Abstract
Objectives
Amyloid deposits are prevalent in osteoarthritis (OA)-affected joints. This study defined the dominant precursor and determined if the deposits affect chondrocyte functions.
Methods
Amyloid deposition in normal and OA human knee cartilage was determined by Congo red staining. Transthyretin (TTR) in cartilage and synovial fluid was analyzed by immunohistochemistry and western blotting. The effects of recombinant amyloidogenic and non-amyloidogenic TTR variants were tested in human chondrocyte cultures.
Results
Normal cartilage from young donors did not contain detectable amyloid deposits but 58% (7/12) of aged normal cartilage and 100% (12/12) of OA cartilage samples showed Congo red staining with green birefringence under polarized light. TTR, located predominantly at the cartilage surfaces, was detected in all OA and a majority of aged, but not young normal cartilage. Chondrocytes and synoviocytes did not contain significant amounts of TTR mRNA. Synovial fluid TTR levels were similar in normal and OA knees. In cultured chondrocytes, only an amyloidogenic TTR variant induced cell death, the expression of proinflammatory cytokines, and extracellular matrix degrading enzymes. The effects of amyloidogenic TTR on gene expression were mediated by in part by Toll-like receptor-4, Receptor for advanced glycation endproducts and p38 MAP kinase. TTR-induced cytotoxicity was inhibited by resveratrol, a plant polyphenol that stabilizes the native tetrameric structure of TTR.
Conclusions
The findings are the first to suggest that TTR amyloid deposition contributes to cell and extracellular matrix damage in articular cartilage in human OA and that therapies designed to reduce TTR amyloid formation might be useful.
Keywords: cartilage, chondrocyte, cytokine
INTRODUCTION
Aging is a major risk factor for the development of many diseases including osteoarthritis (OA) (1) and various forms of amyloidosis (2). In OA, the disease process affects all joint tissues (3) but aging-related changes in articular cartilage appear to occur early, and trigger the tissue remodeling process that is manifested as OA (4). In the amyloidoses, the generation of misfolded proteins or peptides from their soluble precursors, their aggregation and ultimate tissue deposition cause abnormal gene expression, cell death, and organ dysfunction (5). Amyloid arthropathy has been reported in the long-term dialysis-related amyloidosis (6) and associated with multiple myeloma (7).
Approximately 30 human proteins that can form amyloid in vivo have been identified (2). Amyloid deposits bind the dye Congo red resulting in green birefringence under polarized light, and fluoresce with Thioflavin-T and S dyes (8). The presence of Congo red positive deposits in OA-affected articular cartilage is common even in the absence of generalized systemic amyloidosis (9–13).
Transthyretin (TTR) is one of a small number of human amyloid precursors found in OA cartilage (14) and OA synovial fluid (15), the others being immunoglobulin light chains, Apolipoprotein A-I and Apolipoprotein A-II. TTR containing amyloid has also been found in a small number of RA joints (14). TTR is a homotetrameric protein synthesized mainly in the liver and in the choroid plexus of the brain, and it circulates in plasma and cerebrospinal fluid. Wild-type TTR is associated with the syndrome known as senile systemic amyloidosis (SSA), an age-related disease characterized by cardiac TTR deposition (16, 17). There are more than 111 amyloidogenic point mutations in the TTR gene that are the cause of familial amyloid cardiomyopathy (FAC), characterized by primarily cardiac TTR deposition, and familial amyloidotic polyneuropathy (FAP), characterized by TTR deposition in peripheral nerve and heart (18). The variants Val122Ile (V122I) and Val30Met (V30M) are the most common TTR mutations related to FAC and FAP, respectively (19). TTR amyloidogenesis requires the rate-limiting dissociation of the native tetramer into its corresponding monomers. The monomers misfold and initiate the aggregation and amyloidosis cascade in a downhill polymerization process (20).
The prevalence and types of TTR amyloid in aging and OA-affected cartilage have not been established and there is no published information on the source and effects of TTR on cartilage tissue and cells.
MATERIALS AND METHODS
Tissue and cell isolation from human knee joints
Human knee joints from individuals ages 16–94 were obtained at autopsy under approval by the Scripps Human Subjects Committee. Young normal knee joints were harvested at autopsy from 12 donors (age 16 to 48 years, mean ± SD = 31.0 ± 9.1, OA grade 1). Aged normal knee joints were also obtained from 12 donors having no history of joint diseases or overt OA as determined on macroscopic assessment of all joint cartilage surfaces (age 68 to 94, mean ± SD = 75.9 ± 6.3, OA grade 1–2). OA joints were harvested from 12 donors (age from 49 to 90, mean ± SD = 75.6 ± 13.1, OA grade 3–4). Gross morphological grading was performed as described (21). Osteochondral slabs were collected from the weight-bearing area of the femoral condyles for histological analysis.
The remaining cartilage tissue was resected from the subchondral bone for cell and protein isolation (21).
Synovial fluid was collected from each knee through a superolateral approach. The synovial fluid was centrifuged at 14,000×g for 20 minutes, and the supernate was treated with a final concentration of 0.2 mg/mL of hyaluronidase (Sigma) at 37°C for 1 h. Bicinchoninic acid (BCA) protein assay (Pierce) was conducted on protein lysates of cartilage and synovial fluid and samples were diluted with PBS to obtain uniform protein concentrations.
Congo red staining and immunohistochemistry
Each femoral osteochondral slab was fixed in 10% zinc-buffered formalin for 2 days, decalcified in a formic acid decalcifier ‘To Be Decalcified’ (TBD, Thermo Scientific) for 7 days, followed by paraffin embedding. Serial sections (9 µm each) were cut and stained with Congo red (Amyloid Special Stain Kit, Leica). The sections were examined using polarizing light microscopy that reveals apple green birefringence of amyloid (8).
For immunohistochemistry staining of human TTR, sections (4 µm each) were blocked with 10% goat serum for 30 minutes at room temperature. Anti-human TTR (1:400 dilution) from Dako (A0002) was applied with 0.1% Tween 20 and incubated overnight at 4°C. After washing with PBS, the sections were incubated with biotinylated goat anti-rabbit secondary antibody for 30 minutes at room temperature, and then incubated using the Vectastain ABC-AP kit (Vector Laboratories) for 30 minutes. Slides were washed, and sections were incubated with 3,3-diaminobenzidine tetrahydrochloride (DAB) substrate for 5–10 minutes. Specificity controls were obtained by replacing the primary antibody with nonimmune rabbit IgG (1 µg/mL). Staining was absent under these conditions.
Quantitative Western blotting
Western blotting was performed using the LiCor system as described (21) using primary antibodies for human TTR (Dako), phospho-p38, phospho-ERK, phospho-JNK, phospho-c-Jun, and GAPDH (Cell Signaling). Protein of interest integrated intensity values were normalized to those of GAPDH.
Preparation of recombinant TTRs and endotoxin removal
Two recombinant forms of TTR, T119M (non-amyloidogenic) and V122I (amyloidogenic), were prepared and purified as described earlier (22). Endotoxin was removed with Detoxi-Gel Endotoxin Removing Columns (Pierce). LAL Chromogenic Endotoxin Quantitation Kit (Pierce) revealed an endotoxin level in 4 µM TTR preparations below to 0.001 ng/mL. In addition, recombinant TTRs were treated with Polymyxin B (10 µg/mL) for further neutralization of endotoxin, and we confirmed that Polymyxin B completely inhibited the effects of LPS (0.5 ng/mL) on the inflammatory gene expression, but did not inhibit any effect of TTRs.
Chondrocyte isolation and culture
Chondrocytes were isolated and cultured as described previously (23). First passage cells were used in the experiments and cultured (1.0×105 cells/well in 24 well plates) in the presence of 1% calf serum and stimulated with TTR in concentrations ranging from 0.2 to 4 µM. These doses were chosen based on the 3.4–5 µM plasma concentrations of TTR (24).
MAP kinase inhibitors (EMD-Millipore) were used at the following concentrations: 10 µM of SB203580, and 20 µM of PD98059 following pretreatment with these inhibitors for 1 h. Blocking antibodies RAGE (Abcam) was used at a concentration of 10 µg/mL and the TLR inhibitor CLI95 (InvivoGen) was used at a concentration of 1 µg/mL.
TTR cytotoxicity assessment
Resazurin assay is used to measure metabolic activity and cell viability (25). Human chondrocytes were cultured in the presence of recombinant human TTR variants (0.2 to 4 µM) for 24 hours, and resazurin assay was performed to assess cell viability. In this assay, the substrate resazurin is reduced to the soluble and highly fluorescent resorufin formazan by metabolically active cells (26).
Human chondrocytes were treated with recombinant TTRs. After 24 h incubation, 10 µL of resazurin solution (500 µM in PBS) was added, and the cells were incubated for 2.5 h at 37°C. The fluorescence resulting from the reduction of resazurin to resorufin was measured using a multiwall spectrofluorimeter with excitation and emission wavelengths of 530 and 590 nm, respectively (Tecan Safire2, Austria).
Cell death detection
Human chondrocytes were seeded in 96-well plates at a density of 3.0 ×103 cells/well and treated with recombinant TTRs at final concentrations of 4 µM in Opti-MEM with 1% CS. After 72 h incubation, cytoplasmic histone-associated DNA fragments were measured using Cell Death Detection ELISAPLUS Kit (Roche). Cell metabolic activity and apoptotic cell death were expressed as the ratio of the value of TTR-treated cells with respect to the cells treated with vehicle only.
Quantitative real-time PCR
Total RNA was reversed transcribed to complementary DNA using TaqMan® Reverse Transcription Reagents (Applied Biosystems). Quantitative Real-time PCR was carried out using LightCycler 480 Probes Master (Roche). Predesigned primers for human TTR, IL6, iNOS, ADAMTS4, MMP13, and GAPDH were obtained from Applied Biosystems. Results were obtained using LightCycler® 480 Instrument II (Roche).
Statistical analysis
Statistically significant differences in histological analysis were determined using Fisher's exact test. A repeated-measures ANOVA was used for statistical analysis in other experiments. When a significant difference was observed, we used the Bonferroni test to determine significant differences compared to the control condition. The results are reported as mean ± S.D. P values less than 0.05 were considered significant.
RESULTS
Amyloid and TTR deposition in human articular cartilage
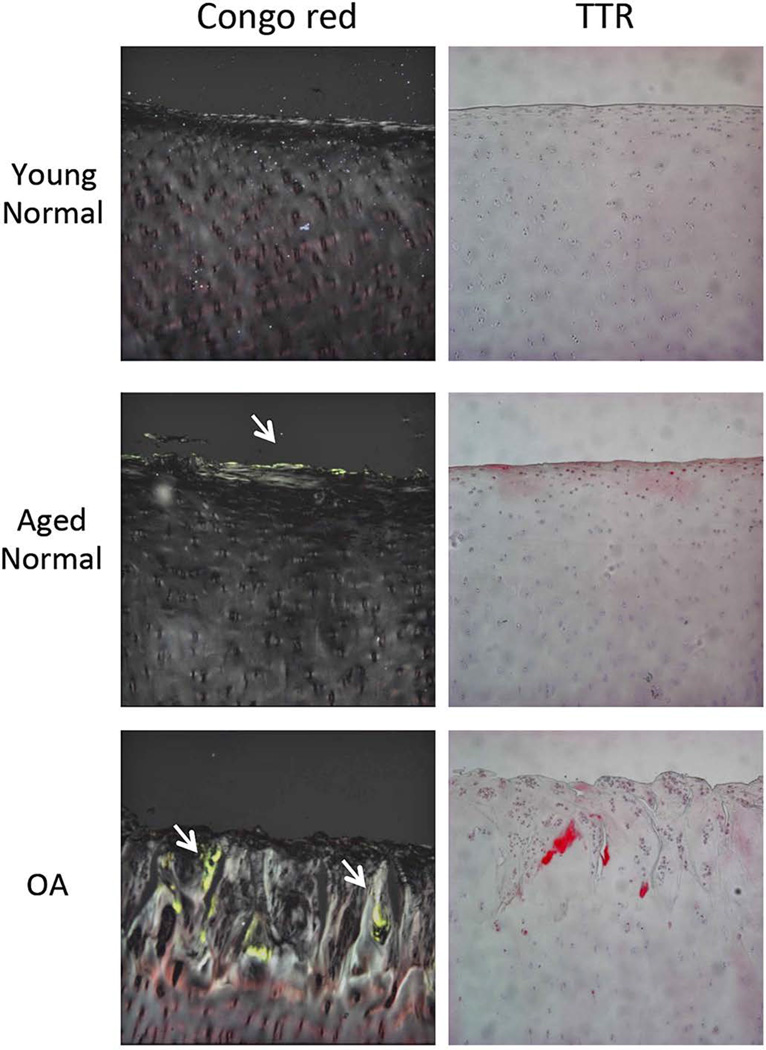
We compared amyloid and TTR deposition between young normal, aged normal and OA human knee cartilage. The results showed that there were significant differences in amyloid deposition as detected by Congo red staining among the 3 groups (Table 1). There was no amyloid deposition in young normal cartilage. In contrast, 58% (7/12) of aged normal cartilage and 100% (12/12) of OA cartilage samples had Congo red staining with green birefringence under polarized light. Similarly, aged normal and OA cartilage showed significantly higher rates of TTR deposition compared to young normal cartilage (Table 1), whereas there was no difference between aged normal and OA cartilage. Amyloid and TTR deposition in aged normal cartilage was observed only in the superficial zone (Figure 1). OA cartilage showed larger areas stained with Congo red and TTR antibody compared to aged normal cartilage, predominantly in areas with fibrillation.
TABLE 1.
Amyloid and TTR deposition in human young normal, aged normal and OA cartilage.
| Group | Age | Sex | OA grade | Congo red | TTR |
|---|---|---|---|---|---|
| Young Normal | 16 | M | 1 | − | − |
| 22 | F | 1 | − | − | |
| 23 | F | 1 | − | − | |
| 24 | M | 1 | − | − | |
| 24 | M | 1 | − | + | |
| 29 | M | 1 | − | − | |
| 33 | M | 1 | − | − | |
| 36 | M | 1 | − | − | |
| 36 | F | 1 | − | − | |
| 40 | M | 1 | − | − | |
| 41 | M | 1 | − | − | |
| 48 | F | 1 | − | − | |
| Aged Normal | 68 | F | 1 | − | + |
| 70 | M | 2 | − | + | |
| 70 | F | 1 | − | − | |
| 74 | M | 1 | − | + | |
| 74 | F | 2 | + | + | |
| 75 | F | 1 | + | − | |
| 76 | M | 1 | + | + | |
| 76 | F | 1 | − | + | |
| 77 | M | 1 | + | + | |
| 78 | F | 2 | + | + | |
| 79 | F | 1 | + | + | |
| 94 | F | 1 | + | + | |
| Osteoarthritis | 49 | F | 3 | + | + |
| 59 | F | 4 | + | + | |
| 62 | F | 3 | + | + | |
| 64 | M | 3 | + | + | |
| 77 | F | 3 | + | + | |
| 80 | M | 3 | + | + | |
| 82 | M | 4 | + | + | |
| 84 | M | 3 | + | + | |
| 84 | F | 4 | + | + | |
| 88 | F | 3 | + | + | |
| 89 | F | 4 | + | + | |
| 90 | F | 4 | + | + | |
Cartilage sections from 12 young normal, 12 aged normal, and 12 OA donors were stained with Congo red and antibodies to human TTR.
The presence of staining is marked as (+).
Figure 1. Amyloid and TTR deposition in human young normal, aged normal and OA cartilage.
Representative images of Congo red staining and Immunohistochemistry for human TTR in young normal, aged normal, and OA cartilage. Arrows indicate green staining of amyloid under polarized light. Images are 10× magnification.
TTR in human cartilage and synovial fluid
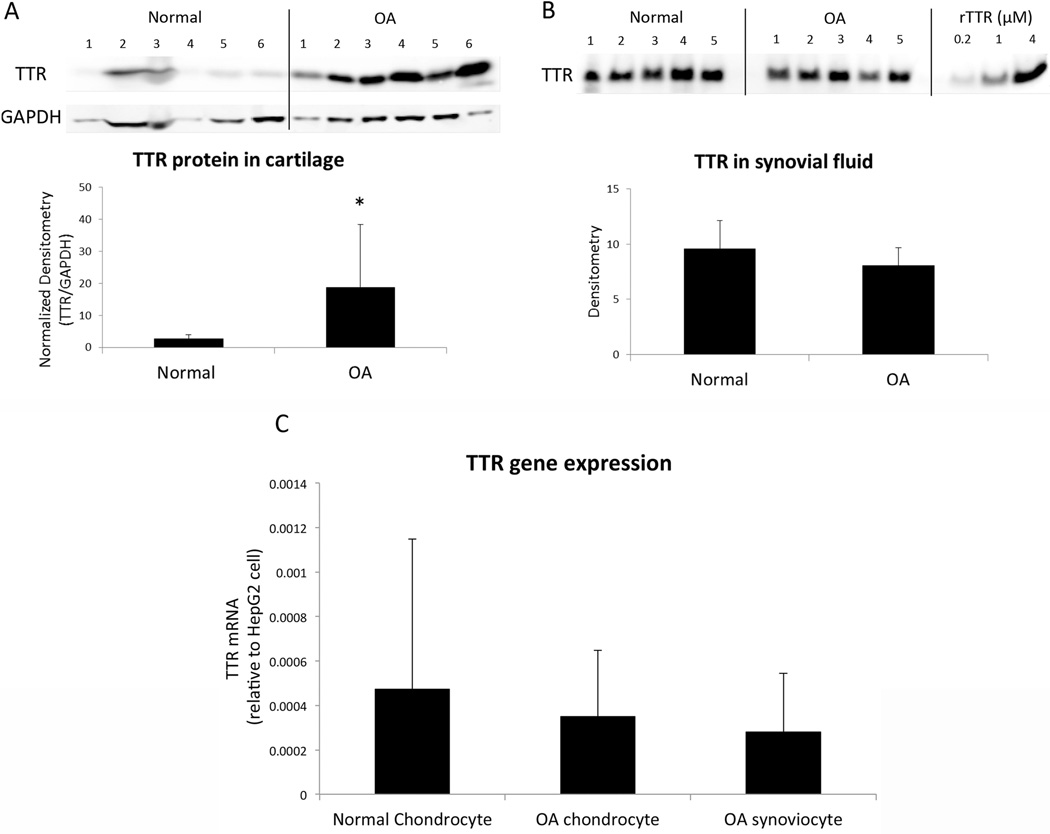
Protein extracts from cartilage and synovial fluid were analyzed using western blotting to compare the levels of TTR between normal and OA joints. TTR protein content in OA cartilage was significantly higher compared to normal tissue (Figure 2A). There was no difference in TTR protein levels in synovial fluid between normal and OA donors (Figure 2B). TTR gene expression in chondrocytes and synoviocytes was very low compared to HepG2 cells derived from the liver, which is the major site of TTR synthesis in humans (27, 28) (Figure 2C). There was no significant difference in TTR mRNA levels between normal and OA chondrocytes.
Figure 2. Comparison of TTR in human cartilage and synovial fluid between young normal and OA donors.
(A) Protein extracts from articular cartilage were normalized by protein content and analyzed by western blotting using antibodies to human TTR. Image and graph show the result of TTR quantification in 6 young normal and 6 OA cartilage samples. * P < 0.05 versus young normal (B) Synovial fluid samples at the same total protein concentration were analyzed by western blotting using antibodies to human TTR. Image and graph show the result of TTR quantification in 5 young normal and 5 OA donors. (C) Human TTR gene expression in chondrocytes from 8 young normal and 8 OA donors and synoviocytes from 2 OA donors was analyzed by real-time PCR. Graph shows chondrocyte TTR gene expression levels relative to TTR gene expression in the hepatoma cell line HepG2.
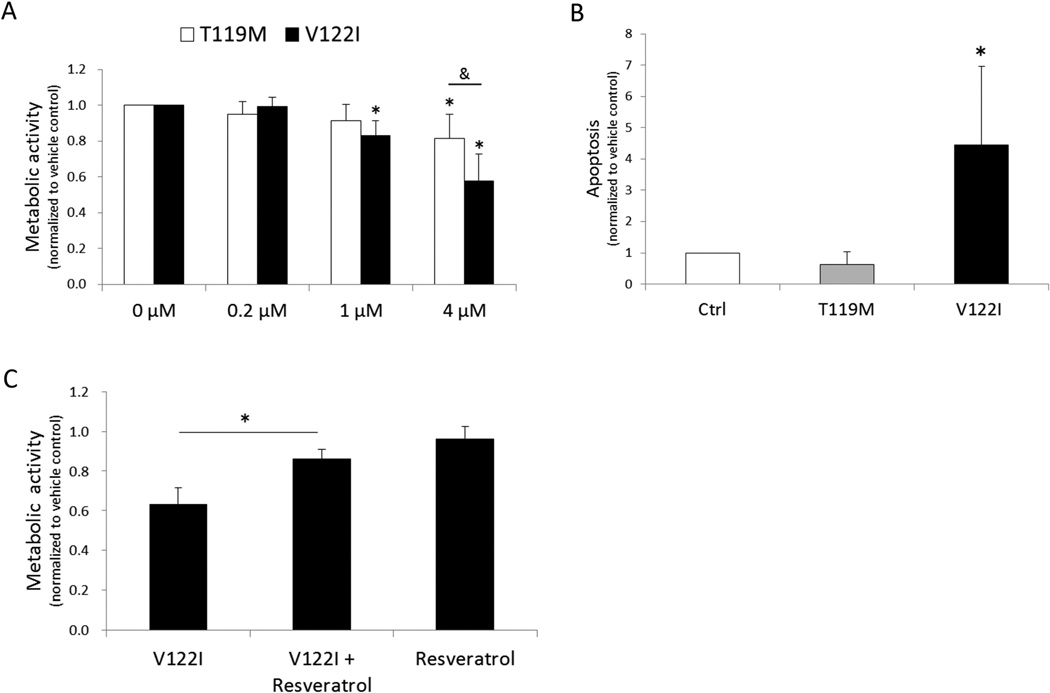
Amyloidogenic TTR reduces cell metabolic activity and induces apoptosis in human chondrocytes
Human chondrocytes were cultured in the presence of recombinant human TTR variants (0.2 to 4 µM) for 24 hours after which the resazurin assay was performed to assess metabolic activity. We used two different forms of recombinant TTR; V122I (amyloidogenic) and T119M (non-amyloidogenic). V122I TTR significantly reduced metabolic activity in a dose dependent manner (Figure 3A). This effect of V122I TTR incubation for 24 h was reversible upon removal of TTR and culture in fresh medium without TTR (data not shown). Culture of chondrocytes with the amyloidogenic TTR variant V122I TTR for 72 h significantly increased apoptotic cell death, while T119M TTR did not (Figure 3B). V122I TTR-induced cytotoxicity was inhibited by co-incubating the protein with resveratrol, a small molecule known to stabilize the native tetrameric structure of TTR and prevent its aggregation and amyloid fibril formation (22) (Figure 3C).
Figure 3. Effects of recombinant TTR variants on cell viability in human chondrocytes.
(A) Chondrocytes were treated with 3 different concentrations of recombinant V122I or T119M TTR (0.2, 1, and 4 µM) for 24 h and cell metabolic activity was assessed by resazurin assay. Graph shows the results from 6 independent experiments using cells from of a total of 6 different normal donors. Values are the mean and SD as the ratio of TTR treated cells to control cells not treated with TTR. *P < 0.05 versus vehicle control. & = P < 0.05 between T119M and V122I. (B) Graph shows the results of a total of 4 donors for apoptosis detection in human chondrocytes treated with 4 µM of TTRs for 72 h. Values are the mean and SD as the ratio of TTR-treated cells to control cells not treated with TTR. * = P < 0.05 versus vehicle control. (C) Chondrocytes were treated with V122I TTR (4 µM) with or without resveratrol for 24 h and cell metabolic activity was assessed by Resazurin assay. Graph shows the results of a total of 5 donors. Values are the mean and SD as the ratio of TTR treated cells to vehicle control cells.
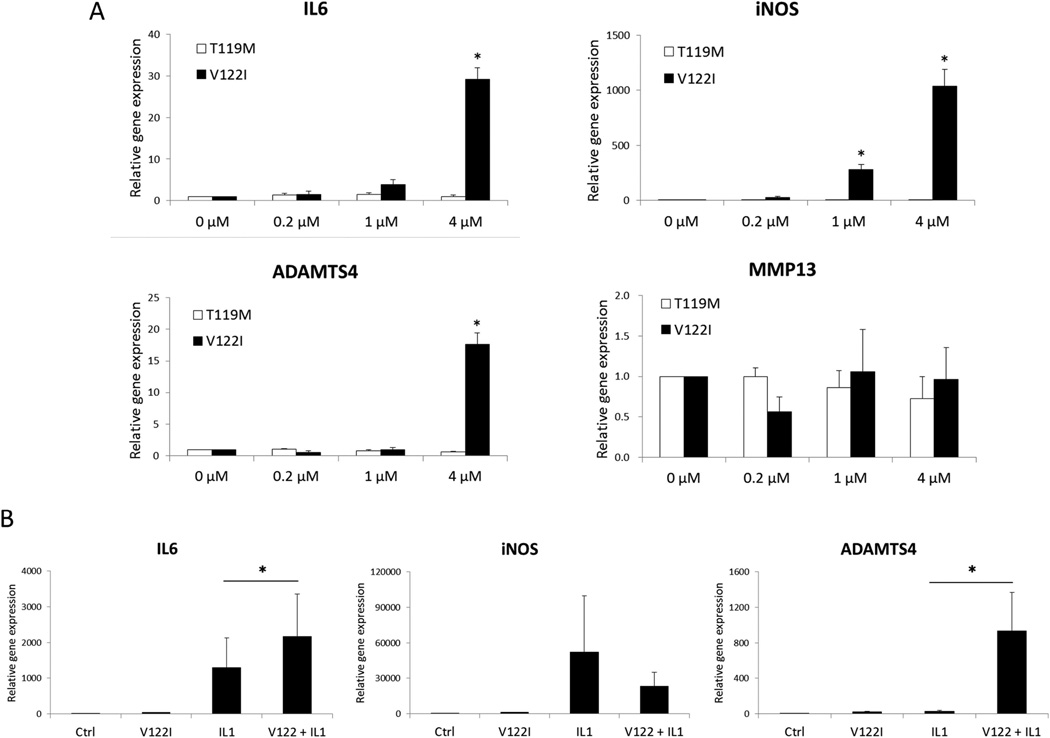
Amyloidogenic TTR induces inflammatory gene expression in human chondrocytes
To determine whether TTRs stimulate the production of mediators of inflammation and cartilage degradation, we treated chondrocytes with the recombinant TTR variants and analyzed changes in gene expression using quantitative PCR. Expression of IL6, iNOS and ADAMTS4, but not that of MMP13, were significantly induced by V122I TTR (Figure 4A). The non-amyloidogenic TTR variant T119M did not induce significant changes in any of the genes analyzed. The IL-1β-mediated increase in IL6 and ADAMTS4 expression were significantly augmented in the presence of the amyloidogenic V122I TTR variant (Figure 4B).
Figure 4. Effects of TTRs on gene expression in human chondrocytes.
(A) Chondrocytes were treated with 3 different concentrations of TTRs (0.2, 1, and 4 µM) for 24 h and gene expression was assessed by real-time PCR. Graph shows the results of a total of 3 donors. * = P < 0.05 versus vehicle control. *P < 0.05 versus vehicle control. (B) Chondrocytes treated with 4 µM of V122I were simultaneously stimulated with IL-1β (0.05 ng/mL) for 24 h. Graph shows the results of a total of 3 donors. Values are the mean and SD. *P < 0.05 in V122I + IL-1β versus IL-1β alone.
TLR4, RAGE and p38 MAPK are involved in TTR stimulation of gene expression
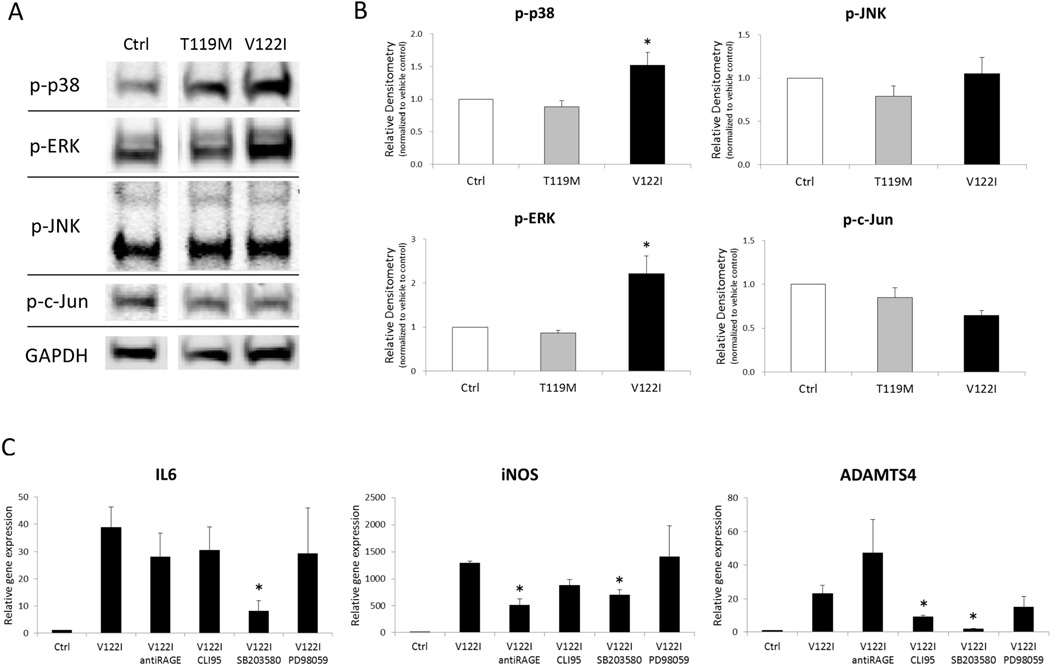
To examine signaling mechanisms involved in amyloidogenic TTR induced gene expression, we measured changes in mitogen-activated protein kinase (MAPK) signaling and tested the effect of inhibitors of MAPK, Toll-like receptor 4 (TLR4) and Receptor for Advanced Glycation End-products (RAGE). The data show that treatment of cells with the amyloidogenic V122I TTR resulted in significant increases in phosphorylation of p38 and ERK, but not JNK and c-Jun (Figure 5A and B). V122I TTR-induced gene expression of IL6, iNOS and ADAMTS4 were significantly inhibited by p38 MAPK inhibitor (SB203580) (Figure 5C). RAGE blocking antibody significantly inhibited iNOS gene expression induced by V122I TTR. On the other hand, the TLR4 inhibitor CLI-95 reduced ADAMTS4 gene expression stimulated by V122I TTR.
Figure 5. Kinases and receptors mediating TTR effects on human chondrocytes.
(A) Representative images of western blotting for phosphorylated MAPK in chondrocytes treated with TTRs. (B) Graph shows the quantification of phosphorylated MAPK in 3 human chondrocyte preparations treated with 4 µM of TTRs for 24 h. Values are the mean and SD as the ratio of TTRs treated cells to vehicle-only treated cells. * = P < 0.05 versus Control. (C) Chondrocytes co-treated with 4 µM of V122I and antibody to RAGE, CLI95, SB203580 and PD98059 for 24 h. Graph shows the changes in gene expression of 3 donors. Values are the mean and SD. *P < 0.05 versus V122I TTR.
DISCUSSION
All human advanced OA cartilage samples were positive for Congo red staining and for TTR. We also analyzed cartilage from a set of elderly donors that did not have a diagnosis of OA or macroscopic evidence of OA-like cartilage damage. Approximately 60% of these samples were positive for Congo red and about 90% were positive for TTR. This finding suggests the possibility that in a fashion similar to that seen in mice transgenic for wild type-human TTR (29), small TTR aggregates deposit before they evolve into fibrillary amyloid structures that become Congo red positive. Other studies have suggested that the prevalence of amyloid deposits in the synovium of OA-affected joints is lower than in our series of cartilage samples (21, 30–32). In a study of patients with carpal tunnel syndrome (CTS), 34% were positive for amyloid and 100% were positive for TTR (33). CTS is a common clinical accompaniment of SSA (34, 35). Our data are consistent with these studies in other tissues, showing a higher percentage of samples that are positive for TTR than for Congo red. It also appears that the prevalence of amyloid and TTR deposition in cartilage is among the highest, and even higher than in CTS where the deposits have been implicated in pathogenesis. Based on this highly prevalent age-dependent TTR deposition, articular cartilage should be included in the tissues that are affected in the SSA syndrome.
The TTR deposits in aged cartilage were exclusively located at the articular cartilage surface or superficial zone (SZ). The earliest aging and OA-related changes in articular cartilage occur at the SZ and include substantial loss of proteoglycans and enzymatic cleavage of type II collagen (36). As the SZ is critical to cartilage and joint homeostasis, TTR deposits could play an important role in initiating the cascade of events that ultimately manifests as OA.
TTR aggregate formation by both, wild type and mutant TTR requires dissociation of the native tetramer into its constituent monomers. A conformational change within the monomer (misfolding) enables the formation of soluble aggregates, which become insoluble as they grow (protofilaments) (37, 38). The insoluble aggregates eventually become amyloid fibrils by the lateral assembly of four protofilaments. Other forms of TTR aggregates can be amorphous and do not bind Congo red. Formation of TTR aggregates can be facilitated by age-related oxidation (39) and nitric oxide-induced modification of TTR (40). Chondrocytes in aged and OA-affected cartilage produce increased levels of oxidants (4). TTR deposition is also accelerated by the presence of certain sulfated glycosaminoglycans (41, 42). The relative levels of glycosaminoglycans, for example the ratio of keratin sulfate to chondroitin sulfate and the sulfation patterns of heparan sulfate, are altered in OA cartilage (43, 44). In addition, age-related oxidation of long-lived cartilage extracellular matrix proteins, such as type II collagen (45) which has a half-life of 117 years may also facilitate TTR aggregate formation (46). Thus, the aged and OA cartilage extracellular matrix is characterized by several modifications that can promote TTR deposition.
Interestingly, an N-terminal truncated form of TTR was detected in sera from OA patients and was among a small number of serum biomarkers that distinguished OA progressors from non-progressors (47). This raises the possibility that proteolytic enzymes that are overproduced in OA and aged joints may facilitate TTR aggregation through this mechanism.
We investigated potential cellular sources of the TTR deposits in aged and OA cartilage. Very low levels of TTR protein were detectable in normal articular cartilage and in cultured chondrocytes and there were no differences in the abundance of TTR mRNA in normal and OA cartilage. These observations suggest that the TTR in aged and OA cartilage originates from extrinsic sources. TTR was detected in synovial fluid but there were no differences in TTR content of normal and OA synovial fluids. In proteomic analyses of human synovial fluid Gobezie et al (48) and Sohn et al (15) identified TTR but there was no apparent difference in levels between OA and normal. The observations that TTR deposits were present in cartilage but chondrocytes do not appear to be a source of TTR, are consistent the deposition of extracellular congophilic material at sites distant from their synthesizing organs that defines the systemic amyloidoses. Collectively, these observations suggest that TTR deposits in cartilage are most likely formed from liver-secreted protein, the major site of TTR synthesis (27, 28), and that it is not differences in the systemic or local TTR levels, but changes in the local tissue environment, that promote TTR aggregation and deposition.
The present study is the first to examine potential functional consequences of TTR deposition in cartilage. Our in vitro model used primary normal human articular chondrocytes that were treated with recombinant amyloidogenic V122I or non-amyloidogenic T119M (49). Recombinant WT TTR was not used because previous studies showed that the purification conditions of WT TTR change its behavior in tissue culture, probably because more than one tetramer conformer can exist in solution (22, 50, 51). Amyloidogenic V122I induced a dose-dependent decrease in cell viability measured by resazurin reduction and caspase activation assays, whereas the naturally occurring stable and non-amyloidogenic T119M TTR variant did not. The type of cell death induced by amyloidogenic TTR was apoptotic and it was attenuated by resveratrol. In our experimental setting, resveratrol was first pre-incubated with V122I TTR to allow it to bind to the protein, before addition of this mixture onto the cells. Thus, the protective effect of resveratrol is most likely related to its ability to stabilize the native tetrameric structure of TTR (22), although it could also be due to its capacity to protect against apoptosis induced by oxidants (52). In chondrocytes, amyloidogenic V122I TTR, but not non-amyloidogenic TTR also led to a dose-dependent increase in the expression of IL6, iNOS and ADAMTS4 mRNAs, while other genes implicated in OA pathogenesis, such as MMP13, were not induced. We also observed a synergistic effect of amyloidogenic V122I TTR with IL-1, a prototypic proinflammatory and catabolic cytokine in cartilage (53). These results demonstrate, for the first time, that amyloidogenic TTR affects chondrocyte survival and gene expression, and may thus contribute to aging-related changes in articular cartilage that provide a major risk factor for the development of OA. Studies of human biopsies of TTR mutant carriers, and in transgenic mice overexpressing human wild type or mutant TTR variants, suggest that cell death and production of inflammatory mediators occurs much earlier than the deposition of amyloid fibrils (54–56).
To further elucidate specificity and mechanisms of TTR effects on chondrocytes, we analyzed cell surface receptors and intracellular signaling pathways. RAGE blocking antibody inhibited the V122I TTR-induced expression of iNOS but not IL6 or ADAMTS4. The TLR4 inhibitor CLI-95 reduced ADAMTS4 expression but neither agent prevented all V122I TTR-induced gene expression changes. RAGE is expressed by chondrocytes and is increased in OA cartilage (57) and mediates the production of metalloproteinases, inflammatory mediators and chondrocyte hypertrophy (58–60). A role for RAGE in mediating the effect of TTR fibrils on neuron-like, Schwann, and endothelial cells has been reported (61, 62). TLR4 is known to be expressed on chondrocytes and can bind several ligands, including advanced glycation end products (63), alarmins (64) and extracellular matrix fragments (65). Our findings that these two receptors may be at least in part mediating the TTR effect on chondrocytes are consistent with their expression and role in signaling in response to other ligands but a role for other receptors can not be excluded. Why the TTR-mediated induction of some of these genes is dependent on one or the other of these receptors requires further analysis. P38 MAP kinase was phosphorylated in response to V122I TTR and a pharmacologic inhibitor of p38 suppressed the V122I TTR induced gene expression, suggesting a prominent role for p38 in mediating the effects of V122I TTR.
Limitations of the present study include that it is correlative in the comparison of the TTR deposition with age and OA status. The in vitro studies are also presenting TTR in a form and context that is not replicating the TTR amyloid fibrils within the cartilage extracellular matrix. Although the in vitro concentrations of TTR used are similar to plasma concentrations, the concentration of TTR aggregates in cartilage tissue is unknown. The present study only tested effects of TTR on chondrocyte function. Other amyloid precursors, such as ApoA, light chains, fibrinogen, gelosin are present in synovial fluid and cartilage (15, 48). It will be important to determine whether these proteins from amyloid and have effects on chondrocytes. In addition to having effects on chondrocyte survival and gene expression, it is also possible that the TTR amyloid deposits in cartilage affect its biomechanical properties. Biomechanical changes in the heart are a mechanisms involved in TTR-amyloid deposition in patients with TTR mutations and cardiomyopathy (66).
CONCLUSION
Collectively, these findings implicate TTR deposition in articular cartilage as a novel mechanism in OA pathogenesis. Recent advances in the development of drug therapies for familial TTR amyloidoses (67–69) may provide new therapeutic options for OA.
Acknowledgments
The authors acknowledge Stuart Duffy and Lilo Creighton for technical assistance.
Funding:
This study was supported by National Institutes of Health grants AG007996 (M.L.) and AG032285 (N.R.), and the Sam and Rose Stein Endowment Fund.
Footnotes
Author contributions:
ML had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study design: ML, YA. Acquisition of data: YA, TM, OA, MO. Analysis and interpretation of data: YA, MO, NR, JB, ML. Manuscript preparation and approval: YA, NR, TM, OA, MO, YI, JB, ML.
Competing interests:
The authors declare that they have no competing financial interests.
Ethics approval:
This study was conducted with the approval of the Scripps Human Subjects Committee.
REFERENCES
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buxbaum JN. The systemic amyloidoses. Curr Opin Rheumatol. 2004;16:67–75. doi: 10.1097/00002281-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 6.Sunk IG, Demetriou D, Szendroedi J, Amoyo L, Raffetseder A, Horl WH, et al. Cartilage biomarkers in hemodialysis patients and the effect of beta2-microglobulin on articular chondrocytes. Osteoarthritis Cartilage. 2008;16:1336–1342. doi: 10.1016/j.joca.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Elsaman AM, Radwan AR, Akmatov MK, Della Beffa C, Walker A, Mayer CT, et al. Amyloid arthropathy associated with multiple myeloma: a systematic analysis of 101 reported cases. Semin Arthritis Rheum. 2013;43:405–412. doi: 10.1016/j.semarthrit.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Reinke AA, Gestwicki JE. Insight into amyloid structure using chemical probes. Chem Biol Drug Des. 2011;77:399–411. doi: 10.1111/j.1747-0285.2011.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bywaters EG, Dorling J. Amyloid deposits in articular cartilage. Ann Rheum Dis. 1970;29:294–306. doi: 10.1136/ard.29.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan MS, Goldenberg DL, Cohen AS, Segal D. The association of amyloid deposits and osteoarthritis. Arthritis Rheum. 1982;25:204–208. doi: 10.1002/art.1780250214. [DOI] [PubMed] [Google Scholar]
- 11.Mitrovic DR, Stankovic A, Quintero M, Ryckewaert A. Amyloid deposits in human knee and hip joints. Rheumatol Int. 1985;5:83–89. doi: 10.1007/BF00270302. [DOI] [PubMed] [Google Scholar]
- 12.Ladefoged C. Amyloid deposits in the knee joint at autopsy. Ann Rheum Dis. 1986;45:668–672. doi: 10.1136/ard.45.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Athanasou NA, Sallie B. Localized deposition of amyloid in articular cartilage. Histopathology. 1992;20:41–46. doi: 10.1111/j.1365-2559.1992.tb00914.x. [DOI] [PubMed] [Google Scholar]
- 14.Niggemeyer O, Steinhagen J, Deuretzbacher G, Zustin J, Ruther W. Amyloid deposition in osteoarthritis of the hip. Arch Orthop Trauma Surg. 2011;131:637–643. doi: 10.1007/s00402-010-1187-z. [DOI] [PubMed] [Google Scholar]
- 15.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornwell GG, 3rd, Sletten K, Johansson B, Westermark P. Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem Biophys Res Commun. 1988;154:648–653. doi: 10.1016/0006-291x(88)90188-x. [DOI] [PubMed] [Google Scholar]
- 17.Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–239. doi: 10.1080/07853890701842988. [DOI] [PubMed] [Google Scholar]
- 18.Ando Y, Nakamura M, Araki S. Transthyretin-related familial amyloidotic polyneuropathy. Arch Neurol. 2005;62:1057–1062. doi: 10.1001/archneur.62.7.1057. [DOI] [PubMed] [Google Scholar]
- 19.Benson MD, Kluve-Beckerman B, Liepnieks JJ, Murrell JR, Hanes D, Uemichi T. Metabolism of amyloid proteins. Ciba Found Symp. 1996;199:104–113. doi: 10.1002/9780470514924.ch7. discussion 13–8. [DOI] [PubMed] [Google Scholar]
- 20.Hurshman AR, White JT, Powers ET, Kelly JW. Transthyretin aggregation under partially denaturing conditions is a downhill polymerization. Biochemistry (Mosc) 2004;43:7365–7381. doi: 10.1021/bi049621l. [DOI] [PubMed] [Google Scholar]
- 21.Akasaki Y, Hasegawa A, Saito M, Asahara H, Iwamoto Y, Lotz MK. Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis. Osteoarthritis Cartilage. 2014;22:162–170. doi: 10.1016/j.joca.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourgault S, Choi S, Buxbaum JN, Kelly JW, Price JL, Reixach N. Mechanisms of transthyretin cardiomyocyte toxicity inhibition by resveratrol analogs. Biochem Biophys Res Commun. 2011;410:707–713. doi: 10.1016/j.bbrc.2011.04.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carames B, Kiosses WB, Akasaki Y, Brinson DC, Eap W, Koziol J, et al. Glucosamine activates autophagy in vitro and in vivo. Arthritis Rheum. 2013;65:1843–1852. doi: 10.1002/art.37977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rappley I, Monteiro C, Novais M, Baranczak A, Solis G, Wiseman RL, et al. Quantification of transthyretin kinetic stability in human plasma using subunit exchange. Biochemistry (Mosc) 2014;53:1993–2006. doi: 10.1021/bi500171j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anoopkumar-Dukie S, Carey JB, Conere T, O'Sullivan E, van Pelt FN, Allshire A. Resazurin assay of radiation response in cultured cells. Br J Radiol. 2005;78:945–947. doi: 10.1259/bjr/54004230. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 27.Felding P, Fex G. Cellular origin of prealbumin in the rat. Biochim Biophys Acta. 1982;716:446–449. doi: 10.1016/0304-4165(82)90040-x. [DOI] [PubMed] [Google Scholar]
- 28.Dickson PW, Howlett GJ, Schreiber G. Rat transthyretin (prealbumin). Molecular cloning, nucleotide sequence, and gene expression in liver and brain. J Biol Chem. 1985;260:8214–8219. [PubMed] [Google Scholar]
- 29.Teng MH, Yin JY, Vidal R, Ghiso J, Kumar A, Rabenou R, et al. Amyloid and nonfibrillar deposits in mice transgenic for wild-type human transthyretin: a possible model for senile systemic amyloidosis. Lab Invest. 2001;81:385–396. doi: 10.1038/labinvest.3780246. [DOI] [PubMed] [Google Scholar]
- 30.Niggemeyer O, Steinhagen J, Zustin J, Ruther W. The value of routine histopathology during hip arthroplasty in patients with degenerative and inflammatory arthritis. Hip Int. 2011;21:98–106. doi: 10.5301/hip.2011.6300. [DOI] [PubMed] [Google Scholar]
- 31.Gu YJ, Ge P, Mu Y, Lu JH, Zheng F, Sun XG. Clinical and laboratory characteristics of patients having amyloidogenic transthyretin deposition in osteoarthritic knee joints. J Zhejiang Univ Sci B. 2014;15:92–99. doi: 10.1631/jzus.B1300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takanashi T, Matsuda M, Yazaki M, Yamazaki H, Nawata M, Katagiri Y, et al. Synovial deposition of wild-type transthyretin-derived amyloid in knee joint osteoarthritis patients. Amyloid. 2013;20:151–155. doi: 10.3109/13506129.2013.803190. [DOI] [PubMed] [Google Scholar]
- 33.Sekijima Y, Uchiyama S, Tojo K, Sano K, Shimizu Y, Imaeda T, et al. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol. 2011;42:1785–1791. doi: 10.1016/j.humpath.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Takei Y, Hattori T, Gono T, Tokuda T, Saitoh S, Hoshii Y, et al. Senile systemic amyloidosis presenting as bilateral carpal tunnel syndrome. Amyloid. 2002;9:252–255. doi: 10.3109/13506120209114102. [DOI] [PubMed] [Google Scholar]
- 35.Takei Y, Hattori T, Tokuda T, Matsuda M, Saitoh S, Hoshii Y, et al. Senile systemic amyloidosis starting as bilateral carpal and left ulnar tunnel syndrome. Intern Med. 2003;42:1050–1051. doi: 10.2169/internalmedicine.42.1050. [DOI] [PubMed] [Google Scholar]
- 36.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly JW. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr Opin Struct Biol. 1998;8:101–106. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 38.Quintas A, Vaz DC, Cardoso I, Saraiva MJ, Brito RM. Tetramer dissociation and monomer partial unfolding precedes protofibril formation in amyloidogenic transthyretin variants. J Biol Chem. 2001;276:27207–27213. doi: 10.1074/jbc.M101024200. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Buxbaum JN, Reixach N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry (Mosc) 2013;52:1913–1926. doi: 10.1021/bi301313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito S, Ando Y, Nakamura M, Ueda M, Kim J, Ishima Y, et al. Effect of nitric oxide in amyloid fibril formation on transthyretin-related amyloidosis. Biochemistry (Mosc) 2005;44:11122–11129. doi: 10.1021/bi050327i. [DOI] [PubMed] [Google Scholar]
- 41.Noborn F, O'Callaghan P, Hermansson E, Zhang X, Ancsin JB, Damas AM, et al. Heparan sulfate/heparin promotes transthyretin fibrillization through selective binding to a basic motif in the protein. Proc Natl Acad Sci U S A. 2011;108:5584–5589. doi: 10.1073/pnas.1101194108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourgault S, Solomon JP, Reixach N, Kelly JW. Sulfated glycosaminoglycans accelerate transthyretin amyloidogenesis by quaternary structural conversion. Biochemistry (Mosc) 2011;50:1001–1015. doi: 10.1021/bi101822y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayliss MT, Osborne D, Woodhouse S, Davidson C. Sulfation of chondroitin sulfate in human articular cartilage. The effect of age, topographical position, and zone of cartilage on tissue composition. J Biol Chem. 1999;274:15892–15900. doi: 10.1074/jbc.274.22.15892. [DOI] [PubMed] [Google Scholar]
- 44.Burkhardt D, Michel BA, Baici A, Kissling R, Theiler R. Comparison of chondroitin sulphate composition of femoral head articular cartilage from patients with femoral neck fractures and osteoarthritis and controls. Rheumatol Int. 1995;14:235–241. doi: 10.1007/BF00262089. [DOI] [PubMed] [Google Scholar]
- 45.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(Pt 1):345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toyama BH, Hetzer MW. Protein homeostasis: live long, won't prosper. Nat Rev Mol Cell Biol. 2013;14:55–61. doi: 10.1038/nrm3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takinami Y, Yoshimatsu S, Uchiumi T, Toyosaki-Maeda T, Morita A, Ishihara T, et al. Identification of potential prognostic markers for knee osteoarthritis by serum proteomic analysis. Biomark Insights. 2013;8:85–95. doi: 10.4137/BMI.S11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gobezie R, Kho A, Krastins B, Sarracino DA, Thornhill TS, Chase M, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Res Ther. 2007;9:R36. doi: 10.1186/ar2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10:160–184. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 50.Reixach N, Deechongkit S, Jiang X, Kelly JW, Buxbaum JN. Tissue damage in the amyloidoses: Transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc Natl Acad Sci U S A. 2004;101:2817–2822. doi: 10.1073/pnas.0400062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorgjerd K, Klingstedt T, Lindgren M, Kagedal K, Hammarstrom P. Prefibrillar transthyretin oligomers and cold stored native tetrameric transthyretin are cytotoxic in cell culture. Biochem Biophys Res Commun. 2008;377:1072–1078. doi: 10.1016/j.bbrc.2008.10.121. [DOI] [PubMed] [Google Scholar]
- 52.Jin H, Liang Q, Chen T, Wang X. Resveratrol protects chondrocytes from apoptosis via altering the ultrastructural and biomechanical properties: an AFM study. PLoS One. 2014;9:e91611. doi: 10.1371/journal.pone.0091611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sousa MM, Cardoso I, Fernandes R, Guimaraes A, Saraiva MJ. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am J Pathol. 2001;159:1993–2000. doi: 10.1016/s0002-9440(10)63050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sousa MM, Fernandes R, Palha JA, Taboada A, Vieira P, Saraiva MJ. Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin Leu55Pro. Am J Pathol. 2002;161:1935–1948. doi: 10.1016/S0002-9440(10)64469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buxbaum JN, Tagoe C, Gallo G, Walker JR, Kurian S, Salomon DR. Why are some amyloidoses systemic? Does hepatic "chaperoning at a distance" prevent cardiac deposition in a transgenic model of human senile systemic (transthyretin) amyloidosis? FASEB J. 2012;26:2283–2293. doi: 10.1096/fj.11-189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, et al. Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheum. 2005;52:2376–2385. doi: 10.1002/art.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yammani RR, Carlson CS, Bresnick AR, Loeser RF. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901–2911. doi: 10.1002/art.22042. [DOI] [PubMed] [Google Scholar]
- 59.Steenvoorden MM, Huizinga TW, Verzijl N, Bank RA, Ronday HK, Luning HA, et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–263. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 60.Cecil DL, Johnson K, Rediske J, Lotz M, Schmidt AM, Terkeltaub R. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J Immunol. 2005;175:8296–8302. doi: 10.4049/jimmunol.175.12.8296. [DOI] [PubMed] [Google Scholar]
- 61.Sousa MM, Du Yan S, Fernandes R, Guimaraes A, Stern D, Saraiva MJ. Familial amyloid polyneuropathy: receptor for advanced glycation end products-dependent triggering of neuronal inflammatory and apoptotic pathways. J Neurosci. 2001;21:7576–7586. doi: 10.1523/JNEUROSCI.21-19-07576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monteiro FA, Cardoso I, Sousa MM, Saraiva MJ. In vitro inhibition of transthyretin aggregate-induced cytotoxicity by full and peptide derived forms of the soluble receptor for advanced glycation end products (RAGE) FEBS Lett. 2006;580:3451–3456. doi: 10.1016/j.febslet.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 63.Chen YJ, Sheu ML, Tsai KS, Yang RS, Liu SH. Advanced glycation end products induce peroxisome proliferator-activated receptor gamma down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products. PLoS One. 2013;8:e66611. doi: 10.1371/journal.pone.0066611. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Schelbergen RF, Blom AB, van den Bosch MH, Sloetjes A, Abdollahi-Roodsaz S, Schreurs BW, et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012;64:1477–1487. doi: 10.1002/art.33495. [DOI] [PubMed] [Google Scholar]
- 65.Campo GM, Avenoso A, Campo S, D'Ascola A, Nastasi G, Calatroni A. Small hyaluronan oligosaccharides induce inflammation by engaging both toll-like-4 and CD44 receptors in human chondrocytes. Biochem Pharmacol. 2010;80:480–490. doi: 10.1016/j.bcp.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 66.Hongo M, Kono J, Yamada H, Misawa T, Tanaka M, Nakatsuka T, et al. Doppler echocardiographic assessments of left ventricular diastolic filling in patients with amyloid heart disease. J Cardiol. 1991;21:391–401. [PubMed] [Google Scholar]
- 67.Penchala SC, Connelly S, Wang Y, Park MS, Zhao L, Baranczak A, et al. AG10 inhibits amyloidogenesis and cellular toxicity of the familial amyloid cardiomyopathy-associated V122I transthyretin. Proc Natl Acad Sci U S A. 2013;110:9992–9997. doi: 10.1073/pnas.1300761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. The transthyretin amyloidoses: from delineating the molecular mechanism of aggregation linked to pathology to a regulatory-agency-approved drug. J Mol Biol. 2012;421:185–203. doi: 10.1016/j.jmb.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–2667. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]