizes to the Golgi apparatus, and participates in a signaling pathway to regulate cell migration and polarization (198).
NF-κB-inducing kinase (NIK), NIK-related kinase (NRK), Traf and Nck-interacting kinase (TNIK), and mishappen/NRK (MINK) are the four members of the GCK-IV sub-group highly-expressed in cardiac and neural tissues (48, 86, 195, 268). NIK and NRK are upstream activators of the MEKK1/MKK4/SAPK and MEKK1/MKK4/JNK signaling cascades, respectively (195). TNIK, through an interaction with a small Rho GTPase induces the disassembly of F-actin (273). Lymphocyte-orientated kinase (LOK), a GCK-V sub-group member, is abundantly found in rapidly proliferating tissues (spleen, placenta, bone marrow) as well as brain, heart, skeletal muscle, colon, kidney, liver, lung, and small intestine. Although not known to trigger any MAPK signaling cascades, LOK does associate with polo-like kinase 1 and may affect cell cycle control (303). Ste20-like kinase (SLK), the second GCK-V member, induces programmed cell death and has a role in cytoskeletal reorganization (34). The four members of GCK-VI sub-group (SPAK, OSR1, STRADα, and STRADβ) will be elaborated on in the next section. Myosin 3A (Myo3A) and Myosin 3B (Myo3B) are unique and unconventional GCK-VII kinases as they have both an N-terminal catalytic domain and a C-terminal actin-based molecular motor. Whereas Myo3A is expressed in the retina, inner ear, pancreas, brain, and testis; Myo3B is only found in the retina, intestine, and testis (62). Inactivating mutations in Myo3A result in non-syndromic progressive hearing loss (302). Finally, the TAO (thousand and one amino acid) kinases constitute the eighth GCK sub-group (GCK-VIII). These kinases are widely expressed in brain, heart, lung, kidney, liver, muscle, placenta, testis, prostate, and ovary. Many upstream signals of other kinases have no effect on the TAO kinases (333). In vitro assays have demonstrated that of the five MEK kinases, only MEK3 and MEK4 are activated by TAO1 (127). TAO1 may also affect the checkpoint function during metaphase-anaphase transition (64).TAO2 activates several MEK kinases and stabilizes microtubules (37, 188). TAO3 was initially named JNK inhibitory kinase because it diminished the response of the JNK/SAPK pathway to human EGF (278).
STRAD α and β pseudokinases
The genetic resemblance of the pseudokinase STRAD proteins places them close or within the GCK-VI subfamily (see the cluster dendrogram represented in Figure 1). Indeed, 90.3% of the catalytic domains of STRAD pseudokinases and SPAK/OSR1 are conserved. However, several key residues are not present in STRAD pseudo-kinases: (1) the conserved threonine residues in the activation segment of SPAK (see Figure 6A); (2) the aspartic acid (D204 in SPAK) residue belonging to the HRD motif in the catalytic loop that forms a hydrogen bond with the threonine residue in the P + 1 loop (T247 in SPAK); (3) the Mg2+ binding site with both the conserved aspartic acid (which typically coordinates two Mg2+ ions) and the conserved phenylalanine of the DFG motif (F223 in SPAK); (4) the critical N-terminal lobe K(E ion pair; (5) the catalytic lysine (K104 in SPAK) which interacts with the γ phosphate of ATP is lost in STRAD alpha; and (6) the conserved salt-bridge interacting glutamic acid (E121 in SPAK) is lost in STRAD beta. All of these changes in the conserved catalytic residues lining the active site were first thought to prevent nucleotide and Mg2+ binding and render the STRAD proteins inactive. However, recent structural studies have shown that pseudokinases are able to bind nucleotides in a regulated manner and allosterically regulate the protein kinases they interact with (see Perspective in Science Signaling (233)). For example, STRAD interaction is critical for the catalytic domain activation of the tumor suppressor kinase LKB1 (334). This occurs through a STRAD-LKB1 interaction that stabilizes the activation loop of LKB1 producing a conformational change allowing substrate binding. Normally, phosphorylation of the activation segment is necessary to trigger this rearrangement in protein kinases.
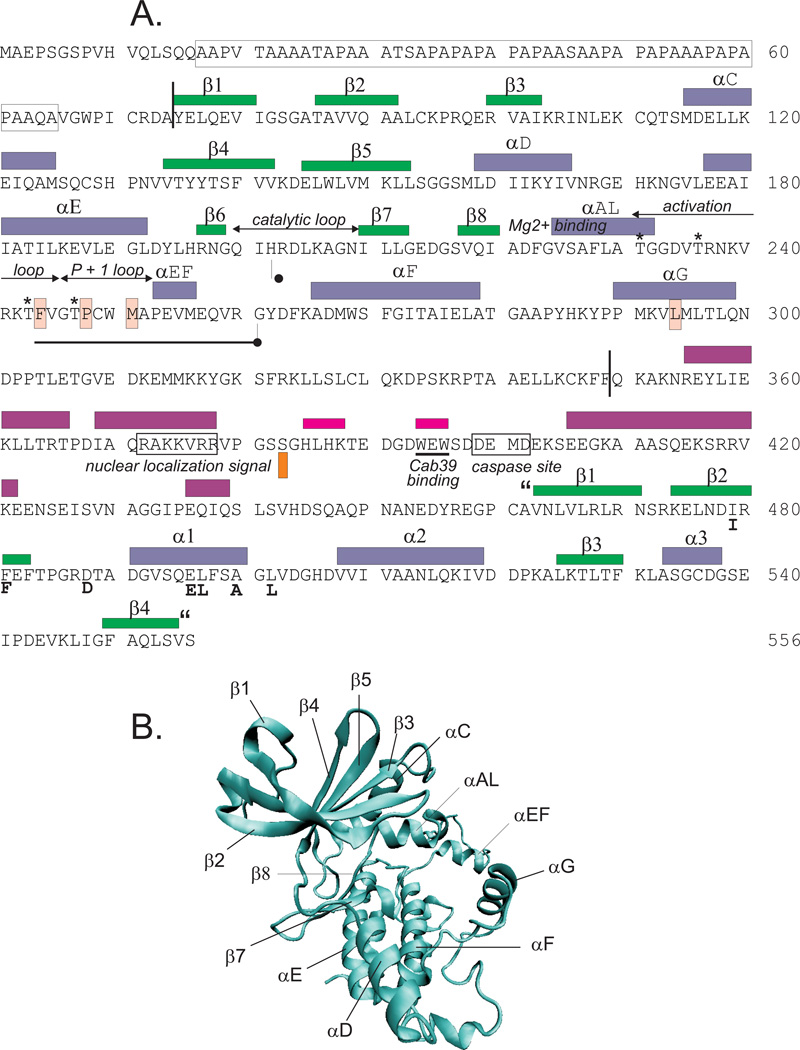
Figure 6. Amino acid sequence and secondary structure of mouse SPAK.
A. The α helices and β sheets (colored in blue and green, respectively) are derived from the crystal structure of the OSR1 catalytic domains (PDB#: 3DAK and 2VWI) and the OSR1 carboxyl-terminal domain (PDB#: 2V3S). The α helices and β sheets (colored in purple) are from modeling based on PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/). The catalytic domain (residues 75 – 349) is indicated by two vertical lines. The positions of the catalytic-, the Mg2+-binding-, activation segment, P + 1-loop, nuclear localization signal, putative caspase and TRAIL cleavage sites are also indicated. The purple β sheet made of WEW (underlined) constitutes the interaction site for Cab39/MO25 proteins. Asterisks highlight four phospho-threonines in the activation segment, one phospho-serine in the catalytic domain, and one additional phospho-serine in the PF1 domain, all phospho-residues that are part of SPAK activation. The PF2 domain is indicated by “quotations”. Light pink boxes highlight four hydrophobic residues that form the hydrophobic substrate pocket or P + 1 pocket. Key carboxy-terminal residues participating in the binding of the target RFx[V/I] peptides are indicated by a bold font. B. Schematic representation of the structure of SPAK. The secondary structure elements were drawn from a SWISS-MODEL-generated PDB file of SPAK catalytic domain based on the OSR1 crystal structure (PDB: 3DAK). Drawing was made using the Visual Molecular Dynamics software (University of Illinois). The α helices and β sheet are highlighted.
III. THE GCK-VI SUBFAMILY: STE20/SPS-1 RELATED PROLINE ALANINE RICH KINASE (SPAK) AND OXIDATIVE STRESS RESPONSE 1 (OSR1)
A. Molecular Discovery of SPAK and OSR1
Western blot analysis of rat brain tissue lysate with a monoclonal antibody targeted against poly(ADP-ribose)polymerase (PARP) detected a novel 66 kDa protein. RT-PCR of rat brain RNA amplified a cDNA fragment encoding a 553-amino acid protein with an estimated size of 60 kDa. Due to the similarity of the catalytic domain with the Ste20 protein kinases and the abundance of proline and alanine residues, this novel protein was also referred to as PASK (proline-alanine-rich Ste20-related kinase) (290). Another novel gene, mapped to the short arm of human chromosome 3, encoded for a 527-amino acid protein with 39% identity to human Ste20/oxidant stress response kinase-1, or SOK1. Based on the possible involvement of this serine/threonine kinase in an oxidative stress response, it was designated as oxidative-stress responsive gene 1 (OSR1) (276). However, cloning of a mouse gene related to the Drosophila pair-rule gene odd-skipped (odd) was also designated Osr1 for odd-skipped related 1 (260). As a result, the Human Genome Organization (HUGO) renamed the Ste20 oxidative-stress responsive kinase OXSR1. However, we will use the widely accepted OSR1 to describe the Ste20-related kinase throughout this review.
A novel kinase sequence with similarity to Ste20 serine/threonine kinases was identified in rat pancreatic beta cells (53). Characterization of this kinase found a region of proline and alanine repeats (PAPA box) upstream of the catalytic domain in the N-terminus. In addition, a nuclear localization signal (RAKKVRR) homologous to the SV40 T antigen and a caspase cleavage motif (DEMD) were identified in the carboxyl-terminal regulatory domain. Based on these characteristics, this protein was named SPAK (Ste20/SPS1-related proline alanine-rich kinase) (130). Amino acid comparison has shown that PASK and SPAK are in fact the same protein, and as the latter has the kinase family name first (i.e. Ste20/SPS1), this name has been used more consistently in subsequent studies (95, 175, 190, 224, 300). Note also that the National Center for Biotechnology Information assigned the name STK39 to human SPAK, whereas OSR1 has yet to be assigned a related name. Overall, mammalian SPAK and OSR1 have 65–67% amino acid identity, with 89% identity in the catalytic domain (36, 224). Alignment of the regulatory domain demonstrated two regions of similarity, designated PF1 (directly after the catalytic domain), and PF2 (terminal 90-amino acid residues) (36).
B. Evolution
For a complete understanding of the mammalian Ste20-related kinases, SPAK and OSR1, it is important to review some of the characteristics of the Ste20 gene in Saccharomyces cerevisiae (yeast). The yeast proteome comprises 117 protein kinases that can be grouped into five major categories: (i) AGC; (ii) CAMK; (iii) CMGC; (iv) Ste11/Ste20; (v) Ste7/MEK. The kinases that cannot fit into these five categories are classified as atypical kinases (246). The Ste11/Ste20 group comprises 13 closely related proteins. Unrooted dendrograms constructed using only the catalytic domain amino acids of these 13 proteins with (Figure 2A) or without (Figure 2B) inclusion of Capsaspora owczarzaki OSR1 (this single cell protozoan being one of the oldest eukaryotes for which the kinase has been sequenced) demonstrates OSR1 being more closely related to Sps1p and Kic1p. When the entire protein is examined, Ste20 and Cla4 (which belong to a separate branch) more closely resemble mammalian PAK kinases, whereas the SPS1p branch more closely resemble GC kinases. Therefore, while SPAK and OSR1 are placed within the mammalian Ste20 family of protein kinases, they are actually more closely related to SPS1p than to Ste20p.
Figure 2. Cluster dendrograms of yeast Ste20/Ste11 kinases.
Dendograms were constructed in absence (A) or presence (B) of protozoan (Capsaspora owczarzaki) OSR1. The amino acid sequences were aligned using VectorNti Suite 6.0 (Invitrogen/Life Technologies), saved as a text file, and then reformatted for use with Promlk, a software component of the Phylogeny Inference Package (PHYLIP) from http://evolution.gs.washington.edu/phylip.html. Length of tree branches can be compared to the reference bar which represents 0.1 amino acid substitutions per site. Note that OSR1 is closest to Saccharomyces cerevisiae Sps1 and Kic1 kinases. Mouse kinase (sequence accession numbers) used: Ksp1 (NP_011950), Bck1 (NP_012440), Ste11 (NP_013466), Ssk22 (NP_009998), Ssk2 (NP_014428), Cdc15 (NP_009411), Sps1 (NP_010811), Kic1 (NP_011970), Ste20 (NP_011856), Skm1 (NP_014528), Cla4 (NP_014101), Sks1 (NP_015299), Vhs1 (NP_010533), and Capsaspora owczarzaki OSR1 (EFW42229).
Mutated genes that alter cell division and make yeast cells sterile are given the prefix Ste (172). A genetic screen, searching for components which mediate the mating response in yeast, identified a DNA fragment with an open-reading frame for a novel protein (Ste20p) with multiple characteristics of serine/threonine kinases. Whiteway and co-workers determined that Ste20p was required in the pheromone receptor-coupled heterotrimeric G-protein response (153). Activation of the pheromone receptor causes G-protein βγ subunits to transmit, via MAP kinases, signals which initiate transcription of target genes that facilitate yeast cell mating and cell cycle G1-phase arrest (58, 174). Identification of OSR1 and SPAK as mammalian homologs to the yeast Ste20p serine/threonine kinase suggests that they may be involved in cell signaling (130, 276). At last count, there were over 30 sequences of OSR1 (from protist to human) and 13 sequences of SPAK (2 birds and 11 mammals) reported in GenBank(. SPAK is likely to have originated from gene duplication during vertebrate evolution, as only a subset of organisms which contain the OSR1 gene also have the SPAK gene (58). Gene duplication arises from the unequal crossing-over during meiosis between misaligned homologous chromosomes. With no selective pressure (i.e. deleterious mutations which effect the organism) on the second copy of the gene, mutations are free to accumulate which may code for a novel function (279, 335). In this case, however, the function of SPAK and OSR1 seems to be similar and possibly redundant in some cell types, whereas specific in others (see section VI). The appearance of 74 additional amino acids rich in proline and alanine residues (i.e. PAPA box) upstream of the catalytic domain is further evidence of a duplicated gene product having acquired novel characteristics. Although the removal of the PAPA box does not seem to affect SPAK activation of NKCC1 (95), it is still unknown if this proline/alanine rich region confers specific novel properties to the kinase.
A BLAST search of the plant genome with the protozoan OSR1 sequence identified a protein kinase from the plant model organism Physcomitrella patens. Amino acid sequence alignment of the catalytic domain of this plant kinase with mouse, C. elegans, and protozoan OSR1 (Figure 3A) revealed a 54–58% identity and 68–73% homology between the plant and animal sequences, respectively (see Figure 3B). This high degree of similarity suggests that this protein is related to proto- and meta-zoan OSR1. Even though the spatial arrangements of the catalytic and regulatory domains are conserved, the sequence of the regulatory domains is highly divergent, indicating that these kinases likely serve separate functions in plant and animal cells. Finally, a BLAST search of bacterial genomes (large biodiversity as represented in Figure 3C) with the protozoan and the plant OSR1 sequences failed to identify any common ancestral kinases.
Figure 3. Evidence for protozoa and plant OSR1 kinase.
A) Amino acid sequence alignment of OSR1 proteins of mouse (NCBI accession number: NP_598746), plant (Physcomitrella patens, XP_001784493), protozoa (Capsaspora owczarzaki, EFW42229), and roundworm (C. elegans, NP_507517). B) Percent identity of OSR1 increases from 55% between plant and higher organisms, to 61% between protozoa and higher organisms, to 71% between C. elegans and mouse. C) Evolutionary tree showing the bacterial branch in blue, the archea branch in green, and the eukaryote branch in red. OSR1 is present in eukaryotes, from protist to fungi to plants to animal cells.
C. Tissue Distribution
Original cloning papers reported wide tissue expression of SPAK and OSR1. Indeed, expression of a 3.7 kb mRNA in Northern blots of rat brain, salivary glands, thymus, heart, lung, spleen, stomach, small and large intestine, adrenal gland, kidney, testis, epididymis, ovary, and uterus were shown by probes created against the carboxyl-terminal half of the SPAK/PASK coding region. Western blotting of rat and mouse tissue lysates with polyclonal antibodies against PASK/SPAK found similar protein distribution as observed with mRNA transcripts (130, 290). In situ hybridization demonstrated SPAK expression in rat embryonic (E14–E15) choroid plexus, myocardium, mesonephron, and dorsal root ganglia. Distinct expression of SPAK was also observed in early pancreatic epithelium and developing gut tube (185). OSR1 mRNA expression was found in lung, kidney, colon, thymus, heart, liver, spleen, skeletal muscle, ovary, leukocyte, small intestine, testis, prostate, placenta, brain, and pancreas (276). Protein expression of OSR1 was found in the soluble, particulate, and nuclear fractions of heart, spleen, liver, kidney, lung, testis, small and large intestine, and stomach (36).
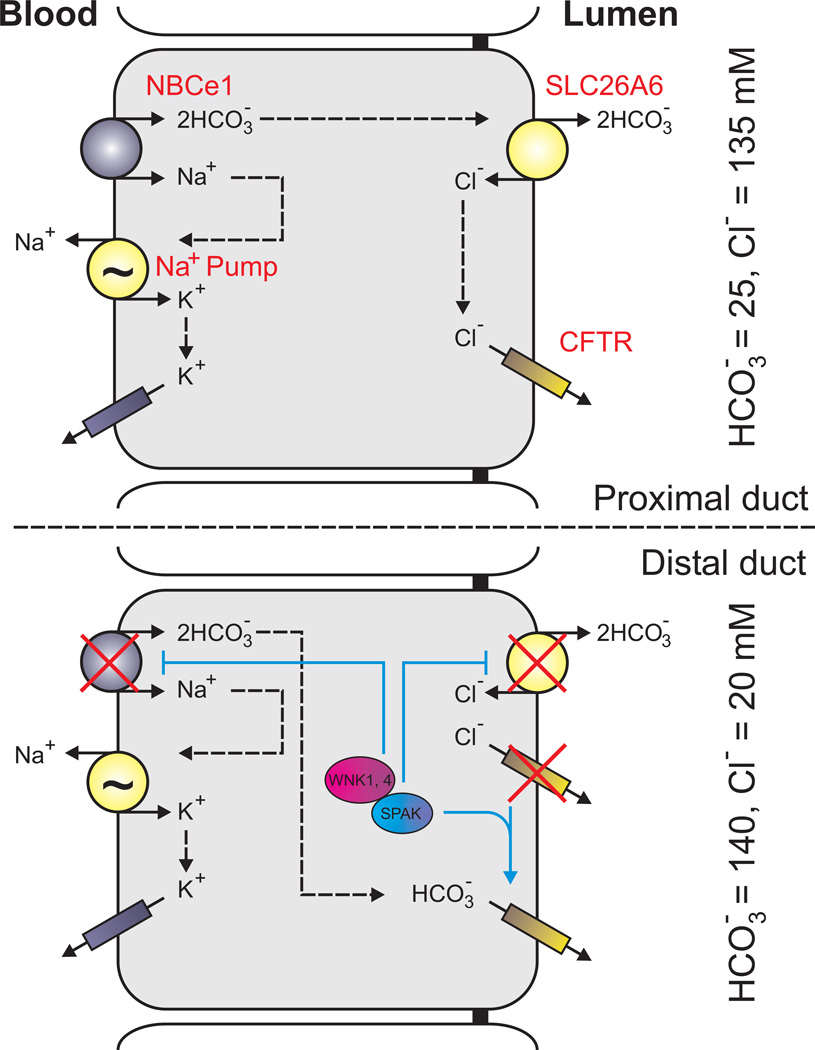
Several studies have also examined the expression of SPAK and OSR1 using conjugated antibodies and immunohistological techniques. In the nervous system, the strongest SPAK immunofluorescent labeling was found in the apical membrane of epithelial choroid plexus cells, followed by cranial nerve nuclei in the brainstem (223, 290). Interestingly, apical localization was lost in choroid plexus isolated from NKCC1 knockout mice, indicating that the kinase is targeted to the membrane by the Na-K-2Cl cotransporter. SPAK is also expressed in both white and grey matter in the spinal cord and highly co-expressed with NKCC1 at the node of Ranvier (sciatic nerve) (223). Note that OSR1 was also found in the same CNS structures, but at a much lower abundance (223). Whether or not this represented a differential between the strength of antibodies is unknown. The same immunohistological studies have found high expression of SPAK in several Cl−-secreting epithelia: gastric gland, sublingual gland, salivary gland (224, 290). Similar to the results found in choroid plexus, the expression of SPAK in salivary gland is co-localized on the basolateral membrane where NKCC1 is highly expressed. Multiple studies have also found SPAK and OSR1 co-localizing with the kidney-specific Na-K-2Cl cotransporter (NKCC2) in the thick ascending limb of Henle and with the Na-Cl cotransporter (NCC) in the distal convoluted tubule (164, 182, 327). More details about renal expression of SPAK and OSR1 is provided in Section VI, sub-section E.
D. Genomic Organization and Isoforms
Mammalian genomic organization is considerably more complex with significant accumulation of repetitive elements and non coding regions compared to the >open= genomes of unicellular fungi. In fact, only 1–2 % of the mammalian genome encodes for proteins (296). Although only three (1, 12, and 13) of the eighteen exons of mouse SPAK (chromosome 2) and mouse OSR1 (chromosome 9) show significant size variations, comparison of the non-coding regions demonstrate that eleven of the seventeen intronic regions have 2–10 fold differences in base pair size (see Figure 4). It is noteworthy that although intronic size variation differs between mouse SPAK and mouse OSR1, the size of introns between mouse and human SPAK and between mouse and human OSR1 is conserved. The catalytic domain is encoded by exons 1–9 and the regulatory domain by exons 10–18. The PF1 and the PF2 regions are encoded by exons 9–11 and exons 14–18, respectively. The non-conserved portion of the regulatory domain is encoded by exons 12–13, coincidentally, one of the two regions with the greatest variation in exon size between SPAK and OSR1.
Figure 4. Genomic organization of mouse SPAK and OSR1.
A) Bar graph of exons 1–17 (exon 18 > 2000 bp) illustrating conservation of exon length between the two Ste20-related kinase genes. B) Bar graph illustrating non-conservation of intronic sequences 1–17 between the two Ste20-related genes. A schematic representation of the kinase is presented in panel A to identify the position of key kinase features.
Different isoforms of a protein are translated from related genes (e.g. KCC2 and KCC3), whereas, alternative splicing events within the same gene produce variants of the same protein isoform (e.g. KCC3a and KCC3b). In humans, 95% of multi-exonic genes are alternatively spliced which greatly increases the biodiversity of proteins encoded by the genome (21, 214). SPAK and OSR1 are considered protein isoforms because they are encoded by different genes, they perform similar functions, and they have small differences in their sequences. During the characterization of signaling pathways involved with intestinal inflammation, a novel SPAK variant was identified and cloned from human colon tissue (323). Analysis of this colonic SPAK variant determined that it lacks the PAPA box upstream of the catalytic domain, as well as residues YELQEV that forms the first β sheet, and the sixth alpha helix (αF) located downstream of the P+1 loop (Figure 5A). Absence of the PAPA box can be explained by the use of an internal donor site (alternative 5' splice junction) in exon 1, resulting in the shortening of the exon. This event, however, does not explain the presence of the last residue of the first exon (isoleucine) in the colonic isoform. Absence of the alpha helix F in the C-lobe as reported by Merlin and co-workers (323) is far more difficult to understand as it would involve early termination of exon 6, followed by splicing in the middle of exon 7 using non-conventional and unusual splice junction sequences. Rather than using the well-conserved exon/intron boundary: CAG gtaa, the colonic variant would utilize an unusual ATG gaac; and rather than using the well-conserved intron/exon boundary: ttttag G, the colonic variant would use non-conserved residues gccatt G. An important question is whether or not this unusual colonic variant with its missing domains is functional. Two pieces of evidence suggest that the PAPA box is not essential for kinase function. First, OSR1 is a functional kinase and, second, removal of the PAPA box from SPAK does not impair the binding and phosphorylation of downstream targets (e.g. NKCC1) (93). This does not, however, imply that the PAPA box has no role or function, just that its role is still currently unknown. The missing amino acids (YELQEV) which form the first β sheet in the catalytic domain could disrupt the folding of the propeller-like structure of the N-lobe and impair kinase function. However, secondary structure modeling of the colonic SPAK variant shows that the first β sheet can be formed by alternative residues (QQAPI, see Figure 5B), thus conserving the folding of the N-lobe. Likewise, the missing alpha helix (αF) could potentially disrupt kinase function as a conserved glutamic acid in αF forms a hydrogen bond with the P+1 loop tryptophan residue (337). Additionally, the Gly261 (mouse sequence) residue which constitutes the domain hinge point is also located within the missing αF sequence (156). Secondary structure modeling of this colonic SPAK variant reveals extension of helix αEF and substitution of Gly261 by Gly281, indicating a possible rescue/conservation of the swapped domain hinge point. Function of the colonic SPAK variant was demonstrated in vitro via autophosphorylation of wild-type but not the shorter colonic SPAK kinase and phosphorylation of myelin basic protein (323), and here in situ (Figure 5C) through activation of NKCC1 in Xenopus laevis oocytes. It is important to note that we did not isolate a de novo SPAK clone from mouse colon, but instead modified our mouse SPAK cDNA to match the reported amino acid sequence of Yan (323).
Figure 5. Characterization of the colonic SPAK isoform.
A) Amino acid alignment between the catalytic domains of full-length SPAK and the colonic isoform. Identical residues are indicated by red font over yellow background. B) Modeling of the first 72 amino acids of the colonic SPAK isoform using PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/). The histogram in blue represents the confidence of the prediction for each amino acid. C = coil, E = strand, H = helix. C) 86Rb influx of mouse NKCC1 co-expressed with mouse WNK4 in Xenopus laevis oocytes with full-length (fl) and colonic (c) SPAK. Bars represent mean ∀ S.E.M (n = 20 – 25 oocytes). The NKCC1-mediated K+ influx is expressed in nmoles/oocyte/hr (unpublished data from Delpire Laboratory).
E. Genetically-Altered Mouse Models
While large animals (e.g. rabbit, dog, and swine) are often preferred for physiological studies, the reproductive cycle, life span and relative ease of genetic manipulation makes the mouse a very useful tool for investigating physiological systems. Several methodologies have been developed to create mice that do not express (knockout), over-express (transgenic), and under-express (hypomorph) proteins of interest, as well as create mice that express mutant forms of proteins (knock-in).
Currently, two SPAK knockout mice have been generated, one conventional (58), and one conditional (327) (see Table 2). The construct for the conventional SPAK knockout mouse was developed by the Wellcome Trust Sanger Institute (Cambridge, UK) using a genomic fragment from the SPAK gene containing the 110 bp exon 6, a tyrosinase minigene, the hprt drug-resistance gene cassette, and the neomycin-resistance gene cassette. Homologous recombination was done in the Delpire laboratory, resulting in the 5' hprt sequence disrupting the coding of SPAK after the duplicate exon 6 (58, 105). The only overt phenotype observed in these conventional knockout mice was a reduced fertility. While behavioral testing identified mild neurological deficits (see section VI, sub-section A and (104)), physiological testing identified renal deficits (discussed in section VI, sub-section E). The second SPAK knockout mouse was generated using a targeting construct containing exons 9 and 10, a neomycin-resistance gene cassette, and homologous loxP and FRT recombination sites. Although the targeting construct was designed using a >floxed= strategy, thus far, only a conventional knockout mouse lacking exon 9/10 has been reported. Similar to the first mouse, the appearance, behavior, and fertility of the heterozygous and homozygous knockout animals of the second line were not overtly different from their wild-type littermates. Physiological testing found the homozygous SPAK knockout mice exhibited a distinct renal phenotype with features typical of human patients with Gitleman Syndrome (discussed in detail in section VI, sub-section E and (327)). In the knockout strategy, disruption of a key exon generally results in the complete absence of the protein of interest. This lack of protein expression has the potential to yield unanticipated developmental and/or compensatory effects that might complicate the analysis of the phenotype. Thus, an alternative strategy to the knockout is to introduce in the gene a mutation that conserves protein expression, but renders the protein inactive. This strategy was used to create a non-functional SPAK knock-in mouse. The targeting construct contained a T-loop threonine to alanine (T243A) mutation in exon 6 and a neomycin-resistance gene cassette flanked by FRT recombination sites. Homologous recombination and excision of the neomycin gene cassette by Flp recombinase replaced the wild-type exon 6 with the T243A mutated exon. This SPAK knock-in mouse is viable and produces homozygous offspring at Mendelian ratios (231). Finally, expression of a protein can be increased in mice by random insertion in the genome of an additional copy of the gene, or of the cDNA encoding the entire open reading frame driven by the native promoter (cloned) or a generic promoter. This transgenic strategy was used to over-express SPAK in the mouse intestine (See section VI, sub-section C, (322)).
Table 2.
Genetically-modified SPAK and OSR1 mouse models.
| Kinase | Chromosome Position of gene |
Exons (Phenotype) |
Targeted Exon |
Mouse | Reference |
|---|---|---|---|---|---|
| SPAK | chromosome 2 | 18 exons | 6 | KO | (58, 182) |
| 68,048,502 – 68,310,325 | 9–10 | KO | |||
| (327) | |||||
| (Higher nociceptive threshold, locomotor phenotype) | |||||
| (Gitelman-like phenotype, hyper-phosphorylation of NKCC2) | |||||
| 6 | T243A KI | (231) | |||
| (Gitelman-like phenotype, no hyper-phosphorylation of NKCC2) | |||||
| N/A | Transgenic | (322) | |||
| OSR1 | chromosome 9 | 18 exons | 15 | KO | (58) |
| 119,147,550 – 119,231,545 | 9–10 | KO | (164) | ||
| (Embryonic lethal) | |||||
| reverse strand | 9–10 | kidney KO | (164) | ||
| (Bartter-like phenotype) | |||||
| 6 | T185A KI | (231) | |||
| (Embryonic lethal) | |||||
See Text for detailed information on phenotypes.
An OSR1 targeted embryonic stem cell was obtained from the UC Davis Mutant Mouse Regional Resource Center (part of the International Gene Trap Consortium). The Center randomly inserts within genes a cDNA encoding a splice acceptor site followed by a β-galactosidase-neomycin fusion cassette (gene trap insertion) which creates fusion transcripts that join the sequences from exons 5' from the insertion site to the β-galactosidase-neomycin marker. Injection of the gene trap construct into C57BL/6J blastocysts produced an ES cell clone (XH-180) with a β-galactosidase/neomycin fusion cassette downstream of exon 15 within the OSR1 gene, thus disrupting the kinase by truncating the last 69 residues. Interestingly, removal of the extreme C-terminus of the OSR1 kinase resulted in embryonic lethality (58) (see Table 2). This strong phenotype suggests either that the large β-galactosidase/neomycin fusion at the carboxyl-terminus of the kinase affects mRNA and/or protein stability, or that the carboxyl-terminus of OSR1 is essential to the function of the kinase. Similar to the strategy used to knockout SPAK, Lin and coworkers also designed a targeting construct to delete exon 9 and 10 of the OSR1 gene (164). Consistent with the results from our laboratory, crossing of their heterozygous OSR1 targeted mice confirmed embryonic lethality of homozygotes between e10.5 and e13.5. As the targeting construct was created with loxP recombination sites around exon 9/10, crossing the OSR1 floxed mouse with a Cre-recombinase transgenic mouse driven by the kidney-specific cadherin gene promoter produced viable kidney-specific OSR1 null mice (see section VI, sub-section E; and (164)). Similar to the SPAK knock-in mouse, an OSR1 knock-in mouse has also been generated by replacing the wild-type exon 6 with an exon containing a T-loop threonine to alanine (T185A) mutation (see Table 2). Again, the knock-in of a non-functional OSR1 allele resulted in homozygous embryonic lethality (231), indicating the necessity of OSR1 early in development as any type of genetic disruption results in a non-viable embryo.
IV. MOLECULAR CHARACTERISTICS OF SPAK AND OSR1
A. Structure and Domains of SPAK and OSR1
Protein domains are stable, compact, three-dimensional structures that fold autonomously (65, 222). Organization of large proteins by structural domains accelerates the individual folding process and is the optimal solution for a large protein to keep hydrophilic residues at their surface while burying hydrophobic residues (106, 107). Multiple domains provide flexibility leading to protein domain dynamics (27). X-ray crystallography of the entire amino-terminal catalytic domain and the last 90 amino acids of the carboxyl-terminal regulatory domain of mouse OSR1 have identified several secondary structures and interacting regions (see Figure 6A). The classic bi-lobal kinase fold of OSR1 has an amino-terminal lobe with five-stranded antiparallel β sheets (β1–β5) (see Figure 6B), and a carboxyl-terminal lobe containing mostly α helices (αC–αF) and 3 additional β sheets (β6–β8) (see Figure 6A) (156, 299)). The conserved lysine residue at the end of the β3 sheet is critical to the binding of an ATP molecule, and the Aspartate-Phenylalanine-Glycine (DFG) sequence between β8 and αAL binds the Mg2+ and positions the ATP molecule for phosphotransfer (1). The DFG sequence also marks the beginning of the activation loop which contains the primary phospho-threonine residue (T243) targeted by WNK kinases (300). There are three hydrophobic residues (F244, P248, and M251) located in the P + 1 loop between αAL and αEF, and one hydrophobic residue (L294) in the αG helix which together form the substrate pocket of the catalytic domain (93).
Crystallographic studies from the van Aalten and Goldsmith laboratories have demonstrated that the catalytic domains of two monomers of human OSR1 dimerize and swap activation segments (156, 299). The domain-swapped region consists of the P+1 loop and helix αEF (region highlighted in Figure 6A, or residues Phe186-Gly203 in human OSR1). Glycine residue 232 in the αAL sub-domain and glycine residue 261 downstream of the αEF sub-domain form the hinge points around the P+1 loop and αEF sub-domain (156). Mutagenesis of the domain-swapped threonine residue within the P+1 loop of SPAK into an alanine (mouse T247A; human T237A) robustly inhibited SPAK from phosphorylating and activating NKCC1 in Xenopus oocytes, indicating that domain swapping and trans-phosphorylation of this residue is vital to SPAK function (93).
Yeast-2-hybrid analysis showed protein-protein interaction between the last 97 amino acids of SPAK and the RFx[V/I] binding motif within KCC3 (224). In order to form crystals of the last 90 amino acids which comprise the conserved carboxyl-terminal PF2 domain of OSR1, the van Aalten laboratory included a GRFQVT peptide sequence found in WNK4. Analysis of the structure identified an elongated negatively charged primary pocket (interaction site of WNK4 peptide) and a secondary hydrophobic pocket (298). Although no crystal structure of the entire kinase has been resolved, examination of full-length OSR1 by small angle X-ray scattering (SAXS) demonstrated an elongated shape in the absence of a WNK4 peptide, and a more compact molecule in the presence of a WNK4 peptide (299). A quantitative surface plasmon resonance binding assay was used to evaluate the effect of site-directed mutagenesis on three groups of residues (bolded in Figure 6A) in the primary pocket which are important in the binding of the biotinylated RFxV peptide sequence of WNK4. The aspartic (D488) and glutamic (E496) acid residues form salt bridges with the peptide arginine residue. The phenylalanine (F481), leucine (L497), alanine (A499), and leucine (L502) residues form a hydrophobic pocket for the benzyl side chain of the peptide phenylalanine residue. We previously demonstrated that mutation of the phenylalanine residue in the RFxV peptide sequence was critical to SPAK recognition (94, 224). Here we show that mutation of F481 in the binding pocket into an alanine also results in loss of WNK4-SPAK activation of NKCC1 in Xenopus laevis oocytes (see Figure 7, unpublished data).
Figure 7. Absence of Function of SPAK F481A mutant.
86Rb influx of mouse NKCC1 co-expressed with mouse WNK4 in Xenopus laevis oocytes with wild-type (wt) and mutant (F481A) forms of SPAK. Bars represent mean ∀ S.E.M (n = 20 – 25 oocytes). The NKCC1-mediated K+ influx is expressed in nmoles/oocyte/hr (unpublished data from Delpire Laboratory).
Interestingly, the WNK4 peptide used to stabilize the crystal structure of the PF2 domain of OSR1 contains a threonine residue right after the valine. A plasmon resonance-based binding assay revealed a significantly lower binding affinity between the phosphorylated WNK4 peptide (i.e. GRFXV-pT) and the primary pocket of OSR1. Additionally, this phosphorylated WNK4 peptide, when transfected to HEK293 cells, did not interact with endogenously expressed SPAK/OSR1 (298). This suggests the possibility of regulating kinase-substrate interaction by phosphorylation of a residue in the interacting domain. This mechanism of regulation, either through autophosphorylation or by another kinase, has not yet been addressed in vivo. It is also important to note that this is relevant for some, but not all, binding partners of SPAK/OSR1 (see Figure 8).
Figure 8. Alignment of 15 SPAK RFx[V/I] motif interacting proteins.
A) Proteins with threonine or serine residues located at positions 5 or 6 in docking motif, thus constituting putative targets for basophilic kinases, are indicated in blue. B) Proteins which do not contain threonine or serine residues at positions 5 or 6 in their docking motif. Note that two motifs do have negatively charged residues located at position 5 or 6.
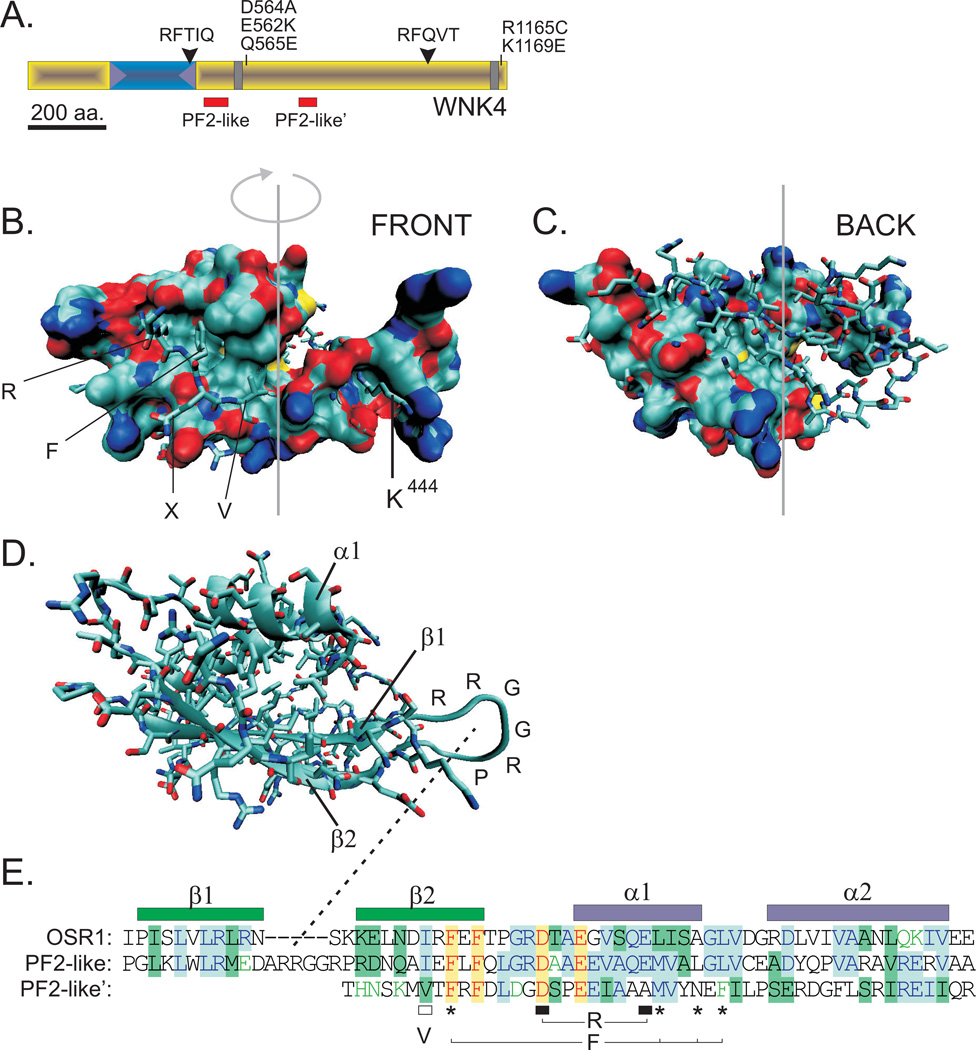
In 2008, we identified a region of the C-terminal regulatory domain of WNK kinases that is homologous to the PF2 domain of SPAK/OSR1 (58). Both this PF2-like region, and a second region of homology (PF2-like’) are located proximal to the catalytic domain (Figure 9A). Modeling the WNK4 PF2-like region using SWISS-MODEL (Swiss Institute of Bioinformatics) and PDB v2s3 (chain A) of OSR1 as a template reveals very high structural similarity for the front halves of the two molecules (Figure 9B). In fact, with the molecular surface of the PF2-like region filled in and the PF2 residues drawn in licorice style, it is clear that the RFx[V/I] peptide perfectly fits into the hydrophobic pocket of WNK4 (see Figure 9B). However, as highlighted by all the residues drawn in licorice style, major differences between the structures are observed in the back halves of the two molecules (Figure 9C). Similar to the SPAK/OSR1 PF2, the structure of the WNK4 PF2-like domain consists of two conserved anti-parallel beta sheets (β1 and β2) and two alpha helices (α1 and α2), and the hydrophobic pocket is formed by residues of β2 and α1 (Figure 9D). Note that the two β sheets are separated by a larger linker consisting of six amino acid residues (R-R-G-G-R-P). Another significant difference between the two structures is the absence of a bulky positive residue (Lys444) in the PF2-like structure, thus creating an additional small hydrophobic pocket (Figure 9B). The two negative charges interacting with the R1 arginine (mWNK4 Asp480 and Glu488), the four hydrophobic residues that accommodate the R2 phenylalanine (mWNK4 Phe473, Met489, Leu492, and Leu494) and the isoleucine that interacts with the R4 valine (mWNK4 Iso471) are all conserved (see Figure 9E). It is important to note that while there is some conservation between the second PF2-like region (PF2-like’), the homology was not strong enough for SWISS-MODEL to provide a structure leaving the relevance of the PF2-like’ domain unknown. However, the possibility that the first PF2-like domain interacts with RFxV peptides has very significant consequences for our understanding of how Ste20-like and WNK kinases regulate ion transport mechanisms. First, the PF2 domain, or its most significant portion, is not unique to SPAK and OSR1 kinases, but also exists in all WNK kinases. Second, the identification of PF2-like motifs in proteins that contain RFxV peptides might provide a molecular mechanism by which proteins can regulate themselves. Indeed, the region proximal to the catalytic domain in WNK1 has been described as an auto-inhibitory domain (315). Third, it is also possible that an intramolecular interaction between RFxV peptides in WNK1 (see Figure 10) and the putative PF2-like domain contributes to kinase inhibition. Competition of this domain with the PF2 domain of SPAK/OSR1 might not only anchor the two kinases, but also possibly relieve auto-inhibition of WNK. Note that in WNK4, a RFTIQ motif is located at the end of the catalytic domain, not too far from the PF2-like domain (see Figures 9A & 10). Whether or not this specific motif interacts with the PF2-like domain is unknown. Finally, the existence of a PF2-like domain in WNK kinases raises the possibility that the kinases might interact directly with the N-terminal tail of cation-chloride cotransporters. This is a significant observation since this has the potential to put in question the kinase signaling cascade and their effects on modulating ion transport.
Figure 9. Evidence for PF2-like domains in WNK4.
A. Schematic representation of WNK4 with location of 2 putative PF2-like domains. B. Front halves of two overlapping structures: a SWISS-MODEL-based structure of the PF2-like motif of WNK4, drawn in surface style, and the OSR1 PF2 domain (PDB: 2v3s), drawn in licorice style. C. Back halves of the same structures after 180° rotation on its vertical axis. D. The PF2-like motif of WNK4 is drawn in cartoon style to highlight the two beta sheets and the two alpha helices. The amino acid linker (R-R-G-G-R-P) between β1 and β2 is highlighted. E. Alignment of sequence of the PF2 domain of OSR1 and the two PF2-like domains of WNK4. The residues that orient and interact with the RFxV peptides are indicated by open and closed bars and stars.
Figure 10. Schematic representation of the WNK kinases.
Each of the four WNK kinases is characterized by short N-terminal region preceding the catalytic domain, and a carboxyl-terminal regulatory domain of variable length. An internal promoter in the WNK1 gene yields a kidney-specific isoform (KS-WNK1) which starts at exon 4a and therefore lacks a functional catalytic domain. Alternative splicing in exon 18 of WNK3 yields a short and long isoform. Single amino acid mutations downstream of the catalytic domain and at the extreme carboxyl-terminal tail of WNK4 which result in PHAII are identified. Multiple putative SPAK/OSR1 interacting motifs (RFx[V/I]) are indicated by labeled arrowheads. An auto-inhibitory domain in WNK1 is identified by a black box and coil-coil domains in WNK1, 3, and 4 are identified by grey boxes.
In Figure 6A, we have also highlighted three additional regions of interest in the PF1 domain. The first is a nuclear localization signal within the second predicted α helix which might target the kinase to the nuclear membrane. Thus far, no functional role for a nuclear localization signal has been determined. The second is a tryptophan - glutamic acid - tryptophan sequence (WEF motif) located in the second predicted β sheet, and which allows interaction with Cab39/MO25 (see Binding Partners/Effectors section). The third region of interest is a putative caspase cleavage site (DEMD), located two residues downstream of the Cab39 binding site. TRAIL-induced cleavage was shown not to occur at this site, but at two downstream sites, leading to inactivation of SPAK and consequent increased cell sensitivity to apoptosis (228).
B. Binding Partners/Effectors
Many proteins interact with SPAK/OSR1 and might therefore be targets of these kinases. Small scale yeast-2-hybrid with alanine scanning mutagenesis identified a 9-amino acid region necessary for interaction between SPAK and the N-terminal domain of mouse K-Cl cotransporter isoform three (KCC3), and both Na-K-2Cl cotransporters (NKCCs)(224). The core of this region is an RFx[V/I] motif which fits into a pocket of the PF2 region of SPAK (223, 300). In a follow-up yeast-2-hybrid screen of a mouse library with the PF2 binding domain of SPAK (amino acids 461–556), our laboratory identified several additional proteins containing this RFx[V/I] motif (223).
Apoptosis Associated Tyrosine Kinase (AATYK)
AATYK was first identified in apoptotic myeloid precursor cells cultured in granulocyte colony-stimulating factor (G-CSF) in the absence of interleukin-3 (IL-3) (101). Subsequent studies have found expression of this novel tyrosine kinase in non-apoptotic cells in all regions of the adult rat brain (e.g. olfactory bulb, forebrain, cortex, midbrain, cerebellum, and pons) (13, 232, 282). AATYK appears to be localized to neuronal cytoplasm and to promote neuronal differentiation and neurite length (13, 232). In addition to the original AATYK1A, a splice alternative (AATYK1B) and two new family members have been cloned (AATYK2 and AATYK3). Each of these new members have an extra N-terminal region consisting of a signal peptide-like sequence and a transmembrane domain region (282, 285). Whereas AATYK1A peripherally associates with the plasma membrane by palmitoylation, the three new family members all insert into the plasma membrane. Although AATYK2 distribution is apparently restricted to the soma, AATYK1 and AATYK3 are present in both neuronal soma and axons (285). The expression of AATYK mRNA and protein was found to increase with postnatal brain development. Over-expression of wild-type AATYK in immature granule cells promoted neurite outgrowth, whereas a tyrosine kinase-defective mutant significantly inhibited outgrowth (284). In cultured cerebellar granule cells, low potassium concentrations result in apoptotic stimulation and a hyper-phosphorylation of AATYK at specific serine residues. The regulation of AATYK phosphorylation is controlled by L-type voltage dependent calcium channel-mediated Ca2+ influx and Ca2+ dependent protein phosphatase activity (126, 283). This observation was confirmed through the use of Ca2+ channel antagonists and activators which resulted in hyper- and hypo-phosphorylation of AATYK, respectively (126). Another yeast-2-hybrid screen of a human brain cDNA library with the Cdk5 activator p35 as a bait identified a fragment of AATYK, suggesting that the tyrosine kinase might be a novel Cdk5/p35 binding and substrate protein (122). Knockout AATYK2 mice have been generated and although the testicular somatic cells appear normal, the germ cells fail to differentiate into elongated spermatids resulting in infertile males (139).
Yeast-2-hybrid analysis identified AATYK1 and AATYK3 as interactors of the PF2 domains of SPAK (223). Surprisingly, co-expression of AATYK with NKCC1 in Xenopus laevis oocytes abolished cotransporter activity to levels observed in the presence of the loop diuretic, bumetanide. Confocal microscopy with an AATYK cDNA construct containing green fluorescent protein determined that the membrane trafficking of NKCC1 was not affected by AATYK. The catalytic activity of the tyrosine kinase was also not necessary for cotransporter inhibition (96). Yeast-2-hybrid and co-immunoprecipitation studies have demonstrated that SPAK and protein phosphatase 1 (PP1) both bind to AATYK. Additionally, the catalytic function of both SPAK and PP1 are necessary for activation/inhibition of NKCC1, respectively. Although PP1 can inhibit NKCC1 activity alone or in the presence of constitutively-active SPAK, AATYK inhibition of the cotransporter was prevented by constitutively-active kinase, suggesting PP1 both directly and indirectly regulates NKCC1 function (90, 96). Altogether, these studies have unmasked a novel regulatory pathway for NKCC1 as the N-terminus of the cotransporter also contains both SPAK and PP1 binding sites. These studies suggest that PP1 not only dephosphorylates and inactivates the cotransporter, but must also dephosphorylate and inactivate the Ste20-related kinase associated with the cotransporter (90, 96).
Gelsolin/Cytoskeleton
An interesting observation from the Piechotta 2003 study was the interaction between gelsolin and SPAK (223). In fact, the initial identification of SPAK in pancreatic beta cells suggested that the non-conserved proline-alanine rich region which precedes the catalytic domain may allow the kinase to interact with cytoskeletal structures such as actin (130). The Na-K-2Cl cotransporter, a target of SPAK/OSR1 phosphorylation, has also been associated with F-actin cytoskeleton and distribution/upregulation of the cotransporter could be blocked by the F-actin stabilizing drug phalloidin (50, 51, 178, 179). Subsequent studies in eel intestinal epithelium demonstrated that regulation of NKCC1 involved both cytoskeleton interactions and protein phosphorylation events. Changes in ion transport in response to both hyper- and hypo-tonic stress required the integrity of both F-actin and microtubules (166). Liedtke and coworkers also demonstrated that increased activity of PKC-delta resulted in activation of airway epithelial NKCC1 through localization and stabilization of actin polymerization (161). The possibility that kinase activity might affect actin polymerization was confirmed in Ehrlich ascites tumor cells which showed that shrinkage activation of NKCC1 was dependent on the cortical F-actin network, myosin II, and myosin light-chain kinase (120). A recent study isolated chondrocytes from articular cartilage to study the effect of exposure to constant changes in extracellular osmolality. Regulatory volume increase in these cells after a hypertonic challenge was mediated by NKCC1 and regulated by the actin cytoskeleton (209).
With-No-Lysine Kinases (WNKs)
The kinase activity of most mammalian serine/threonine protein kinases depends on a conserved lysine residue in sub-domain II of the catalytic domain. However, as the name implies, this key lysine residue has been replaced by a cysteine residue and the kinase activity of WNKs is conferred by another nearby lysine residue (297). Our yeast-2-hybrid screen of a mouse library with the binding domain of SPAK identified WNK4 as a SPAK/OSR1 interactor (223). At the time our screen was performed (2002), the cloning/identification of all the mouse WNK kinases had not yet been completed. One of the interactors found several times in the screen was a protein identified through the NCBI >protein blast as colon cancer antigen 43 (See Table 1 of (223)). We now know, from re-running the blast search, that what was previously >colon cancer antigen 43' was in fact another WNK kinase (WNK2). The original screen, using the PF2 domain of SPAK, fished both WNK2 and WNK4 kinases because they each have a RFx[V/I] binding motif (Figure 10). There are four mammalian WNK kinases (1–4), with WNK1 being the only one possessing four RFx[V/I] motifs. The WNK kinases share 85–90% sequence identity in the catalytic domain, an autoinhibitory region, numerous protein-interaction motifs in their regulatory domain, and tissue-specific expression in the heart and kidney (55, 210, 297, 310, 314).
The second WNK kinase (WNK2) that interacted with SPAK in our yeast-2-hybrid screen is expressed in neurons of the cerebral cortex and cerebellum, and unlike the other three WNKs, is not expressed in the kidney (241). Functional studies using heterologous expression in Xenopus laevis oocytes have shown that WNK2 stimulates NKCC1 activity and inhibits KCC2/KCC4 activity. Immunoprecipitation studies from mouse brain also demonstrated an interaction with a phosphorylated form of SPAK. Taken together, a signaling complex between WNK2-SPAK-NKCC1 likely exists in the brain (241). Human WNK3 has two variants based on alternative splicing in exon 18 (121). The first variant contains a shorter version of exon 18, and the second variant contains an elongated exon 18 (containing an additional 141 bases encoding 47 amino acids). Both variants are present in the brain, whereas only the first variant is found in the kidney (121). In heterologous expression experiments in Xenopus laevis oocytes, the neuronal variant of WNK3 inhibited 22Na+ uptake through NCC, whereas, the renal variant stimulated NCC uptake 2.5 fold. The suppression effect of the neuronal WNK3 on NCC activity is similar to WNK4 (112).
In 2004, we cloned and heterologously expressed mouse WNK4 in Xenopus laevis oocytes and demonstrated that WNK4 lies upstream of SPAK in the regulation of NKCC1 activity (92, 95). Concomitantly, in vitro experiments demonstrated the interaction between SPAK and WNK4 or WNK1 (190, 300). The relationship between the kinases and the cotransporter is described in more detail in section IV, sub-section C. In vitro kinase assays using wild-type and mutant WNK4 proteins (recovered from transfected HEK293 cells) also confirmed that WNK4 binds and phosphorylates OSR1 (3). As expected, inactive or truncated (lacking the carboxyl-terminal domain) WNK4 could not phosphorylate OSR1. Residual phosphorylation of OSR1 was found to be the result of a 40 kDa kinase which co-purified with WNK4. This kinase has not been identified but was shown to be ubiquitous and possibly constitutively-active, as extremely small amounts of this unidentified kinase were able to phosphorylate SPAK and OSR1 (3). A recent study has suggested that along with WNK activity affecting SPAK/OSR1, the two Ste20 kinases might also have a regulatory feedback role on the activity of the WNK kinases (280). Hypotonicity and extracellular K+ concentration have also been shown to act as upstream regulators of WNK1 kinase activity (194). In a study published in 2011, WNK1 was shown to localize to the mitotic spindles during chromosome segregation. Indeed, cells deficient in WNK1 failed to complete cell division suggesting the necessity of the kinase for mitosis and abscission (289). Multiple studies have investigated the role of WNK kinases in salt sensitive hypertension, embryonic organ development, cell signaling, survival, and proliferation (for detailed reviews, see (123, 134, 181, 306)). It is important to note that not all effects mediated by WNK kinases require their catalytic activity, as kinase dead mutants or even regulatory fragments at times are able to recapitulate the physiological effect. Thus, WNK kinases regulate transport through a variety of mechanisms, and only a subset of them involves the stimulation of SPAK/OSR1 signaling pathways. These kinase-dependent and independent effects are discussed thoroughly in a recent review on WNK kinases (181). The relationship between the kinases and the cotransporter is described in more detail in section IV.C. and section VI.E.
Protein Kinase C Isotypes
The twelve protein kinase C (PKC) isotypes (α, βI, βII, γ, δ, ε, η, θ, ζ, ι, μ, ν) can be divided into three subgroups based on structure and co-factor requirements: the Ca+2-dependent conventional PKCs (α, βI, βII, γ), the Ca+2-independent novel PKCs (δ, ε, η, μ, θ); and the insulin/ceramide responsive atypical PKCs (ζ, ι, ν). These isozymes participate in cellular permeability, contraction, migration, proliferation, hypertrophy, apoptosis, and secretion (60). A search of the PKC protein sequences published on the NCBI protein database found SPAK binding motifs in eight of the twelve PKCs (α, βI, βII, δ, ε, η, θ, and ν). Of the four conventional PKCs, three (α, βI, and βII) have a putative SPAK binding motif (KFKI). Four of the five novel PKCs (δ, ε, η, and θ) have [R/K]Fx[V/I] motifs. There are two SPAK binding motifs in PKCδ (RFNI and RFKV); PKCε (RFSV and KFGI); PKCθ (RFKI and RFKV) and three binding motifs in PKCη (two RFGI and one KFNV). The only atypical PKC with a SPAK binding motif is PKCν (KFMV). Of these, only PKCδ and PKCθ have thus far been investigated with respect to SPAK/OSR1.
A yeast-2-hybrid screen found that PKCθ selectively interacts with SPAK. Co-immunoprecipitation of endogenous SPAK from unstimulated Jurkat T cells demonstrated an association with PKCθ, which could be enhanced by 5 minutes of anti-CD3 or phorbol myristate acetate stimulation (160). Purified PKCθ also phosphorylated a GST-SPAK fusion protein in an in vitro kinase assay. Moreover, transfection of Jurkat T-cells with SPAK siRNA inhibited PKCθ-mediated activation of AP-1. In contrast, the PKCθ-mediated activation of NFκB was not affected, indicating pathway specificity. Lastly, SPAK activation by CD3/CD28 co-stimulation was hindered in T cells isolated from knock-out mice deficient in PKCθ. Taken together these data indicate that in T-cells, SPAK is involved in the activation of a transcription factor critical in the production of interleukin-2 (IL-2) and cell proliferation. The residue(s) targeted by PKCθ were mapped using in vitro phosphorylation assays with a constitutively-active PKCθ and wild-type and mutant GST-SPAK fusion proteins. These experiments demonstrated phosphorylation of two serine residues (mouse S321 and S335, see Figure 6A) located towards the end of the catalytic domain, with the majority of the signal occurring on S321. Similar to WNK4 phosphorylation of S383 in the PF1 domain (see next sub-section), disruption of S321 by phosphorylation or mutation, helps the kinase reach an active conformation (Delpire, unpublished data).
Interaction of PKCδ with SPAK was also demonstrated through immunoprecipitation studies in human tracheal epithelial cells (162). Direct binding of inactive or pre-activated PKCδ was then mapped to the regulatory domain of SPAK (259). Three separate experiments demonstrated that PKCδ acts upstream of SPAK in the hyperosmotic-induced activation of NKCC1. First, transfection of human airway epithelial cells with SPAK-specific siRNA did not alter PKCδ activation, but did inhibit activation of the cotransporter. Second, SPAK kinase activity increased in airway epithelial cells after treatment with methoxamine (a PKCδ activator) and decreased after treatment with rottlerin (a PKCδ inhibitor). Finally, an in vitro assay with activated PKCδ resulted in increased SPAK phosphorylation of myelin basic protein (259). Note that other PKC isotypes also have a role in the regulation of NKCC1. Indeed, following a hypoxia/aglycemia treatment, 86Rb uptake studies of bovine brain microvessel endothelial cells (BBMEC) demonstrated a down-regulation of NKCC1 function by both a conventional PKC inhibitor and specific inhibitors for PKCα, PKCβ, and PKCε. Similarly, treatment with a conventional PKC activator resulted in an up-regulation of cotransporter function (329). However, this study did not assess the possible role of SPAK/OSR1 in the PKCα, PKCβ, or PKCε activation of NKCC1. Also note that activation of PKC (or specific PKC isotypes) does not always result in NKCC1 activation, as decreased cell surface expression (retrieval of the cotransporter from the plasma membrane) has been often observed upon PKC activation (76, 193, 277).
Other Known SPAK/OSR1 Interactors
Several additional proteins have been shown to interact with SPAK and/or OSR1. They are the glycoprotein CD46, heat shock protein 105 (Hsp105), the TNF-α receptor (RELT), otoferlin, and calcium binding protein 39 (Cab39). The secretion of interleukin 10 (IL-10) from human CD4+ T helper cells is induced by the human type I glycoprotein, CD46 (141, 142). In turn, IL-10 is critical to self-antigen immune response suppression and the limitation of autoimmunity. The alternative cytosolic domains of CD46 (CYT1 and CYT2) have been shown to interact with SPAK (30), and consequently, knock-down of SPAK in purified CD4+ T cells resulted in diminished production of IL-10 upon stimulation with anti-CD46 antibody. This indicates that the GCK-VI kinase is an intermediate between the CYT1 domain of CD46 and IL-10 production (30).
Hsp105 (the product of the hsph1 gene) was first identified in 1995 through the use of an Hsp105 specific antibody. The authors screened a mouse library for a protein up-regulated in mouse FM3A cells preheated to 42°C (330). Northern blot analysis revealed that Hsp105 mRNA is present in most murine tissues, and is highly expressed in brain. This heat shock protein was shown to regulate the activities of other heat shock proteins (e.g. HSC70/Hsp40) by suppressing their ATPase activity (316, 317). Hsp105 expression is also significantly increased in a variety of cancers (135, 191, 196). In Hela cells, Hsp105 prevents oxidative stress-induced apoptosis by suppressing the activation of caspase-3, caspase-9, and both c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK) (318). Hsp105 belongs to a subfamily consisting of 3 heat shock genes: hsph1, hsp4a, and hsp4a1, distantly related to heat shock protein 70. Hsp4a-like was first identified by differential display analysis as a protein up-regulated by treating mouse inner medullary collecting duct cells with hypertonicity (146). Northern blot of kidney tissue exhibited low cortical expression but high inner and outer medullary expression of hsp4a1. Upon dehydration, mRNA abundance remained unchanged in the cortex, but increased significantly in both outer and inner medulla (146). As for most heat shock proteins, Hsp4a1 mRNA expression was increased by exposing cells to increased (42°C) temperature. To date, the relationship between the heat shock protein and SPAK/OSR1 is unknown. Additional work will be needed to determine whether SPAK/OSR1 phosphorylate HSP105 and influence its function, or HSP105 chaperones the GCK-VI kinases and affects their function.
Two independent studies demonstrated an interaction between SPAK/OSR1 and RELT, a TNF Receptor Expressed in Lymphoid Tissue (46, 228). Another recent study demonstrated that OSR1 complexes with phospholipid scramblase 1 (PLSCR1) only in the presence of RELT. While the precise role of OSR1 in this relationship is still unknown, phosphorylation of the phospholipid scramblase might lead to the enhancement of apoptosis in a variety of cells (45).
Interaction between otoferlin and SPAK was also identified by our yeast-2-hybrid analysis (223). Otoferlin is a mammalian calcium sensor protein with three C2 domains and a single carboxyl-terminal transmembrane domain which suggests an involvement in vesicle membrane fusion. Mutations in otoferlin result in an autosomal recessive, non-syndromic and pre-lingual deafness (186, 293, 331, 332).
Cab39, also called MO25, was first identified as anOSR1 regulator in Drosophila genetic studies (319). Indeed, mutants of Drosphila MO25 and Fray (OSR1 ortholog) showed indistinguishable defects in asymmetric division, indicating that the two proteins operate either together, or in the same pathway. Both proteins are required in embryonic neuroblasts for the proper localization of Miranda, an adaptor protein to Prospero, a cell fate determinant. Thus, improper Miranda localization results in early larval lethality. The study also showed that MO25 and Fray oppose the function of STK11 (also called LKB1), a tumor suppressor gene necessary for definition of the anterior-posterior axis of the Xenopus laevis oocyte (176). Conversely, over-expression of LKB1 resembles the MO25 and Fray phenotype.
Mammalian Cab39 was originally cloned from a two-cell cleavage stage mouse embryo and has been shown to have fundamental functions during both development and in many adult cell types (189). The cloning of the Drosophila ortholog came three years later (200). With the cloning of the entire mouse and human genomes, it is now clear that there are at least two genes that encode Cab39 proteins, each of them producing several transcripts. What is currently known about Cab39 function comes from work performed with Cab39α. In mammalian cells, Cab39α operates as a critical scaffolding subunit stabilizing STK11 (LKB1) (23, 24). The STK11/MO25/STRAD complex, which acts upstream of AMPK protein kinases (168), might also regulate cation-chloride cotransporter function (85). Additional targets for mouse Cab39 include the MST3/MST4/YSK1 kinases which are involved in controlling development and morphogenesis (81). In the same study, it was demonstrated through in vitro experiments that Cab39 binding stimulated SPAK/OSR1 kinase activity by 3–4 fold (81). Cab39 is thought to interact with SPAK at a hydrophobic-acidic-hydrophobic motif (WEW), which is located in the regulatory region of SPAK/OSR1 downstream of the serine residue targeted by WNK4 (see Figure 6A). At this time it is not known if Ca2+ ions modulate the Cab39-SPAK/OSR1 interaction and the SPAK/OSR1 signaling cascade.
Other Potential SPAK/OSR1 Interactors
Our laboratory wondered how many other proteins in the mouse proteome could possibly interact with SPAK/OSR1. Based on twelve putative motifs from SPAK binding partners identified through our yeast-2-hybrid analysis (e.g. NCC, NKCCs, KCCs, WNKs, AATYKs, gelsolin), we expanded our consensus binding motif sequence to: [V/S/G]RFx[V/I]x[V/I/T/S]xx (where x represents any amino acid) and performed an in silico search of the mouse proteome. In addition to the 12 identified SPAK/OSR1 interacting proteins, we found another 119 proteins containing this expanded binding motif. Analysis of the relative frequency of amino acid occurrence at each position in the motif demonstrated a preponderance of serine residues at positions 5 thru 8 (57). Two-thirds of these proteins had an identifiable function ranging from transport to cell signaling to cytoskeletal support. Nearly all of these proteins preferentially had the SPAK/OSR1 binding motif located in their extreme amino- or carboxyl-terminal regions (57). Whether or not the GCK VI kinases truly interact with these proteins remains unknown. Obviously, many factors will contribute to the biological significance of these putative interactions including temporal and spatial co-expression (i.e. same tissue and cellular compartments), presence of upstream activators and inhibitors, and presence of downstream target phosphorylation residues.
C. Functional Reconstitution in Xenopus laevis oocytes
Injection of heterologous mRNA into the oocyte of the African clawed frog (Xenopus laevis) allows the protein products to be studied in a controlled system one component at a time. We and others have observed a low level of bumetanide-sensitive (native NKCC1-mediated) K+ uptake in Xenopus laevis oocytes with only a minor increase upon hypertonic stimulation (95, 224, 272). Over-expression of the amphibian ortholog of the mammalian NKCC1 exhibited a level of isotonic and hypertonic K+ uptake similar to over-expressed mouse NKCC1, confirming that frog oocytes have a limited number of native cotransporters that are already fully active under isotonic conditions (89). Consequently, when the cotransporter is over-expressed by cRNA injection, then the regulators (i.e. kinases) become the limiting factor to increased activation.
Yeast-2-hybrid experiments identified SPAK/OSR1 as in vivo interactors of cation-chloride cotransporters (224) and WNK4 (223). In order to reconstitute the sequence of events leading to in vivo cotransporter activation, several groups have utilized heterologous cRNA expression in the Xenopus laevis oocyte. For instance, co-expression of NKCC1 with wild-type SPAK did not result in cotransporter activation (224), but co-expression of NKCC1 with catalytically-inactive SPAK resulted in decreased NKCC1 function (63). These two observations suggested that a) SPAK is involved in the activation of NKCC1, and b) the wild-type kinase was not active after injection in oocytes. However, activation of NKCC1 could be achieved by co-expressing NKCC1, wild-type SPAK, and wild-type WNK4 (95). It is important to note that these exact same experiments could not be replicated in mammalian cells, as expression of wild-type SPAK alone does result in the activation of the cotransporter (105). This is likely due to the presence of active upstream kinases in these more differentiated cells. The Xenopus laevis oocyte system has identified/confirmed specific residues involved in the protein-protein interaction, phosphorylation, and activation of NKCC1. Similar experiments have been performed in Xenopus oocytes to reconstitute the kinase activation of the kidney-specific Na-K-2Cl cotransporter (NKCC2) (109, 229), the Na-Cl cotransporter (NCC) (208, 250), and kinase inhibition of several K-Cl cotransporters (103, 241).
Along with identifying the kinases involved in the signaling cascade which activates NKCC1, use of the Xenopus laevis oocyte expression system has also provided opportunity to characterize the role of other specific residues in SPAK/OSR1. In 2005, Vitari and coworkers demonstrated that the activation loop threonine residue 185 and the PF1 serine residue 325 in human OSR1 are targets of upstream phosphorylation by WNK4 (300). A concomitant study also demonstrated phosphorylation of the PF1 serine in mouse OSR1 and in rat SPAK by WNK1 (190). When the corresponding threonine and serine residues in mouse SPAK were mutated to mimic phosphorylation (i.e. T243E; S383D), co-expression of this mutant kinase was able to phosphorylate/activate NKCC1 in frog oocytes in the absence of WNK4, demonstrating that this mutant kinase is constitutively-active (91). It is important to note that mutation of either the catalytic loop threonine residue, or the PF1 serine residue alone, were not sufficient to render the kinase constitutively-active. Curiously, mutation of the serine residue into an alanine along with T243E also yielded a constitutively-active kinase, indicating that disruption of the PF1 domain is a necessary component to kinase activation (91). Note that, combining the PF1 serine mutation with mutation of the activation loop threonine into an aspartic acid did not result in cotransporter activation, indicating that an aspartic acid substitution, in contrast to a glutamic acid substitution, does not mimic phosphorylation (91). It is important to recognize that neither of these discoveries could have been made in mammalian cells where endogenous expression of WNK kinases would have masked the effect of expressing these mutant forms of the kinase.
In vivo studies injecting mouse Cab39 into Xenopus laevis oocytes have also uncovered an alternative mechanism of SPAK/OSR1 activation. A recent study comparing the functional activity of a mutant (T197E, S338A) sea urchin ortholog to the constitutively-active mouse OSR1 found that co-expression with WNK4 and NKCC1 cRNA resulted in variable effects ranging from cotransporter inhibition to cotransporter activation (97). However, co-expression of mouse Cab39 cRNA with the mutant sea urchin OSR1 consistently resulted in a four-fold activation of the cotransporter, regardless of WNK4, indicating that Cab39 must somehow stabilize this mutant kinase in an active conformation. We mentioned earlier that the activation loop kinase mutant required the presence of WNK4 for activation, indicating the need for phosphorylation of the PF1 serine (91).However, when the activation loop threonine mutant of SPAK was co-injected with Cab39 cRNA, we observed a three- to five-fold activation of the cotransporter (97), similar to the effect measured in vitro (81). It was concluded that phosphorylation of the PF1 serine by WNK4, or the binding of Cab39 to the same PF1 region (the WEF motif is located only 10 residues away from S383), promotes the active conformation of the kinase. This observation opens the possibility of an alternative activating mechanism where in the presence of Cab39, WNK or non-WNK kinases which only target the activation loop threonine residue, bypass the requirement for phosphorylation of the PF1 serine, and activate SPAK/OSR1.
V. NON-MAMMALIAN STE20-RELATED KINASES
A. Drosophila FRAY
FRAY, the Drosophila ortholog of mammalian OSR1 (58) was initially discovered in an enhancer trap screen (264). The fray gene was cloned to determine the role it plays in the nervous system. FRAY is expressed by peripheral glia and is necessary for normal axonal ensheathment. Null FRAY mutants created with RNA interference (RNAi) died early in larval development and exhibited severe axonal swelling and defasciculation. This phenotype could be rescued by over-expression of fray cDNA in the ensheathing glia of the null FRAY mutants (159). Tissue in situ hybridization demonstrates that subperineurial glia, the cells that provide the paracellular blood nerve barrier in larval Drosophila, express transcripts of Ncc69, a transporter mediating coupled Na+, K+, and Cl− movement. Loss of cotransporter function by RNAi results in a fluid accumulation between glia and axons, indicating an important role for FRAY-Ncc69 in nervous system volume/osmotic homeostasis (158). It is remarkable how conserved the function of these proteins are, as mammalian SPAK/OSR1 and NKCC1 are also expressed in choroid plexus and blood brain barrier (205, 224, 226).
The mechanisms behind asymmetric cell division during neural development are still poorly understood. Both Cab39/MO25, and the serine/threonine kinase, FRAY, have been identified as regulators of neuroblast asymmetric division in Drosophila (319). Indeed, null mo25 and null fray mutants both exhibit defects in the localization of Miranda, a protein that we have introduced when discussing Cab39. Miranda is an adaptor protein to Prospero, a homeodomain transcription factor, which is synthesized in the neuroblast and migrates into the ganglion mother cell (GMC) during mitosis to activate GMC-specific and repress neuroblast-specific gene expression. Miranda targets Prospero to the plasma membrane and is responsible for its apical localization during interphase and migration to the basal membrane during mitosis (for review, see (87)). Thus, MO25 and FRAY likely play a mechanistic role in regulating asymmetric cell division. In addition, the tumor suppressor kinase Lkb1, also introduced before, has been shown to re-distribute MO25 and FRAY from the cytoplasm to the cortex, resulting in the same null mo25-mutant neuroblast phenotype. This suggests that the asymmetric process which MO25 and FRAY regulate is counterbalanced by Lkb1 (319).
B. Caenorhabditis elegans GCK-3
GCK-3, the C. elegans ortholog of mammalian OSR1, was identified by a positive yeast two-hybrid interaction with a RFx[V/I] motif located in the carboxyl-terminal tail of the worm chloride channel, CLH-3b (61). In addition to the evolutionary conservation of the interaction motif, the primary binding pocket (and its critical residues) within the CCT domain (298) is conserved through evolution starting from the protists (see Figure 11). The interaction between GCK-3 and CLH-3b was confirmed by a GST affinity assay (61). As CLH-3b is activated by dephosphorylation (248), the identification of a serine/threonine kinase suggests a novel regulatory pathway in which the kinase inhibits the fast-gating mechanism of the chloride channel. This was confirmed by measurement of robust hyperpolarization-evoked Cl− currents when CLH-3b was expressed alone, and a three- to nine-fold reduction in current density when GCK-3 was co-expressed with CLH-3b in HEK293 cells (61). Mutagenesis experiments combined with electrophysiological examination of the swelling-induced CLH-3b activation identified serine residue 747 as the target of dephosphorylation by the type I protein phosphatases. Mutation of S742 and S747 into glutamate resulted in constitutive, kinase- and swelling-insensitive chloride channels (75). This GCK-3 serine motif with four amino acids between the serine residues is conserved as a threonine motif in mammals (91). The worm excretory cell and worm oocyte both express the clh-3 and gck-3 genes. RNAi of gck-3 in non-maturing worm oocytes resulted in the constitutive activation of endogenous CLH3-b. RNAi of gck-3 in the whole animal dramatically inhibited systemic volume recovery and worm survival after hypertonic shock (39).Taken together, GCK-3 is clearly part of a signaling pathway that controls ClC channel activity and regulation of intracellular Cl−, cell volume, and transepithelial fluid secretion (39, 61, 118).
Figure 11. Conservation of residues in the primary RFxV binding pocket.
Amino acid sequence alignment of the primary RFx[V/I] binding pocket of the single cell eukaryote (Capsaspora), roundworm (C. elegans), fruit fly (Drosophila), and mouse (M. musculus). Green and purple bars are delineating the second β sheet and the first α helix, respectively. Black letters on a blue/green background represent conserved residues. Red letters on a yellow background represent identical residues. Light blue and black letters on a white background represent unique residues. Conserved key carboxyl-terminal residues participating in the binding of the target RFx[V/I] peptides are indicated by a bold font.
Again, the evolutionary conservation in the OSR1/SPAK signaling pathway is remarkable. Indeed, the volume recovery observed in C. elegans, as a result of GCK-3 activity, also involves the only WNK kinase found in the roundworm (WNK-1) (39). Conserved between mammals and the nematode are i) the presence of a RFx[V/I] motif in WNK-1, ii) WNK-1 phosphorylation of GCK-3 at the activation loop threonine and the PF1 domain serine; and iii) effect of alanine substitution of activation loop threonine residue abolishing upstream phosphorylation (118). Null mutations of wnk-1 and gck-3 genes confirm that WNK-1 and GCK-3 activity are required for larval progression, germline formation, spermatogenesis, development of excretory canals, and facilitate chromosome segregation during the first meiotic cell division (74, 118, 148).
VI. PHYSIOLOGICAL ROLES OF SPAK AND OSR1 IN MAMMALIAN SYSTEMS
A. Function in the Nervous System
Brain and Spinal Cord
Expression/function of the Na-K-2Cl and K-Cl cotransporters in the brain have been directly associated with the developmental response to the neurotransmitters, GABA and glycine. In the rodent brain, expression of NKCC1 is highest in newly differentiated neurons in the periventricular zone (212). The cotransporter uses the energy of the Na+ gradient generated by the Na+/K+-ATPase to drive intracellular Cl− to concentrations higher than electrochemical potential equilibrium (4). As a result of high neuronal Cl−, binding of GABA and glycine leads to depolarizing responses (18, 212). By the third post-natal week, neuronal migration has stopped and the intracellular Cl− concentration has significantly decreased. This perinatal period seems to be critical for the development of seizures (68). In fact, bumetanide, a relatively potent inhibitor of NKCC1 has been shown to increase the efficacy of pentobarbital in the prevention of neonatal seizures (67). The decrease of intracellular Cl− after birth is accelerated by the concomitant up-regulated expression of the neuronal K-Cl cotransporter (KCC2) in mature CNS neurons. KCC2 uses the energy of the outward K+ gradient to drive intracellular Cl− below its electrochemical potential equilibrium (169, 217, 243). These well-concerted developmental processes parallel the finely tuned development of the neurotransmitters, GABA and glutamate (17). In the adult brain, KCC2 (and to a lesser degree KCC3, (22)) activity is critical in preventing network hyperexcitability (311, 338). In the spinal cord, KCC2 activity decreases nociception, as down-regulation (42, 131, 187, 199) or pharmacological inhibition of KCC2 (11) leads to increased responses to pain stimuli.
Although a direct relationship between SPAK/OSR1 and NKCC1 has been well established, a similar relationship between the two Ste20-related kinases and the K-Cl cotransporters has only been minimally investigated. The existence of RFx[V/I] motifs in KCC3 (224) and KCC2a (291), heterologous expression studies showing SPAK inhibition of KCC2 function (95), and inhibition of KCC2 in neocortical pyramidal cells, thalamic relay cells, cerebellar granule and Purkinje cells by WNK2 through SPAK (241) are three pieces of evidence suggesting SPAK/OSR1 regulation of K-Cl cotransporters.
Choroid Plexus
Choroid plexus epithelial cells mediate Na+ secretion and K+ reabsorption in cerebrospinal fluid (for a thorough review, see (47)). High expression of NKCC1 (129, 140, 226, 312) and SPAK (224) on the apical membrane indicate that the cotransporter is likely involved in trans-epithelial salt movement (Figure 12A). With the Na+/K+-ATPase also located on the apical membrane, it has been suggested that NKCC1 functions to uncouple the opposing Na+ and K+ movement (226). Indeed, if the pump is the driving force for both Na+ secretion and K+ reabsorption, these two processes will be intimately linked. However, addition of NKCC1 on the apical membrane allows for modulation of the amount of Na+ that is transported in the cerebrospinal fluid (Figure 12B). As mentioned earlier, SPAK localization to the apical membrane disappears in choroid plexus of NKCC1−/− knockout mice, indicating that the kinase is constitutively anchored to the Na-K-2Cl cotransporter in wild-type animals.
Figure 12. Model of Na+ secretion and K+ reabsorption in choroid plexus.
A. Immunolocalization of SPAK protein on apical membrane of choroid plexus epithelial cells. B. Schematic representation of a choroid plexus epithelial cell, showing the basolateral membrane (blood side) on the left, and the apical membrane (CSF side) on the right. The Na+/K+-ATPase on the apical membrane provides the driving force for Na+ secretion and K+ reabsorption. Activity of apical NKCC1 uncouples the movement of Na+ and K+ by recycling Na+ on the apical membrane. The Na+ entry mechanism on the basolateral side is mediated by N a+-bicarbonate cotransporters, NBCn1, NBCn2. The K+ exit mechanisms on the basolateral side are: (1) K+ channels and (2) KCC, a K-Cl cotransporter. SPAK is drawn at the apical membrane, associated with NKCC1.
Blood-Brain Barrier
Vascular endothelial cells with impermeable tight junctions form a blood-brain barrier (BBB) separating circulating blood from the extracellular fluid in the central nervous system. In vitro exposure of these endothelial cells cultured on transwell barriers to astrocyte-conditioned media increased Na-K-2Cl cotransporter and Na-K-ATPase expression by 55% with an asymmetric apical (90%) versus basolateral (10%) distribution of cotransporter protein expression (269, 270). ELISA studies of this astroglial conditioned medium found significant amounts of interleukin-6 (IL-6) (270). As discussed in the intestine sub-section, cytokines regulate mRNA expression of both SPAK and NKCC1. The function of the brain endothelial NKCC1 is to mediate the transport of Na+, Cl−, and water from the blood to the neuronal tissue. Dysregulation of this transport process, such as increased NKCC1 expression and activity, could result in a pathological increase of fluid in the brain. Indeed, middle cerebral artery occlusion, which mimics stroke, results in cerebral edema due to increased NKCC1 expression and activity (26, 206, 207).
Arginine vasopressin (AVP) stimulation through a receptor- and calcium-dependent mechanism (204), glucose and pyruvate deprivation, or hypoxic conditions (84) all rapidly activate p38, JNK, and AMPK in cerebral microvascular endothelial cells and stimulate BBB NKCC1 activity resulting in ischemia-induced edema formation. Inhibitors of these kinase signaling pathways reduce or abolish the stimulation of NKCC activity (301). One of these studies recognized that SPAK and OSR1 are the most direct kinases that phosphorylate and activate NKCC1, and therefore, further study is needed to determine if hypoxia, aglycemia, AVP, or oxygen-glucose deprivation stimulate NKCC1 function through these Ste20-related signaling cascades.
Hypothalamus
Estrogen-receptive neurons in the pre-optic/anterior hypothalamic region of the rat brain are GABAergic (83). Experiments performed in the neonatal hypothalamus have shown that estradiol enhances GABA synthesis, GABA-mediated Ca2+ influx, and modulates GABA-mediated sexual differentiation (219). Total NKCC1 protein expression was shown to be sexually dimorphic (higher in males than females) in perinatal hypothalamus. Administration of estradiol on postnatal day zero did not change expression levels of total NKCC1 or phosphorylated NKCC1 (pNKCC1) one day after birth. However, on the second postnatal day, there was a significant increase in pNKCC1 in hormone-treated females relative to vehicle-treated females and males. Although levels were undetectable at postnatal day zero, a significant level of KCC2 protein was found five days after birth and higher in males relative to females (220). In a recent study from the McCarthy Laboratory, estradiol treatment of newborn rats significantly increased the protein expression levels of SPAK and OSR1. The time-course of this increase in the expression of these two Ste20-related kinases parallels the estradiol-enhanced phosphorylation of NKCC1 found by Perrot-Sinal and co-workers (201).
Sensory Neurons
In contrast to central neurons, NKCC1 expression does not developmentally decrease in sensory (including peripheral) neurons, and therefore, in the absence of KCC2 expression, the intracellular chloride concentration remains high and above the resting membrane potential throughout adulthood (4, 5, 271). The high intracellular chloride concentration in dorsal root ganglion neurons results in depolarizing GABA responses, pre-synaptic inhibition, and filtration of sensory noise (309). Although the intracellular Cl− is already high under physiological conditions, it can further increase upon injury. In fact, the observed increase in NKCC1 activity following peripheral nerve injury has been shown not to involve an increase in expression level of the cotransporter, but rather an increase in phosphorylation of already expressed transporters (225). Studies have also shown that SPAK and OSR1 are enriched in olfactory sensory cilia (117). High kinase expression again correlates with high NKCC1 expression and depolarizing GABA responses which are measured in these olfactory receptor neurons (137, 238). It is important to stress that NKCC1 is not the only mechanism responsible for Cl− accumulation in sensory cilia, as some Cl− accumulation still occurs in NKCC1 knockout mice (197).
As significant portions of the catalytic and regulatory domains of SPAK and OSR1 are similar if not identical, and since the two kinases often have overlapping tissue expression (130, 223, 276, 290, 323), it is possible that they have identical functions. The question of redundancy was addressed in an undifferentiated mouse dorsal root ganglion (DRG) cell line which expresses NKCC1 as well as SPAK and OSR1 (105). Using a combination of semi-quantitative western blot analyses, shRNA, and functional studies, it was found that both kinases are expressed equally in these cells, both participate in the phospho-regulation of NKCC1, and there seemed to be a direct relationship between the amount of kinase expressed in the cells and the activity of the cotransporter. These observations were extended using primary cultured DRG neurons isolated from wild-type and SPAK knockout mice. SPAK knockout mice exhibited a 50% reduction in NKCC1 function, indicating that another kinase (i.e. OSR1) was contributing to NKCC1 phosphorylation. Decreased NKCC1 function in SPAK knockout mice could have also contributed to an increase in the latency of the mice to respond to noxious heat stimuli, and to difficulties in locomotion and balance (104).
Ste20-related Kinases and Neurological Disorders
Autism is a neurodevelopmental disorder affecting an estimated 1 in 150 children. Repetitive behaviors, weak or absent communication skills, and impaired social relationships are features indicative of autism spectrum disorders. A genetic study involving 334 families (a total of 610 subjects) has demonstrated an association between three single nucleotide polymorphisms (SNPs) in STK39 (the human gene encoding SPAK) and autism (234). Significant P values (p < 0.05) were observed with markers rs971257 (marker downstream the STK39 gene), rs1517342 (intron 5–6), and rs1807984 (intron 1–2). The authors of the study also found an interaction between STK39 and the mitochondrial aspartate/glutamate transporter SLC25A12 gene. This interaction at the genetic level indicates a possible functional interaction between the kinase and the mitochondrial amino acid carrier. Another strong association was identified between SLC25a12 SNPs and autism (235). SLC25a12 knockout mice exhibited deficits in myelination and neurofilaments leading to death three weeks after birth (249). In a more recent analysis, however, a lack of association was found between marker rs1807984 and specific core autism symptom domains or with additional specific familial features based on interviews, but the authors stated that this latest analysis did not contradict the previous study (294).
Parkinson's disease is a CNS degenerative disorder characterized by involuntary tremors and postural instability. Death of dopamine-producing cells in the substantia nigra results in the loss of smooth coordinated movements associated with this disease. A genome wide association study to search for rare and common genetic variants conferring risk of Parkinson Disease in a relatively homogenous population of Ashkenazi Jews found a significant association with another intron 5–6 SNP in STK39: marker rs3754775 (intron 5–6). Importantly, STK39 verified in a meta-analysis of datasets from five Parkinson(s disease genome wide association studies performed in the USA and Europe (40). The meta-analysis comprised 5333 patients with Parkinson Disease and 12019 control patients. In this case, marker rs2102808 (5' flanking sequence) was also associated with genetic risk for this neurodegenerative disease. However, as with all association studies, caution should be exercised in ascribing too quickly a definitive disease risk in the absence of additional biological evidence.
Schizophrenia is a psychiatric disorder characterized by severe auditory and visual distortions, behavioral disturbances, and emotional unresponsiveness. Although still poorly understood, the etiology underlying schizophrenia includes both physical (i.e. genetics, neurobiology) and psychological (i.e. environment, social processes) components. A postmortem examination of brain specimens from schizophrenic subjects demonstrated a significant increase in the protein expression of OSR1 and WNK3 in the dorsolateral pre-frontal cortex (9). While no changes in NKCC1 and KCC2 expression levels were observed in the same tissues, it is likely that the increased kinase expression affected the activity of the Na-K-2Cl and K-Cl cotransporters. In fact, increases in NKCC1 and decreases in KCC2 mRNA expression have been found in the hippocampus of schizophrenic individuals (128). KCC2 mRNA expression levels were also significantly lower in the hippocampus of schizophrenic patients who were homozygous for the SNP rs3749034 located in the promoter of the GAD1 gene. Note that GAD1 encodes for the glutamic acid decarboxylase 67 (GAD67), an enzyme that converts glutamate into GABA, and that GABA has been shown to regulate the transcription of KCC2 (100, 167). The fact that both OSR1/WNK3 and NKCC1/KCC2 have been separately linked to schizophrenia suggests that cotransporter activity is likely involved in this neuropsychiatric disorder. It is unfortunate that no similar studies have yet examined the expression of the related K-Cl cotransporter, KCC3, as patients with frame-shift mutations in the SLC12A6 gene also develop psychosis or schizophrenia-like symptoms (6, 82, 150), and KCC3 null mice exhibit a pre-pulse inhibition phenotype (124).
B. Function in the Heart and Vasculature
Although not abundant, SPAK mRNA transcript and protein expression are present in the heart, and thus, SPAK presumably serves a physiological role in the cardiovascular system (130, 290). Heart rate and locomotor activity in the SPAK (T243A) knock-in mouse followed a normal circadian rhythm and was similar to that of a wild-type mouse. However, the mean arterial blood pressure in the inactive SPAK knock-in mouse was significantly lower (discussed below in section VI, sub-section E, and (231)). In many cases, OSR1 expression overlaps with SPAK (105). However, in the heart and skeletal muscle, OSR1 mRNA expression levels are much higher (276). In fact, with the exception of the MAP2 kinases, OSR1 expression in the left ventricle of the heart has the highest protein concentration (~25 ng/mg) of the Ste20-related kinases (12). During the early stages of mouse development, passive diffusion is sufficient for embryonic nutrition. However, yolk sac circulation and development of the heart around e10.5 is necessary to prevent embryonic mortality. Even if the embryo survives past e10.5, inefficient pumping, instability of blood vessel walls, and inappropriate remodeling of the major blood vessels could lead to embryonic mortality (245). The lethality of the conventional homozygous OSR1 knockout was timed between embryonic days 10.5 and 13.5 in the Lin study (164), an observation that is similar to the lethality of WNK1 null mice (313). This suggests that cardiac insufficiency is possibly a leading causative factor. Note, however, that a fraction of animals were shown to die at a later embryonic age, up to E17.5 (58, 231).
The Ste20-related kinases and NKCC1 are both co-expressed in vascular smooth muscle and participate in aortic contractility and blood pressure (102, 184). As expected, total and phosphorylated forms of SPAK were absent in aortic tissues of the SPAK null mouse, and even though the total NKCC1 expression increased, the amount of phosphorylated NKCC1 decreased in aortic tissues (327). This result would suggest that perhaps SPAK is the predominant Ste20-related regulatory kinase of NKCC1 activity. However, examination of aortic tissue from heterozygous global OSR1 knockout mice demonstrated that while the relative protein abundance of total SPAK and NKCC1 was the same as wild-type mice, the abundance of phosphorylated OSR1, SPAK, and NKCC1 was significantly reduced, along with the expression level of total OSR1 (164). Heterozygous OSR1 knockout mice also demonstrated hypotension, indicating that, similar to dorsal root ganglia neurons, SPAK and OSR1 co-regulate NKCC1 and thus, control vascular contractility. These data also suggest that the kinases have additive effects, as elimination of either kinase results in decreased contractility. Another study has identified a novel vascular signaling pathway linking adrenergic receptors with WNK1, SPAK, and NKCC1 (19). Because homozygous WNK1 knockout mice were embryonically lethal, Bergaya and co-workers compared WNK1+/− mice with WNK1+/+ mice. The WNK1+/− mice exhibited a significant decrease in their in vivo blood pressure response, ex vivo vascular contraction after α1-adrenergic receptor activation, and a decrease in the pressure-induced arterial contractile response (19). Finally, for completeness, we will note that WNK4 also affects contractility of blood vessels and blood pressure by decreasing Ca2+ influx mediated by TRPC3 (transient receptor potential canonical 3) (215).
C. Function in the Intestine
Activation of p38 MAPK is a common feature of chronic intestinal inflammatory diseases such as ulcerative colitis and Crohn(s disease (78). In these diseases, p38 MAPK is activated in immune cells (monocytes, macrophages) as well as intestinal microvascular endothelial cells, epithelial cells, and fibroblasts. Activation of p38 MAPK leads to the recruitment of lymphocytes and neutrophils to the site of inflammation and also leads to the stimulation of macrophage producing pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8, and cyclooxygenases 1–3. Activation of p38 MAPK in colonic epithelial cells yield changes in the trans-epithelial resistance, which can be prevented using a p38 MAPK inhibitor (211).
Using degenerate primers corresponding to highly conserved regions of serine/threonine kinases (i.e. subdomains (I, VIb, and VIII)), Merlin and coworkers identified a SPAK-like kinase that is enriched in inflamed human colon tissue (321, 323). This kinase, as discussed earlier, is identical to SPAK but excludes parts of exon 1, 6 and 7, resulting in the absence of the PAPA box and domain IX, a small region of the catalytic domain (Figure 5). Over-expression of colonic SPAK through transfection in Caco2-BBE cells resulted in phosphorylation of p38 MAPK, but not ERK or JNK. These data are compatible with earlier data from Johnston and collaborators in COS7 cells (130). As pro-inflammatory cytokines also lead to activation of p38, Merlin and coworkers examined SPAK mRNA expression in Caco2-BBE cells exposed to IFN-γ, TGF-β, and TNF-α treatment. They showed a 2.5-fold increased SPAK mRNA expression with interferon (323) and TNF-α (320). Note that the precise signaling pathway modulated by SPAK is likely cell-specific, as in hematopoietic cells, SPAK interacts with the TNF receptor RELT (Receptor Expressed in Lymphoid Tissues), leading to activation of both p38 and JNK signaling (228). Transcriptional activation of SPAK in intestinal cells by TGF-β has been linked to the presence of a NF-κB element located some 350 bp upstream of the transcription initiation site (320). Using a luciferase reporter gene assay with a portion of the 5’ region of SPAK, the authors observed stimulation by TNFα. When they inserted constructs with shorter promoter sequences lacking the NF-κB element, the TNF-α stimulation was significantly reduced. Furthermore, mutation of the NF-κB element itself resulted in a 50% decrease of the TNF-α stimulated luciferase signal, indicating that the element is involved in the increased transcription of SPAK observed under inflammation. These studies confirmed increased expression of NF-κB and its translocation to the nucleus, alongside with increased SPAK mRNA and protein levels in mice treated with dextran sodium sulfate to induce colitis (320).
The precise downstream effects of SPAK and p38 MAP kinase in colonic epithelial cells are not fully resolved. Increased SPAK expression has been correlated with a decrease transepithelial barrier resistance. This observation has been made not only in cultured caco2-BBE cells exposed to cytokines (323), but also in mouse colonic mucosa from in mice over-expressing the kinase (322). Conversely, decreased SPAK expression through siRNA resulted in an increase in barrier resistance (322). Altogether, the significant decrease in epithelial barrier resistance measured in transgenic mice over-expressing SPAK is likely to account for the significant aggravation of the inflammation phenotype observed in the dextran sodium sulfate-induced colitis model (322).
Expression of SPAK mRNA levels is also increased in colon tissue isolated from mice treated with hyperosmolarity (321). In this case, induction of SPAK is also mediated at the transcriptional level through the NF-κB element. Similar to the SPAK promoter, the NKCC1 promoter is TATA-less, has SP1 sites that contribute to basal transcriptional activity, and a NF-κB element located some 650 bp upstream of the transcriptional initiation site (236). Furthermore, similar to SPAK, NKCC1 mRNA expression is enhanced by hyperosmolarity (251), IL-6 (270), IL-1and TNF-α (286). As the two proteins interact closely and are therefore part of common functional units, it seems appropriate for their genes to be co-regulated.
D. Function in the Pancreas
The pancreas has dual roles: as an endocrine gland producing glucagon (α cells) and insulin (β cells) necessary for glucose homeostasis (71); and as an exocrine gland secreting a bicarbonate (HCO3−) and salt-rich pancreatic juice containing enzymes to assist in the digestion of carbohydrates, proteins, and fats (154). The classic model for HCO3− secretion across pancreatic acinar cells involves HCO3− entry across the basolateral membrane through a Na+/HCO3− cotransporter (pNBC1) driven by the Na+/K+ ATPase, and HCO3− efflux across the apical membrane, mediated by the coordinated activity of the Cl−/HCO3− exchanger and the cystic fibrosis transmembrane conductance regulator (CFTR) (see Figure 13) (154, 266). Src family kinases and MAP kinases have previously been shown to modulate NBC transport activity in renal epithelial cells (247). In a recent study, Park and coworkers demonstrated that pancreatic duct cells abundantly express the Ste20-related kinases, SPAK/OSR1, and their upstream regulator, WNK1 (216).
Figure 13. Model of bicarbonate secretion in proximal and distal pancreatic duct epithelial cells.
In proximal duct, the luminal bicarbonate concentration is low and the Cl− concentration is high. HCO3− enters the cell through the basolateral NBCe1, driven by the Na+ gradient, whereas HCO3− exits the cell through SLC26a6 driven by the Cl− gradient. In the distal duct, luminal Cl− concentration is low and HCO3− exits the cell through a “SPAK-induced” HCO3−-permeable CFTR. The movement of bicarbonate is driven by the membrane potential.
In 2006, Shirakabe and co-workers identified pNBC1 as a target of phosphorylated IRBIT (IP3 receptor binding protein released upon IP3 binding to the IP3 receptor), and heterologous co-expression of pNBC1 and IRBIT in Xenopus laevis oocytes manifested substantially increased cotransporter activity (257). Thus, IRBIT likely affects function of pNBC1 on the basolateral membrane of pancreatic HCO3− secreting cells. Yang and coworkers (2009) investigated whether IRBIT also regulated CFTR and CFTR-dependent Cl−/HCO3− exchange on the apical membrane. They found that IRBIT also stimulated CFTR and this activation required the PDZ domain of CFTR, as well as, the PEST (Proline/Glutamic Acid/Serine/Threonine-rich) and coiled-coil domains of IRBIT (326). In a follow-up study, the authors determined that WNK1/SPAK or WNK4/SPAK phosphorylation of NBCe1 and CFTR inhibits ductal HCO3− secretion by reducing cell surface expression of both transporters (325). In agreement with the WNK/SPAK pathway reducing NBCe1 and CFTR function, knock-down of the WNK kinases (WNK1, 3, and 4 being expressed) resulted in stimulated ductal secretion (325). Whether the phosphorylation affected forward trafficking or transporter retrieval from the cell surface was not addressed. The SPAK/WNK1 effect on trafficking is interesting and novel. In the case of cation-chloride cotransporters, SPAK phosphorylation is thought to only affect the activity of the transporters already expressed in the plasma membrane. Whereas over-expression of WNK4 has been shown to lead to decreased cell surface expression not only for CFTR (324, 325) and NBCe1B (325), but also for NCC (28) and NKCC1 (132). It is important to note that we have never been able to reproduce the decreased cell surface expression of NKCC1 with WNK4 (95). More detailed studies will be needed to resolve and understand the precise mechanisms by which these kinases affect transporter trafficking. Yang and colleagues also demonstrated that IRBIT governs epithelial secretion by recruiting protein phosphatase 1 to dephosphorylate NBCe1 and CFTR, thus restoring their cell surface expression and HCO3− secretion. It is also possible that IRBIT scaffolded PP1 in proximity of SPAK leading to dephosphorylation and inactivation of the kinase, a mechanism similar to the regulation of NKCC1 by scaffolding both SPAK and PP1 on the cotransporter (90). Whether or not SPAK binds directly to NBCe1 and CFTR is still unknown, however Yang and coworkers have suggested that WNK serves as a scaffold for SPAK binding to the transporter and the channel (325). Finally, in order for IRBIT to bind to both NBCe1 and CFTR, several serine residues need to be phosphorylated, however the identity of the kinase phosphorylating IRBIT is still unknown (8, 325).
It has been argued that neither a 1:1 electroneutral nor 1:2 electrogenic stoichiometry of the anion exchanger on the apical membrane of the acinar duct epithelial cells is sufficient to produce the 140 mmol/L HCO3− that is secreted in the pancreatic duct (154). The Cl−/HCO3− exchanger requires luminal Cl− to drive the secretion of HCO3− into the lumen. However, the luminal Cl− concentration decreases from the proximal to distal end of the pancreatic duct, reducing the driving force and actually shifting the Cl−/HCO3− exchanger from secretion to absorption (Figure 13). As pancreatic HCO3− secretion still occurs in the absence of sufficient luminal Cl−, Park and co-workers (216) have suggested a novel mechanism where the activated WNK1-OSR1/SPAK signaling cascade inhibits HCO3− reabsorption through the anion exchanger, and switches CFTR from a Cl− transporting channel into a HCO3− transporting channel, thus generating bicarbonate-rich fluid in the human pancreatic duct (216). When the intracellular Cl− concentration is low compared to HCO3−, with a Cl−/HCO3− selectivity of CFTR of about 0.5 and a membrane potential of around −60 mV across the apical membrane, CFTR will secrete bicarbonate. While both Yang et al. (325) and Park et al. (216) demonstrated physical association between SPAK and CFTR through immunoprecipitation studies, neither the binding site nor the phospho-residues have yet been mapped. Park and coworkers argue that the signal leading to SPAK activation of CFTR in pancreatic duct cells is a decrease in the intracellular Cl− concentration. Although the activity of both SPAK and OSR1 was shown to be sensitive to Cl− in in vitro kinase assays (93), the identity of the protein which senses and signals changes in intracellular Cl− in cells is still unknown. Clearly, there are many unanswered questions for a more detailed review of the molecular mechanisms of pancreatic fluid and HCO3− secretion, see (155).
E. Different Functional Roles of SPAK and OSR1 in the Kidney
The primary function of the kidney is to preserve electrolyte and water homeostasis critical to normal cell physiology of the whole organism. The thick ascending limb (TAL) of Henle’s loop reabsorbs approximately 25% of the filtered salt load, with an additional 10% being reabsorbed in the distal convoluted tubule (DCT) and the cortical collecting duct (CCD) (for reviews, see (59, 70). These segments of the distal nephron reabsorb salt and divalent cations based on the expression of specific ion transport proteins. Several of these ion transporters are regulated by phosphorylation of specific serine/threonine residues. The identification of the WNK-SPAK/OSR1 signaling cascade (95, 190, 300) has resulted in multiple investigations focusing on the physiological role of these kinases in the mammalian kidney. There are several recent reviews focusing on the role WNK kinases have in regulating salt reabsorption and blood pressure (111, 123, 133, 181). In contrast to peripheral neurons, which seem to interchangeably use SPAK and OSR1 to regulate NKCC1 activity, these Ste20-related kinases have two distinct roles in the Na+-reabsorbing segments of the distal nephron. We have therefore chosen to divide the discussion of this section (and the inherited salt-wasting disorders associated with them) along these segments.
Thick Ascending Limb
The principal role of the TAL is Na+ and water reabsorption. The movement of ions across the apical and basolateral membrane of the epithelial cells secondarily creates an electropositive lumen which constitutes a driving force for the paracellular movement (reabsorption) of calcium and magnesium. The renal isoform of the Na-K-2Cl cotransporter (NKCC2) is expressed in the cells of the TAL, the macula densa, and a short post-macula segment at the transition to the DCT (138, 203). The combination of NKCC2 and a K+ channel (ROMK) on the apical membrane with the basolateral expression of Na+/K+ ATPase and a chloride channel (ClC-Kb) in the TAL epithelium results in net Na+ and Cl− absorption (253). Indeed, in the conventional model of a TAL epithelial cell (see Figure 14), the apical NKCC2 provides the entry mechanism for Na+, K+, and Cl−. The apical ATP-sensitive ROMK recycles K+ and creates an electropositive lumen. The activity of the basolateral Na+/K+-ATPase constitutes the exit mechanism for Na+ and provides the energy for the trans-epithelial Na+ movement. The basolateral Cl− channel, which constitutes the exit mechanism for Cl−, creates an electronegative serosal side. The trans-epithelial potential drives the movement of divalent cations from urine to blood. Improper salt and acid-base homeostasis in the TAL results in Bartter syndrome, a salt-wasting disorder characterized by hypokalemia (low potassium levels due to increased delivery of Na+ to the collecting ducts, which results in increased K+ excretion), increased blood pH (alkalosis), and normal to low blood pressure (15, 221).
Figure 14. Model of salt reabsorption in TAL and DCT.
Schematic representation of the nephron showing glomerulus, proximal tubule (PT), Thick Ascending Limb (TAL), Loop of Henle, Distal Convoluted Tubule (DCT), and Collecting Duct (CD). Model of TAL epithelial cell showing major transporters (NKCC2, ROMK, Na+/K+-ATPase, Cl− channel) involved in salt reabsorption. OSR1 is illustrated as monomers [1], heterodimers [2], and heterotrimeric complexes with a yet to be identified upstream kinase [3]. Model of DCT epithelial cell showing major transporters (NCC, Na+/K+-ATPase) involved in salt reabsorption. SPAK is illustrated as monomers [4], homodimers [5], heterodimers [6], and heterotrimeric complexes [7].
Genetically modified OSR1 mice
As both SPAK and OSR1 are expressed in the TAL, the idea that OSR1 plays a primary role in modulating Na+ reabsorption in this segment comes from genetically modified mouse studies. Indeed, disruption of the SPAK gene was shown to primarily affect the DCT segment (see DCT section, below), and semi-quantitative western blotting and immunofluorescent staining of kidney tissues from these mice demonstrated robust OSR1 expression in the TAL (327). Two independent homozygous OSR1 knockout mice (58, 164) and a homozygous OSR1-T243A knock-in mouse (231) were created and shown to be embryonically lethal (see section III, sub-section E). Genetic analysis of embryos of different stages indicated that the OSR1−/− mice failed to thrive between e10.5 and e13.5 (164). Crossing of a kidney-specific Cre recombinase transgenic mouse with an OSR1 floxed mouse generated a viable kidney-specific (KSP) homozygous OSR1 knockout mouse (164). The KSP-OSR1−/− mouse exhibited Bartter-like syndrome with normal blood pressure, significant hypokalemia, and hypercalciuria. When these KSP-OSR1−/− mice were fed a low Na+ diet, they presented with a systolic hypotension and significantly more Na+ excretion in their urine, indicating markedly reduced Na+ absorption in the TAL. While already hypokalemic, when fed a low K+ diet, these mice exhibited a more pronounced hypokalemia and increased fractional potassium excretion (164).
The Bartter-like syndrome phenotype displayed by the KSP-OSR1−/− mice prompted further examination for both total and phosphorylated levels of OSR1, SPAK, and NKCC2 expression in the TAL. As expected, total OSR1 and phosphorylated OSR1 were absent. The level of phosphorylated NKCC2 was dramatically reduced without a parallel reduction in the level of total cotransporter expression indicating that OSR1 is the predominating Ste20-related kinase in the TAL (164). In contrast, compared to wild-type controls, the expression of phosphorylated forms of both OSR1 and NKCC2 in the kidney tissue lysate of SPAK+/− mice was significantly increased ((327), discussed in detail in the DCT section below).
Milan Hypertensive Rat
The Milan hypertensive rat strain (MHS) develops hypertension as a result of changes in renal tubular Na+ reabsorption (20). These rats have a point mutation in adducin (287) which alters the plasma membrane turnover of the Na+/K+ ATPase and increases the Na+ reabsorption in renal tubule cells (80, 183). Valenti and co-workers found a 58% increase in phosphorylated renal NKCC2 (without an increase in total NKCC2 protein abundance) in MHS hypertensive versus age-matched normotensive rats (32). In addition, there was a clear increase in phosphorylated SPAK immunoprecipitated from MHS renal extracts. Immunofluorescent staining demonstrated a co-localization of phosphorylated SPAK and NKCC2 in the TAL. Chronic exposure to the loop diuretic furosemide reduced the systolic blood pressure of MHS rats, indicating NKCC2 activity contributes to the observed hypertension in these rats (32).
SPAK and OSR1 expression in the mammalian kidney
In order to examine the expression levels of SPAK and OSR1 in the mouse kidney, Alessi and co-workers generated novel antibodies to both kinases and demonstrated specificity for their target epitopes by immunoblot and immunoprecipitation with negligible cross-reactivity. The distribution of SPAK in the nephron was found to be restricted to the medullary and cortical TAL, as well as the DCT. Although, western blots for OSR1 in kidney and testes lysates only had a single band, several bands were observed with an antibody against SPAK (231). Mass-spec analysis of these multiple bands has verified that they all contain portions of the regulatory domain of SPAK. Curiously, tryptic digest of each band never identified the PAPA box region or specific portions of the catalytic domain indicating that mass-spec analysis provides only partial coverage of each fragment and therefore the precise identity/composition of each band/fragment is unknown. Another study demonstrated that a shortened form of SPAK which lacked a catalytic domain is highly expressed in the TAL (182). As mentioned above, lack of OSR1 expression in the TAL significantly reduced the phosphorylation level of NKCC2 in the TAL (164). In the McCormick study, the kidney-specific short form of SPAK exerted a dominant-negative effect on SPAK/OSR1 phosphorylation of NKCC2 in the TAL (182). This potentially explains both the reduced NKCC2 phosphorylation observed in the KSP-OSR1−/− mouse and the increased phosphorylation of the TAL cotransporter observed in the SPAK−/− mouse (see Figure 14). Also in agreement with this hypothesis, is the absence of NKCC2 over-phosphorylation in the SPAK T243A mouse, which can be explained by the normal expression of SPAK fragments in this mouse model (231).
In addition to the Ste20-related kinases, epithelial cells of the TAL, DCT, and collecting duct were shown to also express a sorting protein-related receptor with A-type repeats (SORLA) by immunohistochemistry and in situ hybridization (237). Using a SORLA−/− mouse generated in an earlier study (7), Reiche and co-workers found that although the volume of urine was the same in wild-type and knockout mice, the SORLA−/− mice had a greater fractional excretion of K+ when adequately hydrated, and excretion of Na+ and Cl− was significantly greater when the mice were on water restriction. However, over a 24 h period, the total amount of NaCl excreted was not different between free water and water restriction conditions in knockout animals, indicating impairment of NaCl reabsorption (237). Western blotting demonstrated only a moderate increase in total NCC, ENaC, and NHE expression, but a very significant increase in their level of phosphorylation (activation). However, the principal cotransporter in the TAL (NKCC2) lacked any evidence of phosphorylation with normal abundance levels of the protein. As SORLA is a trafficking protein (7), and a functional interaction with SPAK was demonstrated by immunoprecipitation from transfected HEK293 cells, the authors suggested that loss of cotransporter activity was a result of mistrafficking of SPAK by SORLA (237). Western blotting of renal extracts from SORLA−/− mice exhibited a massive increase in phosphorylated SPAK (without a concomitant increase in total protein abundance), and immunohistological staining showed apical co-localization of the phosphorylated SPAK with NKCC2 in the TAL (237). These results coincide with other studies (164, 182) suggesting that NKCC2 activity in the TAL is predominately regulated by OSR1 phosphorylation. However, if SORLA caused SPAK to be >trapped= in vesicles away from the apical membrane (and NKCC2) then the logical expectation would be an increase in OSR1 phosphorylation of NKCC2. One possibility is that mistrafficking and/or activation of SPAK affects OSR1 activation of NKCC2, ultimately leading to reduced TAL-mediated NaCl reabsorption and therefore increased salt excretion. Based on experimental data from the kidney-specific OSR1 knockout and the global SPAK knockout mice models, we have summarized the regulation of NKCC2 in TAL in Figure 14. We propose that OSR1 forms a domain-swapped dimer, which when activated by a yet to be identified upstream kinase, binds, phosphorylates, and activates NKCC2. Expression of inactive SPAK protein fragments might inhibit this process by competing with active OSR1 for binding sites on the cotransporter. Alternatively, inactive SPAK fragments might inhibit OSR1 function through possible kinase hetero-dimerization.
Distal Convoluted Tubule (DCT)
The Na-Cl cotransporter (NCC) is expressed along the entire DCT segment beginning beyond the post-macula segment expressing NKCC2 and ending at the transition point into the connecting tubule (202, 218, 227, 295). In the conventional model of a DCT epithelial cell (see Figure 15), apical expression of NCC provides the entry mechanism for Na+ and Cl− and basolateral expression of the Na+/K+-ATPase is the exit mechanism for Na+ and the driving force for Na+ reabsorption. Gitelman syndrome is a salt-wasting disorder characterized by reduced K+ levels and reduced pH in the blood (hyperkalemic alkalosis), low Mg2+ levels in the blood (hypomagnesemia), and diminished level of Ca+2 in the urine (hypocalciuria) (110, 258). Pseudohypoaldosteronism type II (PHA type II or Gordon syndrome) is an autosomal-dominant disorder also featuring hyperkalemia and hypertension (310). Because the PHA2 disorder can be treated with thiazide diuretics (180), the deficit likely originates from changes in Na+ transport in the DCT. While Gitelman syndrome is due to mutations in NCC, PHAII is due to deletions in intronic regions of WNK1 or in single amino acid substitutions in WNK4. Because of embryonic lethality, WNK1’s role in modulating salt reabsorption could not be assessed in WNK1 genetically-modified animals. Three of the four PHA type II-linked mutations in WNK4 are found in a highly conserved negatively-charged region downstream of the catalytic domain. In contrast, the role of WNK4 has been studied using different genetically-modified mouse models. First, transgenic mice carrying two wild-type and two Q562E (PHAII) copies of WNK4 demonstrated a PHAII phenotype characterized by high blood pressure, hyperkalemia, hyperchloremia, hypercaliuria, and metabolic acidosis (151). Genetic engineering of a WNK4D561A/+ knock-in mouse also resulted in hypertension, hyperkalemia, and metabolic acidosis (208, 328). A significant increase in the phosphorylation of OSR1, SPAK and NCC was observed in this mouse, indicating enhanced salt reabsorption. In a third study, Uchida and co-workers generated a WNK4 hypomorphic mouse by deleting exon 7 through CRE recombination (208). As expected, reduced expression of wild-type WNK4 resulted in a significant reduction in the phosphorylation of SPAK/OSR1 and NCC. Physiologically, these mice exhibit low blood pressure with increased Na+ and K+ excretion without hypokalemia or metabolic alkalosis when maintained on a low salt diet (208). The resulting increase in Na+ delivery to the collecting duct might explain the increased K+ secretion, however, it is unclear why the blood K+ levels remained constant. The observed decrease in SPAK/OSR1 and NCC phosphorylation in these mice were consistent with the WNK4-SPAK/OSR1-NCC signaling pathway demonstrated in previous in vitro or heterologous expression studies (190, 240). Phosphorylation occurs on several threonine residues that are conserved among the Na+-dependent branch of cation-chloride cotransporters (94, 109, 213, 239). The fourth mouse model generated was a straight WNK4 knockout mouse (33). As anticipated from the data obtained with the PHAII-WNK4 mice, WNK4 null mice developed mild hypokalemia, hypochloremia, metabolic alkalosis, and hypomagnesemia. However Ca2+ excretion was unchanged and there was no significant blood pressure phenotype. (33). The increased NCC function observed in both the transgenic PHAII mouse and the WNK4D561A/+ knock-in mouse was accompanied by an increased transporter density on the apical membrane. The precise mechanism by which SPAK/OSR1 phosphorylation of NCC affects its activity is still unknown. In addition, the mechanism by which WNK4D561A/+ increases phosphorylation of SPAK/OSR1 is also unclear, but the observed phenotype of the knock-in shares many clinical features with PHA type II patients. One possibility is that the regulatory domain of the mutant WNK4 (D561A) might have a dominant-negative effect on wild-type WNK4, thus PHA type II mutations would negate this inhibition and result in stimulation of the WNK-SPAK-NCC signaling cascade. Alternatively, WNK4 could bind to NCC and affects its trafficking (250) in a manner similar to Nedd-4 affecting the trafficking of ENaC (136, 336). In this scenario, PHA type II mutations would disrupt the WNK4-NCC interaction, allow for increased surface expression, and release WNK4 protein to now interact with the Ste20-related kinase.
Figure 15. Regulation of blood volume through distal Na+ reabsorption and blood vessel constriction.
Mild hypovolemia through decreases in the glomerular filtration rate (GFR) results in activation of the renin-angiotensin-aldosterone system. Aldosterone, which is released by the adrenal gland, activates ENaC in the collecting duct and through WNK4/SPAK, also activates the Na-Cl cotransporter in the distal convoluted tubule. AngII activates NCC through SPAK/OSR1, and also affects contractility of blood vessels. Severe hypovolemia (or small rises in plasma osmolarity) leads to the release of arginine-vasopressin (AVP or ADH) from the pituitary gland, resulting in the constriction of blood vessels, insertion of water channels in collecting duct epithelial cells, and activation of NCC (blue arrows).
Experiments performed in Xenopus laevis oocytes have shown that the WNK4 inhibition of NCC function is reversed by angiotensin II (Ang II), a SGK phosphorylation-independent mechanism (250). The use of a catalytically-inactive SPAK (K104R) or a mutant form of NCC lacking a SPAK binding motif confirmed that the Ang II reversal of NCC inhibition by WNK was mediated by SPAK. The two possible mechanisms of WNK4 on NCC (see above) suggest that when WNK4 is inactive, it interacts with NCC directly and negatively affects its function. Alternatively, when activated by Ang II, WNK4 activates SPAK, which in turn phosphorylates and activates NCC (see Figure 15). The effect of Ang II on NCC phosphorylation, verified in mpkDCT cells, was transient and not mediated by aldosterone (250, 275). In contrast, in vivo infusion of eplerenone, an aldosterone receptor blocker, inhibited the long-term effect of Ang II on NCC phosphorylation (275). A detailed study using adrenalectomised rats treated with aldosterone or AngII at low and high concentrations confirmed that both aldosterone and AngII activate NCC through distinct signaling pathways (292). Under Ang II treatment, the increased phosphorylation was seen on NCC residue T53, whereas under aldosterone treatment, it was on residue T58. These data indicate either that separate kinases are involved in phosphorylation of T53 and T58, or that SPAK distinguishes between these residues and phosphorylates them distinctly, depending upon specific signaling cascades. The complex signaling pathways involving WNK/SPAK/OSR1 kinases under high AngII and/or aldosterone states and their effects on the DCT and CD are nicely discussed in relationship to Na+ and K+ handling in different clinical situations in a review by Arroyo and Gamba (10).
Experiments published in 2008 demonstrated that dietary salts (through aldosterone) affected phosphorylation of SPAK and OSR1 and the Na-Cl cotransporter (38). As anticipated, based on our knowledge of kidney physiology, a low Na+ diet significantly increased phosphorylation of SPAK and OSR1, whereas a high Na+ diet resulted in decreased phosphorylation of the kinases. Note that whole kidney lysates were used to assess these phosphorylation levels. The increased phosphorylation levels in the WNK4D561A/+ knock-in mice were, however, insensitive to dietary salt. Abundance and phosphorylation of NCC was also increased in wild-type mice maintained on a low Na+ diet, and elevated (but unregulated) by salts in WNK4D561A/+ knock-in mice. The mice fed with a high Na+ diet demonstrated low aldosterone, and were therefore infused with aldosterone to correct the hormone imbalance. In this condition, the levels of SPAK, OSR1, and NCC function were increased. These data confirm that the salt effect is through the mineralocorticoid hormone.
In addition to aldosterone and angiotensin II, arginine-vasopressin (AVP) is also known to regulate salt retention. AVP is best known for triggering apical membrane insertion of aquaporin-2 in CD epithelial cells facilitating water retention (79). Chronic AVP exposure increases Na+ uptake through NKCC2 (144, 145), NCC (192, 218), and ENaC (69). Using phosphospecific antibodies, Fenton and co-workers demonstrated exclusive staining of phosphorylated NCC at the apical membrane of DCT cells. Acute and chronic treatment of AVP-deficient Brattleboro rats with the V2 receptor-selective vasopressin analog (dAVP) increased the level of phosphorylated NCC two- to three-fold at the apical membrane with no increase in total NCC expression or alteration in the distribution in the DCT. Consistent with other studies, an increased level of NCC phosphorylation was mirrored by increased phosphorylation of SPAK/OSR1 following dAVP treatment (see Figure 15) (218).
Renal sodium reabsorption is also known to be regulated by insulin infusion (54, 281). Uchida and co-workers stimulated mpkDCT cells with insulin and observed a significant dose-dependent increase in both SPAK and NCC phosphorylation. Following intraperitoneal injection of insulin, C57BL/6 mice, maintained on a normal salt diet, exhibited an increase in phosphorylation of SPAK/OSR1 and NCC; however the stimulatory effect of insulin was enhanced in these same mice maintained under a high salt diet (261). Confirmation that insulin was phosphorylating NCC through the WNK4-SPAK signaling cascade was demonstrated by suppression of the insulin effect using siRNA knockdown of WNK4 or SPAK in mpkDCT cells, as well as, SPAK knockout mice and WNK4 hypomorphic mice (261).
Work discussed thus far has demonstrated that SPAK and possibly OSR1 are intermediates between WNK kinases and NCC function. As WNK1, WNK4, and NCC are involved in the control of blood pressure, it seems legitimate to assume that SPAK is also key to blood pressure regulation.
Genome-wide association studies
An Amish Family Diabetes Study that provided a strong genetic association between hypertension and the STK39 gene indicates that SPAK is involved in modulating blood pressure (304). The association was replicated in another Amish and four non-Amish Caucasian cohort studies, reaching a significance level (p < 10−6) in all five studies (n = 7125). Among the 5 polymorphisms that were identified within the associated region of the STK39 gene, rs35929607 A/G variants demonstrated an effect in a luciferase reporter gene assay. The rs35929607 A/G polymorphism was also genotyped in two cohorts of Swedish patients. After stratification for gender, only female carriers of the G-allele exhibited higher systolic and diastolic blood pressure in the cardiovascular cohort (5634 patients) of the >Malmo Diet and Cancer-cardiovascular arm= (MDC-CVA) study but not the >Malmo Preventive Project= (MPP) (77). Although a statistically-significant association was not observed in the MPP cohort, when the data were stratified by gender, again female carriers of the G-allele had a higher prevalence of hypertension. Conversely, the gender differences in Swedish and Chinese cohorts are opposite, as association between two SNPs in the STK39 gene and hypertension was only observed in Han Chinese males but not females (35). Note that no association between blood pressure and risk variants in STK39 (rs6749447, rs3754777, rs35929607) was found in 1372 members from 247 British-Caucasian families (44). As SPAK is a regulator of NCC, we can assume that the STK39 association with high blood pressure is related to NCC function. Unfortunately, no association was found between a large number of SNPs located within the STK39 gene and individuals with good versus poor responses to hydrochlorothiazide treatment (66). In a recent genome-wide association study of African Americans, none of the STK39 SNPs examined were associated with the systolic and diastolic blood pressure phenotypes that were previously reported in other populations (143). Therefore, in the absence of a clear mechanistic relationship between specific STK39 variants and SPAK targets that influence blood pressure, these genome-wide associations studies have to be very cautiously interpreted.
Genetically modified SPAK mice
A second indication that SPAK is involved in modulating blood pressure comes from SPAK knockout (182, 327) and SPAK T243A knock-in mice (231). Indeed, absence of SPAK expression in the DCT leads to a phenotype that is similar to Gitelman(s syndrome, i.e. hypotension with mild hypokalemia, hypomagnesemia, hypocalciuria, and secondary hypoaldosteronism. Some differences exist between SPAK knockout mice generated by two different groups (182, 327). While blood pressure differences were observed under a normal diet in one model (327), it was only under dietary NaCl restriction that hypotension was observed in the other model (182). There were also significant differences between the blood aldosterone levels between wild-type and SPAK knockout mice in the two studies. Indeed, Lin and co-workers demonstrated a significant increase in plasma aldosterone (327), whereas Ellison and co-workers observed no changes in aldosterone levels (182). While Lin and co-workers did not report renin levels, these levels were shown to be elevated in the McCormick study. While a trend towards hypokalemia was observed in both models, this trend was clearly exacerbated under dietary salt restriction. A much different observation was made with NCC knockout mice which maintained their blood K+ levels under both normal diet and dietary salt restriction (252). Finally, the SPAK (T243A) knock-in mouse exhibited a significant decrease in blood pressure with no changes in plasma aldosterone or K+ levels when fed a normal salt diet. Relative hypokalemia was only observed in these mice after Na+ restriction (231). As anticipated for a Gitelman-like phenotype, along with a reduction in total NCC expression, phosphorylation of NCC was greatly reduced in SPAK knockout mice (50% reduction on T53 and 75% reduction on T58 (327)). An interesting difference between the SPAK knockout mouse and the SPAK (T243A) knock-in mouse revolves around the phosphorylation status of NKCC2 in the TAL. Indeed, as briefly discussed in the TAL section, there is published evidence from two distinct groups showing increased phosphorylation of NKCC2 in SPAK knockout mice (182, 327); whereas the phosphorylation state of the cotransporter remained unchanged in the SPAK (T243A) knock-in mice. As the major difference between these two animal models is the presence (knock-in) or absence (knockout) of protein, and Western blot analysis of the SPAK knock-in mouse revealed expression of small SPAK fragments in kidney medulla, it was postulated that SPAK might negatively regulate OSR1 function in this kidney segment (182). This view is consistent with the primary role of OSR1 in this segment, as demonstrated by the phenotype of the kidney-specific OSR1 knockout mouse, and the fact that the small ‘inhibitory’ SPAK fragments would be present in the knock-in, but absent in the knockout. Differential levels of NKCC2 phosphorylation might explain the difference in Ca2+ excretion between the knockout and knock-in animal models.
While there were no noticeable differences in the appearance of DCT cells in the SPAK (T243A) knock-in mice (231), a significant loss in DCT mass was observed in the SPAK knockout mouse (182). This loss of mass might explain the reduction in total NCC expression observed in these mice. The difference between the knock-in and knockout mice indicates that the presence of the protein, even if inactive, plays a role in the morphology of the distal convoluted tubule segment. This observation agrees with previous observations that link the function of NCC with hypo- or hypertrophy of the DCT (151). Indeed, transgenic mice over-expressing wild-type WNK4 (which reduces NCC function) demonstrate a smaller DCT, whereas, transgenic mice overexpressing a PHA II mutant of WNK4 (which increases NCC function) exhibit a larger DCT. However, the precise mechanism by which this DCT rearrangement occurs is not yet fully understood.
As discussed previously, administration of furosemide in SPAK−/− mice result in significant increases in urine output and fractional excretion of Na+ and Cl−. In order for there to be a significant loop diuretic effect, there must have been intact NKCC2 function (i.e. OSR1 phosphorylation of the cotransporter), supporting the view that SPAK is not the kinase that primarily regulates NKCC2 activity in the TAL (327). Although it is difficult to assess the relative expression of SPAK and OSR1 in the different kidney segments due to different properties of individual antibodies, it is however possible to compare the expression levels of SPAK in the membrane of the TAL and DCT within one tissue section. Immunofluorescence studies using a rabbit anti-SPAK polyclonal antibody demonstrated modest expression of full-length SPAK in the TAL compared to the DCT, consistent with the DCT-driven phenotype of the SPAK knockout mouse (182). This observation agrees with the primary role of SPAK in regulating NCC function in the distal convoluted tubule. Thus, in our DCT model (see Figure 14), we propose that SPAK forms domain-swapped dimers which are activated by WNK4, and that SPAK is the major kinase which binds, phosphorylates, and activates NCC. OSR1 is also expressed, although at much lower levels, and might also form heterodimers with SPAK. Alternatively, WNK4 might form a complex with SPAK at the apical membrane cotransporter, instead of shuffling between WNK4 in the cytosol and the cotransporter at the plasma membrane.
F. Role in Cancer Proliferation and Migration
Cancer is characterized by aberrant cell proliferation and migration. The cation-chloride cotransporters have been linked to rapid cell volume changes and maintenance of cell homeostasis (for reviews see (2, 98)). The functional regulation of these transport proteins involves both serine-threonine kinases and protein phosphatases (for review, see (99)). The coordinated effort of cytoskeletal formation and cell volume regulation are responsible for extension and retraction of the lamellipodia of migrating cells (267). As a result, several studies have linked the expression of different cotransporters and their regulatory kinases to malignant cancers (73, 114, 255, 305).
Glioblastomas
Brain tumors that originate from glial cells are primary cancers that are challenging to treat. Unlike other cancers, gliomas do not metastasize through the bloodstream but instead invade and migrate into neighboring brain parenchyma along white matter tracts and blood vessels (108). In order to migrate through narrow extracellular spaces, gliomas modify their cell volume by releasing K+ and Cl− with osmotically obligate water (262, 263). Because of their homeostatic role in cell volume, cation-chloride cotransporters are primary candidates for promoting volume changes associated with cell migration. Multiple K-Cl cotransporter isoforms (1, 3, and 4) have been identified and functionally characterized in a rat glioma cell line (88). Furthermore, inhibition of K-Cl cotransporter activity in these cells completely abrogated regulatory volume decrease (242). Several studies have also demonstrated an integral role for NKCC1 in glioma cell malignancy (72, 114, 115). As the activity of cation-chloride cotransporters is tightly regulated by phosphorylation, it would be of interest to know if cotransporter regulatory kinases are also affected in glioblastomas. Comparisons between malignant brain tumors and control samples showed decreased SPAK and WNK2 and increased WNK3 protein expression (113). A recent study demonstrated that siRNA knockdown of WNK3 in human D54 glioma cells blunted their invasive ability across Transwell barriers, and eliminated bumetanide-sensitive cell migration indicating that WNK3 mediated its effect through the Na-K-2Cl cotransporter (113).
Lymphomas
Dysregulated expression of elements which control tumor-promoting genes in healthy lymphocytes give rise to leukemias and lymphomas. B cell lymphomas, the most frequent lymphocyte malignancy, is characterized by reciprocal chromosome translocation, DNA hypermethylation, and aberrant histone modifications (29, 41, 149, 256). In 2009, hyper-methylation and repressed SPAK expression levels were found in B cell lymphomas. It was also shown that caspase-dependent apoptosis was impaired when SPAK expression was genetically knocked down, indicating that the loss of SPAK might promote increased cell survival and increased resistance to cancer (14).
Lung Cancer
Non-small-cell lung cancers have a 40% – 67% five-year survival rate if caught in the earliest stage of the disease. In a genome-wide analysis of non-small-cell lung tumor tissue performed in patients from Massachusetts General Hospital, and validated in a separate Norwegian cohort, single nucleotide polymorphisms (SNPs) were investigated for their potential prognostic value of overall survival. Of the 74,666 SNPs analyzed, the most significant SNPs were located within introns of serine/threonine kinase 39 (STK39), protocadherin 7 (PCDH7), ataxin 2-binding protein 1 (A2BP1), and eyes absent homolog 2 (EYA2) (125). The SNPs from the STK39 gene were identified as rs10176669 and rs4438452, and were both located within intron 1. However, the study did not determine if SPAK expression was different in these genetic variants.
Prostate Cancer
Androgens play a critical role in the normal development and physiology of the prostate gland (43, 52). Transcription factors, proteases, cyclin-dependent kinases, calcium binding proteins, insulin-like growth factor binding proteins, metabolic enzymes, lipid signaling, and ion homeostasis are just some of the androgen-responsive elements identified in human prostate cancer cells (for review, see (147)). For the purpose of this review, SPAK expression was shown to be significantly increased in a human prostate cancer cell line treated with synthetic androgen R1881 (230). The Ste20-related kinase was identified as part of a cDNA subtraction screen designed to identify novel androgen-responsive genes. The effects of the synthetic androgen on SPAK were dose- and time-dependent, and required both the androgen receptor and protein synthesis. Genistein, a prostate cancer chemo-preventive agent, strongly inhibited SPAK gene expression along with the expression of the androgen-responsive prostate-specific antigen (PSA) (274). Although SPAK expression is modulated by the activity of the androgen-receptor, SPAK is not involved in the phosphorylation/activation of p53, a downstream mediator of androgen/androgen receptor function (244). These data indicate that the kinase might have another role than participating in the response to genotoxic stress. Additionally, reduced SPAK mRNA expression in primary prostate tumors was correlated with a higher incidence of metastases after radical prostatectomy (116).
Breast Cancer
Although there are hundreds of studies demonstrating involvement of kinase activity in breast cancer, only one very recent study has implicated Ste20-related kinase activity. Fra-1 is a member of the Fos protein family which heterodimerizes with Jun family members to form the activating protein 1, a transcription factor which has many important roles in cell growth and tumorigenesis (254). High levels of hyperphosphorylated Fra-1 were found in invasive breast cancer cell lines. Chalbos and coworkers demonstrated that stabilization of Fra-1 by serine/threonine phosphorylation involved PKC theta activity through ERK1/2 and SPAK (16).
VII. QUESTIONS REMAINING TO BE ANSWERED
Although a significant volume of work has accumulated in the fifteen years since the discovery of the mammalian Ste20-related kinases, SPAK and OSR1, there still remain many unanswered questions. Despite our knowing that the WNK kinases are upstream activators of SPAK/OSR1, we are missing the precise details of the signaling cascade. Are all WNK kinases affecting SPAK and OSR1 similarly? Are there any other kinases that fulfill similar roles? We know that after WNK kinases bind to a hydrophobic pocket in the conserved carboxyl terminal domain, they phosphorylate SPAK/OSR1. However, what is still not clear is what happens to SPAK/OSR1 immediately after this phosphorylation event. Crystallographic studies have demonstrated a dimer-swapping of portions of the activation loop. Does this occur before, after, or as a result of WNK phosphorylation? Furthermore, what is the state of WNK kinases after binding and release from SPAK/OSR1. Does the binding/activation of SPAK/OSR1 result in the WNK kinase transitioning to an inactive state, or is it still active and able to bind to another inactive SPAK/OSR1?
It will also be critical to address the role of the PF2-like domain in WNK4. Is this domain only involved in auto-inhibition of the kinase, or is this domain also interacting with RFxV peptides in other proteins? If the latter is true, can the cotransporters or other SPAK targets directly bind the WNK kinases, and what is the functional significance of these interactions?
Several studies have examined the tissue distribution of SPAK/OSR1. In most cases, the expression of these kinases appears to co-localize. Recent studies have demonstrated that SPAK and OSR1 are capable of substituting for each other in the peripheral nervous system (105). However, despite co-expression in the TAL and DCT, they appear to have distinct regulatory targets in the distal nephron. Although in vitro kinase assays and mass-spec studies have demonstrated phosphorylation of specific threonine and serine residues in the catalytic and regulatory domain, respectively, we know there are other residues which could be phosphorylated and affect SPAK/OSR1 function. The identity of the upstream kinase(s) which target these residues is currently unknown, as are the downstream effects of these phosphorylation events. A recent study from our laboratory demonstrated that phosphorylation of the regulatory domain serine residue in mouse SPAK (S383), necessary for kinase activation, could be “replaced” by co-expression with Cab39. The precise nature of how Cab39 achieves this is still unknown.
Finally, the majority of research on these Ste20-related kinases has focused on their regulation of cation-chloride cotransporter activity. However, in 2007, we performed an in silico search for all mouse proteins containing variations of the RFxV binding motif. When we searched the mouse proteome for an extended motif containing [S/G/V]RFx[V/I]xx[V/I/T/S]xx, we found 131 proteins with at least one putative binding motif suggesting that they may be either upstream activators or downstream targets of SPAK/OSR1. Of course by substituting a lysine residue for the arginine in the first position, or adding a leucine residue in the fourth position with [V/I] would expand this list of targets even more. Clearly, SPAK and OSR1 have a significant role in ion transport regulation, but with this expansive list of putative interactors, the importance of these GCK VI kinases is far from complete.
ACKNOWLEGEMENTS
Support for the authors’ research cited in this review came from the National Institute of Neurological Disease and Stroke grant NS36758, the National Institute of General Medical Sciences grant GM74771, American Heart Association Grant-in-Aid 0250101N, and American Heart Association Southeast Affiliate fellowship grant 0625326B.
REFERENCES
- 1.Adams JA. Kinetic and catalytic mechanisms of protein kinases. Chem Rev. 2001;101:2271–2290. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]
- 2.Adragna NC, Fulvio MD, Lauf PK. Regulation of K-Cl cotransport: from function to genes. J Membr Biol. 2004;201:109–137. doi: 10.1007/s00232-004-0695-6. [DOI] [PubMed] [Google Scholar]
- 3.Ahlstrom R, Yu AS. Characterization of the kinase activity of a WNK4 protein complex. Am J Physiol Renal Physiol. 2009;297:F685–F692. doi: 10.1152/ajprenal.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez-Leefmans FJ. Chloride transporters in presynaptic inhibition, pain and neurogenic inflammation. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporter and Channels in the Nervous System: From molecules to diseases. London: Academic Press; 2009. pp. 439–470. [Google Scholar]
- 5.Alvarez-Leefmans FJ, Gamiño SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurons studied with ion-selective microelectrodes. J Physiol (Lond) 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andermann F, Andermann E, Joubert M, Karpati G, Carpenter S, Melancon D. Familial agenesis of the corpus callosum with anterior horn cell disease: a syndrome of mental retardation, areflexia and paraparesis. Trans Amer Neurol Ass. 1972;97:242–244. [Google Scholar]
- 7.Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CA, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci USA. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol Cell. 2006;22:795–806. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Arion D, Lewis DA. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen Psychiatry. 2011;68:21–31. doi: 10.1001/archgenpsychiatry.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arroyo JP, Gamba G. Advances in WNK signaling of salt and potasssium metabolism - clinical implications of basic findings. Am J Nephrol. 2012 doi: 10.1159/000337479. In press. [DOI] [PubMed] [Google Scholar]
- 11.Austin TM, Delpire E. Inhibition of KCC2 in mouse spinal cord neurons leads to hyper-sensitivity to thermal stimulation. Anesth Analg. 2011;113:1509–1515. doi: 10.1213/ANE.0b013e31822e0a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aye TT, Scholten A, Taouatas N, Varro A, Van Veen TA, Vos MA, Heck AJ. Proteome-wide protein concentrations in the human heart. Mol Biosyst. 2010;6:1917–1927. doi: 10.1039/c004495d. [DOI] [PubMed] [Google Scholar]
- 13.Baker SJ, Sumerson R, Reddy CD, Berrebi AS, Flynn DC, Reddy EP. Characterization of an alternatively spliced AATYK mRNA: expression pattern of AATYK in the brain and neuronal cells. Oncogene. 2001;20:1015–1021. doi: 10.1038/sj.onc.1204209. [DOI] [PubMed] [Google Scholar]
- 14.Balatoni CE, Dawson DW, Suh J, Sherman MH, Sanders G, Hong JS, Frank MJ, Malone CS, Said JW, Teitell MA. Epigenetic silencing of Stk39 in B-cell lymphoma inhibits apoptosis from genotoxic stress. Am J Pathol. 2009;175:1653–1661. doi: 10.2353/ajpath.2009.090091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartter FC, Pronove P, Gill JRJ, Maccardle RC. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. Am J Med. 1962;33:811–828. doi: 10.1016/0002-9343(62)90214-0. [DOI] [PubMed] [Google Scholar]
- 16.Belguise K, Milord S, Galtier F, Moquet-Torcy G, Piechaczyk M, Chalbos D. The PKCΘ pathway participates in the aberrant accumulation of Fra-1 protein in invasive ER-negative breast cancer cells. Oncogene. 2012 doi: 10.1038/onc.2011.659. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol (London) 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergaya S, Faure S, Baudrie V, Rio M, Escoubet B, Bonnin P, Henrion D, Loirand G, Achard JM, Jeunemaitre X, Hadchouel J. WNK1 regulates vasoconstriction and blood pressure response to a 1-adrenergic stimulation in mice. Hypertension. 2011;58:439–445. doi: 10.1161/HYPERTENSIONAHA.111.172429. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi G, Baer PG, Fox U, Duzzi L, Pagetti D, Giovannetti AM. Changes in renin, water balance, and sodium balance during development of high blood pressure in genetically hypertensive rats. Circ Res. 1975;36:153–161. doi: 10.1161/01.res.36.6.153. [DOI] [PubMed] [Google Scholar]
- 21.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu Rev Biochem. 2003;72:291–336. doi: 10.1146/annurev.biochem.72.121801.161720. [DOI] [PubMed] [Google Scholar]
- 22.Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, Lemcke B, Horst J, Leuwer R, Pape HC, Volkl H, Hubner CA, Jentsch TJ. Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. Embo J. 2003;22:5422–5434. doi: 10.1093/emboj/cdg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, Prescott AR, Clevers HC, Alessi DR. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. Embo J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudeau J, Scottm JW, Restam N, Deak M, Kieloch A, Komander D, Hardie DG, Prescott AR, van Aalten DM, Alessi DR. Analysis of the LKB1-STRAD-MO25 complex. J Cell Sci. 2004;117:6365–6375. doi: 10.1242/jcs.01571. [DOI] [PubMed] [Google Scholar]
- 25.Boyce KJ, Andrianopoulos A. Ste20-related kinases: effectors of signaling and morphogenesis in fungi. Trends Microbiol. 2011;19:400–410. doi: 10.1016/j.tim.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Brillault J, Lam TI, Rutkowsky JM, Foroutan S, O'Donnell ME. Hypoxia effects on cell volume and ion uptake of cerebral microvascular endothelial cells. Am J Physiol Cell Physiol. 2008;294:C88–C96. doi: 10.1152/ajpcell.00148.2007. [DOI] [PubMed] [Google Scholar]
- 27.Bu Z, Callaway DJ. Proteins move! Protein dynamics and long-range allostery in cell signaling. Adv Protein Chem Struct Biol. 2011;83:163–221. doi: 10.1016/B978-0-12-381262-9.00005-7. [DOI] [PubMed] [Google Scholar]
- 28.Cai H, Cebotaru V, Wang YH, Zhang XM, Cebotaru L, Guggino SE, Guggino WB. WNK4 kinase regulates surface expression of the human sodium chloride cotransporter in mammalian cells. Kidney Int. 2006;69:2162–2170. doi: 10.1038/sj.ki.5000333. [DOI] [PubMed] [Google Scholar]
- 29.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, Suddason T, Lord G, Atkinson JP, Cope A, Hayday A, Kemper C. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmosino M, Rizzo F, Ferrari P, Torielli L, Ferrandi M, Bianchi G, Svelto M, Valenti G. NKCC2 is activated in Milan hypertensive rats contributing to the maintenance of salt-sensitive hypertension. Pflügers Arch Eur J Physiol. 2011;462:281–291. doi: 10.1007/s00424-011-0967-9. [DOI] [PubMed] [Google Scholar]
- 33.Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1200947109. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaar Z, O'Reilly P, Gelman I, Sabourin LA. v-Src-dependent down-regulation of the Ste20-like kinase SLK by casein kinase II. J Biol Chem. 2006;281:28193–28199. doi: 10.1074/jbc.M605665200. [DOI] [PubMed] [Google Scholar]
- 35.Chen LY, Zhao WH, Tian W, Guo J, Jiang F, Jin LJ, Sun YX, Chen KM, An LL, Li GD, Li Q, Li Y, Wu C, Zhao L, Wang WJ, Zheng GY, Li B, Li XQ, Hu J, Tian XL. STK39 is an independent risk factor for male hypertension in Han Chinese. Int J Cardiol. 2012;154:122–127. doi: 10.1016/j.ijcard.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Yazicioglu M, Cobb MH. Characterization of OSR1, a Member of the Mammalian Ste20p/Germinal Center Kinase Subfamily. J Biol Chem. 2004;279:11129–11136. doi: 10.1074/jbc.M313562200. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z, Hutchison M, Cobb MH. Isolation of the protein kinase TAO2 and identification of its mitogen-activated protein kinase/extracellular signal-regulated kinase kinase binding domain. J Biol Chem. 1999;274:28803–28807. doi: 10.1074/jbc.274.40.28803. [DOI] [PubMed] [Google Scholar]
- 38.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kiney Int. 2008;74:1403–1409. doi: 10.1038/ki.2008.451. [DOI] [PubMed] [Google Scholar]
- 39.Choe KP, Strange K. Evolutionarily conserved WNK and Ste20 kinases are essential for acute volume recovery and survival after hypertonic shrinkage in Caenorhabditis elegans. Am J Physiol Cell Physiol. 2007;293:C915–C927. doi: 10.1152/ajpcell.00126.2007. [DOI] [PubMed] [Google Scholar]
- 40.Consortium IPDG. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costello JF, Frühwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomäki P, Lang JC, Schuller DE, Yu L, Bloomfield CD, Caligiuri MA, Yates A, Nishikawa R, Su Huang H, Petrelli NJ, Zhang X, O'Dorisio MS, Held WA, Cavenee WK, Plass C. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- 42.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 43.Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 44.Cunnington MS, Kay C, Avery PJ, Mayosi BM, Koref MS, Keavney B. STK39 polymorphisms and blood pressure: an association study in British Caucasians and assessment of cis-acting influences on gene expression. BMC Med Genet. 2009;10:135. doi: 10.1186/1471-2350-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cusick JK, Mustian A, Jacobs AT, Reyland ME. Identification of PLSCR1 as a protein that interacts with RELT family members. Mol Cell Biochem. 2012;362:55–63. doi: 10.1007/s11010-011-1127-4. [DOI] [PubMed] [Google Scholar]
- 46.Cusick JK, Xu LG, Bin LH, Han KJ, Shu HB. Identification of RELT homologues that associate with RELT and are phosphorylated by OSR1. Biochem Biophys Res Commun. 2006;340:535–543. doi: 10.1016/j.bbrc.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 47.Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda) 2010;25:239–249. doi: 10.1152/physiol.00011.2010. [DOI] [PubMed] [Google Scholar]
- 48.Dan I, Watanabe NM, Kobayashi T, Yamashita-Suzuki K, Fukagaya Y, Kajikawa E, Kimura WK, Nakashima TM, Matsumoto K, Ninomiya-Tsuji J, Kusumi A. Molecular cloning of MINK, a novel member of mammalian GCK family kinases, which is up-regulated during postnatal mouse cerebral development. FEBS Lett. 2000;469:19–23. doi: 10.1016/s0014-5793(00)01247-3. [DOI] [PubMed] [Google Scholar]
- 49.Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 50.D'Andrea L, Lytle C, Matthews JB, Hofman P, Forbush Br, Madara JL. Na:K:2Cl cotransporter (NKCC) of intestinal epithelial cells. Surface expression in response to cAMP. J Biol Chem. 1996;15:28969–28976. doi: 10.1074/jbc.271.46.28969. [DOI] [PubMed] [Google Scholar]
- 51.D'Andrea-Winslow L, Strohmeier GR, Rossi B, Hofman P. Identification of a sea urchin Na(+)/K(+)/2Cl(−) cotransporter (NKCC): microfilament-dependent surface expression is mediated by hypotonic shock and cyclic AMP. J Exp Biol. 2001;204:147–156. doi: 10.1242/jeb.204.1.147. [DOI] [PubMed] [Google Scholar]
- 52.Davies P, Eaton CL. Regulation of prostate growth. J Endocrinol. 1991;131:5–17. doi: 10.1677/joe.0.1310005. [DOI] [PubMed] [Google Scholar]
- 53.DeAizpurua HJ, Cram DS, Naselli G, Devereux L, Dorow DS. Expression of mixed lineage kinase-1 in pancreatic beta-cell lines at different stages of maturation and during embryonic pancreas development. J Biol Chem. 1997;272:16364–16373. doi: 10.1074/jbc.272.26.16364. [DOI] [PubMed] [Google Scholar]
- 54.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol. 2003;23:9208–9221. doi: 10.1128/MCB.23.24.9208-9221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delpire E. The Mammalian family of Sterile20p-like protein kinases. Pflügers Arch. 2009;458:953–967. doi: 10.1007/s00424-009-0674-y. [DOI] [PubMed] [Google Scholar]
- 57.Delpire E, Gagnon KB. Genome-wide analysis of SPAK/OSR1 binding motifs. Physiol Genomics. 2007;28:223–231. doi: 10.1152/physiolgenomics.00173.2006. [DOI] [PubMed] [Google Scholar]
- 58.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 59.Delpire E, Kaplan MR, Plotkin MD, Hebert SC. The Na-(K)-Cl cotransporter family in the mammalian kidney: molecular identification and function(s) Nephrol Dial Transplant. 1996;11:1967–1973. doi: 10.1093/oxfordjournals.ndt.a027081. [DOI] [PubMed] [Google Scholar]
- 60.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279:L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 61.Denton J, Nehrke K, Yin X, Morrison R, Strange K. GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J Gen Physiol. 2005;125:113–125. doi: 10.1085/jgp.200409215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dose AC, Hillman DW, Wong C, Sohlberg L, Lin-Jones J, Burnside B. Myo3A, one of two class III myosin genes expressed in vertebrate retina, is localized to the calycal processes of rod and cone photoreceptors and is expressed in the sacculus. Mol Biol Cell. 2003;14:1058–1073. doi: 10.1091/mbc.E02-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dowd BF, Forbush B. PASK (Proline-Alanine-rich STE20-related Kinase), a Regulatory Kinase of the Na-K-Cl Cotransporter (NKCC1) J Biol Chem. 2003;278:27347–27353. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- 64.Draviam VM, Stegmeier F, Nalepa G, Sowa ME, Chen J, Liang A, Hannon GJ, Sorger PK, Harper JW, Elledge SJ. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signalling. Nat Cell Biol. 2007;9:556–564. doi: 10.1038/ncb1569. [DOI] [PubMed] [Google Scholar]
- 65.Drenth J, Jansonius JN, Koekoek R, Swen HM, Wolthers BG. Structure of papain. Nature. 1968;218:929–932. doi: 10.1038/218929a0. [DOI] [PubMed] [Google Scholar]
- 66.Duarte JD, Lobmeyer MT, Wang Z, Chapman AB, Gums JG, Langaee TY, Boerwinkle E, Turner ST, Johnson JA. Lack of association between polymorphisms in STK39, a putative thiazide response gene, and blood pressure response to hydrochlorothiazide. Pharmacogenet Genomics. 2010;20:516–519. doi: 10.1097/FPC.0b013e32833b5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dzhala VI, Brumback AC, Staley K. Bumetanide Enhances Phenobarbital Efficacy in a Neonatal Seizure Model. Ann Neurol. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 68.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 69.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol. 2000;279:F46–F53. doi: 10.1152/ajprenal.2000.279.1.F46. [DOI] [PubMed] [Google Scholar]
- 70.Ecelbarger CA, Tiwari S. Sodium transporters in the distal nephron and disease implications. Curr Hypertens. 2006;8:158–165. doi: 10.1007/s11906-006-0013-z. [DOI] [PubMed] [Google Scholar]
- 71.Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995;186:629–637. [PMC free article] [PubMed] [Google Scholar]
- 72.Ernest NJ, Sontheimer H. Extracellular glutamine is a critical modulator for regulatory volume increase in human glioma cells. Brain Res. 2007;1144:231–238. doi: 10.1016/j.brainres.2007.01.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ernest NJ, Weaver AK, Van Duyn LB, Sontheimer HW. Relative contribution of chloride channels and transporters to regulatory volume decrease in human glioma cells. Am J Physiol Cell Physiol. 2005;288:C1451–C1460. doi: 10.1152/ajpcell.00503.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Falin RA, Miyazaki H, Strange K. C. elegans STK39/SPAK ortholog-mediated inhibition of ClC anion channel activity is regulated by WNK-independent ERK kinase signaling. Am J Physiol Cell Physiol. 2011;300:C624–C635. doi: 10.1152/ajpcell.00343.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falin RA, Morrison R, Ham AJ, Strange K. Identification of regulatory phosphorylation sites in a cell volume- and Ste20 kinase-dependent ClC anion channel. J Gen Physiol. 2009;133:29–42. doi: 10.1085/jgp.200810080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farokhzad OC, Sagar GD, Mun EC, Sicklick JK, Lotz M, Smith JA, Song JC, O'Brien TC, Sharma CP, Kinane TB, Hodin RA, Matthews JB. Protein kinase C activation downregulates the expression and function of the basolateral Na+/K+/2Cl(−) cotransporter. J Cell Physiol. 1999;181:489–498. doi: 10.1002/(SICI)1097-4652(199912)181:3<489::AID-JCP13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 77.Fava C, Danese E, Montagnana M, Sjögren M, Almgren P, Engström G, Nilsson P, Hedblad B, Guidi GC, Minuz P, Melander O. Serine/threonine kinase 39 is a candidate gene for primary hypertension especially in women: results from two cohort studies in Swedes. J Hypertens. 2011;29:484–491. doi: 10.1097/HJH.0b013e328342b2c1. [DOI] [PubMed] [Google Scholar]
- 78.Feng YJ, Li YY. The role of p38 mitogen-activated protein kinase in the pathogenesis of inflammatory bowel disease. J Dig Dis. 2011;12:327–332. doi: 10.1111/j.1751-2980.2011.00525.x. [DOI] [PubMed] [Google Scholar]
- 79.Fenton RA, Moeller HB. Recent discoveries in vasopressin-regulated aquaporin-2 trafficking. Prog Brain Res. 2008;170:571–579. doi: 10.1016/S0079-6123(08)00444-5. [DOI] [PubMed] [Google Scholar]
- 80.Ferrandi M, Salardi S, Parenti P, Ferrari P, Bianchi G, Braw R, Karlish SJ. Na+/K+/Cl(−)-cotransporter mediated Rb+ fluxes in membrane vesicles from kidneys of normotensive and hypertensive rats. Biochem Biophys Acta. 1990;102:13–20. doi: 10.1016/0005-2736(90)90377-z. [DOI] [PubMed] [Google Scholar]
- 81.Filippi BM, de los Heros P, Mehellou Y, Navratilova I, Gourlay R, Deak M, Plater L, Toth R, Zeqiraj E, Alessi DR. MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. Embo J. 2011;30:1730–1741. doi: 10.1038/emboj.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Filteau M-J, Pourcher E, Bouchard RH, Baruch P, Mathieu J, Bédart F, Simard N, Vincent P. Corpus callosum agenesis and psychosis in Andermann syndrome. Arch Neurol. 1991;48:1275–1280. doi: 10.1001/archneur.1991.00530240079027. [DOI] [PubMed] [Google Scholar]
- 83.Flügge G, Oertel WH, Wuttke W. Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology. 1986;43:1–5. doi: 10.1159/000124500. [DOI] [PubMed] [Google Scholar]
- 84.Foroutan S, Brillault J, Forbush B, O'Donnell ME. Moderate-to-severe ischemic conditions increase activity and phosphorylation of the cerebral microvascular endothelial cell Na+-K+-Cl− cotransporter. Am J Physiol Cell Physiol. 2005;289:C1492–C1501. doi: 10.1152/ajpcell.00257.2005. [DOI] [PubMed] [Google Scholar]
- 85.Fraser SA, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, Levidiotis V, Kemp BE, Power DA. Regulation of the renal-specific Na+-K+-2Cl− co-transporter NKCC2 by AMP-activated protein kinase (AMPK) Biochem J. 2007;405:85–93. doi: 10.1042/BJ20061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu CA, Shen M, Huang BC, Lasaga J, Payan DG, Luo Y. TNIK, a novel member of the germinal center kinase family that activates the c-Jun N-terminal kinase pathway and regulates the cytoskeleton. J Biol Chem. 1999;274:30729–30737. doi: 10.1074/jbc.274.43.30729. [DOI] [PubMed] [Google Scholar]
- 87.Fuerstenberg S, Broadus J, Doe CQ. Asymmetry and cell fate in the Drosophila embryonic CNS. Int J Dev Biol. 1998;42:379–383. [PubMed] [Google Scholar]
- 88.Gagnon KB, Adragna NC, Fyffe RE, Lauf PK. Characterization of glial cell K-Cl cotransport. Cell Physiol Biochem. 2007;20:121–130. doi: 10.1159/000104160. [DOI] [PubMed] [Google Scholar]
- 89.Gagnon KB, Delpire E. Molecular determinants of hyperosmotically activated NKCC1-mediated K+/K+ exchange. J Physiol (Lond) 2010;588:3385–3396. doi: 10.1113/jphysiol.2010.191932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gagnon KB, Delpire E. Multiple pathways for protein phosphatase 1 (PP1) regulation of Na-K-2Cl Cotransporter (NKCC1) Function. The N-terminal Tail of the Na-K-2Cl cotransporter serves as a regulatory scaffold for Ste20-related proline/alanine-rich kinase (SPAK) and PP1. J Biol Chem. 2010;285:14115–14121. doi: 10.1074/jbc.M110.112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gagnon KB, Delpire E. On the substrate recognition and negative regulation of SPAK, a kinase modulating Na+-K+-2Cl− cotransport activity. Am J Physiol Cell Physiol. 2010;299:C614–C620. doi: 10.1152/ajpcell.00074.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gagnon KB, England R, Delpire E. The activity of cation-chloride cotransporters is modulated by two kinases: SPAK and WNK4. FASEB J. 2005;19:A1155. [Google Scholar]
- 93.Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol. 2006;26:689–698. doi: 10.1128/MCB.26.2.689-698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gagnon KB, England R, Delpire E. A single binding motif is required for SPAK activation of the Na-K-2Cl cotransporter. Cell Physiol Biochem. 2007;20:131–142. doi: 10.1159/000104161. [DOI] [PubMed] [Google Scholar]
- 95.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-chloride cotransporters is modulated by the interaction of two kinases: SPAK and WNK4. Am J Physiol Cell Physiol. 2006;290:C134–C142. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- 96.Gagnon KB, England R, Diehl L, Delpire E. Apoptosis associated tyrosine kinase scaffolding of protein phosphatase 1 and SPAK reveals a novel pathway for Na-K-2C1 cotransporter regulation. Am J Physiol (Cell Physiol) 2007;292:C1809–C1815. doi: 10.1152/ajpcell.00580.2006. [DOI] [PubMed] [Google Scholar]
- 97.Gagnon KB, Rios K, Delpire E. Functional insights into the activation mechanism of Ste20-related kinases. Cell Physiol Biochem. 2011;28:1219–1230. doi: 10.1159/000335854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gamba G. Molecular Physiology and Pathophysiology of Electroneutral Cation-Chloride Cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 99.Gamba G, Garbarini NJ, Delpire E. Regulation of cation-chloride cotransporters. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporter and Channels in the Nervous System: From molecules to diseases. London: Academic Press; 2009. pp. 357–381. [Google Scholar]
- 100.Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 101.Gaozza E, Baker SJ, Vora RK, Reddy EP. AATYK: a novel tyrosine kinase induced during growth arrest and apoptosis of myeloid cells. Oncogene. 1997;15:3127–3135. doi: 10.1038/sj.onc.1201575. [DOI] [PubMed] [Google Scholar]
- 102.Garg P, Martin CF, Elms SC, Gordon FJ, Wall SM, Garland CJ, Sutliff RL, O'Neill WC. Effect of the Na-K-2Cl cotransporter NKCC1 on systemic blood pressure and smooth muscle tone. Am J Physiol Heart Circ Physiol. 2007;292:H2100–H2105. doi: 10.1152/ajpheart.01402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garzón-Muvdi T, Pacheco-Alvarez D, Gagnon KB, Vázquez N, Ponce-Coria J, Moreno E, Delpire E, Gamba G. WNK4 kinase is a negative regulator of K+-Cl− cotransporters. Am J Physiol Renal Physiol. 2007;292:F1197–F1207. doi: 10.1152/ajprenal.00335.2006. [DOI] [PubMed] [Google Scholar]
- 104.Geng Y, Byun N, Delpire E. Behavioral Analysis of Ste20 Kinase SPAK Knockout Mice. Behavioural Brain Res. 2010;208:377–382. doi: 10.1016/j.bbr.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Geng Y, Hoke A, Delpire E. The Ste20 kinases SPAK and OSR1 regulate NKCC1 function in sensory neurons. J Biol Chem. 2009;284:14020–14028. doi: 10.1074/jbc.M900142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.George RA, Heringa J. SnapDRAGON: a method to delineate protein structural domains from sequence data. J Mol Biol. 2002;316:839–851. doi: 10.1006/jmbi.2001.5387. [DOI] [PubMed] [Google Scholar]
- 107.George RA, Lin K, Heringa J. Scooby-domain: prediction of globular domains in protein sequence. Nucl Acids Res. 2005;33:W160–U163. doi: 10.1093/nar/gki381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. doi: 10.1097/00006123-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 109.Gimenez I, Forbush B. Regulatory phosphorylation sites in the NH2 terminus of the renal Na-K-Cl cotransporter (NKCC2) Am J Physiol Renal Physiol. 2005;289:F1341–F1345. doi: 10.1152/ajprenal.00214.2005. [DOI] [PubMed] [Google Scholar]
- 110.Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians. 1966;79:221–235. [PubMed] [Google Scholar]
- 111.Glover M, O'shaughnessy KM. SPAK and WNK kinases: a new target for blood pressure treatment? Curr Opin Nephrol Hypertens. 2011;20:16–22. doi: 10.1097/MNH.0b013e32834132bc. [DOI] [PubMed] [Google Scholar]
- 112.Glover M, Zuber AM, O'Shaughnessy KM. Renal and brain isoforms of WNK3 have opposite effects on NCCT expression. J Am Soc Nephrol. 2009;20:1314–1322. doi: 10.1681/ASN.2008050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haas BR, Cuddapah VA, Watkins S, Rohn KJ, Dy TE, Sontheimer H. With-No-Lysine Kinase 3 (WNK3) stimulates glioma invasion by regulating cell volume. Am J Physiol Cell Physiol. 2011;301:C1150–C1160. doi: 10.1152/ajpcell.00203.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haas BR, Sontheimer H. Inhibition of the Sodium-Potassium-Chloride Cotransporter Isoform-1 reduces glioma invasion. Cancer Res. 2010;70:5597–5606. doi: 10.1158/0008-5472.CAN-09-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamann S, Herrera-Perez JJ, Zeuthen T, Alvarez-Leefmans FJ. Cotransport of water by the Na+-K+-2Cl(−) cotransporter NKCC1 in mammalian epithelial cells. J Physiol. 2010;588:4089–4101. doi: 10.1113/jphysiol.2010.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hendriksen PJ, Dits NF, Kokame K, Veldhoven A, van Weerden WM, Bangma CH, Trapman J, Jenster G. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 2006;66:5012–5020. doi: 10.1158/0008-5472.CAN-05-3082. [DOI] [PubMed] [Google Scholar]
- 117.Hengl T, Kaneko H, Dauner K, Vocke K, Frings S, Mohrlen F. Molecular components of signal amplification in olfactory sensory cilia. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0909032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hisamoto N, Moriguchi T, Urushiyama S, Mitani S, Shibuya H, Matsumoto K. Caenorhabditis elegans WNK-STE20 pathway regulates tube formation by modulating ClC channel activity. EMBO Rep. 2008;9:70–75. doi: 10.1038/sj.embor.7401128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoffman GR, Cerione RA. Flipping the switch: the structural basis for signaling through the CRIB motif. Cell. 2000;102:403–406. doi: 10.1016/s0092-8674(00)00045-3. [DOI] [PubMed] [Google Scholar]
- 120.Hoffmann EK, Pedersen SF. Shrinkage insensitivity of NKCC1 in myosin II-depleted cytoplasts from Ehrlich ascites tumor cells. Am J Physiol Cell Physiol. 2007;292:C1854–C1866. doi: 10.1152/ajpcell.00474.2006. [DOI] [PubMed] [Google Scholar]
- 121.Holden S, Cox J, Raymond FL. Cloning, genomic organization, alternative splicing and expression analysis of the human gene WNK3 (PRKWNK3) Gene. 2004;335 doi: 10.1016/j.gene.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 122.Honma N, Asada A, Takeshita S, Enomoto M, Yamakawa E, Tsutsumi K, Saito T, Satoh T, Itoh H, Kaziro Y, Kishimoto T, Hisanaga S. Apoptosis-associated tyrosine kinase is a Cdk5 activator p35 binding protein. Biochem Biophys Res Commun. 2003;310:398–404. doi: 10.1016/j.bbrc.2003.08.143. [DOI] [PubMed] [Google Scholar]
- 123.Hoorn EJ, Nelson JH, McCormick JA, Ellison DH. The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol. 2011;22:605–614. doi: 10.1681/ASN.2010080827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Howard HC, Mount DB, Rochefort D, Byun N, Dupré N, Lu J, Fan X, Song L, Rivière J-B, Prévost C, Welch R, England R, Zhan FQ, Mercado A, Siesser WB, George AL, Horst J, Simonati A, McDonald MP, Bouchard J-P, Mathieu J, Delpire E, Rouleau GA. Mutations in the K-Cl cotransporter KCC3 cause a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat Genet. 2002;32:384–392. doi: 10.1038/ng1002. [DOI] [PubMed] [Google Scholar]
- 125.Huang YT, Heist RS, Chirieac LR, Lin X, Skaug V, Zienolddiny S, Haugen A, Wu MC, Wang Z, Su L, Asomaning K, Christiani DC. Genome-wide analysis of survival in early-stage non-small-cell lung cancer. J Clin Oncol. 2009;27:2660–2667. doi: 10.1200/JCO.2008.18.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hughes JP, Ward DR, Facci L, Richardson JC, Skaper SD. Apoptosis-associated tyrosine kinase and neuronal cell death. Neurochem Res. 2010;35:588–597. doi: 10.1007/s11064-009-0103-9. [DOI] [PubMed] [Google Scholar]
- 127.Hutchison M, Berman KS, Cobb MH. Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J Biol Chem. 1998;273:28625–28632. doi: 10.1074/jbc.273.44.28625. [DOI] [PubMed] [Google Scholar]
- 128.Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, Bigelow LB, Weinberger DR, Kleinman JE. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johanson CE, Sweeney SM, Parmelee JT, Epstein MH. Cotransport of sodium and chloride by the adult mammalian choroid plexus. Am J Physiol (Cell Physiol) 1990;258:C211–C216. doi: 10.1152/ajpcell.1990.258.2.C211. [DOI] [PubMed] [Google Scholar]
- 130.Johnston AM, Naselli G, Gonez LJ, Martin RM, Harrison LC, Deaizpurua HJ. SPAK, a Ste20/SPS1-related kinase that activates the p38 pathway. Oncogene. 2000;19:4290–4297. doi: 10.1038/sj.onc.1203784. [DOI] [PubMed] [Google Scholar]
- 131.Jolivalt CG, Lee CA, Ramos KM, Calcutt NA. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain. 2008;140:48–57. doi: 10.1016/j.pain.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kahle KT, Gimenez I, Hassan H, Wilson FH, Wong RD, Forbush B, Aronson PS, Lifton RP. WNK4 regulates apical and basolateral Cl− flux in extrarenal epithelia. Proc Natl Acad Sci USA. 2004;101:2064–2069. doi: 10.1073/pnas.0308434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kahle KT, Rinehart J, Lifton RP. Phosphoregulation of the Na-K-2Cl and K-Cl cotransporters by the WNK kinases. Biochem Biophys Acta. 2010;1802:1150–1158. doi: 10.1016/j.bbadis.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 135.Kai M, Nakatsura T, Egami H, Senju S, Nishimura Y, Ogawa M. Heat shock protein 105 is overexpressed in a variety of human tumors. Oncol Rep. 2003;10:1777–1782. [PubMed] [Google Scholar]
- 136.Kamynina E, Debonneville C, Bens M, Vandewalle A, Staub O. A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J. 2001;15 doi: 10.1096/fj.00-0191com. [DOI] [PubMed] [Google Scholar]
- 137.Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. J Neurosci. 2004;24:7931–7938. doi: 10.1523/JNEUROSCI.2115-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kaplan MR, Plotkin MD, Lee W-S, Xu Z-C, Lytton J, Hebert SC. Apical localization of the Na-K-2Cl cotransporter, rBSC1, on rat thick ascending limbs. Kidney Int. 1996;49:40–47. doi: 10.1038/ki.1996.6. [DOI] [PubMed] [Google Scholar]
- 139.Kawa S, Ito C, Toyama Y, Maekawa M, Tezuka T, Nakamura T, Nakazawa T, Yokoyama K, Yoshida N, Toshimori K, Yamamoto T. Azoospermia in mice with targeted disruption of the Brek/Lmtk2 (brain-enriched kinase/lemur tyrosine kinase 2) gene. Proc Natl Acad Sci USA. 2006;103:19344–19349. doi: 10.1073/pnas.0603603103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keep RF, Xiang J, Betz AL. Potassium cotransport at the rat choroid plexus. Am J Physiol (Cell Physiol) 1994;267:C1616–C1622. doi: 10.1152/ajpcell.1994.267.6.C1616. [DOI] [PubMed] [Google Scholar]
- 141.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 142.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 143.Kidambi S, Ghosh S, Kotchen JM, Grim CE, Krishnaswami S, Kaldunski ML, Cowley AWJ, Patel SB, Kotchen TA. Non-replication study of a genome-wide association study for hypertension and blood pressure in African Americans. BMC Med Genet. 2012;13:27. doi: 10.1186/1471-2350-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol. 1999;276:F96–F103. doi: 10.1152/ajprenal.1999.276.1.F96. [DOI] [PubMed] [Google Scholar]
- 145.Knepper MA, Kim GH, Fernandez-Llama P, Ecelbarger CA. Regulation of thick ascending limb transport by vasopressin. J Am Soc Nephrol. 1999;10:628–634. doi: 10.1681/ASN.V103628. [DOI] [PubMed] [Google Scholar]
- 146.Kojima R, Randall J, Brenner BM, Gullans SR. Osmotic stress protein 94 (Osp94). A new member of the Hsp110/SSE gene subfamily. J Biol Chem. 1996;271:12327–12332. doi: 10.1074/jbc.271.21.12327. [DOI] [PubMed] [Google Scholar]
- 147.Koochekpour S. Androgen receptor signaling and mutations in prostate cancer. Asian J Androl. 2010;12:639–657. doi: 10.1038/aja.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kupinski AP, Müller-Reichert T, Eckmann CR. The Caenorhabditis elegans Ste20 kinase, GCK-3, is essential for postembryonic developmental timing and regulates meiotic chromosome segregation. Dev Biol. 2010;344:758–771. doi: 10.1016/j.ydbio.2010.05.505. [DOI] [PubMed] [Google Scholar]
- 149.Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 150.Labrisseau A, Vanasse M, Brochu P, Jasmin G. The andermann syndrome: agenesis of the corpus callosum associated with mental retardation and progressive sesorimotor neuronopathy. Can J Neurol Sci. 1984;11:257–261. doi: 10.1017/s0317167100045509. [DOI] [PubMed] [Google Scholar]
- 151.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 152.Lamson RE, Winters MJ, Pryciak PM. Cdc42 regulation of kinase activity and signaling by the yeast p21-activated kinase Ste20. Mol Cell Biol. 2002;22:2939–2951. doi: 10.1128/MCB.22.9.2939-2951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Leberer E, Dignard D, Harcus D, Thomas DY, Whiteway M. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signaling components. EMBO J. 1992;11:4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee MG, Muallem S. Physiology of duct cell secretion. In: Beger H, Buchler M, Kozarek R, et al., editors. Pancreas: an integrated textbook of basic science, medicine, and surgery. Oxford, England: Blackwell Publishing; 2008. pp. 78–90. [Google Scholar]
- 155.Lee MG, Ohana E, Park HW, Yang D, Muallem S. Molecular Mechanism of Pancreatic and Salivary Gland Fluid and HCO3 Secretion. Physiol Rev. 2012;92:39–74. doi: 10.1152/physrev.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee SJ, Cobb MH, Goldsmith EJ. Crystal structure of domain-swapped STE20 OSR1 kinase domain. Protein Sci. 2009;18:304–313. doi: 10.1002/pro.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 158.Leiserson WM, Forbush B, Keshishian H. Drosophila glia use a conserved cotransporter mechanism to regulate extracellular volume. Glia. 2011;59:320–332. doi: 10.1002/glia.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leiserson WM, Harkins EW, Keshishian H. Fray, a drosophila serine/threonine kinase homologous to mammalian PASK, is required for axonal ensheatment. Neuron. 2000;28:793–806. doi: 10.1016/s0896-6273(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 160.Li Y, Hu J, Vita R, Sun B, Tabata H, Altman A. SPAK kinase is a substrate and target of PKCtheta in T-cell receptor-induced AP-1 activation pathway. Embo J. 2004;23:1112–1122. doi: 10.1038/sj.emboj.7600125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liedtke CM, Hubbard M, Wang X. Stability of actin cytoskeleton and PKC-delta binding to actin regulate NKCC1 function in airway epithelial cells. Am J Physiol Cell Physiol. 2003;284:C487–C496. doi: 10.1152/ajpcell.00357.2002. [DOI] [PubMed] [Google Scholar]
- 162.Liedtke CM, Wang X, Smallwood ND. Role for protein phosphatase 2A in the regulation of Calu-3 epithelial Na+-K+-2Cl−, type 1 co-transport function. J Biol Chem. 2005;280:25491–25498. doi: 10.1074/jbc.M504473200. [DOI] [PubMed] [Google Scholar]
- 163.Lin JL, Chen HC, Fang HI, Robinson D, Kung HJ, Shih HM. MST4, a new Ste20-related kinase that mediates cell growth and transformation via modulating ERK pathway. Oncogene. 2001;20:6559–6569. doi: 10.1038/sj.onc.1204818. [DOI] [PubMed] [Google Scholar]
- 164.Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, Sytwu HK, Sohara E, Uchida S, Sasaki S, Yang SS. Impaired phosphorylation of Na+-K+-2Cl− cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proc Natl Acad Sci U S A. 2011;108:17538–17543. doi: 10.1073/pnas.1107452108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ling P, Lu TJ, Yuan CJ, Lai MD. Biosignaling of mammalian Ste20-related kinases. Cell Signal. 2008;20:1237–1247. doi: 10.1016/j.cellsig.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 166.Lionetto MG, Pedersen SF, Hoffmann EK, Giordano ME, Schettino T. Roles of the cytoskeleton and of protein phosphorylation events in the osmotic stress response in eel intestinal epithelium. Cell Physiol Biochem. 2002;12:163–178. doi: 10.1159/000066276. [DOI] [PubMed] [Google Scholar]
- 167.Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- 168.Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–834. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol. 1999;39:558–568. [PubMed] [Google Scholar]
- 170.Lu TJ, Lai WY, Huang CY, Hsieh WJ, Yu JS, Hsieh YJ, Chang WT, Leu TH, Chang WC, Chuang WJ, Tang MJ, Chen TY, Lu TL, Lai MD. Inhibition of cell migration by autophosphorylated mammalian sterile 20-like kinase 3 (MST3) involves paxillin and protein-tyrosine phosphatase-PEST. J Biol Chem. 2006;281:38405–38417. doi: 10.1074/jbc.M605035200. [DOI] [PubMed] [Google Scholar]
- 171.Ma X, Zhao H, Shan J, Long F, Chen Y, Zhang Y, Han X, Ma D. PDCD10 interacts with Ste20-related kinase MST4 to promote cell growth and transformation via modulation of the ERK pathway. Mol Biol Cell. 2007;18:1965–1978. doi: 10.1091/mbc.E06-07-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mackay V, Manney TR. Mutations affecting sexual conjugation and related processes in Saccharomyces cerevisiae. II. Genetic analysis of nonmating mutants. Genetics. 1974;76:273–288. doi: 10.1093/genetics/76.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Manning G, Plowman GD, Hunter T, Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends Biochem Sci. 2002;27:514–520. doi: 10.1016/s0968-0004(02)02179-5. [DOI] [PubMed] [Google Scholar]
- 174.Marsh L, Neiman AM, Herskowitz I. Signal transduction during pheromone response in yeast. Annu Rev Cell Biol. 1991;7:699–728. doi: 10.1146/annurev.cb.07.110191.003411. [DOI] [PubMed] [Google Scholar]
- 175.Marshall WS, Ossum CG, Hoffmann EK. Hypotonic shock mediation by p38 MAPK, JNK, PKC, FAK, OSR1 and SPAK in osmosensing chloride secreting cells of killifish opercular epithelium. J Exp Biol. 2005;208:1063–1077. doi: 10.1242/jeb.01491. [DOI] [PubMed] [Google Scholar]
- 176.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 177.Matsui Y, Nakano N, Shao D, Gao S, Luo W, Hong C, Zhai P, Holle E, Yu X, Yabuta N, Tao W, Wagner T, Nojima H, Sadoshima J. Lats2 is a negative regulator of myocyte size in the heart. Circ Res. 2008;103:1309–1318. doi: 10.1161/CIRCRESAHA.108.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Matthews JB, Awtrey CS, Madara JL. Microfilament-dependent activation of Na+/K+/2Cl− cotransport by cAMP in intestinal epithelial monolayers. J Clin Invest. 1992;90:1608–1613. doi: 10.1172/JCI116030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Matthews JB, Smith JA, Tally KJ, Awtrey CS, Nguyen H, Rich J, Madara JL. Na-K-2Cl cotransport in intestinal epithelial cells. Influence of chloride efflux and F-actin on regulation of cotransporter activity and bumetanide binding. J Biol Chem. 1994;269:15703–15709. [PubMed] [Google Scholar]
- 180.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab. 2002;87:3248–3254. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 181.McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev. 2011;91:177–219. doi: 10.1152/physrev.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang C-L, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK Isoform Switch Modulates Renal Salt Transport and Blood Pressure. Cell Metabolism. 2011;14:352–364. doi: 10.1016/j.cmet.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Melzi ML, Syrén ML, Assael BM, Sereni F, Aperia A. Increased renal tubular Na-K-ATPase activity in Milan hypertensive rats in the prehypertensive period. Pediatr Nephrol. 1991;5:700–703. doi: 10.1007/BF00857877. [DOI] [PubMed] [Google Scholar]
- 184.Meyer JW, Flagella M, Sutliff RL, Lorenz JN, Nieman ML, Weber CS, Paul RJ, Shull GE. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral Na(+)-K(+)-2Cl(−) cotransporter. Am J Physiol Heart Circ Physiol. 2002;283:H1846–H1855. doi: 10.1152/ajpheart.00083.2002. [DOI] [PubMed] [Google Scholar]
- 185.Miao N, Fung B, Sanchez R, Lydon J, Barker D, Pang K. Isolation and expression of PASK, a serine/threonine kinase during rat embryonic development, with special emphasis on the pancreas. J Histochem Cytochem. 2000;48:1391–1400. doi: 10.1177/002215540004801009. [DOI] [PubMed] [Google Scholar]
- 186.Migliosi V, Modamio-Høybjør S, Moreno-Pelayo MA, Rodríguez-Ballesteros M, Villamar M, Tellería D, Menéndez I, Moreno F, Del Castillo I. Q829X, a novel mutation in the gene encoding otoferlin (OTOF), is frequently found in Spanish patients with prelingual non-syndromic hearing loss. J Med Genet. 2002;39:502–506. doi: 10.1136/jmg.39.7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miletic G, Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain. 2008;137:532–539. doi: 10.1016/j.pain.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mitsopoulos C, Zihni C, Garg R, Ridley AJ, Morris JD. The prostate-derived sterile 20-like kinase (PSK) regulates microtubule organization and stability. J Biol Chem. 2003;278:18085–18091. doi: 10.1074/jbc.M213064200. [DOI] [PubMed] [Google Scholar]
- 189.Miyamoto H, Matsushiro A, Nozaki M. Molecular cloning of a novel mRNA sequence expressed in cleavage stage mouse embryos. Mol Reprod Dev. 1993;34:1–7. doi: 10.1002/mrd.1080340102. [DOI] [PubMed] [Google Scholar]
- 190.Moriguchi T, Urushiyama S, Hisamoto N, Iemura SI, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 191.Muchemwa FC, Nakatsura T, Fukushima S, Nishimura Y, Kageshita T, Ihn H. Differential expression of heat shock protein 105 in melanoma and melanocytic naevi. Melanoma Res. 2008;18:166–171. doi: 10.1097/CMR.0b013e3282fe9a16. [DOI] [PubMed] [Google Scholar]
- 192.Mutig K, Saritas T, Uchida S, Kahl T, Borowski T, Paliege A, Bohlick A, Bleich M, Shan Q, Bachmann S. Short-term stimulation of the thiazide-sensitive Na+-Cl− cotransporter by vasopressin involves phosphorylation and membrane translocation. Am J Physiol Renal Physiol. 2010;298:F502–F509. doi: 10.1152/ajprenal.00476.2009. [DOI] [PubMed] [Google Scholar]
- 193.Mykoniatis A, Shen L, Fedor-Chaiken M, Tang J, Tang X, Worrell RT, Delpire E, Turner JR, Matlin KS, Bouyer P, Matthews JB. Phorbol 12-myristate 13-acetate-induced endocytosis of the Na-K-2Cl cotransporter in MDCK cells is associated with a clathrin-dependent pathway. Am J Physiol Cell Physiol. 2010;298:C85–C97. doi: 10.1152/ajpcell.00118.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Naito S, Ohta A, Sohara E, Ohta E, Rai T, Sasaki S, Uchida S. Regulation of WNK1 kinase by extracellular potassium. Clin Exp Nephrol. 2011;15:195–202. doi: 10.1007/s10157-010-0378-9. [DOI] [PubMed] [Google Scholar]
- 195.Nakano K, Yamauchi J, Nakagawa K, Itoh H, Kitamura N. NESK, a member of the germinal center kinase family that activates the c-Jun N-terminal kinase pathway and is expressed during the late stages of embryogenesis. J Biol Chem. 2000;275:20533–20539. doi: 10.1074/jbc.M001009200. [DOI] [PubMed] [Google Scholar]
- 196.Nakatsura T, Senju S, Yamada K, Jotsuka T, Ogawa M, Nishimura Y. Gene cloning of immunogenic antigens overexpressed in pancreatic cancer. Biochem Biophys Res Commun. 2001;281:936–944. doi: 10.1006/bbrc.2001.4377. [DOI] [PubMed] [Google Scholar]
- 197.Nickell WT, Kleene NK, Gesteland RC, Kleene SJ. Neuronal chloride accumulation in olfactory epithelium of mice lacking NKCC1. J Neurophysiol. 2006;95:2003–2006. doi: 10.1152/jn.00962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nogueira E, Fidalgo M, Molnar A, Kyriakis J, Force T, Zalvide J, Pombo CM. SOK1 translocates from the Golgi to the nucleus upon chemical anoxia and induces apoptotic cell death. J Biol Chem. 2008;283:16248–16258. doi: 10.1074/jbc.M709724200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nomura H, Sakai A, Nagano M, Umino M, Suzuki H. Expression changes of cation chloride cotransporters in the rat spinal cord following intraplantar formalin. Neurosci Res. 2006;56:435–440. doi: 10.1016/j.neures.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 200.Nozaki M, Onishi Y, Togashi S, Miyamoto H. Molecular characterization of the Drosophila Mo25 gene, which is conserved among Drosophila, mouse, and yeast. DNA Cell Biol. 1996;15:505–509. doi: 10.1089/dna.1996.15.505. [DOI] [PubMed] [Google Scholar]
- 201.Nugent BM, Valenzuela CV, Simons TJ, McCarthy MM. Kinases SPAK and OSR1 Are Upregulated by Estradiol and Activate NKCC1 in the Developing Hypothalamus. J Neurosci. 2012;32:593–598. doi: 10.1523/JNEUROSCI.5415-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Obermüller N, Bernstein P, Velazquez H, Reilly R, Moser D, Ellison DH, Bachmann S. Expression of the thiazide-sensitive Na-Cl cotransporter in rat and human kidney. Am J Physiol (Renal Physiol) 1995;269:F900–F910. doi: 10.1152/ajprenal.1995.269.6.F900. [DOI] [PubMed] [Google Scholar]
- 203.Obermüller N, Kunchaparty S, Ellison DH, Bachmann S. Expression of the Na-K-2Cl cotransporter by macula densa and thick ascending limb cells of rat and rabbit nephron. J Clin Invest. 1996;98:635–640. doi: 10.1172/JCI118834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.O'Donnell ME, Duong V, Suvatne J, Foroutan S, Johnson DM. Arginine vasopressin stimulation of cerebral microvascular endothelial cell Na-K-Cl cotransporter activity is V1 receptor and [Ca] dependent. Am J Physiol Cell Physiol. 2005;289:C283–C292. doi: 10.1152/ajpcell.00001.2005. [DOI] [PubMed] [Google Scholar]
- 205.O'Donnell ME, Lam TI, Tran L, Anderson SE. The role of the blood-brain barrier Na-K-2Cl cotransporter in stroke. Adv Exp Med Biol. 2004;559:67–75. doi: 10.1007/0-387-23752-6_6. [DOI] [PubMed] [Google Scholar]
- 206.O'Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood-brain barrier Na-K-Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2006;26:1234–1249. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- 207.O'Donnell ME, Tran L, Lam TI, Liu XB, Anderson SE. Bumetanide inhibition of the blood-brain barrier Na-K-Cl cotransporter reduces edema formation in the rat middle cerebral artery occlusion model of stroke. J Cereb Blood Flow Metab. 2004;24:1046–1056. doi: 10.1097/01.WCB.0000130867.32663.90. [DOI] [PubMed] [Google Scholar]
- 208.Ohta A, Rai T, Yui N, Chiga M, Yang SS, Lin SH, Sohara E, Sasaki S, Uchida S. Targeted disruption of the Wnk4 gene decreases phosphorylation of Na-Cl cotransporter, increases Na excretion and lowers blood pressure. Hum Mol Genet. 2008;18:3978–3986. doi: 10.1093/hmg/ddp344. [DOI] [PubMed] [Google Scholar]
- 209.Ong SB, Shah D, Qusous A, Jarvis SM, Kerrigan MJ. Stimulation of regulatory volume increase (RVI) in avian articular chondrocytes by gadolinium chloride. Biochem Cell Biol. 2010;88:505–512. doi: 10.1139/o09-179. [DOI] [PubMed] [Google Scholar]
- 210.O'Reilly M, Marshall E, Speirs HJ, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Assoc Nephrol. 2003;14:2447–2456. doi: 10.1097/01.asn.0000089830.97681.3b. [DOI] [PubMed] [Google Scholar]
- 211.Oshima T, Sasaki M, Kataoka H, Miwa H, Takeuchi T, Joh T. Wip1 protects hydrogen peroxide-induced colonic epithelial barrier dysfunction. Cell Mol Life Sci. 2007;64:3139–3147. doi: 10.1007/s00018-007-7268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Owens DF, Boyce LH, Davis MBE, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pacheco-Alvarez D, Cristóbal PS, Meade P, Moreno E, Vazquez N, Muñoz E, Díaz A, Juárez ME, Giménez I, Gamba G. The Na+:Cl− cotransporter is activated and phosphorylated at the amino-terminal domain upon intracellular chloride depletion. J Biol Chem. 2006;281:28755–28763. doi: 10.1074/jbc.M603773200. [DOI] [PubMed] [Google Scholar]
- 214.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 215.Park HW, Kim JY, Choi SK, Lee YH, Zeng W, Kim KH, Muallem S, Lee MG. Serine-threonine kinase with-no-lysine 4 (WNK4) controls blood pressure via transient receptor potential canonical 3 (TRPC3) in the vasculature. Proc Natl Acad Sci USA. 2011;108:10750–10755. doi: 10.1073/pnas.1104271108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Park HW, Nam JH, Kim JY, Namkung W, Yoon JS, Lee JS, Kim KS, Venglovecz V, Gray MA, Kim KH, Lee MG. Dynamic regulation of CFTR bicarbonate permeability by [Cl−]i and its role in pancreatic bicarbonate secretion. Gastroenterology. 2010;139:620–631. doi: 10.1053/j.gastro.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 217.Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem. 1996;271:16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 218.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA. Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int. 2010;78:160–169. doi: 10.1038/ki.2010.130. [DOI] [PubMed] [Google Scholar]
- 219.Perrot-Sinal TS, Davis AM, Gregerson KA, Kao JP, McCarthy MM. Estradiol enhances excitatory gamma-aminobutyric acid-mediated calcium signaling in neonatal hypothalamic neurons. Endocrinology. 2001;142:2238–2243. doi: 10.1210/endo.142.6.8180. [DOI] [PubMed] [Google Scholar]
- 220.Perrot-Sinal TS, Sinal CJ, Reader JC, Speert DB, McCarthy MM. Sex differences in the chloride cotransporters, NKCC1 and KCC2, in the developing hypothalamus. J Neuroendocrinol. 2007;19:302–308. doi: 10.1111/j.1365-2826.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- 221.Peters M, Jeck N, Reinalter S, Leonhardt A, Tönshoff B, Klaus GG, Konrad M, Seyberth HW. Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med. 2002;112:183–190. doi: 10.1016/s0002-9343(01)01086-5. [DOI] [PubMed] [Google Scholar]
- 222.Phillips DC. The three-dimensional structure of an enzyme molecule. Sci Am. 1966;215:78–90. doi: 10.1038/scientificamerican1166-78. [DOI] [PubMed] [Google Scholar]
- 223.Piechotta K, Garbarini NJ, England R, Delpire E. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl− cotransporter in the nervous system: Evidence for a scaffolding role of the kinase. J Biol Chem. 2003;278:52848–52856. doi: 10.1074/jbc.M309436200. [DOI] [PubMed] [Google Scholar]
- 224.Piechotta K, Lu J, Delpire E. Cation-chloride cotransporters interact with the stress-related kinases SPAK and OSR1. J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 225.Pieraut S, Matha V, Sar C, Hubert T, Méchaly I, Hilaire C, Mersel M, Delpire E, Valmier J, Scamps F. NKCC1 phosphorylation stimulates neurite growth of injured adult sensory neurons. J Neurosci. 2007;27:6751–6759. doi: 10.1523/JNEUROSCI.1337-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na+-K+-2Cl− cotransporter BSC2 in the nervous system. Am J Physiol Cell Physiol. 1997;272:C173–C183. doi: 10.1152/ajpcell.1997.272.1.C173. [DOI] [PubMed] [Google Scholar]
- 227.Plotkin MD, Kaplan MR, Verlander JW, Lee W-S, Brown D, Poch E, Gullans SR, Hebert SC. Localization of the thiazide sensitive Na-Cl cotransporter, rTSC, in the rat kidney. Kid Int. 1996;50:174–183. doi: 10.1038/ki.1996.300. [DOI] [PubMed] [Google Scholar]
- 228.Polek TC, Talpaz M, Spivak-Kroizman T. The TNF receptor, RELT, binds SPAK and uses it to mediate p38 and JNK activation. Biochem Biophys Res Commun. 2006;343:125–134. doi: 10.1016/j.bbrc.2006.02.125. [DOI] [PubMed] [Google Scholar]
- 229.Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, Juárez P, Muñoz E, Michel G, Bobadilla NA, Gimenez I, Lifton RP, Hebert SC, Gamba G. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Qi H, Labrie Y, Grenier J, Fournier A, Fillion C, Labrie C. Androgens induce expression of SPAK, a STE20/SPS1-related kinase, in LNCaP human prostate cancer cells. Mol Cell Endocrinol. 2001;182:181–192. doi: 10.1016/s0303-7207(01)00560-3. [DOI] [PubMed] [Google Scholar]
- 231.Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O'Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med. 2010;2:63–75. doi: 10.1002/emmm.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Raghunath M, Patti R, Bannerman P, Lee CM, Baker S, Sutton LN, Phillips PC, DC R. A novel kinase, AATYK induces and promotes neuronal differentiation in a human neuroblastoma (SH-SY5Y) cell line. Mol Brain Res. 2000;77:151–162. doi: 10.1016/s0169-328x(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 233.Rajakulendran T, Sicheri F. Allosteric protein kinase regulation by pseudokinases: insights from STRAD. Sci Signal. 2010;3:pe8. doi: 10.1126/scisignal.3111pe8. [DOI] [PubMed] [Google Scholar]
- 234.Ramoz N, Cai G, Reichert JG, Silverman JM, Buxbaum JD. An analysis of candidate autism loci on chromosome 2q24-q33: evidence for association to the STK39 gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1152–1158. doi: 10.1002/ajmg.b.30739. [DOI] [PubMed] [Google Scholar]
- 235.Ramoz N, Reichert JG, Smith CJ, Silverman JM, Bespalova IN, Davis KL, Buxbaum JD. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry. 2004;161:662–669. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- 236.Randall J, Thorne T, Delpire E. Partial cloning and characterization of Slc12a2: the gene encoding the secretory Na+-K+-2Cl− cotransporter. Am J Physiol (Cell Physiol) 1997;273:C1267–C1277. doi: 10.1152/ajpcell.1997.273.4.C1267. [DOI] [PubMed] [Google Scholar]
- 237.Reiche J, Theilig F, Rafiqi FH, Carlo AS, Militz D, Mutig K, Todiras M, Christensen EI, Ellison DH, Bader M, Nykjaer A, Bachmann S, Alessi D, Willnow TE. SORLA/SORL1 functionally interacts with SPAK to control renal activation of Na(+)-K(+)-Cl(−) cotransporter 2. Mol Cell Biol. 2010;30:3027–3037. doi: 10.1128/MCB.01560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Reisert J, Lai J, Yau KW, Bradley J. Mechanism of the excitatory Cl− response in mouse olfactory receptor neurons. Neuron. 2005;45:553–561. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci. 2008;121:3293–3304. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- 240.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 241.Rinehart J, Vázquez N, Kahle KT, Hodson CA, Ring AM, Gulcicek EE, Louvi A, Bobadilla NA, Gamba G, Lifton RP. WNK2 kinase is a novel regulator of essential neuronal cation-chloride cotransporters. J Biol Chem. 2011;286:30171–30180. doi: 10.1074/jbc.M111.222893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ringel F, Plesnila N. Expression and functional role of potassium-chloride cotransporters (KCC) in astrocytes and C6 glioma cells. Neurosci Lett. 2008;442:219–223. doi: 10.1016/j.neulet.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 243.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 244.Rokhlin OW, Scheinker VS, Taghiyev AF, Bumcrot D, Glover RA, Cohen MB. MicroRNA-34 mediates AR-dependent p53-induced apoptosis in prostate cancer. Cancer Biol Ther. 2008;7:1288–1296. doi: 10.4161/cbt.7.8.6284. [DOI] [PubMed] [Google Scholar]
- 245.Rossant J. Mouse mutants and cardiac development: new molecular insights into cardiogenesis. Circ Res. 1996;78:349–353. doi: 10.1161/01.res.78.3.349. [DOI] [PubMed] [Google Scholar]
- 246.Rubenstein EM, Schmidt MC. Mechanisms regulating the protein kinases of Saccharomyces cerevisiae. Eukaryot Cell. 2007;6:571–583. doi: 10.1128/EC.00026-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ruiz OS, Robey RB, Qiu YY, Wang LJ, Li CJ, Ma J, Arruda JA. Regulation of the renal Na-HCO(3) cotransporter. XI. Signal transduction underlying CO(2) stimulation. Am J Physiol Renal Physiol. 1999;277:F580–F586. doi: 10.1152/ajprenal.1999.277.4.F580. [DOI] [PubMed] [Google Scholar]
- 248.Rutledge E, Denton J, Strange K. Cell cycle- and swelling-induced activation of a Caenorhabditis elegans ClC channel is mediated by CeGLC-7alpha/beta phosphatases. J Cell Biol. 2002;158:435–444. doi: 10.1083/jcb.200204142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sakurai T, Ramoz N, Barreto M, Gazdoiu M, Takahashi N, Gertner M, Dorr N, Gama Sosa MA, De Gasperi R, Perez G, Schmeidler J, Mitropoulou V, Le HC, Lupu M, Hof PR, Elder GA, Buxbaum JD. Slc25a12 disruption alters myelination and neurofilaments: a model for a hypomyelination syndrome and childhood neurodevelopmental disorders. Biol Psychiatry. 2010;67:887–894. doi: 10.1016/j.biopsych.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA. 2009;106:4384–4389. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Schliess F, Schäfer C, vom Dahl S, Fischer R, Lordnejad MR, Häussinger D. Expression and regulation of the Na(+)/K(+)/2Cl(−) cotransporter NKCC1 in rat liver and human HuH-7 hepatoma cells. Arch Biochem Biophys. 2002;401:187–197. doi: 10.1016/S0003-9861(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 252.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE. Phenotype resembling Gitelman's syndrome in mice lacking the apical Na+- Cl− cotransporter of the distal convoluted tubule. J Biol Chem. 1998;273:29150–29155. doi: 10.1074/jbc.273.44.29150. [DOI] [PubMed] [Google Scholar]
- 253.Seyberth HW, Schlingmann KP. Bartter- and Gitelman-like syndromes: salt-losing tubulopathies with loop or DCT defects. Pediatr Nephrol. 2011;26:1789–1802. doi: 10.1007/s00467-011-1871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 255.Shen MR, Chou CY, Hsu KF, Hsu YM, Chiu WT, Tang MJ, Alper SL, Ellory JC. KCl cotransport is an important modulator of human cervical cancer growth and invasion. J Biol Chem. 2003;278:39941–39950. doi: 10.1074/jbc.M308232200. [DOI] [PubMed] [Google Scholar]
- 256.Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- 257.Shirakabe K, Priori G, Yamada H, Ando H, Horita S, Fujita T, Fujimoto I, Mizutani A, Seki G, Mikoshiba K. IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO3− cotransporter 1 (pNBC1) Proc Natl Acad Sci USA. 2006;103:9542–9547. doi: 10.1073/pnas.0602250103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Simon DB, Nelson-Williams C, Johnson Bia M, Ellison D, Karet FE, Morey Molina A, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitelman HJ, Lifton RP. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nature Genetics. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 259.Smith L, Smallwood N, Altman A, Liedtke CM. PKC delta acts upstream of SPAK in the activation of NKCC1 by hyperosmotic stress in human airway epithelial cells. J Biol Chem. 2008;283:22147–22156. doi: 10.1074/jbc.M801752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.So PL, Danielian PS. Cloning and expression analysis of a mouse gene related to Drosophila odd-skipped. Mech Dev. 1999;84:157–160. doi: 10.1016/s0925-4773(99)00058-1. [DOI] [PubMed] [Google Scholar]
- 261.Sohara E, Rai T, Yang SS, Ohta A, Naito S, Chiga M, Nomura N, Lin SH, Vandewalle A, Ohta E, Sasaki S, Uchida S. Acute insulin stimulation induces phosphorylation of the Na-Cl cotransporter in cultured distal mpkDCT cells and mouse kidney. PLoS One. 2011;6:e24277. doi: 10.1371/journal.pone.0024277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sontheimer H. Ion channels and amino acid transporters support the growth and invasion of primary brain tumors. Mol Neurobiol. 2004;29:61–71. doi: 10.1385/MN:29:1:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sontheimer H. An unexpected role for ion channels in brain tumor metastasis. Exp Biol Med (Maywood) 2008;233:779–791. doi: 10.3181/0711-MR-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics. 1999;153:135–177. doi: 10.1093/genetics/153.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Stegert MR, Hergovich A, Tamaskovic R, Bichsel SJ, Hemmings BA. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol Cell Biol. 2005;25:11019–11029. doi: 10.1128/MCB.25.24.11019-11029.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 267.Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 268.Su YC, Han J, Xu S, Cobb M, Skolnik EY. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. EMBO J. 1997;16:1279–1290. doi: 10.1093/emboj/16.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Sun D, Lytle C, O'Donnell ME. Astroglial cell-induced expression of Na-K-Cl cotransporter in brain microvascular endothelial cells. Am J Physiol (Cell Physiol) 1995;269:C1506–C1512. doi: 10.1152/ajpcell.1995.269.6.C1506. [DOI] [PubMed] [Google Scholar]
- 270.Sun D, Lytle C, O'Donnell ME. IL-6 secreted by astroglial cells regulates Na-K-Cl cotransport in brain microvessel endothelial cells. Am J Physiol Cell Physiol. 1997;272:C1829–C1835. doi: 10.1152/ajpcell.1997.272.6.C1829. [DOI] [PubMed] [Google Scholar]
- 271.Sung K-W, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA-receptor mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Suvitayavat W, Dunham PB, Haas M, Rao MC. Characterization of the proteins of the intestinal Na+-K+-2Cl− cotransporter. Am J Physiol (Cell Physiol) 1994;267:C375–C384. doi: 10.1152/ajpcell.1994.267.2.C375. [DOI] [PubMed] [Google Scholar]
- 273.Taira K, Umikawa M, Takei K, Myagmar BE, Shinzato M, Machida N, Uezato H, Nonaka S, Kariya K. The Traf2- and Nck-interacting kinase as a putative effector of Rap2 to regulate actin cytoskeleton. J Biol Chem. 2004;279:49488–49496. doi: 10.1074/jbc.M406370200. [DOI] [PubMed] [Google Scholar]
- 274.Takahashi Y, Hursting SD, Perkins SN, Wang TC, Wang TT. Genistein affects androgen-responsive genes through both androgen- and estrogen-induced signaling pathways. Mol Carcinog. 2006;45:18–25. doi: 10.1002/mc.20153. [DOI] [PubMed] [Google Scholar]
- 275.Talati G, Ohta A, Rai T, Sohara E, Naito S, Vandewalle A, Sasaki S, Uchida S. Effect of angiotensin II on the WNK-OSR1/SPAK-NCC phosphorylation cascade in cultured mpkDCT cells and in vivo mouse kidney. Biochem Biophys Res Commun. 2010;393:844–848. doi: 10.1016/j.bbrc.2010.02.096. [DOI] [PubMed] [Google Scholar]
- 276.Tamari M, Daigo Y, Nakamura Y. Isolation and characterization of a novel serine threonine kinase gene on chromosome 3p22-21.3. J Hum Genet. 1999;44:116–120. doi: 10.1007/s100380050121. [DOI] [PubMed] [Google Scholar]
- 277.Tang J, Bouyer P, Mykoniatis A, Buschmann M, Matlin KS, Matthews JB. Activated PKC{delta} and PKC{epsilon} inhibit epithelial chloride secretion response to cAMP via inducing internalization of the Na+-K+-2Cl− cotransporter NKCC1. J Biol Chem. 2010;285:34072–34085. doi: 10.1074/jbc.M110.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tassi E, Biesova Z, Di Fiore PP, Gutkind JS, Wong WT. Human JIK, a novel member of the STE20 kinase family that inhibits JNK and is negatively regulated by epidermal growth factor. J Biol Chem. 1999;274:33287–33295. doi: 10.1074/jbc.274.47.33287. [DOI] [PubMed] [Google Scholar]
- 279.Taylor JS, Raes J. Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet. 2004;38:615–643. doi: 10.1146/annurev.genet.38.072902.092831. [DOI] [PubMed] [Google Scholar]
- 280.Thastrup JO, Rafiqi FH, Vitari AC, Pozo-Guisado E, Deak M, Mehellou Y, Alessi DR. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J. 2012;441 doi: 10.1042/BJ20111879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Tiwari S, Riazi S, Ecelbarger CA. Insulin's impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol. 2007;293:F974–F984. doi: 10.1152/ajprenal.00149.2007. [DOI] [PubMed] [Google Scholar]
- 282.Tomomura M, Fernandez-Gonzales A, Yano R, Yuzaki M. Characterization of the apoptosis-associated tyrosine kinase (AATYK) expressed in the CNS. Oncogene. 2001;20:1022–1032. doi: 10.1038/sj.onc.1204210. [DOI] [PubMed] [Google Scholar]
- 283.Tomomura M, Furuichi T. Apoptosis-associated tyrosine kinase (AATYK) has differential Ca2+-dependent phosphorylation states in response to survival and apoptotic conditions in cerebellar granule cells. J Biol Chem. 2005;280:35157–35163. doi: 10.1074/jbc.M500353200. [DOI] [PubMed] [Google Scholar]
- 284.Tomomura M, Hasegawa Y, Hashikawa T, Tomomura A, Yuzaki M, Furuichi T, Yano R. Differential expression and function of apoptosis-associated tyrosine kinase (AATYK) in the developing mouse brain. Brain Res Mol Brain Res. 2003;112:103–112. doi: 10.1016/s0169-328x(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 285.Tomomura M, Morita N, Yoshikawa F, Konishi A, Akiyama H, Furuichi T, Kamiguchi H. Structural and functional analysis of the apoptosis-associated tyrosine kinase (AATYK) family. Neuroscience. 2007;148:510–521. doi: 10.1016/j.neuroscience.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 286.Topper JN, Wasserman SM, Anderson KR, Cai J, Falb D, Gimbrone MAJ. Expression of the bumetanide-sensitive Na-K-Cl cotransporter BSC2 is differentially regulated by fluid mechanical and inflammatory cytokine stimuli in vascular endothelium. J Clin Invest. 1997;99:2941–2949. doi: 10.1172/JCI119489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Tripodi G, Valtorta F, Torielli L, Chieregatti E, Salardi S, Trusolino L, Menegon A, Ferrari P, Marchisio PC, Bianchi G. Hypertension-associated point mutations in the adducin alpha and beta subunits affect actin cytoskeleton and ion transport. J Clin Invest. 1996;97:2815–2822. doi: 10.1172/JCI118737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tu H, Wigler M. Genetic evidence for Pak1 autoinhibition and its release by Cdc42. Mol Cell Biol. 1999;19:602–611. doi: 10.1128/mcb.19.1.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tu SW, Bugde A, Luby-Phelps K, Cobb MH. WNK1 is required for mitosis and abscission. Proc Natl Acad Sci USA. 2011;108:1385–1390. doi: 10.1073/pnas.1018567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ushiro H, Tsutsumi T, Suzuki K, Kayahara T, Nakano K. Molecular cloning and characterization of a novel Ste20-related protein kinase enriched in neurons and transporting epithelia. Arch Biochem Biophys. 1998;355:233–240. doi: 10.1006/abbi.1998.0736. [DOI] [PubMed] [Google Scholar]
- 291.Uvarov P, Ludwig A, Markkanen M, Pruunsild P, Kaila K, Delpire E, Timmusk T, Rivera C, Airaksinen MS. A Novel N-terminal Isoform of the Neuron-Specific K-Cl cotransporter KCC2. J Biol Chem. 2007;282:30570–30576. doi: 10.1074/jbc.M705095200. [DOI] [PubMed] [Google Scholar]
- 292.van der Lubbe N, Lim CH, Fenton RA, Meima ME, Jan Danser AH, Zietse R, Hoorn EJ. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 2011;79:66–76. doi: 10.1038/ki.2010.290. [DOI] [PubMed] [Google Scholar]
- 293.Varga R, Kelley PM, Keats BJ, Starr A, Leal SM, Cohn E, Kimberling WJ. Non-syndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J Med Genet. 2003;40:45–50. doi: 10.1136/jmg.40.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Vavolizza RD, Schmeidler J, Ramoz N, Buxbaum JD, Smith CJ, Silverman JM. The Effect of an Autism-Associated Polymorphism in the STK39 Gene on the Autism Symptom Domains. J Autism Dev Disord. 2012;42:319–320. doi: 10.1007/s10803-011-1226-9. [DOI] [PubMed] [Google Scholar]
- 295.Velazquez H, Naray-Fejes-Toth A, Silva T, Andujar E, Reilly RF, Desir GV, Ellison DH. Rabbit distal convoluted tubule coexpresses NaCl cotransporter and 11 beta-hydroxysteroid dehydrogenase II mRNA. Kidney Int. 1998;54:464–472. doi: 10.1046/j.1523-1755.1998.00036.x. [DOI] [PubMed] [Google Scholar]
- 296.Venter JC, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 297.Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene. 2001;20:5562–5569. doi: 10.1038/sj.onc.1204726. [DOI] [PubMed] [Google Scholar]
- 298.Villa F, Goebel J, Rafiqi FH, Deak M, Thastrup J, Alessi DR, van Aalten DMF. Structural insights into the recognition of substrates and activators by the OSR1 kinase. EMBO Rep. 2007;8:839–845. doi: 10.1038/sj.embor.7401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Villa FMD, Alessi DR, van Aalten DM. Structure of the OSR1 kinase, a hypertension drug target. Proteins. 2008;73:1082–1087. doi: 10.1002/prot.22238. [DOI] [PubMed] [Google Scholar]
- 300.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome, phosphorylate and active SPAK and OSR1 protein kinases. Biochem J. 2005;391:17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wallace BK, Foroutan S, O'Donnell ME. Ischemia-induced stimulation of Na-K-Cl cotransport in cerebral microvascular endothelial cells involves AMP kinase. Am J Physiol Cell Physiol. 2011;301:C316–C326. doi: 10.1152/ajpcell.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Walsh T, Walsh V, Vreugde S, Hertzano R, Shahin H, Haika S, Lee MK, Kanaan M, King MC, Avraham KB. From flies' eyes to our ears: mutations in a human class III myosin cause progressive nonsyndromic hearing loss DFNB30. Proc Natl Acad Sci U S A. 2002;99:7518–7523. doi: 10.1073/pnas.102091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Walter SA, Cutler RE, Jr, Martinez R, Gishizky M, Hill RJ. Stk10, a new member of the polo-like kinase kinase family highly expressed in hematopoietic tissue. J Biol Chem. 2003;278:18221–18228. doi: 10.1074/jbc.M212556200. [DOI] [PubMed] [Google Scholar]
- 304.Wang Y, O'Connell JR, McArdle PF, Wade JB, Dorff SE, Shah SJ, Shi X, Pan L, Rampersaud E, Shen H, Kim JD, Subramanya AR, Steinle NI, Parsa A, Ober CC, Welling PA, Chakravarti A, Weder AB, Cooper RS, Mitchell BD, Shuldiner AR, Chang YP. Whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci U S A. 2009;106:226–231. doi: 10.1073/pnas.0808358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Watkins S, Sontheimer H. Hydrodynamic cellular volume changes enable glioma cell invasion. J Neurosci. 2011;31:17250–17259. doi: 10.1523/JNEUROSCI.3938-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Welling P, Chang C, Delpire E, Wade J. Multigene kinase network, kidney transport and salt in essential hypertension. Kid Int. 2010;77:1063–1069. doi: 10.1038/ki.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wells CM, Jones GE. The emerging importance of group II PAKs. Biochem J. 2010;425:465–473. doi: 10.1042/BJ20091173. [DOI] [PubMed] [Google Scholar]
- 308.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 309.Willis WD. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- 310.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 311.Woo N-S, Lu J, England R, McClellan R, Dufour S, Mount DB, Deutch AY, Lovinger DM, Delpire E. Hyper-excitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus. 2002;12:258–268. doi: 10.1002/hipo.10014. [DOI] [PubMed] [Google Scholar]
- 312.Wu Q, Delpire E, Hebert SC, Strange K. Functional demonstration of Na-K-2Cl cotransporter activity in isolated, polarized choroid plexus cells. Am J Physiol Cell Physiol. 1998;275:C1565–C1572. doi: 10.1152/ajpcell.1998.275.6.C1565. [DOI] [PubMed] [Google Scholar]
- 313.Xie J, Wu T, Xu K, Huang IK, Cleaver O, Huang CL. Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice. Am J Pathol. 2009;175:1315–1327. doi: 10.2353/ajpath.2009.090094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275:16795–16801. doi: 10.1074/jbc.275.22.16795. [DOI] [PubMed] [Google Scholar]
- 315.Xu B-e, Min X, Stippec S, Lee B-H, Goldsmith EJ, Cobb MH. Regulation of WNK1 by an Autoinhibitory Domain and Autophosphorylation. J Biol Chem. 2002;277:48456–48462. doi: 10.1074/jbc.M207917200. [DOI] [PubMed] [Google Scholar]
- 316.Yamagishi N, Ishihara K, Hatayama T. Hsp105alpha suppresses Hsc70 chaperone activity by inhibiting Hsc70 ATPase activity. J Biol Chem. 2004;279:41727–41733. doi: 10.1074/jbc.M407947200. [DOI] [PubMed] [Google Scholar]
- 317.Yamagishi N, Nishihori H, Ishihara K, Ohtsuka K, Hatayama T. Modulation of the chaperone activities of Hsc70/Hsp40 by Hsp105alpha and Hsp105beta. Biochem Biophys Res Commun. 2000;272:850–855. doi: 10.1006/bbrc.2000.2864. [DOI] [PubMed] [Google Scholar]
- 318.Yamagishi N, Saito Y, Hatayama T. Mammalian 105 kDa heat shock family proteins suppress hydrogen peroxide-induced apoptosis through a p38 MAPK-dependent mitochondrial pathway in HeLa cells. FEBS J. 2008;275:4558–4570. doi: 10.1111/j.1742-4658.2008.06598.x. [DOI] [PubMed] [Google Scholar]
- 319.Yamamoto Y, Izumi Y, Matsuzaki F. The GC kinase Fray and Mo25 regulate Drosophila asymmetric divisions. Biochem Biophys Res Commun. 2008;366:212–218. doi: 10.1016/j.bbrc.2007.11.128. [DOI] [PubMed] [Google Scholar]
- 320.Yan Y, Dalmasso G, Nguyen HT, Obertone TS, Charrier-Hisamuddin L, Sitaraman SV, Merlin D. Nuclear factor-kappaB is a critical mediator of Ste20-like proline-/alanine-rich kinase regulation in intestinal inflammation. Am J Pathol. 2008;173:1013–1028. doi: 10.2353/ajpath.2008.080339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yan Y, Dalmasso G, Nguyen HT, Obertone TS, Sitaraman SV, Merlin D. Ste20-related proline/alanine-rich kinase (SPAK) regulated transcriptionally by hyperosmolarity is involved in intestinal barrier function. PLoS One. 2009;4:e5049. doi: 10.1371/journal.pone.0005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yan Y, Laroui H, Ingersoll SA, Ayyadurai S, Charania M, Yang S, Dalmasso G, Obertone TS, Nguyen H, Sitaraman SV, Merlin D. Overexpression of Ste20-Related Proline/Alanine-Rich Kinase Exacerbates Experimental Colitis in Mice. J Immunol. 2011;187:1496–1505. doi: 10.4049/jimmunol.1002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yan Y, Nguyen H, Dalmasso G, Sitaraman SV, Merlin D. Cloning and characterization of a new intestinal inflammation-associated colonic epithelial Ste20-related protein kinase isoform. Biochim Biophys Acta. 2007;1769:106–116. doi: 10.1016/j.bbaexp.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yang CL, Liu X, Paliege A, Zhu X, Bachmann S, Dawson DC, Ellison DH. WNK1 and WNK4 modulate CFTR activity. Biochem Biophys Res Comm. 2007;353:535–540. doi: 10.1016/j.bbrc.2006.11.151. [DOI] [PubMed] [Google Scholar]
- 325.Yang D, Li Q, So I, Huang CL, Ando H, Mizutani A, Seki G, Mikoshiba K, Thomas PJ, Muallem S. IRBIT governs epithelial secretion in mice by antagonizing the WNK/SPAK kinase pathway. J Clin Invest. 2011;121:956–965. doi: 10.1172/JCI43475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Yang D, Shcheynikov N, Zeng W, Ohana E, So I, Ando H, Mizutani A, Mikoshiba K, Muallem S. IRBIT coordinates epithelial fluid and HCO3− secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J Clin Invest. 2009;119:193–202. doi: 10.1172/JCI36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol. 2010;21:1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metabolism. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 329.Yang T, Roder KE, Bhat GJ, Thekkumkara TJ, Abbruscato TJ. Protein kinase C family members as a target for regulation of blood-brain barrier Na,K,2Cl-cotransporter during in vitro stroke conditions and nicotine exposure. Pharm Res. 2006;23:291–302. doi: 10.1007/s11095-005-9143-2. [DOI] [PubMed] [Google Scholar]
- 330.Yasuda K, Nakai A, Hatayama T, Nagata K. Cloning and expression of murine high molecular mass heat shock proteins, HSP105. J Biol Chem. 1995;270:29718–29723. doi: 10.1074/jbc.270.50.29718. [DOI] [PubMed] [Google Scholar]
- 331.Yasunaga S, Grati M, Chardenoux S, Smith TN, Friedman TB, Lalwani AK, Wilcox ER, Petit C. OTOF encodes multiple long and short isoforms: genetic evidence that the long ones underlie recessive deafness DFNB9. Am J Hum Genet. 2000;67:591–600. doi: 10.1086/303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yasunaga S, Grati M, Cohen-Salmon M, El-Amraoui A, Mustapha M, Salem N, El-Zir E, Loiselet J, Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nature Genet. 1999;21:363–369. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]
- 333.Yustein JT, Xia L, Kahlenburg JM, Robinson D, Templeton D, Kung HJ. Comparative studies of a new subfamily of human Ste20-like kinases: homodimerization, subcellular localization, and selective activation of MKK3 and p38. Oncogene. 2003;22:6129–6141. doi: 10.1038/sj.onc.1206605. [DOI] [PubMed] [Google Scholar]
- 334.Zeqiraj E, Filippi BM, Deak M, Alessi DR, van Aalten DM. Structure of the LKB1-STRAD-MO25 complex reveals an allosteric mechanism of kinase activation. Science. 2009;326:1707–1711. doi: 10.1126/science.1178377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zhang J. Evolution by gene duplication: an update. Trends Ecol Evol. 2003;18:292–298. [Google Scholar]
- 336.Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. J Biol Chem. 2007;282 doi: 10.1074/jbc.M611329200. [DOI] [PubMed] [Google Scholar]
- 337.Zhou T, Raman M, Gao Y, Earnest SCZ, Machius M, Cobb MH, Goldsmith EJ. Crystal structure of the TAO2 kinase domain: activation and specificity of a Ste20p MAP3K. Structure. 2004;12:1891–1900. doi: 10.1016/j.str.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 338.Zhu L, Polley N, Mathews GC, Delpire E. NKCC1 and KCC2 Prevent Hyperexcitability in the Mouse Hippocampus. Epilepsy Res. 2008;79:201–212. doi: 10.1016/j.eplepsyres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]