Abstract
Combinations of aminoglycosides and loop diuretics have been known to have a synergistic effect in ototoxic injury. Because murine hair cells are relatively resistant to ototoxicity compared to those of other mammals, investigators have turned to combination therapies to create ototoxic lesions in the mouse inner ear. In this paper, we perform a systematic comparison of hearing thresholds, hair cell damage and monocyte migration into the mouse cochlea after kanamycin versus combined kanamycin/furosemide and explore the pathophysiology of enhanced hair cell loss in aminoglycoside ototoxicity in the presence of loop diuretic. Combined kanamycin-furosemide resulted in elevation of threshold not only in the high frequencies, but across all frequencies with more extensive loss of outer hair cells when compared to kanamycin alone. The stria vascularis was severely atrophied and stellate cells in the spiral limbus were missing in kanamycin-furosemide exposed mice while these changes were not observed in mice receiving kanamycin alone. Monocytes and macrophages were recruited in large numbers to the spiral ligament and spiral ganglion in these mice. Combination therapy resulted in a greater number of macrophages in total, and many more macrophages were present further apically when compared to mice given kanamycin alone. Combined kanamycin-furosemide provides an effective method of addressing the relative resistance to ototoxicity that is observed in most mouse strains. As the mouse becomes increasingly more common in studies of hearing loss, and combination therapies gain popularity, recognition of the overall effects of combined aminoglycoside-loop diuretic therapy will be critical to interpretation of the interventions that follow.
1. Introduction
Aminoglycoside antibiotics have long been known to have ototoxic effects. These antibiotics, including gentamicin, tobramycin, kanamycin, streptomycin, amikacin, and neomycin, are associated with hearing loss and vestibular dysfunction due to hair cell loss. Some of the aminoglycoside antibiotics, such as gentamicin, have a more vestibulotoxic profile, while others, such as kanamycin, have a more cochleotoxic profile. For those aminoglycosides that are cochleotoxic, their effects target the high frequency portion of the cochlea, and in mammals, they affect outer hair cells while sparing inner hair cells. In current practice, gentamicin remains the most commonly prescribed intravenous aminoglycoside antibiotic and is sometimes associated with vestibular impairment. Hair cell loss after aminoglycoside ototoxicity is permanent, and our interventions for vestibular and hearing dysfunction after aminoglycoside ototoxicity are limited. Despite the various studies that have examined the protective effects of antioxidant therapies and other strategies against aminoglycoside ototoxicity, none of these proposed agents is an accepted therapy for hearing loss prevention (Bock et al., 1983; Campbell et al., 2007; Jiang et al., 2005; Kawamoto et al., 2004; Rybak and Whitworth, 2005).
Aminoglycoside antibiotics continue to be popular in their use as an ototoxic agent in animal research to study the process of hair cell survival, death and regeneration. Many prior studies have demonstrated the predictable effects of aminoglycoside antibiotics administered both in vivo and in vitro with experimental animals (Gratacap et al.,1985; Richardson and Russell,1991; Stone et al.,1996; Weisleder and Rubel, 1993). Currently, aminoglycosides are used in research to eradicate hair cells in studies of hair cell regeneration and animal models of cochlear implantation (Ryugo et al., 2010; Shepherd et al., 1994; Warchol, 2010). In many of these studies, the goal of aminoglycoside administration is to eradicate hair cells and subsequently test an intervention targeting the remaining supporting cells or spiral ganglion cells. However, when aminoglycoside antibiotics are administered in vivo, complete loss of hair cells is not achieved, and low frequency hearing remains. In the case of mice, aminoglycosides are particularly ineffective in eliminating hair cells (Wu et al., 2001). As our research community expands the use of mice in studies of deafness, combination drug therapy to achieve hair cell loss has become routine. In recent work, loop diuretics have been used to augment the ototoxic effect of aminoglycoside antibiotics and eliminate hair cells in mice as well as in other mammals (Agterberg et al., 2008; Oesterle et al., 2008; Taylor et al., 2008; Xu et al., 1993).
Morphologic changes in the mouse cochlea that occur after the combined use of these two ototoxins have not been formally studied. Here, we compare the effect of aminoglycoside alone with the effect of aminoglycoside in combination with furosemide to 1. Characterize the difference in residual hearing function between these two regimens 2. Characterize the difference in hair cell susceptibility and other sensory and non-sensory cell susceptibility and 3. Determine whether there is an inflammatory response in the cochlea after cell damage from either aminoglycoside or aminoglycoside + loop diuretic.
2. Materials and methods
2.1. Experimental animals
Wild-type C57Bl6 male mice were bred within our facility from founders purchased from Jackson Laboratories, Bar Harbor, ME. Despite their predisposition to age-related hearing loss, we have used these mice in numerous studies of ototoxicity and acoustic trauma because of a variety of transgenic reagents available on this mouse background. All experiments were conducted prior to 16 weeks of age at which time high frequency hearing loss is observed in these mice due to aging (Hequembourg and Liberman, 2001). Prior to this age, C57Bl6 demonstrate minimal age-related threshold changes. All animal protocols described were approved by the Institute for Animal Care and Use Committee.
2.2. Drug administration
Kanamycin sulfate was purchased from USB Corporation (Cleveland, OH, USA) and prepared at 45 mg/ml in saline. Furosemide (10 mg/ml, Hospira Inc., Lake Forest, IL, USA) was diluted to 5 mg/ml in sterile saline. A dose of 900 mg/kg body weight kanamycin was given every 12 h for 15 consecutive days by intra-peritoneal (IP) injection. IP injections of furosemide at 50 mg/kg were performed every morning, 20 min following the injection of kanamycin for 15 consecutive days. A prior regimen using twice daily injections of furosemide was abandoned due to unacceptably high rate of mortality (greater than 50%). All mice were 8 weeks of age at the initiation of the 15-day course of therapy and were 10 weeks of age at the end. Control mice received IP saline injections twice daily for 15 consecutive days.
2.3. Auditory brainstem response (ABR)
Two weeks after the end of drug administration when the mice were 12 weeks of age, auditory evoked brainstem responses (ABR) were performed on all of the mice. Mice were anesthetized with intraperitoneal xylazine (20 mg/kg) and ketamine (100 mg/kg). ABRs were evoked through tone pips and recorded via subcutaneous electrodes placed in the ipsilateral pinna and vertex, with the ground electrode placed by the tail. The stimuli were 5-msec tone pips (0.5-msec rise-fall with a cos2 onset envelope, delivered at 40/s). The response was amplified (×10,000), filtered (100 Hz–3 kHz), and averaged using BioSig computer software (System 3; Tucker-Davis Technologies). The sound level was raised in 5-dB steps from 15-dB SPL to 99-dB SPL. At each sound level,1024 responses were averaged, with stimulus polarity alternated. Response waveforms were rejected if the peak-to-peak voltage exceeded 15 μV. We tested responses at 5.6, 8, 11, 16, 22.63, 32, 45.2, and 64 kHz for ABR threshold. Threshold was determined by a single observer, who noted the lowest sound level at which a recognizable waveform was seen on a screen of tracings stacked from lowest to highest sound level. Waveforms were confirmed as auditory evoked responses by their increasing amplitude and decreasing latency with increasing sound intensity of the stimulus. If hearing threshold was not detected at 99 dB, a threshold value of 100 dB was assigned. Differences in hearing thresholds between mouse groups were compared and analyzed using one-way ANOVA. A change in threshold was considered significant if p < 0.05.
2.4. Histological preparation
Immediately after ABR, animals were injected with a lethal dose of anesthetic agent and perfused via transcardiac puncture with 4.0% paraformaldehyde in 0.01 M phosphate buffer. Both petrous temporal bones were extracted and the round and oval windows opened to allow intralabyrinthine perfusion of fixative. After overnight fixation at 4 °C, cochleas were decalcified in a saturated solution of EDTA in phosphate buffer for 5 days at 4 °C.
2.5. Immunohistochemistry
Decalcified cochleas were immersed in cryoprotection solution (20% glycerol in phosphate buffer), frozen in 30% sucrose on dry ice, and cut into 30 μm serial sections from round window to oval window on a horizontal sliding microtome. Sections were washed in phosphate-buffered saline (PBS; pH 7.4), dried onto slides, incubated in 10% normal goat serum in 0.1% Triton X-100 for 1 h and placed in 1:8000 rat anti-mouse CD45 (Serotec, Oxford, UK) overnight at room temperature followed by goat anti-rat AlexaFluor 594 at 1:5000 (Molecular Probes, Eugene, OR). Rat anti-mouse CD45 is an antibody raised against common leukocyte antigen; this antigen is present on all white blood cells. CD45 indicates a cell’s derivation from bone marrow and identifies monocytes, macrophages, dendritic cells, and microglia, as well as granulocytes, such as neutrophils and basophils as well as lymphocytes and natural killer cells. After immunolabeling, the sections were then rinsed and coverslipped. Control sections were processed with no primary antibody to detect background labeling.
For counting CD45+ cells, four sections for each cochlea were selected from the series on the basis of their proximity to the modiolus, high quality staining and intact histology and were viewed on an Olympus BX51 microscope with a 40× objective. Approximately 20% of the total organ was sampled in these counts. The cochlea was divided into four regions (lower basal, upper basal, lower apical, and upper apical turns) and each region was assigned a mean frequency on the tonotopic map of the mouse cochlea. The observer examined the entire thickness of the slide by focusing up and down through the depth of the tissue and counted CD45+ cells for those cells with an identifiable nucleus with CD45 expression on the cell surface.
2.6. Morphometric analysis
Decalcified right cochleas were postfixed in osmium (1% OsO4 in dH2O) for 60 min. Cochleas were dehydrated through a series of graded ethanols ending in 100% propylene oxide and subsequently embedded in Araldite resin (see protocol in Wang et al., 2002). The plastic-embedded cochleas were seriallysectioned at 40 μm in a plane parallel to the axis of the modiolus. Each section was numbered, oriented, mounted, and coverslipped. For each subject, the cochlear spiral was reconstructed using Neurolucida software (MicroBright-Field, Colchester, VT). Using the tunnel of Corti as the reference point, we mapped each profile of the cochlear duct that contained sensory cells in three-dimensional space. From these three-dimensional reconstructed data, the distance of each cochlear profile from the basal tip was computed using custom software. The cochlear location in space was then converted into frequency according to frequency-map data described by Ehret (1983) which was fit to a mathematical equation f (kHz) = 3.109 × (10(100–d)×0.0142–0.7719), where d = percent distance from the cochlear base.
2.6.1. Hair cells
A standard cytocochleogram was prepared for each ear using high-power oil-immersion objectives and Nomarski optics. In every section through the cochlear duct, the number of present and absent hair cells was assessed throughout the entire section thickness. Evaluation of both the nuclear and cuticular regions was used to make these assessments. Outer hair cell stereocilia could not reliably be resolved at the light microscopic level, but inner hair cell stereocilia could clearly be seen. Presence or absence of the outer hair cell was determined by the presence of a nucleus within the Deiter cup, the presence of a cuticular plate superior to that nucleus and the integrity of the reticular lamina across the apical surface of the organ of Corti. Statistical analysis was performed on the effect of the different drug regimens using one-way ANOVA. P values of <0.05 were considered statistically significant.
2.6.2. Spiral ligament and spiral limbus fibrocytes
In every section through the cochlear duct, we estimated the percentage of remaining fibrocytes. Estimates of cell loss were performed with a 40× objective, and were based on the presence of cell nuclei and were compared to the cell density seen in control ears.
3. Results
High frequency hearing loss is observed after kanamycin and profound hearing loss is seen after combined kanamycin-furosemide
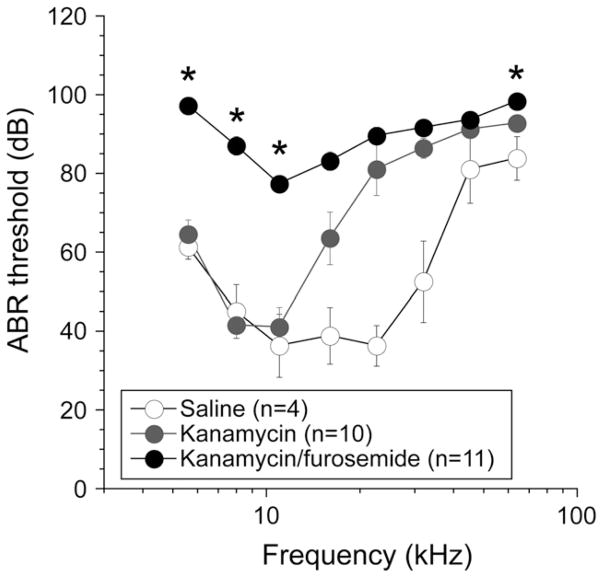
Fig. 1 demonstrates ABR thresholds after saline, kanamycin and kanamycin-furosemide elicited by tone pips from 5 to 64 kHz. These animals had been treated twice daily with kanamycin in the kanamycin-only group and with an additional once daily dose of furosemide in the combined therapy group. This treatment was repeated for 15 consecutive days, and mice were then allowed to recover for two weeks. Hearing thresholds were obtained four weeks after the initial dose. Age-matched mice that received 15 days of kanamycin twice daily experienced average threshold elevations of approximately 20 dB at 16 kHz, 35 dB at 22 kHz and maximum threshold elevation at 32 kHz and higher. Combined administration of kanamycin and furosemide caused near-maximal threshold shift across the entire hearing spectrum in all mice with some preservation of hearing at 11 kHz, where threshold was approximately 50 dB higher than in control mice. While kanamycin does cause a substantial hearing deficit, kanamycin combined with loop diuretic extends the hearing loss into the low frequencies.
Fig. 1.
ABR threshold after saline, kanamycin, and kanamycin-furosemide injection. ABR thresholds were elevated at 16 kHz and higher after kanamycin, while thresholds were elevated at all tested frequencies after kanamycin-furosemide injections. Figure icons represent means and error bars represent standard errors of the mean (SEM). Asterisks demonstrate thresholds at which the difference between kanamycin and kanamycin-furosemide were statistically significantly different as evaluated by one-way ANOVA test with p < 0.05.
Outer hair cell loss after kanamycin is confined to the basal turn while kanamycin/furosemide ablates outer hair cells through the apical turn
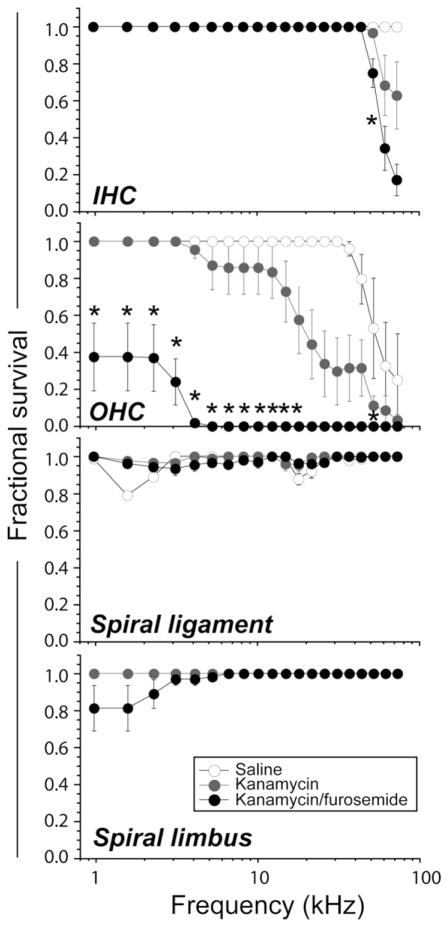
Outer hair cells are the main target of aminoglycoside ototoxicity and clearly we demonstrate that this is true in both kanamycin and kanamycin-furosemide exposed mice. The upper two graphs in Fig. 2 demonstrate quantitative measurements of inner and outer hair cell survival after kanamycin and kanamycin-furosemide. The inner hair cells were preserved in both regimens except for a consistent loss of inner hair cells confined to the far basal end of the cochlea. Approximately 60% of inner hair cells survived in the far basal region of the cochlea after kanamycin administration while only 20% of inner hair cells survived in this same region after combination kanamycin-furosemide. Other than this tiny fraction of the cochlear duct, all inner hair cells were well preserved in both conditions.
Fig. 2.
Cytocochleograms after saline, kanamycin, and kanamycin-furosemide injection. A loss of outer hair cells was seen in the extreme base in saline-injected control animals. This degree of high frequency hair cell loss has been reported and is expected in the C57Bl6 mouse strain. Mild to moderate inner hair cell (IHC) damage was seen in high frequencies after kanamycin and kanamycin-furosemide injection. Moderate to severe outer hair cell (OHC) damage was seen in 14 kHz or higher frequency after kanamycin injection, whereas almost all OHC disappeared after kanamycin-furosemide injection. Mild spiral ligament damage was seen in the control mice, and there was no difference among three groups. Three out of 11 mice exhibited spiral limbus damage after kanamycin-furosemide injection. Figure icons represent means, and error bars represent SEM. Asterisks demonstrate regions in which the difference between hair cell survival in kanamycin and kanamycin-furosemide treated groups were statistically significantly different as evaluated by one-way ANOVA with p < 0.05.
Outer hair cell survival was severely affected in both regimens and was markedly worse after combination therapy. After kanamycin alone, outer hair cell loss was first noted in the 10 kHz region and progressed basally. The outer hair cells in the apical turn survived in every specimen (Fig. 2). After combination therapy, only 40% of the outer hair cells in the apex survived after kanamycin/ furosemide. The remainder of outer hair cells from the 4 kHz region to the base were completely eradicated. In both regimens, hair cells were severely depleted in the high frequencies, but as Fig. 2 illustrates, the percentage of surviving hair cells was lower at all frequencies in the kanamycin-furosemide group. Kanamycin with a 15-day twice daily regimen eradicated only one third to one quarter of mouse outer hair cells; neither kanamycin nor kanamycin combined with furosemide at these doses was effective in eliminating inner hair cells.
Cytocochleograms representing damage in the spiral ligament and the spiral limbus are also shown in Fig. 2. A significant loss of spiral limbus fibrocytes was observed after combination kanamycin/furosemide. The interdental cells of the spiral limbus were not affected. This damage in the spiral limbus was not observed in controls or in mice given kanamycin alone. The loss of fibrocytes in the apical turn of the spiral limbus has been described in the past in noise injury, but not in aminoglycoside or loop diuretic ototoxicity. There was no significant loss of spiral ligament fibrocytes observed in either control or drug-treated animals.
Micrographs of the mouse cochlea demonstrate qualitative differences in cochlear injury after kanamycin and combination kanamycin-furosemide
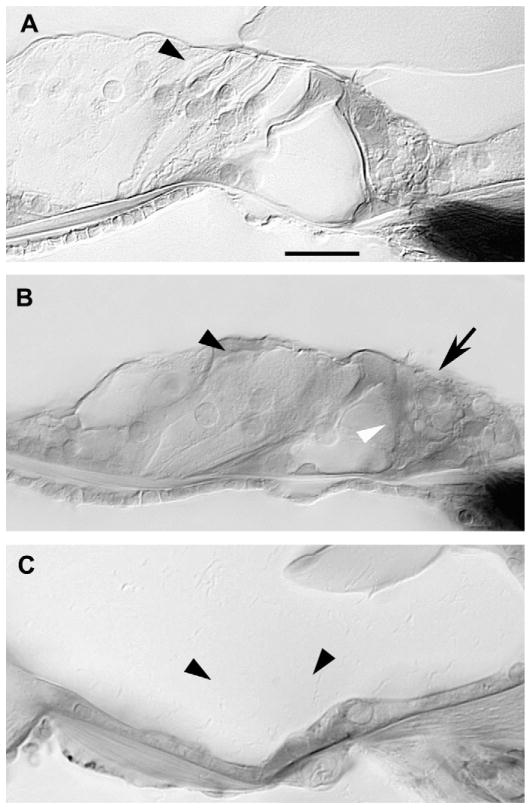
Fig. 3 demonstrates representative sections from the organ of Corti in kanamycin and kanamycin/furosemide treated mice. The top panel shows normal appearing outer hair cells in the apical turn after kanamycin (Fig. 3A). The inner hair cells are present, stereocilia are visible, the tunnel of Corti and spaces of Nuel are intact, and outer hair cells are present and normal appearing. The combination of kanamycin-furosemide killed nearly all outer hair cells in the apex (Fig. 3B). The tunnel of Corti is filled with cellular debris, the Deiter cell layer is disorganized and the outer hair cells and their nuclei are absent. The reticular lamina is intact and thus the separation between endolymph and perilymph is maintained. The architecture of the organ of Corti is preserved despite loss of the individual outer hair cells. The height of the tunnel of Corti is normal and the pillar cells are present. The basal cytoplasm of inner hair cells in these specimens is vacuolated, suggestive of injury to the terminals during tissue processing. In the basal portion of the cochlea, outer hair cells are extensively damaged in both kanamycin alone and combination kanamycin-furosemide treated ears. In some animals after combination kanamycin-furosemide, the entire organ of Corti is absent in the lower basal turn, and a squamous, undifferentiated epithelium is left as in Fig. 3C. Two out of eleven mice treated with combination kanamycin-furosemide demonstrated this denuded flat epithelium. There appears to be a loss of all supporting cells and pillar cells as well as a loss of hair cells. The derivation and the future evolution of these squamous-appearing epithelial cells are unclear. The final outcome characterized by a flat epithelium as shown in Fig. 3C was observed in a minority of the cochleas that were examined after kanamycin-furosemide treatment.
Fig. 3.
Complete outer hair cell loss was seen after combination kanamycin/furosemide injection. A: After kanamycin alone, outer hair cells (arrowhead) were preserved in lower apical turn. B: After combined kanamycin and furosemide, outer hair cells (black arrowhead) degenerated, pillar cells (white arrowhead) appear somewhat collapsed, but inner hair cells (arrow) were preserved in lower apical turn. C: Normal structures of the organ of Corti (arrowhead) were replaced by a flat epithelium in the lower basal turn after kanamycin and furosemide injection. Scale bar = 50 μm in A applies to A–C.
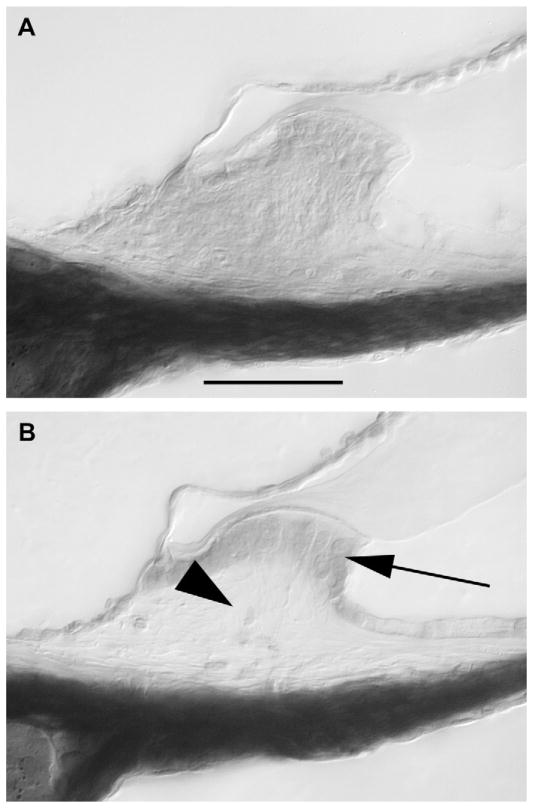
In the spiral ligament, mild loss of type IV fibrocytes was seen in the control mice as is characteristic in C57Bl6 mice, and there was no perceptible change in the spiral ligament associated with either kanamycin or combination kanamycin-furosemide. In the spiral limbus, there are normally two populations of cells: the interdental cells that secrete the tectorial membrane and the stellate cells or fibrocytes that comprise the central portion of the spiral limbus. We observed severe degeneration of the stellate cells in the spiral limbus in the upper apical turn in three out of eleven mice after kanamycin-furosemide (Fig. 4). In the other eight mice, the spiral limbus was completely normal. The interdental cells did not demonstrate susceptibility to either regimen. The injury caused by kanamycin-furosemide in the limbus caused either complete loss of all stellate cells or complete preservation of these cells. There were no specimens that showed an intermediate response.
Fig. 4.
Degeneration of the spiral limbus was seen after kanamycin and furosemide injection. A: Saline injection. B: Kanamycin-furosemide injection. Stellate cells (fibrocytes) were damaged in the apical turn of the spiral limbus after kanamycin injection (B, arrowhead). Scale bar = 100 μm in A applies to A–B.
The stria vascularis was noted to be severely atrophic in the mice exposed to combination kanamycin-furosemide (Fig. 5). Strial atrophy was observed through all turns of the cochlea. Neither the kanamycin-treated animals nor the control animals demonstrated any appreciable changes in the morphology of the stria vascularis under light microscopy. With the thick plastic sections used for this analysis, we could not determine the specific cell type that was affected and responsible for strial atrophy seen in the kanamycin-furosemide group.
Fig. 5.
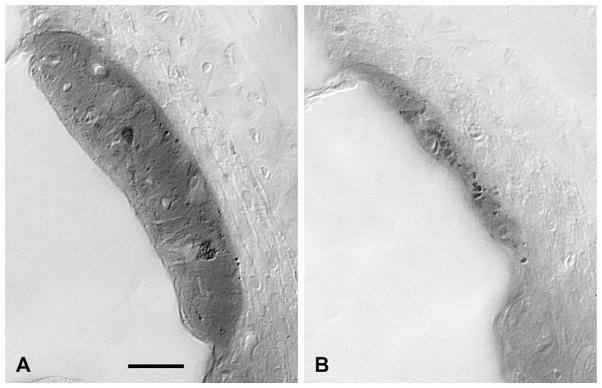
Stria vascularis in the lower apical turn after kanamycin-furosemide injection. A: Saline injection. B: Kanamycin-furosemide injection. Stria vascularis was shrunken after kanamycin-furosemide injection. Scale bar = 50 μm in A applies to A–B.
Combination kanamycin-furosemide recruits more monocytes and macrophages to the mouse cochlea than kanamycin alone
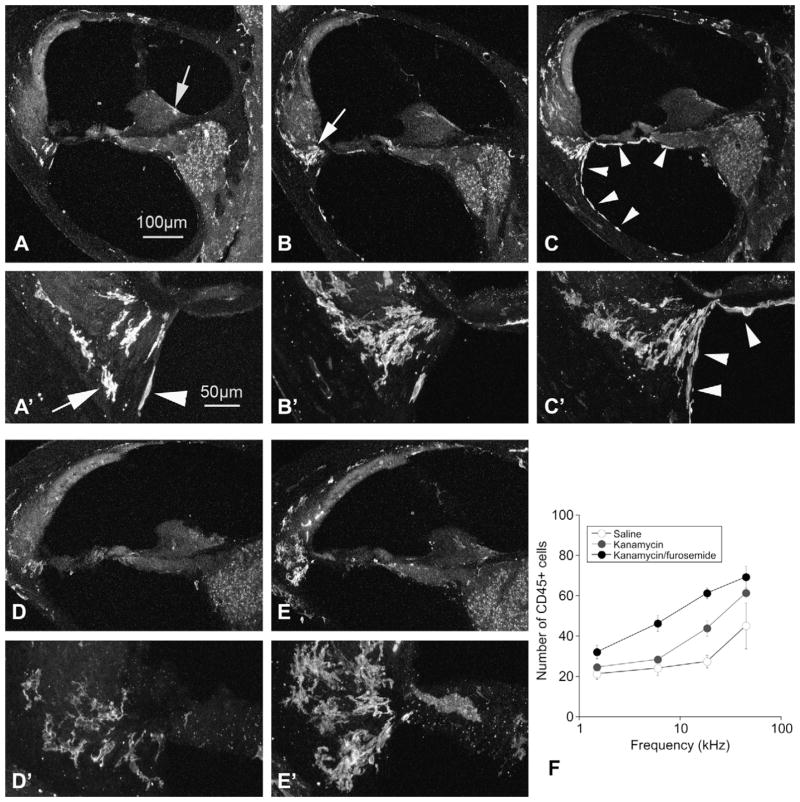
Leukocytes in the membranous labyrinth were identified by immunohistochemistry for CD45 (common leukocyte antigen). In aminoglycoside-treated mice, cochlear leukocytes, previously shown to be specifically monocytes and macrophages, were observed in large numbers in the lower portion of the spiral ligament, the spiral limbus and within the spiral ganglion. In control animals, a sparse collection of CD45+ cells was present in the lower spiral ligament, the spiral limbus and the spiral ganglion (shown in Fig. 6A). After kanamycin, there was a significant increase in cochlear leukocytes which were concentrated in the lower portion of the spiral ligament (Fig. 6B). The mice treated with kanamycin and furosemide had the largest number of cochlear macrophages, observed primarily in the spiral ligament, the spiral ganglion and in the scala tympani on the undersurface of the basilar membrane (see Fig. 6C, white arrows). These cells appear to be in the process of migrating outside of their resting location in the spiral ligament. In the apical turn, there are sparse cochlear leukocytes present in the spiral ligament after kanamycin alone (Fig. 6D), compared with mice receiving kanamycin-furosemide where there are large numbers of these cells in the basal and apical turn.
Fig. 6.
CD45 immunohistochemistry demonstrates migratory mononuclear phagocytes after saline (A), kanamycin (B and D), and kanamycin-furosemide (C and E). Figures A, B, and C show the basal turn; D and E demonstrate the apical turn. High magnification images are shown in A′, B′, C′, D′ and E′. A: After saline, there are few cochlear mononuclear phagocytes that are present in the spiral limbus (arrow in A), in the spiral ligament (see arrow in A′) and in the scala tympani (arrowhead in A′). B: After kanamycin, there are more CD45 positive cells in the spiral ligament, concentrated in the type IV fibrocyte region (arrow in B). C: CD45+ cells after kanamycin-furosemide were significantly increased when compared to kanamycin alone. There are notably more phagocytic cells present in the scala tympani (arrowheads in C). D: In the apical turn, there are few macrophages seen in the kanamycin alone treated ears (D and D′). E: After combination kanamycin-furosemide, there is an abundance of cochlear mononuclear phagocytes extending well into the apex as seen in E and E′. F: The number of CD45+ cells is quantified across the cochlear duct in mice exposed to saline, kanamycin and kanamycin-furosemide in F. (Scale bar in A also applies for figures B, C, D, and E. Scale bar in A′ applies for B′, C′, D′, and E′).
The number of macrophages found in each 30 μm cochlear section is shown in Fig. 6F. In this figure, four locations along the cochlear duct were designated for cell counts (upper and lower apical turn and upper and lower basal turn) and the average frequency represented by each of these half turns was used to localize these cochlear macrophages on the frequency axis. In the kanamycin-treated mice, there is an increase in the number of cochlear mononuclear phagocytes, particularly in the upper and lower basal turns. In mice treated with combination kanamycin-furosemide, the number of cochlear macrophages was even greater than in mice exposed to kanamycin alone, and this response extended into the low frequency region where kanamycin-furosemide caused hair cell loss and kanamycin alone did not. There was no significant increase in cochlear macrophages observed in the stria vascularis, although there was a consistent and reproducible population of macrophages that resided in the stria and appeared to have an active role in ingesting dead cells in both control and damaged cochleas (data not shown).
4. Discussion
Hearing and vestibular loss have been observed in patients treated with aminoglycoside antibiotics since the use of streptomycin for treatment of tuberculosis in the 1940s (Schatz et al., 1944). Since then, research efforts to improve our understanding of ototoxicity have been performed primarily in birds, guinea pigs, and in organ culture of neonatal mice (Brummett, 1981; Cheng et al., 2005; Cunningham et al., 2002; Dai et al., 2006; Forge and Li, 2000; Gratacap et al., 1985; Hirose et al., 1997; Jiang et al., 2005; Karasawa et al., 2008; Richardson and Russell, 1991; Rizzi and Hirose, 2007; Wang et al., 2003). Recently, scientists have turned to in vivo experiments in mice to study molecular mechanisms of hair cell susceptibility to ototoxicity. In part, because mice are exceptionally resistant to aminoglycoside ototoxicity, more and more investigators are using a combination of aminoglycoside and loop diuretic to study loss of hair cells in research applications (Hartman et al., 2009; Oesterle et al., 2008; Taylor et al., 2008; Versnel et al., 2007; Xu et al., 1993). Here, we describe important quantitative and qualitative effects of combination therapy with loop diuretic and aminoglycoside in C57Bl6 mice. In our study, repeated doses of kanamycin given twice daily with furosemide once daily, resulted in no substantial loss of inner hair cells and near total loss of outer hair cells. In addition, kanamycin/furosemide combination therapy affected the stria vascularis and spiral limbus, findings that we did not observe in animals given kanamycin alone.
Because mice are resistant to ototoxic hair cell loss, extremely high doses or frequently repeated, prolonged administration of drugs have been used. Two recent manuscripts have demonstrated a single dose regimen using ten times the standard dose of loop diuretic after a 1000 mg/kg injection of kanamycin (Oesterle et al., 2008; Taylor et al., 2008). By their report, nearly all outer hair cells are gone within 48 h of drug administration and inner hair cells also are susceptible at later time points. Different inbred strains (CBA/ CaJ and Swiss Webster) and younger mice (4–20 weeks in Oesterle et al. (2008), 3 weeks in Taylor et al. (2008)) were used in these studies. CBA/CaJ mice have been shown to be more susceptible to aminoglycoside ototoxicity than C57Bl6 mice (Wu et al., 2001). The differences in hair cell susceptibility could also be attributed to greater hair cell vulnerability at earlier ages; our mice were exposed at 7–8 weeks of life compared to 3–4 weeks in prior studies. The slight disparity between our findings of outer hair cell survival at the apex and complete inner hair cell preservation and the prior reported findings of total loss of outer and significant loss of inner hair cells could be explained by their loop diuretic dose being tenfold higher, the use of a different mouse strain, and the difference in age at the time of exposure.
The mechanism by which loop diuretics exacerbate aminoglycoside-induced hearing loss and outer hair cell damage is unclear. However, based on what is known about loop diuretics, we can offer hypotheses on potential contributors to this effect. Marginal cells are the primary cells in the cochlea that express Na+/ K+/2Cl− cotransporters which are the target of loop diuretics, and they play a vital role in the ionic homeostasis of the endolymph. These channels are present on the basolateral surface of the marginal cells (Sakaguchi et al., 1998). When these channels are blocked by loop diuretics, the intrastrial space expands due to accumulation of sodium in this space and passive retention of free water (Higashiyama et al., 2003; Santi and Lakhani, 1983). There is a simultaneous drop in the endocochlear potential (EP) when this cotransporter is inhibited (Asakuma and Snow, 1980; Azuma et al., 2002; Sewell, 1984). As the EP drops, there is loss of the electrochemical gradient that drives the transduction current through hair cells, and hearing thresholds increase (Alam et al., 1998; Brummett et al., 1979). These changes after loop diuretic are reversible but may be critical in the timely resolution of aminoglycoside entry into the endolymph. In the guinea pig, the largest drop in EP was observed 2 min after injection of intravenous loop diuretic (Pike and Bosher, 1980). Subsequently, the EP rapidly recovered and by 180 min after drug administration, the EP returned to normal.
The cell types in the stria vascularis that are affected by loop diuretics have been carefully described, but results reported in the literature are not consistent. Loop diuretics, as a single agent, are known to cause short-term changes in the morphology of the stria vascularis. Some have observed swelling of marginal cells with shrinkage of intermediate cells (Pike and Bosher,1980). Others have reported shrinkage of marginal cells and swelling of intermediate cells (Rybak, 1993; Rybak et al., 1991; Santi and Lakhani, 1983). It is possible that at various times after loop diuretic, there is alternate shrinkage and swelling of marginal and intermediate cell types. The earlier mentioned study by Taylor et al. also reported changes after combination kanamycin/diuretic administration of strial atrophy and shrinkage of marginal cells. Expansion of the intrastrial space is a common finding in most studies. Permanent changes in strial morphology have not been observed in mice given furosemide alone (Arnold et al., 1981; Lang et al., 2003; Rybak, 1993).
While strial atrophy has been reported in guinea pigs receiving prolonged courses of gentamicin in prior studies (Forge et al.,1987), long term strial changes were not apparent in our mice two weeks after 15 days of twice daily kanamycin treatment as a single agent. In this current study, we did observe that atrophy and thinning of the stria vascularis were long-term sequelae of combination kanamycin-furosemide exposure. A prior study using freeze fracture and thin sectioning demonstrated that the stria vascularis in guinea pigs treated with aminoglycosides was significantly thinner than normal and less structurally complex compared to control animals (Forge, 1981). Other studies have also noted the effect of strial atrophy after chronic aminoglycoside administration; however, strial atrophy in aminoglycoside ototoxicity has been shown to cause little change the endocochlear potential (Forge et al., 1987; Komune and Snow, 1982). Others have shown using fluorescently tagged aminoglycoside administered via intraperitoneal route that aminoglycosides enter the endolymph primarily through blood vessels in the stria and accumulate in the cytoplasm of marginal cells preferentially over intermediate and basal cells (Wang and Steyger, 2009). In our study using light microscopy only, we did not observe changes in strial morphology after administration of aminoglycosides as a single agent.
The mechanism of uptake of aminoglycosides from the vascular space into sensory cells has also been carefully studied, and investigators have demonstrated the presence of aminoglycosides in a number of cochlear cell types including the sensory cells of the cochlea and the stria vascularis, spiral ligament, and spiral limbus (Dai and Steyger, 2008; de Groot et al., 1990; Hashino et al., 2000; Mihelic-Rapp and Giebel, 1996). Certain cells that demonstrate uptake of the drug are susceptible whereas others can absorb the drug and remain resistant. Also, concentration of divalent cations, such as calcium and magnesium appear to affect aminoglycoside uptake into the hair cell and hair cell susceptibility to aminoglycoside in zebrafish (Coffin et al., 2009). It is apparent that the amount of gentamicin uptake per cell is not a key factor in determining which cells are susceptible to the drug, as others have shown that numerous cells that avidly incorporate aminoglycoside remain resistant to ototoxicity, such as the type I and III fibrocytes and apical hair cells (Imamura and Adams, 2003; Tran Ba Huy et al., 1983). The mechanism behind spiral limbus susceptibility to kanamycin/furosemide is uncertain, but could be due to bystander injury of the spiral limbus after uptake and disposal of these toxic agents. There is currently no evidence to suggest what role the spiral limbus stellate cells play in ototoxicity.
Aminoglycosides, when administered alone, primarily affect the hair cells in the basal turn. While this observation has been noted for many decades, the specific mechanism behind the susceptibility of high frequency hair cells is not entirely clear. One theory that has been proposed is that the concentration gradient of aminoglycosides favors its accumulation in the basal turn (Dai and Steyger, 2008, 2006; Hiel et al., 1992). With the addition of a loop diuretic, the concentration of aminoglycoside in the perilymph and endolymph increases and could possibly diffuse further into the apical turn. Some studies have shown that aminoglycosides are maintained at a higher concentration in the serum, cerebrospinal fluid (CSF) and perilymph after coadministration with loop diuretic (Ohtani et al., 1978; Tran Ba Huy et al., 1983). One theory regarding the pharmacokinetics of kanamycin after loop diuretic is that kanamycin is not effectively cleared through the typical renal mechanisms after loop diuretic (Ohtani et al., 1978). Others have proposed that loop diuretics facilitate entry of aminoglycoside into the endolymph while not affecting concentration of the drug in the perilymph (Tran Ba Huy et al., 1983). In this later paper, administration of both aminoglycoside and loop diuretic was given by continuous IV infusion, while most other studies describe a single injection given either intravenously or intraperitoneally. Thus, drug levels in the serum, CSF and ear fluids may be difficult to compare between studies. One case report documents an increase in the aminoglycoside peak and trough by approximately five-fold when given in combination with loop diuretic (Kaka et al., 1984). This finding would support the observation reported in the study by Ohtani et al. demonstrating that loop diuretics heightened drug levels in various compartments within the animal. Unfortunately, the studies that document the effect of loop diuretics on the pharmacokinetics of aminoglycoside clearance are sparse.
Finally, in this study, we noted the appearance of a large population of inflammatory cells in the inner ear after ototoxic injury. A larger number of macrophages appeared in cochleas exposed to combination therapy when compared to kanamycin alone. Inflammatory cell recruitment to the inner ear is an event that has only recently been noted in ototoxicity. Aminoglycoside ototoxicity has been shown to occur via classic apoptotic mechanisms characterized by the elaboration of reactive oxygen species, the presence of activated caspases in dying hair cells, and morphologic features of pyknosis and fragmentation of nuclei (Cunningham et al., 2002, 2004; Forge and Li, 2000; Hirose et al., 1997; Matsui et al., 2002; Tabuchi et al., 2007). Furthermore, the inhibition of caspases have been shown to inhibit hair cell death after aminoglycoside exposure in the utricle in vitro (Matsui et al., 2002). In such forms of apoptotic cell death, inflammation and recruitment of leukocytes is considered a rare event. However, it is clear that monocytes and macrophages do enter the cochlea during ototoxic injury and these cells collect the ligament and the spiral ganglion as seen after acoustic injury (Hirose et al., 2005). Macrophages migrate further apically in mice that receive combined therapy when compared to those mice receiving kanamycin alone, possibly due to chemotactic factors produced by damaged hair cells in the apical region. Despite significant atrophy and cell loss in the stria vascularis, we do not observe large numbers of macrophages in this area. This apparent lack of macrophages in the stria could be due to decreased permeability of strial endothelial cells to migrating leukocytes or to rapid reentry of macrophages back into circulation, leading to a shorter transit time in the intrastrial spaces. Why strial damage or atrophy does not induce an accumulation of macrophages, despite the apparent vascularity of this structure, is unclear.
After hair cell injury, supporting cells are believed to be involved in phagocytosis of debris in the sensory epithelium (Abrashkin et al., 2006; Li et al., 1995). We postulate that while supporting cells may play a role in cell clearance, professional phagocytes recruited to the cochlea might also contribute to clearance of hair cell debris. We did not observe direct evidence of macrophages engulfing hair cells in the light microscopic survey performed here, but the role of tissue macrophages in other organs is entirely consistent with this possibility. Macrophage migration could exacerbate ototoxicity, either due to bystander effects or due to activation of inflammatory mediators that follow monocyte and macrophage recruitment to the inner ear. It is also possible that monocytes and macrophages play a protective role after ototoxicity. Monocytes and macrophages are known to produce cytokines and growth factors that are important for epithelial repair in other organs and could play a similar role in the inner ear. At this time, we have no evidence to suggest that cochlear macrophage recruitment is either beneficial or detrimental after hair cell injury from ototoxicity. Exactly what role is played by recruited monocytes to the site of cochlear injury is currently unknown and is the subject of ongoing investigation.
In this study, cochlear macrophages are not observed in large numbers in the organ of Corti. Theyare located primarily in the lateral wall and in the spiral ganglion and adherent to the walls of the scala tympani. We hypothesize that the high potassium content of the endolymph is a hostile environment for macrophages and therefore, they are seen very rarely in the scala media. If the high potassium concentration is unfavorable for macrophages survival, how they remain viable in the intrastrial spaces is unclear. It is apparent from prior work that macrophages and monocytes are consistently observed in the stria vascularis, particularly surrounding the strial capillaries.
Fixed and stained tissue sections provide only brief snapshots of cochlear macrophages at a specific time and place. If hair cell clearance by macrophages occurs quickly and sporadically through brief extensions of filapodia into the sensory epithelium from cell bodies in scala tympani, it could be difficult to capture these cells in the act of ingesting a dying hair cell. To address these questions, we are pursuing other methods of imaging cochlear macrophages interacting with injured hair cells. Cytokines and growth factors secreted by macrophages could substantially affect the final outcome of the injured cochlea. To summarize, we propose that both supporting cells and recruited macrophages may contribute to clearance of damaged hair cells and that macrophages may play an important role in repair and recovery of the sensory epithelium by modulating the inflammatory response to injury. We propose that circulating monocytes are recruited to the ear not only for their phagocytic function, but also to summon other downstream events that eventually lead to resolution of inflammatory cell recruitment and repair.
Acknowledgments
We thank Dr. Grahame Kidd for his assistance with confocal microscopy and Elizabeth H. Shick for her technical assistance. This work was funded by NIH grant K08 DC005761 and a grant from the Triple T Foundation and by Nancy Lerner Fisher.
References
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, Beyer LA, Gong TW, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hear Res. 2006;218:20–29. doi: 10.1016/j.heares.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Agterberg MJ, Versnel H, de Groot JC, Smoorenburg GF, Albers FW, Klis SF. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008;244:25–34. doi: 10.1016/j.heares.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Alam SA, Ikeda K, Kawase T, Kikuchi T, Katori Y, Watanabe K, Takasaka T. Acute effects of combined administration of kanamycin and furosemide on the stria vascularis studied by distortion product otoacoustic emission and transmission electron microscopy. Tohoku J Exp Med. 1998;186:79–86. doi: 10.1620/tjem.186.79. [DOI] [PubMed] [Google Scholar]
- Arnold W, Nadol JB, Jr, Weidauer H. Ultrastructural histopathology in a case of human ototoxicity due to loop diuretics. Acta Otolaryngol. 1981;91:399–414. doi: 10.3109/00016488109138521. [DOI] [PubMed] [Google Scholar]
- Asakuma S, Snow JB., Jr Effects of furosemide and ethacrynic acid on the endocochlear direct current potential in normal and kanamycin sulfate-treated guinea pigs. Otolaryngol Head Neck Surg. 1980;88:188–193. [PubMed] [Google Scholar]
- Azuma H, Takeuchi S, Higashiyama K, Ando M, Kakigi A, Nakahira M, Yamakawa K, Takeda T. Bumetanide-induced enlargement of the intercellular space in the stria vascularis requires an active Na+-K+-ATPase. Acta Otolaryngol. 2002;122:816–821. [PubMed] [Google Scholar]
- Bock GR, Yates GK, Miller JJ, Moorjani P. Effects of N-acetylcysteine on kanamycin ototoxicity in the guinea pig. Hear Res. 1983;9:255–262. doi: 10.1016/0378-5955(83)90030-8. [DOI] [PubMed] [Google Scholar]
- Brummett RE. Effects of antibiotic–diuretic interactions in the guinea pig model of ototoxicity. Rev Infect Dis. 1981;3 (Suppl):S216–S223. [PubMed] [Google Scholar]
- Brummett RE, Brown RT, Himes DL. Quantitative relationships of theototoxic interaction of kanamycin and ethacrynic acid. Arch Otolaryngol. 1979;105:240–246. doi: 10.1001/archotol.1979.00790170010003. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SS, Larsen DL, Mitchell DL, El-Azizi M, Verhulst SJ, Hughes LF. Prevention of noise-and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Coffin AB, Reinhart KE, Owens KN, Raible DW, Rubel EW. Extracellular divalent cations modulate aminoglycoside-induced hair cell death in the zebrafish lateral line. Hear Res. 2009;253:42–51. doi: 10.1016/j.heares.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW. Caspase activation in hair cells of the mouse utricle exposed to neomycin. J Neurosci. 2002;22:8532–8540. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurobiol. 2004;60:89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- Dai CF, Steyger PS. A systemic gentamicin pathway across the stria vascularis. Hear Res. 2008;235:114–124. doi: 10.1016/j.heares.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai CF, Mangiardi D, Cotanche DA, Steyger PS. Uptake of fluorescent gentamicin by vertebrate sensory cells in vivo. Hear Res. 2006;213:64–78. doi: 10.1016/j.heares.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JC, Meeuwsen F, Ruizendaal WE, Veldman JE. Ultrastructural localization of gentamicin in the cochlea. Hear Res. 1990;50:35–42. doi: 10.1016/0378-5955(90)90031-j. [DOI] [PubMed] [Google Scholar]
- Ehret G. Peripheral anatomy and physiology II. In: Willott J, editor. The Auditory Psychobiology of the Mouse. Charles C. Thomas; Springfield, IL: 1983. pp. 169–200. [Google Scholar]
- Forge A. Freeze-fracture studies of the stria vascularis following administration of ethacrynic acid to guinea pigs. Scand Audiol Suppl. 1981;14 (Suppl):173–183. [PubMed] [Google Scholar]
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139:97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Forge A, Wright A, Davies SJ. Analysis of structural changes in the stria vascularis following chronic gentamicin treatment. Hear Res. 1987;31:253–265. doi: 10.1016/0378-5955(87)90195-x. [DOI] [PubMed] [Google Scholar]
- Gratacap B, Charachon R, Stoebner P. Results of an ultrastructural study comparing stria vascularis with organ of Corti in guinea pigs treated with kanamycin. Acta Otolaryngol. 1985;99:339–342. doi: 10.3109/00016488509108920. [DOI] [PubMed] [Google Scholar]
- Hartman BH, Basak O, Nelson BR, Taylor V, Bermingham-McDonogh O, Reh TA. Hes5 expression in the postnatal and adult mouse inner ear and the drug-damaged cochlea. J Assoc Res Otolaryngol. 2009;10:321–340. doi: 10.1007/s10162-009-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashino E, Shero M, Salvi RJ. Lysosomal augmentation during aminoglycoside uptake in cochlear hair cells. Brain Res. 2000;887:90–97. doi: 10.1016/s0006-8993(00)02971-1. [DOI] [PubMed] [Google Scholar]
- Hequembourg S, Liberman MC. Spiralligamentpathology:a majoraspectofage-relatedcochleardegenerationinC57BL/6 mice. J Assoc Res Otolaryngol. 2001;2:118–129. doi: 10.1007/s101620010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiel H, Schamel A, Erre JP, Hayashida T, Dulon D, Aran JM. Cellular and subcellular localization of tritiated gentamicin in the guinea pig cochlea following combined treatment with ethacrynic acid. Hear Res. 1992;57:157–165. doi: 10.1016/0378-5955(92)90148-g. [DOI] [PubMed] [Google Scholar]
- Higashiyama K, Takeuchi S, Azuma H, Sawada S, Yamakawa K, Kakigi A, Takeda T. Bumetanide-induced enlargement of the intercellular space in the stria vascularis critically depends on Na+ transport. Hear Res. 2003;186:1–9. doi: 10.1016/s0378-5955(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. 2005;489:180–194. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- Hirose K, Hockenbery DM, Rubel EW. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear Res. 1997;104:1–14. doi: 10.1016/s0378-5955(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Imamura S, Adams JC. Changes in cytochemistry of sensory and nonsensory cells in gentamicin-treated cochleas. J Assoc Res Otolaryngol. 2003;4:196–218. doi: 10.1007/s10162-002-2037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Schacht J. NF-kappaB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. J Neurosci Res. 2005;79:644–651. doi: 10.1002/jnr.20392. [DOI] [PubMed] [Google Scholar]
- Kaka JS, Lyman C, Kilarski DJ. Tobramycin-furosemide interaction. Drug Intell Clin Pharm. 1984;18:235–238. doi: 10.1177/106002808401800310. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Wang Q, Fu Y, Cohen DM, Steyger PS. TRPV4 enhances the cellular uptake of aminoglycoside antibiotics. J Cell Sci. 2008;121:2871–2879. doi: 10.1242/jcs.023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J, Raphael Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther. 2004;9:173–181. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Komune S, Snow JB., Jr Nature of the endocochlear dc potential in kanamycin-poisoned guinea pigs. Arch Otolaryngol. 1982;108:334–338. doi: 10.1001/archotol.1982.00790540006002. [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Effects of chronic furosemide treatment and age on cell division in the adult gerbil inner ear. J Assoc Res Otolaryngol. 2003;4:164–175. doi: 10.1007/s10162-002-2056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Nevill G, Forge A. Two modes of hair cell loss from the vestibular sensory epithelia of the guinea pig inner ear. J Comp Neurol. 1995;355:405–417. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Ogilvie JM, Warchol ME. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–1227. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihelic-Rapp M, Giebel W. A new immunohistochemical method for the detection of gentamicin in inner ear fluid compartments. Eur Arch Otorhinolaryngol. 1996;253:411–416. doi: 10.1007/BF00168493. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani I, Ohtsuki K, Omata T, Ouchi J, Saito T. Potentiation and its mechanism of cochlear damage resulting from furosemide and aminoglycoside antibiotics. ORL J Otorhinolaryngol Relat Spec. 1978;40:53–63. doi: 10.1159/000275386. [DOI] [PubMed] [Google Scholar]
- Pike DA, Bosher SK. The time course of the strial changes produced by intravenous furosemide. Hear Res. 1980;3:79–89. doi: 10.1016/0378-5955(80)90009-x. [DOI] [PubMed] [Google Scholar]
- Richardson GP, Russell IJ. Cochlear cultures as a model system for studying aminoglycoside induced ototoxicity. Hear Res. 1991;53:293–311. doi: 10.1016/0378-5955(91)90062-e. [DOI] [PubMed] [Google Scholar]
- Rizzi MD, Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2007;15:352–357. doi: 10.1097/MOO.0b013e3282ef772d. [DOI] [PubMed] [Google Scholar]
- Rybak LP. Ototoxicity of loop diuretics. Otolaryngol Clin North Am. 1993;26:829–844. [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005;10:1313–1321. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth C, Scott V. Comparative acute ototoxicity of loop diuretic compounds. Eur Arch Otorhinolaryngol. 1991;248:353–357. doi: 10.1007/BF00169028. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Baker CA, Montey KL, Chang LY, Coco A, Fallon JB, Shepherd RK. Synaptic plasticity after chemical deafening and electrical stimulation of the auditory nerve in cats. J Comp Neurol. 2010;518:1046–1063. doi: 10.1002/cne.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N, Crouch JJ, Lytle C, Schulte BA. Na-K-Cl cotransporter expression in the developing and senescent gerbil cochlea. Hear Res. 1998;118:114–122. doi: 10.1016/s0378-5955(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Santi PA, Lakhani BN. The effect of bumetanide on the stria vascularis: a stereological analysis of cell volume density. Hear Res. 1983;12:151–165. doi: 10.1016/0378-5955(83)90103-x. [DOI] [PubMed] [Google Scholar]
- Schatz A, Bugie E, Sa W. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. Proc Soc Exp Biol Med. 1944;55:66–69. doi: 10.1097/01.blo.0000175887.98112.fe. [DOI] [PubMed] [Google Scholar]
- Sewell WF. The effects of furosemide on the endocochlear potential and auditory-nerve fiber tuning curves in cats. Hear Res. 1984;14:305–314. doi: 10.1016/0378-5955(84)90057-1. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Matsushima J, Martin RL, Clark GM. Cochlear pathology following chronic electrical stimulation of the auditory nerve: II. Deafened kittens Hear Res. 1994;81:150–166. doi: 10.1016/0378-5955(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Stone JS, Leano SG, Baker LP, Rubel EW. Hair cell differentiation in chick cochlear epithelium after aminoglycoside toxicity: in vivo and in vitro observations. J Neurosci. 1996;16:6157–6174. doi: 10.1523/JNEUROSCI.16-19-06157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Pak K, Chavez E, Ryan AF. Role of inhibitor of apoptosis protein in gentamicin-induced cochlear hair cell damage. Neuroscience. 2007;149:213–222. doi: 10.1016/j.neuroscience.2007.06.061. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Nevill G, Forge A. Rapid hair cell loss: a mouse model for cochlear lesions. J Assoc Res Otolaryngol. 2008;9:44–64. doi: 10.1007/s10162-007-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Ba Huy P, Manuel C, Meulemans A, Sterkers O, Wassef M, Amiel C. Ethacrynic acid facilitates gentamicin entry into endolymph of the rat. Hear Res. 1983;11:191–202. doi: 10.1016/0378-5955(83)90078-3. [DOI] [PubMed] [Google Scholar]
- Versnel H, Agterberg MJ, de Groot JC, Smoorenburg GF, Klis SF. Time course of cochlear electrophysiology and morphology after combined administration of kanamycin and furosemide. Hear Res. 2007;231:1–12. doi: 10.1016/j.heares.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both amino-glycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Steyger PS. Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J Assoc Res Otolaryngol. 2009;10:205–219. doi: 10.1007/s10162-009-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME. Sensory regeneration in the vertebrate inner ear: differences at the levels of cells and species. Hear Res. 2010 doi: 10.1016/j.heares.2010.05.004. (Epub ahead of print May 19, 2010) [DOI] [PubMed] [Google Scholar]
- Weisleder P, Rubel EW. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol. 1993;331:97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hear Res. 2001;158:165–178. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]
- Xu SA, Shepherd RK, Chen Y, Clark GM. Profound hearing loss in the cat following the single co-administration of kanamycin and ethacrynic acid. Hear Res. 1993;70:205–215. doi: 10.1016/0378-5955(93)90159-x. [DOI] [PubMed] [Google Scholar]