Abstract
Objective
To describe factors associated with pressure ulcers in individuals with spina bifida (SB) enrolled in the National Spina Bifida Patient Registry (NSBPR).
Design
Unbalanced longitudinal multicenter cohort study.
Setting
Nineteen SB clinics.
Participants
Individuals with SB (N=3153) enrolled in 19 clinic sites that participate in the NSBPR.
Interventions
Not applicable.
Main Outcome Measures
Pressure ulcer status (yes/no) at the annual visit between 2009 and 2012.
Results
Of 3153 total participants, 19% (n=603) reported ulcers at their most recent annual clinic visit. Seven factors–level of lesion, wheelchair use, urinary incontinence, shunt presence, above the knee orthopedic surgery, recent surgery, and male sex–were significantly associated with the presence of pressure ulcers. Of these factors, level of lesion, urinary incontinence, recent surgery, and male sex were included in the final logistic regression model. The 3 adjusting variables–SB type, SB clinic, and age group–were significant in all analyses (all P<.001).
Conclusions
By adjusting for SB type, SB clinic, and age group, we found that 7 factors–level of lesion, wheelchair use, urinary incontinence, shunt presence, above the knee orthopedic surgery, recent surgery, and male sex–were associated with pressure ulcers. Identifying key factors associated with the onset of pressure ulcers can be incorporated into clinical practice in ways that prevent and enhance treatment of pressure ulcers in the population with SB.
Keywords: Pressure ulcer, Rehabilitation, Risk factors, Spinal cord injuries
Spina bifida (SB) is a neural tube defect that occurs when the spinal column of a developing fetus does not close properly in utero. It affects approximately 3.1 individuals per 10,000 children and adolescents in 10 regions of the United States according to a population-based study.1 Myelomeningocele is the most severe form of SB, in which the vertebrae and spinal canal do not close before birth. Meningocele and lipomyelomeningocele are milder forms. Resulting sequelae of an open neural tube defect may include impairments in mobility, cognition, urinary and fecal continence, and an accumulation of secondary conditions, which may require numerous medical and surgical interventions throughout the life span.2
Pressure ulcers result from prolonged pressure to soft tissue, skin, and muscle. They may occur in individuals who have impairments in sensation or motor function. Each year, more than 2.5 million people develop pressure ulcers in the United States.3 Between 1999 and 2005, the overall pressure ulcer prevalence rate was 15% for hospitalized individuals in 9 international pressure ulcer surveys,4 and 4% in hospitalized children in 2003.5 In individuals with SB, skin wounds are reported as one of the primary diagnoses associated with hospitalizations.6 Pressure ulcers can lead to serious complications such as infection, sepsis, leg amputation, and even death.
The annual prevalence rate of pressure ulcers and other causes of skin breakdown in individuals with myelomeningocele reported in the literature for all ages is between 15% and 77%,7-11 and in adults with SB it is 34%,12 a much higher rate than in the general population. The wide variations in the rates may be attributed to the differences in population age and inclusion criteria over different studies.
Although there are many published articles describing risk factors for skin breakdown including pressure ulcers, there has been a lack of studies focusing exclusively on this topic in the population with SB. A recent cohort study over a 13-year period found that age, wheelchair use, bare feet, obesity, and reduced executive functioning are key risk factors for wound development.13 Another small questionnaire-based study of 87 adults with myelomeningocele showed a higher prevalence of pressure ulcers in individuals with memory deficits, Chiari II malformation, and sensory deficit.14 For young adults with myelomeningocele, the number of ulcers has also been found to be associated with motor and educational level.15
Understanding factors associated with pressure ulcers in this population is critical to help them maintain or improve their health. Using results from a prospective study with an adequate sample size will provide a scientific basis to help influence clinical care. This study aims to explore factors associated with pressure ulcers in individuals enrolled in the National Spina Bifida Patient Registry (NSBPR) in the United States between 2009 and 2012.
Methods
Study population
The sample is composed of participants with SB from 19 clinic sites who participated in the NSBPR between 2009 and 2012. After institutional review board approval and obtaining informed consent/assent from parents and patients, the clinics collected longitudinal data on individuals with SB.16 At the initial visit, basic demographic and diagnostic information as well as information on surgical procedures were collected from each patient. At the initial visit and each subsequent annual visit, information on insurance status, anthropometric measurements, surgeries and procedures, education and employment, and treatments and outcomes was also collected. Depending on when a patient was enrolled, a patient may have had from 1 to 4 submitted annual reports. By the end of 2012, a total of 3738 participants were enrolled in the NSBPR. Of these, 585 participants aged <2 years at enrollment were excluded because mobility status is variable in this age group and for this reason they were not typically evaluated as a cohort. The final analytical data set was composed of 3153 participants’ 5593 annual reports (see supplemental tables S1 and S2, available online only at http://www.archives-pmr.org/).
Variables
SB type and level of lesion
Participants were classified into 2 SB types: myelomeningocele or non-myelomeningocele, which included the diagnoses of meningocele, lipomyelomeningocele, or fatty filum. Each patient's level of lesion was determined on their both right and left sides on the basis of the presence of antigravity strength of muscle groups: hip flexors, quadriceps, dorsiflexors, and plantar flexors. The functional level of lesion was classified into 5 categories–1, sacral, 2, low-lumbar, 3, mid-lumbar, 4, high-lumbar, and 5, thoracic in the order of increasing severity–and used as a continuous variable (1–5) in the analysis. For asymmetric level of lesions, the higher spinal level (ie, more severe) was selected.
Pressure ulcer status
At each annual visit, participants were asked if there have been any pressure ulcers in the past 12 months or since the previous clinic visit.
Surgeries
Six surgical types were considered as potential factors. Chiari II decompression and shunt placement surgeries that occurred from birth to the time of the annual visit were considered as (history) binary indicators. Orthopedic, urology, and tethered cord release surgeries that occurred from the 12 months before enrollment to the time of each annual visit were considered as (partial history) binary indicators. Orthopedic surgeries were divided into 2 categories: above the knee and below the knee. Procedures addressing scoliosis, kyphosis, hip flexion contracture, and hip subluxation/dislocation were classified as above the knee procedures. Correction of knee flexion contracture, external tibial torsion, ankle valgus deformity, equinus contracture, clubfoot deformity, congenital vertical talus, and congenital deformity of foot were considered as below the knee procedures. Lastly, to consider the effect of recent hospitalization on pressure ulcers, a summary variable reflecting any recent surgery since the previous annual visit was also created as a binary variable (yes/no).
Bladder/bowel incontinence
Participants were asked if they were wet during the day with or without interventions for bladder incontinence and if they experienced involuntary stool leakage for bowel incontinence. If they responded yes to either question, they were assigned to the incontinence group for bladder and bowel, respectively.
Ambulatory ability
Participants were grouped into 4 ambulatory categories and then regrouped into 2 groups: community ambulators or wheelchair users. Community ambulators were defined as those who are able to walk indoors and outdoors for most of their activities regardless of whether they use assistive device or braces. Individuals in this group could use a wheelchair, but only for long trips in the community. Otherwise, individuals were classified as wheelchair users.
Insurance type, age group, and race/ethnicity
Medicaid, Medicare, and state high-risk health care plans were classified into public insurance, and all other types of insurance were classified as private. Participants were classified into 1 of 3 groups at each annual visit: group 1 included patients aged 2 to <10 years, group 2 included patients aged 10 to 20 years, and group 3 included patients aged >20 years; age group was treated as a continuous variable (Groups 1–3) in the analysis. With regard to race/ethnicity, participants were classified into non-Hispanic white, non-Hispanic black, Hispanic or Latino, or Asian.
Data analysis
The study population was described by their general characteristics at the most recent annual clinic visit. To examine any association between pressure ulcer status and a candidate factor, multiple logistic regressions were run treating pressure ulcer status as a dependent variable and any given factor as a primary independent variable by using all data. SB type, SB clinic, and age groups were adjusted in the analyses, as we assumed that the variation of ulcers would be explained by SB types, ages, and the unobserved clinic level effects; for example, clinics may treat wounds differently or have a patient population that differs significantly from that in other clinics. We note that a clinic with a sample of size n=42 was set as the reference. To account for repeated measurements per patient over multiple years, generalized estimating equation (GEE) analysis was performed with a compound symmetry correlation structure. The GEE is robust for the misspecification of the covariance structure and is more efficient for large sample sizes compared to the number of repeated measures. For the factors that had significant associations (P<.05) in the analyses, the adjusted prevalence of pressure ulcers was calculated over various age groups by SB type.
In addition, we developed the final model to select the joint model, balancing model complexity, and model fit by applying quasi-likelihood information criteria (QIC).17 The QIC method is a model selection method for GEE models. Including all the significant factors initially, the final model was developed as the one that led to the smallest QIC by comparing several GEE candidate models. All data analyses were performed with SAS version 9.3.18,a
Results
Pressure ulcer prevalence
The final analytical data set was composed of 3153 participants with at least 1 completed initial and annual visit report forms. During the study period, 1036 pressure ulcers occurred in 825 participants (26%). Table 1 lists the general characteristics of participants by pressure ulcer status at the most recent annual clinic visit. Approximately 19% (n=603) of participants reported a pressure ulcer in the past 12 months. The myelomeningocele group had a higher prevalence of pressure ulcers. The prevalence of pressure ulcers increased with a higher level of lesions, gradually increasing from 7% (n=63) in participants with sacral level lesions to 31% (n=169) in participants with thoracic level lesions. In comparison with community ambulators, wheelchair users had an 11% higher prevalence of pressure ulcers. Participants with bladder incontinence were 1.1% more likely to experience pressure ulcers than did participants with bowel incontinence, and overall, ulcers were more common in participants with any incontinence. The prevalence of pressure ulcers was higher in those with a history of neurologic and orthopedic surgeries, non-Hispanic black participants, men, those aged ≥10 years, and those with public insurance only. The mean age was >6.3±2.1 years for patients aged 2 to <10 years, 14.9±2.8 years for patients aged 10 to 20 years, and 27.8±8.8 years for patients aged >20 years.
Table 1.
Sample distribution of factors at the most recent annual clinic visit by pressure ulcer status
| Pressure Ulcer Status |
|||
|---|---|---|---|
| Factor | n (%) | Yes | No |
| 3153 (100) | 603 (19.1) | 2550 (80.9) | |
| SB type | |||
| Myelomeningocele | 2566 (81.4) | 553 (21.5) | 2013 (78.5) |
| Others | 587 (18.6) | 50 (8.5) | 537 (91.5) |
| Level of lesion* | |||
| Sacral | 886 (28.1) | 63 (7.1) | 823 (92.9) |
| Low-lumbar | 565 (17.9) | 89 (15.8) | 476 (84.3) |
| Mid-lumbar | 867 (27.5) | 215 (24.8) | 652 (75.2) |
| High-lumbar | 285 (9.0) | 67 (23.5) | 218 (76.5) |
| Thoracic | 550 (17.4) | 169 (30.7) | 381 (69.3) |
| Wheelchair use | |||
| Yes | 1421 (45.1) | 360 (25.3) | 1061 (74.7) |
| No | 1732 (54.9) | 243 (14.0) | 1489 (86.0) |
| Incontinence | |||
| Urinary | |||
| Yes | 1851 (58.7) | 375 (20.3) | 1476 (79.7) |
| No | 1302 (41.3) | 228 (17.5) | 1074 (82.5) |
| Stool | |||
| Yes | 1662 (52.7) | 319 (19.2) | 1343 (80.8) |
| No | 1491 (47.3) | 284 (19.1) | 1207 (81.0) |
| Neurologic surgery | |||
| Chiari II | |||
| decompression† | |||
| Yes | 235 (7.5) | 64 (27.2) | 171 (72.8) |
| No | 2918 (92.6) | 539 (18.5) | 2379 (81.5) |
| Shunt† | |||
| Yes | 2077 (65.9) | 473 (22.8) | 1604 (77.2) |
| No | 1076 (34.1) | 130 (12.1) | 946 (87.9) |
| Tethered cord | |||
| release‡ | |||
| Yes | 142 (4.5) | 31 (21.8) | 111 (78.2) |
| No | 3011 (95.5) | 572 (19.0) | 2439 (81.0) |
| Orthopedic surgery‡ | |||
| Above the knee | |||
| Yes | 120 (3.8) | 35 (29.2) | 85 (70.8) |
| No | 3033 (96.2) | 568 (18.7) | 2465 (81.3) |
| Below the knee | 211 (6.7) | 50 (23.7) | 161 (76.3) |
| Yes | |||
| No | 2942 (93.3) | 553 (18.8) | 2389 (81.2) |
| Urology surgery‡ | |||
| Yes | 285 (9.0) | 60 (21.0) | 225 (79.0) |
| No | 2868 (91.0) | 543 (18.9) | 2325 (81.1) |
| Recent surgery | |||
| Yes | 649 (20.6) | 149 (23.0) | 500 (77.0) |
| No | 2504 (79.4) | 454 (18.1) | 2050 (81.9) |
| Race/ethnicity | |||
| Non-Hispanic white | 2059 (65.3) | 413 (20.1) | 1646 (79.9) |
| Non-Hispanic black | 256 (8.1) | 59 (23.1) | 197 (76.9) |
| Hispanic or Latino | 651 (20.7) | 103 (15.8) | 548 (84.2) |
| Asian | 130 (4.1) | 14 (10.8) | 116 (89.2) |
| Unknown | 57 (1.8) | 14 (24.6) | 43 (75.4) |
| Sex | |||
| Male | 1494 (47.4) | 299 (20.0) | 1195 (80.0) |
| Female | 1659 (52.6) | 304 (18.3) | 1355 (81.7) |
| Age group (y)* | |||
| 2–<10 | 1177 (37.3) | 144 (12.2) | 1033 (87.8) |
| 10–20 | 1420 (45.0) | 321 (22.6) | 1099 (77.4) |
| >20 | 556 (17.6) | 138 (24.8) | 418 (75.2) |
| Insurance type | |||
| Public | 2066 (65.5) | 414 (20.0) | 1652 (80.0) |
| Private | 1087 (34.5) | 189 (17.4) | 898 (82.6) |
NOTE. Values are n (%). The mean ages for each age group were 6.3±2.1, 14.9±2.8, 27.8±8.8y in an order.
Treated as a continuous variable in the main analysis.
Any surgery history from birth to the last annual visit.
Any surgery history from enrollment to the last annual visit.
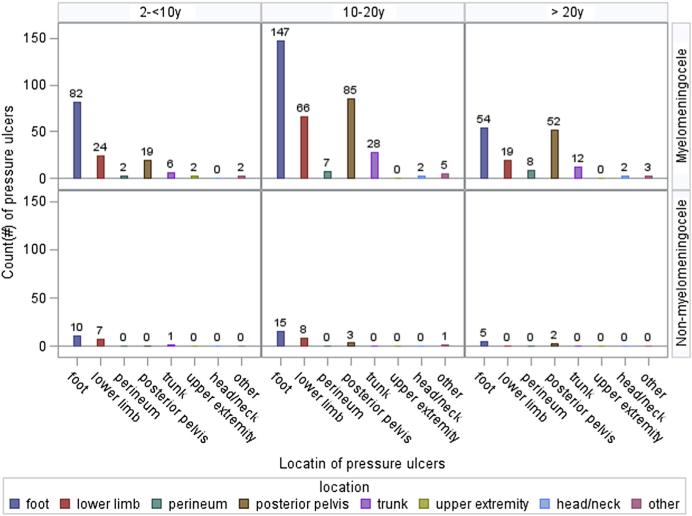
The location of ulcers was most frequent in the areas of the foot, lower limb, or posterior pelvis for all age groups and SB types (fig 1). Participants with myelomeningocele had more ulcers than did participants with non-myelomeningocele. Although participants aged 2 to <10 years had more ulcers on the lower limb than on the posterior pelvis, those aged ≥10 years had more ulcers on the posterior pelvis than on the lower limb.
Fig 1.
Count (#) of pressure ulcers by location for myelomeningocele (top) and others (bottom) at the most recent annual clinic visit.
Pressure ulcer factors
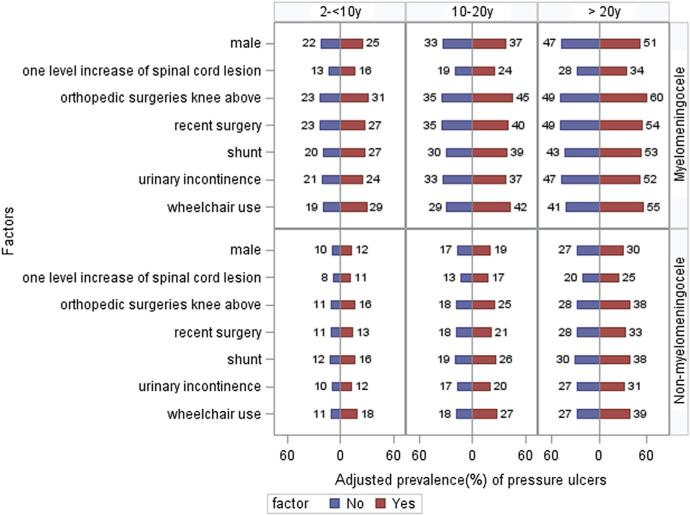
The results of multiple logistic regression using the GEE are summarized in table 2. Adjusting for SB type, SB clinic, and age groups, the factors significantly associated with pressure ulcers were level of lesion, ambulation status, bladder incontinence, shunt placement, above the knee orthopedic surgery, recent surgery, and male sex. For each increase in the functional level of lesion, the odds of pressure ulcers were increased by 34% (P<.001). The odds were 74% higher (P<.001) in wheelchair users and 22% higher (P=.02) in participants with bladder incontinence. The odds of a pressure ulcer were increased by 49% (P<.001) in participants with a history of shunt placement and by 53% (P=.02) in participants with a history of above the knee orthopedic surgery. In men, the odds were 17% (P=.04) higher than those in women. No significant associations were found between pressure ulcer and bowel incontinence, Chiari II decompression, urology surgery, tethered cord release, below the knee orthopedic surgery, race/ethnicity, or insurance type. The 3 adjusting variables–SB type, SB clinic, and age groups–were all significant in all analyses (data not shown). To facilitate the interpretation of these results, we illustrated the adjusted prevalence of the pressure ulcer status for the 7 significantly associated factors by their values (yes/no) from the fitted model over various age groups in figure 2. Overall, the prevalence of pressure ulcers in participants with myelomeningocele was higher than that in participants with non-myelomeningocele. Also, as the age increased within both myelomeningocele and non-myelomeningocele groups, the prevalence of pressure ulcers increased gradually. For example, in wheelchair users with myelomeningocele, the pressure ulcer prevalence was 29% in the age group of 2 to 10 years, 42% in the age group of 10–20 years, and 55% in the age group of >20 years. In their peers with non-myelomeningocele conditions, the pressure ulcer prevalence was 18%, 27%, and 39%, respectively. In participants with myelomeningocele with shunt placement, the adjusted prevalence of pressure ulcers was 7% higher in the age group of 2 to 10 years, 9% higher in the age group of 10 to 20 years, and 10% higher in the age group of >20 years as compared with those who were not shunted.
Table 2.
OR (95% CI) from multiple logistic regression models and the final model, controlling for SB type, SB clinic, and age groups
| Multiple Logistic Regression Models |
Final Model |
|||
|---|---|---|---|---|
| Factor | OR (95% CI) | P | OR (95% CI) | P |
| Level of lesion | 1.34 (1.27–1.43) | <.001 | 1.34 (1.26–1.42) | <.001 |
| Wheelchair use | 1.74 (1.47–2.06) | <.001 | NA | |
| Urinary incontinence | 1.22 (1.04–1.43) | .02 | 1.15 (0.98–1.35) | .09 |
| Stool incontinence | 1.05 (0.90–1.23) | .51 | NA | |
| Chiari II decompression | 1.08 (0.83–1.40) | .57 | NA | |
| Tethered cord release | 1.21 (0.85–1.73) | .29 | NA | |
| Shunt | 1.49 (1.17–1.90) | <.001 | NA | |
| Above the knee orthopedic surgery | 1.53 (1.06–2.20) | .02 | NA | |
| Below the knee orthopedic surgery knee | 1.23 (0.93–1.63) | .14 | NA | |
| Urology surgery | 1.24 (0.97–1.60) | .09 | NA | |
| Recent surgery | 1.26 (1.06–1.49) | .01 | 1.18 (0.99–1.40) | .06 |
| Race/ethnicity | NA | |||
| Non-Hispanic white | Reference | NA | NA | |
| Non-Hispanic black | 0.97 (0.72–1.30) | .84 | NA | |
| Hispanic or Latino | 0.91 (0.72–1.16) | .44 | NA | |
| Asian | 0.96 (0.59–1.55) | .85 | NA | |
| Sex: male | 1.17 (1.00–1.37) | .04 | 1.18 (1.01–1.39) | .04 |
| Public insurance | 1.15 (0.97–1.36) | .11 | NA | |
Abbreviation: NA, not applicable.
Fig 2.
Adjusted prevalence (%) of pressure ulcers by associated factors over various age groups for myelomeningocele (top) and others (bottom), adjusting SB clinic. Adjusted prevalence was calculated using GEE analysis. Each model included the factor of interest, age group, SB type, and SB clinic.
Our final model included level of lesion, urinary incontinence, recent surgery, and male sex, adjusting for SB type, SB clinic, and age groups (see table 2). Higher level of lesion and male sex including SB type, age group, and SB clinic were still significantly associated with pressure ulcers (all P<.05): the odds of pressure ulcer were 55% higher for the myelomeningocele group (odds ratio [OR], 1.55; 95% confidence interval [CI], 1.17–2.04) and 68% higher for one age group increase (OR, 1.68; 95% CI, 1.48–1.92) (not shown in table 2). The ORs for 18 clinics versus the referred clinic are presented in figure 3 (ORs, 0.08–1.16). The ORs excluding 1.00 in the 95% CI are significant.
Fig 3.
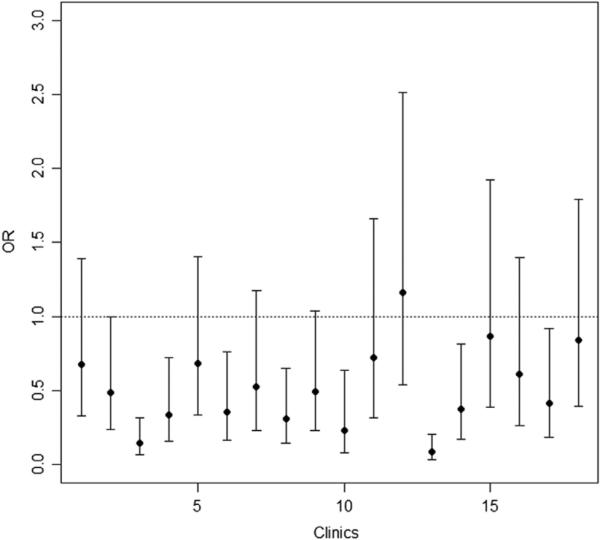
OR (95% CI) for SB clinics in the final model.
Discussion
Pressure ulcers were more common in individuals with a higher level of lesions in the study, which is consistent with the findings of Ottolini et al.13 In general, higher lesion levels are generally associated with higher degrees of paralysis and sensory loss, which may explain the higher rates of pressure ulcers. Wheelchair use was also associated with ulcers, which is not surprising because prolonged sitting and immobility may predispose to pressure ulcers. Among demographic factors, male sex was associated with the occurrence of pressure ulcers. Similar findings for higher risk of ulcers in men were reported among palliative home care clients,19 adults in acute care hospitals,20 and individuals with spinal cord injury,21 where a higher level of care requirements19 and poor nutrition20 compared to women were discussed as possible reasons. After neurological surgeries, we found that ulcers were more likely to occur in the shunted group.13 Bladder incontinence was another factor associated with ulcers, whereas bowel incontinence was not. Work by others has suggested that urinary incontinence may be a more accurate predictor than fecal incontinence in individuals with spinal cord injury,22,23 and it has been well demonstrated that incontinence and moisture are strongly associated with pressure ulcers. For orthopedic surgeries, only above the knee procedures were related to ulcers. Although the interactions of mobility status, orthopedic surgery, bracing, and pressure ulcers are likely quite complex, it is possible that individuals undergoing orthopedic procedures above the knee are more vulnerable to pressure ulcers because their mobility may be more limited than those who have procedures exclusively below the knee. Any recent surgery was also related to ulcers. This may indicate a hospital-acquired pressure ulcer related to some posthospitalization circumstance. This cannot be clearly explained using these data because the date of the pressure ulcer occurrence was unknown.
Interestingly, a few studies reported that shunt, wheelchair use, sex,14 and level of lesion24 were not associated with pressure ulcers in individuals with SB, which was in contrast to our findings of this study. However, these studies may lack the statistical power to reveal the risk factors for ulcers because of small sample sizes (n=87 or 66), and the lack of significance in small studies should not be considered as evidence that the factors are not a risk factor.
In all analyses, we adjusted for SB type, age groups, and SB clinic, and these estimates were all statistically significant. As expected, individuals with myelomeningocele are more limited in their ambulation than those of the non-myelomeningocele group; pressure ulcers were more prevalent in those with myelomeningocele.11,25 The significant relation of the age to pressure ulcers corresponds with findings of a previous study,26 but in our study, ulcers are most common in adults aged >20 years, not in adolescents. Interestingly, the SB clinic is significant, possibly because of clinical variations in diagnosis, skin inspection, patient/parent education, and pressure ulcer treatments. For example, in some clinics, skin care specialists may check the patients during their regular visit, but in other clinics, ulcers may be reported by patients only when asked by clinicians.
Study limitations
Our study has several limitations. First, participants were not asked to record exact dates of their surgeries and pressure ulcers; therefore, some surgical procedures potentially occurred after participants developed pressure ulcers, although all occurred in the same year. Second, although pressure ulcers reported by a participant and by examination are different entities, we could not separate these from our data. Third, although long-term prospective data are well suited to address causality of the factors, we could review NSBPR data only over a 4-year period at this time. However, annual data continue to be collected up to 2019 (the currently funded period of this longitudinal data collection) including exact dates of the surgical procedures extracted from medical records, so the risk factors for ulcers may be better understood in a future analysis. Fourth, the impact of mobility category on pressure ulcers was assessed with only wheelchair use, whereas orthoses and other devices can also cause pressure or shear that can lead to ulcers. Fifth, we did not distinguish between factors related to initial ulcers versus those related to recurrence of ulcers. Ulcer recurrences were previously found in 53% of individuals with SB.13 We also did not distinguish between factors related to multiple ulcers. We expect that future studies with more (longitudinal) data will address these issues better. Sixth, the prevalence of pressure ulcers is possibly underestimated for the general population with SB because participants may not report their ulcers during an annual visit unless they were routinely examined by clinicians. Finally, we did not adjust for multiple testing such as Bonferroni correction because this study was exploratory in nature and we did not posit a strong assumption of no association between pressure ulcers and a factor.
Conclusions
Recent pressure ulcers were present in 19% of the total sample at the mean age of 14 years, present in 22% for myelomeningocele and 9% for non-myelomeningocele. Factors with statistically significant associations with pressure ulcers were level of lesion, wheelchair use, urinary incontinence, presence of shunt, recent surgery, and male sex. Of these, level of lesion, urinary incontinence, recent surgery, and sex were also included in the final model. Approximately 75% to 85% of individuals born with SB are now surviving into adulthood, likely owing to advances in medical and surgical care.27,28 This elevates the importance of dealing with the morbidity associated with pressure ulcers in a longer living population. When considering prevention and treatments for pressure ulcers, more importance should be placed on the factors identified in these models of association.
Supplementary Material
Acknowledgments
We thank all members of the National Spina Bifida Patient Registry (NSBPR) Coordinating Committee who have participated in the development of the NSBPR: William Walker, MD, Seattle Children's Hospital; Kathryn Smith, RN, DrPH, Children's Hospital Los Angeles; Kurt Freeman, PhD, ABPP, Oregon Health & Science University; Pamela Wilson, MD, Children's Hospital Colorado; Kathleen Sawin, PhD, CPNP-PC, FAAN, Children's Hospital of Wisconsin; Jeffrey Thomson, MD, Connecticut Children's Medical Center; Heidi Castillo, MD, Cincinnati Children's Hospital Medical Center; Timothy Brei, MD, Riley Hospital for Children; David Joseph, MD, Children's Hospital of Alabama; Elaine Pico, MD, Children's Hospital and Research Center at Oakland; Mitul Kapadia, MD, University of California, San Francisco; Robin Bowman, MD, Ann & Robert H. Lurie Children's Hospital of Chicago; John Wiener, MD, Duke University; Paula Peterson, PNP, Primary Children's Medical Center; Mark Dias, MD, Pennsylvania State University, Hershey Medical Center; Karen Ratliff-Schaub, MD, Nationwide Children's Hospital; Brad Dicianno, MD, University of Pittsburgh; James Chinarian, MD, Wayne State University; and The Spina Bifida Association.
Supported by the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA (grant no. 1UO1DDD000744.01). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
List of abbreviations
- CI
confidence interval
- GEE
generalized estimating equation
- NSBPR
National Spina Bifida Patient Registry
- OR
odds ratio
- QIC
quasi-likelihood information criteria
- SB
spina bifida
Footnotes
Ward is consultant to Centers for Disease Control and Prevention.
Disclosures: none.
Supplier
SAS Institute Inc.
References
- 1.Shin M, Besser LM, Siffel C, et al. Congenital Anomaly Multistate Prevalence and Survival Collaborative. Prevalence of spina bifida among children and adolescents in 10 regions in the United States. Pediatrics. 2010;126:274–9. doi: 10.1542/peds.2009-2084. [DOI] [PubMed] [Google Scholar]
- 2.Dicianno BE, Kurowski BG, Yang JM, et al. Rehabilitation and medical management of the adult with spina bifida. Am J Phys Med Rehabil. 2008;87:1027–50. doi: 10.1097/PHM.0b013e31818de070. [DOI] [PubMed] [Google Scholar]
- 3.Berlowitz D, VanDeusen Lukas C, Parker V, et al. Preventing pressure ulcers in hospitals: a toolkit for improving quality of care. Agency for Healthcare Research and Quality; Rockville: Apr, 2011. Available at: http://www.ahrq.gov/professionals/systems/long-term-care/resources/pressure-ulcers/pressureulcertoolkit/index.html. Accessed July 12, 2014. [Google Scholar]
- 4.Vangilder C, Macfarlane GD, Meyer S. Results of nine international pressure ulcer prevalence surveys: 1989 to 2005. Ostomy Wound Manage. 2008;54:40–54. [PubMed] [Google Scholar]
- 5.McLane KM, Bookout K, McCord S, McCain J, Jefferson LS. The 2003 national pediatric pressure ulcer and skin breakdown prevalence survey: a multisite study. J Wound Ostomy Continence Nurs. 2004;31:168–78. doi: 10.1097/00152192-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Dicianno BE, Wilson R. Hospitalizations of adults with spina bifida and congenital spinal cord anomalies. Arch Phys Med Rehabil. 2010;91:529–35. doi: 10.1016/j.apmr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhoef M, Barf HA, Post MW, van Asbeck FW, Gooskens RH, Prevo AJ. Secondary impairments in young adults with spina bifida. Dev Med Child Neurol. 2004;46:420–7. doi: 10.1017/s0012162204000684. [DOI] [PubMed] [Google Scholar]
- 8.Farley T, Vines C, McCluer S, Stefans V, Hunter J. Secondary disabilities in Arkansas with spina bifida. Eur J Pediatr Surg. 1994;4:39–40. [PubMed] [Google Scholar]
- 9.Diaz Llopis I, Bea Munoz M, Martinez Agullo E, Lopez Martinez A, GarciaAymerich V, Forner Valero JV. Ambulation in patients with myelomeningocele: a study of 1500 patients. Paraplegia. 1993;31:28–32. doi: 10.1038/sc.1993.5. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto GA, Lamers JV, Shurtleff DB. Skin breakdown in patients with myelomeningocele. Arch Phys Med Rehabil. 1983;64:20–3. [PubMed] [Google Scholar]
- 11.Curtis BH, Brightman E. Spina bifida: a follow-up of ninety cases. Conn Med. 1962;26:145–50. [Google Scholar]
- 12.McCann JP, McDonnell GV. A ten-year review of adults with spina bifida attending a specialist clinic. Eur J Ped Surg. 2003;13:S50. [Google Scholar]
- 13.Ottolini K, Harris AB, Amling JK, Kennelly AM, Phillips LA, Tosi LL. Wound care challenges in children and adults with spina bifida: an open-cohort study. J Pediatr Rehabil Med. 2013;6:1–10. doi: 10.3233/PRM-130231. [DOI] [PubMed] [Google Scholar]
- 14.Plaum PE, Riemer G, Frøslie KF. Risk factors for pressure sores in adult patients with myelomeningocele—a questionnaire-based study. Cerebrospinal Fluid Res. 2006;3:14. doi: 10.1186/1743-8454-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmood D, Dicianno B, Bellin M. Self-management, preventable conditions and assessment of care among young adults with myelomeningocele. Child Care Health Dev. 2011;37:861–5. doi: 10.1111/j.1365-2214.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 16.Thibadeau JK, Ward EA, Soe MM, et al. Testing the feasibility of a National Spina Bifida Patient Registry. Birth Defects Res A Clin Mol Teratol. 2013;97:36–41. doi: 10.1002/bdra.23094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001;57:120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 18.SAS Institute Inc . SAS/STAT 9.3 user's guide. SAS Institute Inc; Cary: 2011. [Google Scholar]
- 19.Brink P, Smith TF, Linkewich B. Factors associated with pressure ulcers in palliative home care. J Palliat Med. 2006;9:1369–75. doi: 10.1089/jpm.2006.9.1369. [DOI] [PubMed] [Google Scholar]
- 20.Fisher AR, Wells G, Harrison MB. Factors associated with pressure ulcers in adults in acute care hospitals. Adv Skin Wound Care. 2004;17:80–90. doi: 10.1097/00129334-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Vidal J, Sarrias M. An analysis of the diverse factors concerned with the development of pressure sores in spinal cord injured patients. Paraplegia. 1991;29:261–7. doi: 10.1038/sc.1991.37. [DOI] [PubMed] [Google Scholar]
- 22.Byrne DW, Salzberg CA. Major risk factors for pressure ulcers in the spinal cord disabled: a literature review. Spinal Cord. 1996;34:255–63. doi: 10.1038/sc.1996.46. [DOI] [PubMed] [Google Scholar]
- 23.Salzberg CA, Byrne DW, Cayten CG, van Niewerburgh P, Murphy JG, Viehbeck M. A new pressure ulcer risk assessment scale for individuals with spinal cord injury. Am J Phys Med Rehabil. 1996;75:96–104. doi: 10.1097/00002060-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Ong LC, Lim YN, Sofiah A. Malaysian children with spina bifida: relationship between functional outcome and level of lesion. Singapore Med J. 2002;43:12–7. [PubMed] [Google Scholar]
- 25.Harris MB, Banta JV. Cost of skin care in the myelomeningocele population. J Pediatr Orthop. 1990;10:355–61. doi: 10.1097/01241398-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Jones ML, Mathewson CS, Adkins VK, Ayllon T. Use of behavioral contingencies to promote prevention of recurrent pressure ulcers. Arch Phys Med Rehabil. 2003;84:796–802. doi: 10.1016/s0003-9993(02)04943-2. [DOI] [PubMed] [Google Scholar]
- 27.Bowman RM, McLone DG, Grant JA, Tomita T, Ito JA. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. 2001;34:114–20. doi: 10.1159/000056005. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee S. Transition to adulthood in spina bifida: changing roles and expectations. ScientificWorldJournal. 2007;7:1890–5. doi: 10.1100/tsw.2007.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.