Abstract
Oxidative stress plays a role in atherosclerotic diseases such as coronary artery disease (CAD), and much attention has been paid to antioxidant foods. The relationships between the consumption of vegetables and fruits and atherosclerotic diseases have been reported in many epidemiological studies showing a reduced risk of such diseases. In addition to the antioxidant vitamins C and E, green and yellow vegetables contain abundant quantities of carotenoids and polyphenols. The consumption of carotenoids and vitamins C and E has been shown to be inversely associated with CAD. However, supplementation with beta-carotene and vitamins C and E shows no beneficial effect, but rather mortality is increased with beta-carotene and vitamin E supplements. Therefore, it is recommended to consume vegetables and fruits, but vitamin supplementation is not recommended. Many epidemiological studies also report that higher consumption of fish, rich in n-3 polyunsaturated fatty acids (PUFAs), is associated with a lower risk of CAD and stroke. Antiatherosclerotic effects of n-3 PUFAs include reduced platelet aggregation, triglyceride-lowering effect, anti-inflammatory effect, and plaque stabilization, but the anti-inflammatory effect is principally responsible for preventing atherosclerosis. It is recommended to consume fish at least twice a week in patients without CAD and to consider n-3 PUFA supplements in patients with documented CAD. Regarding soy products, soy protein consumption reduces low-density-lipoprotein cholesterol and triglyceride levels. Isoflavone, a polyphenol contained in soybeans, has antiatherosclerotic property because it has a structure similar to that of estrogen and bonds with estrogen receptors. High consumption of isoflavone has been reported to be associated with a reduced risk of CAD and stroke only in women, but the preventative effect of soy products in the general population has not yet been clarified. Thus, many epidemiological studies report the promising effects of antioxidant foods, but there are many unclear points remaining with regard to the contribution of the nutritional elements found in antioxidant foods to the prevention of atherosclerotic diseases.
Keywords: antioxidants, fruits, seafoods, soy products, vegetables
Introduction
The oxidative stress that occurs during the breakdown of the oxidant/antioxidant balance within our body plays a major role in the development of atherosclerotic diseases such as coronary artery disease (CAD) and stroke. While lipid- lowering therapy with statins is commonly used for the treatment for atherosclerotic disease, a great deal of attention has been paid to antioxidant foods, which can contribute to the maintenance of the oxidant/antioxidant balance. A wide range of antioxidants exists within foods, with the function of these antioxidants in relation to atherosclerosis having also been widely reported.
Vegetables and Fruits
Vegetables and fruits are well known to be important sources of carbohydrates, dietary fiber, antioxidant vitamins, and minerals, along with carotenoids, polyphenols, and other phytochemicals. The relationships between eating vegetables and fruits and preventing CAD and stroke have been demonstrated in many epidemiological studies showing a reduced risk of such diseases. In a prospective cohort study by Liu et al.1 among 39,876 female health professionals, assessing the associations between vegetable and fruit consumption and risk of cardiovascular diseases, including CAD and stroke, the relative risk between those with the lowest vegetable and fruit consumption (median value: 2.6 servings/day) and those with the highest consumption (median value: 10.2 servings/day) was 1.0 and 0.68, respectively. This study suggested a protective effect of vegetables and fruits against CAD, especially myocardial infarction (MI). Another study by Joshipura et al.2 among 42,148 men and 84,251 women also demonstrated a relative risk for CAD of 0.80 in the highest quintile of vegetable and fruit consumption and a 4% lower risk for each one-serving per day increase in such consumption. In their study, the consumption of green leafy vegetables and vitamin C-rich fruits and vegetables contributed most to the protective effect of vegetables and fruits. Furthermore, He et al.3 conducted a meta-analysis of eight cohort studies to evaluate the relationship between the vegetable and fruit consumption and the risk of stroke. They showed that compared with the group of people who consumed less than three servings per day of vegetables and fruits, there was a reduced relative risk of stroke of 0.89 in the group with three to five servings per day and 0.74 in the group with more than five servings per day (Table 1). Therefore, the consumption of vegetables and fruits is recognized to be inversely associated with the risk of atherosclerotic diseases such as CAD and stroke.
Table 1.
Risk of stroke (95% confidence interval) for three to five servings and more than five servings of fruits and vegetables per day compared with less than three servings (adapted from He FJ et al.3).
| FRUITS AND VEGETABLE INTAKE (SERVINGS PER DAY) | ||
|---|---|---|
| 3–5 | >5 | |
| Joshipura et al (men) | 0.77 (0.49–1.20) | 0.78 (0.57–1.06) |
| Joshipura et al (women) | 0.89 (0.66–1.20) | 0.70 (0.58–0.85) |
| Hirvonen et al | 0.85 (0.78–0.93) | 0.74 (0.58–0.95) |
| Bazzano et al | 0.94 (0.83–1.07) | 0.70 (0.55–0.89) |
| Johnsen et al | 0.86 (0.66–1.12) | 0.73 (0.54–0.99) |
| Sauvaget et al | 0.90 (0.82–0.99) | 0.75 (0.69–0.82) |
| Steffen et al | 1.24 (0.96–1.61) | 0.94 (0.54–1.63) |
| Keli et al | 0.82 (0.54–1.24) | 0.75 (0.45–1.24) |
| Gillman et al | 0.60 (0.39–0.92) | 0.49 (0.30–0.79) |
| Pooled relative risk | 0.89 (0.83–0.97) | 0.74 (0.69–0.79) |
In addition to the antioxidant vitamins C and E, green and yellow vegetables contain abundant quantities of carotenoids, such as beta-carotene, and polyphenols, such as anthocyanin, which are believed to contribute to the prevention of atherosclerotic diseases. We previously reported both red and green perilla, which are popular vegetables in Japan and China, to be very rich in polyphenols and to have strong antioxidant activity against low-density lipoprotein (LDL) oxidation.4 Law et al.5 conducted a meta-analysis of 11 cohort studies to evaluate the relationships between dietary consumption of carotenoids and vitamins C and E contained in vegetables and fruits and the risk of CAD. They demonstrated the consumption of carotenoids and vitamins C and E to be inversely associated with CAD and showed the risk of CAD to be 15%, 12%, and 12% lower at the 90th than at the 10th centiles of carotenoid, vitamin C, and vitamin E consumption, respectively. These epidemiological studies conducted many randomized trials of antioxidant supplements to assess the effects on primary and secondary prevention of CAD and stroke. However, a randomized, placebo- controlled intervention trial,6 wherein 9,541 patients at high risk for cardiovascular events were administered either vitamin E (800 international units [IU]/day) or placebo, reported no preventative effect of vitamin E on major cardiovascular events, but rather the vitamin E group had higher incidence of heart failure. Furthermore, Bjelakovic et al.7 conducted a meta-analysis of 68 randomized trials with 232,606 participants to evaluate the effect of antioxidant supplements on all-cause mortality. They demonstrated that vitamins C and E and beta-carotene supplements given singly or combined with other supplements had no beneficial effect, and mortality was significantly increased with beta-carotene and vitamin E supplements. The cause of increased mortality with antioxidant supplements remains unclear, but some specific patient subgroups may receive benefit from such supplements. According to the report by Levy et al.8, supplementation with vitamins C and E showed significant benefit on the progression of coronary artery stenosis in haptoglobin 1 allele homozygotic women, but not in those with haptoglobin 2 allele, suggesting that the relative benefit or harm of vitamin supplements on CAD may depend on such haptoglobin types. Therefore, the American Heart Association (AHA) published a statement in 20069 recommending the consumption of vegetables and fruits, especially green and yellow vegetables, but not recommending antioxidant vitamin supplementation, to prevent atherosclerotic diseases such as CAD and stroke.
Fruits, especially citrus fruits, contain a large quantity of flavonoids, as well as antioxidant vitamin C and carotenoids. In particular, oranges and grapefruits contain large quantities of hesperidin and naringin. The report by Esmaillzadeh et al.10 on the eating habits of middle-aged women indicated that subjects with healthy eating habits (consumption of large quantities of fruits, vegetables, legumes, and fish and consumption of low quantity of meats high in saturated fatty acids) had a notably reduced risk of metabolic syndrome, with the consumption of fruits making a particularly significant contribution to this reduction. Williams et al.11 also showed that high consumption of fruits had negative correlation with obesity and triglyceride levels, along with positive correlation with high-density-lipoprotein cholesterol levels. Moreover, Knekt et al.12 reported the risk of stroke to be reduced by 20% in subjects with high consumption of hesperidin and naringin. Consuming fruits, along with green and yellow vegetables, is recognized to be beneficial in preventing atherosclerotic diseases.
Seafoods
Epidemiological studies implemented in the 1970s13,14 demonstrated an extremely low incidence of CAD among the indigenous people of Greenland, Eskimos, who consume mainly fish and seals rich in n-3 polyunsaturated fatty acids (PUFAs) (Fig. 1). As a result, the importance of n-3 PUFAs, that is, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), came to be noted. EPA is found in high concentrations in fish oil of sardines, along with DHA in tuna and bonito. Many epidemiological studies demonstrated higher fish consumption to be associated with a lower risk of CAD and stroke.15–17 Among Japanese populations, along with the association between high consumption of fish and low mortality due to CAD, Iso et al.17 reported an inverse association between fish consumption and risk of CAD. Moreover, in a rural district of Japan, the incidence of CAD was shown to be lower in fishing areas than in the mercantile and farming areas.18
Figure 1.
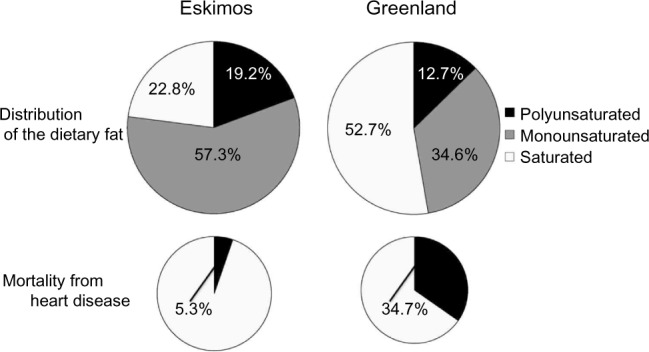
Dietary fat in Eskimo and Greenland divided by fat types and mortality from heart disease. Adapted from Bang and Dyerberg J et al.13,14
PUFAs are important constituents of cell membrane phospholipids, and the phospholipids of inflammatory cells are rich in n-6 PUFA arachidonic acid (AA), with low amounts of n-3 PUFAs EPA and DHA. AA acts as a source of proinflammatory eicosanoids, whereas both EPA and DHA reduce AA-derived eicosanoids and proinflammatory cytokines and produce anti-inflammatory resolvins and protectins.19 The balance between EPA (or DHA) and AA is important for regulating the production of proinflammatory and anti-inflammatory mediators. The high consumption of fish, rich in EPA and DHA, changes the PUFA composition of inflammatory cells, which leads to higher contents of EPA and DHA at the expense of AA.19 Proposed factors that may account for the antiatherosclerotic effects of n-3 PUFAs include reduced platelet aggregation, lowering of serum triglycerides and blood pressure, reduced expression of adhesion molecules, anti-inflammatory effect, and plaque stabilization (Table 2).19–21 However, accumulating evidences suggest that the anti-inflammatory effect of n-3 PUFAs is principally responsible for preventing atherosclerotic diseases.19,20,22 Matsumoto et al.22 showed that EPA administration suppresses atherosclerotic lesions with less macro phages and more collagen in apolipoprotein E-deficient mice. A randomized, controlled trial of patients undergoing carotid endarterectomy also showed n-3 PUFA supplementation to result in less inflammation and increased stability in carotid plaques.23
Table 2.
Factors involved in CHD risk that are affected by n-3 fatty acids (adapted from Harris et al.21).
| FACTOR | EFFECT |
|---|---|
| Serum TG concentration | ↓ |
| Production of chemoattractants | ↓ |
| Production of growth factors | ↓ |
| Cell surface expression of adhesion molecules | ↓ |
| Production of inflammatory eicosanoids | ↓ |
| Blood pressure | ↓ |
| Endothelial relaxation | ↑ |
| Thrombosis | ↓ |
| Cardiac arrhythmias | ↓ |
| Heart rate variability | ↑ |
| Atherosclerotic plaque stability | ↑ |
To assess the effect of n-3 PUFA supplements on the prevention of cardiovascular events, several randomized trials were conducted. A randomized clinical trial (Japan Eicosapentaenoic acid Lipid Intervention Study [JELIS]) with 1.8 g/day of EPA in 18,645 Japanese patients with hyperlipemia reported 19% reduction in major coronary events in the EPA group.24 Another randomized trial (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico [GISSI]-Prevenzione) with 1 g/day of n-3 PUFAs in 11,323 patients surviving recent MI also showed 30% reduction in cardiovascular mortality.25 Therefore, the AHA statement published in 20069 recommended the consumption of fish, preferably oily fish, at least twice a week in patients without CAD and to consider n-3 PUFA supplementation in patients with documented CAD. However, one of the potential problems arising from excessive intake of n-3 PUFAs is the occurrence of bleeding complications due to inhibited platelet aggregation. Increased incidence of brain hemorrhage has in fact been reported among Inuit people who consume large amounts of EPA and DHA.26 Therefore, consumption of n-3 PUFAs at more than 10 g/day should be avoided.
In addition to EPA and DHA, astaxanthin is another substance contained in fish, which may play a role in the prevention of atherosclerosis. Astaxanthin is a type of carotenoid, similar to lycopene in tomatoes and beta-carotene in carrots. It is present mainly in the red muscle meat of Salmonidae, the shells of crustaceans, and the body surface of red sea bream. Astaxanthin is a strong antioxidant, with a particularly strong ability to eliminate singlet oxygen, said to be 6,000 times that of vitamin C and 500 times that of vitamin E. We previously showed that the consumption of 3.6 mg/day for 2 weeks prolonged LDL lag time, suggesting the inhibition of LDL oxidation by astaxanthin.27 Moreover, Yoshida et al.28 reported the consumption of astaxanthin (6–18 mg/day) for 12 weeks to reduce serum triglyceride levels and to increase high-density-lipoprotein (HDL) cholesterol and adiponectin levels in 61 subjects with mild hyperlipidemia.
Soy Products
The fatty acid composition of lipids included in soybeans is different from that of lipids contained in meats, and soybeans contain a much greater quantity of PUFAs than saturated fatty acids. The amino acid composition of soy protein is also different from that of animal proteins. A meta-analysis of 38 clinical trials to assess the effect of soy protein consumption on serum lipid levels demonstrated 13% reduction in LDL-cholesterol levels and 11% reduction in triglyceride levels, with no significant increase in HDL-cholesterol levels.29 Furthermore, isoflavone, which is a type of polyphenol contained in soybeans, is expected to have antiatherosclerotic property because it has a similar structure to estrogen and can bond with estrogen receptors. In a large cohort study in Japanese populations (Japan Public Health Center-based Prospective Study), high consumption of isoflavone was shown to be associated with a reduced risk of CAD and stroke in women, but not in men.30 However, in a randomized controlled trial on postmenopausal women in the USA, soy protein supplementation (25 g/day) was reported to reduce subclinical atherosclerosis progression, assessed as carotid artery intima-media thickness progression, by 16% relative to the placebo group, but this treatment effect was not statistically significant.31 Only among the subgroup of women who were in the <5 years postmenopausal stage, soy protein reduced carotid thickness progression by 68%, with borderline significance. Therefore, because the preventative effect of soy protein or isoflavone supplementation has not yet been proven in regard to atherosclerotic diseases, further research is required.
Conclusion
While many epidemiological studies have reported the promising effects of antioxidant foods, there are still many unclear points remaining in regard to the contribution of individual substances of antioxidant foods to the prevention of atherosclerotic diseases. As shown in Table 3, AHA recommends the consumption of fish, especially oily fish, as well as a diet rich in vegetables and fruits, for athero-sclerotic disease risk reduction.9 While further research is needed in regard to the individual effects of these nutritional elements on atherosclerosis, it is also necessary to focus on the effectiveness of eating a combination of nutritional elements. Food is not simply an indulgence but is required to maintain good health. It should be remembered that it is important to balance the foods we incorporate into our daily lifestyle.
Table 3.
AHA diet and lifestyle recommendations for cardiovascular disease risk reduction (adapted from Lichtenstein AH et al.9).
| • Balance calorie intake and physical activity to achieve or maintain a healthy body weight. |
| • Consume a diet rich in vegetables and fruits. |
| • Choose whole-grain, high-fiber foods. |
| • Consume fish, especially oily fish, at least twice a week. |
| • Limit your intake of saturated fat to <7% of energy, trans-fat to <1% of energy, and cholesterol to <300 mg/day by choosing lean meats and vegetable alternatives; selecting fat-free (skin), 1%-fat, and low-fat dairy products; and minimizing intake of partially hydrogenated fats. |
| • Minimize your intake of beverages and foods with added sugars. |
| • Choose and prepare foods with little or no salt. |
| • If you consume alcohol, do so in moderation. |
Notes: When you eat food that is prepared outside of the home, follow the AHA Diet and Lifestyle Recommendations.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: YM, ES. Analyzed the data: ES, YM. Contributed to the writing of the manuscript: YM, ES. Agree with manuscript results and conclusions: KK, YM, ES. Jointly developed the structure and arguments for the paper: YM, ES. Made critical revisions and approved final version: ES. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Liu S, Manson JE, Lee IM, et al. Fruit and vegetable intake and risk of cardiovascular disease: the women’s health study. Am J Clin Nutr. 2000;72:922–8. doi: 10.1093/ajcn/72.4.922. [DOI] [PubMed] [Google Scholar]
- 2.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–14. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 3.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet. 2006;367:320–6. doi: 10.1016/S0140-6736(06)68069-0. [DOI] [PubMed] [Google Scholar]
- 4.Saita E, Kishimoto Y, Tani M, et al. Antioxidant activities of perilla frutescens against low-density lipoprotein oxidation in vitro and in human subjects. J Oleo Sci. 2012;61:113–20. doi: 10.5650/jos.61.113. [DOI] [PubMed] [Google Scholar]
- 5.Law MR, Morris JK. By how much does fruit and vegetable consumption reduce the risk of ischemic heart disease. Eur J Clin Nutr. 1998;52:549–56. doi: 10.1038/sj.ejcn.1600603. [DOI] [PubMed] [Google Scholar]
- 6.HOPE and HOPE-TOO Trial Investigators Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–47. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 7.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 8.Levy AP, Friedenberg P, Lotan R, et al. The effect of vitamin therapy on the progression of coronary artery atherosclerosis varies by haptoglobin type in postmenopausal women. Diabetes Care. 2004;27:925–30. doi: 10.2337/diacare.27.4.925. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 10.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr. 2007;85:910–8. doi: 10.1093/ajcn/85.3.910. [DOI] [PubMed] [Google Scholar]
- 11.Williams DE, Prevost AT, Whichelow MJ, Cox BD, Day NE, Wareham NJ. A cross-sectional study of dietary patterns with glucose intolerance and other features of the metabolic syndrome. Br J Nutr. 2000;83:257–66. doi: 10.1017/s0007114500000337. [DOI] [PubMed] [Google Scholar]
- 12.Knekt P, Kumpulainen J, Jarvinen R, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. 2002;76:560–8. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 13.Bang HO, Dyerberg J, Sinclair HM. The composition of the eskimo food in north western greenland. Am J Clin Nutr. 1980;33:2657–61. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- 14.Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978;2:117–9. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- 15.Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285:304–12. doi: 10.1001/jama.285.3.304. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–21. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 17.Iso H, Kobayashi M, Ishihara J, et al. JPHC Study Group. Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese: the Japan public health center-based (JPHC) study cohort 1. Circulation. 2006;113:195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Azuma A, Kuribayashi T, Sugihara H, Okuda S, Nakagawa M. Serum fatty acid levels, dietary style and coronary heart disease in three neigh-boring areas in Japan: the Kumihama study. Br J Nutr. 2003;89:267–72. doi: 10.1079/BJN2002747. [DOI] [PubMed] [Google Scholar]
- 19.Calder PC. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. 2012;56:1073–80. doi: 10.1002/mnfr.201100710. [DOI] [PubMed] [Google Scholar]
- 20.Momiyama Y. Association between serum omega-3 to omega-6 polyunsaturated fatty acid ratio and cardiovascular events in a general Japanese population. Atherosclerosis. 2013;231:281–2. doi: 10.1016/j.atherosclerosis.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 21.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart desease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto M, Sata M, Fukuda D, et al. Orally administered eicosapentaenoic acid reduces and stabilizes atherosclerotic lesions in ApoE-deficient mice. Atherosclerosis. 2008;197:524–33. doi: 10.1016/j.atherosclerosis.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Cawood AL, Ding R, Napper FL, et al. Eicosapentaenoic acid from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212:252–9. doi: 10.1016/j.atherosclerosis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama M, Origasa H, Matsuzaki M, et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 25.Marchioli R, Barzi F, Bomba E, et al. GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the GISSI-Prevenzione. Circulation. 2002;105:1897–903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 26.Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–74. Acta Med Scand. 1980;208:401–6. [PubMed] [Google Scholar]
- 27.Iwamoto T, Hosoda K, Hirano R, et al. Inhibition of low-density lipoprotein oxidation by astaxanthin. J Atheroscler Thromb. 2000;7:216–22. doi: 10.5551/jat1994.7.216. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida H, Yanai H, Ito K, et al. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010;209:520–3. doi: 10.1016/j.atherosclerosis.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–82. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 30.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the japan public health center-based (JPHC) study cohort I. Circulation. 2007;116:2553–62. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 31.Hodis HN, Mack WJ, Kono N, et al. Women’s Isoflavone Soy Health Research Group Isoflavone soy protein supplementation and atherosclerosis progression in healthy postmenopausal women: a randomized controlled trial. Stroke. 2011;42:3168–75. doi: 10.1161/STROKEAHA.111.620831. [DOI] [PMC free article] [PubMed] [Google Scholar]


