Abstract
As typical biofoulers, barnacles possess hard shells and cause serious biofouling problems. In this study, we analyzed the protein component of the barnacle Amphibalanus (= Balanus) amphitrite shell using gel-based proteomics. The results revealed 52 proteins in the A. Amphitrite shell. Among them, 40 proteins were categorized into 11 functional groups based on KOG database, and the remaining 12 proteins were unknown. Besides the known proteins in barnacle shell (SIPC, carbonic anhydrase and acidic acid matrix protein), we also identified chorion peroxidase, C-type lectin-like domains, serine proteases and proteinase inhibitor proteins in the A. Amphitrite shell. The sequences of these proteins were characterized and their potential functions were discussed. Histology and DAPI staining revealed living cells in the shell, which might secrete the shell proteins identified in this study.
Introduction
Biomineralization is a widespread phenomenon in organisms. The variety of solid inorganic structures formed by biomineralization are used for support, protection, mastication, gravity perception, and various other functions [1].
The barnacle, which is a crustacean, has a hard shell that is a result of biomineralization. Unlike other crustaceans (for instance, shrimp and crab), for which the shells are periodically shed and rebuilt for the purposes of growth, regeneration, metamorphosis, and reproduction [2, 3], barnacle shells grow continously throughout their life, and only the interior cuticle is moltted to make more space for the softbody. The barnacle shell consists of a chitin-protein microfibril framework and is mineralized with calcite [4]. The inner lamina of barnacle shell consists of parallel calcified layers separated by organic sheets, and these sheets show autofluorescence and consist of chitin surrounded by proteoglycans and other minor proteins [5].
So far, only a few studies have focused on the protein component of barnacle shells. Khalifa et al. [4] revealed highly acidic proteins in the matrix components of the Amphibalanus (= Balanus) amphitrite shell. A glycoprotein, namely settlement inducing protein complex (SIPC) is also present in barnacle shell [6–8]. Khandeparker and Anil [9] charaterized arthropodin protein complex from barnacel extracts and identified two undescribed subunits (66-kDa and 98-kDa) in barnacle shell. In the basal plate of Magabalanus rosa, Kamino's group solubilized the proteins of secondary cement and found that the cement is composed of at least two distinct groups of proteins (formic-acid-insoluble proteins and highly hydoxylated proteins) [10]. Later, they further identifed several cememts proteins (e.g. Mrcp-68k, Mrcp-100k, Mrcp-52k [11] and Mrcp-19k [12]) from the basal plate.
In the present study, we sought to profile the chemical and protein components of the adult shell of the typical intertidal barnacle A. amphitrite using X-ray fluorescence (XRF) analysis and gel-based proteomics. An understanding of the proteome and clarification of its roles in the barnacle shell will contribute more information to practical engineering processes and the synthesis of novel materials [13].
Results
X-ray fluorescence analysis of the barnacle shell
The XRF analysis revealed calcium as the major component of the A. amphitrite shell, occupying more than 92% of both the weight and molar percentages. Small amounts of Na, Mg and Sr were also detected in the shell (Table 1 and S1 Fig).
Table 1. Results for the Amphibalanus amphitrite shell using the XRF-technique (quantitative analysis).
| Element | Line | Energy | ms% | mol% | K | Net | Error% |
|---|---|---|---|---|---|---|---|
| Na | K | 1.04 | 1.3994 | 2.4013 | 0.0030933 | 870 | 13.1503 |
| Mg | K | 1.25 | 0.9925 | 1.6104 | 0.0035363 | 2016 | 2.0118 |
| S | K | 2.31 | 1.3228 | 1.6275 | 0.0185220 | 51170 | 0.0549 |
| Cl | K | 2.62 | 1.3704 | 1.5249 | 0.0184698 | 41130 | 0.1255 |
| Ca | K | 3.69 | 93.8160 | 92.3411 | 0.7592769 | 1638546 | 0.1926 |
| Sr | K | 14.14 | 1.0989 | 0.4948 | 0.0184209 | 63678 | 0.1099 |
Proteomics analysis of the barnacle shell
The total protein from whole barnacle shell was extracted in acetic acid, 1% SDS buffer, and 10% SDS buffer sequentially. Three fractions were collected and analyzed independently. The PAGE gel revealed clear bands for the extracts from the barnacle shell (Fig 1). The combination of MS results from all three fractions led to the identification of a total of 52 proteins in our transcriptome database (S2 Fig). Among these proteins, 20, 3, and 12 were uniquely present in the acetic acid, 1% SDS, and 10% SDS fractions, respectively. Six proteins were shared by all three fractions. Based on the KOG database, 40 proteins were categorized into 11 functional groups; the remaining 12 proteins were considered as hypothetical proteins (S1 Table).
Fig 1. PAGE image showing protein extracts of the Amphibalanus amphitrite shell.
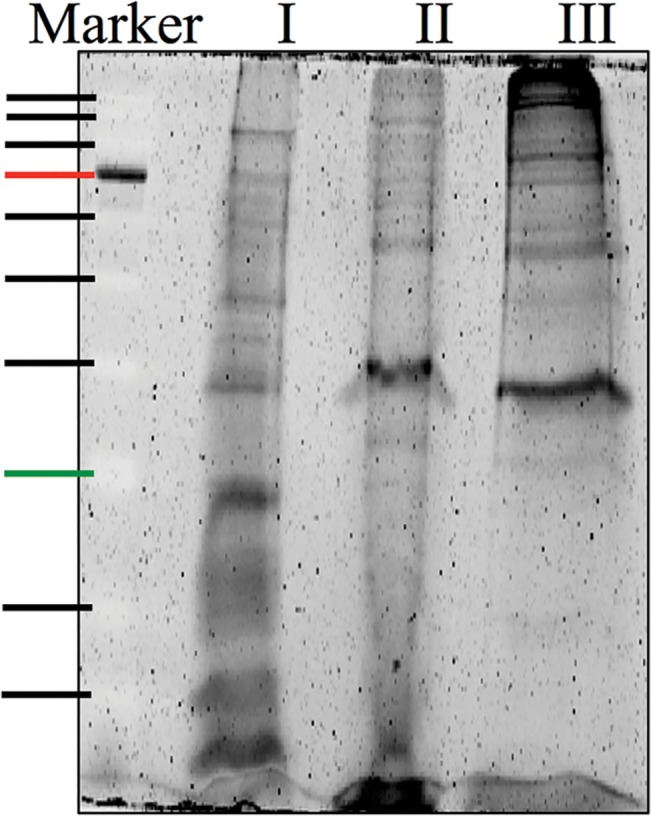
The gel was stained by SYPRO Ruby dye (Invitrogen) and the later coomassie blue staining confirmed the molecular weight of each band in the marker. Lane I: acetic acid fraction; Lane II: 1% SDS fraction; Lane III: 10% SDS fraction. Marker from top to bottom: 170, 130, 93, 70, 53, 41, 30, 22, 14 and 9 kDa.
Settlement-inducing protein complex
The settlement-inducing protein complex (SIPC) is a glycoprotein and has been previously detected in the A. amphitrite shell by Western blot analysis [6]. MS analysis detected SIPC in both the 1% and 10% SDS fractions but not in the acetic acid fraction.
Shell matrix proteins
An acidic shell matrix protein (coded by CL6615.Contig1_Ba_mix) was identified in the acetic acid fraction (Fig 2). This protein did not retrieve any known proteins using Blastp or tBlastn in NCBI. The partial sequence (381 amino acids) was used to calculate the most dominant amino acids, where were Asp (41.2%), Glu (21.8%) and Gly (14.2%) (S2 Table). The pI was estimated at 2.68. Compared with Asprich from A. rigida, this protein displayed an identity of 38.6% and a similarity of 49.0%; a slightly lower similarity (31.8%) and identity (43.7%) were obtained for this protein compared to Aspein from P. fucata.
Fig 2. Comparison of an Asp-rich shell matrix protein from Amphibalanus amphitrite with Asprich (Atrina rigida; AAU04808.1) and Aspein (Pinctada fucata; AB094512).
The Asp-rich shell matrix protein from A. amphitrite mainly contained Asp punctuated with Glu or Gly, which are labeled in bold with a single underline. Asprich includes three poly(Asp) motifs (presented in block with a single underline) and two DEAD motifs (presented in bold with a double underline). Aspein consists of mostly poly(Asp) blocks punctuated with Ser-Gly dipeptides (presented in bold with a single underline).
Another protein coded by the contig CL4062.Contig1_Ba_mix was detected in all three fractions. This protein was annotated as similar to a known matrix protein called Prisilkin-39 with an identity of 44%. Based on its partial sequence, this protein had a high ratio of Gly (19.4%), Tyr (20.8%) and Ser (11.8%) in sequence component. Similarly to oyster Prisilkin-39 (ACJ06766.1, pI = 8.83), this protein has a basic isoelectric point (pI = 9.75).
Serine proteases and serine protease inhibitor
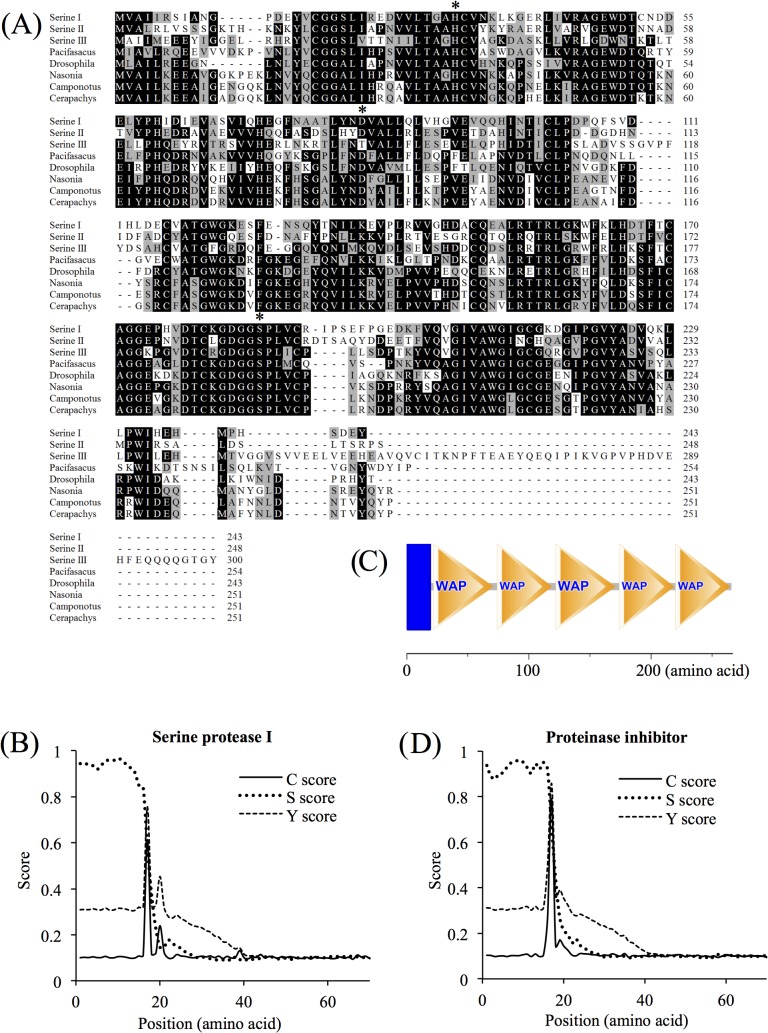
In the 1% and 10% SDS fractions, 3 different proteins were revealed to contain a trypsin-like serine protease domain by motif scanning. These proteins were named serine protease I, II, and III (Table 2). Among them, serine protease I and II were much longer than serine protease III. After alignment, the serine protease domains of these three proteins revealed a high similarity to the serine protease from Pacifasacus, Drosophila, Nasonia, Camponotus and Cerapachys (Fig 3A). Three catalytic triad active-site residues (H, D and S) [14] were all conserved in these three serine protease isoforms (Fig 3A). Prediction of the signal peptide revealed that the first 16 amino acids (MMRWVLLASLAALASS) were a signal peptide of serine protease I. However, no signal peptide was detected in serine protease II or III (Fig 3B).
Table 2. Physical and chemical parameters of three serine protease isoforms and chorionic proteinase inhibitor in Amphibalanus amphitrite.
| Serine Protease I | Serine protease II | Serine protease III | chorionic proteinase inhibitor | |
|---|---|---|---|---|
| Contig | CL563.Contig1 | CL1641.Contig1 | CL200.Contig3 | CL5577.Contig1 |
| Present fractions | 10% SDS | 10% SDS | 1% SDS | acetic acid |
| Sequence length (AA) | 659 | 890 | 300 | 274 |
| MW (kDa) | 66.0 | 91.5 | 33.2 | 29.6 |
| pI | 4.88 | 5.74 | 5.44 | 7.29 |
| Instability index | 30.38 (stable) | 34.28 (stable) | 44.00 (unstable) | 40.25 (unstable) |
| Signal peptide | yes | no | no | yes |
Fig 3. Sequence analysis of serine proteases and chorionic proteinase inhibitor from Amphibalanus amphitrite.
(A). Alignment of three serine proteases from A. amphitrite (I, II, and III) with Pacifastacus leniusculus (ACB41380), Drosophila sechellia (XP002036473.1), Nasonia vitripennis (NP001155060), Camponotus floridanus (EFN72618.1), and Cerapachys biroi (EZA53191.1). The three catalytic triad active-site residues (H, D and S) were all conserved in the three serine protease isoforms from A. amphitrite and marked with an asterisk. (B). Signal peptide prediction in serine protease I from A. amphitrite. The first 16 amino acids were predicted to be a signal peptide. C-score (raw cleavage site score), S-score (signal peptide score) and Y-score (combined cleavage site score) were calculated using the SingalP 4.1 Server. (C). Motif scanning revealed that the chorionic proteinase inhibitor from A. amphitrite contained 1 transmembrane domain (blue rectangle) and 5 WAP (whey acidic protein) domains (yellow triangle). (D). Signal peptide prediction suggested that the first 16 residues represented a signal peptide in the chorionic proteinase inhibitor.
In the acetic acid fraction, the protein coded by the contig CL5577.Contig1_Ba_mix displayed 29% similarity with the chorionic proteinase inhibitor from Tribolodon hakonensis. Although the identification score of this protein was only 35, MS2 detected 11 peptides matching with this protein. Based on the sequence in our transcriptome database, 5 continuous whey acidic protein (WAP) domains were present in the corresponding protein (Fig 3C). The WAP domain possesses peptidase inhibitor activity [15], and therefore, this contig was considered to be a protease inhibitor in the barnacle shell. The first 16 amino acids (MASWIVLLLLAPAISA) were predicted as a signal peptide using the SignalP 4.1 Server (Fig 3D).
Shell hardening-related proteins
In the barnacle shell, three homologs of carbonic anhydrase were detected. The first one (coded by CL15286.Contig1_Ba_mix; A. amphitrite I) was detected in all three fractions. Its partial sequence, containing 278 amino acid residues, showed 30% identity with alpha-carbonic anhydrase from Daphnia pulex (EFX81683.1). The second (coded by CL10121.Contig1_Ba_mix; A. amphitrite II) and third (coded by Unigene27659_Ba_mix; A. amphitrite III) proteins both showed 26% and 30% identity with D. pulex alpha-carbonic anhydrase, respectively, and were found in the 1% and 10% SDS fractions. All three putative histidine residues that function as zinc ligands [16] were conserved in carbonic anhydrase II, similarly to the majority of the residues forming the hydrogen-bond network to zinc-bound solvent molecules (Fig 4). However, for carbonic anhydrase I and III, two of the three histidines that function as zinc ligands were mutated (Fig 4).
Fig 4. Alignment of the carbonic anhydrase from Amphibalanus amphitrite (I, II and III), Daphnia pulex (EFX81683.1), Danio rerio (NP571185.1) and Hyriopsis cumingii (AHY35316.1).
Putative histidine residues that function as zinc ligands are marked with filled circles above the residues. The residues forming the hydrogen-bonded network to zinc-bound solvent molecules are depicted by open circles.
A protein (coded by CL8390.Contig1_Ba_mix) identified in all three extractions showed 34% identity to the chorion peroxidase from the pea aphid Acyrthosiphon pisum (XP001946672.2) and 40% identity to that from the Chinese mitten crab Eriocheir sinensis (also named as peroxinectin; ADF87945.1). One putative integrin-binding motif was located at the C terminus of this protein, and this motif was mutated to YGD (Tyr-Gly-Asp) rather than the canonical sequence RGD (Arg-Gly-Asp) or KGD (Lys-Gly-Asp) (Fig 5).
Fig 5. Alignment of the deduced chorion peroxidase/peroxinectin from Amphibalanus amphitrite, Acyrthosiphon pisum (XP001946672.2), Eriocheir sinensis (ADF87945.1), Macrophthalmus japonicas (AID47197.1), and Penaeus monodon (AAL05973.1).
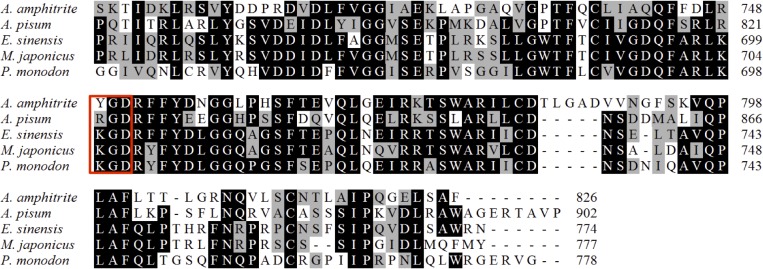
The putative integrin-binding motif is indicated by a red box, and in A. amphitrite, this motif was mutated to YGD (Tyr-Gly-Asp) rather than the canonical sequence RGD (Arg-Gly-Asp) or KGD (Lys-Gly-Asp).
Immune-related protein
Two proteins containing a C-type lectin-like domain were detected in the shell of A. amphitrite. Among them, the protein coded by CL14971.Contig1_Ba_mix was present in all three fractions, while the protein coded by Unigene5283_Ba_mix was detected in the 1% SDS fraction. After searching NCBI, both proteins were found to be the most similar to the mannose-binding protein from the mud crab Scylla paramamosain, with a similarity of 22.5% and 18.8%, respectively. Alignment with the C-type lectin-like domains from other animals revealed that both C-type lectin-like domains found in this study included a "QPD" motif in the Ca2+-binding site and 2 or 4 conserved cysteine residues (Fig 6).
Fig 6. Alignment of C-type lectin-like domains from Amphibalanus amphitrite (I and II), Scylla paramamosain (ADF27340.1), Fenneropenaeus indicus (ADV17348.1), Litopenaeus vannamei (AGV68681.1), and Danio rerio (XP005172687.1).

Similarly to S. paramamosain and F. indicus, the typical "EPD" or "EPN" motif in both C-type lectin-like domains (I and II) was mutated into "QPD" in A. amphitrite (boxed in red). At least two and four cysteines were conserved in the C-type lectin-like domains I and II, respectively. These conserved cysteines are indicated by arrows.
HE and DAPI staining
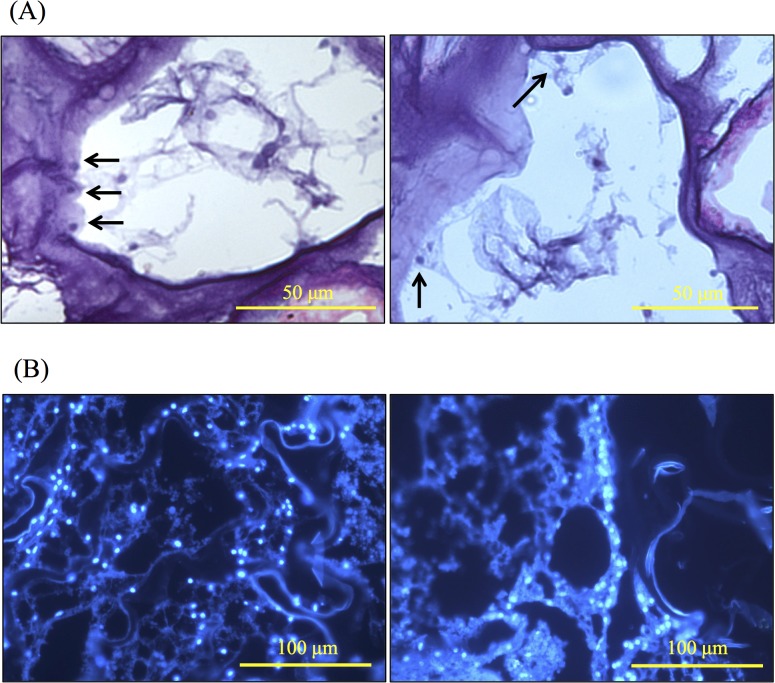
To evaluate the presence of living cells in the barnacle shell, decalcified shells were cut into thin sections and stained with HE or DAPI. At the lower part of the shell paries (just above the basal suture), HE staining revealed cell-shaped structures that were attached to the surfaces of the small inner channels around lacunaes (Fig 7A), and DAPI staining showed cell nucleuses (Fig 7B).
Fig 7. Hematoxylin-eosin (A) and DAPI staining (B) showing cells in histological sections of the Amphibalanus amphitrite shell.
Arrows identify potential cell structures.
Discussion
Chemical component of the barnacle shell
The mineral of barnacle shell is calcite [17] that is the most stable polymorph of calcium carbonate. In this study, the barnacle shell displayed effervescence in 5% acetic acid during decalcification, suggesting the presence of carbonate cement. The XRF analysis revealed high concentration of Ca in the A. amphitrite shell, which is similar to the shell of mollusks [18, 19], but different from some other crustacean shells, such as shrimp [20], crab, and lobster [21], in which Ca represents only 12–27.8% of the weight of the exoskeleton. A high content of Ca might contribute to the rigidity of the barnacle shell and subsequently protect the soft body.
Gordon et al. [22] showed that Na abundance in the Balanus shells varied from 0.16% to 0.50%, and was proportional to environmental salinity; Mn abundance ranged from 0.008% to 0.38%, and was related to the Mn concentration in the local seawater. In this study, Na represented 1.40% of weight percentage, but Mn was not detected. These results are different from Gordon et al. [22], which might be attributed to the related concentrations in the local environment.
In the shells of the barnacle Balanus balanoides and Elminius modestus, the ratio of Sr:Ca ranges from 7×10−3 to 9×10−3 [23]. In this study, the ratio of Sr:Ca was equal to 1.17×10−2, similarly to those in B. balanoides and E. modestus, indicating that ratio of Sr:Ca might be relatively constant among different barnacle species.
Proteomics analysis of the barnacle shell
Because shell proteins are often insoluble, highly acidic, and interact with minerals to form complexes, it is difficult to extract and purify them directly [24]. In the present study, to minimize the interference of carbonate minerals, barnacle shells were soaked in 5% acetic acid. Subsequently, the residues were extracted using a high concentration of urea, SDS, and DTT. These strong buffers should dissolve as many proteins as possible.
The proteins present in the acetic acid fraction should be relatively hydrophilic, whereas the proteins in the 1% and 10% SDS fractions should be more hydrophobic or cross-linked by disulfide bonds. Six proteins were shared by all three fractions, which suggested that these proteins might be abundant in the shell or present in different forms. For a given protein, some molecules might form complexes with other proteins or substrates, and a strong buffer may be required to dissolve these complexes; in contrast, other molecules might be freely present in epithelium cells and soluble in the acetic acid fraction.
Settlement-inducing protein complex
SIPC is not only a waterborne pheromone that induces the gregarious settlement of barnacle larvae [7], but also a contact pheromone of importance in species recognition [25], since it can be detected in the footprints deposited by cyprids [6] and the adult shells [8]. SIPC is expressed in the cuticles of both nauplius and cypris larvae as well as the adult, indicating that it is produced by the epidermal cells that secrete the cuticle [8]. The presence of SIPC in the barnacle shell suggested epidermal cells in the barnacle shell.
Shell matrix proteins
Unusual acidic proteins, such as Aspein and Asprich, have previously been identified in the shell matrix of mollusks [26, 27]. Aspein from the pearl oyster Pinctada fucata is rich in Asp (57.6%), Gly (15.5%), and Ser (12.8%) residues (S2 Table), with an isoelectric point (pI) of 1.45. Its sequence is almost completely occupied by poly(Asp) blocks punctuated with Ser-Gly dipeptides [26] (Fig 2). Asprich from the bivalve Atrina rigida is composed of 60.3% acidic amino acids (48.6% Asp and 11.7% Glu), with Gly occupying approximately 5.4% of the amino acid sequence (S2 Table). Its average pI is equal to 3.1 [27]. In Asprich, a domain with DEAD repeats displaying Mg2+-binding capability was identified [27]. To the best of our knowledge, both Aspein and Asprich have been only reported in bivalves, and not in any crustacean.
In this study, the protein coded by the contig CL6615.Contig1_Ba_mix was identified to be an acidic shell matrix protein, which displayed considerable similarity to the Asprich from A. rigida and the Aspein from P. fucata. However, the high similarity of the sequence alignment was not sufficient to confirm that this protein was Asprich or Aspein, because all of them were phylogenetically uninformative. All of these proteins contained extensive low-complexity regions (rich in Asp, Glu, or Gly), and phylogenetic alignment of these proteins would lead to false-positive results [28]. Based on the amino acid composition and sequence alignment, this acidic shell matrix protein was much more similar to Asprich than to Aspein. Nevertheless, it lacked either the typical "polyD punctured with SG" structure in Aspein (Fig 2) or the conserved DEAD motif in Asprich (Fig 2). Thus, we considered it to be a novel acidic shell matrix protein. Asp is associated with calcium-binding and possibly magnesium-binding activity through interactions with the carboxylate groups of Asp [27]. Poly(Glu) domains are responsible for protein aggregation [29]. High contents of Asp and poly(Glu) in the acidic shell matrix protein indicated that this protein might participate in the formation of the shell structure.
Prisilkin-39 was first detected in the shell of P. fucata [30], and then its homolog was found in fish (Cynoglossus semilaevis, XP008334378) and insect (Nasonia vitripennis, XP003424571). This protein is a highly repetitive protein with a high composition of Gly (29.72%), Tyr (23.26%), and Ser (18.60%) residues [30]. In the shell of P. fucata, Prisilkin-39 binds tightly with chitin, an insoluble polysaccharide that forms the highly structured framework of the shell and is involved in chitinous framework building. It also participates in the regulation of crystal growth during prismatic layer mineralization [30]. In the present study, the protein coded by the contig CL4062.Contig1_Ba_mix was identified to be Prisilkin-39 with an identity of 44%, and has a basic isoelectric point, indicating that this protein might bind to chitin and provide a complementary function to the acidic shell matrix protein mentioned above, forming the basis of the barnacle shell.
Serine protease and proteinase inhibitor protein
Barnacle shell is considered to consist of only acellular biominerals. It is puzzling to detect proteases and proteinase inhibitor proteins in the shell. However, this is not an isolated observation [31]. These proteins/domains have been identified in the shell matrix of the abalone H. refescens, H. asinia, and H. laevigata, the pearl oyster P. margaritifera and P. fucata, the sea urchin Strongylocentrotus purpuratus as well as chicken eggs (Gallus gallus domesticus) (summarized in [31]).
Similar to trypsin and prostate-specific antigen (PSA), serine proteases are likely synthesized as a preproenzyme containing an N-terminal signal peptide followed by a short activation peptide and an enzymatic domain at the C-terminal end [14]. In barnacle, shell formation is in a dynamically steady state balanced by shell construction and molting. The trypsin-like serine protease might, similar to that in human bone [32], function to digest extracellular matrix proteins for shell resorption. As a cofactor, serine protease cascades also participate in the proteolytic activation of prophenoloxidase (proPO), which is a reaction implicated in melanotic encapsulation, wound healing, and protein cross-linking [33]. These serine proteases might also play a role in the protection in the barnacle shell. Moreover, serine proteases might also take a role in the hardening process of barnacle shell, as these proteases have already been shown to participate in cement polymerization [34].
Purified proteinase inhibitor proteins from the nacre of the oyster P. margaritifera revealed specific inhibitory activity to serine proteases [35]. Thus, these inhibitor proteins might play a role in either protection of proteins involved in shell formation or in defense against parasites, or both [35]. In this study, the chorionic proteinase inhibitor might have similar functions, either regulating the shell formation or functioning in immunity. Moreover, the chorionic protease inhibitor found in the barnacle shell had 5 WAP domains, suggesting that chorionic protease inhibitor might have negative effects on crystal formation, as 3-repeated WAP domains in the perlwapin from the sea snail H. laevigata shell inhibited the growth of certain crystallographic planes during biomineralization [36].
Carbonic anhydrase
Carbonic anhydrase, that catalyzes the hydration of CO2 to produce HCO3 - and H+, is essential for the calcification process. It has been identified in several calcifying epithelia as well as some extracellular skeletal matrices (such as crustacean calcium storage concretions and mollusk shells) [37]. In the barnacle shell, three homologs of carbonic anhydrase were detected, which might be associated with the production of bicarbonate ions to precipitate calcium carbonate; it might also be possible that carbonic anhydrase produces protons and then induces the dissolution of calcium carbonate [37]. For carbonic anhydrase I and III, two of the three histidines that function as zinc ligands were mutated, revealing that carbonic anhydrases I and III might function in other ways or possess different activities.
Chorion peroxidase
Chorion peroxidase participates in the hardening process (cuticular tanning) of the chorion in the mosquito Aedes aegypti by catalyzing chorion protein crosslinking through dityrosine formation [38]. A contig (CL8390.Contig1_Ba_mix) in all three fractions was identified as chorion peroxidase in the barnacle shell, which has a mutated motif of YGD rather than RGD or KGD. This mutated YGD motif also displays the ability to bind integrin [39]. In the barnacle shell, the outer surface is covered by a cuticle, and the organic components of the shelly layers represent part of the endocuticle [40]. It is possible that chorion peroxidase may function in the cuticle hardening process in the shell.
C-type lectins
C-type lectins or C-type lectin-like proteins/domains are widely present in biominerals [41]. These conserved domains are mainly responsible for calcium-dependent carbohydrate binding. In addition, they bind selectively to various ligands, including lipids, proteins and inorganic compounds (such as CaCO3 and ice) [42]. They are also involved in the recognition and clearance of microbial agents in both vertebrate and invertebrate immunity [43]. In the barnacle Megabalanus rosa, C-type lectins participate in the mineralization and defense in shells [44]. In vitro studies revealed that C-type lectins inhibited the crystal nucleation of aragonite and regulated the morphology and growth orientation of calcite through binding to the surface of the crystal to stop its growth [45]. In this study, C-type lectin-like domains in the shell of A. amphitrite might also has the activity in the regulation of crystal formation.
Moreover, the EPN and QPD motif is important for mannose and galactose binding, respectively [46]. In the present study, two proteins containing a C-type lectin-like domain were detected in the shell of A. amphitrite. The presence of a conserved QPD motif suggested that these two domains in A. amphitrite might be important for the detection of galactose in bacteria and participate in the immune defense mechanisms.
HE and DAPI staining of barnacle shell
As previously reported, epithelial cells were present in the exoskeletons of crustaceans and the shells of mollusks. Further studies confirmed their participation in the shell formation processes [47, 48]. In barnacle shells, the regions of growth comprise the basal suture (the joint between the lower outer edge of the basis and lower parts of the paries), the downward protruding region of the sheath, the margins of the opercular plates, the radii and the alae [40]. Later histological studies observed elongated cuticle-secreting cells in the basal suture, and variously modified hypodermal cells underlying the basis, the opercular membrane, and the shell plates [40]. Recently, Gohad et al. [49] found chloride epithelia at the basis and parietal plates of adult barnacles using silver staining microscopic techniques. In the present study, HE and DAPI staining revealed cell-shaped structures attaching to the surfaces of the inner channels around lacunae, which is consistent with the findings by Gohad et al. [49]. Proteomics analysis identified 9 cytoskeletal proteins and several metabolism-related intracellular proteins such as ATP synthase and fructose 1, 6-bisphosphate aldolase, which are considered to only function intracellularly. These results further confirmed the existence of epithelia in the shell parietes.
Conclusions
Using XRF analysis, we found that Na, Mg, Ca, and Sr represented 1.4%, 1.0%, 93.8%, and 1.1% of weight percentage in the A. amphitrite shell. Proteomics results detected 52 proteins in the barnacle shell. Based on KOG database, 40 proteins were categorized into 11 functional groups. Besides some known shell proteins (SIPC, acidic shell matrix proteins, and carbonic anhydrase), we also detected a basic shell matrix protein (Prisilkin-39), C-type lectin-like domains, chorion peroxidase, serine proteases and a proteinase inhibitor protein in the barnacle shell. The HE and DAPI staining revealed living cells in the shells, which might secrete the shell proteins.
Materials and Methods
Ethics Statement
No specific permit is required for the present study in Hong Kong.
The place for barnacle collection does not belong to any national parks, protected areas or private lands. Barnacle collection there does not involve any endangered or protected species or contravene any environmental protection law.
Barnacle collection and shell preparation
Adults of Amphibalanus amphitrite were collected from Sai Kung Pier, Hong Kong (22°22'53''N, 114°16'32''E). After the removal of soft tissues and basal plates, the outer and inner surfaces of the shell were carefully brushed with 0.22 μm-filtered seawater (FSW). Next, the shells were immersed in sodium hypochlorite (active chlorine≥6.25%) at 4°C overnight to eliminate trace contamination of soft tissue. After flushing with Mill-Q water and air-drying, the shells were ready for further analysis.
X-ray fluorescence analysis
Bleached shells were ground into powder, heated in an oven at 110°C for 10 hours to remove moisture, and finally examined using an X-ray fluorescence (XRF) spectrometer (JEOL JSX-3201Z) to analyze the chemical components of the barnacle shell.
Decalcification and protein extraction
Shells were ground into fine particles in liquid nitrogen and then decalcified in 5% acetic acid at 4°C overnight. After centrifugation at 10,000 g for 20 min, the pellet was further washed twice in 5% acetic acid. All of the supernatants were pooled together as acetic acid fraction.
The pellets containing acid-insoluble proteins were first extracted in 1% SDS extraction buffer (8 M urea, pH 7.4, 0.5 M DTT, 1% SDS and commercial protease inhibitor, Roche, German) and then further extracted in 10% SDS extraction buffer (8 M urea, pH 7.4, 0.5 M DTT, 10% SDS and commercial protease inhibitor, Roche, German). Following each extraction, the pellets were washed twice in the same buffer used.
Polyacrylamide gel electrophoresis and in-gel digestion
The protein concentrations were determined using a RC-DC protein assay kit (Bio-Rad, CA, USA). All of the samples were loaded on 12% polyacrylamide gel (PAGE). For each fraction, 80 μg of total protein was loaded in the gel. After coomassie blue staining, the lane of each fraction was cut into 10 fragments for total protein profiling. The in-gel digestion procedure has been described by Zhang et al. [50].
Mass spectrometric analysis and Mascot searching
After a clean-up procedure using ZipTip C18 peptide tips (Millipore, MA),the peptides were analyzed using an electrospray ionization (ESI)-quadrupole time-of-flight (Q-TOF) mass spectrometer (QSTAR XL, Applied Biosystems/Sciex, ON, Canada). Another biological replicate of sample was prepared using sample procedure and finally analyzed using a LTQ Velos Dual-Pressure Ion Trap Mass Spectrometer (coupled with LC and ETD source, Thermo Scientific). The protocols for Q-TOF and LTQ-MS analysis have been described by Han et al. [51] and Liu et al. [52], respectively. Each sample was injected twice and the results were combined together.
The raw data files generated by ESI-QqTOF were converted into.pkl format using ProteinLynx (v2.2.5, waters). MASCOT daemon (v2.2) was applied to search the peptide information against our in-house transcriptome database of A. amphitrite [53]. Decoy sequences generated by Trans-Proteomic Pipeline (TPP) were added in the database [54] and the protein identification false discovery rate (FDR) cutoff was set at 0.01.
Bioinformatics analysis
The proteins were categorized into different functional groups via a tBLASTx search against the KOG database [55]. Potential motifs in each gene were scanned using the SMART website (http://smart.embl-heidelberg.de). The physical and chemical parameters of the proteins were estimated using the ProtParam tool available on the ExPASy Bioinformatics Resource Portal (http://web.expasy.org). The identity and similarity between proteins were calculated using SIAS (http://imed.med.ucm.es/Tools/sias.html). Signal peptides were predicted online using the SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/).
Histological sections, hematoxylin-eosin and DAPI staining
The bleached shells were fixed in 4% PFA/PBS solution at 4°C overnight and then decalcified in 0.5 M EDTA (pH 8.0) at 4°C until they became soft. After three washes with PBS, the samples were dehydrated in gradient concentrations of ethanol, infiltrated in xylene and embedded in wax. The samples were cut into 4-μm-thick sections and mounted onto glass slides. After dewaxing using xylene, the sections were rehydrated in gradient concentrations of ethanol followed by PBS.
The HE staining was performed using a HE staining kit (Jiancheng Biotech., Nanjing, China). Subsequently, the sections were sealed using DPX mountant for histology (Sigma) and observed under a light microscopy.
Rehydrated sections were stained with 300 nM DAPI for 30 min, and then washed with PBS for 4×15 min. Next, the sections were sealed and observed under a fluorescent microscopy.
Supporting Information
(TXT)
High concentrations of Ca, Cl, S, Sr, Mg and Na elements were detected in the barnacle shell.
(TIFF)
In total, 52 proteins were identified in all three fractions, and 20, 3, and 12 proteins were uniquely detected in the acetic acid, 1% SDS and 10% SDS fractions, respectively.
(TIFF)
The Genbank access number and protein description identify protein matches from the NCBI database. The NCBI score and E-value are obtained from the tBLASTx searching. The protein score is derived from the combined scores of all observed mass spectra that can be matched to amino acid sequences within that protein. Protein matches gives the number of mass spectra assigned to this protein. ✓ means present in that fraction.
(PDF)
(PDF)
Data Availability
All the protein sequences mentioned in this study are enclosed in the Supporting Information files.
Funding Statement
This work was supported by a grant (DY125-15-T-02) from the China Ocean Mineral Resources Research and Development Association, an award from the King Abdullah University of Science and Technology (SA-C0040/UK-C0016) and grants (GRF661611, GRF662413) from the Research Grants Council of the Hong Kong Special Administrative Region to PYQ as well as a grant from the National Natural Science Foundation of China (31460092) to LSH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shafer TH, McCartney MA, Faircloth LM. Identifying exoskeleton proteins in the blue crab from an expressed sequence tag (EST) library. Integr Comp Biol. 2006;46(6):978–90. 10.1093/icb/icl022 [DOI] [PubMed] [Google Scholar]
- 2. Marin F, Luquet G. Molluscan shell proteins. C R Palevol. 2004;3(6):469–92. [Google Scholar]
- 3. Glazer L, Sagi A. On the involvement of proteins in the assembly of the crayfish gastrolith extracellular matrix. Invertebr Reprod Dev. 2012;56(1):57–65. [Google Scholar]
- 4. Khalifa GM, Weiner S, Addadi L. Mineral and matrix components of the operculum and shell of the barnacle Balanus amphitrite: calcite crystal growth in a hydrogel. Cryst Growth Des. 2011;11(11):5122–30. [Google Scholar]
- 5. Fernández MS, Vergara I, Oyarzún A, Arias JI, Rodríguez R, Wiff JP, et al. Extracellular matrix molecules involved in barnacle shell mineralization. Mat Res Soc Symp Proc. 2002;724:N1.2. [Google Scholar]
- 6. Matsumura K, Nagano M, Kato-Yoshinaga Y, Yamazaki M, Clare AS, Fusetani N. Immunological studies on the settlement–inducing protein complex (SIPC) of the barnacle Balanus amphitrite and its possible involvement in larva–larva interactions. Proc R Soc Lond B Biol Sci. 1998;265(1408):1825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsumura K, Nagano M, Fusetani N. Purification of a larval settlement-inducing protein complex (SIPC) of the barnacle, Balanus amphitrite . J Exp Zool. 1998;281:12–20. [Google Scholar]
- 8. Dreanno C, Kirby RR, Clare AS. Locating the barnacle settlement pheromone: spatial and ontogenetic expression of the settlement-inducing protein complex of Balanus amphitrite . Proc R Soc B. 2006;273:2721–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khandeparker L, Anil AC. Role of conspecific cues and sugars in the settlement of cyprids of the barnacle, Balanus amphitrite . J Zool. 2011;284:206–14. [Google Scholar]
- 10. Kamino K, Odo S, Maruyama T. Cement proteins of the acorn barnacle, Megabalanus rosa . Biol Bull. 1996;190:403–9. [DOI] [PubMed] [Google Scholar]
- 11. Kamino K, Inoue K, Maruyama T, Takamatsu N, Harayama S, Shizuri Y. Barnacle cement proteins importance of disulfide bonds in their insolubility. J Biol Chem. 2000;275:27360–5. [DOI] [PubMed] [Google Scholar]
- 12. Urushida Y, Nakano M, Matsuda S, Inoue N, Kanai S, Kitamura N, et al. Identification and functional charasterization of a novel barnacle cement protein. FEBS J. 2007;274:4336–46. [DOI] [PubMed] [Google Scholar]
- 13. Arias JL, Fernández MS. Polysaccharides and proteoglycans in calcium carbonate-based biomineralization. Chem Rev. 2008;108(11):4475–82. 10.1021/cr078269p [DOI] [PubMed] [Google Scholar]
- 14. Nelson PS, Gan L, Ferguson C, Moss P, Gelinas R, Hood L, et al. Molecular cloning and characterization of prostase, an androgen-regulated serine protease with prostate-restricted expression. Proc Natl Acad Sci. 1999;96(6):3114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hennighausen LG, Sippel AE. Mouse whey acidic protein is a novel member of the family of ‘four-disulfide core’ proteins. Nucleic Acids Res. 1982;10(8):2677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukuzawa H, Fujiwara S, Yamamoto Y, Dionisio-Sese ML, Miyachi S. cDNA cloning, sequence, and expression of carbonic anhydrase in Chlamydomonas reinhardtii: regulation by environmental CO2 concentration. Proc Natl Acad Sci. 1990;87(11):4383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khalifa GM, Weiner S, Addadi L. Mineral and matrix components of the operculum and shell of the barnacle Balanus amphitrite: calcite crystal growth in a hydrogel. Cryst Growth Des. 2011;11:5122–5130. [Google Scholar]
- 18. Zhang C, Zhang R. Matrix proteins in the outer shells of molluscs. Mar Biotechnol. 2006;8(6):572–86. [DOI] [PubMed] [Google Scholar]
- 19. Yoon G-L, Kim B-T, Kim B-O, Han S-H. Chemical–mechanical characteristics of crushed oyster-shell. Waste Manag. 2003;23(9):825–34. [DOI] [PubMed] [Google Scholar]
- 20. Rødde RH, Einbu A, Vårum KM. A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydr Polym. 2008;71(3):388–93. [Google Scholar]
- 21. Boßelmann F, Romano P, Fabritius H, Raabe D, Epple M. The composition of the exoskeleton of two crustacea: The American lobster Homarus americanus and the edible crab Cancer pagurus . Thermochimica Acta. 2007;463(1):65–8. [Google Scholar]
- 22. Gordon CM, Carr RA, Larson RE. The influence of environmental factors on the sodium and manganese content of barnacle shells. Limnol Oceanogr. 1970;15:461–6. [Google Scholar]
- 23. Bourget E. Environmental and structural control of trace elements in barnacle shells. Mar Biol. 1974;28:27–36. [Google Scholar]
- 24. Gotliv BA, Addadi L, Weiner S. Mollusk shell acidic proteins: in search of individual functions. ChemBioChem. 2003;4(6):522–9. [DOI] [PubMed] [Google Scholar]
- 25. Dreanno C, Kirby RR, Clare AS. Involvement of the barnacle settlement-inducing protein complex (SIPC) in species recognition at settlement. J Exp Mar Bio Ecol. 2007;351:276–82. [Google Scholar]
- 26. Tsukamoto D, Sarashina I, Endo K. Structure and expression of an unusually acidic matrix protein of pearl oyster shells. Biochem Biophys Res Commun. 2004;320(4):1175–80. [DOI] [PubMed] [Google Scholar]
- 27. Gotliv BA, Kessler N, Sumerel JL, Morse DE, Tuross N, Addadi L, et al. Asprich: A novel aspartic acid‐rich protein family from the prismatic shell matrix of the bivalve Atrina rigida . ChemBioChem. 2005;6(2):304–14. [DOI] [PubMed] [Google Scholar]
- 28. Korf I, Yandell M, Bedell J. Blast: O'Reilly Media, Inc.; 2003. [Google Scholar]
- 29. Marie B, Trinkler N, Zanella-Cleon I, Guichard N, Becchi M, Paillard C, et al. Proteomic identification of novel proteins from the calcifying shell matrix of the Manila clam Venerupis philippinarum . Mar Biotechnol. 2011;13(5):955–62. 10.1007/s10126-010-9357-0 [DOI] [PubMed] [Google Scholar]
- 30. Kong Y, Jing G, Yan Z, Li C, Gong N, Zhu F, et al. Cloning and characterization of Prisilkin-39, a novel matrix protein serving a dual role in the prismatic layer formation from the oyster Pinctada fucata . J Biol Chem. 2009;284(16):10841–54. 10.1074/jbc.M808357200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marie B, Marie A, Jackson DJ, Dubost L, Degnan BM, Milet C, et al. Proteomic analysis of the organic matrix of the abalone Haliotis asinina calcified shell. Proteome Sci. 2010;8:54 10.1186/1477-5956-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kinoshita S, Wang N, Inoue H, Maeyama K, Okamoto K, Nagai K, et al. Deep sequencing of ESTs from nacreous and prismatic layer producing tissues and a screen for novel shell formation-related genes in the pearl oyster. PLoS One. 2011;6(6):e21238 10.1371/journal.pone.0021238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta . Immunol Rev. 2004;198(1):97–105. [DOI] [PubMed] [Google Scholar]
- 34. Dickinson GH, Vega IE, Wahl KJ, Orihuela B, Beyley V, Rodriguez EN, et al. Barnacle cement: a polymerization model based on evolutionary concepts. J Exp Biol. 2009;212:3499–510. 10.1242/jeb.029884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bédouet L, Duplat D, Marie A, Dubost L, Berland S, Rousseau M, et al. Heterogeneity of proteinase inhibitors in the water-soluble organic matrix from the oyster nacre. Mar Biotechnol. 2007;9(4):437–49. [DOI] [PubMed] [Google Scholar]
- 36. Treccani L, Mann K, Heinemann F, Fritz M. Perlwapin, an abalone nacre protein with three four-disulfide core (whey acidic protein) domains, inhibits the growth of calcium carbonate crystals. Biophys J. 2006;91(7):2601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marie B, Luquet G, Bédouet L, Milet C, Guichard N, Medakovic D, et al. Nacre calcification in the freshwater mussel Unio pictorum: carbonic anhydrase activity and purification of a 95 kDa calcium‐binding glycoprotein. ChemBioChem. 2008;9(15):2515–23. 10.1002/cbic.200800159 [DOI] [PubMed] [Google Scholar]
- 38. Han Q, Li G, Li J. Purification and characterization of chorion peroxidase from Aedes aegypti eggs. Arch Biochem Biophys. 2000;378(1):107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Robinson CM, Zhou X, Rajaiya J, Yousuf MA, Singh G, DeSerres JJ, et al. Predicting the next eye pathogen: analysis of a novel adenovirus. mBio. 2013;4(2):e00595–12. 10.1128/mBio.00595-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crisp DJ, Bourget E. Growth in barnacles. Adv Mar Biol. 1985;22:199–244. [Google Scholar]
- 41. Mann K, Siedler F. Amino acid sequences and phosphorylation sites of emu and rhea eggshell C-type lectin-like proteins. Comp Biochem Physiol Biochem Mol Biol. 2006;143(2):160–70. [DOI] [PubMed] [Google Scholar]
- 42. Zelensky AN, Gready JE. Comparative analysis of structural properties of the C‐type‐lectin‐like domain (CTLD). Proteins Struct Funct Bioinfor. 2003;52(3):466–77. [DOI] [PubMed] [Google Scholar]
- 43. Wu C, Charoensapsri W, Nakamura S, Tassanakajon A, Söderhäll I, Söderhäll K. An MBL-like protein may interfere with the activation of the proPO-system, an important innate immune reaction in invertebrates. Immunobiology. 2013;218(2):159–68. 10.1016/j.imbio.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 44. Kamiya H, Jimbo M, Yako H, Muramoto K, Nakamura O, Kado R, et al. Participation of the C-type hemolymph lectin in mineralization of the acorn barnacle Megabalanus rosa . Mar Biol. 2002;140(6):1235–40. [Google Scholar]
- 45. Matsubara H, Hayashi T, Ogawa T, Muramoto K, Jimbo M, Kamiya H. Modulating effect of acorn barnacle C‐type lectins on the crystallization of calcium carbonate. Fish Sci. 2008;74(2):418–24. [Google Scholar]
- 46. Li M, Li C, Ma C, Li H, Zuo H, Weng S, et al. Identification of a C-type lectin with antiviral and antibacterial activity from pacific white shrimp Litopenaeus vannamei . Dev Comp Immunol. 2014;46(2):231–40. 10.1016/j.dci.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 47. Marie B, Joubert C, Tayalé A, Zanella-Cléon I, Belliard C, Piquemal D, et al. Different secretory repertoires control the biomineralization processes of prism and nacre deposition of the pearl oyster shell. Proc Natl Acad Sci. 2012;109(51):20986–91. 10.1073/pnas.1210552109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wade N, Goulter KC, Wilson KJ, Hall MR, Degnan BM. Esterified astaxanthin levels in lobster epithelia correlate with shell colour intensity: potential role in crustacean shell colour formation. Comp Biochem Physiol Biochem Mol Biol. 2005;141(3):307–13. [DOI] [PubMed] [Google Scholar]
- 49. Gohad NV, Dickinson GH, Orihuela B, Rittschof D, Mount AS. Visualization of putative ion-transporting epithelia in Amphibalanus amphitrite using correlative microscopy: potential function in osmoregulation and biomineralization. J Exp Mar Bio Ecol. 2009;380:88–98. [Google Scholar]
- 50. Zhang Y, Xu Y, Arellano SM, Xiao K, Qian PY. Comparative proteome and phosphoproteome analyses during cyprid development of the barnacle Balanus (= Amphibalanus) amphitrite . J Proteome Res. 2010;9(6):3146–57. 10.1021/pr1000384 [DOI] [PubMed] [Google Scholar]
- 51. Han Z, Sun J, Zhang Y, He F, Xu Y, Matsumura K, et al. iTRAQ-based proteomic profiling of the barnacle Balanus amphitrite in response to the antifouling compound meleagrin. J Proteome Res. 2013;12(5):2090–100. 10.1021/pr301083e [DOI] [PubMed] [Google Scholar]
- 52. Liu X, Hu Y, Pai P-J, Chen D, Lam H. Label-free quantitative proteomics analysis of antibiotic response in Staphylococcus aureus to oxacillin. J Proteome Res. 2014;13(3):1223–33. 10.1021/pr400669d [DOI] [PubMed] [Google Scholar]
- 53. Chen Z-F, Zhang H, Wang H, Matsumura K, Wong YH, Ravasi T, et al. Quantitative proteomics study of larval settlement in the barnacle Balanus amphitrite . PLoS One. 2014;9(2):e88744 10.1371/journal.pone.0088744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Deutsch EW, Mendoza L, Shteynberg D, Farrah T, Lam H, Tasman N, et al. A guided tour of the Trans‐Proteomic Pipeline. Proteomics. 2010;10(6):1150–9. 10.1002/pmic.200900375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics. 2003;4(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TXT)
High concentrations of Ca, Cl, S, Sr, Mg and Na elements were detected in the barnacle shell.
(TIFF)
In total, 52 proteins were identified in all three fractions, and 20, 3, and 12 proteins were uniquely detected in the acetic acid, 1% SDS and 10% SDS fractions, respectively.
(TIFF)
The Genbank access number and protein description identify protein matches from the NCBI database. The NCBI score and E-value are obtained from the tBLASTx searching. The protein score is derived from the combined scores of all observed mass spectra that can be matched to amino acid sequences within that protein. Protein matches gives the number of mass spectra assigned to this protein. ✓ means present in that fraction.
(PDF)
(PDF)
Data Availability Statement
All the protein sequences mentioned in this study are enclosed in the Supporting Information files.