Abstract
Background and Purpose
General anesthesia (GA) for endovascular therapy (EVT) of acute ischemic stroke (AIS) may be associated with worse outcomes.
Methods
The IMS III trial randomized patients within 3hrs of AIS onset to IV t-PA±EVT. GA use within 7hrs of stroke onset was recorded per protocol. Good outcome was defined as 90day mRS≤2. A multivariable analysis adjusting for dichotomized NIHSS (8-19 versus ≥20), age, and time from onset to groin puncture was performed.
Results
Four hundred thirty-four patients were randomized to EVT, 269(62%) were treated under local anesthesia and 147(33.9%) under GA; 18(4%) were undetermined. The two groups were comparable except for median baseline NIHSS (16 local anesthesia versus 18 [GA], p<0.0001). The GA group was less likely to achieve a good outcome (adjusted-RR 0.68, CI 0.52-0.90; p=0.0056) and had increased in-hospital mortality (adjusted-RR 2.84, CI 1.65-4.91; p=0.0002). Those with medically indicated GA had worse outcomes (adjusted RR 0.49, CI 0.30-0.81, p=0.005) and increased mortality (RR 3.93, CI 2.18-7.10; p<0.0001) with a trend for higher mortality with routine GA. There was no significant difference in the adjusted risks of SAH (p=0.32) or symptomatic ICH (p=0.37).
Conclusions
GA was associated with worse neurological outcomes and increased mortality in the EVT arm; this was primarily true among patients with medical indications for GA. Relative risk estimates, though not statistically significant, suggest reduced risk for SAH and sICH under local anesthesia. Although the reasons for these associations are not clear, these data support the use of local anesthesia when possible during EVT.
Keywords: Anesthesia, Ischemic Stroke, Endovascular Treatment, Thrombolysis Subject Codes: Acute Cerebral Infarction, Emergency Treatment of Stroke
INTRODUCTION
The Interventional Management of Stroke (IMS) III trial was the largest randomized open-label trial of endovascular therapy (EVT) following intravenous (IV) thrombolysis for acute ischemic stroke (AIS). The trial was stopped early due to futility, which in combination with data from other trials has resulted in a reassessment of the utility of EVT as an adjunct treatment for IV thrombolysis treated patients, despite superior reperfusion with EVT.1-3 To better understand the discrepancy between superior reperfusion and similar clinical outcomes, it is important to investigate factors associated with EVT that may positively or negatively affect clinical outcome. Although newer device technologies have garnered the majority of attention, another potentially important factor contributing to outcomes is periprocedural patient management such as blood pressure (BP), glucose, and temperature management, all of which are linked with stroke outcomes.4 The choice of procedural anesthesia may also be important.
Several retrospective registries have shown worse outcomes in patients treated with EVT under general anesthesia (GA) as compared to local anesthesia or conscious sedation (will be collectively referred to as LA in this manuscript). The largest of these was a multi-center retrospective study of 980 patients that found that GA was associated with poor outcome at 90 days and increased mortality.5 In a retrospective analysis of 75 patients from the IMS II study, patients treated with lesser degrees of anesthesia fared better with improved neurological outcomes and lower mortality.6 These data were retrospective and have been controversial as there are proponents of GA who cite increased safety during the procedure as the primary indication, although the retrospective data have shown that the risk of subarachnoid hemorrhage (SAH) or symptomatic intracerebral hemorrhage (sICH) has been equivalent or lower with LA.5
The IMS III trial afforded an opportunity to study the possible impact of anesthesia on EVT outcomes in a planned analysis. The following primary hypotheses were tested in this study: 1) GA is associated with poorer outcomes, 2) there is a difference in the risk of SAH and sICH in patients undergoing GA compared to LA and 3) GA is associated with longer time to EVT initiation.
Methods
The IMS III trial was a multicenter, randomized, open-label trial of EVT following IV thrombolysis in patients with moderate to severe AIS treated within 3 hours of stroke onset sponsored by the National Institute of Neurological Disorders and Stroke. The protocol, patient selection criteria, treatment approaches, and final results have been previously published.1, 7 Randomization and analysis were stratified by severity, defined according to National Institutes of Health Stroke Scale (NIHSS) ≤19 or ≥20. The primary clinical outcome was a modified Rankin Scale (mRS) score of ≤2 at 90 days, performed by blinded investigators. Recanalization success was defined as a Thrombolysis in Cerebral Infarction (TICI) score ≥2.8 The primary safety endpoints were death, SAH and sICH (defined as any intracranial hemorrhage within 24±6 hours of randomization temporally related to a decline in neurological status as well as new or worsening neurologic symptoms in the judgment of the clinical investigator and which may have warranted medical intervention). Local institutional review board approval was obtained at all centers.
Periprocedural anesthesia was defined as GA if the subject underwent endotracheal intubation within 7 hours of stroke onset. This time period was chosen to capture all patients who were intubated before or during procedure because the protocol mandated initiation of the angiographic procedure within 5 hours and completion within 7 hours after stroke onset. All other EVT subjects were defined as having undergone LA, regardless of whether or not they received conscious sedation. Subjects were intubated based upon the judgment of the treating team and not per protocol. The primary reason for intubation was categorized by each investigator as: 1) “routine practice” (i.e. routine practice to intubate and use GA prior to EVT) or 2) “medically indicated” (i.e. concern for ability to protect airway/aspiration risk, cardiopulmonary deterioration, signs of herniation/increased intracranial pressure, inadequate pain control or agitation, or other). The study protocol did not mandate one approach over the other. The endovascular approach was previously described.7
Statistical Analysis
Subjects with intubation status unknown (n=18) were excluded from the analysis. For consistency with the primary publication, an unfavorable outcome was imputed for subjects with mRS missing or obtained outside of window. The generalized linear model was used to test the association between GA and outcome, with the log link used in order to produce relative risk estimates. Confidence intervals (CI) provided are 95% intervals. Because the limited sample size and small number of safety events, particularly with respect to sICH, limited the number of potential covariate adjustments, these were pre-specified based on clinical relevance rather than selected according to statistical significance. For models of good outcome and in-hospital mortality, adjusted models included severity stratum, age, and time from onset to groin puncture (TOG); models of safety outcomes were adjusted only for mechanical embolectomy. Procedural times are described via mean±SD; the effect of GA on these times is assessed via t-test.
Results
A total of 434 patients were randomized to the EVT arm of IMS III. General anesthesia was utilized in 147(33.9%) patients. The GA and LA cohorts were comparable in baseline demographics, medical comorbidities, time to t-PA, TOG, time from IV t-PA initiation to EVT initiation, time from onset to recanalization, 40-minute post t-PA bolus systolic BP (SBP), and occlusion side (Table 1). The LA cohort tended to have lower NIHSS scores (median 16 vs. 18, p <0.0001) and slightly lower incidence of internal carotid artery occlusion (p=0.06). Intubation was associated with stroke severity as measured by NIHSS quartiles (≤14, 15-19, 20-24, ≥25, p-value<0.0001) as well as by baseline Alberta Stroke Program Early CT Score (ASPECTS) (p-value=0.0395). Reperfusion success (TICI 2-3) was achieved in 76.4% of the GA cohort versus 72.8% of the LA cohort, p=0.48 (Table 2).
Table 1.
Patient and Procedural Details
| Endovascular Therapy (N=434)1 | Intravenous t-PA Only (N=222)2 | |||||
|---|---|---|---|---|---|---|
| Endotracheal Intubation Status | All Intubated | Routine Intubation | Medically Indicated Intubation | Not Intubated | Intubated | Not Intubated |
| (N=147) | (N=76) | (N=71) | (N=269) | (N=17) | (N=196) | |
| Demographics, N (%) | ||||||
| Age (range) | 69 (23-83) | 67 (31-82) | 72 (23-83) | 69 (27-89) | 70 (52-81) | 68 (23-84) |
| Female | 72 (49) | 35 (46.1) | 37 (52.1) | 133 (49.4) | 8 (47.1) | 89 (45.4) |
| Caucasian | 126 (85.7) | 67 (88.2) | 59 (83.1) | 223 (82.9) | 16 (94.1) | 167 (85.2) |
| Clinical History, N (%) | ||||||
| Hypertension | 108 (73.5) | 49 (64.5) | 59 (83.1) | 196 (72.9) | 15 (88.2) | 148 (75.5) |
| Diabetes Mellitus | 37 (25.2) | 16 (21.1) | 21 (29.6) | 51 (19.0) | 4 (23.5) | 45 (23.0) |
| Atrial Fibrillation | 60 (40.8) | 29 (38.2) | 31 (43.7) | 89 (33.1) | 5 (29.4) | 63 (32.1) |
| Congestive Heart Failure | 19 (12.9) | 8 (10.5) | 11 (15.5) | 29 (10.8) | 1 (5.9) | 28 (14.3) |
| Hyperlipidemia | 65 (44.2) | 30 (39.5) | 35 (49.3) | 142 (52.8) | 7 (41.2) | 100 (51.0) |
| Prior Antiplatelet Use | 65 (44.2) | 30 (39.5) | 35 (49.3) | 117 (43.5) | 8 (47.1) | 94 (48.0) |
| Clinical Status, mean (range) | ||||||
| NIHSS (median) | 18 (7-40) | 16 (7-40) | 20 (11-40) | 16 (7-29) | 21 (10-30) | 16 (8-30) |
| ASPECTS | 7.5 (0-10) | 8 (0-10) | 7 (0-10) | 8 (0-10) | 8 (1-10) | 8 (1-10) |
| Glucose (mmol/L) | 6.8 (3.9-23.3) | 6.5 (3.9-23.3) | 7 (4.7-19.7) | 6.5 (3-21.5) | 6.8 (5.4-14.4) | 6.6 (2.6-21.8) |
| Onset to Intravenous t-PA (min) | 117 (29-189) | 115 (36-189) | 120 (29-180) | 119.5 (25-200) | 124 (74-180) | 119.5 (44-194) |
| Onset to Groin Puncture (min) | 210 (110-315) | 210 (115-304) | 212.5 (110-315) | 206 (80-341) | 200.5 (149-252)§ | |
| Onset to Reperfusion (min, median [IQ]) | 332 (280-376) | 343 (267-371) | 329 (283-377) | 333 (285-374) | ||
| 40 min Systolic Blood Pressure | 142.5 (87-208) | 144.5 (97-195) | 141.5 (87-208) | 150 (85-226) | 149.5 (84-174) | 145 (66-215) |
| Left Hemisphere | 82 (55.8) | 42 (55.3) | 40 (56.3) | 131 (48.7) | 12 (70-6) | 89 (45.4) |
| Occlusion Location, N (%)3 | ||||||
| Internal Carotid Artery | 34 (26.6) | 19 (28.8) | 15 (24.2) | 33 (17.7)* | ||
| Middle Cerebral Artery Trunk | 53 (41.4) | 27 (40.9) | 26 (41.9) | 77 (41.4)♮ | ||
| Middle Cerebral Artery Branch | 36 (28.1) | 18 (27.3) | 18 (29.0) | 68 (36.6) | ||
| Vertebro-basilar | 5 (3.9) | 2 (3.0) | 3 (4.8) | 6 (3.2) | ||
| Endovascular Approach, N (%)4 | ||||||
| Standard Microcatheter | 42 (32.6) | 16 (23.9) | 26 (41.9) | 94 (49.5) | ||
| EKOS™ | 13 (10.1) | 9 (13.4) | 4 (6.5) | 9 (4.7) | ||
| Merci™ | 39 (30.2) | 18 (26.9) | 21 (33.9) | 47 (24.7) | ||
| Penumbra™ | 27 (20.9) | 20 (29.9) | 7 (11.3) | 27 (14.2) | ||
| Other | 8 (6.2) | 4 (6.0) | 4 (6.5) | 13 (6.8) | ||
18 subjects with unknown intubation status were excluded from analysis
9 subjects with unknown intubation status were excluded from analysis
108 subjects excluded from percentage denominator; 97 with no endovascular therapy, 5 with occlusion not identified on angiogram.
97 subjects with no endovascular therapy excluded from percentage denominator.
2 patients received endovascular therapy
p=0.06
An additional 2(0.7%) patients had anterior cerebral artery occlusion
NIHSS= National Institutes of Health Stroke Scale, ASPECTS- Alberta Stroke Program Early CT Score, t-PA- tissue plasminogen activator, IQ- interquartile range
Table 2.
Outcomes
| Endovascular Therapy (N=434)1 | Intravenous t-PA Only (N=222)2 | |||||
|---|---|---|---|---|---|---|
| Endotracheal Intubation Status | All Intubated | Routine Intubation | Medically Indicated Intubation | Not Intubated | Intubated | Not Intubated |
| N(%) | (N=147) | (N=76) | (N=71) | (N=269) | (N=17) | (N=196) |
| TICI 2-33 | 94 (76.4) | 51 (79.7) | 43 (72.9) | 131 (72.8) | - | - |
| Modified Rankin Scale ≤2 | 45 (30.6) | 31 (40.8) | 14 (19.7) | 129 (48) | 0 (0) | 84 (42.9) |
| In-hospital Death | 34 (23.1) | 10 (13.2) | 24 (33.8) | 20 (7.4) | 7 (41.2) | 27 (13.8) |
| Symptomatic Intracerebral Hemorrhage | 12 (8.2) | 5 (6.6) | 7 (9.9) | 13 (4.8) | 2 (11.8) | 10 (5.1) |
| Subarachnoid Hemorrhage4 | 23 (16.0) | 11 (14.7) | 12 (17.4) | 23 (8.9) | 1 (7.1) | 10 (5.4) |
18 subjects with unknown intubation status were excluded from analysis
9 subjects with unknown intubation status were excluded from analysis
113 subjects with unknown TICI score excluded from percentage denominator.
29 subjects with unknown subarachnoid hemorrhage status excluded from percentage denominator; 14 endovascular subjects and 15 IV tPA only subjects.
t-PA- tissue plasminogen activator, TICI- Thrombolysis In Cerebral Infarction recanalization grade
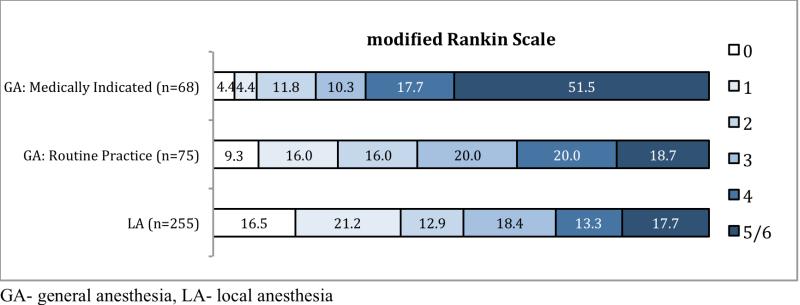
Good outcome was achieved in 129/269(48.0%) LA patients and in 45/147(30.6%) GA patients (Table 2). The GA group was significantly less likely to achieve a good outcome (relative risk [RR] 0.64, CI 0.49-0.84; p=0.0013) (Figure 1). There was a significant association between intubation and in-hospital death with 23.1% (34/147) mortality in the GA cohort and 7.4% (20/269) in the LA cohort (RR 3.11, CI 1.86-5.20; p<0.0001). When adjusted for severity stratum (NIHSS ≤19 or NIHSS ≥20), age, and TOG, there remained a significant negative association between GA and good outcomes (RR 0.68, CI 0.52-0.90; p=0.0056) and in-hospital mortality (RR 2.84, CI 1.65-4.91; p=0.0002) (Table 3).
Figure.
Distribution of modified Rankin Scale scores of disability at 3 months.
Table 3.
Adjusted outcomes analysis
| Endovascular Therapy (N=434)1 | ||||
|---|---|---|---|---|
| Endotracheal Intubation Status | All Intubated | Routine Intubation | Medically Indicated Intubation | Not Intubated |
| N(%) | (N=147) | (N=76) | (N=71) | (N=269) |
| Modified Rankin Scale ≤2 | RR 0.68, CI 0.52-0.90, p=0.0056 | RR 0.80, CI 0.60-1.06, p=0.12 | RR 0.49, CI 0.30-0.81, p=0.005 | 1 |
| In-hospital Death | RR 2.84, CI 1.65-4.91, p=0.0002 | RR 1.82, CI 0.87-3.77, p=0.11 | RR 3.93, CI 2.18-7.10, p<0.0001 | 1 |
| Symptomatic Intracerebral Hemorrhage | RR 1.45, CI 0.64-3.27, p=0.37 | 1 | ||
| Subarachnoid Hemorrhage | RR 1.34, CI 0.76-2.38, p=0.32 | 1 | ||
18 subjects with unknown intubation status were excluded from analysis
Compared to LA, medically indicated GA was associated with lower probability of a good outcome (adjusted RR 0.49, CI 0.30-0.81; p=0.005) and increased mortality (adjusted RR 3.93, CI 2.18-7.10; p<0.0001) (Table 3). Differences between routine GA and LA subjects did not reach statistical significance for either good outcome (adjusted RR 0.80, CI 0.60 -1.06; p=0.12) or mortality (adjusted RR 1.82, CI 0.87-3.77; p=0.11). There was higher in-hospital mortality among the medically indicated GA cohort than the routine cohort (adjusted RR 2.16, CI 1.09-4.29; p=0.0274) but the difference in good outcomes did not reach significance (adjusted RR 0.62, CI 0.36-1.07; p=0.0840). The results were not substantively affected by adjusting for a more detailed severity designation (NIHSS <=14, 15-19, 20-24, >=25).
There was a significant association between GA and SAH (RR 1.79, CI 1.04-3.08; p=0.035). This relationship was confounded by endovascular approach. After adjustment for mechanical embolectomy the association was not significant (adjusted RR 1.34, CI 0.76-2.38; p=0.32), Table 3. The association between GA and sICH did not reach statistical significance (RR 1.69, CI 0.79-3.61; p=0.18), even after adjustment for mechanical embolectomy (adjusted RR 1.45, CI 0.64-3.27; p=0.37).
An on treatment analysis by exclusion of the 97 (23.3%) patients in the EVT arm who did not receive any EVT did not significantly change the results. General anesthesia was still associated with reduction in good outcomes (RR 0.53, p=0.0003) and an increase of inhospital mortality (RR 2.95, p<0.0001) but the association with risk of SAH (RR 1.45, p=0.21) or sICH (RR 1.47, p=0.35) was not statistically significant. These findings were maintained after adjustment.
In both the unadjusted and adjusted analyses, there was insufficient evidence to conclude that there was a significant difference in good outcomes between the LA (48%) and routine GA (40.8%) cohorts compared to IV t-PA alone (42.9%). However in-hospital mortality was significantly reduced in the LA cohort compared to IV therapy (adjusted RR 0.56, CI 0.33-0.97; p=0.0002). The medically indicated GA cohort had lower probability of good outcomes compared to IV therapy (adjusted RR 0.57, CI 0.35-0.93, p=0.0154) and significantly increased mortality (adjusted RR 2.0, CI 1.23-3.26, p=0.0002).
Discussion
The use of GA in the EVT arm in the IMS III Trial was associated with worse neurological outcomes and increased mortality. There was a 17% absolute difference in the proportion of patients with good outcomes in favor of LA that remained essentially unchanged when adjusted for NIHSS severity stratum, age, and TOG. In-hospital mortality was approximately 3-fold higher in the GA group. The difference between GA and LA was primarily seen in subjects for whom GA was deemed medically necessary as compared to routine practice, although the trend lines for all outcomes and endpoints favored LA even compared to the routine GA patients. These differences in outcomes are clinically significant and consistent with the findings of recent retrospective series.5, 6, 9 The largest of these was a multicenter retrospective study of 980 IAT patients, 44% of whom were treated under GA.5 In that study GA was associated with increased odds of poor outcome (mRS≥3, OR 2.33, 95%CI 1.63-3.44; p<0.0001) and mortality (OR 1.68, 95%CI 1.23-2.30; p<0.0001).
The exact reasons why GA appears to be associated with worse outcomes is likely multifactorial. One possibility is hemodynamic perturbations, especially hypotension associated with the induction of GA.10 In a retrospective single center study of 96 EVT patients, Davis et al found a negative association between GA use and good outcomes (15% probability of good outcomes versus 60% in the LA patients).11 They also found an association of good outcomes with SBP>140mmHg, the presence of which was negatively correlated with GA use, leading them to postulate that the deleterious effects of GA were due to the changes in SBP. This association is supported by another retrospective study of 216 EVT patients, 60% of whom were treated with GA and the remainder with conscious sedation using dexmedetomidine. This study found greater variations in BP in the GA group and that higher procedural BP was associated with better outcomes.12 In this current study the closest preprocedural BP reading collected, the SBP 40 minutes after the initiation of IV t-PA, was numerically higher in the LA cohort although the difference was not statistically significant. A higher pre-anesthesia BP could be associated with improved outcomes and could explain some of the findings in this study but that is purely conjectural because no other peri-procedural readings were collected.11, 12
The mechanism by which reduction in BP could worsen outcomes is unknown but is likely via a reduction in cerebral blood flow to the ischemic penumbra potentiating the extent of injury.13 Additionally increases in cerebral venous pressure, which have been noted to occur with general anesthesia and endotracheal intubation, could potentiate the effect of lower BP.14 Future trials should study the effect of BP on outcomes and its possible interaction with type of anesthesia.5, 6, 9 There are theoretical concerns of neurotoxicity of certain anesthetic agents but only a prospective study comparing different agents can confirm or disprove this effect.15
A major weakness of the retrospective studies published to date is that the reasons for initiating anesthesia were not known and the association between GA and poor outcomes could have been due to underlying medical comorbidities or stroke severity that necessitated GA. We attempted to control for as many factors as possible but the small number of events limited the number of variables that we could assess. Although the median NIHSS was slightly lower in the LA group, a finding seen in some of the earlier series, the difference in outcomes in favor of LA persisted after adjustment for the dichotomized severity stratum (NIHSS ≤19 versus ≥20).5, 9 Davis et al. also found that GA patients had more severe strokes than LA patients, but in their institution GA was reserved “for patients who cannot cooperate and those with acute critical events, such as airway obstruction”.11 In IMS III we attempted to control for this variable by differentiating the GA cohort into those intubated as part of “routine practice” and those intubated due to a “medical indication”. The worst outcomes were in those who had a “medical indication” This would suggest that sicker patients (i.e. cardiopulmonary failure) do worse, perhaps due to their underlying medical conditions or an interaction with GA. However the differences in medical comorbidities were not significant between the GA and LA cohorts. Additionally the GA patients tended to have higher NIHSS scores, but the differences were unlikely significant enough to account for the major differences in outcomes and a 3 fold higher mortality in the GA cohort compared to LA. A major limitation of this study is the fact that “medically indicated” was broadly defined in the protocol and included patients with cardiopulmonary deterioration, neurological deterioration with concern for the patient's ability to predict the airway and those with inadequate pain control or agitation. In retrospect, separating these indications for intubation may have helped to better clarify which group(s) of patients had worse outcomes related to the underlying medical condition rather than some possible effect from GA. It is known that some centers perform GA as “medically indicated” in patients with aphasia or who are unable to follow commands.11 In IMS III this approach is suggested by the higher proportion of left hemispheric strokes in the GA group as a whole (Table 1). This practice at some centers may explain in part the relatively higher NIHSS in that group since the NIHSS is biased towards higher scores in dominant hemisphere patients.16 The differences could also be accounted for in the lower proportion of patients with angiographic occlusion in the LA group but the on treatment analysis was not different.
In addition to hemodynamic perturbations, GA may affect outcomes by masking neurological deterioration or headache during the EVT procedure, which could lead to an adjustment of the endovascular approach and avoidance of a major complication such as vessel perforation.5, 17, 18 A contrary point of view is that GA may be safer by preventing patient movement which could lead to wire perforation of one of the cerebral vessels. Our theory that there would be a difference in the primary safety measures of sICH and SAH has not been corroborated by the findings. This is in keeping with the findings from other studies that the risk of sICH was the same or lower in the LA group.5, 6, 9, 11 Therefore there is to date no evidence that GA is safer than LA.19-20, 21 Additionally, GA has been associated with significantly higher treatment costs. In a preplanned analysis of costs in the IMS III trial the average cost of EVT with GA was $46,444 compared with a cost of $30,350 for EVT with LA.22
With the rapid rise of retrievable stents as the new standard of interventional care, the applicability of our results to patients treated with such devices is unclear since a minority (N=7) of patients in IMS III was treated with retrievable stents. However a recent retrospective series of patients treated only with Solitaire FR™ (Covidien Inc., Irvine CA) and analyzed for the effect of anesthesia found a significant negative effect of GA compared to LA.23 In that study the odds ratio for good outcome was 1.3 [1.01–1.6], p=0.04 in favor of LA when adjusted for anterior circulation strokes and electively intubated patients only. The OR for mortality with GA was 3.3 (1.6–7.1, p=0.001). This study was a follow-up to the earlier study examining the effect of GA by Abou-Chebl and colleagues and both datasets were garnered from essentially the same centers and operators with the major difference being the use of retrievable stents in the latter study. This suggests that the effect of GA may be independent of the type of devices used.23 Further substantiating this assumption is that an analysis of the recent MR CLEAN trial, which was comparable to IMS III but included use of retrievable stents in 97% of endovascular patients, also showed that there was an association with better outcomes (mRS≤2) in patients treated with LA (OR 2.79 (1.70 - 4.59).24(Berkhemer OA, Impact of General Anaesthesia On Treatment Effect in the MR CLEAN Trial, Oral Presentation, ISC, Feb 13, 2015, Nashville, TN) Importantly, the three recent trials of endovascular thrombectomy where GA was rarely used (9% in ESCAPE) provide empirical evidence that the procedure can be safely performed under LA a large majority of the time.25
Surprisingly GA was not associated with significantly longer treatment times.26 One possible explanation is that when all aspects of EVT preparation are relatively slow, the impact of GA is not perceptible. As these processes become more efficient the time needed for induction of GA may become increasingly important. However we did not adjust for case complexity, time of day, etc., factors which could prolong the measured time to groin puncture. These data suggest that in IMS III the observed negative association with GA was unlikely to have been purely due to a delay in treatment.
A final important finding of this study is that compared to IV thrombolysis only patients, those receiving EVT without GA had lower in-hospital mortality. Also the medically indicated GA cohort, although small, had significantly worse outcomes and higher mortality compared to the IV thrombolysis patients. In combination with other subgroup analyses such as the cohort of patients who had computerized tomographic angiography proven occlusion, these findings may help in the design and patient selection for future trials of EVT: e.g. future trials may exclude patients likely to need GA for EVT, increasing their power to detect a benefit.
This study has several limitations. Firstly, not all data were available in all patients. Secondly, although there was a distinction in reasons for intubation, the definition of “medically indicated” was not pre-specified and could have been open to interpretation as discussed above. Furthermore we did not collect information on the exact timing of the intubation for GA. If a substantial number of patients were intubated post-operatively for a complication within 7 hours of stroke onset the results would have been heavily biased against the use of GA. Although anecdotal and post-hoc, discussions with the investigators at the highest enrolling centers suggest that the overwhelming majority of patients were intubated pre-procedure. The study may have been underpowered to detect a statistically significant difference between routine GA and LA. Finally BP data during the induction of anesthesia and the EVT procedure were not collected.
In conclusion, in IMS III, GA use in the EVT arm was associated with worse neurological outcomes and increased mortality. The worst outcomes were in the patients with medically indicated GA, although there was a trend for worse outcomes in those patients treated with GA as part of routine care compared to LA. General anesthesia was not safer than LA in terms of hemorrhage risk. Lastly, GA was not associated with a significant delay in treatment. This is the first prospective study to evaluate a technical aspect of EVT and its effect on outcomes and strongly supports the standardization of EVT procedural details in future trials. These data do not address the potential mechanisms of the GA effect but confirm that there is an effect that should be studied in a prospective, randomized trial. Based on these data, the only data from a prospective study, LA is a viable, safe, and cost-effective option for periprocedural patient management in patients without a clear medical indication for intubation.
Supplementary Material
Acknowledgments
We would like to thank the IMS III enrolling centers and investigators (see Data Supplement for a listing)
Funding: National Institute of Neurological Disorders and Stroke
Footnotes
Anesthesia and IV+IA therapy outcomes in IMS III.
Clinical Trial Registration: http://clinicaltrials.gov. Unique Identifier: NCT00359424
Disclosures: Abou-Chebl: None
Yeatts: Consultant/Advisory Board: Genentech
Yan: None.
Cockroft: Other: Covidien, Actuated Medical.
Goyal: Consultant/Advisory Board: Covidien.
Jovin: Grant, non-financial, and other support: Fundació Ictus Malaltia Vascular; Non-financial support: Covidien/Medtronic; Personal fees: Silk Road Medical and Air Liquide; Non-financial support: Covidien/Medtronic and Stryker Neurovascular outside the submitted work
Khatri: Research Support: Genentech, Penumbra; DSMB Member: Biogen
Meyers: None.
Spilker: None
Sugg: Speakers’ Bureau: Genentech.
Wartenberg: Speakers’ Bureau: Bard Medical.
Tomsick: Research Grant: Covidien.
Broderick: Research Grant: Genentech; Research Support: Concentric, Cordis, Ekos;
Travel to Australian stroke conference: Boerhinger Ingelheim
Hill: Research Grant: Covidien; Consultant/Advisory Board: Merck
References
- 1.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-pa versus t-pa alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams HP, Jr., del ZG, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the american heart association/american stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: The american academy of neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Chebl A, Lin R, Hussain MS, Jovin TG, Levy EI, Liebeskind DS, et al. Conscious sedation versus general anesthesia during endovascular therapy for acute anterior circulation stroke: Preliminary results from a retrospective, multicenter study. Stroke. 2010;41:1175–1179. doi: 10.1161/STROKEAHA.109.574129. [DOI] [PubMed] [Google Scholar]
- 6.Nichols C, Carrozzella J, Yeatts S, Tomsick T, Broderick J, Khatri P. Is periprocedural sedation during acute stroke therapy associated with poorer functional outcomes? J Neurointerv Surg. 2010;2:67–70. doi: 10.1136/jnis.2009.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khatri P, Hill MD, Palesch YY, Spilker J, Jauch EC, Carrozzella JA, et al. Methodology of the interventional management of stroke iii trial. Int J Stroke. 2008;3:130–137. doi: 10.1111/j.1747-4949.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higashida R, Furlan A, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol. 2003;14:S493–S494. doi: 10.1097/01.rvi.0000084592.53089.4e. [DOI] [PubMed] [Google Scholar]
- 9.Jumaa MA, Zhang F, Ruiz-Ares G, Gelzinis T, Malik AM, Aleu A, et al. Comparison of safety and clinical and radiographic outcomes in endovascular acute stroke therapy for proximal middle cerebral artery occlusion with intubation and general anesthesia versus the nonintubated state. Stroke. 2010;41:1180–1184. doi: 10.1161/STROKEAHA.109.574194. [DOI] [PubMed] [Google Scholar]
- 10.Leonardi-Bee J, Bath PMW, Phillips SJ, Sandercock PAG, Group ISTC Blood pressure and clinical outcomes in the international stroke trial. Stroke. 2002;33:1315–1320. doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 11.Davis MJ, Menon BK, Baghirzada LB, Campos-Herrera CR, Goyal M, Hill MD, et al. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology. 2012;116:396–405. doi: 10.1097/ALN.0b013e318242a5d2. [DOI] [PubMed] [Google Scholar]
- 12.Whalin MK, Lopian S, Wyatt K, Sun CH, Nogueira RG, Glenn BA, et al. Dexmedetomidine: A safe alternative to general anesthesia for endovascular stroke treatment. J Neurointerv Surg. 2014;6:270–275. doi: 10.1136/neurintsurg-2013-010773. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Effect of blood pressure and diabetes on stroke in progression. Lancet. 1994;344:156–159. doi: 10.1016/s0140-6736(94)92757-x. [DOI] [PubMed] [Google Scholar]
- 14.Artru AA, Powers K, Doepfner P. Csf, sagittal sinus, and jugular venous pressures during desflurane or isoflurane anesthesia in dogs. J of Neurosurgical Anesthesiology. 1994;6:239–248. doi: 10.1097/00008506-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Messick JM, Jr., Newberg LA, Nugent M, Faust RJ. Principles of neuroanesthesia for the nonneurosurgical patient with cns pathophysiology. Anesth Analg. 1985;64:143–174. [PubMed] [Google Scholar]
- 16.Yoo AJ, Romero J, Hakimelahi R, Nogueira RG, Rabinov JD, Pryor JC, et al. Predictors of functional outcome vary by the hemisphere of involvement in major ischemic stroke treated with intra-arterial therapy: A retrospective cohort study. BMC Neurol. 2010;10:25. doi: 10.1186/1471-2377-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molina CA, Selim MH. General or local anesthesia during endovascular procedures: Sailing quiet in the darkness or fast under a daylight storm. Stroke. 2010;41:2720–2721. doi: 10.1161/STROKEAHA.110.595447. [DOI] [PubMed] [Google Scholar]
- 18.Abou-Chebl A, Krieger DW, Bajzer CT, Yadav JS. Intracranial angioplasty and stenting in the awake patient. J Neuroimaging. 2006;16:216–223. doi: 10.1111/j.1552-6569.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 19.Shi ZS, Liebeskind DS, Loh Y, Saver JL, Starkman S, Vespa PM, et al. Predictors of subarachnoid hemorrhage in acute ischemic stroke with endovascular therapy. Stroke. 2010;41:2775–2781. doi: 10.1161/STROKEAHA.110.587063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kase CS, Furlan AJ, Wechsler LR, Higashida RT, Rowley HA, Hart RG, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: The proact ii trial. Neurology. 2001;57:1603–1610. doi: 10.1212/wnl.57.9.1603. [DOI] [PubMed] [Google Scholar]
- 21.Vora NA, Gupta R, Thomas AJ, Horowitz MB, Tayal AH, Hammer MD, et al. Factors predicting hemorrhagic complications after multimodal reperfusion therapy for acute ischemic stroke. AJNR. 2007;28:1391–1394. doi: 10.3174/ajnr.A0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson KN, Simpson AN, Mauldin PD, Hill MD, Yeatts SD, Spilker JA, et al. Drivers of costs associated with reperfusion therapy in acute stroke: The interventional management of stroke iii trial. Stroke. 2014;45:1791–1798. doi: 10.1161/STROKEAHA.113.003874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abou-Chebl A, Zaidat OO, Castonguay AC, Gupta R, Sun CH, Martin CO, et al. North american solitaire stent-retriever acute stroke registry: Choice of anesthesia and outcomes. Stroke. 2014;45:1396–1401. doi: 10.1161/STROKEAHA.113.003698. [DOI] [PubMed] [Google Scholar]
- 24.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 25.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann O, Hug A, Bosel J, Petersen JJ, Hartmann M, Rohde S, et al. Fast-track intubation for accelerated interventional stroke treatment. Neurocrit Care. 2012;17:354–360. doi: 10.1007/s12028-012-9671-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.